- 1Beijing Key Laboratory of Learning and Cognition, Department of Psychology, The Collaborative Innovation Center for Capital Education Development, Capital Normal University, Beijing, China
- 2Beijing Gese Technology Co., Ltd., Beijing, China
- 3Department of Psychology, Shandong Normal University, Jinan, China
Although previous evidence has suggested that there is a genetic link between schizophrenia and creativity, the specific genetic variants that underlie the link are still largely unknown. To further explore the potential genetic link between schizophrenia and creativity, in a sample of 580 healthy Han Chinese subjects, this study aimed to (1) validate the role of Neuregulin 1 (NRG1) rs6994992 (one schizophrenia risk variant that has been previously linked to creativity in the European population) in the relationship between schizophrenia and creativity and (2) explore the associations between 10 other schizophrenia risk variants and creativity. For NRG1 rs6994992, the result validated its association with creativity measures. However, since NRG1 rs6994992 is not a schizophrenia risk variant in the Han Chinese population, the validated association suggested that ethnic difference may exist in the relationship between NRG1 rs6994992, schizophrenia and creativity. For other schizophrenia risk variants, the result only demonstrated a nominal association between ZNF536 rs2053079 and creativity measures which would not survive correction for multiple testing. No association between polygenic risk score for these 10 schizophrenia risk variants and creativity measures was observed. In conclusion, this study provides limited evidence for the associations between these schizophrenia risk variants and creativity in healthy Han Chinese subjects. Future studies are warranted to better understand the potential genetic link between schizophrenia and creativity.
Introduction
John Forbes Nash, the Father of Game Theory, had suffered from schizophrenia. He once said, “I wouldn’t have had good scientific ideas if I had thought more normally”. Along similar lines, many of the creative luminaries throughout history (e.g., Pink Floyd frontman Syd Barrett) have been associated with schizophrenia (Neel Burton, 2015). Do these anecdotes suggest that, at least in some cases, there is a link between schizophrenia and creativity?
Actually, the relationship between schizophrenia and creativity has been of particular interest for psychologists for a long time, and previous studies has provided mixed evidence reporting both positive and negative relationships. While some studies shown that people with schizophrenia are more creative and creative people are often associated with an elevated level of schizotypy and psychosis proneness (Burch et al., 2006; Karksson, 2010; Fink et al., 2014), there has been evidence showing the opposite results (Nemoto et al., 2005; Kyaga et al., 2013; Son et al., 2015). To further clarify the relationship, Acar et al. (2018) recently conducted a meta-analysis with 200 effect sizes obtained from 42 studies, and the result demonstrated that schizophrenia and creativity seem to have an inverted-U relationship, in which mild expression of schizophrenia symptoms (one or two minor signs of symptoms) may facilitate creativity but more severe signs of the symptoms would be detrimental to creativity.
Although the link between schizophrenia and creativity has received empirical support, there has been one important question remains to be answered; that is, how and by what mechanisms schizophrenia and creativity are correlated. The “shared vulnerability model” may help to answer this question. Based on findings from neuroimaging and genetic studies, Carson (2011) proposed the “shared vulnerability model” to explain the relationship between psychopathology and creativity. According to the model, psychiatric disorders (e.g., schizophrenia) and creativity may share specific overlapping cognitive processes; and more importantly, there might be a genetic link between psychiatric disorders and creativity. This model provided a new perspective to understand the link between schizophrenia and creativity, and highlighted the potential effects of schizophrenia risk variants on creativity.
Previous genetic epidemiological studies have indicated that schizophrenia is highly heritable. Due to the great attention that schizophrenia has attracted and the availability of large samples, a series of risk variants have recently been identified (Shi et al., 2009, 2011; Stefansson et al., 2009; Lee et al., 2013; Li et al., 2017). According to the “common disease/common variant” hypothesis, the risk alleles of these risk variants are also carried by healthy subjects in general populations and may thus impact brain development and cognitive functions of normal healthy subjects.
Consistent with this hypothesis, by examining the association between Neuregulin 1 gene (NRG1) and creativity, Kéri (2010) investigated for the first time the association between schizophrenia risk variants and creativity in healthy subjects. In a sample of healthy European subjects, Kéri (2010) showed that the TT genotype of NRG1 rs6994992 polymorphism, which has been previously related to schizophrenia risk (Wei et al., 2007; Kéri et al., 2009), was associated with higher creative achievements. This study provided the first direct evidence for the association between schizophrenia risk variant and creativity in healthy subjects, and suggested that NRG1 rs6994992 might be among the many risk variants that underlie the genetic link between schizophrenia and creativity. However, it is worth noting that, although NRG1 rs6994992 has been widely recognized as an important schizophrenia risk variant (Li et al., 2006; Jeremy et al., 2008; Buonanno, 2010), recent studies have also suggested an ethnic difference in the effect of NRG1 rs6994992 on schizophrenia (i.e., the relationship between NRG1 rs6994992 and schizophrenia was mainly observed in the European population, but not in the Han Chinese population) (Zhao et al., 2004; Mostaid et al., 2016). Considering this fact, to further validate whether NRG1 rs6994992 underlies the genetic link between schizophrenia and creativity, it is necessary to reevaluate the association between NRG1 rs6994992 and creativity in other populations. Thus, the first aim of this study was to clarify the relationship between NRG1 rs6994992, schizophrenia and creativity by validating the association between NRG1 rs6994992 and creativity in healthy subjects of Han Chinese population in which NRG1 rs6994992 is not a schizophrenia risk variant.
Moreover, as mentioned above, although attempts have been made to directly investigate the association between schizophrenia risk variants and creativity, this subject of research has not received enough attention in the literature. Until now, besides NRG1 rs6994992, the associations between other schizophrenia risk variants and creativity have not yet been explored, and it is therefore still unknown whether there have been other schizophrenia risk variants that underlie the genetic link between schizophrenia and creativity. With this in mind, the second aim of this study was to explore whether other reported schizophrenia risk variants are associated with creativity in healthy Han Chinese subjects.
Materials and Methods
Subjects
Subjects aged 18–55 years predominantly from Beijing, China, were recruited through Internet and social media advertisements. The initial inclusion criteria were: (1) written informed consent; (2) parents and grandparents were Han Chinese; (3) aged 18–55 years; (4) no self-reported history of neurological or psychiatric disorders (including substance abuse or dependence); (5) no current psychoactive medications. Recruitment began in November 2017 and ended in September 2018. A total of 624 subjects were enrolled into this study. Among these eligible subjects, 44 were excluded because of discordant gender information, low quality genotyping data (genotyping failure in more than 1% of genetic variants in Illumina Infinium Global Screening Array), genetic relatedness with other subjects, or missing phenotype data, leaving 580 subjects (310 males, 270 females) as the final sample. The subjects had a mean age of 26.43 years (SD = 5.82). Written informed consent forms were obtained from all subjects. This study was approved by the Ethics Committee of Psychology Department of Capital Normal University.
Measures of Creativity
Two alternative-uses tasks (“comb” and “toothpick”) were used in the present study to measure creativity. In these tasks, subjects were given 3 min to list as many uses as possible for each common object. Each participant’s responses were scored for the three core dimensions of creativity (fluency, flexibility, and originality). Specifically, the fluency score is the number of responses. The flexibility score is the number of categories used in one’s responses. The originality score is the number of unusual responses (given by less than 5% of all subjects). Finally, a total score was calculated by summing the scores of all three dimensions. Three trained graduate students from Capital Normal University rated all the responses.
Genetic Variants Selection and Genotyping
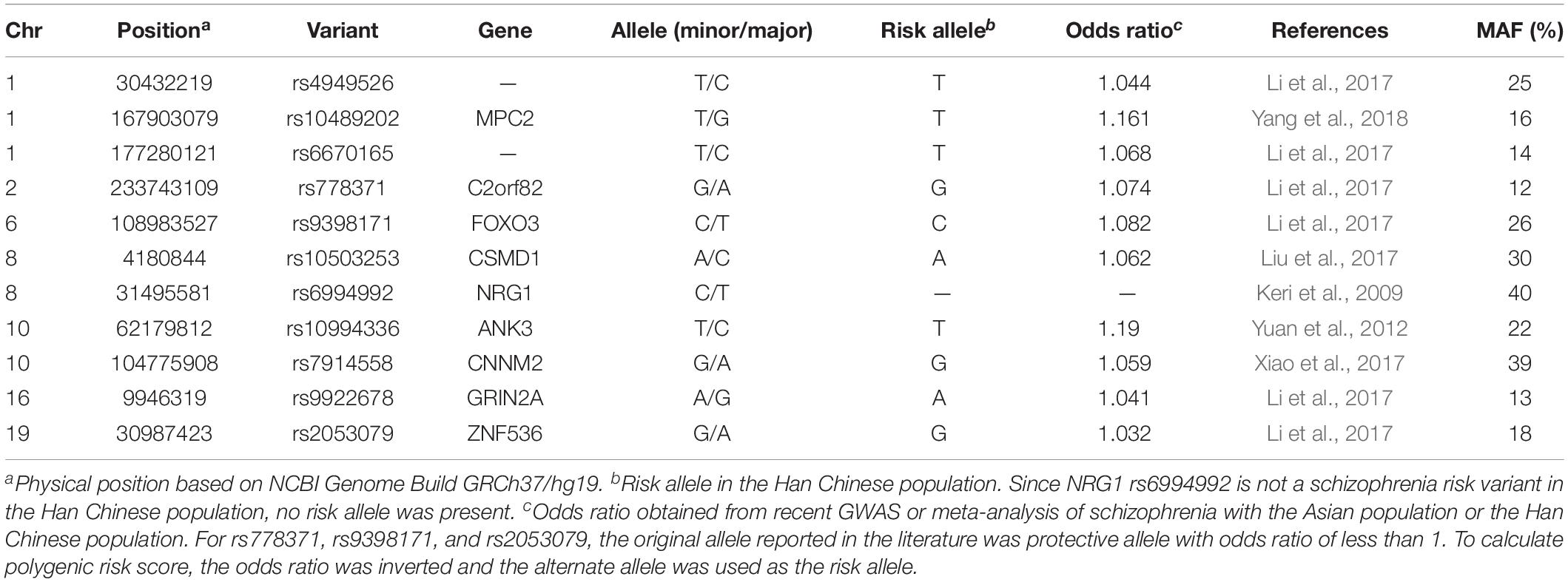
Besides NRG1 rs6994992, 10 other schizophrenia risk variants were also selected and genotyped in this study. The selection of these schizophrenia risk variants was mainly based on previous association studies of schizophrenia. The inclusion criteria was as follows: (a) risk variants that have been implicated in recent GWAS or meta-analysis of schizophrenia with the Asian population or the Han Chinese population (Yuan et al., 2012; Li et al., 2017; Liu et al., 2017; Xiao et al., 2017; Yang et al., 2018); (b) minor allele frequency (MAF) ≥5% in the Han Chinese population based on the 1000 Genomes Project database; (c) known or promising importance in neural developments and brain functions related to creativity.
Genomic DNA extracted from saliva samples was genotyped using an Illumina Infinium Global Screening Array (Illumina, San Diego, CA, United States) at Beijing Gese Technology Co., Ltd., All 11 genetic variants passed the quality control criteria of call rate >95%, MAF > 10%, and consistency with Hardy-Weinberg equilibrium (p > 0.05, Fisher exact test). The overview of information on all studied schizophrenia risk variants is provided in Table 1.
Statistical Analysis
To adjust for the possible presence of population stratification, principal component analysis (based on quality controlled and linkage disequilibrium pruned data of 132,888 variants from Illumina Infinium Global Screening Array) was applied using EIGENSOFT v7.2.1 software (Price et al., 2006). The first three principal components as well as gender and age (Matud et al., 2007; Palmiero et al., 2014, 2017; Palmiero, 2015) were included as covariates in all the genetic analyses mentioned below. Single variant analysis under additive genetic model was performed using linear regression in PLINK v1.07 software (Purcell et al., 2007). Polygenic risk score for schizophrenia risk variants was calculated by adding a given value for each variant with an associated risk allele. Weighted risk score was calculated using the scoring option implemented in PLINK. Each score was given a weight according to the natural log transformed odds ratio provided for each risk variant. Odds ratios for risk variants were obtained from recent GWAS or meta-analysis of schizophrenia with the Asian population or the Han Chinese population (see reference in Table 2). The unweighted risk score was calculated as the number of risk alleles counted for each subject. NRG1 rs6994992 was excluded from this analysis because it is not a risk variant in the Han Chinese population. Linear regression was used to test the associations between polygenic risk score and creativity measures. Power calculation for single variant analysis was performed using the QUANTO program (Knight, 2004).
Results
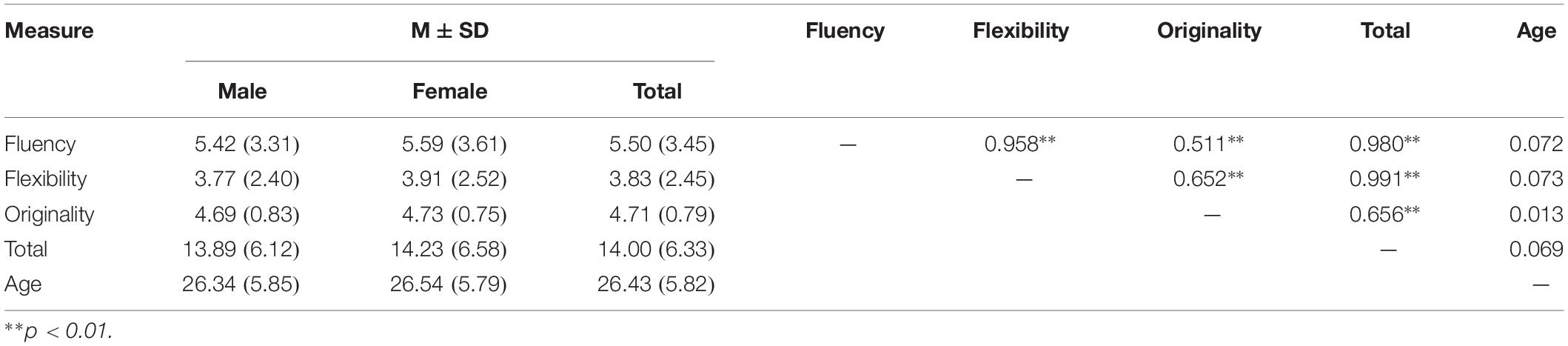
Descriptive statistics and inter-correlations are shown in Table 2. Significant correlations were observed between the four indexes of creativity measures, while no significant effect of gender and age was observed.
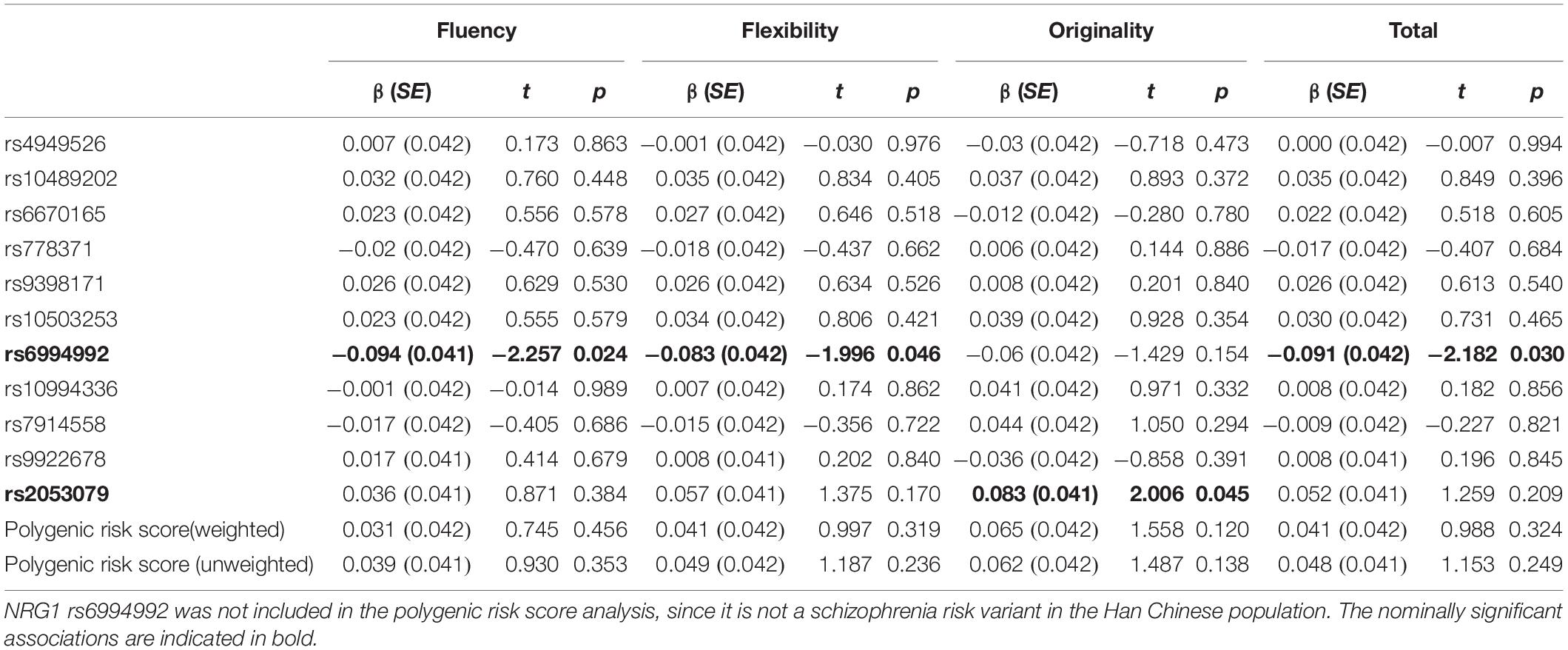
Table 3 summarizes the results of the single variant analysis and polygenic risk score analysis. Two SNPs showed nominal associations with creativity measures. Specifically, the NRG1 rs6994992 showed nominal associations with fluency (effect size R2 = 0.009), flexibility (effect size R2 = 0.007) and the total score (effect size R2 = 0.008), with the T allele being associated with higher scores; the ZNF536 rs2053079 showed a nominal association with originality (effect size R2 = 0.007), with the A allele being associated with higher scores. Power calculation showed that, for common genetic variants (MAF > 10%) under the additive model, our sample has 80% power to detect effect size (R2) greater than 0.0135 (with α = 0.05). As shown in Table 3, the result of polygenic risk score analysis showed that neither the weighted polygenic risk score nor the unweighted polygenic risk score was associated with the four indexes of creativity measures.
Discussion
Although the debate over the relationship between schizophrenia and creativity has continued in the literature, recent evidence has suggested that there might be a genetic link between schizophrenia and creativity. In this study, to explore the potential genetic link, 11 schizophrenia risk variants were genotyped and tested for their associations with creativity in a sample of healthy Han Chinese subjects.
Of the 11 schizophrenia risk variants, only NRG1 rs6994992 has been previously examined for its association with creativity (Kéri, 2010). The NRG1 encodes a key developmental growth factor that involved in neural development, neurotransmission and synaptic plasticity (Harrison and Law, 2006). The rs6994992 polymorphism is located in the type IV promoter region of NRG1 and the T allele of rs6994992 has been shown to be associated with increased transcript levels of type IV NRG1 in brains of both healthy and schizophrenic patients (Law et al., 2006). To demonstrate the role of rs6994992 in schizophrenia, many studies have been conducted to investigate the association between NRG1 rs6994992 and schizophrenia in different ethnic populations (e.g., Stefansson et al., 2002, 2003; Zhao et al., 2004; Petryshen et al., 2005; Li et al., 2006). Although the overall results generally supported the association between NRG1 rs6994992 and schizophrenia, there has also been evidence suggested an ethnic difference in the relationship between NRG1 rs6994992 and schizophrenia, with the association mainly being present in the European populations (Li et al., 2006; Mostaid et al., 2016; Jagannath et al., 2017). Specially, in the Asian population, several studies have suggested that NRG1 rs6994992 may not be a schizophrenia risk variant. For example, using both case-control and family-based designs, Zhao et al. (2004) and Li et al. (2006) showed that NRG1 rs6994992 was not associated with schizophrenia in the Han Chinese population. In samples of the Japanese and the Korean populations, Iwata et al. (2003) and Kim et al. (2006) showed that NRG1 rs6994992 was not associated with schizophrenia. Similar results were also obtained by three meta-analysis studies examining the association between NRG1 and schizophrenia (Li et al., 2006; Mostaid et al., 2016; Jagannath et al., 2017). Moreover, since schizophrenia and other psychiatric disorders may have shared genetic etiology (Berrettini, 2000; Bramon and Sham, 2001; Cardno et al., 2002; Craddock et al., 2009; Huang et al., 2010; Cross-Disorder Group of the Psychiatric Genomics Consortium Lee et al., 2013), several studies have also investigated the association between NRG1 rs6994992 and other psychiatric disorders such as bipolar disorder (e.g., Green et al., 2005; Cassidy et al., 2006; Thomson et al., 2007; Georgieva et al., 2008; Goes et al., 2009). While findings from these studies tended to support for the role of NRG1 rs6994992 (mainly as part of haplotype) in bipolar disorder, all these studies were conducted in the European population. According to the best of our knowledge, only a few studies have investigated the association between NRG1 and bipolar disorder in the Asian population (including the Han Chinese population), and none examined NRG1 rs6994992 (e.g., Crisafulli et al., 2013; Cao et al., 2014; Wen et al., 2016). Thus, whether NRG1 rs6994992 was also related to bipolar disorder in the Asian population remains to be elusive.
As for the relationship between NRG1 rs6994992 and creativity, Kéri (2010) first found that NRG1 rs6994992 was associated with creativity in a sample of healthy European subjects, and suggested that NRG1 rs6994992 may be one risk variant that underlies the genetic link between schizophrenia and creativity. In this study, the association between NRG1 rs6994992 and creativity was replicated in a sample of healthy Han Chinese subjects. However, since NRG1 rs6994992 was not a schizophrenia risk variant in the Han Chinese population, it was expected that no association between NRG1 rs6994992 and creativity would observed in our sample if NRG1 rs6994992 underlies the genetic link between schizophrenia and creativity. Therefore, the results of this study raised new questions about the role of NRG1 rs6994992 in the relationship between schizophrenia and creativity, and suggested ethnic differences may also exist in the relationship between NRG1 rs6994992, schizophrenia and creativity.
Ethnic difference has particular relevance for understanding the genetic basis of complex diseases and traits since many genes that are known to have been affected by natural selection are functionally important (Pickrell et al., 2009; Hofer et al., 2012; Field et al., 2016). The level of ethnic difference shown by diverse ethnic populations is related to allele frequency differences of genetic variants (Lan et al., 2007; Mattei et al., 2009). Because different populations are subject to distinct environments, certain environmental conditions may act through selective pressure to alter the frequencies of genetic variants and result in population-specific allele frequencies (Cavalli-Sforza et al., 1994; Chikhi et al., 1998; Cavalli-Sforza and Feldman, 2003; Myles et al., 2008; Hancock et al., 2011; Corona et al., 2013). Several studies have highlighted the importance of population-specific allele frequencies in gene expression and suggested that allele frequency differences of regulatory genetic variants (e.g., NRG1 rs6994992 located in promoter region of NRG1) may significantly influence gene expression and thus lead to differences in the genetic basis of complex diseases and traits across ethnic populations (Goddard et al., 2000; Ioannidis et al., 2004; Spielman et al., 2007; Myles et al., 2008).
In the case of NRG1 rs6994992, given that significant allele frequency differences were observed for the risk T allele between the Han Chinese population (60%) and the European population (37%), it is possible that such population-specific allele frequencies may account for some of the reasons for the ethnic difference in the relationship between NRG1 rs6994992, schizophrenia and creativity (i.e., allele frequency may moderate the relationship between NRG1 rs6994992, schizophrenia and creativity). Based on our results and the high frequency of the T allele in the Han Chinese population, it seems that while the association between NRG1 rs6994992 and schizophrenia is more subject to allele frequency of the T allele (the risk for schizophrenia mediated by the T allele might be masked by the high frequency of the T allele in the Han Chinese population), the association between NRG1 rs6994992 and creativity is not affected by allele frequency of the T allele and thus more consistent across different ethnic populations. And this consistency is supported by recent neuroimaging findings that NRG1 rs6994992 was associated with creativity-related brain structures and function in healthy subjects of different ethnic populations. For example, in healthy European subjects it has been shown that NRG1 rs6994992 was associated with differences in frontal brain structures in both gray and white matter (Hall et al., 2006; Mcintosh et al., 2008; Knickmeyer et al., 2014). And more recently, in healthy Chinese subjects NRG1 rs6994992 has been shown to be related to both structures and functional connectivity of frontal and temporal lobes, hippocampus and angular gyrus (Zhang et al., 2019).
Besides NRG1 rs6994992, 10 other schizophrenia risk variants were also investigated in this study and the results showed that only ZNF536 rs2053079 was nominally associated with creativity. Because ZNF536 rs2053079 has not been previously examined for its association with creativity, our result provides the first evidence for the association of this schizophrenia risk variant with creativity in healthy subjects. ZNF536 encodes a highly conserved novel zinc finger protein that is most abundant in the brain and negatively regulates neuron differentiation (Qin et al., 2009). Recent findings have suggested that ZNF536 may play an essential role in development of forebrain neurons (Thyme et al., 2019). The rs2053079 polymorphism is located in the intron region of ZNF536. Although the exact biological function of rs2053079 has not been established, this intron variant has been repeatedly implicated in recent large-scale GWAS of schizophrenia and creativity-related cognitive functions (Davies et al., 2018; Lee et al., 2018). Based on this evidence, it is possible that ZNF536 rs2053079 may play a role in the relationship between schizophrenia and creativity. However, it should also be noted that, owing to the very limited statistical power in this study, ZNF536 rs2053079 only showed nominal association with creativity. Since underpowered studies have increased risk of false positive results, this result may suffer from type I error. This coincides with the fact that the nominal associations between ZNF536 rs2053079 and creativity would not survive correction for multiple testing. Therefore, rather than hypothesis testing, the finding concerning the association between ZNF536 rs2053079 and creativity should be more viewed as hypothesis generating, replication in independent samples of different ethnic populations is warranted.
Evidence from recent GWAS has revealed that most complex diseases and traits are highly polygenic and influenced by hundreds or even thousands of variants with small effects (Manolio et al., 2009; Gratten et al., 2014; Robinson et al., 2014). To account for the polygenic nature and to increase statistical power to detect weak associations of small effects, the polygenic score approach has recently been proposed and applied in genetic studies of complex diseases and traits (Purcell et al., 2009; Dudbridge and Wray, 2013). For example, the polygenic score approach has been used by the Psychiatric Genomics Consortium and the International Schizophrenia Consortium to investigate major depression and schizophrenia (Lee et al., 2013). And more recently, Power et al. (2015) demonstrated that the polygenic risk scores for schizophrenia and bipolar disorder would predict artistic society membership or creative profession. In this study, to test the accumulated effect of the 11 schizophrenia risk variants, the polygenic score approach was also employed to examine the association between the polygenic risk score of these risk variants and creativity. However, the results showed that the polygenic risk score was not associated with creativity, indicating limited contribution of the accumulated effect of these risk variants to creativity. But anyway, given the highly polygenic nature of schizophrenia and creativity, the polygenic score approach held the promise to fully elucidate the potential genetic link between schizophrenia and creativity, and should be encouraged in future studies.
Several limitations of this study should be considered. First, some important background information concerning the subjects that may bias the results was not included in this study. In this study, self-report history of psychiatric disorders was used as an inclusion criterion of the subjects; however, such exclusion criteria may be insufficient. Under such inclusion criteria (without structured interview), subjects with undiagnosed psychiatric disorders (e.g., undiagnosed mood disorders) known to affect creativity as well as subjects with relatives of psychiatric disorders patients were likely to be included in this study, which may confound the results. Moreover, other important variables (e.g., educational level and IQ level) that were likely to influence the relationship between schizophrenia risk variants and creativity were not included in this study. For future study, the inclusion of these important variables as well as the application of more strict inclusion criteria would help to clarify the findings of this study and lead to a more accurate interpretation of the results. Second, as mentioned above, due to the small sample size, this study may not provide adequate power to detect weak associations of very small effect sizes. According to power calculation of single genetic variant analysis, for common genetic variants (MAF 10%), our sample only has sufficient (80% power) to detect effect size (R2) greater than 0.0135 (with α = 0.05), thus the possibility of type I and type II error cannot be excluded. It is important for future studies to validate these results in larger samples. Third, only very limited numbers of schizophrenia risk variants were examined in this study, other crucial risk variants were not included. Since both schizophrenia and creativity are highly polygenic, the potential genetic link between schizophrenia and creativity may rely on complex networks and interactions of multiple risk variants. To better reveal the genetic contribution of schizophrenia risk variants to creativity, such complex networks and interactions should be considered and examined in future studies. Forth, only divergent thinking test was used in this study as a measure of creativity, which may not accurately reflect subjects’ real creativity. Although divergent thinking is critical to creativity, creativity itself is a multi-aspects concept and each aspect may represent a unique and complex trait. Besides divergent thinking, it is also necessary for future studies to investigate the associations between schizophrenia risk variants and other components of creativity (e.g., creative problem solving, creative personality, and creative achievement). Fifth, the potential interactions between schizophrenia risk variants and environment factors were not examined in this study. It has been well acknowledged that both schizophrenia and creativity are determined by the complex interplays of genes and environmental factors (Tsuang, 2000; Kandler et al., 2016). To fully elucidate the link between schizophrenia and creativity, it is reasonable for future studies to include environmental factors (e.g., education background and family environment) and focus on the potential effects of gene-environment interactions on the relationship between schizophrenia and creativity.
CONCLUSION
In conclusion, this study provides limited evidence for the associations between these schizophrenia risk variants and creativity in healthy Han Chinese subjects. Future studies are warranted to better understand the potential genetic link between schizophrenia and creativity.
Members of the GeseDNA Research Team
Leilei Zhang, Qian Sun, Zenan Dou, Yanmeng Zou, Shan Guan, Hoyin Lo, and Lingzhi Song, Beijing Gese Technology Co., Ltd., Beijing, China.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
This study was carried out in accordance with the recommendations of local ethics guidelines, Ethics Committee of Psychology Department of Capital Normal University with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Ethics Committee of Psychology Department of Capital Normal University.
Author Contributions
DW, QG, TG, and GeseDNA Research Team collected the data. DW, SZ, JZ, and JL were involved in the conception and design of the work. DW and SZ contributed in writing the main text of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31871093 and 31671124), CNU’s Capacity Building for Sci-Tech Innovation – Fundamental Scientific Research Funds (025-185305000), the Beijing Municipal Commission of Education (TJSH20161002801), and the Beijing Brain Initiative of Beijing Municipal Science and Technology Commission.
Conflict of Interest
TG and GeseDNA Research Team were employed by company Beijing Gese Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the help and support provided by Dr. Xiaofei Wu. We thank all the members of Insight & Creativity Lab for their help.
References
Acar, S., Chen, X., and Cayirdag, N. (2018). Schizophrenia and creativity: a meta-analytic review. Schizophr. Res 195, 23–31. doi: 10.1016/j.schres.2017.08.036
Berrettini, W. H. (2000). Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol. Psychiatry 48, 531–538. doi: 10.1016/S0006-3223(00)00883-0
Bramon, E., and Sham, P. C. (2001). The common genetic liability between schizophrenia and bipolar disorder: a review. Curr. Psychiatry Rep. 3, 332–337. doi: 10.1007/s11920-001-0030-1
Buonanno, A. (2010). The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res. Bull. 83, 122–131. doi: 10.1016/j.brainresbull.2010.07.012
Burch, G. S. J., Pavelis, C., Hemsley, D. R., and Corr, P. J. (2006). Schizotypy and creativity in visual artists. Br. J. Psychol. 97, 177–190. doi: 10.1348/000712605X60030
Cao, L., Deng, W., Guan, L., Yang, Z., Lin, Y., Ma, X., et al. (2014). Association of the 3′ region of the neuregulin 1 gene with bipolar I disorder in the Chinese Han population. J. Affect. Disord. 162, 81–88. doi: 10.1016/j.jad.2014.03.037
Cardno, A. G., Rijsdijk, F. V., Sham, P. C., Murray, R. M., and McGuffin, P. (2002). A twin study of genetic relationships between psychotic symptoms. Am. J. Psychiatry 159, 539–545. doi: 10.1176/appi.ajp.159.4.539
Carson, S. H. (2011). Creativity and psychopathology: a shared vulnerability model. Can. J. Psychiatry 56, 144–153. doi: 10.1177/070674371105600304
Cassidy, F., Roche, S., Claffey, E., and McKeon, P. (2006). First family-based test for association of neuregulin with bipolar affective disorder. Mol. Psychiatry 11, 706–707.
Cavalli-Sforza, L., Menozzi, P., and Piazza, A. (1994). The History and Geography of Human Genes. Princeton, NJ: Princeton university press.
Cavalli-Sforza, L. L., and Feldman, M. W. (2003). The application of molecular genetic approaches to the study of human evolution. Nat. Genet. 33, 266–275. doi: 10.1038/ng1113
Chikhi, L., Destro-Bisol, G., Bertorelle, G., Pascali, V., and Barbujani, G. (1998). Clines of nuclear DNA markers suggest a largely Neolithic ancestry of the European gene pool. Proc. Natl. Acad. Sci. U.S.A. 95, 9053–9058. doi: 10.1073/pnas.95.15.9053
Corona, E., Chen, R., Sikora, M., Morgan, A. A., Patel, C. J., Ramesh, A., et al. (2013). Analysis of the genetic basis of disease in the context of worldwide human relationships and migration. PLoS Genet. 9:e1003447. doi: 10.1371/journal.pgen.1003447
Craddock, N., O’Donovan, M. C., and Owen, M. J. (2009). Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophr. Bull. 35, 482–490. doi: 10.1093/schbul/sbp020
Crisafulli, C., Chiesa, A., Han, C., Lee, S.-J., Balzarro, B., Andrisano, C., et al. (2013). Case-control association study of 36 single-nucleotide polymorphisms within 10 candidate genes for major depression and bipolar disorder. Psychiatry Res. 209, 121–123. doi: 10.1016/j.psychres.2012.11.009
Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee, S. H., Ripke, S., Neale, B. M., Faraone, S. V., Purcell, S. M., et al. (2013). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 45, 984–994. doi: 10.1038/ng.2711
Davies, G., Lam, M., Harris, S. E., Trampush, J. W., Luciano, M., Hill, W. D., et al. (2018). Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun. 9:2098. doi: 10.1038/s41467-018-04362-x
Dudbridge, F., and Wray, N. R. (2013). Power and predictive accuracy of polygenic risk scores. PLoS Genet. 9:e1003348. doi: 10.1371/journal.pgen.1003348
Field, Y., Boyle, E. A., Telis, N., Gao, Z., Gaulton, K. J., Golan, D., et al. (2016). Detection of human adaptation during the past 2000 years. Science 354, 760–764. doi: 10.1126/science.aag0776
Fink, A., Weber, B., Koschutnig, K., Benedek, M., Reishofer, G., Ebner, F., et al. (2014). Creativity and schizotypy from the neuroscience perspective. Cogn. Affect. Behav. Neurosci. 14, 378–387. doi: 10.3758/s13415-013-0210-6
Georgieva, L., Dimitrova, A., Ivanov, D., Nikolov, I., Williams, N. M., Grozeva, D., et al. (2008). Support for neuregulin 1 as a susceptibility gene for bipolar disorder and schizophrenia. Biol. Psychiatry 64, 419–427. doi: 10.1016/j.biopsych.2008.03.025
Goddard, K. A. B., Hopkins, P. J., Hall, J. M., and Witte, J. S. (2000). Linkage disequilibrium and allele-frequency distributions for 114 single-nucleotide polymorphisms in five populations. Am. J. Hum. Genet. 66, 216–234. doi: 10.1086/302727
Goes, F. S., Willour, V. L., Zandi, P. P., Belmonte, P. L., MacKinnon, D. F., Mondimore, F. M., et al. (2009). Family-based association study of Neuregulin 1 with psychotic bipolar disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150, 693–702. doi: 10.1002/ajmg.b.30895
Gratten, J., Wray, N. R., Keller, M. C., and Visscher, P. M. (2014). Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat. Neurosci. 17, 782–790. doi: 10.1038/nn.3708
Green, E. K., Raybould, R., Macgregor, S., Gordon-Smith, K., Heron, J., Hyde, S., et al. (2005). Operation of the schizophrenia susceptibility gene, neuregulin 1, across traditional diagnostic boundaries to increase risk for bipolar disorder. Arch Gen. Psychiatry 62, 642–648. doi: 10.1001/archpsyc.62.6.642
Hall, J., Whalley, H. C., Job, D. E., Baig, B. J., McIntosh, A. M., Evans, K. L., et al. (2006). A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat. Neurosci. 9, 1477–1478. doi: 10.1038/nn1795
Hancock, A. M., Witonsky, D. B., Alkorta-Aranburu, G., Beall, C. M., Gebremedhin, A., Sukernik, R., et al. (2011). Adaptations to climate-mediated selective pressures in humans. PLoS Genet. 7:e1001375. doi: 10.1371/journal.pgen.1001375
Harrison, P. J., and Law, A. J. (2006). Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol. Psychiatry 60, 132–140. doi: 10.1016/j.biopsych.2005.11.002
Hofer, T., Foll, M., and Excoffier, L. (2012). Evolutionary forces shaping genomic islands of population differentiation in humans. BMC Genomics 13:107. doi: 10.1186/1471-2164-13-107
Huang, J., Perlis, R. H., Lee, P. H., Rush, A. J., Fava, M., Sachs, G. S., et al. (2010). Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am. J. Psychiatry 167, 1254–1263. doi: 10.1176/appi.ajp.2010.09091335
Ioannidis, J. P., Ntzani, E. E., and Trikalinos, T. A. (2004). ‘acial’differences in genetic effects for complex diseases. Nat. Genet. 36, 1312–1318. doi: 10.1038/ng1474
Iwata, N., Suzuki, T., Ikeda, M., Kitajima, T., Yamanouchi, Y., Inada, T., et al. (2003). No association with the neuregulin 1 haplotype to Japanese schizophrenia. Mol. Psychiatry. 9, 126–127. doi: 10.1038/sj.mp.4001456
Jagannath, V., Theodoridou, A., Gerstenberg, M., Franscini, M., Heekeren, K., Correll, C. U., et al. (2017). Prediction analysis for transition to schizophrenia in individuals at clinical high risk for psychosis: the relationship of DAO, DAOA, and NRG1 variants with negative symptoms and cognitive deficits. Front. Psychiatry. 8:292. doi: 10.3389/fpsyt.2017.00292
Jeremy, H., Whalley, H. C., Job, D. E., Baig, B. J., Mcintosh, A. M., Evans, K. L., et al. (2008). A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat. Neurosci. 9, 1477–1478. doi: 10.1038/nn1795
Kim, J. W., Lee, Y.-S., Cho, E.-Y., Jang, Y. L., Park, D. Y., Choi, K.-S., et al. (2006). Linkage and association of schizophrenia with genetic variations in the locus of neuregulin 1 in Korean population. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 141B, 281–286. doi: 10.1002/ajmg.b.30209
Kandler, C., Riemann, R., Angleitner, A., Spinath, F. M., and Penke, L. (2016). The nature of creativity: the roles of genetic factors, personality traits, cognitive abilities, and environmental sources. J. Pers. Soc Psychol. 111, 230–249. doi: 10.1037/pspp0000087
Karksson, J. L. (2010). Genetic association of giftedness and creativity with schizophrenia. Hereditas 66, 177–181. doi: 10.1111/j.1601-5223.1970.tb02343.x
Kéri, S. (2010). Genes for psychosis and creativity: a promoter polymorphism of the neuregulin 1 gene is related to creativity in people with high intellectual achievement. Psychol. Sci. 20, 1070–1073. doi: 10.1111/j.1467-9280.2009.02398.x
Keri, S., Kiss, I., and Kelemen, O. (2009). Effects of a neuregulin 1 variant on conversion to schizophrenia and schizophreniform disorder in people at high risk for psychosis. Mol. Psychiatry 14, 118–119. doi: 10.1038/mp.2008.1
Kéri, S., Kiss, I., Seres, I., and Kelemen, O. (2009). A polymorphism of the neuregulin 1 gene (SNP8NRG243177/rs6994992) affects reactivity to expressed emotion in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 418–420. doi: 10.1002/ajmg.b.30812
Knickmeyer, R. C., Jiaping, W., Hongtu, Z., Xiujuan, G., Sandra, W., Hamer, R. M., et al. (2014). Common variants in psychiatric risk genes predict brain structure at birth. Cereb. Cortex 24, 1230–1246. doi: 10.1093/cercor/bhs401
Knight, J. (2004). A survey of current software for genetic power calculations. Hum. Genomics 1, 225–227. doi: 10.1186/1479-7364-1-3-225
Kyaga, S., Landén, M., Boman, M., Hultman, C. M., Långström, N., and Lichtenstein, P. (2013). Mental illness, suicide and creativity: 40-year prospective total population study. J. Psychiatric. Res. 47, 83–90. doi: 10.1016/j.jpsychires.2012.09.010
Lan, Q., Shen, M., Garcia-Rossi, D., Chanock, S., Zheng, T., Berndt, S. I., et al. (2007). Genotype frequency and F ST analysis of polymorphisms in immunoregulatory genes in Chinese and Caucasian populations. Immunogenetics 59, 839–852. doi: 10.1007/s00251-007-0253-3
Law, A. J., Lipska, B. K., Cynthia Shannon, W., Hyde, T. M., Straub, R. E., Ryota, H., et al. (2006). Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc. Natl. Acad. Sci. U.S.A. 103, 6747–6752. doi: 10.1073/pnas.0602002103
Lee, J. J., Wedow, R., Okbay, A., Kong, E., Maghzian, O., Zacher, M., et al. (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121. doi: 10.1038/s41588-018-0147-3
Lee, S. H., Ripke, S., Neale, B. M., Faraone, S.V., Purcell, S.M., Perlis, R. H., et al. (2013). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 45, 984–994. doi: 10.1038/ng.2711
Li, D., Collier, D. A., and He, L. (2006). Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum. Mol. Genet. 15, 1995–2002. doi: 10.1093/hmg/ddl122
Li, Z., Chen, J., Yu, H., He, L., Xu, Y., Zhang, D., et al. (2017). Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat Genet. 49, 1576–1583. doi: 10.1038/ng.3973
Liu, W., Liu, F., Xu, X., and Bai, Y. (2017). Replicated association between the European GWAS locus rs10503253 at CSMD1 and schizophrenia in Asian population. Neurosci. Lett. 647, 122–128. doi: 10.1016/j.neulet.2017.03.039
Manolio, T. A., Collins, F. S., Cox, N. J., Goldstein, D. B., Hindorff, L. A., Hunter, D. J., et al. (2009). Finding the missing heritability of complex diseases. Nature 461, 747–753. doi: 10.1038/nature08494
Mattei, J., Parnell, L. D., Lai, C. Q., Garciabailo, B., Xian, A., Shen, J., et al. (2009). Disparities in allele frequencies and population differentiation for 101 disease-associated single nucleotide polymorphisms between Puerto Ricans and non-Hispanic whites. BMC Genetics 10:45. doi: 10.1186/1471-2156-10-45
Matud, M. P., Rodríguez, C., and Grande, J. (2007). Gender differences in creative thinking. Pers. Individ. Dif. 43, 1137–1147. doi: 10.1016/j.paid.2007.03.006
Mcintosh, A., Moorhead, T. D., Lymer, G., Munoz-Maniega, S., Mckirdy, J., Sussmann, J., et al. (2008). The effects of a neuregulin 1 variant on white matter density and integrity. Mol. Psychiatry 13, 1054–1059. doi: 10.1038/sj.mp.4002103
Mostaid, M. S., Lloyd, D., Liberg, B., Sundram, S., Pereira, A., Pantelis, C., et al. (2016). Neuregulin-1 and schizophrenia in the genome-wide association study era. Neurosci. Biobehav. Rev. 68, 387–409. doi: 10.1016/j.neubiorev.2016.06.001
Myles, S., Dan, D., Barrett, J., Stoneking, M., and Timpson, N. (2008). Worldwide population differentiation at disease-associated SNPs. BMC Med. Genomics 1:22. doi: 10.1186/1755-8794-1-22
Neel Burton, M. D. (2015). Mad Genius: Schizophrenia and Creativity. Available at: https://www.psychologytoday.com/us/blog/hide-and-seek/201509/mad-genius-schizophrenia-and-creativity.
Nemoto, T., Mizuno, M., and Kashima, H. (2005). Qualitative evaluation of divergent thinking in patients with schizophrenia. Behav. Neurol. 16, 217–224. doi: 10.1155/2005/386932
Palmiero, M. (2015). The effects of age on divergent thinking and creative objects production: a cross-sectional study. High Abil. Stud. 26, 93–104. doi: 10.1080/13598139.2015.1029117
Palmiero, M., Di Giacomo, D., and Passafiume, D. (2014). Divergent thinking and age-related changes. Creat. Res. J. 26, 456–460. doi: 10.1080/10400419.2014.961786
Palmiero, M., Nori, R., and Piccardi, L. (2017). Verbal and visual divergent thinking in aging. Exp. Brain Res. 235, 1021–1029. doi: 10.1007/s00221-016-4857-4
Petryshen, T., Middleton, F., Kirby, A., Aldinger, K., Purcell, S., Tahl, A., et al. (2005). Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol. Psychiatry 10, 366–374. doi: 10.1038/sj.mp.4001608
Pickrell, J. K., Coop, G., Novembre, J., Kudaravalli, S., Li, J. Z., Absher, D., et al. (2009). Signals of recent positive selection in a worldwide sample of human populations. Genome Res. 19, 826–837. doi: 10.1101/gr.087577.108
Power, R. A., Steinberg, S., Bjornsdottir, G., Rietveld, C. A., Abdellaoui, A., Nivard, M. M., et al. (2015). Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat. Neurosci. 18, 953–955. doi: 10.1038/nn.4040
Price, A. L., Patterson, N. J., Plenge, R. M., Weinblatt, M. E., Shadick, N. A., and Reich, D. (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909. doi: 10.1038/ng1847
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575. doi: 10.1086/519795
Purcell, S. M., Wray, N. R., Stone, J. L., Visscher, P. M., O’Donovan, M. C., Sullivan, P. F., et al. (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752. doi: 10.1038/nature08185
Qin, Z., Ren, F., Xu, X., Ren, Y., Li, H., Wang, Y., et al. (2009). ZNF536, a novel zinc finger protein specifically expressed in the brain, negatively regulates neuron differentiation by repressing retinoic acid-induced gene transcription. Mol. Cell Biol. 29, 3633–3643. doi: 10.1128/MCB.00362-09
Robinson, M. R., Wray, N. R., and Visscher, P. M. (2014). Explaining additional genetic variation in complex traits. Trends Genet. 30, 124–132. doi: 10.1016/j.tig.2014.02.003
Shi, J., Levinson, D. F., Duan, J., Sanders, A. R., Zheng, Y., Pe’Er, I., et al. (2009). Common variants on chromosome 6p22. 1 are associated with schizophrenia. Nature 460, 753–757. doi: 10.1038/nature08192
Shi, Y., Li, Z., Xu, Q., Wang, T., Li, T., Shen, J., et al. (2011). Common variants on 8p12 and 1q24. 2 confer risk of schizophrenia. Nat. Genet. 43, 1224–1227. doi: 10.1038/ng.980
Son, S., Kubota, M., Miyata, J., Fukuyama, H., Aso, T., Urayama, S. I., et al. (2015). Creativity and positive symptoms in schizophrenia revisited: structural connectivity analysis with diffusion tensor imaging. Schizophr. Res. 164, 221–226. doi: 10.1016/j.schres.2015.03.009
Spielman, R. S., Bastone, L. A., Burdick, J. T., Morley, M., Ewens, W. J., and Cheung, V. G. (2007). Common genetic variants account for differences in gene expression among ethnic groups. Nat. Genet. 39, 226–231. doi: 10.1038/ng1955
Stefansson, H., Ophoff, R. A., Steinberg, S., Andreassen, O. A., Cichon, S., Rujescu, D., et al. (2009). Common variants conferring risk of schizophrenia. Nature 460, 744–747. doi: 10.1038/nature08186
Stefansson, H., Petursson, H., Sigurdsson, E., Steinthorsdottir, V., Bjornsdottir, S., Sigmundsson, T., et al. (2002). Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 71, 877–892. doi: 10.1086/342734
Stefansson, H., Sarginson, J., Kong, A., Yates, P., Steinthorsdottir, V., Gudfinnsson, E., et al. (2003). Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am. J. Hum. Genet. 72, 83–87. doi: 10.1086/345442
Thomson, P., Christoforou, A., Morris, S., Adie, E., Pickard, B., Porteous, D., et al. (2007). Association of Neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Mol. Psychiatry 12, 94–104. doi: 10.1038/sj.mp.4001889
Thyme, S. B., Pieper, L. M., Li, E. H., Pandey, S., Wang, Y., Morris, N. S., et al. (2019). Phenotypic landscape of schizophrenia-associated genes defines candidates and their shared functions. Cell 177, 478.–491. doi: 10.1016/j.cell.2019.01.048
Tsuang, M. (2000). Schizophrenia: genes and environment. Biol. Psychiatry 47, 210–220. doi: 10.1016/S0006-3223(99)00289-9
Wei, T., Yanhong, W., Bert, G., Jingshan, C., Michael, D., Harrison, P. J., et al. (2007). Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J. Biol. Chem. 282, 24343–24351. doi: 10.1074/jbc.m702953200
Wen, Z., Chen, J., Khan, R. A. W., Song, Z., Wang, M., Li, Z., et al. (2016). Genetic association between NRG1 and schizophrenia, major depressive disorder, bipolar disorder in Han Chinese population. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 171, 468–478. doi: 10.1002/ajmg.b.32428
Xiao, X., Luo, X.-J., Chang, H., Liu, Z., and Li, M. (2017). Evaluation of European schizophrenia GWAS loci in Asian populations via comprehensive meta-analyses. Mol. Neurobiol. 54, 4071–4080. doi: 10.1007/s12035-016-9990-3
Yang, Y., Wang, L., Li, L., Li, W., Zhang, Y., Chang, H., et al. (2018). Genetic association and meta-analysis of a schizophrenia GWAS variant rs10489202 in East Asian populations. Transl. Psychiatry 8:144. doi: 10.1038/s41398-018-0211-x
Yuan, A., Yi, Z., Wang, Q., Sun, J., Li, Z., Du, Y., et al. (2012). ANK3 as a risk gene for schizophrenia: new data in han Chinese and meta analysis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 997–1005. doi: 10.1002/ajmg.b.32107
Zhang, W., Cai, S., Huang, K., Lv, Y., Kang, Y., Wang, Q., et al. (2019). Association between schizophrenia risk allele dosage of rs6994992 and whole-brain structural and functional characteristics. Psychiat. Res. Neuroim. doi: 10.1016/j.pscychresns.2019.05.005 [Epub ahead of print].
Keywords: schizophrenia, creativity, genes, divergent thinking, risk variants
Citation: Wang D, Guo T, Guo Q, Zhang S, Zhang J, Luo J and GeseDNA Research Team (2019) The Association Between Schizophrenia Risk Variants and Creativity in Healthy Han Chinese Subjects. Front. Psychol. 10:2218. doi: 10.3389/fpsyg.2019.02218
Received: 20 March 2019; Accepted: 17 September 2019;
Published: 01 October 2019.
Edited by:
Drozdstoy Stoyanov Stoyanov, Plovdiv Medical University, BulgariaReviewed by:
Fanglin Guan, Xi’an Jiaotong University Health Science Center, ChinaOkan Caliyurt, Trakya University, Turkey
Copyright © 2019 Wang, Guo, Guo, Zhang, Zhang, Luo and GeseDNA Research Team. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingting Guo, dGluZ3RpbmcuZ3VvQGdlc2VkbmEuY29t; Shun Zhang, eWlueGluZ3JlbjE5ODZAaG90bWFpbC5jb20=; Jinghuan Zhang, emhhbmdqaW5naHVhbkBzZG51LmVkdS5jbg==; Jing Luo, bHVvakBwc3ljaC5hYy5jbg==
 Dan Wang
Dan Wang Tingting Guo2*
Tingting Guo2* Jing Luo
Jing Luo