- 1Department of Biological Psychology, Clinical Psychology, and Psychotherapy, Institute of Psychology, University of Würzburg, Würzburg, Germany
- 2Department of Psychology, Education and Child Studies, Erasmus School of Social and Behavioural Sciences, Erasmus University Rotterdam, Rotterdam, Netherlands
- 3Center of Mental Health (ZEP), University Hospital of Würzburg, Würzburg, Germany
- 4Department of Medical Psychology and Sociology, Medical Faculty, University of Augsburg, Augsburg, Germany
Acceptance-based regulation of pain, which focuses on the allowing of pain and pain related thoughts and emotions, was found to modulate pain. However, results so far are inconsistent regarding different pain modalities and indices. Moreover, studies so far often lack a suitable control condition, focus on behavioral pain measures rather than physiological correlates, and often use between-subject designs, which potentially impede the evaluation of the effectiveness of the strategies. Therefore, we investigated whether acceptance-based strategies can reduce subjective and physiological markers of acute pain in comparison to a control condition in a within-subject design. To this end, participants (N = 30) completed 24 trials comprising 10 s of heat pain stimulation. Each trial started with a cue instructing participants to welcome and experience pain (acceptance trials) or to react to the pain as it is without employing any regulation strategies (control trials). In addition to pain intensity and unpleasantness ratings, heart rate (HR) and skin conductance (SC) were recorded. Results showed significantly decreased pain intensity and unpleasantness ratings for acceptance compared to control trials. Additionally, HR was significantly lower during acceptance compared to control trials, whereas SC revealed no significant differences. These results demonstrate the effectiveness of acceptance-based strategies in reducing subjective and physiological pain responses relative to a control condition, even after short training. Therefore, the systematic investigation of acceptance in different pain modalities in healthy and chronic pain patients is warranted.
Introduction
Pain is an unpleasant sensory and emotional experience (Merskey and Bogduk, 2016), sometimes even referred to as an emotion that involves a physical sensation (Price, 1999; Wieser and Pauli, 2016). Thus, it is not surprising that emotion regulation (ER) strategies (Gross, 1998), which address the modification of affective experiences, are also capable of modulating the perception of pain (Masedo and Esteve, 2007; Braams et al., 2012; Kohl et al., 2013; Hampton et al., 2015). Numerous studies on commonly used ER strategies such as reappraisal and distraction (Gross, 2002; John and Gross, 2004) already demonstrated effective reductions of negative emotions (McRae et al., 2010; Kanske et al., 2011; Webb et al., 2012; Schönfelder et al., 2014) and pain (Van Damme et al., 2008; Verhoeven et al., 2011; Lapate et al., 2012; Hampton et al., 2015). The ability to regulate emotions was shown to correlate with the successful regulation of heat pain stimuli (Lapate et al., 2012), suggesting a general regulation skill for both emotion and pain.
A special case of ER are acceptance-based strategies, which are defined as the embracing of emotions or situations without judging or avoiding them (Hayes et al., 1999; Hofmann and Asmundson, 2008; Braams et al., 2012). The concept of acceptance-based strategies derives from the Acceptance and Commitment Therapy (ACT), a “third wave” cognitive and behavioral treatment approach, which focuses on contextual and experiential changes (Hayes et al., 2006; Hayes and Hofmann, 2017). The general goal of ACT is to increase psychological flexibility – the ability to stay present in the moment and to change or persist value-based behavior (Hayes et al., 2006). Acceptance (Hayes et al., 1999; Hofmann et al., 2009; Braams et al., 2012; Kohl et al., 2013) involves the active and aware embrace of events and is one of six core ACT processes underlying psychological flexibility (Hayes et al., 2006). Two closely related ACT processes and widely used conceptualizations of acceptance-based strategies in emotion and pain regulation research are mindfulness (“being present” and “non-judgmental”) (Braams et al., 2012; Kohl et al., 2013) and cognitive defusion (“decrease in believability of or attachment to an event”) (Kohl et al., 2013).
Even though acceptance-based strategies do not aim at the reduction of emotions or pain, various studies showed that they can alter the pain experience and therefore can be considered a regulation strategy (Kohl et al., 2012). Furthermore, there is an ongoing debate (Hofmann and Asmundson, 2008; Liverant et al., 2008; Hofmann et al., 2009; Wolgast et al., 2011) about the classification of acceptance within the process model of ER by Gross (Gross, 1998), suggesting that acceptance includes both antecedent- and response-focused components (for additional information on the conceptualization of acceptance, see Supplementary Material).
Previous studies found that acceptance-based strategies modulate behavioral pain measures such as pain threshold (PT) and tolerance more profoundly than other ER strategies – designed along the process model of ER – such as suppression of pain-related responses (Masedo and Esteve, 2007; Braams et al., 2012), reappraisal of the pain stimulus (Kohl et al., 2013), and distraction from pain (McMullen et al., 2008; Jackson et al., 2012; Moore et al., 2015). Similarly, so called control-based protocols, which are conceptualized as the exact opposite of ACT (Keogh et al., 2005) by instructing participants to ignore the pain stimulation and stop thinking about it, were found to be less effective in pain tolerance tasks than acceptance-based protocols (Keogh et al., 2005). A meta-analysis by Kohl et al. (2012) suggests that acceptance-based strategies compared to other regulation strategies are especially successful in increasing pain tolerance, while findings involving subjective pain measures such as pain intensity are less clear: acceptance-based strategies led to either decreased pain intensity compared to suppression (Masedo and Esteve, 2007) and control-based protocols (Gutierrez et al., 2004; Keogh et al., 2005), showed no difference when compared to distraction (McMullen et al., 2008; Moore et al., 2015), reappraisal (Kohl et al., 2013), or control-based protocols (Hayes et al., 1999; Paez-Blarrina et al., 2008a, b), or were even less effective than distraction (Kohl et al., 2013).
Most importantly, previous studies often used pre-to-post measurements or control conditions containing either spontaneous coping (Masedo and Esteve, 2007; Evans et al., 2014; Forsyth and Hayes, 2014) or no instructions at all (McMullen et al., 2008; Paez-Blarrina et al., 2008a; Braams et al., 2012). This might have led to an unsystematic use of ill-defined strategies and thus compromised the results. Some studies (Gutierrez et al., 2004; Keogh et al., 2005; Paez-Blarrina et al., 2008a; Kohl et al., 2013) even used no control condition at all, which makes it difficult to determine the actual effectiveness of a regulation strategy. Therefore, we chose to develop and include a neutral control condition to ascertain the effectiveness of acceptance-based strategies.
Only one study so far (Braams et al., 2012) implemented physiological measures to capture the effectiveness of acceptance-based strategies in modulating autonomous pain responses but used a between-subject design. A within-subject design might be better suited to account for potential inter-individual variance regarding physiological responses and regulation skills, which we consequently applied in our study.
In the present study, we compared an acceptance-based strategy with a carefully introduced control condition, where participants should not use any strategies, in a within-subject design. We complemented subjective measures of pain (intensity, unpleasantness) with psychophysiological pain responses (heart rate, HR; skin conductance, SC) (Rhudy et al., 2009; Loggia et al., 2011).
Our main goal was to test the successful reduction of experimentally induced pain by acceptance-based regulation. Thus, we hypothesized the acceptance-based strategy to result in decreased pain ratings and pain-evoked HR and SC responses compared to the control condition.
Methods
Participants
An optimal sample size of 27 participants was calculated a priori using G∗Power (Faul et al., 2009) assuming a medium to large effect size of Cohen’s d of 0.5 (Braams et al., 2012), alpha error of 0.05 (one-tailed paired t-test) and power of 0.8 (Kohl et al., 2013). Potential drop-out was considered and 31 (17 women) participants were recruited via an online platform by the University of Würzburg. They received either course credit or €10 for participation. Participants did not take any central nervous or pain medication and had no current or prior history of chronic pain (self-report). One participant indicated close to no pain sensation (pain intensity: M = 0.67, pain unpleasantness: M = 0.33; VAS 0–100) throughout the entire experiment and was therefore excluded from the final analysis. Thus, 30 participants (16 women; age M = 25.37, SD = 3.58) remained in the statistical analysis. The experimental procedure was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of the medical faculty of the University of Würzburg. All subjects gave written informed consent before participating.
Thermal Pain Stimulation
Pain stimuli were delivered via a thermal stimulator with an active thermode area of 25 × 50 mm (Somedic SenseLab AB, Sösdala, Sweden). The thermode was attached to the volar forearm of the non-dominant hand. We assessed the individual pain threshold (PT) using the method of adjustment (Horn-Hofmann and Lautenbacher, 2015) to take individual differences in pain sensitivity (Nielsen et al., 2009) into account. For that, we instructed participants to adjust the thermode’s temperature – starting at 35°C – by pressing two different buttons (± 0.5°C/keystroke; maximum temperature 49°C) until they reached a level of thermal sensation that went from hot to just painful. This procedure was repeated three times and the average of all three temperatures was used as the final PT (M = 44.87°C, SD = 2.06). During practice trials and the main experiment, pain stimuli were calibrated to the individual PT plus 1°C (target temperature) to achieve a moderate but painful stimulation (Lautenbacher et al., 1995; Horn et al., 2012; Reicherts et al., 2016). Heat stimulation started at a baseline temperature of 10°C below PT and rose at a rate of 5°C/s. Thus, the thermode reached the target temperature after 2.2 s. The target temperature was presented for 10 s. Afterward, the thermode cooled down in 2.2 s to the baseline temperature. The pain stimulus duration of 10 s was chosen following similar experimental designs (Lapate et al., 2012; Prins et al., 2014; Hampton et al., 2015) and was supposed to give the participants sufficient time to engage in the strategy. To prevent habituation to the pain stimulus, the position of the thermode was changed after the PT procedure, after the practice trials and after each 6th trial of the main experiment (starting position was counterbalanced across participants).
Instructions
The acceptance-based strategy was conceptualized along three core ACT processes, namely acceptance, mindfulness and cognitive defusion. Participants were instructed that acceptance involves the allowing of any experiences (acceptance) (Hayes et al., 2006) without further evaluation (mindfulness) (Braams et al., 2012). When participants saw the word “ACCEPT” on the screen, they should let their feelings run their natural course, allow themselves to stay with their emotions (Hofmann et al., 2009) and might employ the “clouds in the sky”-metaphor (Kohl et al., 2013) as a method of detachment from pain (defusion) and to facilitate understanding of the strategy. In the control condition “PERCEIVE,” participants were instructed to sense the pain as it is and not use any strategies. To underscore the distinction between conditions, instructions were briefly summarized: whenever the word “ACCEPT” appeared on the screen, participants should apply the acceptance-based strategy, while no strategy should be used when the word “PERCEIVE” appeared.
Measures
Pain Ratings
Participants were instructed about the distinction of pain intensity and pain unpleasantness using the radio metaphor by Price et al. (1983). During the experiment, participants rated the heat pain stimuli using a digitized visual analog scale (VAS) presented on the screen, ranging from 0 = no pain/not unpleasant at all to 100 = maximum pain/extremely unpleasant, respectively.
Heart Rate
To measure electrocardiography (ECG), three electrodes were attached on the torso of the participant (right collarbone, left lower costal arch, left lower side of the torso). The continuous raw ECG-signal was sampled with 250 Hz, using a V-Amp amplifier and Brain Vision Recorder, V-Amp Edition 1.10, recording software (both Brain Products Inc., Munich, Germany). The signal was filtered (High cut-off: 30 Hz, Notch filter: 50 Hz) (Boucsein, 2012), R-waves were automatically detected and manually checked, the inter-beat intervals were calculated and then converted into the continuous HR (Koers et al., 1999) by the Vision Analyzer software (BrainProducts, Munich, Germany). HR signal was baseline corrected relative to 1 s interval before visual cue onset.
The effectiveness of ER might underlie temporal characteristics such as different strategy onsets, but only few studies have considered temporal dynamics so far (Dan-Glauser and Gross, 2011, 2015; Pavlov et al., 2014; Koval et al., 2015). To capture these, 25 1-s time bins were calculated (Dan-Glauser and Gross, 2015; Koval et al., 2015) by averaging intervals of 1 s, starting at cue onset (second 0) and ending with the offset of the fixation cross (second 25). A broad time interval was analyzed to capture potentially delayed psychophysiological responding following heat pain administration (Loggia et al., 2011). One participant was excluded from psychophysiological analyses due to bad data quality.
Skin Conductance
SC was recorded using two 8 mm Ag/AgCl surface electrodes (electrode gel: 0.5% NaCl) attached to the thenar and hypothenar eminence of the participant’s non-dominant hand. Similar to the ECG signal, the SC signal was sampled with 250 Hz, with constant application of 0.5 V. The signal was filtered (High cut-off: 1 Hz, Notch filter: 50 Hz) (Boucsein, 2012) and baseline corrected relative to 1 s interval before visual cue onset via Vision Analyzer software (BrainProducts, Munich, Germany). Again, 25 1-s bins were calculated to capture potential variations across trial duration, equally to the HR analysis. One participant was excluded from psychophysiological analyses due to bad data quality.
Questionnaires
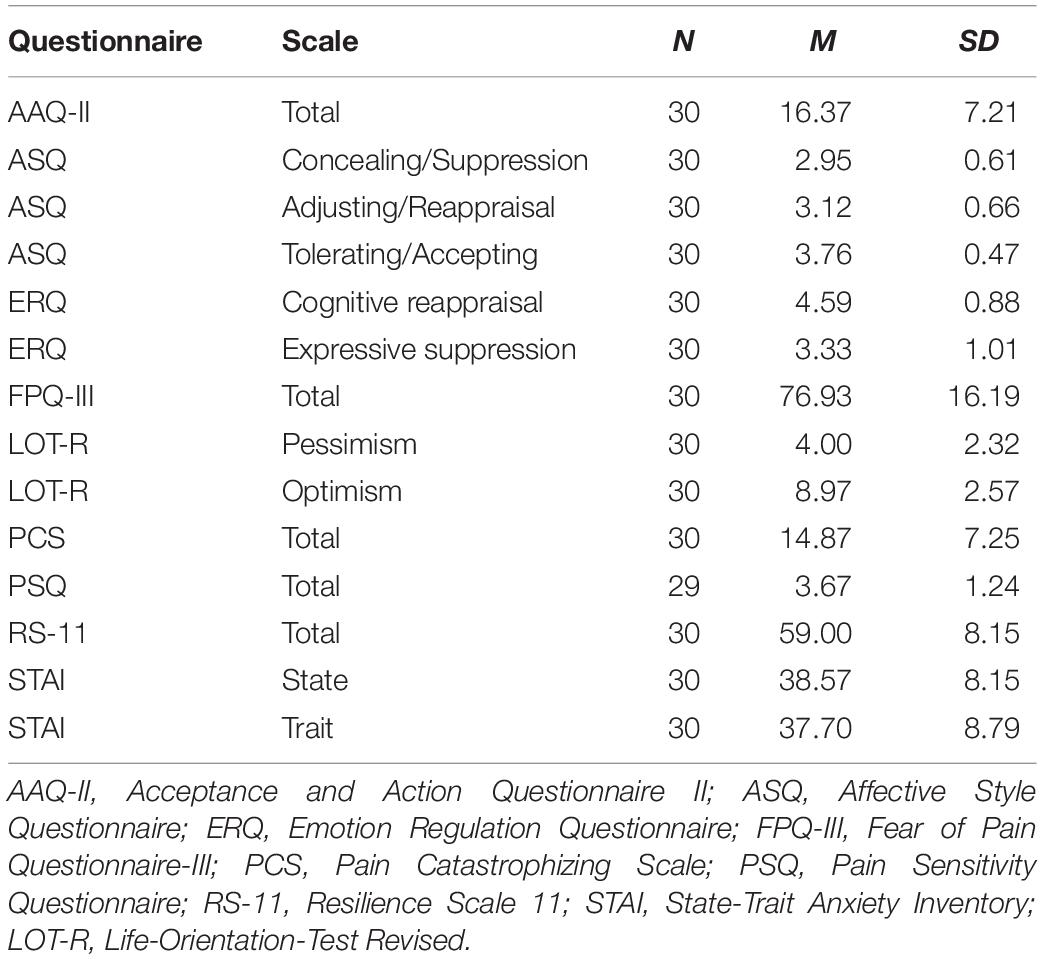
Participants completed several questionnaires addressing habitually preferred ER styles [AAQ-II (Bond et al., 2011; Hoyer and Gloster, 2013), ASQ (Hofmann and Kashdan, 2010; Graser et al., 2012), ERQ (Gross and John, 2003; Abler and Kessler, 2009)], negative affect [STAI (Laux et al., 1981; Spielberger et al., 1983)], attitudes toward pain [FPQ-III (McNeil and Rainwater, 1998; Baum et al., 2013), PCS (Sullivan et al., 1995; Meyer et al., 2008), PSQ (Ruscheweyh et al., 2009)], optimism [LOT-R (Scheier et al., 1994; Glaesmer et al., 2008)] and resilience [RS-11 (Wagnild and Young, 1993; Schumacher et al., 2005)], which are supposed to affect pain and emotion processing, respectively (Rhudy and Meagher, 2000; Rhudy et al., 2004; Forys and Dahlquist, 2007; Geers et al., 2010; Hanssen et al., 2013; Boselie et al., 2014; Hampton et al., 2015; Moore et al., 2015; Biggs et al., 2016; Wieser and Pauli, 2016; Goubert and Trompetter, 2017; Hemington et al., 2017; Hinkle and Quiton, 2019). Questionnaires on ER styles were filled out before the experiment. All remaining questionnaires were presented after the experiment. Mean questionnaire scores and standard deviations are shown in Table 1.
Regulation Ratings/Manipulation Check
After each acceptance trial, participants rated how well they were able to regulate pain by applying the strategy (VAS 0–100; 0 = not at all; 100 = very well). As participants should not regulate pain in the control condition, no regulation ratings were taken. After the experiment, participants filled out a manipulation check survey (MCS) asking on a 9-point rating scale how clear the instructions were (1 = unclear, 9 = clear), how easily they could be implemented (1 = not at all, 9 = very well) and whether participants tried to distract themselves from pain during the main experiment (1 = not at all, 9 = very much).
Procedure
Participants were informed about the details of the experiment and signed a written informed consent. They filled out questionnaires (STAI-S, ERQ, ASQ, and FAH-II) and answered a sociodemographic survey. As soon as they completed the questionnaires, the individual PT was assessed. Afterward, the electrodes for ECG and SC measures were attached. Participants received written standardized instructions on a screen describing the two experimental conditions (“ACCEPT” vs. “PERCEIVE”) and practiced each of them twice. The experimenter made sure that participants fully understood the instructions before starting the main experiment. Participants were separated from the experimenter by a folding screen and interacted with the experimenter solely for the relocation of the thermode. Each trial started with a central fixation cross on a gray screen. After 5 s, either the word “ACCEPT” or “PERCEIVE” appeared in the middle of the screen (cue onset), indicating the two conditions, respectively. The cue remained on the screen for 20 s before disappearing (cue offset). Five seconds after cue onset, the pain stimulation started. After cue and pain offset, a fixation cross was presented for 5 s, followed by the pain intensity and unpleasantness ratings, and the regulation ratings (acceptance only). The subsequent interstimulus interval varied between 15 and 18 s (randomly). The experiment consisted of 24 randomized trials (12 per condition, no more than two trials of the same condition in a row). After the experiment, participants filled out the remaining questionnaires (FPQ-III, PSQ, PCS, STAI-T, LOT-R, RS-11) and the MCS. The experimental procedure was controlled using the software Presentation (Version 17.2, Neurobehavioral Systems Inc., Albany, CA, United States).
Statistical Analysis
Pain ratings (intensity and unpleasantness) were analyzed separately with pairwise t-tests comparing the acceptance vs. control condition. Pain intensity and unpleasantness ratings were compared with each other using pairwise t-tests of z-standardized difference scores between control and acceptance condition. Cohen’s dav was used as a measure of effect size (Cohen, 1988) as recommended by Lakens (2013). For analysis of HR and SC, we used a repeated-measures ANOVA with the within-factor condition (acceptance vs. control) and the within-factor time (twenty-five 1-s bins) and reported partial eta-squared ηp2. In case the assumption of sphericity was violated (Mauchly), the Greenhouse-Geisser correction was applied. Post hoc comparisons of different factor levels were realized using pairwise t-tests. Pearson correlations were conducted to explore the association of pain ratings during the acceptance-based strategy and questionnaire scores (ERQ, ASQ, AAQ-II, STAI, PCS, FPQ-III, PSQ, LOT-R, and RS-11). The regulation ratings were analyzed using a repeated-measures ANOVA with the within-factor trials (4 levels) by averaging three successive trials. Significance level was defined as p < 0.05.
Results
Pain Ratings
Analysis of pain intensity revealed a significant effect of condition, t(29) = 3.23, p = 0.003, dav = 0.217, indicating lower pain intensity ratings for the acceptance compared to the control condition. Similarly, analysis of pain unpleasantness revealed a significant effect of condition, t(29) = 5.26, p < 0.001, dav = 0.484, indicating reduced pain unpleasantness ratings for the acceptance vs. control condition. Mean pain intensity and unpleasantness ratings are shown in Figure 1. Analysis of standardized difference scores yielded a stronger regulatory effect of acceptance for unpleasantness than for intensity pain ratings, t(29) = -3.09, p = 0.004, dav = -0.486.
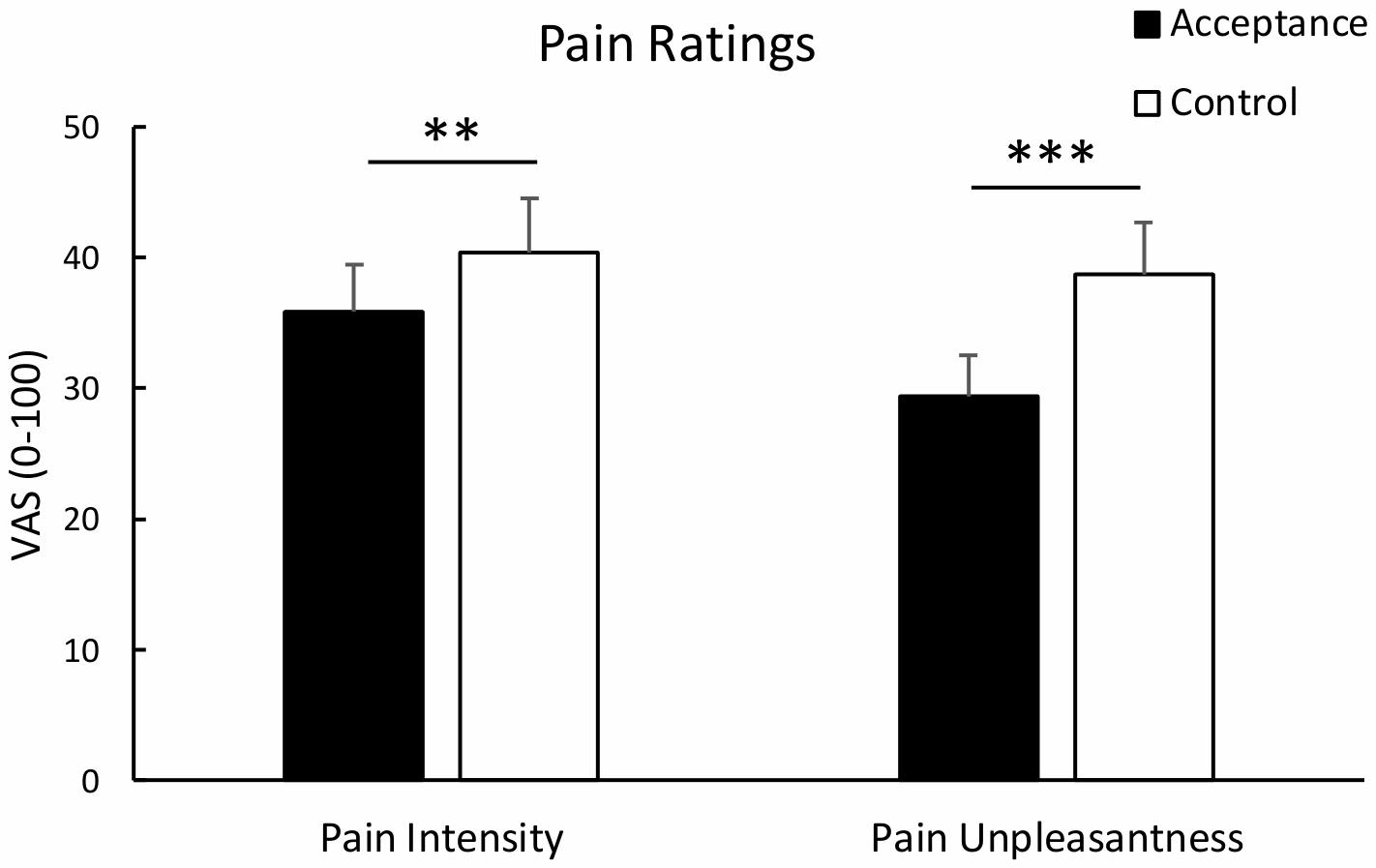
Figure 1. Mean pain intensity and unpleasantness ratings with standard error bars for the acceptance and control condition. Both pain intensity and pain unpleasantness were significantly lower in the acceptance than the control condition. **p < 0.01; ***p < 0.001.
Heart Rate
Analysis of HR revealed no significant main effect of condition, F(1, 28) = 0.76, p = 0.390, ηp2 = 0.027, but a significant main effect of time, F(3.96, 110.98) = 17.14, p < 0.001, ηp2 = 0.380 and a significant interaction of condition and time, F(6.34, 177.56) = 2.46, p = 0.024, ηp2 = 0.081. Post hoc analyses revealed lower HR for the acceptance condition compared to the control condition [second 20, t(28) = -2.10, p = 0.045; second 21, t(28) = -2.22, p = 0.035; second 22, t(28) = -2.00, p = 0.056; second 23, t(28) = -2.03, p = 0.052; second 24, t(28) = -2.12, p = 0.043, 25; t(28) = -1.73, p = 0.094]. The mean time course for both conditions is shown in Figure 2.
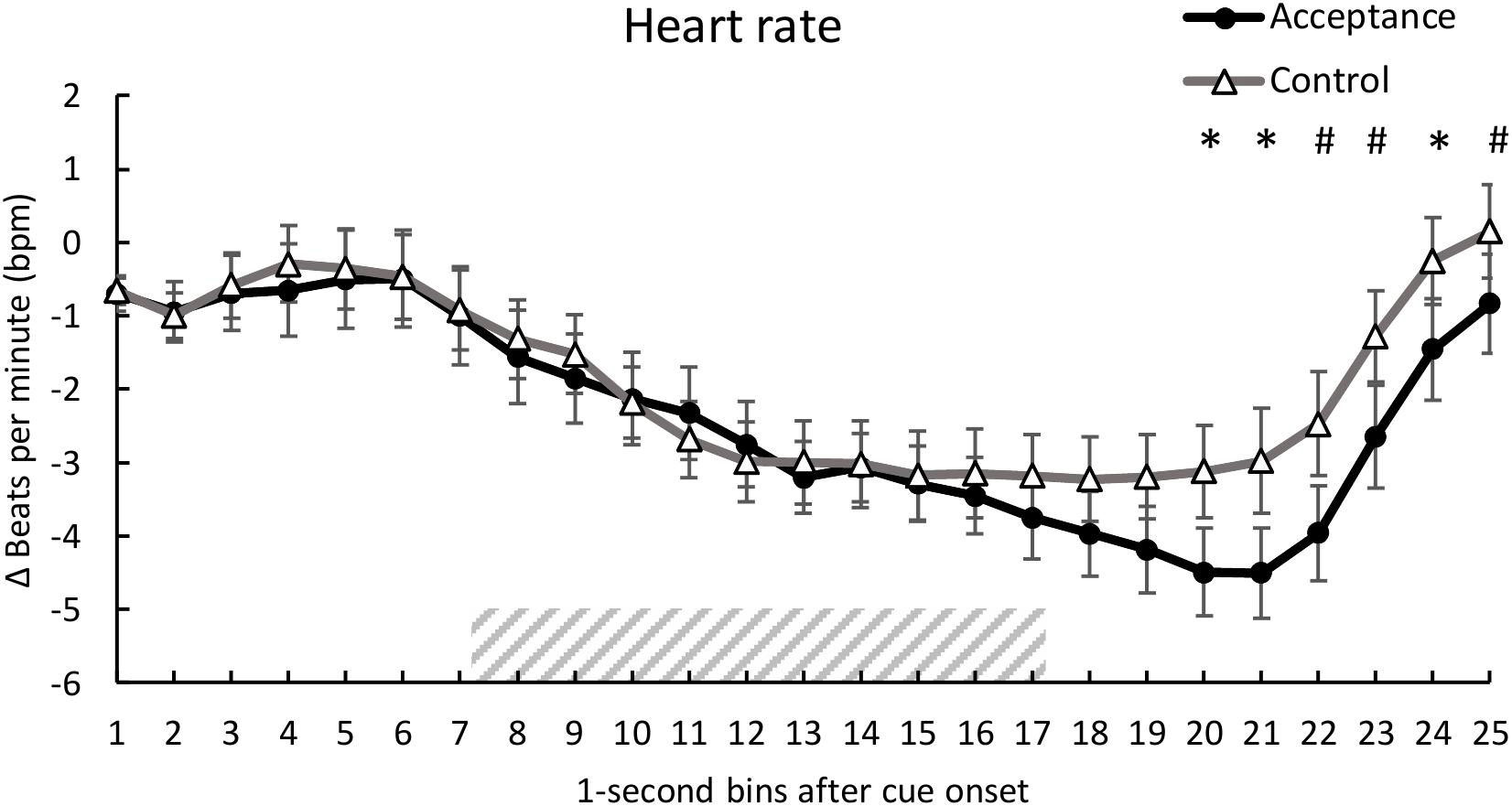
Figure 2. Mean time course (1-s bins) of the heart rate (baseline-corrected 1 s before cue onset) with standard error bars for the acceptance and control trials. The dashed area represents the 10-s heat pain stimulus (7.2 s until 17.2 s after cue onset). There was a significantly lower HR for acceptance compared to the control trials during seconds 20, 21, and 24 of the trial. *p < 0.05; #p < 0.10.
Skin Conductance
Analysis of SC showed no significant main effect of condition, F(1, 28) = 0.10, p = 0.920, ηp2 < 0.01. A significant main effect of time was found, F(1.94, 54.35) = 4.01, p < 0.001, ηp2 = 0.125, indicating a SC reaction to the heat pain stimulus (see Figure 3). There was no significant interaction between condition and time, F(2.97, 83.20) = 0.30, p = 0.846, ηp2 = 0.01.
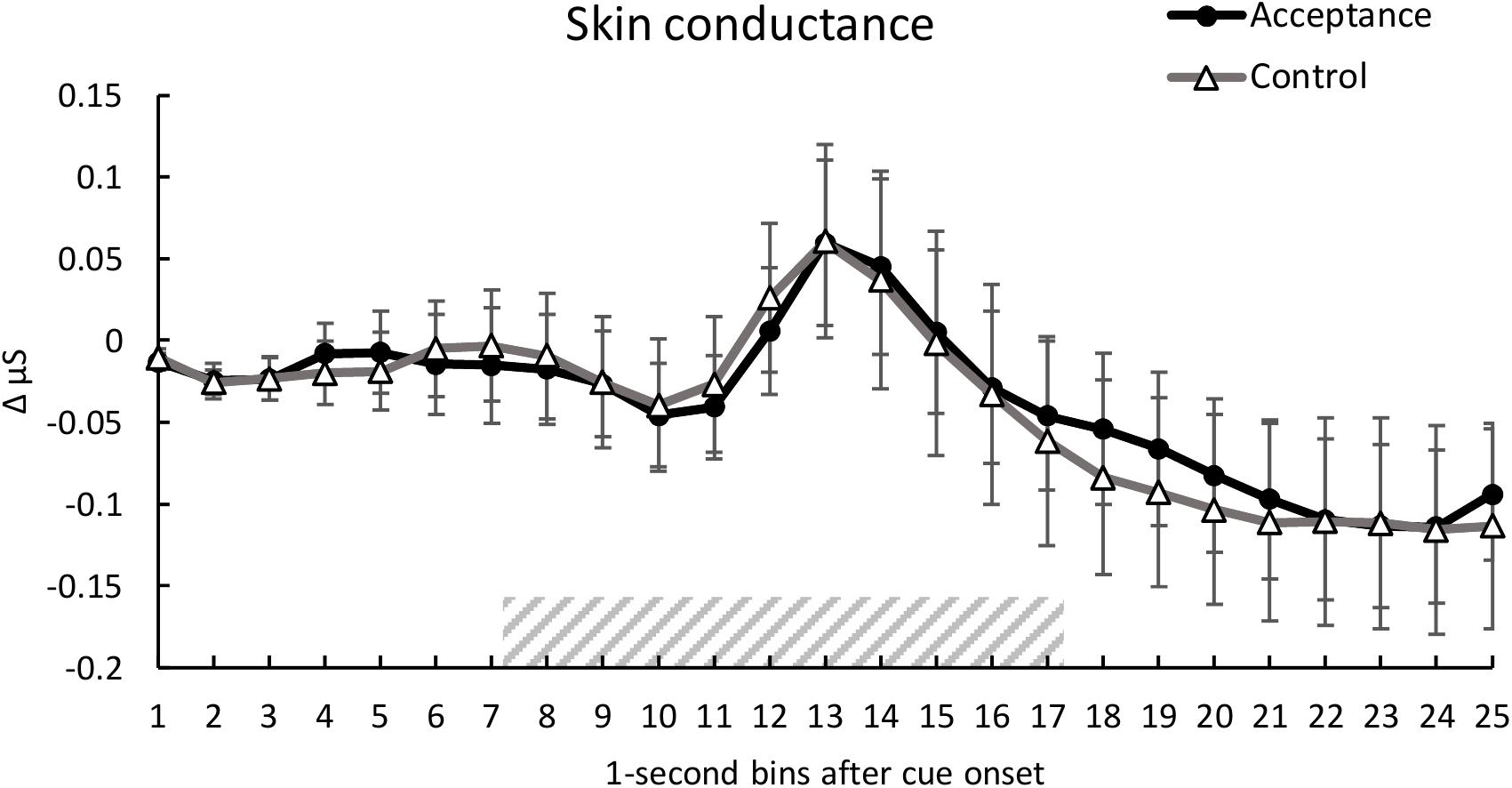
Figure 3. Mean time course (1-s bins) of the skin conductance (baseline-corrected 1 s before cue onset) with standard error bars for the acceptance and control trials. The dashed area represents the 10-s heat pain stimulus (7.2 s until 17.2 s after cue onset). There were no significant differences between the two conditions over time.
Correlations of Pain Ratings and ER Style Questionnaires
Correlation analysis revealed no significant associations between pain ratings of the acceptance condition and ER style questionnaire scores (ERQ subscales reappraisal and suppression; ASQ subscales suppression, reappraisal and accepting; AAQ-II total score) nor the resilience scale RS-11 total score; all ps > 0.063. There were also no significant correlations between the acceptance ratings and the remaining questionnaire scores (STAI state & trait, PCS total, FPQ-III total, PSQ total, LOT-R optimism & pessimism; all ps > 0.083).
Regulation Ratings/Manipulation Check
Analysis of regulation ratings did not show a significant change over time, F(3, 87) = 2.48, p = 0.066, ηp2 = 0.079. However, there was a trend indicating better subjective regulatory performance toward the end of the experiment: Trials 1–3: M = 60.04, SD = 19.72; trials 4–6: M = 58.53, SD = 19.40; trials 7–9: M = 61.34, SD = 16.45; trials 10–12: M = 64.67, SD = 17.79.
In the MCS, participants rated the instructions of acceptance, M = 7.80, SD = 1.10, and the control condition, M = 8.33, SD = 0.84, as rather clear and easy to implement (acceptance: M = 6.87, SD = 1.38; control M = 7.97, SD = 1.19). Further, participants did not distract themselves from heat pain (M = 3.43, SD = 2.11).
Discussion
In the present study, we found that an acceptance-based pain regulation strategy led to a reduced perception of acute heat pain compared to a carefully instructed control condition as indicated by pain intensity and unpleasantness ratings. Also, HR was significantly lower during acceptance-based regulation of pain, while SC responses showed no significant difference between conditions. The present study demonstrates that acceptance-based strategies can modulate subjective and physiological correlates of pain in healthy controls even after brief practice.
Modulation of Pain Ratings by the Acceptance-Based Strategy
Acceptance compared to the control condition led to significantly reduced pain ratings, replicating previous findings (Gutierrez et al., 2004; Keogh et al., 2005; Masedo and Esteve, 2007; Paez-Blarrina et al., 2008a, b; Braams et al., 2012; Kohl et al., 2013). Especially pain unpleasantness was sensitive for the use of the acceptance-based strategy, as indicated by the significant difference across pain rating dimensions.
The pronounced modulation of the affective component of pain is in line with the theoretical foundation of acceptance-based strategies, which aim at changing the behavioral and emotional pain responses rather than its sensory experience (Hayes et al., 1999; Masedo and Esteve, 2007; Kohl et al., 2013). Nevertheless, we found that accepting the heat pain stimulation also decreased sensory aspects of pain. These results resemble the findings by Prins et al. (2014) who showed that a brief mindfulness induction (comprising acceptance-based strategies) led to stronger reductions of pain unpleasantness than pain intensity but only in high pain catastrophizers. The authors point out that the aim of mindfulness is not the reduction of symptoms but instead modifying the experience of the symptoms (Chiesa and Serretti, 2011; Prins et al., 2014), which is likely also the case in acceptance-based strategies.
Kohl et al. (2012) concluded in their meta-analysis that acceptance-based strategies probably are most effective at modulating behavioral pain measures whereas findings concerning pain ratings are rather inconsistent. This heterogeneity might be due to the combination of a pain tolerance task and the subsequent needless measure of pain ratings. One study (Kohl et al., 2013), for instance, found elevated pain tolerance markers and higher pain intensity ratings for a pain acceptance condition. Some previous studies instead demonstrated elevated pain tolerance while pain ratings remained unaffected (Hayes et al., 1999; Keogh et al., 2005) or even were reduced (Masedo and Esteve, 2007). Only one study (Braams et al., 2012) showed reduced pain ratings when investigating acceptance-based strategies by using brief pain stimuli instead of pain tolerance tasks. Future studies should incorporate both subjective pain processing and behavioral pain measures.
Effects of the Acceptance-Based Strategy on Physiological Pain Responses
In our study, we recorded HR and SC as psychophysiological pain responses (Loggia et al., 2011). Contrary to our hypothesis, analysis of SC did not show a significant difference between the acceptance and control condition. However, we found general SC responses following the pain stimulation around 6 s after pain onset, similar to previous studies (Breimhorst et al., 2011). According to the meta-analysis by Kohl et al. (2012), findings regarding the influence of acceptance-based strategies on physiological correlates of emotion and pain are mixed. Several studies investigating acceptance-based strategies in the context of emotion regulation did not find any effects on HR (Eifert and Heffner, 2003; Dunn et al., 2009; Erisman and Roemer, 2010) or SC (Eifert and Heffner, 2003; Campbell-Sills et al., 2006; Erisman and Roemer, 2010). This indicates that accepting a negative affective state, which might also include pain, does not necessarily reduce physiological arousal (Kohl et al., 2012). Loggia et al. (2011) found that HR was a better predictor of pain ratings than SC, which might explain the different effects of the acceptance-based strategy on SC and HR in the present study.
We found the HR to be significantly lower in the acceptance compared to the control condition during cue offset, 3 s after the 10 s pain stimulation. This might indicate that acceptance-based strategies take some time to evolve their effect. Dan-Glauser and Gross (Dan-Glauser and Gross, 2015) did not find any differences between an acceptance-based and a control condition on negative emotions (8 s picture presentation) and concluded that acceptance-based strategies step in rather late in the emotion formation process (Gross, 1998). Similarly, our results might also reflect a later onset of acceptance-based strategy effects on pain. Alternatively, the more pronounced deceleration of the HR in the acceptance condition could reflect a faster recovery from pain. Temporal dynamics in subjective and physiological measures might become more evident in a longer tonic pain stimulation. Thus, different pain durations should be incorporated in future research. Furthermore, larger sample sizes might be helpful in investigating physiological responses, especially SC signals.
The questions remain, whether more training of acceptance-based strategies (Erisman and Roemer, 2010) and more detailed instructions (McMullen et al., 2008) might lead to even clearer subjective and physiological effects. Future research should systematically vary the amount of training prior to the experiment to detect critical aspects underlying the successful use of acceptance-based strategies.
Limitations and Outlook
The present results showed that the use of acceptance-based pain regulation was associated with reductions of subjective and physiological pain responses. The effect of acceptance on psychophysiological pain measures might be further explored using different pain stimulation intensities and modalities or endogenous pain inhibitory indices (Horn-Hofmann et al., 2018). Furthermore, it might be worthwhile comparing an acceptance-based strategy with other well-established regulation strategies such as reappraisal or distraction to identify shared and unique processes involved in the regulation of pain. Given potential gender differences in pain processing and coping (Fillingim et al., 2009), it would be interesting to address them in future pain regulation studies providing sufficiently large sample sizes.
We carefully instructed participants to follow all experimental instructions, and their compliance is supported by both our results and manipulation check. Nevertheless, we cannot completely rule out the use of acceptance during the control condition or alternative coping strategies (Cioffi and Holloway, 1993). In future studies, more detailed post experimental surveys and additional measures of experimental adherence should be employed to detect potential confounds.
An expectancy toward a certain outcome plays a crucial role in the effectiveness of mindfulness and acceptance-based strategies (Brown and Jones, 2010; Zeidan et al., 2012), hence eliminating its effect would be difficult let alone meaningless. However, it would be interesting for future research to capture participants’ expectations regarding the effectiveness of pain regulation strategies systematically.
Although HR and SC serve as reliable psychophysiological indicators of pain responses (Rhudy et al., 2009; Loggia et al., 2011), they undoubtedly capture only a small portion of the processes involved in emotion and pain regulation (Kohl et al., 2012). HR variability, for instance, is a well-established measure of ER (Appelhans and Luecken, 2006) and might be a promising index for the regulation of pain unpleasantness (Appelhans and Luecken, 2008). However, analyzing HR variability would be at the expense of capturing temporal dynamics as its calculation requires prolonged intervals (Shaffer and Ginsberg, 2017).
In the present study, we did not continuously measure subjective pain to avoid distraction from the pain stimulation and to prevent disruption of strategy usage. Nevertheless, continuous ratings [e.g., with rating dials (Hutcherson et al., 2005)] in ER research reliably measured ongoing emotions without interfering with them or the strategy application (Hutcherson et al., 2005; Dan-Glauser and Gross, 2011). Incorporating continuous pain ratings might be a promising tool for future regulation research.
We did not find any associations between ER styles or other psychological factors such as anxiety or pain sensitivity and the effectiveness of the acceptance-based strategy in modulating pain. Yet, individual differences in preferred ER styles could still play a critical role in the effectiveness of pain regulation strategies. This might be especially relevant for research on chronic pain since the habitual use of maladaptive ER strategies, like experiential suppression, could represent a risk factor for pain chronification (Koechlin et al., 2018). Thus, studies using larger sample sizes are necessary to explore the role of psychological traits for pain regulation.
Future research should consider translating similar experimental designs – including carefully prepared control conditions – to chronic pain populations, providing a deeper understanding of the mechanisms involved in successful pain acceptance and advance the development of psychological interventions for chronic pain.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of the medical faculty of the University of Würzburg. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VH organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. All authors wrote sections of the manuscript, contributed to conception and design of the study, manuscript revision, and read and approved the submitted version.
Funding
This publication was funded by the German Research Foundation (DFG) and supported by the Open Access Publication Fund of the University of Würzburg. This work was supported by the Evangelisches Studienwerk e.V. and conducted within the doctoral project “Resilience factors in pain processing.” This work was also supported by the Research Group “Emotion and Behavior,” FOR 605, Wi2714/3-2, which was sponsored by the German Research Foundation (DFG). This project was realized with the support of the structured doctoral programs “Graduate School of Life Sciences (GSLS)” by the University of Würzburg and “Biopsychology of Pain and Emotions” by the Universities of Würzburg and Bamberg.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Christian Kaiser for assisting in data collection and preparation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.01514/full#supplementary-material
Abbreviations
ACT, Acceptance and Commitment Therapy; ECG, electrocardiograph; ER, emotion regulation; HR, heart rate; MCS, manipulation check survey; PT, pain threshold; SC, skin conductance; VAS, visual analog scale.
References
Abler, B., and Kessler, H. (2009). Emotion Regulation Questionnaire - Eine deutschsprachige Fassung des, E. R.Q von Gross und John. Diagnostica 55, 144–152. doi: 10.1026/0012-1924.55.3.144
Appelhans, B. M., and Luecken, L. J. (2006). Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 10, 229–240. doi: 10.1037/1089-2680.10.3.229
Appelhans, B. M., and Luecken, L. J. (2008). Heart rate variability and pain: associations of two interrelated homeostatic processes. Biol. Psychol. 77, 174–182. doi: 10.1016/j.biopsycho.2007.10.004
Baum, C., Schneider, R., Keogh, E., and Lautenbacher, S. (2013). Different stages in attentional processing of facial expressions of pain: a dot-probe task modification. J. Pain 14, 223–232. doi: 10.1016/j.jpain.2012.11.001
Biggs, E. E., Meulders, A., and Vlaeyen, J. W. S. (2016). “The neuroscience of pain and fear,” in The Neuroscience of Pain, Stress, and Emotion: Psychological and Clinical Implications, eds M. A. Flaten and M. al’Absi (London: Elsevier), 133–157. doi: 10.1016/b978-0-12-800538-5.00007-8
Bond, F. W., Hayes, S. C., Baer, R. A., Carpenter, K. M., Guenole, N., Orcutt, H. K., et al. (2011). Preliminary psychometric properties of the acceptance and action questionnaire–II: a revised measure of psychological inflexibility and experiential avoidance. Behav. Ther. 42, 676–688. doi: 10.1016/j.beth.2011.03.007
Boselie, J. J., Vancleef, L. M., Smeets, T., and Peters, M. L. (2014). Increasing optimism abolishes pain-induced impairments in executive task performance. Pain 155, 334–340. doi: 10.1016/j.pain.2013.10.014
Boucsein, W. (2012). Methods of electrodermal recording. Electrodermal Act. 2012, 87–258. doi: 10.1007/978-1-4614-1126-0_2
Braams, B. R., Blechert, J., Boden, M. T., and Gross, J. J. (2012). The effects of acceptance and suppression on anticipation and receipt of painful stimulation. J. Behav. Ther. Exp. Psychiatry 43, 1014–1018. doi: 10.1016/j.jbtep.2012.04.001
Breimhorst, M., Sandrock, S., Fechir, M., Hausenblas, N., Geber, C., and Birklein, F. (2011). Do intensity ratings and skin conductance responses reliably discriminate between different stimulus intensities in experimentally induced pain? J. Pain 12, 61–70. doi: 10.1016/j.jpain.2010.04.012
Brown, C. A., and Jones, A. K. P. (2010). Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain 150, 428–438. doi: 10.1016/j.pain.2010.04.017
Campbell-Sills, L., Barlow, D. H., Brown, T. A., and Hofmann, S. G. (2006). Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behav. Res. Ther. 44, 1251–1263. doi: 10.1016/j.brat.2005.10.001
Chiesa, A., and Serretti, A. (2011). Mindfulness-based interventions for chronic pain: a systematic review of the evidence. J. Altern. Complement. Med. 17, 83–93. doi: 10.1089/acm.2009.0546
Cioffi, D., and Holloway, J. (1993). Delayed costs of suppressed pain. J. Pers. Soc. Psychol. 64, 274–282. doi: 10.1037/0022-3514.64.2.274
Cohen, J. (1988). The Concepts of Power Analysis. Statistical power analysis for the Behavioral Sciences, 2 Edn. New York, NY: Lawrence Erlbaum Associates, 1–17.
Dan-Glauser, E. S., and Gross, J. J. (2011). The temporal dynamics of two response-focused forms of emotion regulation: experiential, expressive, and autonomic consequences. Psychophysiology 48, 1309–1322. doi: 10.1111/j.1469-8986.2011.01191.x
Dan-Glauser, E. S., and Gross, J. J. (2015). The temporal dynamics of emotional acceptance: experience, expression, and physiology. Biol. Psychol. 108, 1–12. doi: 10.1016/j.biopsycho.2015.03.005
Dunn, B. D., Billotti, D., Murphy, V., and Dalgleish, T. (2009). The consequences of effortful emotion regulation when processing distressing material: a comparison of suppression and acceptance. Behav. Res. Ther. 47, 761–773. doi: 10.1016/j.brat.2009.05.007
Eifert, G. H., and Heffner, M. (2003). The effects of acceptance versus control contexts on avoidance of panic-related symptoms. J. Behav. Ther. Exp. Psychiatry 34, 293–312. doi: 10.1016/j.jbtep.2003.11.001
Erisman, S. M., and Roemer, L. (2010). A preliminary investigation of the effects of experimentally induced mindfulness on emotional responding to film clips. Emotion 10, 72–82. doi: 10.1037/a0017162
Evans, D. R., Eisenlohr-Moul, T. A., Button, D. F., Baer, R. A., and Segerstrom, S. C. (2014). Self-regulatory deficits associated with unpracticed mindfulness strategies for coping with acute pain. J. Appl. Soc. Psychol. 44, 23–30. doi: 10.1111/jasp.12196
Faul, F., Erdfelder, E., Buchner, A., and Lang, A. G. (2009). Statistical power analyses using G∗Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160. doi: 10.3758/Brm.41.4.1149
Fillingim, R. B., King, C. D., Ribeiro-Dasilva, M. C., Rahim-Williams, B., and Riley, J. L. III (2009). Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain 10, 447–485. doi: 10.1016/j.jpain.2008.12.001
Forsyth, L., and Hayes, L. L. (2014). The effects of acceptance of thoughts, mindful awareness of breathing, and spontaneous coping on an experimentally induced pain task. Psychol. Rec. 64, 447–455. doi: 10.1007/s40732-014-0010-6
Forys, K. L., and Dahlquist, L. M. (2007). The influence of preferred coping style and cognitive strategy on laboratory-induced pain. Health Psychol. 26, 22–29. doi: 10.1037/0278-6133.26.1.22
Geers, A. L., Wellman, J. A., Fowler, S. L., Helfer, S. G., and France, C. R. (2010). Dispositional optimism predicts placebo analgesia. J. Pain 11, 1165–1171. doi: 10.1016/j.jpain.2010.02.014
Glaesmer, H., Hoyer, J., Klotsche, J., and Herzberg, P. Y. (2008). The German version of the Life-Orientation-Test (LOT-R) for dispositional optimism and pessimism. Z. Gesundheitspsychol. 16, 26–31. doi: 10.1026/0943-8149.16.1.26
Goubert, L., and Trompetter, H. (2017). Towards a science and practice of resilience in the face of pain. Eur. J. Pain 21, 1301–1315. doi: 10.1002/ejp.1062
Graser, J., Bohn, C., Kelava, A., Schreiber, F., Hofmann, S. G., and Stangier, U. (2012). Affective Style Questionnaire - deutsche Fassung. Diagnostica 58, 100–111. doi: 10.1026/0012-1924/a000056
Gross, J. J. (1998). The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 2, 271–299. doi: 10.1037/1089-2680.2.3.271
Gross, J. J. (2002). Emotion regulation: affective, cognitive, and social consequences. Psychophysiology 39, 281–291. doi: 10.1017/S0048577201393198
Gross, J. J., and John, O. P. (2003). Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 85, 348–362. doi: 10.1037/0022-3514.85.2.348
Gutierrez, O., Luciano, C., Rodriguez, M., and Fink, B. C. (2004). Comparison between an acceptance-based and a cognitive-control-based protocol for coping with pain. Behav. Ther. 35, 767–783. doi: 10.1016/s0005-7894(04)80019-4
Hampton, A. J., Hadjistavropoulos, T., Gagnon, M. M., Williams, J., and Clark, D. (2015). The effects of emotion regulation strategies on the pain experience: a structured laboratory investigation. Pain 156, 868–879. doi: 10.1097/j.pain.0000000000000126
Hanssen, M. M., Peters, M. L., Vlaeyen, J. W. S., Meevissen, Y. M. C., and Vancleef, L. M. G. (2013). Optimism lowers pain: evidence of the causal status and underlying mechanisms. Pain 154, 53–58. doi: 10.1016/j.pain.2012.08.006
Hayes, S. C., Bissett, R. T., Korn, Z., Zettle, R. D., Rosenfarb, I. S., Cooper, L. D., et al. (1999). The impact of acceptance versus control rationales on pain tolerance. Psychol. Rec. 49, 33–47. doi: 10.1007/BF03395305
Hayes, S. C., and Hofmann, S. G. (2017). The third wave of cognitive behavioral therapy and the rise of process-based care. World Psychiatry 16, 245–246. doi: 10.1002/wps.20442
Hayes, S. C., Luoma, J. B., Bond, F. W., Masuda, A., and Lillis, J. (2006). Acceptance and commitment therapy: model, processes and outcomes. Behav. Res. Ther. 44, 1–25. doi: 10.1016/j.brat.2005.06.006
Hemington, K. S., Cheng, J. C., Bosma, R. L., Rogachov, A., Kim, J. A., and Davis, K. D. (2017). Beyond negative pain-related psychological factors: resilience is related to lower pain affect in healthy adults. J. Pain 18, 1117–1128. doi: 10.1016/j.jpain.2017.04.009
Hinkle, C. E., and Quiton, R. L. (2019). Higher dispositional optimism predicts lower pain reduction during conditioned pain modulation. J Pain 20, 161–170. doi: 10.1016/j.jpain.2018.08.006
Hofmann, S. G., and Asmundson, G. J. G. (2008). Acceptance and mindfulness-based therapy: new wave or old hat? Clin. Psychol. Rev. 28, 1–16. doi: 10.1016/j.cpr.2007.09.003
Hofmann, S. G., Heering, S., Sawyer, A. T., and Asnaani, A. (2009). How to handle anxiety: the effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behav. Res. Ther. 47, 389–394. doi: 10.1016/j.brat.2009.02.010
Hofmann, S. G., and Kashdan, T. B. (2010). The Affective Style Questionnaire: development and psychometric properties. J. Psychopathol. Behav. Assess. 32, 255–263. doi: 10.1007/s10862-009-9142-4
Horn, C., Blischke, Y., Kunz, M., and Lautenbacher, S. (2012). Does pain necessarily have an affective component? Negative evidence from blink reflex experiements. Pain Res. Manag. 17, 15–24. doi: 10.1155/2012/478019
Horn-Hofmann, C., Kunz, M., Madden, M., Schnabel, E. L., and Lautenbacher, S. (2018). Interactive effects of conditioned pain modulation and temporal summation of pain-the role of stimulus modality. Pain 159, 2641–2648. doi: 10.1097/j.pain.0000000000001376
Horn-Hofmann, C., and Lautenbacher, S. (2015). Modulation of the startle reflex by heat pain: does threat play a role? Eur. J. Pain 19, 216–224. doi: 10.1002/ejp.539
Hoyer, J., and Gloster, A. T. (2013). Psychologische Flexibilität messen: der Fragebogen zu Akzeptanz und Handeln, I. I. Verhaltenstherapie 23, 42–44. doi: 10.1159/000347040
Hutcherson, C. A., Goldin, P. R., Ochsner, K. N., Gabrieli, J. D., Barrett, L. F., and Gross, J. J. (2005). Attention and emotion: does rating emotion alter neural responses to amusing and sad films? Neuroimage 27, 656–668. doi: 10.1016/j.neuroimage.2005.04.028
Jackson, T., Yang, Z., Li, X., Chen, H., Huang, X., and Meng, J. (2012). Coping when pain is a potential threat: the efficacy of acceptance versus cognitive distraction. Eur. J. Pain 16, 390–400. doi: 10.1002/j.1532-2149.2011.00019.x
John, O. P., and Gross, J. J. (2004). Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. J. Pers. 72, 1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x
Kanske, P., Heissler, J., Schönfelder, S., Bonger, A., and Wessa, M. (2011). How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex 21, 1379–1388. doi: 10.1093/cercor/bhq216
Keogh, E., Bond, F. W., Hanmer, R., and Tilston, J. (2005). Comparing acceptance- and control-based coping instructions on the cold-pressor pain experiences of healthy men and women. Eur. J. Pain 9, 591–598. doi: 10.1016/j.ejpain.2004.12.005
Koechlin, H., Coakley, R., Schechter, N., Werner, C., and Kossowsky, J. (2018). The role of emotion regulation in chronic pain: a systematic literature review. J. Psychosom. Res. 107, 38–45. doi: 10.1016/j.jpsychores.2018.02.002
Koers, G., Mulder, L. J., and van der Veen, F. (1999). The computation of evoked heart rate and blood pressure. J. Psychophysiol. 13:83. doi: 10.1027//0269-8803.13.2.83
Kohl, A., Rief, W., and Glombiewski, J. A. (2012). How effective are acceptance strategies? A meta-analytic review of experimental results. J. Behav. Ther. Exp. Psychiatry 43, 988–1001. doi: 10.1016/j.jbtep.2012.03.004
Kohl, A., Rief, W., and Glombiewski, J. A. (2013). Acceptance, cognitive restructuring, and distraction as coping strategies for acute pain. J. Pain 14, 305–315. doi: 10.1016/j.jpain.2012.12.005
Koval, P., Butler, E. A., Hollenstein, T., Lanteigne, D., and Kuppens, P. (2015). Emotion regulation and the temporal dynamics of emotions: effects of cognitive reappraisal and expressive suppression on emotional inertia. Cogn. Emot. 29, 831–851. doi: 10.1080/02699931.2014.948388
Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and, A. N.OVAs. Front. Psychol. 4:863. doi: 10.3389/fpsyg.2013.00863
Lapate, R. C., Lee, H., Salomons, T. V., van Reekum, C. M., Greischar, L. L., and Davidson, R. J. (2012). Amygdalar function reflects common individual differences in emotion and pain regulation success. J. Cogn. Neurosci. 24, 148–158. doi: 10.1162/jocn_a_00125
Lautenbacher, S., Roscher, S., and Strian, F. (1995). Tonic pain evoked by pulsating heat: temporal summation mechanisms and perceptual qualities. Somatosens Mot. Res. 12, 59–70. doi: 10.3109/08990229509063142
Laux, L., Glanzmann, P., Schaffner, P., and Spielberger, C. D. (1981). Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisung. Weinheim: Beltz Test GmbH.
Liverant, G. I., Brown, T. A., Barlow, D. H., and Roemer, L. (2008). Emotion regulation in unipolar depression: the effects of acceptance and suppression of subjective emotional experience on the intensity and duration of sadness and negative affect. Behav. Res. Ther. 46, 1201–1209. doi: 10.1016/j.brat.2008.08.001
Loggia, M. L., Juneau, M., and Bushnell, M. C. (2011). Autonomic responses to heat pain: heart rate, skin conductance, and their relation to verbal ratings and stimulus intensity. Pain 152, 592–598. doi: 10.1016/j.pain.2010.11.032
Masedo, A. I., and Esteve, M. R. (2007). Effects of suppression, acceptance and spontaneous coping on pain tolerance, pain intensity and distress. Behav. Res. Ther. 45, 199–209. doi: 10.1016/j.brat.2006.02.006
McMullen, J., Barnes-Holmes, D., Barnes-Holmes, Y., Stewart, I., Luciano, C., and Cochrane, A. (2008). Acceptance versus distraction: brief instructions, metaphors and exercises in increasing tolerance for self-delivered electric shocks. Behav. Res. Ther. 46, 122–129. doi: 10.1016/j.brat.2007.09.002
McNeil, D. W., and Rainwater, A. J. (1998). Development of the Fear of Pain Questionnaire-III. J. Behav. Med. 21, 389–410. doi: 10.1023/a:1018782831217
McRae, K., Hughes, B., Chopra, S., Gabrieli, J. D. E., Gross, J. J., and Ochsner, K. N. (2010). The neural bases of distraction and reappraisal. J. Cogn. Neurosci. 22, 248–262. doi: 10.1162/jocn.2009.21243
Merskey, H., and Bogduk, N. (2016). IASP Task Force on Taxonomy Part III: Pain terms, a current list with Definitions and notes on Usage. IASP Task Force Taxon 1994. Washington, DC: IASP, 209–214.
Meyer, K., Sprott, H., and Mannion, A. F. (2008). Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J. Psychosom. Res. 64, 469–478. doi: 10.1016/j.jpsychores.2007.12.004
Moore, H., Stewart, I., Barnes-Holmes, D., Barnes-Holmes, Y., and McGuire, B. E. (2015). Comparison of acceptance and distraction strategies in coping with experimentally induced pain. J. Pain Res. 8, 139–151. doi: 10.2147/JPR.S58559
Nielsen, C. S., Staud, R., and Price, D. D. (2009). Individual differences in pain sensitivity: measurement, causation, and consequences. J. Pain 10, 231–237. doi: 10.1016/j.jpain.2008.09.010
Paez-Blarrina, M., Luciano, C., Gutierrez-Martinez, O., Valdivia, S., Ortega, J., and Rodriguez-Valverde, M. (2008a). The role of values with personal examples in altering the functions of pain: comparison between acceptance-based and cognitive-control-based protocols. Behav. Res. Ther. 46, 84–97. doi: 10.1016/j.brat.2007.10.008
Paez-Blarrina, M., Luciano, C., Gutierrez-Martinez, O., Valdivia, S., Rodriguez-Valverde, M., and Ortega, J. (2008b). Coping with pain in the motivational context of values - Comparison between an acceptance-based and a cognitive control-based protocol. Behav. Modif. 32, 403–422. doi: 10.1177/0145445507309029
Pavlov, S. V., Reva, N. V., Loktev, K. V., Tumyalis, A. V., Korenyok, V. V., and Aftanas, L. I. (2014). The temporal dynamics of cognitive reappraisal: cardiovascular consequences of downregulation of negative emotion and upregulation of positive emotion. Psychophysiology 51, 178–186. doi: 10.1111/psyp.12159
Price, D. D., Mcgrath, P. A., Rafii, A., and Buckingham, B. (1983). The validation of visual analog scales as ratio scale measures for chronic and experimental pain. Pain 17, 45–56. doi: 10.1016/0304-3959(83)90126-4
Prins, B., Decuypere, A., and Van Damme, S. (2014). Effects of mindfulness and distraction on pain depend upon individual differences in pain catastrophizing: an experimental study. Eur. J. Pain 18, 1307–1315. doi: 10.1002/j.1532-2149.2014.491.x
Reicherts, P., Gerdes, A. B. M., Pauli, P., and Wieser, M. J. (2016). Psychological placebo and nocebo effects on pain rely on expectation and previous experience. J. Pain 17, 203–214. doi: 10.1016/j.jpain.2015.10.010
Rhudy, J. L., France, C. R., Bartley, E. J., McCabe, K. M., and Williams, A. E. (2009). Psychophysiological responses to pain: further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiology 46, 939–948. doi: 10.1111/j.1469-8986.2009.00835.x
Rhudy, J. L., Grimes, J. S., and Meagher, M. W. (2004). Fear-induced hypoalgesia in humans: effects on low intensity thermal stimulation and finger temperature. J. Pain 5, 458–468. doi: 10.1016/j.jpain.2004.08.001
Rhudy, J. L., and Meagher, M. W. (2000). Fear and anxiety: divergent effects on human pain thresholds. Pain 84, 65–75. doi: 10.1016/s0304-3959(99)00183-9
Ruscheweyh, R., Marziniak, M., Stumpenhorst, F., Reinholz, J., and Knecht, S. (2009). Pain sensitivity can be assessed by self-rating: development and validation of the pain sensitivity questionnaire. Pain 146, 65–74. doi: 10.1016/j.pain.2009.06.020
Scheier, M. F., Carver, C. S., and Bridges, M. W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a re-evaluation of the Life Orientation Test. J. Pers. Soc. Psychol. 67, 1063–1078. doi: 10.1037//0022-3514.67.6.1063
Schönfelder, S., Kanske, P., Heissler, J., and Wessa, M. (2014). Time course of emotion-related responding during distraction and reappraisal. Soc. Cogn. Affect. Neurosci. 9, 1310–1319. doi: 10.1093/scan/nst116
Schumacher, J., Leppert, K., Gunzelrnann, T., Strauß, B., and Brähler, E. (2005). Die Resilienzskala - Ein Fragebogen zur Erfassung der psychischen Widerstandsfähigkeit als Personmerkmal. [The Resilience Scale - A questionnaire to assess resilience as a personality characteristic.]. Z. Klin. Psychol. Psychiatr. Psychother. 53, 16–39.
Shaffer, F., and Ginsberg, J. P. (2017). An Overview of Heart Rate variability Metrics and Norms. Front. Public Health 5:258. doi: 10.3389/fpubh.2017.00258
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., and Jacobs, G. A. (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.
Sullivan, M. J. L., Bishop, S. R., and Pivik, J. (1995). The Pain Catastrophizing Scale: development and validation. Psychol. Assess. 7, 524–532. doi: 10.1037/1040-3590.7.4.524
Van Damme, S., Crombez, G., Van De Wever, K. N., and Goubert, L. (2008). Is distraction less effective when pain is threatening? An experimental investigation with the cold pressor task. Eur. J. Pain 12, 60–67. doi: 10.1016/j.ejpain.2007.03.001
Verhoeven, K., Van Damme, S., Eccleston, C., Van Ryckeghem, D. M. L., Legrain, V., and Crombez, G. (2011). Distraction from pain and executive functioning: an experimental investigation of the role of inhibition, task switching and working memory. Eur. J. Pain 15, 866–873. doi: 10.1016/j.ejpain.2011.01.009
Wagnild, G. M., and Young, H. M. (1993). Development and psychometric evaluation of the Resilience Scale. J. Nurs. Meas. 1, 165–178.
Webb, T. L., Miles, E., and Sheeran, P. (2012). Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol. Bull. 138:775. doi: 10.1037/a0027600
Wieser, M. J., and Pauli, P. (2016). “Neuroscience of pain and emotion,” in The Neuroscience of Pain, Stress, and Emotion: Psychological and Clinical Implications, eds M. A. Flaten and M. al’Absi (Amsterdam: Elsevier), 3–27. doi: 10.1016/b978-0-12-800538-5.00001-7
Wolgast, M., Lundh, L.-G., and Viborg, G. (2011). Cognitive reappraisal and acceptance: an experimental comparison of two emotion regulation strategies. Behav. Res. Ther. 49, 858–866. doi: 10.1016/j.brat.2011.09.011
Keywords: pain regulation, emotion regulation, acceptance, cognitive strategies, acute pain, acceptance-based strategy, psychological modulation of pain, pain ratings
Citation: Haspert V, Wieser MJ, Pauli P and Reicherts P (2020) Acceptance-Based Emotion Regulation Reduces Subjective and Physiological Pain Responses. Front. Psychol. 11:1514. doi: 10.3389/fpsyg.2020.01514
Received: 27 February 2020; Accepted: 08 June 2020;
Published: 30 June 2020.
Edited by:
Florin Dolcos, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Darren J. Edwards, Swansea University, United KingdomSebastian Ocklenburg, Ruhr University Bochum, Germany
Copyright © 2020 Haspert, Wieser, Pauli and Reicherts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Haspert, valentina.haspert@uni-wuerzburg.de
 Valentina Haspert
Valentina Haspert Matthias J. Wieser
Matthias J. Wieser Paul Pauli
Paul Pauli Philipp Reicherts
Philipp Reicherts