
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol., 08 October 2019
Sec. Quantitative Psychology and Measurement
Volume 10 - 2019 | https://doi.org/10.3389/fpsyg.2019.02147
 Chiara Marzorati1,2*
Chiara Marzorati1,2* Dario Monzani1,2
Dario Monzani1,2 Ketti Mazzocco1,2
Ketti Mazzocco1,2 Francesca Pavan3
Francesca Pavan3 Massimo Monturano3
Massimo Monturano3 Gabriella Pravettoni1,2
Gabriella Pravettoni1,2Background: This study aims to validate and evaluate the psychometric properties and measurement invariance of the Italian version of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30), which is a measure of quality of life (QoL) for lung cancer patients after surgery.
Methods: A total of 167 lung cancer patients completed the Italian version of the EORTC QLQ-C30 questionnaire at 30 days after they received a lobectomy. The factor structure of this scale was assessed by performing confirmatory factor analysis (CFA). Measurement invariance was evaluated by considering differential item functioning (DIF) due to age, gender, and type of surgery (i.e., robot- or not robot-assisted).
Results: The CFA demonstrated the validity of the factor structure of the EORTC QLQ-C30 in assessing overall health and eight distinct subscales of adverse events and functioning. Moreover, the results highlighted a minimal DIF with only trivial consequences on measurement invariance. Specifically, the DIF did not affect the mean differences of latent scores of QoL between patients undergoing robot-assisted surgery or traditional surgery.
Conclusion: These findings supported the validity and suitability of the EORTC QLQ-C30 for the assessment of QoL in lung cancer patients of diverse ages and genders undergoing lobectomy with or without robot-assisted surgery.
Patient reported outcomes (PROs) have become important factors in cancer care to measure the patient’s perception of the health status, including treatment side effects, functional impairments, and health-related QoL (U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research et al., 2006; Mercieca-Bebber et al., 2018). Through the QoL assessment, it would be possible to obtain a more complete framework of the medical condition, especially for diseases requiring long-term care services. Among the large amount of developed instruments to evaluate patient well-being, the EORTC QLQ-C30 is the most used tool for assessing QoL in cancer-specific patients (Iravani et al., 2018). The EORTC QLQ-C30 consists of 30 self-reported questions assessing different aspects of patient functioning, global health status, and cancer-related symptoms. More specifically, it is composed of five multi-item functional scales (role, physical, cognitive, emotional, and social functioning), three multi-item symptom scales (fatigue, pain, and nausea and vomiting), individual items concerning common symptoms in cancer patients (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties), and two questions assessing overall QoL. All of the multi-item scales and single-item measures range in a score from 0 to 100, where a high score represents a higher response level. Thus, a high score for a functional scale implicates a healthy level of functioning, while a high score for a symptom scale represents a worse level of symptoms (Aaronson et al., 1993). The EORTC QLQ-C30 has been translated in over 110 languages and validated in many countries in different samples of cancer patients (Bjordal et al., 2000; EORTC Quality of Life Group, 2019). According to a cross-cultural project on a large and heterogeneous sample, the EORTC QoL Group reported robust measurement properties across various countries and languages (Scott et al., 2006, 2007). In Italy, the questionnaire has been validated only in breast and colon cancer patients (Apolone et al., 1998; Mosconi et al., 2002; Winters et al., 2014). At the same time, other authors investigated the applicability of the EORTC QLQ-C30 structure, and positively demonstrated its invariance across different cancer sites (Costa et al., 2015). Despite these psychometric properties, few scientific articles performed factor analysis for validating this tool in lung cancer patients, a clinical area in which the EORTC QLQ-C30 is the most used instrument to report patient well-being through the different phases of disease (Damm et al., 2013). To our knowledge, no published articles investigated the psychometric properties and the measurement invariance of the Italian version of the EORTC QLQ-C30 in lung cancer patients. In fact, only four studies measured QoL in Italian lung cancer patients through the administration of the EORTC QLQ-C30. Two of them were international studies and involved several countries, with all of them focusing on non-small cell lung cancer (Di Maio et al., 2004; Maione et al., 2005; Koller et al., 2017; Wood et al., 2019).
The purpose of the current study was to evaluate the factor structure proposed by Costa et al. (2015) for the EORTC QLQ-C30 in a sample of postoperative lung cancer patients who underwent lobectomy surgery. Moreover, its measurement invariance across patients of varying age, gender, and undergoing robotic or traditional surgery was also evaluated. The testing of measurement invariance is a necessary step to further evaluate any inter-individual differences.
An Italian sample of 167 patients with lung cancer who were also undergoing lobectomy were recruited for the value-based project1 at the European Institute of Oncology in Milan between October 2015 and October 2017. Patients were included in the study if they: (1) were diagnosed with lung cancer, (2) were native Italian speakers, (3) referred to the value-based project, and (4) did not have neurological or psychopathological problems. They completed the EORTC QLQ-C30 after 30 days from surgery (Aaronson et al., 1993; Apolone et al., 1998). During the doctor’s post-operative visit, a trained nurse distributed the questionnaire to the patients and they completed it using paper and pencil. Informed consent was provided and signed by each participant. Participation in the study was voluntary and at each moment, patients could withdraw their consent. The study was developed in accordance with the principles stated in the Declaration of Helsinki (59th WMA General Assembly, Seoul, 2008) and was approved by the European Institute of Oncology Ethical Committee at the European Institute of Oncology, Milan, Italy.
All statistical analyses were performed using the maximum likelihood with robust standard errors (MLR) estimation method with Mplus 8.2 (Muthen and Muthén, 2017). The MLR estimator is robust to strong departures from univariate and multivariate normality of observed variables. The EORTC QLQ-C30 comprises nine multiple-item dimensions and six single items. In a first step, the proposed model for the EORTC QLQ-C30 was assessed through CFA. Specifically, as reported in Figure 1, the measurement model included the nine multiple item dimensions of physical functioning (five items), role functioning (two items), emotional functioning (four items), social functioning (two items), cognitive functioning (two items), pain (two items), fatigue (three items), nausea and vomiting (two items), and overall health and QoL (two items). Following Costa et al. (2015), the six single-item dimensions (i.e., dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties) were omitted from the tested model. For ease of interpretation, the covariances among latent dimensions of QoL were not reported in the figure, but they were all estimated in the analyses.
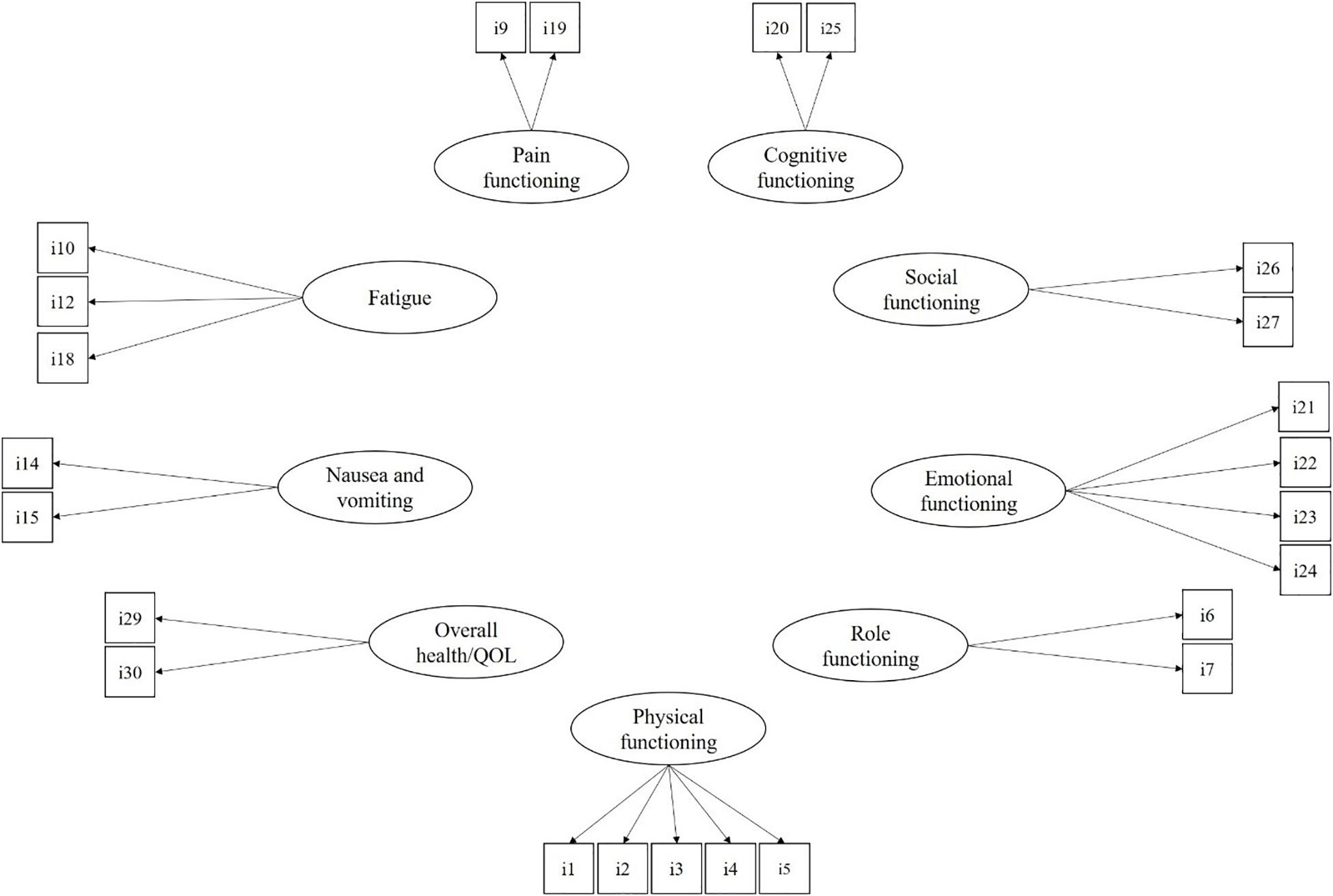
Figure 1. The measurement model for the EORTC QLQ-C30. For ease of interpretation, covariances among latent factors are not reported but estimated in the CFA model.
Model fit was assessed by considering five main fit indices. Specifically, a good-fitting model was indicated by a non-significant χ2, a RMSEA below 0.06, a SRMR <0.80, a CFI, and a TLI >0.90 (Hu and Bentler, 1999). Moreover, the 90% confidence interval for RMSEA was considered to test the null hypothesis of poor model fit. Specifically, a good-fitting model was indicated by the upper limit <0.08 and the lower limit close to zero. Finally, we considered the PCLOSE as well, a one-sided test of the null hypothesis that the model has a close fit (i.e., RMSEA equals 0.05). P-value >0.50 indicated a good-fitting model (Jöreskog and Sörbom, 1996).
Then, measurement invariance was evaluated by considering DIF. DIF is a prerequisite for a valid and meaningful comparison of levels of QoL across gender, age, and type of surgery (robot-assisted vs. traditional surgery). Specifically, a MIMIC was performed to assess differences in the measurement model due to age, gender, and type of surgery. A MIMIC model was performed because it has specific advantages over multiple-group CFA (MCFA) in evaluating measurement invariance. Specifically, compared to MCFA, the MIMIC model permits to: assess differences in the measurement model due to several confounding variables; simultaneously evaluate the role of dichotomous (i.e., robot-assisted vs. not robot-assisted surgery and gender) and continuous variables (i.e., age); include directly in the model continuous variables without median-splitting, mean-splitting, or subjective categorization; and test measurement invariance even with small sample size. Thus, mainly because of the low sample size, we preferred the MIMIC model over the MCFA to assess the structural invariance of the EORTC QLQ-C30. In the last decade, MIMIC model had been adopted to validly test measurement invariance of self-report measure of QoL in asthma (Mora et al., 2009) and pediatric patients (Stevanovic et al., 2016), life satisfaction (Jang et al., 2017), dispositional optimism (Steca et al., 2017), protective behavioral strategies (Treloar et al., 2014), adolescent burnout (Li et al., 2013), and depression (Skule et al., 2014).
The MIMIC model included the measurement model (i.e., the EORTC QLQ-C30 factor structure) plus a structural model assessing DIF. This structural model estimated the effect of covariates of gender, age, and type of surgery on latent dimensions of QoL and, thus, evaluated differences in these latent factors due to the three considered covariates. The structural model included the direct effects of these three covariates on items as well. In a first step, these direct effects fixed at zero. Then, modification indices were examined to ascertain whether the estimation of any of these direct effects would improve model fit. Estimation of direct effects was performed with a stepwise approach: the constraint that resulted in the greatest change of χ2 (i.e., highest value of the modification index) were firstly estimated. We then continued at freely estimating one direct effect at time until any modification was relevant (i.e., Δχ2 > 3.84). Each significant direct effect was interpreted as an indication of DIF: the likelihood to endorse an item was conditional to the specific covariate involved in the direct effect. For example, if the direct effect of age on item 1 was significant and positive, then the likelihood of endorsing this item differed between patients of different age and, specifically, younger people had lower chance to endorse this item. Thus, measurement invariance may be strongly impaired when high degree of DIF is ascertained. Age was treated as a continuous variable, whereas gender (i.e., male = 0; female = 1) and type of surgery (i.e., not robot-assisted surgery = 0; robot-assisted surgery = 1) were binary variables.
Participants had a mean age of 66.69 ± 7.70 and 100 (59.9%) of them were males. The sample underwent lobectomy surgical procedure (N = 54; 32.3% with robot-assisted surgery; N = 113; 67.7% with not robot-assisted surgery). Other clinical variables are reported in Table 1.
Descriptive statistics of item response (mean, standard deviation, and minimum and maximum) are reported in Table 2.
The proposed measurement model for the EORTC QLQ-C30 showed a good fit [χ2(216, N = 167) = 301.48; RMSEA = 0.05; 90% CI of RMSEA = 0.04–0.06; PCLOSE = 0.555; CFI = 0.95; TLI = 0.93; SRMR = 0.05]. The standardized loadings are displayed in Table 3. As reported, all the items had significant loadings and high loadings ranging from 0.36 to 1.08, except for i5 (λ = 0.17; SE = 0.07; p < 0.05) and i15 (λ = 0.13; SE = 0.12; p > 0.05). Specifically, while high scores of pain, fatigue, nausea and vomiting, and physical, role, cognitive, emotional, and social functioning indicated high levels of impairment, high values of overall health and QoL denoted high levels of health-related QoL.
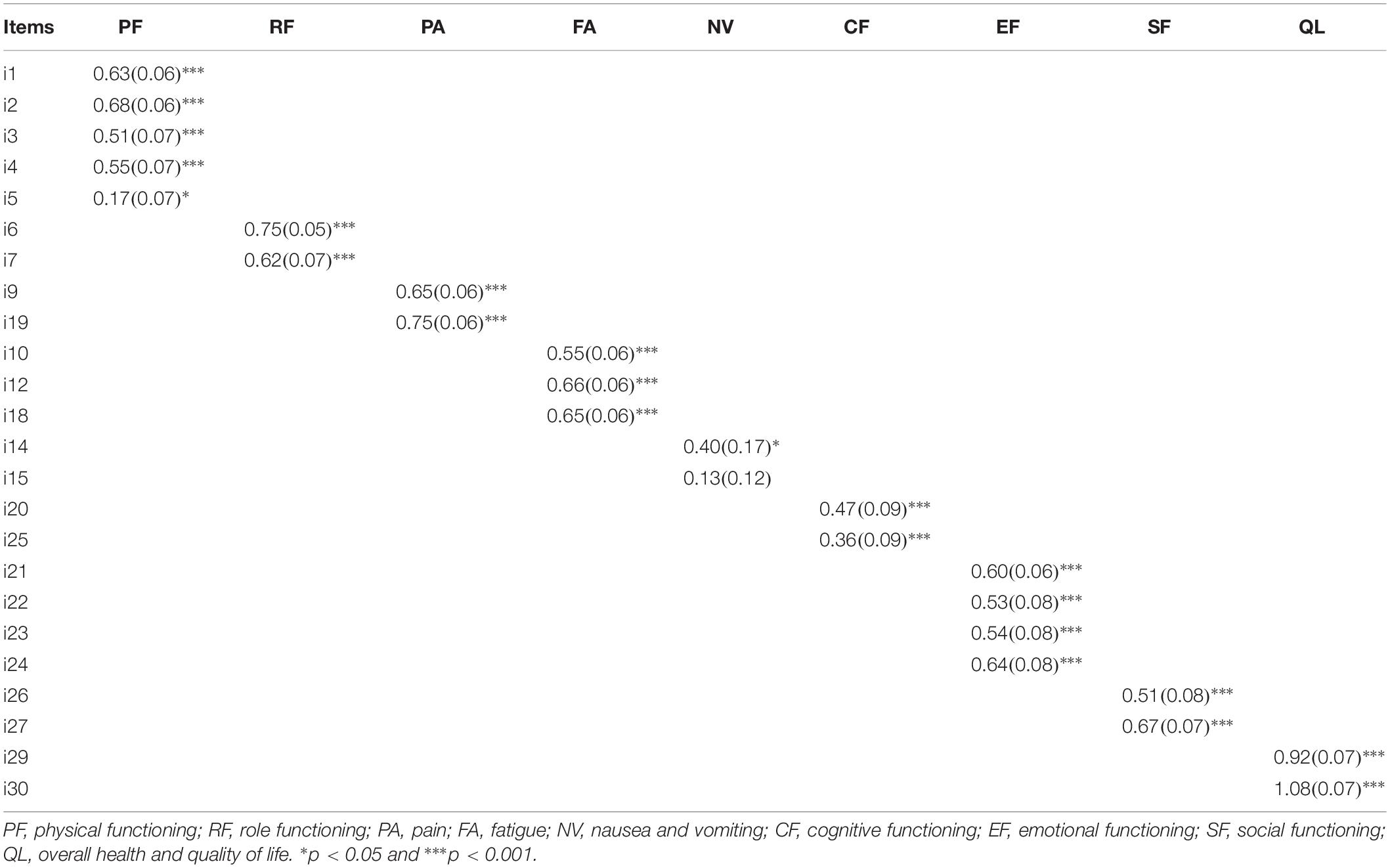
Table 3. Standardized factors loading, standard errors, and significance for the measurement model of the EORTC QLQ-C30.
Table 4 displays correlations among the nine latent dimensions of QoL. Significant correlation coefficients ranged from 0.24 to 0.85 in absolute values. These correlations could be interpreted as measure of effect size of the associations among latent factors. Following suggestion by Cohen (1988), the magnitude of these coefficients was interpreted as: weak (>0.10), moderate (>0.30), and strong (>0.50). Specifically, weak associations were reported between cognitive functioning and physical functioning (r = 0.29), emotional functioning and nausea/vomiting (r = 0.29), and nausea/vomiting and health-related QoL (r = −0.24). A grand total of 18 correlations were large in magnitude. Physical functioning and fatigue were the latent dimensions displaying the higher number of strong correlations with other factors of QoL. Specifically, physical functioning displayed strong associations with pain (r = 0.54), fatigue (r = 0.85), health-related QoL (r = −0.67), role (r = 0.77), cognitive (r = 0.51), emotional (r = 0.50), and social functioning (r = 0.50). Fatigue showed strong associations with health-related QoL (r = −0.68), pain (r = 0.63), nausea/vomiting (r = 0.51), physical (r = 0.85), role (r = 0.76), cognitive (r = 0.59), emotional (r = 0.62), and social functioning (r = 0.51). Finally, role functioning was the latent dimension of QoL most strongly associated with health-related QoL (r = −0.72).

Table 4. Correlations (and their significance) among the nine latent dimensions of the EORTC QLQ-C30.
After entering age, gender, and type of surgery in the model, goodness of fit slightly remained substantially unchanged [χ2(261, N = 167) = 385.65; RMSEA = 0.05; 90% CI of RMSEA = 0.04–0.06; PCLOSE = 0.299; CFI = 0.93; TLI = 0.91; SRMR = 0.05]. The standardize factor loadings ranged from 0.15 to 1.01. Some significant influences of the three covariates on latent factors of QoL were reported. Specifically, type of surgery was responsible for differences in nausea/vomiting (β = −0.52; SE = 0.22; p < 0.05), pain (β = −0.32; SE = 0.15; p < 0.05), and physical (β = −0.39; SE = 0.15; p < 0.01), role (β = −0.46; SE = 0.16; p < 0.01), cognitive (β = −0.31; SE = 0.15; p < 0.05), and social functioning (β = −0.36; SE = 0.15; p < 0.05).
The inspection of modification indices suggested that model fit would be improved by freely estimated the direct effect of age on item 1 (β = −0.03; SE = 0.01; p < 0.001). After the estimation of this effect, the model still showed a good fit [χ2(260, N = 167) = 368.42; RMSEA = 0.05; 90% CI of RMSEA = 0.04–0.06; PCLOSE = 0.491; CFI = 0.94; TLI = 0.92; SRMR = 0.05]. No other modification indices were relevant.
After controlling for this DIF, some significant influences of the three covariates on latent factors of QoL were reported. Specifically, these influences were the same as the ones reported in the previous MIMIC model (i.e., the model not freely estimating direct effects of covariates on items). Specifically, type of surgery was responsible for differences in nausea/vomiting (β = −0.52; SE = 0.22; p < 0.05), pain (β = −0.32; SE = 0.15; p < 0.05), and physical (β = −0.38; SE = 0.15; p < 0.01), role (β = −0.46; SE = 0.16; p < 0.01), cognitive (β = −0.31; SE = 0.15; p < 0.05), and social functioning (β = −0.36; SE = 0.15; p < 0.05). The only exception was that age directly influenced physical functioning (β = 0.03; SE = 0.01; p < 0.01). Thus, by comparing this final model with the previous one we may conclude that any bias due to DIF is only minimal and not accounting for DIF it may have only trivial consequences for the assessment of physical functioning (i.e., the magnitudes of age differences in physical functioning were comparable across the two models).
This study represents an evaluation of the dimensionality and measurement invariance of the Italian version of the EORTC QLQ-C30 in a sample of patients with lung cancer who underwent lobectomy surgery. Our results demonstrated the validity of the factor structure proposed by Costa et al. (2015) and thus suggested that the EORTC QLQ-C30 could be used as a valid measure of QoL in lung cancer patients undergoing lobectomy. In a previous study, Costa et al. (2015) proposed and supported this measurement model in a sample of cancer patients coming from 14 countries all over the World and considering all the types of cancer (breast, colorectal, gynecological, head and neck, lung, esophagus/stomach, and prostate cancer). Compared to a previous trial on lung cancer patients assessing the changes in QoL over time (Pompili et al., 2018), this study represents the first attempt on an Italian sample to evaluate the dimensionality and interindividual differences of patients’ QoL with different sociodemographic and clinical characteristics. Another study (Maringwa et al., 2011) analyzed previously DIF on advanced cancer patients, while the present validation article was conducted on lung cancer patients with a primitive diagnosis.
The questionnaire comprises nine different dimensions. While one factor assesses “overall health and health-related QoL,” the remaining eight factors measure distinct symptoms and functioning, namely nausea/vomiting, pain, fatigue for the symptoms’ subscales, and physical, role, emotional, cognitive, and social functioning for the functioning subscales. All the nine subscales were significantly and strongly loaded by their relative items. The only exception was the nausea and vomiting dimension: one out of its two items exhibited a non-significant and very low loading on its factor. Further research is needed to better assess the validity of this subscale in evaluating symptoms of nausea and vomiting in lung cancer patients and, if necessary, to develop more reliable items to evaluate this kind of adverse events.
Moreover, this study is the first one to evaluate the psychometric properties of the Italian version of the EORTC QLQ-C30 in lung cancer patients and assess its measurement invariance and DIF due to age, gender, and robot-assisted versus not robot-assisted surgery. The presence of measurement invariance is one of the necessary steps in efficient and reliable evaluation of interindividual differences in QoL within samples of lung cancer patients and it represents a prerequisite to validly compare levels of overall health across patients of different genders and genders undergoing lobectomy with or without robot-assisted surgery.
Our main results attested that only one item displayed a trivial DIF. Specifically, compared to younger patients, the elderly were more likely to endorse Item 1 (i.e., “Do you have any trouble doing strenuous activities, like carrying a heavy shopping bag or a suitcase?”) on a four-point scale (i.e., 1 = “Not at all”; 2 = “A little”; 3 = “Quite a bit”; and 4 = “Very much”). However, the magnitude of this DIF was very small.
Finally, the last step in the evaluation of DIF involved the assessment of mean differences of nine latent scores of QoL across patients of different gender, varying age, and underwent robot-assisted or traditional surgery. The main aim of this analysis was to ascertain whether not controlling for DIF may lead to consequences for the assessment of QoL (i.e., mean differences in QoL differ when controlling or not controlling or DIF). These results highlighted that the DIF had only an irrelevant effect on the estimation of differences in latent means of QoL among patients. Accidentally, the results coming from this last step also highlighted that younger patients displayed higher levels of physical functioning than elderly ones and that robot-assisted surgery may promote better QoL 1 month after surgery. Specifically, compared to patients undergoing traditional surgery, people treated with robot-assisted surgery displayed lower pain, nausea and vomiting, as well as better physical, role, cognitive, and social functioning. This latter result is consistent with empirical evidence showing that lung cancer patients treated with robotic thoracic surgery reported a reduced postoperative pain and complications, fewer functional impairments, and a lower need of blood transfusions (Cheng et al., 2007; Nasir et al., 2014). However, it’s noteworthy that the main aim of this analysis was to assess the magnitude and the influence of DIF on mean differences of the nine latent scores of QoL; we did not aim at assessing differences due to age, gender, and type of surgery on patients’ QoL. Moreover, since we did not balance the baseline characteristics (i.e., QoL itself) between patient underwent robot-assisted or traditional surgery, these results may not be interpreted in a casual way.
Current results may be considered in light of some main limitations. Specifically, it was not possible to test convergent and/or divergent validity of the EORTC QLQ-C30 due to a lack of other self-report measures of patients’ well-being. Nevertheless, a previous Italian validation of the questionnaire reported a substantial convergent validity (Apolone et al., 1998), even though not in lung cancer patients. Finally, these statistical analyses must be taken with caution due to the relatively small sample size. Specifically, as highlighted by Kline (2015), the median of typical sample sizes in structural equation modeling studies is about 200 cases. Thus, our sample size of 167 lung cancer patients is slightly below this common standard. However, lower sample sizes are commonly recruited when the specific population being studied is restricted in size and it is difficult to reach higher sample sizes (Kline, 2015). Thus, while the low sample size may represent a limit of our study, this size is a direct consequence of our target population. Because of this small sample, structural invariance of the EORTC QLQ-C30 was assessed by performing MIMIC model and DIF analysis which, compared to MCFA, permit to better test measurement invariance even with small sample size. Future research collecting larger samples would be needed to further assess the factor structure of the EORTC QLQ-C30 in lung cancer patients underwent lobectomy with or without robot-assisted surgery.
Despite these limitations, our findings attested the goodness of the nine-factor structure of the Italian version of the EORTC QLQ-C30 in lung cancer patients and its measurement invariance in assessing QoL in patients with varying ages genders undergoing lobectomy with or without robot-assisted surgery. This is also the first study validating a QoL questionnaire on lung cancer patients. In fact, other scales have not been already validated among Italian lung cancer samples. Additionally, the EORTC QLQ-C30 assesses more dimensions related to a cancer diagnosis than other questionnaires. As a practical consequence, we advise that nine distinct scores of overall health, pain, fatigue, nausea/vomiting, physical, social, role, emotional, and cognitive functioning should be computed for evaluating lung cancer patients’ QoL in future research and clinical practice. The valid and reliable assessment of adverse events and functioning in lung cancer patients is a relevant and prognostic factor in patient’s recovery. In fact, patient survival is highly affected by treatment side-effects such as fatigue, loss of appetite, dyspnea, and coughing, as well as physical, psychological, cognitive, and social functioning (Efficace et al., 2006; Braun et al., 2011; Polanski et al., 2016). The EORTC QLQ-C30 may help healthcare stakeholders in measuring and monitoring QoL in both clinical and research fields. In particular, QoL in lung cancer has been studied to understand patients’ health status during processes aimed to stop smoking and how it may influence patients’ preferences in medical decision-making. It was also used to better investigate possible long-term effects of rumination on patients’ recovery and well-being (Gorini et al., 2018; Masiero et al., 2019). In a patient-centered approach, the measurement of QoL would be also important to assess how individual differences and cognitive processes may influence patient well-being in different medical conditions (Pravettoni and Gorini, 2011; Cutica et al., 2014; Arnaboldi et al., 2015).
In conclusion, the EORTC QLQ-C30 is a useful and valid self-report tool and it can be used to assess interindividual differences of QoL in lung cancer patients in both clinical and research contexts.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by the European Institute of Oncology IRCCS, Milan, Italy. The patients/participants provided their written informed consent to participate in this study.
KM, CM, MM, FP, and GP: concept and design. FP and CM: acquisition of the data. CM and DM: statistical analysis, interpretation of the data, and manuscript writing. CM, DM, KM, and GP: drafting revision of the manuscript for important intellectual content. MM and GP: supervision. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CFA, confirmatory factor analysis; CFI, comparative fit index; DIF, differential item functioning; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire-Core 30 (QLQ-C30); MCFA, multiple-group factor analysis; MIMIC, multiple indicators–multiple causes; MLR, robust maximum likelihood; PCLOSE, probability of close fit; QoL, quality of life; RMSEA, root mean square error of approximation; SRMR, standardized root mean square residual; TLI, tucker–lewis index.
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J., et al. (1993). The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 85, 365–376.
Apolone, G., Filiberti, A., Cifani, S., Ruggiata, R., and Mosconi, P. (1998). Evaluation of the EORTC QLQ-C30 questionnaire: a comparison with SF-36 health survey in a cohort of Italian long-survival cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 9, 549–557. doi: 10.1023/a:1008264412398
Arnaboldi, P., Santoro, L., Mazzocco, K., Oliveri, S., Maggioni, A., and Pravettoni, G. (2015). The paradox of pelvic exenteration: the interaction of clinical and psychological variables. Int. J. Gynecol. Cancer 25, 1534–1540. doi: 10.1097/IGC.0000000000000523
Bjordal, K., de Graeff, A., Fayers, P. M., Hammerlid, E., van Pottelsberghe, C., Curran, D., et al. (2000). A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur. J. Cancer 36, 1796–1807. doi: 10.1016/s0959-8049(00)00186-6
Braun, D. P., Gupta, D., and Staren, E. D. (2011). Quality of life assessment as a predictor of survival in non-small cell lung cancer. BMC Cancer 11:353. doi: 10.1186/1471-2407-11-353
Cheng, D., Downey, R. J., Kernstine, K., Stanbridge, R., Shennib, H., Wolf, R., et al. (2007). Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2, 261–292. doi: 10.1097/IMI.0b013e3181662c6a
Cohen, J. D. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Cambridge, MA: Academic Press. doi: 10.1234/12345678
Costa, D. S. J., Aaronson, N. K., Fayers, P. M., Pallant, J. F., Velikova, G., and King, M. T. (2015). Testing the measurement invariance of the EORTC QLQ-C30 across primary cancer sites using multi-group confirmatory factor analysis. Qual. Life Res. 24, 125–133. doi: 10.1007/s11136-014-0799-0
Cutica, I., Vie, G. M., and Pravettoni, G. (2014). Personalised medicine: the cognitive side of patients. Eur. J. Intern. Med. 25, 685–688. doi: 10.1016/J.EJIM.2014.07.002
Damm, K., Roeske, N., and Jacob, C. (2013). Health-related quality of life questionnaires in lung cancer trials: a systematic literature review. Health Econ. Rev. 3:15. doi: 10.1186/2191-1991-3-15
Di Maio, M., Gridelli, C., Gallo, C., Manzione, L., Brancaccio, L., Barbera, S., et al. (2004). Prevalence and management of pain in Italian patients with advanced non-small-cell lung cancer. Br. J. Cancer 90, 2288–2296. doi: 10.1038/sj.bjc.6601810
Efficace, F., Bottomley, A., Smit, E. F., Lianes, P., Legrand, C., Debruyne, C., et al. (2006). Is a patient’s self-reported health-related quality of life a prognostic factor for survival in non-small-cell lung cancer patients? A multivariate analysis of prognostic factors of EORTC study 08975. Ann. Oncol. 17, 1698–1704. doi: 10.1093/annonc/mdl183
EORTC Quality of Life Group (2019). Quality of Life Group Website. Available at: https://qol.eortc.org/ (accesed September, 2019).
Gorini, A., Riva, S., Marzorati, C., Cropley, M., and Pravettoni, G. (2018). Rumination in breast and lung cancer patients: preliminary data within an Italian sample. Psychooncology 27, 703–705. doi: 10.1002/pon.4468
Hu, L., and Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 6, 1–55. doi: 10.1080/10705519909540118
Iravani, K., Jafari, P., Akhlaghi, A., and Khademi, B. (2018). Assessing whether EORTC QLQ-30 and FACT-G measure the same constructs of quality of life in patients with total laryngectomy. Health Qual. Life Outcomes 16:183. doi: 10.1186/s12955-018-1012-x
Jang, S., Kim, E. S., Cao, C., Allen, T. D., Cooper, C. L., Lapierre, L. M., et al. (2017). Measurement invariance of the satisfaction with life scale across 26 countries. J. Cross. Cult. Psychol. 48, 560–576. doi: 10.1177/0022022117697844
Jöreskog, K., and Sörbom, D. (1996). Lisrel 8: User’s Reference Guide. Lincolnwood, IL: Scientific Software International.
Koller, M., Hjermstad, M. J., Tomaszewski, K. A., Tomaszewska, I. M., Hornslien, K., Harle, A., et al. (2017). An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Ann. Oncol. 28, 2874–2881. doi: 10.1093/annonc/mdx453
Li, B., Wu, Y., Wen, Z., and Wang, M. (2013). Adolescent student burnout inventory in Mainland China. J. Psychoeduc. Assess. 32, 227–235. doi: 10.1177/0734282913508246
Maione, P., Perrone, F., Gallo, C., Manzione, L., Piantedosi, F. V., Barbera, S., et al. (2005). Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter italian lung cancer in the elderly study. J. Clin. Oncol. 23, 6865–6872. doi: 10.1200/JCO.2005.02.527
Maringwa, J. T., Quinten, C., King, M., Ringash, J., Osoba, D., Coens, C., et al. (2011). Minimal important differences for interpreting health-related quality of life scores from the EORTC QLQ-C30 in lung cancer patients participating in randomized controlled trials. Support. Care Cancer 19, 1753–1760. doi: 10.1007/s00520-010-1016-5
Masiero, M., Lucchiari, C., Mazzocco, K., Veronesi, G., Maisonneuve, P., Jemos, C., et al. (2019). E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine Tob. Res. 21, 119–126. doi: 10.1093/ntr/nty047
Mercieca-Bebber, R., King, M. T., Calvert, M. J., Stockler, M. R., and Friedlander, M. (2018). The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 9, 353–367. doi: 10.2147/PROM.S156279
Mora, P. A., Contrada, R. J., Berkowitz, A., Musumeci-Szabo, T., Wisnivesky, J., and Halm, E. A. (2009). Measurement invariance of the mini asthma quality of life questionnaire across African-American and Latino adult asthma patients. Qual. Life Res. 18, 371–380. doi: 10.1007/s11136-009-9443-9
Mosconi, P., Apolone, G., Barni, S., Secondino, S., Sbanotto, A., and Filiberti, A. (2002). Quality of life in breast and colon cancer long-term survivors: an assessment with the EORTC QLQ-C30 and SF-36 questionnaires. Tumori 88, 110–116. doi: 10.1177/030089160208800206
Muthen, L. K., and Muthén, B. O. (2017). MPlus User’ s Guide. Eighth version. Los Angeles, CA: Muthén & Muthén, doi: 10.1111/j.1600-0447.2011.01711.x
Nasir, B. S., Bryant, A. S., Minnich, D. J., Wei, B., and Cerfolio, R. J. (2014). Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann. Thorac. Surg. 98, 203–208. doi: 10.1016/j.athoracsur.2014.02.051
Polanski, J., Jankowska-Polanska, B., Rosinczuk, J., Chabowski, M., and Szymanska-Chabowska, A. (2016). Quality of life of patients with lung cancer. Onco. Targets. Ther. 9, 1023–1028. doi: 10.2147/OTT.S100685
Pompili, C., Velikova, G., Franks, K., Absolom, K., Callister, M., Robson, J., et al. (2018). EORTC QLQ-C30 summary score reliably detects changes in QoL three months after anatomic lung resection for non-small cell lung cancer (NSCLC). Lung Cancer 123, 149–154. doi: 10.1016/s0169-5002(18)30207-1
Pravettoni, G., and Gorini, A. (2011). A P5 cancer medicine approach: why personalized medicine cannot ignore psychology. J. Eval. Clin. Pract. 17, 594–596. doi: 10.1111/j.1365-2753.2011.01709.x
Scott, N., Fayers, P., Aaronson, N., Bottomley, A., de Graeff, A., Groenvold, M., et al. (2007). The use of differential item functioning analyses to identify cultural differences in responses to the EORTC QLQ-C30. Qual. Life Res. 16, 115–129. doi: 10.1007/s11136-006-9120-1
Scott, N. W., Fayers, P. M., Bottomley, A., Aaronson, N. K., de Graeff, A., Groenvold, M., et al. (2006). Comparing translations of the EORTC QLQ-C30 using differential item functioning analyses. Qual. Life Res. 15, 1103–1115. doi: 10.1007/s11136-006-0040-x
Skule, C., Ulleberg, P., Lending, H. D., Berge, T., Egeland, J., Brennen, T., et al. (2014). Depressive symptoms in people with and without alcohol abuse: factor structure and measurement invariance of the beck depression inventory (BDI-II) across groups. PLoS One 9:e88321. doi: 10.1371/journal.pone.0088321
Steca, P., Monzani, D., Pierobon, A., Avvenuti, G., Greco, A., and Giardini, A. (2017). Measuring dispositional optimism in patients with chronic heart failure and their healthcare providers: the validity of the Life Orientation Test-Revised. Patient Prefer. Adherence 11, 1497–1503. doi: 10.2147/PPA.S139522
Stevanovic, D., Atilola, O., Vostanis, P., Pal Singh Balhara, Y., Avicenna, M., Kandemir, H., et al. (2016). Cross-cultural measurement invariance of adolescent self-report on the pediatric quality of life inventoryTM 4.0. J. Res. Adolesc. 26, 687–695. doi: 10.1111/jora.12218
Treloar, H. R., Martens, M. P., and McCarthy, D. M. (2014). Testing measurement invariance of the protective behavioral strategies scale in college men and women. Psychol. Assess. 26, 307–313. doi: 10.1037/a0034471
U.S. Department of Health and Human Services Fda Center for Drug Evaluation and Research, U.S. Department of Health and Human Services Fda Center for Biologics Evaluation and Research, and U.S. Department of Health and Human Services Fda Center for Devices and Radiological Health (2006). Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual. Life Outcomes 4:79. doi: 10.1186/1477-7525-4-79
Winters, Z. E., Balta, V., Thomson, H. J., Brandberg, Y., Oberguggenberger, A., Sinove, Y., et al. (2014). Phase III development of the European organization for research and treatment of cancer quality of life questionnaire module for women undergoing breast reconstruction. Br. J. Surg. 101, 371–382. doi: 10.1002/bjs.9397
Keywords: lung cancer, EORTC QLQ-C30, quality of life, validity, assessment, measurement invariance
Citation: Marzorati C, Monzani D, Mazzocco K, Pavan F, Monturano M and Pravettoni G (2019) Dimensionality and Measurement Invariance of the Italian Version of the EORTC QLQ-C30 in Postoperative Lung Cancer Patients. Front. Psychol. 10:2147. doi: 10.3389/fpsyg.2019.02147
Received: 15 May 2019; Accepted: 05 September 2019;
Published: 08 October 2019.
Edited by:
Michela Balsamo, Università degli Studi “G. d’Annunzio” Chieti–Pescara, ItalyCopyright © 2019 Marzorati, Monzani, Mazzocco, Pavan, Monturano and Pravettoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Marzorati, Y2hpYXJhLm1hcnpvcmF0aUBpZW8uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.