- 1School of Psychology, Southwest University, Chongqing, China
- 2Key Laboratory of Cognition and Personality, Ministry of Education, Chongqing, China
- 3School of Psychology, University of Sussex, Brighton, United Kingdom
- 4Sackler Centre for Consciousness Science, University of Sussex, Brighton, United Kingdom
Individuals with autistic traits are those who present in the normal population with characteristics of social, communication, personality, and cognitive impairments but do not meet the clinical threshold for autism spectrum disorder (ASD). Most studies have focused on the abnormalities in ASD patients rather than on individuals with autistic traits. In this study, we focused on the behaviors of a large sample (N = 401) of Chinese individuals with different levels of autistic traits, measured using the Autism Spectrum Quotient, and applied voxel-based morphometry (VBM) to determine their association to differences in brain structure. The results mainly showed that the correlation between gray matter volume (GMV) and gray matter density of the brain and the Autism Spectrum Quotient was significant in these regions: the right middle frontal gyrus, which are involved in social processing and social reasoning; the left parahippocampal gyrus, which is involved in socioemotional behaviors and unconscious relational memory encoding; and the right superior parietal lobule, which are involved in cognitive control and the ability to show attention to detail. These findings reveal that people with autistic traits in the normal population have atypical development in GMV and gray matter density, which may affect their social functioning and communication ability.
Introduction
Autism spectrum disorder (ASD) consists of neurodevelopmental symptoms characterized by core deficits in social functioning but also extending to other cognitive differences (Assaf et al., 2010). The idea of a spectrum captures both the heterogeneity within ASD itself (e.g., from low- to high-functioning intelligence) and the principle of continuity of the symptom profile within the general population itself, in which individuals may exhibit autistic traits to a greater or lesser extent. For instance, the parents of autistic children often report increased levels of autistic traits which are not severe enough to merit a formal diagnosis (Wheelwright et al., 2010). Variation in the level of autistic tendencies is often measured using the Autism Spectrum Quotient (Baron-Cohen et al., 2001). This is a self-report measure that asks about the presence of a range of traits and behaviors commonly seen in autism, including poor social understanding, problems in attention switching, greater attention to detail, poor imagination, and poor communication skills. There has been considerable previous research exploring the brain differences between ASD and controls using functional imaging (Pelphrey et al., 2005; Ikeda et al., 2018) and structural imaging techniques (Critchley et al., 2000; Grelotti et al., 2002; Belmonte et al., 2004; Rojas et al., 2006; Deruelle et al., 2008). However, the study of brain-based individual differences in autistic traits within the general population has received comparatively less attention. This is an important complementary approach that may also have certain advantages. For instance, the overall level of intellectual functioning (a potential confound) is likely to be more homogenous among a student-based neurotypical sample than an ASD sample. The present study examines differences in gray matter linked to autistic traits using a Chinese version of the Autism Spectrum Quotient (AQ) as well as provides important evidence about the neural basis of autistic traits that will potentially contribute to a wider discussion about how autism should be diagnosed and characterized (Pang et al., 2018).
Gray matter volume (GMV) represents the absolute amount of gray matter (Good et al., 2001; Mechelli et al., 2005), whereas gray matter density (GMD) represents the relative concentration of gray matter structures in spatially warped images (i.e., the proportion of gray matter relative to all tissue types within a region) (Mechelli et al., 2005). Focusing on both absolute GMV and GMD may help our understanding of the mechanism of the brain and the individual’s autistic traits.
Previous studies on GMV in ASDs have shown inconsistent results. The existence of studies mostly focused on frontal and temporal regions which are responsible for emotional control and social communication (Hyde et al., 2010; Fan et al., 2012; Morein-Zamir et al., 2016; Patriquin et al., 2016) had long been controversial. First, some studies reported increased GMV in the frontal gyrus (Hyde et al., 2010; Kaiser et al., 2010; Toal et al., 2010; Ecker et al., 2012), while some studies reported decreased GMV in the frontal gyrus (Abell et al., 1999; McAlonan et al., 2005; Takeuchi et al., 2014a; Mori et al., 2015; D’Angelo et al., 2016). Second, some studies reported increased GMV in the temporal gyrus (Abell et al., 1999; Ecker et al., 2012; Lai et al., 2013), while decreased GMV in the temporal gyrus was also reported (Abell et al., 1999; Toal et al., 2010; D’Angelo et al., 2016). There are also studies showing the subcortical brain areas like the basal ganglia extending to the thalamus and the ventral striatum (Takeuchi et al., 2014b). These inconsistent results suggested that the mechanisms of the brain in individuals are still remain unknown. There might exist some other brain regions that closely relate to the autistic traits like the parietal and the parahippocampal regions.
Similar results were obtained in the frontal and the cingulate regions when measuring GMD. A review of a voxel-based morphometry (VBM) study found that GMD decreased in the right paracingulate sulcus and the left inferior frontal gyrus within adults with high-functioning autism (Brambilla, 2003). Another study which explored the relationship between autism and schizophrenia patients within the gray matter and the white matter found that the autism group demonstrated bilateral prefrontal and anterior cingulate increases in contrast with the prefrontal and the left temporal reductions in schizophrenia (Katz et al., 2016). In the three important psychiatric spectra – schizophrenia spectrum disorder, ASD, and obsessive–compulsive disorder – it was found that the GMD of patients did not develop randomly but rather followed identifiable decreased patterns of coalteration in the lateral prefrontal cortex, the ventromedial prefrontal, the orbitofrontal cortex, and the cingulate regions (Cauda et al., 2018). There was also increased GMD shown in the brain regions in autism researches. The neural correlates of executive function in autistic spectrum disorders have shown significant increase in the middle frontal gyrus (MFG) compared with the control groups. Moreover, in individuals with ASD, increased frontal GMD and increased functional activation shared the same anatomical location (Schmitz et al., 2006). Additionally, in a joint behavioral and neuroimaging study of somatosensory and premotor, GMD was significantly higher in the right motor cortex (precentral gyrus) of those with ASD compared to controls (Winter, 2016).
ASD has special characteristics, mainly referring to social deficits, communication disabilities, and repetitive and stereotyped behaviors (Hadjikhani et al., 2005; Troyb et al., 2016). These changes in behavior and mental health are thought to be etiological factors reflected by brain maturation and anatomy (Belmonte et al., 2004; Schmeisser and Boeckers, 2017). While people with autistic traits have not obtained enough attention at a clinical level, they do have an impact on their emotion control, social communication, and interaction (Sucksmith et al., 2011). Individuals with autistic traits undertake the same social responsibilities as normal individuals but psychologically suffer more pain; they are doing the same job as everyone else but taking up more cognitive resources (Sucksmith et al., 2011; Lai et al., 2013). Research into autistic tendencies may result, to some extent, in helping this group ease their burdens and obtain a better, healthier life and therefore preventing a regression to sub-optimal clinical health conditions (Guan and Zhao, 2015). The atypical changes in GMV and GMD, especially in the frontal lobe, the lingual gyrus, the occipital gyrus, the anterior cingulate cortex, the insula, and the parahippocampus during childhood, even to adulthood, within individuals with autistic traits may reflect that these brain regions of the human brain play important roles in cognitive functions, which affect an individual’s brain and behavioral development. As mentioned above, these studies of VBM have been mostly demonstrated on ASD patients but have rarely focused on individuals varying in their levels of autistic traits, especially in the Chinese sample. Therefore, in this study, the participants underwent structural MRI scans after performing an AQ test. The AQ scores were then assessed in relation to GMV and GMD after brain scanning. We hypothesized that the GMV and the GMD of the frontal lobe and the parietal lobe would increase as AQ scores increased and the parahippocampal gyrus (PHG) would decrease as AQ scores increased in individuals with autistic traits since they require more cognitive resources to perform the same work or task as a neurotypical person. This may also be because gray matter maturation is abnormal, while normal gray matter development increases at earlier ages, followed by sustained loss starting around puberty (Gogtay and Thompson, 2010), which may also lead to atypical gray matter development.
Materials and Methods
Standard Protocol Approvals, Registration, and Consent
This study and the experimental procedure were approved by the Brain Imaging Center Institutional Review Board of Southwest University of China. In accordance with the Declaration of Helsinki (World Medical Association [WMA], 1991), all participants provided written informed consent and received payment for their time.
Participants
Four hundred and one individuals (111 men, aged 18–26 years, mean = 21.04 years, standard deviation = 1.27) participated in this research as part of our project investigating associations among genes, brain imaging, and mental health (Liu et al., 2017). Before the experiment, we collected the sample’s basic information to exclude subjects with potential mental disorder; two trained and experienced graduate students in the School of Psychology performed the Structured Clinical Interview for the DMS-IV; all participants included in this study did not meet the DMS-IV criteria for psychiatric disorders and did not use drugs that can affect brain functions. In addition, a self-report checklist was used by all participants to exclude any of following criteria: serious brain trauma, substance abuse, hypertension, or cardiovascular disease. All participants were right-handed, had normal vision, got reasonable payment, and were undergraduates at Southwest University.
Assessment of AQ
The AQ is a quantitative measure of autistic traits in the general population (Baron-Cohen et al., 2001). The Chinese version of AQ (Lau et al., 2013) was used in this study which consists of the social skill, communication, attention switching, imagination, and attention to detail subscales contained within 50 statements, to which the participants responded on a four-point Likert scale: “definitely agree” or “slightly agree” responses scored one point, while “slightly disagree” or “definitely disagree” responses scored one point in reverse options. In half of the statements, the diagnostic answer is “agree,” and in the other half “disagree.” One point is awarded for each diagnostic answer which results in a continuous distribution of scores in the population sample. The total score ranges from 0 to 50 points, with higher scores suggesting a greater magnitude of autistic traits. Currently available data from research on the properties of this scale indicate that the measurement reliability for the total score is satisfactory (Austin, 2005; Hurst et al., 2007; Hoekstra et al., 2008; Ingersoll et al., 2011; Kloosterman et al., 2011). In the present study, we focused on analyzing the total AQ score.
Image Acquisition
A 3-T Siemens Trio MRI scanner (Siemens Medical, Erlangen, Germany) was used to gather images. Then, high-resolution T1-weighted structural images (repetition time = 1,900 ms, inversion time = 900 ms, flip angle = 9°, echo time = 2.52 ms, 256 × 256 matrix, 176 slices, 1.0 mm slice thickness, and voxel size = 1 × 1 × 1 mm3) were collected, on which a magnetization-prepared rapid gradient echo (MPRAGE) sequence was used.
MRI Preprocessing
The structural magnetic resonance (MR) images were processed with SPM81 implemented in MATLAB R2014a (MathWorks Inc., Natick, MA, United States). First, every magnetic resonance image was displayed in SPM8 to monitor artifacts and obvious anatomical abnormalities. Then, VBM was performed with diffeomorphic anatomical registration using exponentiated lie algebra (DARTEL) (Ashburner, 2007). The new segment toolbox from SPM8 was applied to every T1-weighted MR image to extract tissue maps corresponding to gray matter, white matter, and cerebrospinal fluid in the native space. The DARTEL template creation toolbox was used to improve intersubject alignment. The resliced images of the gray and white matter were registered to a subject-specific template, and subsequently the normalization function in the DARTEL toolbox was used to normalize the individual images of gray and white matter to the MNI space (1.5 mm isotropic voxels). Finally, each subject’s gray and white matter maps were warped using their corresponding smoothed (10-mm full-width at half-maximum Gaussian kernel) and reversible deformation parameters to the custom template space and then to the MNI standard space. GMV images were modulated by calculating the Jacobian determinants derived from the special normalization step and by multiplying each voxel by the relative change in volume.
Statistical Analysis
We applied multiple linear regression to identify the brain regions whose GMV and GMD were associated with individual differences in the AQ within SPM8. In this study, a customized binary mask was used to avoid the partial volume effect by including voxels with a gray matter value >0.2. All subsequent statistical analyses were conducted in this mask. To remove potential confounds, we used age and total GMV and GMD mean values as nuisance covariates. Clusters with continuous suprathreshold voxels (p < 0.001) were initially identified within the custom mask and within the AlphaSim correction for multiple-comparison in DPABI. Many studies used smoothness kernel in preprocessing to estimate the biggest cluster size with AlphaSim correction; however, the effective smoothness is bigger than the applied because the pre-smoothed image has implicit smoothness. Simply inputting Gaussian smoothing kernel that was applied during preprocessing to AlphaSim is incorrect (Bennett et al., 2009). DPABI prevents that kind of errors by performing AlphaSim correction based on the estimated effective smoothness (Yang et al., 2016). The minimum cluster size in AlphaSim correction of GMV-positive and GMV-negative was k >192. The minimum cluster size in AlphaSim correction of GMD positive was k >50, respectively. The p-maps were thresholded to yield an expected p-value of <0.05, voxel-wise p < 0.005.
Results
Descriptive Statistics
The demographic data and behavioral results are shown in Table 1. The mean AQ score of the current sample was 19.58, and the standard deviation was 5.27. The difference in AQ between gender are shown in Supplementary Table S1.
Correlations Between GMV and AQ Score
After entering age, gender, and global volumes of gray matter as covariates into the regression model, a multiple regression analysis revealed that the AQ (total) score had a significant positive association with the GMV in the MFG and the middle occipital gyrus (MNI coordinates: 10, 33, 1.5, T = 3.90; 30, -88.5, 0, T = 3.22). Additionally, the AQ score had a significant negative association with the GMV in the left parahippocampal gyrus (MNI coordinates: -13.5, 3, -27, T = -3.79) (see Figure 1 and Table 2).
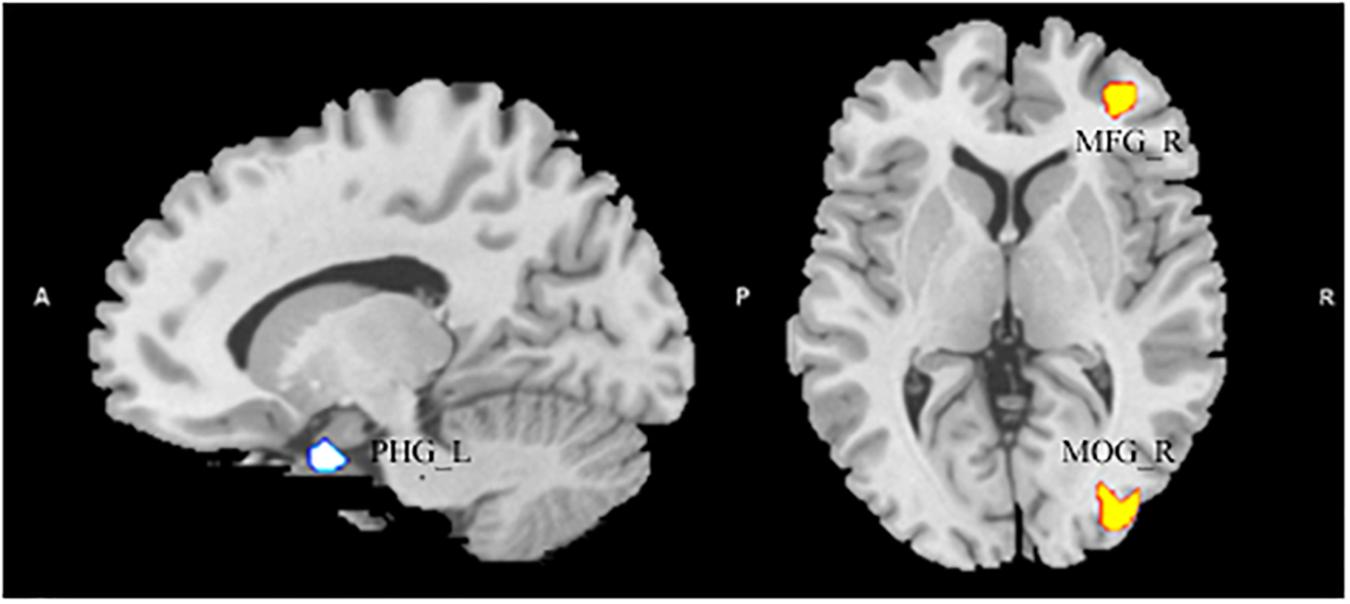
Figure 1. The significant positive correlation between gray matter volume (GMV) and Autism Spectrum Quotient score showing areas in the right middle frontal gyrus and in the middle occipital gyrus, and had a significant negative association with the GMV in the left parahippocampal gyrus. Alphasim-corrected cluster level p < 0.05, voxel-wise level p < 0.005.

Table 2. The brain regions in gray matter volume significantly correlated with Autism Spectrum Quotient.
Correlations Between GMD and AQ Score
After entering the age, the gender, and the global density of gray matter as covariates into the regression model, a multiple regression analysis revealed that the AQ (total) score had a significant positive association with the GMD in the left superior frontal gyrus (SFG), right precentral gyrus, and negative in the right superior parietal lobule (SPL) (MNI coordinates: 25.5, -6, 63, T = 3.60; -45, -1.5, 52.5, T = 3.33; 13.5, -73.5, 58.5; T = -3.18) (see Figure 2 and Table 3).
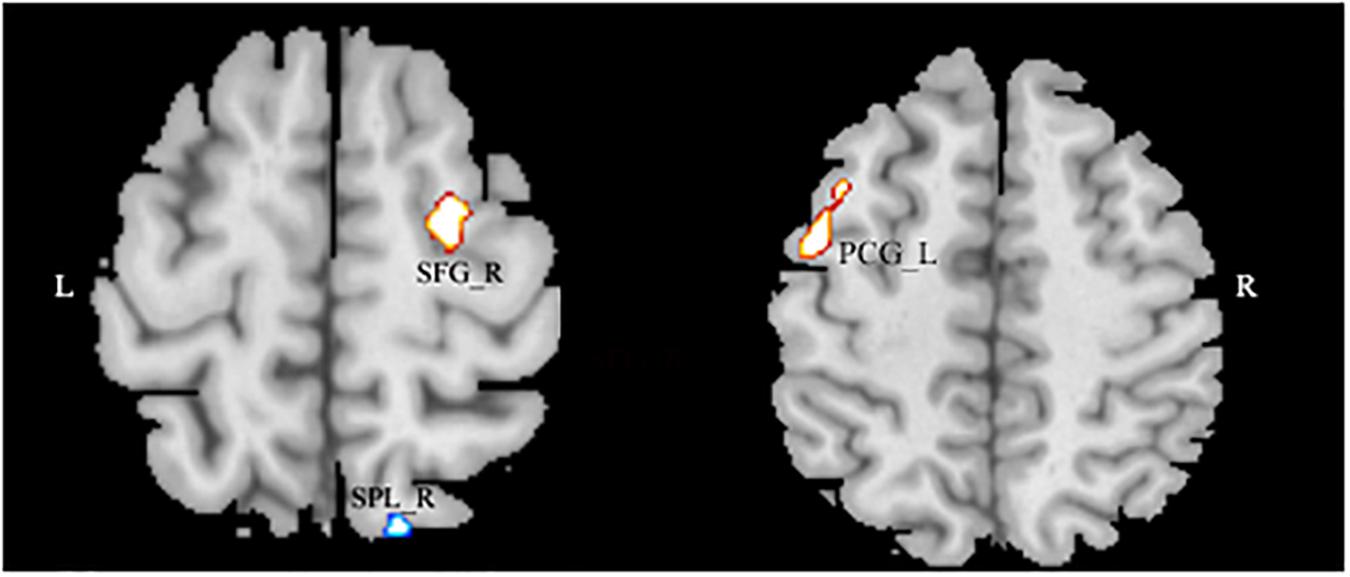
Figure 2. The significant negative correlation between gray matter volume and Autism Spectrum Quotient score showing areas in the left superior frontal gyrus and in the right precentral gyrus, and negative in the right superior parietal lobule. Alphasim-corrected cluster level p < 0.05, voxel-wise level p < 0.005.
We also explore the associations between the sub-dimensions of AQ and the brain areas of GMV and GMD and found significant associations in social skill, attention switching, and communication parts (see Supplementary Table S2).
Discussion
In this study, we investigated associations between the GMV and the GMD of brain structures and the AQ of individuals with autistic traits. Our VBM analysis results showed that the AQ (total) score had a significant positive association with GMV in the right MFG and the right middle occipital gyrus and a negative association to left PHG. The AQ (total) score had a significant positive correlation with the GMD in the left SFG and the right precentral gyrus and a negative association with the right SPL and the right MFG. These findings point to disturbances of brain growth and maturation as an important pathomechanism. The atypical development in GMV and GMD in the brain structures of individuals with autistic traits may explain the abnormal neuro and social behaviors to some degree.
In the present study, we focused on the brain regions that are closely related to the autistic traits. First, the AQ (total) score was significantly and positively correlated with gray matter in an extensive region that included the right MFG and SFG of gray matter, which is in line with a previous study and involved in social communication and interaction (Hyde et al., 2010; Fan et al., 2012; Marsh et al., 2014; Morein-Zamir et al., 2016; Patriquin et al., 2016; Stevenson et al., 2018); these two regions are also known to be involved in planning, flexibility, executive functioning, and working memory in ASD (Zelazo and Müller, 2002; Hill, 2004; Jurado and Rosselli, 2007; Craig et al., 2016). These findings collectively suggested atypical development in the structure and functions of the brain; indeed neuropsychological and neuroimaging studies performed thus far have suggested the association between people with autistic traits and increases in MFG and SFG volumes which may be influenced by executive dysfunction and social communication deficits (Booth et al., 2003; Corbett et al., 2009; Vanegas and Davidson, 2015; Craig et al., 2016). People with autistic traits who have normal intelligence, normal lifestyle, and seemingly normal social interactions are burdened with more social pressure, which may be due to their social executive dysfunction (Bayliss and Tipper, 2005; Christ et al., 2010). The discrepancy between social communication situational pressures and actual social abilities is consequent to the compensatory strategies of adults with autistic traits or of clinical ASD patients that bring task performance to ceiling levels (Schneider et al., 2013, 2014). The atypical development in the MFG and SFG could reflect a lack of pruning during the normal growth spurt, leading to excessive preservation of unneeded increases. Such an effect would certainly lead to abnormal structure between individuals with autistic traits and brain regions.
Second, the significant decrease in the left PHG of GMV was consistent with previous neuroimaging findings in adults and children with autism (Page et al., 2009; Kosaka et al., 2010; Mueller et al., 2013; Yu et al., 2019), Moreover, severely restricted and repetitive behaviors were associated with the PHG (Monk et al., 2009; Weng et al., 2010; Hau et al., 2019). These two brain regions are implicated in unconscious relational memory encoding, autobiographical memory (Tanweer et al., 2010; Duss et al., 2014), and socioemotional behaviors (Dawson, 1991; Yang et al., 2016; Puiu et al., 2018) that are abnormal in individuals with autistic traits and in patients with clinical ASD, such as understanding the mental state of others, emotion processing, and language (Barrett et al., 2007; Lartseva et al., 2015; Grecucci et al., 2016). As previously reported, individuals with autistic traits could communicate with others in a normal way but are burdened with more social pressure (El Kaliouby et al., 2006). Individuals with autistic traits show deficits in social communication or social avoidance and do not have compensatory strategies, which influence the relationship between cognitive functions and neural system (Senju et al., 2011; Low and Watts, 2013). Additionally, individuals with autistic traits usually remembered more adverse events like social rejection, childhood trauma, and daily interpersonal stress (Sebastian, 2015). Young adults have the ability to integrate emotional information into a proper degree that need wholesome neurological development (Sebastian, 2015). Neuroimaging evidence is now showing that improvements in social cognition during young adults are underpinned by the ongoing development in relevant regions (Blakemore, 2008). This may be the reason for the decrease in GMV of the left PHG as reflected in the current study and may explain atypical behaviors such as poor communication disorder and repetitive behaviors within individuals with autistic traits.
Third, we also found that individuals with autistic traits had a decrease in GMD in the right SPL which was inconsistent with previous study, which might be the case given that the large sample has more weight in this study than others. The SPL is important in cognitive control and attention to detail (Koechlin and Hyafil, 2007; Kompus et al., 2009; Qiu et al., 2018). To identity brain regions that differed in activity during social and non-social orienting, Greene’s study found that the ASD group demonstrated significant activation in the SPL (Greene et al., 2011). In a facial processing research, improved facial affected recognition performance which was accompanied by higher activation of the right SPL (Bolte et al., 2006), which indicated that the decrease of SPL in the present study within individuals with autistic traits might influence social cognition deficits. The SPL also composed the frontal–parietal control (FPC) network (Koechlin et al., 1999; Kompus et al., 2009; Mundy, 2018), and deficits to this phenomenon in brain regions may explain the non-social difficulties in individuals with autistic traits, such as repetitive, poorly controlled, and poor goal-directed action (Takarae et al., 2014; de Wit, 2018). The FPC system has been identified as supporting cognitive control and decision-making processing (Vincent et al., 2008). Relative to typical individuals, the social failure of individuals with autistic traits to process information globally might be argued to follow from problems in shifting between local and global processing (Bogousslavsky et al., 1987; Frith, 2004; Liss et al., 2008; Van Eylen et al., 2018), and a failure of cognitive control may be the neural basis of the autistic traits in these individuals (Vartanian et al., 2018). These atypical social cognitive functions relative to SPL was found within individuals with autistic traits in this study, indicating that social cognition deficit not only influences people’s behavior strongly but also results in a unique neuroanatomical structure. Above all, these atypical developments in brain structures, such as the MFG SFG, the left PHG, and the SPL, may play a role in social and attention abilities associated with brain function.
Conclusion
In summary, the present study replicated a previous study and broadened our understanding of the neural mechanisms underlying autistic traits within young adults. We found that the MFG, the SFG, the PHG, and the SPL brain regions play an important role in individuals with autistic traits; these brain regions are involved in some cognitive function deficits like social communication, cognitive control, attention to detail, and socioemotional behaviors. These abnormalities are consistent in young adults with autistic traits, which are reflected in specific brain regions. The current study that interpret individuals with autistic traits can help young people know themselves and integrate into their social life better in some degree.
Limitations
The current study investigates the brain areas of GMV and GMD in a large sample of young adults with autistic traits. The results showed that social communication, cognitive control, and some other brain functions are linked to brain areas in GMV and GMD, which replicated a previous study and broadened our understanding of the neural mechanism underlying autistic traits. Meanwhile, there are also deficiencies in the following three aspects: first, our autistic traits sample are all university students; this may have a bias in it compared to average level. Second, in our sample, there is a difference between genders. In a future study, we will further explore this difference using a larger sample wherein there is balancing of gender difference between men and women. Third, we want to further explore the neural mechanism using more different brain types, like resting-state functional connectivity and task-based functional connectivity, among others.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Brain Imaging Center Institutional Review Board of Southwest University of China. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
QJ conceived the experiments. YY and ZR conducted the experiments, analyzed the results, and carried on manuscript writing. JW provided the AQ questionnaire and proposed many constructive advices on the manuscript. All authors reviewed the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31771231), Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0520), Social Science Planning Project of Chongqing (2018PY80) and Fundamental Research Funds for the Central Universities (SWU119007), Chang Jiang Scholars Program, National Outstanding Young People Plan, Chongqing Talent Program.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.00523/full#supplementary-material
Footnotes
References
Abell, F., Krams, M., Ashburner, J., Passingham, R., Friston, K., Frackowiak, R., et al. (1999). The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport 10, 1647–1651. doi: 10.1097/00001756-199906030-00005
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. doi: 10.1016/j.neuroimage.2007.07.007
Assaf, M., Jagannathan, K., Calhoun, V. D., Miller, L., Stevens, M. C., Sahl, R., et al. (2010). Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage 53, 247–256. doi: 10.1016/j.neuroimage.2010.05.067
Austin, E. J. (2005). Personality correlates of the broader autism phenotype as assessed by the Autism Spectrum Quotient (AQ). Pers. Individ. Differ. 38, 451–460. doi: 10.1016/j.paid.2004.04.022
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., and Clubley, A. E. (2001). The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31:13.
Barrett, L. F., Lindquist, K. A., and Gendron, M. (2007). Language as context for the perception of emotion. Trends Cogn. Sci. 11, 327–332.
Bayliss, A. P., and Tipper, S. P. (2005). Gaze and arrow cueing of attention reveals individual differences along the autism spectrum as a function of target context. Br. J. Psychol. 96(Pt 1), 95–114. doi: 10.1348/000712604X15626
Belmonte, M. K., Allen, G., Beckel-Mitchener, A., Boulanger, L. M., Carper, R. A., and Webb, S. J. (2004). Autism and abnormal development of brain connectivity. J. Neurosci. 24, 9228–9231. doi: 10.1523/jneurosci.3340-04.2004
Bennett, C. M., Wolford, G. L., and Miller, M. B. (2009). The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 4, 417–422. doi: 10.1093/scan/nsp053
Blakemore, S.-J. (2008). The social brain in adolescence. Nat. Rev. Neurosci. 9:267. doi: 10.1038/nrn2353
Bogousslavsky, J., Miklossy, J., Deruaz, J. P., Assal, G., and Regli, F. (1987). Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J. Neurol. Neurosurg. Psychiatr. 50, 607–614. doi: 10.1136/jnnp.50.5.607
Bolte, S., Hubl, D., Feineis-Matthews, S., Prvulovic, D., Dierks, T., and Poustka, F. (2006). Facial affect recognition training in autism: can we animate the fusiform gyrus? Behav. Neurosci. 120, 211–216. doi: 10.1037/0735-7044.120.1.211
Booth, R., Charlton, R., Hughes, C., and Happe, F. (2003). Disentangling weak coherence and executive dysfunction: planning drawing in autism and attention-deficit/hyperactivity disorder. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 358, 387–392. doi: 10.1098/rstb.2002.1204
Brambilla, P. (2003). Brain anatomy and development in autism: review of structural MRI studies. Brain Res. Bull. 61, 557–569. doi: 10.1016/j.brainresbull.2003.06.001
Cauda, F., Nani, A., Costa, T., Palermo, S., Tatu, K., Manuello, J., et al. (2018). The morphometric co-atrophy networking of schizophrenia, autistic and obsessive spectrum disorders. Hum. Brain Mapp. 39, 1898–1928. doi: 10.1002/hbm.23952
Christ, S. E., Kanne, S. M., and Reiersen, A. M. (2010). Executive function in individuals with subthreshold autism traits. Neuropsychology 24, 590–598. doi: 10.1037/a0019176
Corbett, B. A., Constantine, L. J., Hendren, R., Rocke, D., and Ozonoff, S. (2009). Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 166, 210–222. doi: 10.1016/j.psychres.2008.02.005
Craig, F., Margari, F., Legrottaglie, A. R., Palumbi, R., de Giambattista, C., and Margari, L. (2016). A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 12, 1191–1202. doi: 10.2147/NDT.S104620
Critchley, H. D., Daly, E. M., Bullmore, E. T., Williams, S. C. R., Van Amelsvoort, T., Robertson, D. M., et al. (2000). The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain 123, 2203–2212. doi: 10.1093/brain/123.11.2203
D’Angelo, D., Lebon, S., Chen, Q., Martin-Brevet, S., Snyder, L. G., Hippolyte, L., et al. (2016). Defining the effect of the 16p11.2 duplication on cognition, behavior, and medical comorbidities. JAMA Psychiatry 73, 20–30. doi: 10.1001/jamapsychiatry.2015.2123
Dawson, G. (1991). VIII A psychobiological perspective on the early socio-emotional development of children with autism. Models Integr. 3:207.
de Wit, S. (2018). The balance between goal-directed and habitual action control in disorders of compulsivity. Goal Direct. Decis. Mak. 2018, 331–365. doi: 10.1016/b978-0-12-812098-9.00015-2
Deruelle, C., Hubert, B., Santos, A., and Wicker, B. (2008). Negative emotion does not enhance recall skills in adults with autistic spectrum disorders. Autism Res. 1, 91–96. doi: 10.1002/aur.13
Duss, S. B., Reber, T. P., Hanggi, J., Schwab, S., Wiest, R., Muri, R. M., et al. (2014). Unconscious relational encoding depends on hippocampus. Brain 137(Pt 12), 3355–3370. doi: 10.1093/brain/awu270
Ecker, C., Suckling, J., Deoni, S. C., Lombardo, M. V., Bullmore, E. T., Baron-Cohen, S., et al. (2012). Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Archiv. Gen. Psychiatr. 69, 195–209. doi: 10.1001/archgenpsychiatry.2011.1251
El Kaliouby, R., Picard, R., and Baron-Cohen, S. (2006). Affective computing and autism. Ann. N. Y. Acad. Sci. 1093, 228–248.
Fan, J., Bernardi, S., Van Dam, N. T., Anagnostou, E., Gu, X., Martin, L., et al. (2012). Functional deficits of the attentional networks in autism. Brain Behav. 2, 647–660. doi: 10.1002/brb3.90
Frith, C. (2004). Is autism a disconnection disorder? Lancet Neurol. 3:577. doi: 10.1016/s1474-4422(04)00875-0
Gogtay, N., and Thompson, P. M. (2010). Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn. 72, 6–15. doi: 10.1016/j.bandc.2009.08.009
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J., and Frackowiak, R. S. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14, 21–36. doi: 10.1006/nimg.2001.0786
Grecucci, A., Rubicondo, D., Siugzdaite, R., Surian, L., and Job, R. (2016). Uncovering the social deficits in the autistic brain. A source-based morphometric study. Front. Neurosci. 10:388. doi: 10.3389/fnins.2016.00388
Greene, D. J., Colich, N., Iacoboni, M., Zaidel, E., Bookheimer, S. Y., and Dapretto, M. (2011). Atypical neural networks for social orienting in autism spectrum disorders. Neuroimage 56, 354–362. doi: 10.1016/j.neuroimage.2011.02.031
Grelotti, D. J., Gauthier, I., and Schultz, R. T. (2002). Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev. Psychobiol. 40, 213–225. doi: 10.1002/dev.10028
Guan, J., and Zhao, X. (2015). Sub-threshold autistic traits in normal population: its concept, structure and influencing factors. Adv. Psychol. Sci. 23:1599. doi: 10.3724/sp.j.1042.2015.01599
Hadjikhani, N., Joseph, R. M., Snyder, J., and Tager-Flusberg, H. (2005). Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb. Cortex 16, 1276–1282. doi: 10.1093/cercor/bhj069
Hau, J., Aljawad, S., Baggett, N., Fishman, I., Carper, R. A., and Müller, R. A. (2019). The cingulum and cingulate U-fibers in children and adolescents with autism spectrum disorders. Hum. Brain Mapp. 40, 3153–3164. doi: 10.1002/hbm.24586
Hoekstra, R. A., Bartels, M., Cath, D. C., and Boomsma, D. I. (2008). Factor structure, reliability and criterion validity of the autism-spectrum quotient (AQ): a study in Dutch population and patient groups. J. Autism Dev. Disord. 38, 1555–1566. doi: 10.1007/s10803-008-0538-x
Hurst, R. M., Mitchell, J. T., Kimbrel, N. A., Kwapil, T. K., and Nelson-Gray, R. O. (2007). Examination of the reliability and factor structure of the Autism Spectrum Quotient (AQ) in a non-clinical sample. Pers. Individ. Differ. 43, 1938–1949. doi: 10.1016/j.paid.2007.06.012
Hyde, K. L., Samson, F., Evans, A. C., and Mottron, L. (2010). Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum. Brain Mapp. 31, 556–566. doi: 10.1002/hbm.20887
Ikeda, T., Hirai, M., Sakurada, T., Monden, Y., Tokuda, T., Nagashima, M., et al. (2018). Atypical neural modulation in the right prefrontal cortex during an inhibitory task with eye gaze in autism spectrum disorder as revealed by functional near-infrared spectroscopy. Neurophotonics 5:035008. doi: 10.1117/1.NPh.5.3.035008
Ingersoll, B., Hopwood, C. J., Wainer, A., and Brent Donnellan, M. (2011). A comparison of three self-report measures of the broader autism phenotype in a non-clinical sample. J. Autism Dev. Disord. 41, 1646–1657. doi: 10.1007/s10803-011-1192-2
Jurado, M. B., and Rosselli, M. (2007). The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev. 17, 213–233. doi: 10.1007/s11065-007-9040-z
Kaiser, M. D., Hudac, C. M., Shultz, S., Lee, S. M., Cheung, C., Berken, A. M., et al. (2010). Neural signatures of autism. Proc. Natl. Acad. Sci. U.S.A. 107, 21223–21228. doi: 10.1073/pnas.1010412107
Katz, J., d’Albis, M. A., Boisgontier, J., Poupon, C., Mangin, J. F., Guevara, P., et al. (2016). Similar white matter but opposite grey matter changes in schizophrenia and high-functioning autism. Acta Psychiatr. Scand. 134, 31–39. doi: 10.1111/a.12579
Kloosterman, P. H., Keefer, K. V., Kelley, E. A., Summerfeldt, L. J., and Parker, J. D. (2011). Evaluation of the factor structure of the Autism-Spectrum Quotient. Pers. Individ. Differ. 50, 310–314.
Koechlin, E., Basso, G., Pietrini, P., Panzer, S., and Grafman, J. (1999). The role of the anterior prefrontal cortex in human cognition. Nature 399:148. doi: 10.1038/20178
Koechlin, E., and Hyafil, A. (2007). Anterior prefrontal function and the limits of human decision-making. Science 318, 594–598. doi: 10.1126/science.1142995
Kompus, K., Hugdahl, K., Ohman, A., Marklund, P., and Nyberg, L. (2009). Distinct control networks for cognition and emotion in the prefrontal cortex. Neurosci. Lett. 467, 76–80. doi: 10.1016/j.neulet.2009.10.005
Kosaka, H., Omori, M., Munesue, T., Ishitobi, M., Matsumura, Y., Takahashi, T., et al. (2010). Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. Neuroimage 50, 1357–1363. doi: 10.1016/j.neuroimage.2010.01.085
Lai, M.-C., Lombardo, M. V., Chakrabarti, B., and Baron-Cohen, S. (2013). Subgrouping the autism “spectrum”: reflections on DSM-5. PLoS Biol. 11:e1001544. doi: 10.1371/journal.pbio.1001544
Lartseva, A., Dijkstra, T., and Buitelaar, J. K. (2015). Emotional language processing in autism spectrum disorders: a systematic review. Front. Hum. Neurosci. 8:991. doi: 10.3389/fnhum.2014.00991
Lau, W. Y.-P., Gau, S. S.-F., Chiu, Y.-N., Wu, Y.-Y., Chou, W.-J., Liu, S.-K., et al. (2013). Psychometric properties of the Chinese version of the Autism Spectrum Quotient (AQ). Res. Dev. Disabil. 34, 294–305. doi: 10.1016/j.ridd.2012.08.005
Liss, M., Mailloux, J., and Erchull, M. J. (2008). The relationships between sensory processing sensitivity, alexithymia, autism, depression, and anxiety. Pers. Individ. Differ. 45, 255–259. doi: 10.1016/j.paid.2008.04.009
Liu, W., Wei, D., Chen, Q., Yang, W., Meng, J., Wu, G., et al. (2017). Longitudinal test-retest neuroimaging data from healthy young adults in southwest China. Sci. Data 4:170017. doi: 10.1038/sdata.2017.17
Low, J., and Watts, J. (2013). Attributing false beliefs about object identity reveals a signature blind spot in humans’ efficient mind-reading system. Psychol. Sci. 24, 305–311. doi: 10.1177/0956797612451469
Marsh, R., Horga, G., Parashar, N., Wang, Z., Peterson, B. S., and Simpson, H. B. (2014). Altered activation in fronto-striatal circuits during sequential processing of conflict in unmedicated adults with obsessive-compulsive disorder. Biol. Psychiatry 75, 615–622. doi: 10.1016/j.biopsych.2013.02.004
McAlonan, G. M., Cheung, V., Cheung, C., Suckling, J., Lam, G. Y., Tai, K. S., et al. (2005). Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain 128(Pt 2), 268–276. doi: 10.1093/brain/awh332
Mechelli, A., Price, C. J., Friston, K. J., and Ashburner, J. (2005). Voxel-based morphometry of the human brain: methods and applications. Curr. Med. Imaging Rev. 1, 105–113. doi: 10.2174/1573405054038726
Monk, C. S., Peltier, S. J., Wiggins, J. L., Weng, S. J., Carrasco, M., Risi, S., et al. (2009). Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage 47, 764–772. doi: 10.1016/j.neuroimage.2009.04.069
Morein-Zamir, S., Voon, V., Dodds, C. M., Sule, A., van Niekerk, J., Sahakian, B. J., et al. (2016). Divergent subcortical activity for distinct executive functions: stopping and shifting in obsessive compulsive disorder. Psychol. Med. 46, 829–840. doi: 10.1017/S0033291715002330
Mori, K., Toda, Y., Ito, H., Mori, T., Mori, K., Goji, A., et al. (2015). Neuroimaging in autism spectrum disorders: 1H-MRS and NIRS study. J. Med. Invest. 62, 29–36. doi: 10.2152/jmi.62.29
Mueller, S., Keeser, D., Samson, A. C., Kirsch, V., Blautzik, J., Grothe, M., et al. (2013). Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal mri study. PLoS One 8:e67329. doi: 10.1371/journal.pone.0067329
Mundy, P. (2018). A review of joint attention and social-cognitive brain systems in typical development and autism spectrum disorder. Eur. J. Neurosci. 47, 497–514. doi: 10.1111/ejn.13720
Page, L. A., Rubia, K., Deeley, Q., Daly, E., Toal, F., Mataix-Cols, D., et al. (2009). A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Res. 174, 202–209. doi: 10.1016/j.pscychresns.2009.05.002
Pang, Y., Lee, C. M., Wright, M., Shen, J., Shen, B., and Bo, J. (2018). Challenges of case identification and diagnosis of autism spectrum disorders in China: a critical review of procedures, assessment, and diagnostic criteria. Res. Autism Spectr. Disord. 53, 53–66. doi: 10.1016/j.rasd.2018.06.003
Patriquin, M. A., DeRamus, T., Libero, L. E., Laird, A., and Kana, R. K. (2016). Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum. Brain Mapp. 37, 3957–3978. doi: 10.1002/hbm.23288
Pelphrey, K. A., Morris, J. P., and McCarthy, G. (2005). Neural basis of eye gaze processing deficits in autism. Brain 128(Pt 5), 1038–1048. doi: 10.1093/brain/awh404
Puiu, A. A., Wudarczyk, O., Goerlich, K. S., Votinov, M., Herpertz-Dahlmann, B., Turetsky, B., et al. (2018). Impulsive aggression and response inhibition in attention-deficit/hyperactivity disorder and disruptive behavioral disorders: findings from a systematic review. Neurosci. Biobehav. Rev. 90, 231–246. doi: 10.1016/j.neubiorev.2018.04.016
Qiu, L., Su, J., Ni, Y., Bai, Y., Zhang, X., Li, X., et al. (2018). The neural system of metacognition accompanying decision-making in the prefrontal cortex. PLoS Biol. 16:e2004037. doi: 10.1371/journal.pbio.2004037
Rojas, D. C., Peterson, E., Winterrowd, E., Reite, M. L., Rogers, S. J., and Tregellas, J. R. (2006). Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry 6:56. doi: 10.1186/1471-244X-6-56
Schmeisser, M. J., and Boeckers, T. M. (2017). Translational Anatomy and Cell Biology of Autism Spectrum Disorder, Vol. 224. Berlin: Springer.
Schmitz, N., Rubia, K., Daly, E., Smith, A., Williams, S., and Murphy, D. G. (2006). Neural correlates of executive function in autistic spectrum disorders. Biol. Psychiatry 59, 7–16. doi: 10.1016/j.biopsych.2005.06.007
Schneider, D., Nott, Z. E., and Dux, P. E. (2014). Task instructions and implicit theory of mind. Cognition 133, 43–47. doi: 10.1016/j.cognition.2014.05.016
Schneider, D., Slaughter, V. P., Bayliss, A. P., and Dux, P. E. (2013). A temporally sustained implicit theory of mind deficit in autism spectrum disorders. Cognition 129, 410–417. doi: 10.1016/j.cognition.2013.08.004
Sebastian, C. L. (2015). Social cognition in adolescence: social rejection and theory of mind. Psicol. Educ. 21, 125–131. doi: 10.1016/j.pse.2015.08.004
Senju, A., Southgate, V., Snape, C., Leonard, M., and Csibra, G. (2011). Do 18-month-olds really attribute mental states to others? A critical test. Psychol. Sci. 22, 878–880. doi: 10.1177/0956797611411584
Stevenson, R. A., Sun, S. Z., Hazlett, N., Cant, J. S., Barense, M. D., and Ferber, S. (2018). Seeing the forest and the trees: default local processing in individuals with high autistic traits does not come at the expense of global attention. J. Autism Dev. Disord. 48, 1382–1396. doi: 10.1007/s10803-016-2711-y
Sucksmith, E., Roth, I., and Hoekstra, R. A. (2011). Autistic traits below the clinical threshold: re-examining the broader autism phenotype in the 21st century. Neuropsychol. Rev. 21, 360–389. doi: 10.1007/s11065-011-9183-9
Takarae, Y., Luna, B., Minshew, N. J., and Sweeney, J. A. (2014). Visual motion processing and visual sensorimotor control in autism. J. Intern. Neuropsychol. Soc. 20, 113–122. doi: 10.1017/s1355617713001203
Takeuchi, H., Taki, Y., Nouchi, R., Sekiguchi, A., Kotozaki, Y., Miyauchi, C. M., et al. (2014a). Regional gray matter density is associated with achievement motivation: evidence from voxel-based morphometry. Brain Struct. Funct. 219, 71–83. doi: 10.1007/s00429-012-0485-3
Takeuchi, H., Taki, Y., Sassa, Y., Hashizume, H., Sekiguchi, A., Fukushima, A., et al. (2014b). Regional gray matter volume is associated with empathizing and systemizing in young adults. PLoS One 9:e84782. doi: 10.1371/journal.pone.0084782
Tanweer, T., Rathbone, C. J., and Souchay, C. (2010). Autobiographical memory, autonoetic consciousness, and identity in asperger syndrome. Neuropsychologia 48, 900–908. doi: 10.1016/j.neuropsychologia.2009.11.007
Toal, F., Daly, E. M., Page, L., Deeley, Q., Hallahan, B., Bloemen, O., et al. (2010). Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol. Med. 40, 1171–1181. doi: 10.1017/S0033291709991541
Troyb, E., Knoch, K., Herlihy, L., Stevens, M. C., Chen, C.-M., Barton, M., et al. (2016). Restricted and repetitive behaviors as predictors of outcome in autism spectrum disorders. J. Autism Dev. Disord. 46, 1282–1296. doi: 10.1007/s10803-015-2668-2
Van Eylen, L., Boets, B., Steyaert, J., Wagemans, J., and Noens, I. (2018). Local and global visual processing in autism spectrum disorders: influence of task and sample characteristics and relation to symptom severity. J. Autism Dev. Disord. 48, 1359–1381. doi: 10.1007/s10803-015-2526-2
Vanegas, S. B., and Davidson, D. (2015). Investigating distinct and related contributions of weak central coherence, executive dysfunction, and systemizing theories to the cognitive profiles of children with autism spectrum disorders and typically developing children. Res. Autism Spectr. Disord. 11, 77–92. doi: 10.1016/j.rasd.2014.12.005
Vartanian, O., Beatty, E. L., Smith, I., Blackler, K., Lam, Q., and Forbes, S. (2018). One-way traffic: the inferior frontal gyrus controls brain activation in the middle temporal gyrus and inferior parietal lobule during divergent thinking. Neuropsychologia 118(Pt A), 68–78. doi: 10.1016/j.neuropsychologia.2018.02.024
Vincent, J. L., Kahn, I., Snyder, A. Z., Raichle, M. E., and Buckner, R. L. (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 100, 3328–3342. doi: 10.1152/jn.90355.2008
Weng, S.-J., Wiggins, J. L., Peltier, S. J., Carrasco, M., Risi, S., Lord, C., et al. (2010). Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 1313, 202–214. doi: 10.1016/j.brainres.2009.11.057
Wheelwright, S., Auyeung, B., Allison, C., and Baron-Cohen, S. (2010). Defining the broader, medium and narrow autism phenotype among parents using the Autism Spectrum Quotient (AQ). Mol. Autism 1:10. doi: 10.1186/2040-2392-1-10
Winter, T. J. (2016). Neuromechanisms of Bimanual Coordination in the Autistic Brain: A Joint Behavioural and Neuroimaging Study. Master thesis, University of Otago, Dunedin.
World Medical Association [WMA] (1991). Declaration of Helsinki. Law, medicine & health care: a publication of the Amercian society of law & medicine. Gastroenterol. Japon. 26, 269–270.
Yang, X., Si, T., Gong, Q., Qiu, L., Jia, Z., Zhou, M., et al. (2016). Brain gray matter alterations and associated demographic profiles in adults with autism spectrum disorder: a meta-analysis of voxel-based morphometry studies. Austr. N. Zeal. J. Psychiatr. 50, 741–753. doi: 10.1177/0004867415623858
Yu, H., Meng, Y.-J., Li, X.-J., Zhang, C., Liang, S., Li, M., et al. (2019). Common and distinct patterns of grey matter alterations in borderline personality disorder and bipolar disorder: voxel-based meta-analysis. Br. J. Psychiatr. 215, 1–9. doi: 10.1192/bjp.2019.44
Keywords: autism spectrum traits, young adults, gray matter volume, gray matter density, voxel-based morphometry
Citation: Yaxu Y, Ren Z, Ward J and Jiang Q (2020) Atypical Brain Structures as a Function of Gray Matter Volume (GMV) and Gray Matter Density (GMD) in Young Adults Relating to Autism Spectrum Traits. Front. Psychol. 11:523. doi: 10.3389/fpsyg.2020.00523
Received: 14 September 2019; Accepted: 05 March 2020;
Published: 08 April 2020.
Edited by:
Yiping Zhong, Hunan Normal University, ChinaReviewed by:
Poppy Watson, University of New South Wales, AustraliaYuka Kotozaki, Tohoku University, Japan
Copyright © 2020 Yaxu, Ren, Ward and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Jiang, cWl1ajMxOEBzd3UuZWR1LmNu
†These authors have contributed equally to this work
 Yu Yaxu
Yu Yaxu Zhiting Ren1,2†
Zhiting Ren1,2† Jamie Ward
Jamie Ward Qiu Jiang
Qiu Jiang