
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 07 October 2020
Sec. Women's Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.574669
This article is part of the Research Topic Breast Cancer in Young Women View all 18 articles
 Benedetta Bonardi1,2†
Benedetta Bonardi1,2† Claudia Massarotti3†
Claudia Massarotti3† Marco Bruzzone4
Marco Bruzzone4 Oranite Goldrat5
Oranite Goldrat5 Giorgia Mangili2
Giorgia Mangili2 Paola Anserini3
Paola Anserini3 Stefano Spinaci6
Stefano Spinaci6 Luca Arecco7,8
Luca Arecco7,8 Lucia Del Mastro7,9
Lucia Del Mastro7,9 Marcello Ceppi4
Marcello Ceppi4 Isabelle Demeestere1,5‡
Isabelle Demeestere1,5‡ Matteo Lambertini7,8*‡
Matteo Lambertini7,8*‡Background: The co-administration of letrozole during controlled ovarian stimulation (COS) with gonadotropins is used to limit the potentially harmful effects of a supra-physiological rise in estrogen levels on hormone-sensitive cancers. However, the efficacy and safety of adding letrozole to COS remain debated.
Methods: This is a systematic review and meta-analysis of published studies that compared the efficacy and safety of COS with co-administration of letrozole vs. COS without letrozole in all patient populations. A secondary analysis was done including only the studies in breast cancer patients. The primary efficacy endpoint was the number of retrieved mature Metaphase II (MII) oocytes. Secondary efficacy and safety endpoints were total number of oocytes, maturation rate, fertilization rate, number of cryopreserved embryos, peak estradiol levels, progesterone levels, and total gonadotropin dose. Data for each endpoint were reported and analyzed thorough mean ratio (MR) with 95% confidence interval (CI).
Results: A total of 11 records were selected including 2,121 patients (990 patients underwent COS with letrozole and 1,131 COS without letrozole). The addition of letrozole to COS did not have any negative effect on the number of mature oocytes collected (MR = 1.00, 95% CI = 0.87–1.16; P = 0.967) and the other efficacy endpoints. COS with letrozole was associated with significantly decreased peak estradiol levels (MR = 0.28, 95% CI = 0.24–0.32; P < 0.001). Similar results were observed in the secondary analysis including only breast cancer patients.
Conclusions: These findings are reassuring on the efficacy and safety of COS with gonadotropins and letrozole and are particularly important for fertility preservation in women with hormone-sensitive cancers.
Over the last years, cancer death rate has been continuously dropping thanks to improvement in screening techniques and therapies ensuring early diagnosis and increased survival (1). Life-saving treatments such as chemotherapy or radiotherapy have several potential long-term adverse effects including gonadotoxicity (2–8). The subsequent risk of treatment-related infertility and the loss of ovarian endocrine function represent important causes of distress for patients who are diagnosed during their reproductive years (9–11). Therefore, scientific societies strongly recommend fertility consultation before initiation of anticancer treatments in all patients of childbearing age (12–15).
In the last decades, oocyte and embryo cryopreservation have become standard procedures for fertility preservation (12–15). In order to increase the chance for success, controlled ovarian stimulation (COS) with high doses of gonadotropins is needed to maximize the number of oocytes retrieved and stored (16). COS exposes women to supra-physiological estrogen levels, raising concerns about the safety of the procedure in patients with hormone-sensitive cancers (17, 18). The use of both letrozole and tamoxifen was proposed, alongside classic COS protocols, to avoid unnecessary and potentially harmful effects of the rise in estrogen levels on the cancer (19, 20). Letrozole is an aromatase inhibitor that blocks androgen conversion into estrogen and it is used “off label” in infertility treatment in many countries, especially as ovulation inductor for women with either anovulatory cycles (21), including those with polycystic ovary syndrome (PCOS) (22), or unexplained infertility before planned intercourses or intra-uterine insemination (IUI) (23). Co-treatment with letrozole was proposed also alongside the COS for in vitro fertilization (IVF) in infertile women (24). However, the warning letter published by the original manufacturer still limits its general acceptance. Indeed, the safety concerns related to an increased number of reported malformations in pregnancies resulting from protocols that included letrozole were based only on a single abstract (including 150 babies from 130 pregnancies, compared to a large group of spontaneous low-risk pregnancies) and never confirmed by larger and methodologically sounder studies (25–28). Therefore, the concerns related to potential risks of congenital malformations have been dispelled by the scientific community, but the warning remains.
In terms of efficacy, some studies showed that letrozole co-administration was associated with comparable or even better oocyte yield than traditional protocols, without increasing serum estradiol levels (19, 29, 30), while others have demonstrated a reduction in the number of growing follicles, oocytes retrieved, and pregnancies as well as an increased incidence of cycle cancellations (31, 32). Moreover, data are not homogeneous, with most studies comparing COS with letrozole in oncologic patients to infertile women or donors as controls (29, 33, 34).
Because of the aforementioned controversial data about this important issue, we performed a systematic review and meta-analysis to clarify the efficacy and safety of adding letrozole to COS for IVF.
This was a quantitative synthesis of studies that compared the efficacy and safety of COS with co-administration of letrozole (letrozole cohort) vs. COS without letrozole (no-letrozole cohort).
The primary efficacy endpoint was the number of retrieved mature Metaphase II (MII) oocytes. Secondary efficacy endpoints were total number of retrieved oocytes, maturation rate, and fertilization rate. Other secondary safety endpoints were peak estradiol levels, total gonadotropin dose, and length of the stimulation.
Pregnancy rate, live birth rate, relapse rate, and disease-free survival in cancer patients, adverse events, and progesterone levels were other pre-planned endpoints of interest. However, they could not be analyzed due to lack of data among the included studies.
As secondary analysis, the role of COS with or without letrozole was investigated specifically in the breast cancer patient population. All the analyses were repeated by including only the three studies that included breast cancer patients in both the letrozole and no-letrozole cohorts (35–37).
A systematic literature search of PubMed was conducted to identify studies investigating protocols of COS with letrozole compared to those without letrozole. The search was not limited to studies about cancer patients who needed to cryopreserve their oocytes or embryos, but included also infertile patients and COS for elective fertility preservation. The search was restricted to full papers written in English and reporting original data; no restriction in terms of year of publication was applied. The final date of search was March 31, 2020. The terms used for the search strategy were “letrozole,” “aromatase inhibitor,” “controlled ovarian stimulation,” “fertility preservation,” “cancer,” “breast cancer,” “oocyte vitrification,” and “oocyte freezing.” Boolean operators were used to connect specific search keywords.
The effective combination of search terms was designed and organized by one reviewer (BB) and discussed with two other reviewers (ID and ML). The titles and abstracts obtained from the search were analyzed independently by two reviewers (BB and ML), and a third author (ID) evaluated the search results in order to apply the eligibility criteria.
Records eligible for this analysis had the following features: (a) studies comparing COS with or without letrozole; (b) in the experimental group, letrozole had to be included for the whole COS. Records with the following characteristics were excluded: (a) studies in which letrozole was given only for a few days and not for the whole duration of COS; (b) studies that used letrozole only for ovulation induction; (c) studies written in languages other than English; (d) studies without control group; and (e) studies that compared COS with letrozole vs. COS plus other drugs.
Two investigators (BB and ML) independently extracted data from all the eligible studies. From each eligible record, the following variables were collected: first author, year of publication, sample size and type of COS (letrozole and no letrozole), patients' characteristics (indications, age), characteristics of COS cycle (trigger method, estradiol level at triggering, total gonadotropin dose, and number of days of stimulation), efficacy outcomes (number of mature MII oocytes, total number of oocytes retrieved, number of cryopreserved mature oocytes, maturation rate, fertilization rate, number of cryopreserved embryos, and pregnancy rate/live birth rate), relapse rate and disease-free survival (in cancer patients), adverse events, and progesterone levels when available.
Mean values with standard deviation or odds ratios (ORs) with 95% confidence intervals (CIs) were collected for all endpoints of interest (number of MII oocytes, total number of collected oocytes, maturation rate, fertilization rate, peak estradiol levels, total gonadotropin dose, and length of the stimulation). Statistical analysis was conducted with a random-effects model.
In order to analyze each endpoint and to compare the performances of COS with or without letrozole, data were studied via mean ratios (MRs), 95% CI, and P-values. A MR value >1 indicates that for a specific endpoint, the letrozole cohort has higher values while a MR < 1 means that the study favors standard COS without letrozole.
P < 0.05 were considered statistically significant. To evaluate heterogeneity among studies, I2 values and relative P-values were also reported. A sensitivity analysis for each endpoint was performed to assess if the results were mostly driven by one or more studies.
The search strategy returned 625 records: after applying the inclusion and exclusion criteria, 15 records were potentially eligible for this meta-analysis (Figure 1). Among them, three records were excluded because they referred to the same study: in two cases [Goldrat et al. (38) vs. Goldrat et al. (34) and Cakman et al. (39) vs. Quinn et al. (36)], the article with the most updated data was selected (34, 36); for the other case [Haas et al. (24) vs. Haas et al. (40)], the least recent paper was included because of a larger sample size and the reporting of endpoints considered in the present meta-analysis (24). One article was excluded because it did not provide the required data for statistical analysis (41).
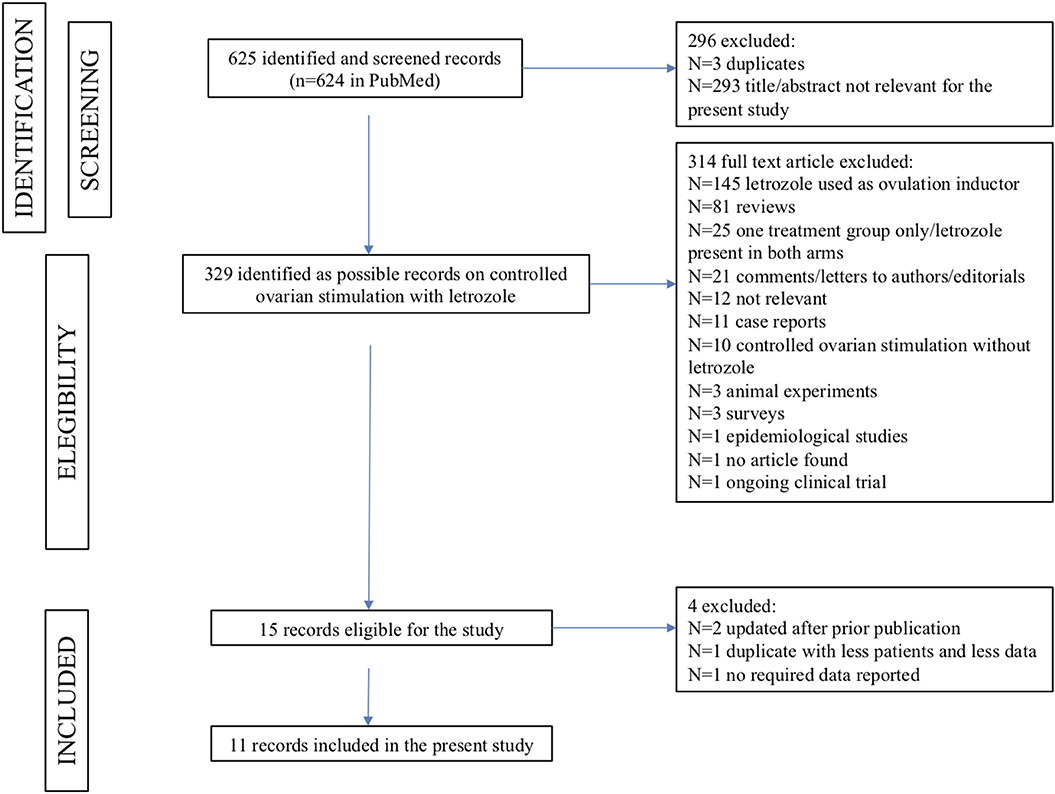
Figure 1. The PRISMA flowchart summarizing the process for identifying the records to include in the present meta-analysis.
Therefore, a total of 11 records were selected for the current meta-analysis, including 2,121 patients, of whom 990 underwent COS with letrozole and 1,131 underwent COS without letrozole (24, 29, 33–37, 42–45). Six studies were conducted in cancer patients only (35–37, 42, 43, 45); one study in infertile patients only (24). COS with letrozole in cancer patients was compared to COS without letrozole in infertile controls in two studies (29, 34), to COS without letrozole in healthy elective fertility preservation patients in another study (33), and to both cancer patients and healthy elective fertility preservation patients in another study (44).
The main characteristics of the included studies are summarized in Table 1.
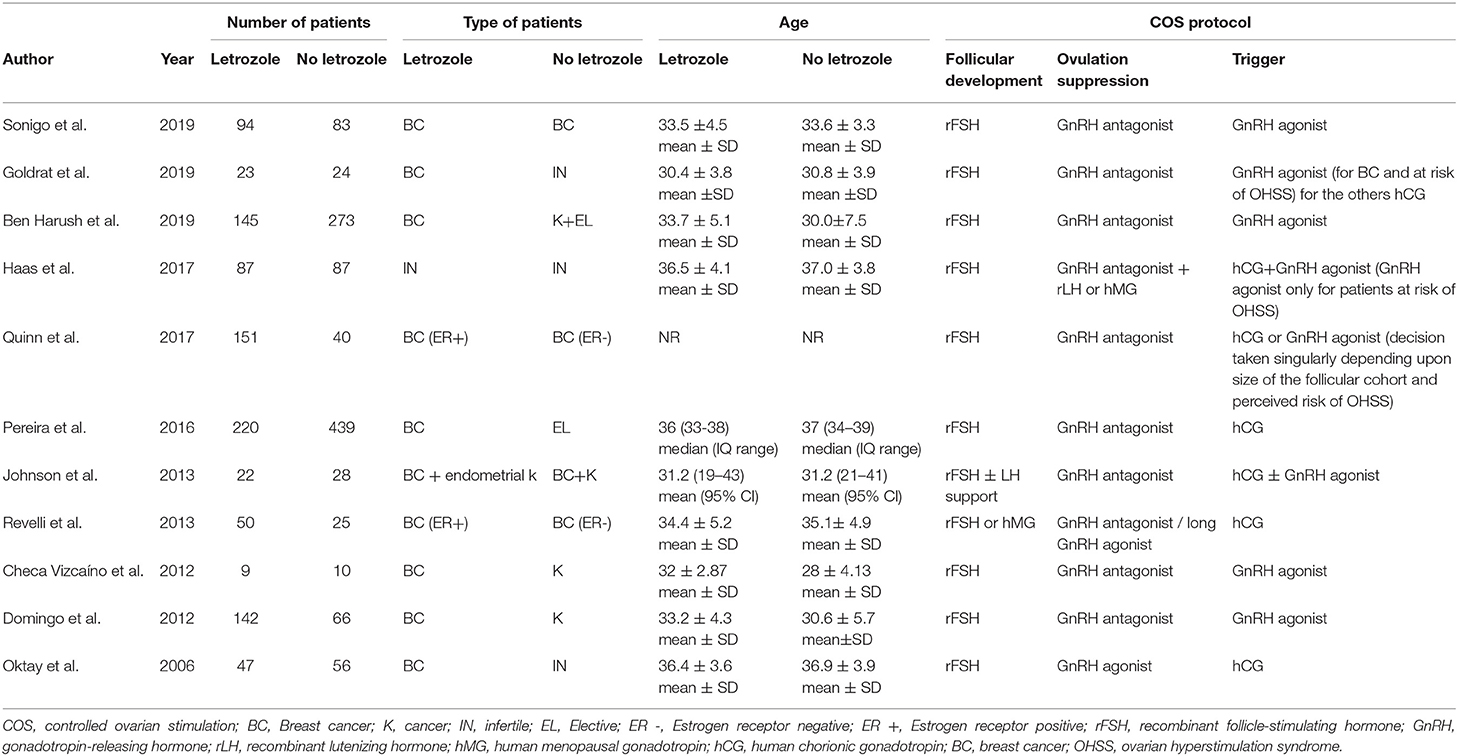
Table 1. Main characteristics of the included studies and type of protocol of controlled ovarian stimulation.
The total number of MII oocytes, as quantitative marker of efficacy, was reported in nine studies (24, 29, 34–37, 42–44). No difference between the letrozole and no-letrozole cohorts was found with a MR value of 1.00 (95% CI = 0.87–1.16; P = 0.967; Figure 2). Heterogeneity was high (I2 = 68.6%; P = 0.001). Sensitivity analysis is reported in Supplementary Table 1.
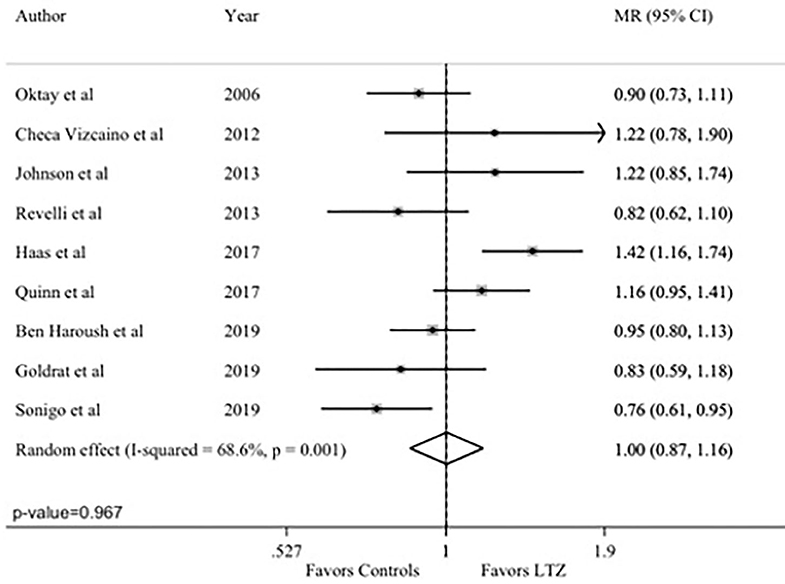
Figure 2. Primary endpoint: number of Metaphase II (MII) oocytes. MR, mean ratios; CI, confidence intervals; LTZ, letrozole.
The total number of retrieved oocytes was reported in all studies (24, 29, 33–37, 42–45). No difference between the letrozole and no-letrozole cohorts was found (MR = 1.04; 95% CI = 0.93–1.17; P = 0.493; Figure 3A). Heterogeneity was high (I2 = 73.8%; P < 0.001). Sensitivity analysis is reported in Supplementary Table 2.
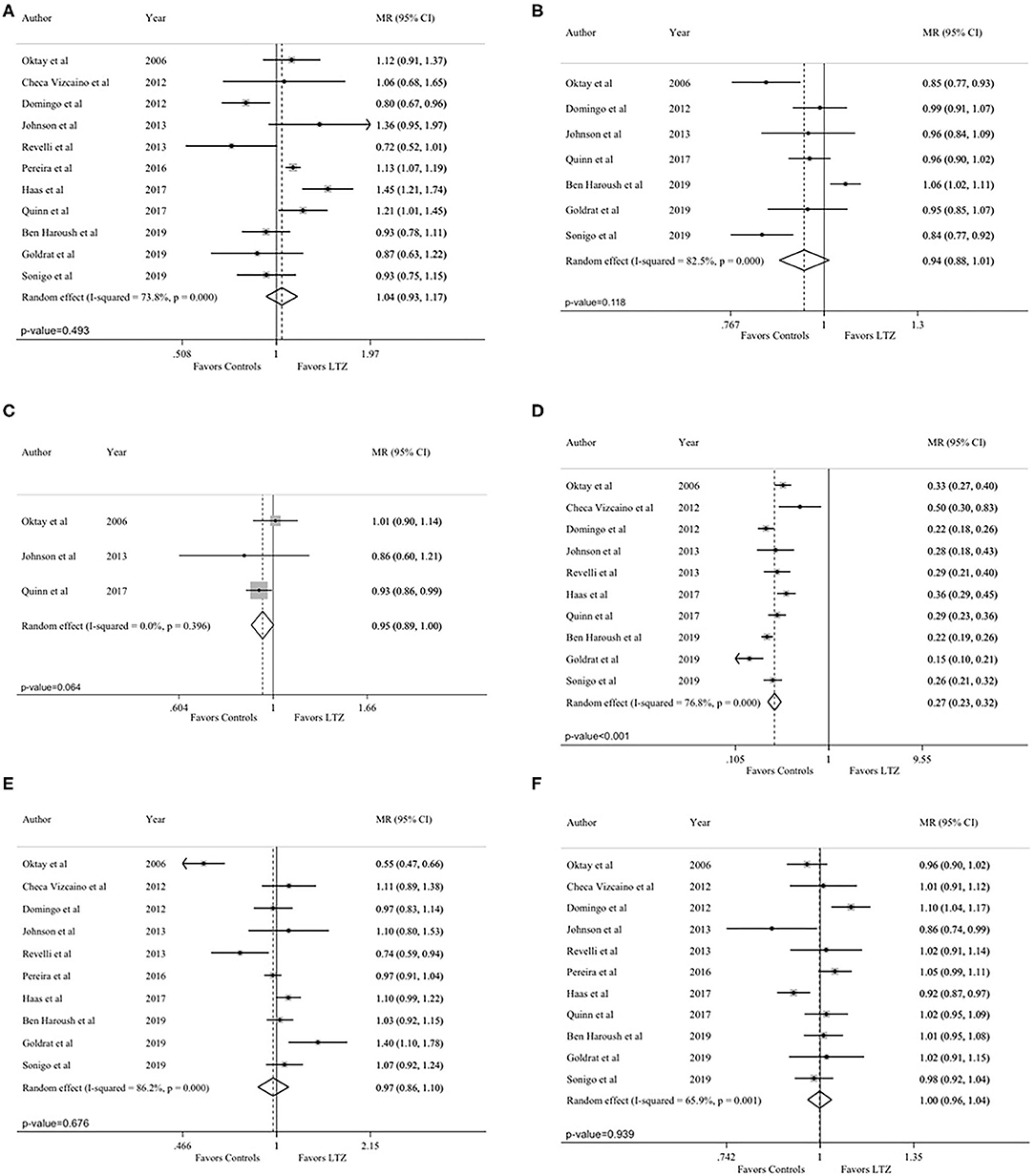
Figure 3. Secondary endpoints: (A) total number of retrieved oocytes; (B) maturation rate; (C) fertilization rate; (D) peak estradiol levels; (E) total gonadotropin dose; (F) length of the stimulation. MR, mean ratios; CI, confidence intervals; LTZ, letrozole.
Seven studies reported on maturation rate (29, 34, 36, 37, 43–45). Higher maturation rate was observed in the no-letrozole cohort; however, the difference was not statistically significant (MR = 0.94, 95% CI = 0.88–1.01, P = 0.118, Figure 3B). Heterogeneity was high (I2 = 82.5%; P < 0.001). Sensitivity analysis is reported in Supplementary Table 3.
Fertilization rate was reported in three studies (29, 36, 43). A higher fertilization rate was observed in the no-letrozole cohort; however, the difference was not statistically significant (MR = 0.95, 95% CI = 0.89–1.00, P = 0.064; Figure 3C). No heterogeneity was observed (I2 = 0.0%; P = 0.396). Sensitivity analysis is reported in Supplementary Table 4.
Peak estradiol levels were reported in 10 studies (24, 29, 34–37, 42–45). Estradiol levels were significantly lower in the letrozole cohort (MR = 0.27, 95% CI = 0.23–0.32; P < 0.001; Figure 3D). Heterogeneity was high (I2 = 76.8%; P < 0.001). Sensitivity analysis is reported in Supplementary Table 5.
Total gonadotropin dose was reported in 10 studies (24, 29, 33–35, 37, 42–45). No statistically significant difference was observed between the letrozole and no-letrozole cohorts (MR = 0.97, 95% CI = 0.86–1.10, P = 0.676; Figure 3E). Heterogeneity was high (I2 = 86%, P < 0.001). Sensitivity analysis is reported in Supplementary Table 6.
Length of the stimulation was reported in all included studies (24, 29, 33–37, 42–45). There was no difference between the letrozole and no-letrozole cohorts (MR =1.00, 95% CI = 0.96–1.04, P = 0.939; Figure 3F). Heterogeneity was high (I2 = 65.9%; P = 0.001). Sensitivity analysis is reported in Supplementary Table 7.
All the analyses were repeated by including only the three articles comparing breast cancer patients in both the letrozole and no-letrozole cohorts (35–37). Based on data availability, four endpoints (number of MII oocytes, total number of oocytes, length of the stimulation, and peak estradiol levels) could be analyzed. The observed results were consistent with those of the primary analysis. No difference between the letrozole and no-letrozole cohorts was observed in terms of total number of MII oocytes (MR = 0.90; 95% CI = 0.68–1.20; P = 0.482; Figure 4A; I2 = 76.9% and P = 0.013), total oocytes retrieved (MR = 0.96; 95% CI = 0.73–1.26; P = 0.771; Figure 4B; I2 = 76.2%; P = 0.015), and length of the stimulation (MR = 1.00, 95% CI = 0.96–1.04, P = 0.985; Figure 4C; I2 = 0.0%; P = 0.666). Peak estradiol levels were significantly lower in the letrozole group as compared to the no-letrozole group (MR = 0.28, 95% CI = 0.24–0.32; P < 0.001; Figure 4D; I2 = 0.0% and P = 0.778).
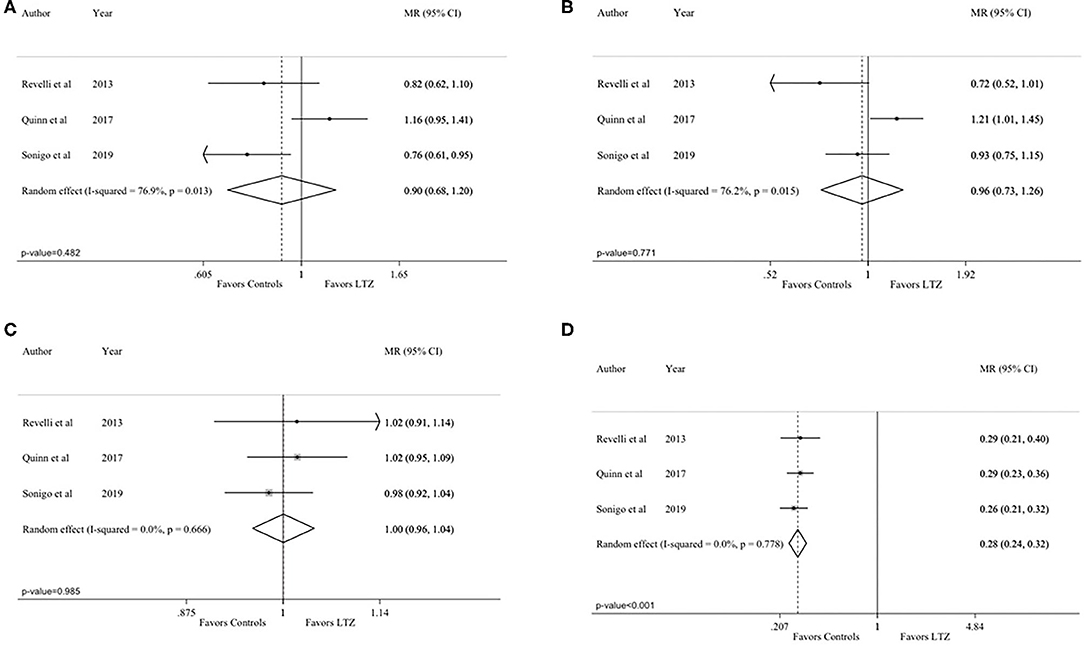
Figure 4. Analysis including only the studies comparing breast cancer patients in both the letrozole and no-letrozole groups: (A) number of Metaphase II (MII) oocytes; (B) total number of retrieved oocytes; (C) length of the stimulation; (D) peak estradiol levels. MR, mean ratios; CI, confidence intervals; LTZ, letrozole.
While letrozole as an ovulation inductor is well-known and widely used (21, 22, 46), its role alongside a COS protocol is less studied and therefore less used in infertile patients, due to conflicting results and the safety warning of the producer. However, in the last years, it has become the standard of care for COS in patients with hormone-sensitive cancers to avoid potentially harmful supra-physiological estradiol levels (47), which are the main reason for oncologists to oppose oocyte or embryo cryopreservation (18). However, the evidence on the use of COS protocol that include letrozole is based on few observational studies, most of them with a small sample size and heterogeneous in nature.
The present meta-analysis aimed to assess the efficacy and safety of letrozole co-administration during COS. It showed that the addition of letrozole to COS does not have a negative effect on the number of mature oocytes collected and on other efficacy endpoints, while it is associated with significantly decreased peak estradiol levels which may be of great importance particularly in patients with hormone-sensitive cancers.
A high heterogeneity among studies was observed in the majority of the analysis. This may be due to study design (none was a randomized trial), their low sample size, the different cohorts of patients included also in terms of age, as well as the non-homogeneous COS protocols. For example, Ben Haroush et al. included in the no-letrozole cohort healthy women who elected to have their oocyte cryopreserved for social reasons and very young patients (26.5 ± 7.1 years) with non-hormone-sensitive cancers (44). Both these groups of women are expected to be high-responders, but surprisingly, the authors reported similar number of retrieved oocytes between groups with a slightly higher maturation rate in favor of the breast cancer cohort. The sensitivity analysis reported in Supplementary Table 3 showed that, after excluding the study by Ben Haroush et al., maturation rate results become statistically significant in favor of the no-letrozole cohort, supporting that this study strongly weights on the final statistical results for this parameter.
The study design, and specifically the choice of the controls, is the feature associated with the highest risk of bias for the included studies. By comparing results between cancer patients and healthy infertile women with the latter probably having a worse prognosis at start, a selection bias becomes impossible to avoid, especially in an observational study. Using healthy patients who elected to have their fertility preserved for social reasons is probably a more accurate choice; however, literature is not univocal on ovarian response to COS in cancer patients before gonadotoxic therapies, not excluding a worse ovarian reserve even before starting anticancer therapies (48, 49). Study comparing cancer patients in both study groups usually had smaller sample size and did not exclude potential bias due to the impact of the cancer type. Only three studies included exclusively breast cancer patients in both study cohorts. To specifically investigate the performance of COS with or without letrozole in breast cancer patients, we performed a secondary analysis that showed no influence of letrozole on all the evaluated efficacy endpoints. However, some issues remain to be clarified also in this setting. For example, BRCA-mutated women, which are described by some reports as less fertile (50, 51), have more frequently hormone receptor-negative cancers; therefore, they are more likely to be included in the no-letrozole cohorts. Recent data, demonstrating the safety of pregnancy in breast cancer survivors with germline BRCA pathogenic variants (52), further highlight the need to pursue with additional research efforts to define the optimal fertility preservation approaches in these patients. Only one study in this meta-analysis compared infertile patients both in the letrozole and no-letrozole cohorts (24). The authors hypothesized a beneficial effect of letrozole on ovarian response because of an androgen-mediated increase of FSH receptors on granulosa cells, as it was seen in primates (53). Their results in terms of number of oocytes and blastocysts obtained are promising, but the study design is retrospective and it analyzed only 174 IVF cycles. A well-designed randomized trial would be better suited to confirm or deny their findings.
Another important potential explanation for the heterogeneity among studies is the ovulation trigger criteria that were used. In an earlier study, Oktay et al. showed lower oocyte maturation rates when trigger was achieved at a leading follicle size of 17 mm (29). Once trigger was done at a follicular size of 19–21 mm, maturation rates improved. In several studies included in our meta-analysis, ovulation trigger was performed in both groups either at 17 mm or “when appropriate” (24, 35, 37, 44). This issue may account for the lower oocyte maturation rate in the letrozole cohort as compared to the non-letrozole cohort.
Importantly, the core outcome of fertility research should be the live birth rate being the ultimate chance that a specific treatment gives a patient the possibility to have a baby. Unfortunately, a meta-analysis on this outcome is not yet possible. Only one study included in our metanalysis reported this outcome in both the letrozole and no-letrozole cohorts (43). In this study, out of 50 patients, only six returned to thaw the embryos, one in the letrozole cohort and five in the no-letrozole cohort. For the patients who received COS with letrozole, one twin pregnancy via gestational carrier was obtained; it was complicated by pre-eclampsia, and two babies were born pre-term with a cesarean section. Among the five patients who received COS without letrozole only, three had their embryos transferred (the embryos did not survive the thawing for the other two). One patient used a gestational carrier, the pregnancy had no complications, and the baby was delivered vaginally at term. The other two patients had singleton pregnancies: one was complicated by pre-term labor but managed to deliver at term vaginally; the other was complicated by a baby large for gestational age and was delivered at term via cesarean section (43). Notably, utilization rate of cryopreserved material in cancer patients is reported to be quite low [around 10–23% for frozen embryos (54–56) and 5% for frozen oocytes (57–59)], considering that these women, also those who need to use their cryopreserved oocytes or embryos to have a pregnancy, have to complete their oncological therapies before. With time, more data on the utilization of such material will become available.
In terms of safety concerns, peak estradiol level was lower in the letrozole cohort. These data indirectly confirm the possible protective mechanism of letrozole for patients who are affected by hormone-sensitive cancers including breast tumors. Breast cancer patients who undergo COS with letrozole before starting chemotherapy does not appear to have higher risk of recurrence than those who do not undergo any fertility preservation procedure (60). The safety of this approach has also been shown for patients undergoing neoadjuvant chemotherapy, although the evidence is more limited in this setting (60, 61). The length of the stimulation is another safety parameter; indeed, this is of particular importance for cancer patients who need to start life-saving oncological treatments as soon as possible. Our study shows that standard protocols for COS with or without letrozole have the same stimulation length. Due to the paucity of information reported in the included articles, safety data remain largely incomplete. More evidence is needed on oncological outcomes (i.e., relapse rate, disease-free survival, adverse events, and delay in chemotherapy start) (62) as well as progesterone levels during COS (38, 63).
In conclusion, letrozole co-administration during COS resulted to be as effective as standard COS but with significantly decreased peak estradiol levels, suggesting its increased safety for patients with hormone-sensitive cancers. Although current data are reassuring, more studies, including randomized controlled trials, are needed to finally prove the efficacy and safety of letrozole co-administration during COS, particularly among cancer patients. Moreover, long-term outcomes in terms of both efficacy and safety should be strongly encouraged to be collected.
The data extracted from the original publications and supporting the conclusions of this article will be made available by the authors, without undue reservation.
ID and ML contributed to the conception and design of the study. BB, ID, and ML performed the literature search, study selection, and data extraction that were then reviewed by all authors. MB and MC performed the statistical analysis. The results were interpreted by BB, CM, ID, and ML The initial manuscript was drafted by BB, CM, ID, and ML. All authors revised the manuscript critically for important intellectual content and approved it.
This work was partially funded by grants from the Italian Ministry of Health 5 × 1,000 funds 2017 (Italy) and Fonds National de la Recherche Scientifique (FNRS, Belgium). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
LDM acted as consultant for Roche, Novartis, Eisai, Pfeizer, Astrazeneca, Ipsen, Eli Lilly, MSD, Seattle Genetics and Genomic Health outside the submitted work. ID acted as consultant for Roche and received speaker honoraria from Novartis. ML acted as a consultant for Roche and Novartis, and received honoraria from Theramex, Takeda, Roche, Lilly, Pfizer and Novartis outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ML acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at Université Libre de Bruxelles (ULB) in Brussels (Belgium) during the conduction of this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.574669/full#supplementary-material
Supplementary Table 1. Sensitivity analysis for the endpoint “mature Metaphase II (MII) oocytes.”
Supplementary Table 2. Sensitivity analysis for the endpoint “total number of retrieved oocytes.”
Supplementary Table 3. Sensitivity analysis for the endpoint “maturation rate.”
Supplementary Table 4. Sensitivity analysis for the endpoint “fertilization rate.”
Supplementary Table 5. Sensitivity analysis for the endpoint “peak estradiol levels.”
Supplementary Table 6. Sensitivity analysis for the endpoint “total gonadotropin dose.”
Supplementary Table 7. Sensitivity analysis for the endpoint “length of the stimulation.”
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
2. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American society of clinical oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. (2006) 24:2917–31. doi: 10.1200/JCO.2006.06.5888
3. Adriaens I, Smitz J, Jacquet P. The current knowledge on radiosensitivity of ovarian follicle development stages. Hum Reprod Update. (2009) 15:359–77. doi: 10.1093/humupd/dmn063
4. Lambertini M, Del Mastro L, Pescio MC, Andersen CY, Azim HA, Peccatori FA, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. (2016) 14:1. doi: 10.1186/s12916-015-0545-7
5. Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update. (2019) 25:673–93. doi: 10.1093/humupd/dmz027
6. Lambertini M, Olympios N, Lequesne J, Calbrix C, Fontanilles M, Loeb A, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-müllerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol. (2019) 9:575. doi: 10.3389/fonc.2019.00575
7. Dolmans MM, Lambertini M, Macklon KT, Almeida Santos T, Ruiz-Casado A, Borini A, et al. EUropean REcommendations for female FERtility preservation (EU-REFER): a joint collaboration between oncologists and fertility specialists. Crit Rev Oncol Hematol. (2019) 138:233–40. doi: 10.1016/j.critrevonc.2019.03.010
8. Arecco L, Perachino M, Damassi A, Latocca MM, Soldato D, Vallome G, et al. Burning questions in the oncofertility counseling of young breast cancer patients. Breast Cancer. (2020) 14:1178223420954179. doi: 10.1177/1178223420954179
9. Letourneau JM, Smith JF, Ebbel EE, Craig A, Katz PP, Cedars MI, et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer. (2012) 118:4579–88. doi: 10.1002/cncr.26649
10. Lambertini M, Fontana V, Massarotti C, Poggio F, Dellepiane C, Iacono G, et al. Prospective study to optimize care and improve knowledge on ovarian function and/or fertility preservation in young breast cancer patients: results of the pilot phase of the PREgnancy and Fertility (PREFER) study. Breast. (2018) 41:51–6. doi: 10.1016/j.breast.2018.06.012
11. Ruggeri M, Pagan E, Bagnardi V, Bianco N, Gallerani E, Buser K, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European Centers. Breast. (2019) 47:85–92. doi: 10.1016/j.breast.2019.07.001
12. Ethics Committee of the American Society for Reproductive Medicine. electronic address: ASRM@asrm.org fertility preservation and reproduction in patients facing gonadotoxic therapies: an ethics committee opinion. Fertil Steril. (2018) 110:380–6. doi: 10.1016/j.fertnstert.2018.05.034
13. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. (2020). doi: 10.1016/j.annonc.2020.09.006
14. Lambertini M, Cinquini M, Moschetti I, Peccatori FA, Anserini P, Valenzano Menada M, et al. Temporary ovarian suppression during chemotherapy to preserve ovarian function and fertility in breast cancer patients: a GRADE approach for evidence evaluation and recommendations by the Italian association of medical oncology. Eur J Cancer. (2017) 71:25–33. doi: 10.1016/j.ejca.2016.10.034
15. Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline Update. J Clin Oncol. (2018) 36:1994–2001. doi: 10.1200/JCO.2018.78.1914
16. Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod. (2011) 26:1768–74. doi: 10.1093/humrep/der106
17. Lambertini M, Pescio MC, Viglietti G, Goldrat O, Mastro LD, Anserini P, et al. Methods of controlled ovarian stimulation for embryo/oocyte cryopreservation in breast cancer patients. Expert Rev Quality Life Cancer Care. (2017) 2:47–59. doi: 10.1080/23809000.2017.1270760
18. Lambertini M, Di Maio M, Pagani O, Curigliano G, Poggio F, Del Mastro L, et al. The BCY3/BCC 2017 survey on physicians' knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. (2018) 42:41–9. doi: 10.1016/j.breast.2018.08.099
19. Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. (2005) 23:4347–53. doi: 10.1200/JCO.2005.05.037
20. Meirow D, Raanani H, Maman E, Paluch-Shimon S, Shapira M, Cohen Y, et al. Tamoxifen co-administration during controlled ovarian hyperstimulation for in vitro fertilization in breast cancer patients increases the safety of fertility-preservation treatment strategies. Fertil Steril. (2014) 102:488–95.e3. doi: 10.1016/j.fertnstert.2014.05.017
21. Wang R, Kim BV, van Wely M, Johnson NP, Costello MF, Zhang H, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta-analysis. BMJ. (2017) 356:j138. doi: 10.1136/bmj.j138
22. Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. (2018) 5:CD010287. doi: 10.1002/14651858.CD010287.pub3
23. Polyzos NP, Tzioras S, Badawy AM, Valachis A, Dritsas C, Mauri D. Aromatase inhibitors for female infertility: a systematic review of the literature. Reprod Biomed Online. (2009) 19:456–71. doi: 10.1016/j.rbmo.2009.06.008
24. Haas J, Bassil R, Meriano J, Samara N, Barzilay E, Gonen N, et al. Does daily co-administration of letrozole and gonadotropins during ovarian stimulation improve IVF outcome? Reprod Biol Endocrinol. (2017) 15:70. doi: 10.1186/s12958-017-0288-8
25. Haas J, Casper RF. In vitro fertilization treatments with the use of clomiphene citrate or letrozole. Fertil Steril. (2017) 108:568–71. doi: 10.1016/j.fertnstert.2017.08.017
26. Tulandi T, Martin J, Al-Fadhli R, Kabli N, Forman R, Hitkari J, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. (2006) 85:1761–5. doi: 10.1016/j.fertnstert.2006.03.014
27. Sharma S, Ghosh S, Singh S, Chakravarty A, Ganesh A, Rajani S, et al. Congenital malformations among babies born following letrozole or clomiphene for infertility treatment. PLoS ONE. (2014) 9:e108219. doi: 10.1371/journal.pone.0108219
28. Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. (2008) 26:2630–5. doi: 10.1200/JCO.2007.14.8700
29. Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. (2006) 91:3885–90. doi: 10.1210/jc.2006-0962
30. Arya S, Kupesic-Plavsic S, Mulla ZD, Dwivedi AK, Crisp Z, Jose J, et al. Ovulation induction and controlled ovarian stimulation using letrozole gonadotropin combination: a single center retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. (2017) 218:123–8. doi: 10.1016/j.ejogrb.2017.09.023
31. Kamath MS, Maheshwari A, Bhattacharya S, Lor KY, Gibreel A. Oral medications including clomiphene citrate or aromatase inhibitors with gonadotropins for controlled ovarian stimulation in women undergoing in vitro fertilisation. Cochrane Database Syst Rev. (2017) 11:CD008528. doi: 10.1002/14651858.CD008528.pub3
32. Hembram M, Biswas R, Jain A. A study of controlled ovarian stimulation with clomiphene citrate or letrozole in combination with gonadotropins and IUI in unexplained infertility. J Hum Reprod Sci. (2017) 10:173–7. doi: 10.4103/jhrs.JHRS_120_16
33. Pereira N, Hancock K, Cordeiro CN, Lekovich JP, Schattman GL, Rosenwaks Z. Comparison of ovarian stimulation response in patients with breast cancer undergoing ovarian stimulation with letrozole and gonadotropins to patients undergoing ovarian stimulation with gonadotropins alone for elective cryopreservation of oocytes†. Gynecol Endocrinol. (2016) 32:823–6. doi: 10.1080/09513590.2016.1177013
34. Goldrat O, Van Den Steen G, Gonzalez-Merino E, Dechène J, Gervy C, Delbaere A, et al. Letrozole-associated controlled ovarian hyperstimulation in breast cancer patients vs. conventional controlled ovarian hyperstimulation in infertile patients: assessment of oocyte quality related biomarkers. Reprod Biol Endocrinol. (2019) 17:3. doi: 10.1186/s12958-018-0443-x
35. Revelli A, Porcu E, Levi Setti PE, Delle Piane L, Merlo DF, Anserini P. Is letrozole needed for controlled ovarian stimulation in patients with estrogen receptor-positive breast cancer? Gynecol Endocrinol. (2013) 29:993–6. doi: 10.3109/09513590.2013.819083
36. Quinn MM, Cakmak H, Letourneau JM, Cedars MI, Rosen MP. Response to ovarian stimulation is not impacted by a breast cancer diagnosis. Hum Reprod. (2017) 32:568–74. doi: 10.1093/humrep/dew355
37. Sonigo C, Sermondade N, Calvo J, Benard J, Sifer C, Grynberg M. Impact of letrozole supplementation during ovarian stimulation for fertility preservation in breast cancer patients. Eur J Obstet Gynecol Reprod Biol X. (2019) 4:100049. doi: 10.1016/j.eurox.2019.100049
38. Goldrat O, Gervy C, Englert Y, Delbaere A, Demeestere I. Progesterone levels in letrozole associated controlled ovarian stimulation for fertility preservation in breast cancer patients. Hum Reprod. (2015) 30:2184–9. doi: 10.1093/humrep/dev155
39. Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. (2015) 27:215–21. doi: 10.1097/GCO.0000000000000180
40. Haas J, Bassil R, Gonen N, Meriano J, Jurisicova A, Casper RF. The VEGF and PEDF levels in the follicular fluid of patients co- treated with LETROZOLE and gonadotropins during the stimulation cycle. Reprod Biol Endocrinol. (2018) 16:54. doi: 10.1186/s12958-018-0367-5
41. Kim JH, Kim SK, Lee HJ, Lee JR, Jee BC, Suh CS, et al. Efficacy of random-start controlled ovarian stimulation in cancer patients. J Korean Med Sci. (2015) 30:290–5. doi: 10.3346/jkms.2015.30.3.290
42. Checa Vizcaíno MA, Corchado AR, Cuadri MESI, Comadran MG, Brassesco M, Carreras R. The effects of letrozole on ovarian stimulation for fertility preservation in cancer-affected women. Reprod Biomed Online. (2012) 24:606–10. doi: 10.1016/j.rbmo.2012.02.020
43. Johnson LNC, Dillon KE, Sammel MD, Efymow BL, Mainigi MA, Dokras A, et al. Response to ovarian stimulation in patients facing gonadotoxic therapy. Reprod Biomed Online. (2013) 26:337–44. doi: 10.1016/j.rbmo.2013.01.003
44. Ben-Haroush A, Wertheimer A, Klochendler E, Sapir O, Shufaro Y, Oron G. Effect of letrozole added to gonadotropins in controlled ovarian stimulation protocols on the yield and maturity of retrieved oocytes. Gynecol Endocrinol. (2019) 35:324–7. doi: 10.1080/09513590.2018.1534950
45. Domingo J, Guillén V, Ayllón Y, Martínez M, Muñoz E, Pellicer A, et al. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril. (2012) 97:930–4. doi: 10.1016/j.fertnstert.2012.01.093
46. Costello M, Garad R, Hart R, Homer H, Johnson L, Jordan C, et al. A review of first line infertility treatments and supporting evidence in women with polycystic ovary syndrome. Med Sci (Basel). (2019) 7.:95 doi: 10.3390/medsci7090095
47. Lambertini M, Goldrat O, Clatot F, Demeestere I, Awada A. Controversies about fertility and pregnancy issues in young breast cancer patients: current state of the art. Curr Opin Oncol. (2017) 29:243–52. doi: 10.1097/CCO.0000000000000380
48. Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, et al. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma–evaluation by using antimüllerian hormone and retrieved oocytes. Fertil Steril. (2012) 98:141–4. doi: 10.1016/j.fertnstert.2012.04.021
49. Lekovich J, Lobel ALS, Stewart JD, Pereira N, Kligman I, Rosenwaks Z. Female patients with lymphoma demonstrate diminished ovarian reserve even before initiation of chemotherapy when compared with healthy controls and patients with other malignancies. J Assist Reprod Genet. (2016) 33:657–62. doi: 10.1007/s10815-016-0689-1
50. Lambertini M, Goldrat O, Toss A, Azim HA, Peccatori FA, Ignatiadis M, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients. Cancer Treat Rev. (2017) 59:61–70. doi: 10.1016/j.ctrv.2017.07.001
51. Turan V, Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update. (2020) 26:43–57. doi: 10.1093/humupd/dmz043
52. Lambertini M, Ameye L, Hamy A-S, Zingarello A, Poorvu PD, Carrasco E, et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J Clin Oncol. (2020) 38:3012–23. doi: 10.1200/JCO.19.02399
53. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. (1999) 84:2951–6. doi: 10.1210/jcem.84.8.5929
54. Barcroft J, Dayoub N, Thong KJ. Fifteen year follow-up of embryos cryopreserved in cancer patients for fertility preservation. J Assist Reprod Genet. (2013) 30:1407–13. doi: 10.1007/s10815-013-0024-z
55. Dolmans MM, Hollanders de Ouderaen S, Demylle D, Pirard C. Utilization rates and results of long-term embryo cryopreservation before gonadotoxic treatment. J Assist Reprod Genet. (2015) 32:1233–7. doi: 10.1007/s10815-015-0533-z
56. Luke B, Brown MB, Missmer SA, Spector LG, Leach RE, Williams M, et al. Assisted reproductive technology use and outcomes among women with a history of cancer. Hum Reprod. (2016) 31:183–9. doi: 10.1093/humrep/dev288
57. Martinez M, Rabadan S, Domingo J, Cobo A, Pellicer A, Garcia-Velasco JA. Obstetric outcome after oocyte vitrification and warming for fertility preservation in women with cancer. Reprod Biomed Online. (2014) 29:722–8. doi: 10.1016/j.rbmo.2014.09.002
58. Druckenmiller S, Goldman KN, Labella PA, Fino ME, Bazzocchi A, Noyes N. Successful oocyte cryopreservation in reproductive-aged cancer survivors. Obstet Gynecol. (2016) 127:474–80. doi: 10.1097/AOG.0000000000001248
59. Diaz-Garcia C, Domingo J, Garcia-Velasco JA, Herraiz S, Mirabet V, Iniesta I, et al. Oocyte vitrification vs. ovarian cortex transplantation in fertility preservation for adult women undergoing gonadotoxic treatments: a prospective cohort study. Fertil Steril. (2018) 109:478–485.e2. doi: 10.1016/j.fertnstert.2017.11.018
60. Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. (2016) 101:1364–71. doi: 10.1210/jc.2015-3878
61. Letourneau JM, Wald K, Sinha N, Juarez-Hernandez F, Harris E, Cedars MI, et al. Fertility preservation before breast cancer treatment appears unlikely to affect disease-free survival at a median follow-up of 43 months after fertility-preservation consultation. Cancer. (2020) 126:487–95. doi: 10.1002/cncr.32546
62. Lambertini M, Fontanella C. How reliable are the available safety data on hormonal stimulation for fertility preservation in young women with newly diagnosed early breast cancer? Breast Cancer Res Treat. (2018) 168:773–4. doi: 10.1007/s10549-017-4654-1
Keywords: fertility, controlled ovarian stimulation, letrozole, gonadotropins, breast cancer
Citation: Bonardi B, Massarotti C, Bruzzone M, Goldrat O, Mangili G, Anserini P, Spinaci S, Arecco L, Del Mastro L, Ceppi M, Demeestere I and Lambertini M (2020) Efficacy and Safety of Controlled Ovarian Stimulation With or Without Letrozole Co-administration for Fertility Preservation: A Systematic Review and Meta-Analysis. Front. Oncol. 10:574669. doi: 10.3389/fonc.2020.574669
Received: 20 June 2020; Accepted: 01 September 2020;
Published: 07 October 2020.
Edited by:
Luigi Formisano, University of Naples Federico II, ItalyReviewed by:
Barbara Buonomo, European Institute of Oncology (IEO), ItalyCopyright © 2020 Bonardi, Massarotti, Bruzzone, Goldrat, Mangili, Anserini, Spinaci, Arecco, Del Mastro, Ceppi, Demeestere and Lambertini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matteo Lambertini, matteo.lambertini@unige.it
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.