- 1Department of Psychology, University of Otago, Dunedin, New Zealand
- 2Ara Institute of Canterbury, Christchurch, New Zealand
- 3Department of Biochemistry, University of Otago, Dunedin, New Zealand
Background: To understand the genetic underpinnings of emotion, researchers have studied genetic variants in the oxytocin system, a hormone and neurotransmitter important to socio-emotional functioning. The oxytocin receptor gene (OXTR) variant rs53576 has been associated with emotional traits such as positive affect and related constructs such as optimism and self-esteem. Individuals carrying the A allele (AG and AA genotypes) of rs53576 have been found to score lower in these traits when compared to GG homozygotes, although not always. Given recent mixed evidence regarding this polymorphism, replication of these associations is critical.
Methods: Using a cross-sectional design, the present study tested the association between rs53576 and a wide variety of emotional traits and states in a sample of 611 young adults ages 18 – 25 of various ethnicities (European, Asian, Māori/Pacific Islander, other). Participants completed standard trait measures of positive and negative affect, depressive symptoms, life engagement, psychological well-being, optimism, and self-esteem. They also completed state measures of positive and negative affect and life engagement for 13-days using Internet daily diaries.
Results: Controlling for ethnicity and gender, variation at the OXTR variant rs53576 obtained from blood samples was not related to any of the emotional traits or states. This null finding occurred despite measuring emotions in “near to real time” using daily diaries and having sufficient power to detect a medium effect size difference between homozygous genotype groups.
Conclusion: These findings suggest that variation at the rs53576 locus may not be as involved in emotional differences as initial studies suggested.
Introduction
Oxytocin is a neurotransmitter and hormone that regulates a variety of social and emotional behaviors including trust and maternal bonding (Campbell, 2010). Oxytocin also plays a role in emotional processes by attenuating the stress response and promoting feelings of calmness and well-being (Kirsch et al., 2005; Uvnäs-Moberg et al., 2005; Campbell, 2010; IsHak et al., 2011). The oxytocin receptor appears to be an important neurochemical mechanism linking oxytocin to emotion (IsHak et al., 2011). Rodent studies show a higher concentration of oxytocin receptors in the central amygdala that inhibit amygdala activity when oxytocin is introduced (Huber et al., 2005). Oxytocin receptors have also been discovered on serotonergic neurons in the raphe nuclei that facilitate serotonin release and may drive anxiolytic effects of oxytocin (Yoshida et al., 2009).
In light of these mechanisms, genetic differences in the oxytocin receptor gene (OXTR) may influence emotional differences between people. One previously studied variant of OXTR is rs53576, a single nucleotide polymorphism located in intron 3 (Wu et al., 2005). The A (minor) allele of rs53576 has been associated with impaired social and maternal behaviors (e.g., Rodrigues et al., 2009; Kogan et al., 2011; Riem et al., 2011). Similarly, the G allele of rs53576 has been associated with greater sociality in people (Li et al., 2015). However, evidence for the association between rs53576 and emotional traits is inconsistent.
Several studies have tested for associations between rs53576 and emotional traits. A study of 289 German adults found that men with the AA genotype reported lower trait positive affect compared to men with the AG or GG genotypes; however, only 13 men had the AA genotype so results should be interpreted cautiously (Lucht et al., 2009). Saphire-Bernstein et al. (2011) found that among a sample of 344 mixed-ethnicity adults, both men and women carriers of the A allele (AA, AG) reported lower trait optimism, self-esteem, and mastery, as well as higher self-reported depression compared to GGs. But a larger sample of 1229 Caucasian women showed no such association between rs53576 and optimism (Cornelis et al., 2012). Moreover, an earlier case control study (92 cases/ 192 controls) found that unipolar depression was more likely among patients with the GG genotype of rs53576 – not the AA or AG genotype (Costa et al., 2009), which was replicated in another recent case control study (Costa et al., 2017). Similarly, a large population based study (n = 1185) found AA men had lower depression symptoms (vs. GG or GA), although this genotype was not overly represented among suicide victims (n = 763) (Wasilewska et al., 2017). Finally, a meta-analysis reported that rs53576 was unrelated to depression but was related to elevated general sociality for the GG genotype (Li et al., 2015). Taken together, these studies paint a mixed picture of the relationship between rs53576 and emotion.
Given the high likelihood for false-positives in genetic association studies, it is important to replicate genetic associations such as the OXTR rs53576 and emotion, hence our aim here. We measured a variety of emotion constructs at the trait level (positive and negative affect, depressive symptoms, life engagement, psychological well-being, optimism, and self-esteem) and state level (positive and negative affect, life satisfaction) using daily diaries in light of evidence that “near to real time” emotion reports may be especially sensitive for detecting genetic association with emotion (Conner and Barrett, 2012; Finan et al., 2012).
Materials and Methods
Participants and Procedure
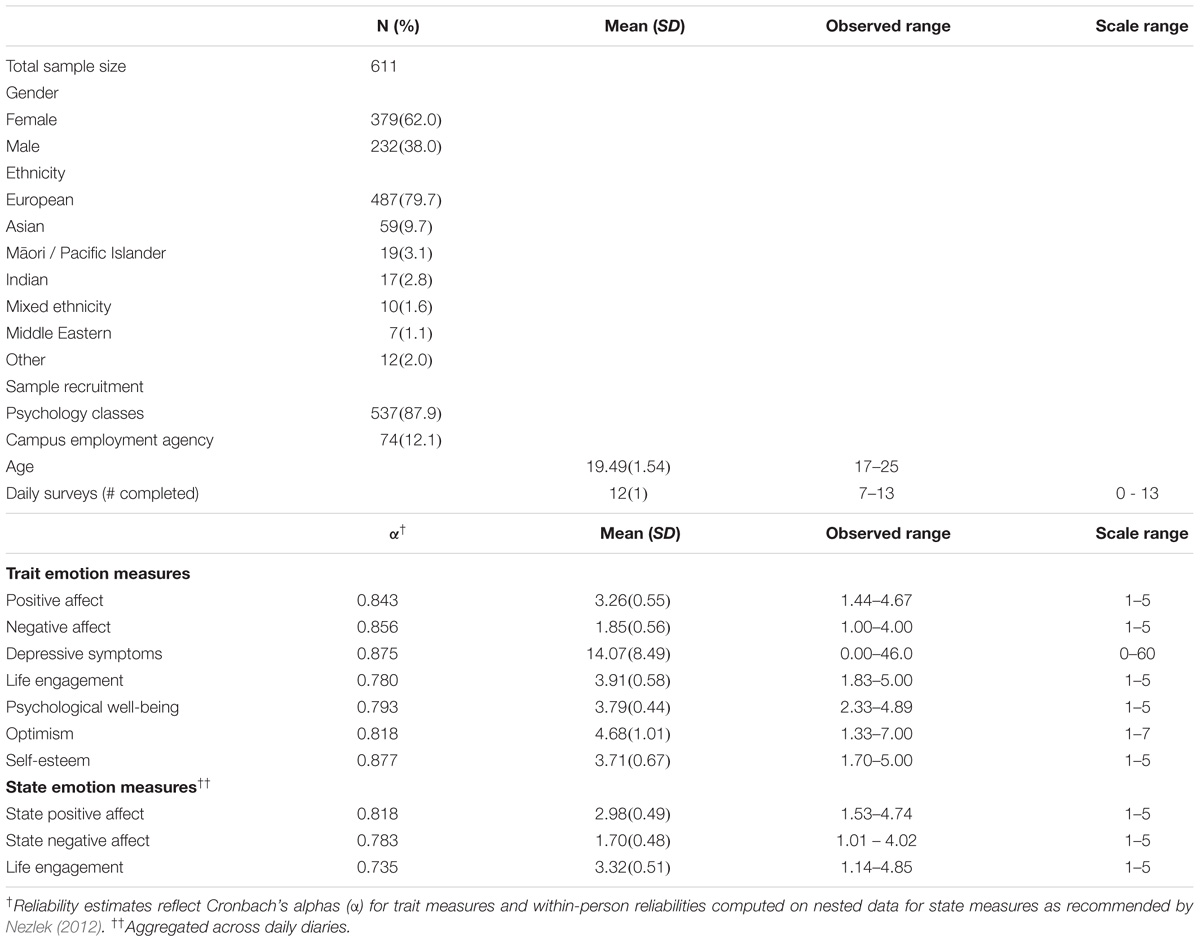
Participants were 611 young adults studying at the University of Otago, New Zealand (see Table 1, demographics). The data were collected in 2011 and 2012 as part of the Daily Life Study, and carried out in accordance with the recommendations and approval of the University of Otago Human Ethics Committee (10/777). All participants gave written informed consent in accordance with the Declaration of Helsinki. Participants completed a baseline survey with demographic questions and trait measures in private cubicles, completed an Internet daily diary survey each day between 3 and 8 pm for 13 consecutive days, gave a non-fasting 22 ml venous blood sample at an on-campus clinic (4 people gave a saliva sample), and completed a final survey.
Measures
The baseline survey measured trait positive affect and negative affect using an affective circumplex measure (Barrett and Russell, 1999) in which participants rated nine positive affect items (enthusiastic, excited, energetic, happy, cheerful, pleased, calm, content, relaxed) and nine negative affect items (irritable, angry, hostile, nervous, anxious, tense, dejected, sad, unhappy) for how they “generally feel” from 1 (not at all) to 5 (extremely). Depressive symptoms were measured using the 20-item Center for Epidemiological Studies Depression Scale (Radloff, 1977) with items answered “in the last week” on a 4-point scale from 0 (never or rarely a symptom occurs) to 3 (most or all of the time it occurs), and optimism using the 6-item Life Orientation Test – Revised (Carver et al., 2010) with items answered on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree). The final survey measured psychological well-being (18-item Psychological Well-being Scale; Ryff and Keyes, 1995), life engagement (6-item Life Engagement Test; Scheier et al., 2006), and self-esteem (10-item Rosenberg Self-Esteem Scale; Rosenberg, 1965) with all items answered on 5-point scales from 1 (not at all) to 5 (extremely). The daily diary survey measured state positive affect and state negative affect using the circumplex scale as above and state life engagement using the Life Engagement Test as above, with items rated for how they felt “today” on a scale from 1 (not at all) to 5 (extremely).
Genotyping
Genotypes for the OXTR variant rs53576 were obtained from blood (n = 607) or saliva samples (n = 4). DNA was extracted from peripheral white blood cells using a guanidine-HCl-based procedure with chloroform extraction (McKinney et al., 2010) and genotyped for the rs53576 genotype (GG, AG, or AA) using a Life Technologies TaqMan 5 nuclease assay (probe id C_3290335_10) and performed according to manufacturer’s instructions. Fourteen percent of samples were repeated with 100% concordance in genotype.
Statistical Analyses and Power
Analysis of covariance (ANCOVA) tested for genotype differences in each emotion measure while controlling for ethnicity and gender. The three genotype groups (GG, GA, and AA) served as the primary predictor. Ethnicity was coded using a set of three dummy variables (European as reference group 000, compared with Asian 100, Māori/Pacific Islander 010, and Other 001). Gender was coded 0 for men and 1 for women. Gender differences in genotype effects were tested in follow up analyses by modeling the gender x genotype interaction and also by running the analyses for men and women separately. The p-value was set to less than 0.005 using a Bonferroni correction (0.05/10 primary tests) to adjust for multiple hypothesis testing. Based on an estimated sample prevalence of GG and AA (43 and 15%) (estimated from Lucht et al., 2009; and Saphire-Bernstein et al., 2011), at least 480 participants were needed to have 0.8 power to detect a medium size effect (d = 0.5) between GG and AA at α = 0.005. We found a lower prevalence of AAs in our sample compared to initial estimates, but with a final sample of 611 we still had 0.84 power to detect a medium size difference between the homozygotes at α = 0.005.
Results
The genotype distributions for the OXTR rs53576 variant were GG 275 (45%), AG 262 (42.9%), and AA 74 (12.1%), which were in Hardy Weinberg Equilibrium (χ2 = 0.90, p = 0.344). The AA genotype was the least common of the three genotypes, reflecting a frequency f(A) of 0.336 for the A allele. The A allele was more common among Asian participants [f(A) = 0.610] than European participants [f(A) = 0.308, χ2 = 50.86, p < 0.001], Māori/Pacific Island participants [f(A) = 0.317, χ2 = 14.02, p = 0.001] or other ethnicities [f(A) = 0.283, χ2 = 22.43, p < 0.001].
Table 2 presents the emotion results. There was no evidence of genotype differences in any of the emotion measures that exceeded the Bonferroni adjustment of p < 0.005. There were no significant gender × genotype interactions, although when analyzing men and women separately, AA women reported higher self-esteem (Madjusted = 3.89) than AG or GG women [AG, Madjusted = 3.59; GG, Madjusted = 3.60; F(df) = 3.189, p = 0.042], but this association did not exceed the Bonferroni adjustment of p < 0.005.
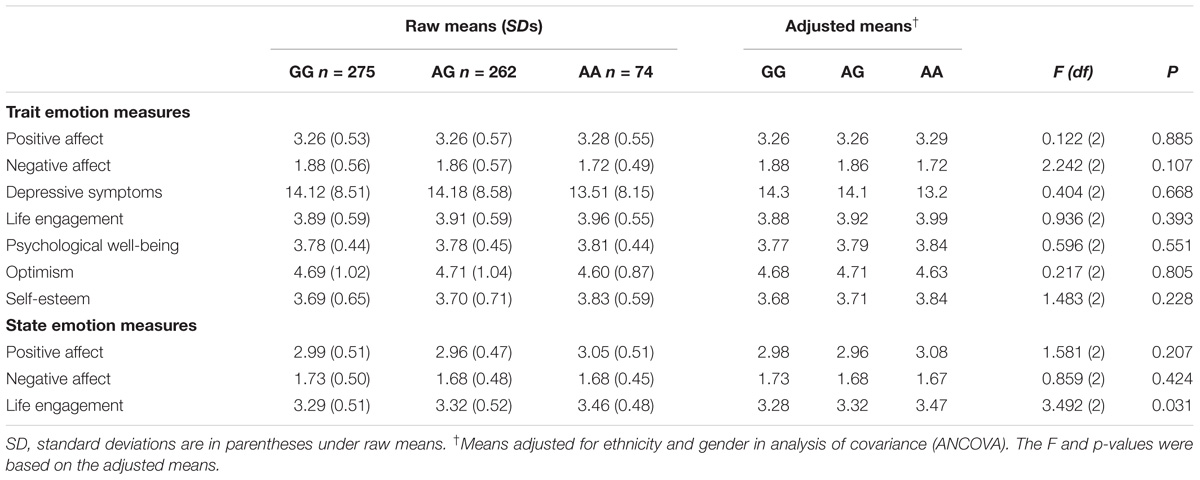
Table 2. Emotional Traits and States for each OXTR rs53576 Genotype: Raw Means, Standard Deviations, and ANCOVA Results.
Discussion
Variation at the OXTR rs53576 locus was not associated with self-reported emotion in our sample. Even with data gathered in real time, we still found no evidence of genetic association. The present findings are similar to the null findings from Li et al’s. (2015) meta-analysis and Cornelis et al. (2012) who found no association between rs53576 and optimism, but contrast with findings from Lucht et al. (2009) who found lower trait positive affect in AA men, and with Saphire-Bernstein et al. (2011) who found lower optimism, mastery, self-esteem and depression in A carriers. We do not know what factors account for these differences, but sample characteristics including ethnic ancestry and environmental factors could play a role. Our sample, like that of Cornelis et al. (2012), consisted mainly of European participants who have a lower frequency of the A allele e.g., [f(A) = 0.336 in our sample], whereas the Saphire-Bernstein et al. (2011) sample included more Asian participants who have a higher frequency of the A allele [f(A) = 0.624]. However, ethnicity differences may not fully explain the differences because Saphire-Bernstein et al. (2011) controlled for ethnicity in secondary analyses and found the genetic association was reduced but not eliminated. Cornelis et al. (2012) suggested that associations might be more evident in younger rather than middle aged samples; however, our sample was young, ages 18 to 25 (mean age 19).
Main effect analyses could mask gene-environment and gene-gene interactions, such that the expression of rs53576 may be conditional upon life stress or cultural norms (Kim et al., 2011; Sasaki et al., 2011; McQuaid et al., 2013; LeClair et al., 2016) or conditional upon other genotypes. Sample differences in stress could possibly interact with rs53576 to produce variability in results. Participants from Saphire-Bernstein et al. (2011) were recruited for a study of stress and coping and could be at higher stress levels than our participants, which could accentuate genotype effects. However, participants in the Nurses’ Health Study had moderate levels of stress (Feskanich et al., 2002) yet showed no genotype differences in emotion as a function of rs53576 (Cornelis et al., 2012). It is notable that the largest studies of rs53576 and emotion to date – Cornelis et al. (2012); Wasilewska et al. (2017), and ours – showed either null results or mixed results. Larger sample sizes may have reduced the likelihood of false-positives (Ioannidis et al., 2001; Pickrell et al., 2011).
Genetic variants with demonstrable effects on brain processes are stronger candidates; however, in the case of OXTR rs53576, we can find no direct functional evidence of how variation at the rs53576 locus influences processes or oxytocin expression in the brain. Only associative evidence exists. For example, neuroimaging research has found significant brain differences associated with rs53576 (e.g., reduced volume in hypothalamic gray matter and reduced volume and functional connectivity in the amygdala for A alleles by Tost et al., 2010; reduced amygdala volume and reduced connectivity for female A alleles by Wang et al., 2014). However, as noted by Tost et al. (2010), this evidence does not directly support a role for the oxytocin receptor. It is only correlational and therefore it cannot be ruled out that other variants in linkage disequilibrium may actually be driving observed associations. Possible candidates include another OXTR variant or a variant of the vasopressin gene neighboring OXTR (Gimpl and Fahrenholz, 2001).
We encourage replication in larger samples with greater power to detect small effect sizes. We also encourage future exploration of gene-environment and gene-gene interactions (e.g., Windle and Mrug, 2015). However, the present null findings, coupled with the null or mixed results of prior work and the lack of functional evidence for rs53576 in relation to emotion, suggest that future research on emotion should consider other variants besides rs53576 that may influence the emotional pathways of oxytocin such as rs6449182 in the CD38 gene, which regulates oxytocin release (Jin et al., 2007), and has been linked to emotional bonding in couples (Algoe and Way, 2014).
Author Contributions
TC and KM conceived the research. KM, MC, BR, and JF collected the data. AP-G, RT, MM, and TM processed the blood samples and genotyped the DNA and saliva samples. TC and KM wrote the article with assistance from JF, BR, MC, and TM.
Funding
This research was supported by a University of Otago Research grant to TC.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank members of the 2011 and 2012 Daily Experiences Lab for help with data collection, Hadyn Youens for assistance with programming, and the Merriman lab in the Department of Biochemistry for DNA processing and genotyping (Marilyn Merriman, Mandy Phipps-Green, and Ruth Topless).
References
Algoe, S. B., and Way, B. M. (2014). Evidence for a role of the oxytocin system, indexed by genetic variation in CD38, in the social bonding effects of expressed gratitude. Soc. Cogn. Affect. Neurosci. 9, 1855–1861. doi: 10.1093/scan/nst182
Barrett, L. F., and Russell, J. A. (1999). The structure of current affect: controversies and emerging consensus. Curr. Dir. Psychol. Sci. 8, 10–14. doi: 10.1111/1467-8721.00003
Campbell, A. (2010). Oxytocin and human social behaviour. Pers. Soc. Psychol. Rev. 14, 281–295. doi: 10.1177/1088868310363594
Carver, C. S., Scheier, M. F., and Segerstrom, S. C. (2010). Optimism. Clin. Psychol. Rev. 30, 879–889. doi: 10.1016/j.cpr.2010.01.006
Conner, T. S., and Barrett, L. F. (2012). Trends in ambulatory self-report: the role of momentary experience in psychosomatic medicine. Psychosom. Med. 74, 327–337. doi: 10.1097/PSY.0b013e3182546f18
Cornelis, M. C., Glymour, M. M., Chang, S.-C., Tchetgen, E. J. T., Liang, L., Koenen, K. C., et al. (2012). Oxytocin receptor (OXTR) is not associated with optimism in the nurses’ health study. Mol. Psychiatry 17, 1157–1159. doi: 10.1038/mp.2011.178
Costa, B., Pini, S., Baldwin, D. S., Silove, D., Manicavasagar, V., Abelli, M., et al. (2017). Oxytocin receptor and G-protein polymorphisms in patients with depression and separation anxiety. J. Affect. Disord. 218, 365–373. doi: 10.1016/j.jad.2017.03.056
Costa, B., Pini, S., Gabelloni, P., Abelli, M., Lari, L., Cardini, A., et al. (2009). Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology 34, 1506–1514. doi: 10.1016/j.psyneuen.2009.05.006
Feskanich, D., Kastrup, J. L., Marshall, J. R., Colditz, G. A., Stampfer, M. J., Willett, W. C., et al. (2002). Stress and suicide in the nurses’ health study. J. Epidemiol. Community Health 56, 95–98. doi: 10.1136/jech.56.2.95
Finan, P. H., Tennen, H., Thoemmes, F., Zautra, A. J., and Davis, M. C. (2012). Ambulatory monitoring in the genetics of psychosomatic medicine. Psychosom. Med. 74, 349–355. doi: 10.1097/PSY.0b013e3182544a74
Gimpl, G., and Fahrenholz, F. (2001). The oxytocin receptor system: structure, function and regulation. Psychol. Rev. 81, 629–683. doi: 10.1152/physrev.2001.81.2.629
Huber, D., Vienante, P., and Stoop, R. (2005). Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308, 245–248. doi: 10.1126/science.1105636
Ioannidis, J. P., Ntzani, E. E., Trikalinos, T. A., and Contopoulos-Ioannidis, D. G. (2001). Replication validity of genetic association studies. Nat. Genet. 29, 306–309. doi: 10.1038/ng749
IsHak, W. W., Kahloon, M., and Fakhry, H. (2011). Oxytocin role in enhancing well-being: a literature review. J. Affect. Disord. 130, 1–9. doi: 10.1016/j.jad.2010.06.001
Jin, D., Liu, H. X., Torashima, T., Nagai, T., Lopatina, O., Shnayder, N. A., et al. (2007). CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45. doi: 10.1038/nature05526
Kim, H. S., Sherman, D. K., Mojaverian, T., Sasaki, J. Y., Park, J., Suh, E. M., et al. (2011). Gene-culture interaction: oxytocin receptor polymorphism (OXTR) and emotion regulation. Soc. Psychol. Pers. Sci. 2, 665–672. doi: 10.1177/1948550611405854
Kirsch, P., Esslinger, C., Chen, Q., Mier, D., Lis, S., Siddhanti, S., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005
Kogan, A., Saslow, L. R., Impett, E. A., Oveis, C., Keltner, D., and Saturn, S. R. (2011). Thin-slicing study of the oxytocin receptor (OXTR) gene and the evaluation and expression of the prosocial disposition. PNAS 108, 19189–19192. doi: 10.1073/pnas.1112658108
LeClair, J., Sasaki, J. Y., Ishii, K., Shinada, M., and Kim, H. S. (2016). Gene–culture interaction: influence of culture and oxytocin receptor gene (OXTR) polymorphism on loneliness. Cult. Brain 4, 21–37. doi: 10.1007/s40167-016-0034-7
Li, J., Zhao, Y., Li, R., Broster, L. S., Zhou, C., and Yang, S. (2015). association of oxytocin receptor gene (OXTR) rs53576 polymorphism with sociality: a meta-analysis. PLoS One 10:e0131820. doi: 10.1371/journal.pone.0131820
Lucht, M. J., Barnow, S., Sonnenfeld, C., Rosenberger, A., Grabe, H. J., Schroeder, W., et al. (2009). Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subjects. Prog. Neuro Psychopharmacol. Biol. Psychiatry 33, 860–866. doi: 10.1016/j.pnpbp.2009.04.004
McKinney, C., Fanciulli, M., Merriman, M. E., Phipps-Green, A., Alizadeh, B. Z., Koeleman, B. P., et al. (2010). Association of variation in Fcgamma receptor 3B gene copy number with rheumatoid arthritis in Caucasian samples. Ann. Rheum. Dis. 69, 1711–1716. doi: 10.1136/ard.2009.123588
McQuaid, R. J., McInnis, O. A., Stead, J. D., Matheson, K., and Anisman, H. (2013). A paradoxical association of an oxytocin receptor gene polymorphism: early-life adversity and vulnerability to depression. Front. Neurosci. 7:128. doi: 10.3389/fnins.2013.00128
Nezlek, J. (2012). “Multilevel modeling analyses of diary-style data,” in Handbook of Research Methods for Studying Daily Life, eds M. R. Mehl and T. S. Conner (New York, NY: Guilford Press), 357–383.
Pickrell, J., Barrett, J., MacArthur, D., and Jostins, L. (2011). Size Matters, and Other Lessons From Medical Genetics, [Web Log Message]. Available at: http://www.genomesunzipped.org/2011/11/size-matters-and-other-lessons-from-medical-genetics.php
Radloff, L. S. (1977). The CES-D Scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401. doi: 10.1177/014662167700100306
Riem, M. M., Pieper, S., Out, D., Bakersman-Kranenburg, M. J., and van IJzendoorn, M. H. (2011). Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. SCAN 6, 294–300. doi: 10.1093/scan/nsq035
Rodrigues, S. M., Saslow, L. R., Garcia, N., John, O. P., and Keltner, D. (2009). Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. PNAS 106, 21437–21441. doi: 10.1073/pnas.0909579106
Rosenberg, M. (1965). Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press. doi: 10.1515/9781400876136
Ryff, C. D., and Keyes, C. L. M. (1995). The structure of psychological well-being revisted. J. Pers. Soc. Psychol. 69, 719–727. doi: 10.1037/0022-3514.69.4.719
Saphire-Bernstein, S., Way, B. M., Kim, H. S., Sherman, D. K., and Taylor, S. E. (2011). Oxytocin receptor gene (OXTR) is related to psychological resources. PNAS 108, 15118–15122. doi: 10.1073/PNAS.1113137108
Sasaki, J. Y., Kim, H. S., and Xu, J. (2011). Religion and well-being: the moderating role of culture and the oxytocin receptor (OXTR) gene. J. Cross Cult. Psychol. 42, 1394–1405. doi: 10.1177/0022022111412526
Scheier, M. F., Wrosch, C., Baum, A., Cohen, S., Martire, L. M., Matthews, K. A., et al. (2006). The life engagement test: assessing purpose in life. J. Behav. Med. 29, 291–298. doi: 10.1007/s10865-005-9044-1
Tost, H., Kolachana, B., Hakimi, S., Lemaitre, H., Verchinski, B. A., Mattay, V. S., et al. (2010). A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalimbic-limbic structure and function. PNAS 107, 13936–13941. doi: 10.1073/pnas.1003296107
Uvnäs-Moberg, K., Arn, I., and Magnusson, D. (2005). The psychobiology of emotion: the role of the oxytocinergic system. Int. J. Behav. Med. 12, 59–65. doi: 10.1207/s15327558ijbm1202-3
Wang, J., Qin, W., Liu, B., Zhou, Y., Wang, D., Zhang, Y., et al. (2014). Neural mechanisms of oxytocin receptor gene mediating anxiety-related temperament. Brain Struct. Funct. 219, 1543–1554. doi: 10.1007/s00429-013-0584-9
Wasilewska, K., Pawlak, A., Kostrzewa, G., Sobczyk-Kopcioł, A., Kaczorowska, A., Badowski, J., et al. (2017). OXTR polymorphism in depression and completed suicide—A study on a large population sample. Psychoneuroendocrinology 77, 84–89. doi: 10.1016/j.psyneuen.2016.12.003
Windle, M., and Mrug, S. (2015). Hypothesis-driven research for G × E interactions: the relationship between oxytocin, parental divorce during adolescence, and depression in young adulthood. Front. Psychol. 6:1322. doi: 10.3389/fpsyg.2015.01322
Wu, S., Jia, M., Ruan, Y., Liu, J., Guo, Y., Shuang, M., et al. (2005). Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. J. Biol. Psychol. 58, 74–77. doi: 10.1016/j.biopsych.2005.03.013
Keywords: oxytocin, genetics, genes, rs53576, emotion, mental health, well-being, mood
Citation: Conner TS, McFarlane KG, Choukri M, Riordan BC, Flett JAM, Phipps-Green AJ, Topless RK, Merriman ME and Merriman TR (2018) The Oxytocin Receptor Gene (OXTR) Variant rs53576 Is Not Related to Emotional Traits or States in Young Adults. Front. Psychol. 9:2548. doi: 10.3389/fpsyg.2018.02548
Received: 09 October 2018; Accepted: 28 November 2018;
Published: 11 December 2018.
Edited by:
Sarah Whittle, The University of Melbourne, AustraliaReviewed by:
Carina Sauer, Central Institute for Mental Health, GermanyJoshua A. Wilt, Case Western Reserve University, United States
Copyright © 2018 Conner, McFarlane, Choukri, Riordan, Flett, Phipps-Green, Topless, Merriman and Merriman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamlin S. Conner, dGNvbm5lckBwc3kub3RhZ28uYWMubno=
 Tamlin S. Conner
Tamlin S. Conner Karma G. McFarlane1
Karma G. McFarlane1 Maria Choukri
Maria Choukri Benjamin C. Riordan
Benjamin C. Riordan Jayde A. M. Flett
Jayde A. M. Flett Ruth K. Topless
Ruth K. Topless Tony R. Merriman
Tony R. Merriman