- 1College of Medicine, QU Health, Qatar University, Doha, Qatar
- 2Biomedical Research Center, Qatar University, Doha, Qatar
Breast and cervical cancers comprise 50% of all cancers during pregnancy. In particular, gestational breast cancer is considered one of the most aggressive types of cancers, which is a rare but fatal disease. However, the incidence of this type of cancer is increasing over the years and its prevalence is expected to rise further as more women delay childbearing. Breast cancer occurring after pregnancy is generally triple negative with specific characterizations of a poorer prognosis and outcome. On the other hand, it has been pointed out that this cancer is associated with a specific group of genes which can be used as precise targets to manage this deadly disease. Indeed, combination therapies consisting of gene-based agents with other cancer therapeutics is presently under consideration. We herein review recent progress in understanding the development of breast cancer during pregnancy and their unique subtype of triple negative which is the hallmark of this type of breast cancer.
Introduction
Breast cancer is the most common type of cancer in females affecting more than 2.1 million women and causing more than half a million deaths annually (1). The etiology of breast cancer is complex and heterogeneous with numerous pathological characteristics; these directly correlate with available treatment options and disease prognosis (2). Based on microarray and unsupervised cluster analysis studies, breast cancer is classified into four molecular subtypes with distinct gene expression patterns and clinical outcomes (3). These subtypes include luminal (A and B), human epidermal growth factor receptor 2 (HER2)-type, and triple-negative breast cancers (TNBC) (4–6).
TNBC possesses molecular characteristics and clinical aggressiveness that is analogous to that of basal-like cancer (7). TNBC lacks estrogen receptor (ER), progesterone receptor (PR), and HER2 expression and accounts for ~15% of all breast cancer cases (7). More intriguingly, the described subtype of breast cancer is reportedly associated with high-grade invasive ductal carcinomas and, when compared with other subtypes, TNBC was found to be larger with higher metastatic propensity to lungs, brain and other visceral organs.
Since the majority of basal-like cancers are also TNBC and more than 80% of TNBC are basal-like breast cancers, it has been postulated that TNBC and basal-like phenotypes are essentially analogous (8). Using gene expression profiling, the molecular heterogeneity of TNBC was well defined. One study subclassified TNBC into six molecular subtypes including basal-like 1, basal-like 2, immunomodulatory, mesenchymal-like, mesenchymal stem-like, and luminal androgen receptor (LAR) subtype (9). Furthermore, TNBC molecular subtyping revealed three subtypes, LAR, basal-like with low immune response and high M2-like macrophages and basal-enriched with high immune response and low M2-like macrophages (10). Despite histological differences, the metastatic characteristics of the highlighted TNBC subtypes remain comparable (11). In addition to the metastatic potential of TNBC, it is vital to note that once TNBC metastasizes the window between relapse and death becomes very narrow (12). Dent et al. reports that patients with TNBC were more likely to experience significant relapses and higher rates of death when compared with women suffering from other types of breast cancers (12). The same group also reports a four folds increase in the likelihood of visceral metastasis in TNBC patients when compared with other types of breast cancer (13).
Breast cancer risk factors are various; nonetheless, a strong association between pregnancy and breast cancer has been well established (14, 15). Although early age pregnancy is considered generally protective against breast cancer, this protection is deferred. Nevertheless, the period immediately subsequent to pregnancy is characterized by a risk of breast cancer development (16). During the last 30 years, diagnosis of cancer during pregnancy has become more common due to the present trend of delaying pregnancy or childbearing to an older age (17). Pregnancy-associated breast cancer (PABC) is an upcoming issue; in this review, we aim at illustrating recent advances in understanding the development and progression of PABC and their associated genes with emphasis on TNBC to review current and potential management options.
Gestational cancer is defined as cancer diagnosed during pregnancy or the first postpartum year (18). Pregnancy-associated melanoma, breast and cervical cancers are the most common malignancies during pregnancy; both cervical and breast cancers account for 50% of all gestational cancers (19). Hematological cancers including leukemia and lymphoma comprise 25% of gestational cancer cases, while ovarian, thyroid and colon cancers are less common (19).
Pregnancy-associated breast cancer (PABC), also known as “gestational breast cancer” is defined as breast cancer diagnosed either during pregnancy or up to one year postnatal (20) and affects around 1 in 3,000 pregnant women (21). In comparison with nulliparous women, breast cancer in pregnant women is histologically similar; approximately 75%–90% of the tumors are invasive ductal carcinomas with no-special-type (NST) (21–26). While, invasive lobular carcinoma (ILC) and other histological types are uncommon in patients with PABC (23, 27–29). Previous studies have showed that postpartum period is linked to a higher risk of developing more aggressive, high-grade breast cancer (14, 16, 23, 26, 30–32) with high tumor nuclear grade (29, 33, 34) and poorly differentiated tumors (24). PABC is also associated with lymphovascular invasion (22, 23, 33), more frequent lymph node involvement and larger tumor size (21–23, 27, 35–40). Similar to nulliparous women, PABC tends to commonly metastasize to lung, liver, brain, and skeletal system (41). Women with PABC have a poorer clinical outcome and disease-free survival with a higher mortality rate compared with nulliparous women (42–45).
With regards to steroid receptors, the previous data showed that estrogen and progesterone play major roles in breast tumorigenesis (46–48), and their effects on breast cells are mediated by their respective receptors, the ER and the PR (49, 50). Earlier studies evaluated tumor histology as well as the prognostic and predictive markers (ER, PR, HER‐2/neu, p53, and Ki‐67) in PABC; in comparison to age-matched non-pregnant women, their findings show that PABC exhibit lower expression of ER/PR and higher expression Ki‐67, p53 HER2 (23, 25, 26, 43, 51–53). However, a study by Shousha showed that during pregnancy or early lactation, the expression of HER-2/neu was negative; however HER2 expression was noticed after delivery or at the end of lactation indicating suppression of HER-2/neu expression during pregnancy and lactation (32). Low ER positivity was observed in women with PABC, plausibly due to decreased ER levels during pregnancy (22, 51, 54, 55). It has been indicated that increased estrogen levels can aid in preventing ER-positive tumors (56). Furthermore, multiparous women (≥3 live births) who never breastfed were at a higher risk of ER–/PR– breast cancers compared with multiparous women with a history of breastfeeding (57). A study by Harvell et al. analyzed the presence of breast cancer subtypes in PABC and found that the presence of Luminal A, Luminal B, Her2-positive, TNBC, and basal-like subtypes in PABC (58). Other studies also confirmed TNBC, Luminal B and HER2-positive as the most common subtypes among PABC while luminal A subtype was rare (25, 52, 59–61) (Table 1).
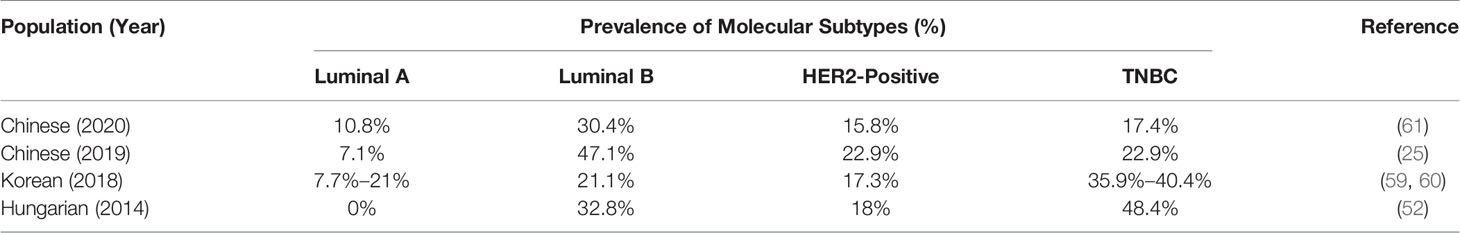
Table 1 Prevalence of molecular subtypes of breast cancer in pregnancy-associated breast cancer (PABC).
Several risk factors have been associated with PABC including hormonal changes, immune suppression during pregnancy as well as diagnostic challenges related to increased postpartum breast density and subsequent breast cancer diagnosis (14, 30). Breast involution is considered an important risk factor due to its shared features with pro-inflammatory microenvironment (26, 62–64), thus, providing suitable grounds for tumor growth and spread (65). Possible mechanisms of PABC include breast differentiation and involution (14, 66, 67). Following lactation, breast remodeling is a regulated program that involves the stimulation of fibroblasts, endothelial cells and immune cells. These cells then activate breast cells enhancing wound closure and remodeling of damaged tissue leading to the growth and development of transformed cells (14, 66). Moreover, an in-vitro and in-vivo study showed that involuting breast can assist the growth of existing tumor cells (66). In-vivo data showed that weaning-induced involution maintained ductal development of normal cells, however, in tumor cells they promoted invasion. Intriguingly, Yang et al. reported that early age at menarche, nulliparity, and late age at first birth increased the risk of luminal A breast cancer without any association with TNBC (68). On the contrary, all highlighted factors were identified as risk factor for TNBC in several other studies (69–74). Women’s race was also identified as a risk factor for TNBC. For instance, in comparison with white women, African-American women were found to be at a higher risk for TNBC, especially at a young age (<45 years) (71, 75, 76). A study by Ma et al. showed a protective effect of breastfeeding against development of TNBC (69). While data from the African American breast cancer epidemiology and Risk (AMBER) Consortium, showed that breastfeeding decreased the risk of TNBC associated with multiparity (77). Several other studies have revealed a significant correlation between PABC and high-grade breast cancers (16, 78–80); high grade morphology can be linked with PABC up to 10 years following pregnancy.
PABCs frequently display a higher incidence of the TNBC phenotype in comparison with cancers affecting nulliparous women. TNBCs comprise around 30%–40% of all PABC cases (52, 81) and are more likely to occur in recent pregnancy associated (within 1-2 years) breast cancers (52, 81). However, another study showed that TNBC risk can be present beyond 2 years postpartum; being one of the reasons for overall poor prognosis that characterizes tumors detected after pregnancy (16).
Molecular Features of Pregnancy-Associated Breast Cancer
To further understand the carcinogenic molecular pathways effected during pregnancy, leading to breast cancer development, it is crucial to investigate associated gene deregulation patterns as well as mutations and their role in breast cancer development. In comparison with normal epithelium, in PABC several hormone target genes regulating the mitotic phase were overexpressed (58); four of these genes MKI6, AURKA, BIRC5, and MMP11 are included in the Oncotype DX (82). Furthermore, the expression of tumor suppressor, p63 was downregulated in PABC; its expression correlates with enhanced invasion and aggressive feature of PABC (58, 83).
Azim et al. aimed at identifying the effects of pregnancy and involution on certain gene expression patterns in breast cancer cells compared with normal breast tissue (84). The authors found that the expression of PD-1, PD-L1, and gene sets related to SRC, IGF1, and β-catenin were higher compared with non-parous breast cancer females. However, this difference in the expression did not reach statistical significance. Therefore, in order to confirm this important finding with high statistical significance, more studies and larger patient sample sizes are necessary, which may lead to important therapeutic avenues based on these gene targets. During pregnancy, in response to growth hormones, expression of ER, PR, and IGF-1 is elevated and is linked with increase in breast cancer cell proliferation (14). In this context, in-vivo studies showed loss of PD-L1 to correlate with fetal resorption and increase in fetal lethality (85, 86), hence the expression pattern of PD-1 and its ligand PD-L1 in PABC was assessed. Another study analyzed PD-1 and PD-L1 expression in both tumor-infiltrating lymphocytes (TILs) in PABC and nulliparous women; PD-L1 was strongly elevated in PABC TILs in comparison with controls, independent of tumor characteristics (87). On the other hand, in TILs PD-1 was expressed in both PABC and nulliparous women (87). Research has shown that high stromal TILs and PD-L1 expression to be frequently present in TNBC (88). A similar study by Acs et al. (2017) assessed PD-1, PD-L1, and CTLA-4 expression in PABC and non-PABC women; the expression of PD-1, PD-L1 was seen in peritumoral lymphocytes, however there was no expression of CTLA-4 and elevated PD-L1 expression was associated with early-onset of breast cancer and poor prognosis (89).
Furthermore, certain pathways were also found to be highly activated in parous breast cancer females including the G protein-coupled receptor (GPCR) and the serotonin receptor signaling pathways (84). GPCR signaling pathway plays a vital role in multiple cellular processes and mediates the activation of around 3% of the genes (90). Consequently, any aberration within this pathway may contribute to various diseases including cardiac, inflammatory and neoplastic (91). However, GPCRs are large family of receptors and only two of these receptors (CXCR4 and GPR30) were found to be highly expressed in breast cancers (84). The upregulations of these genes may induce breast cancer growth and metastasis (92). Moreover, activation of those GPCRs happens upon their binding to their ligands, which triggers the subsequent Ca2+ mobilization and kinase cascade activation leading to the induction of the expression of genes that are crucial for cellular growth (93).
The interplay between pregnancy and breast cancer has been an intriguing research topic over the years (94–96). Schedin et al. showed that many alterations- both inflammatory and non-inflammatory- occur in postpartum breasts causing a tumor inducing microenvironment (14). To further illustrate the mechanisms through which this takes place, Asztalos et al. reported that the involution process works to restore the status of breast tissue -prior to pregnancy- by inducing apoptosis, detachment of cells from the basement membrane (97), and other inflammation related events (62). The created inflammatory environment may initiate and contribute to the progression of breast cancer, especially through promoting tumor cell proliferation (14, 98). More importantly, a multitude of studies report different genetic patterns of PABC as compared to those detected in nulliparous women (16, 52, 81). Notably, estrogens can also bind to a known subtype of GPCRS, G protein estrogen receptor (GPER), thus contributing to breast cancer initiation and progression (99). The upregulation of serotonin induces tumorigenesis through induction of cellular proliferation (100). Furthermore, serotonin receptor pathway helps in the regulation of the expression of cathepsin S (CTSS) and is highly expressed in several cancer subtypes in which it correlates with their progression (101). The aggressiveness of pregnancy associated-TNBC, its poor prognosis and lack of treatment modalities makes it pivotal to study its genetic patterns and identify novel treatment options.
Molecular Features of Pregnancy Associated Triple-Negative Breast Cancers
Several variations are mainly ascribed to TNBC subgroup that was reportedly more predominant in PABCs (16, 52, 81); however, the exact mechanism by which pregnancy induces TNBC is yet to be fully elucidated. Among the different subtypes of breast cancer, CTSS is involved in invasion and is highly expressed in TNBC (102). Another factor that might contribute to the poor prognosis of TNBC is that TNBC increases the levels tryptophan-2,3-dioxygenase (TDO2) enzyme through inflammatory signals (103). TDO2 is a critical enzyme in catabolism of tryptophan, and its upregulation increases the production of tryptophan metabolites that exhibit antiapoptotic effects in TNBC cells (102).
Nevertheless, studies that specifically addressed the impact of pregnancy on development of TNBC are scarce. Asztalos et al. found a unique gene expression pattern for a specific set of genes in parous females who developed breast cancer in comparison to nulliparous breast cancer females. Differently expressed genes included 14 genes such as CXCL1, CXCL12, ELN, ERBB2, ESR1, FBN1, IL1A, IL8, MMP12, MMP2, PGR, TGFB3, THBS1, and TIMP2. Four of these genes (CXCL1, IL1A, IL8, MMP12) were upregulated, while the remaining 10 genes were down regulated in TNBC. Notably, downregulation of three of these genes (ESR1, PGR, ERBB2) are features of TNBC (104). Three of the upregulated genes (CXCL1, IL1A, IL8) are involved in inflammatory responses. Furthermore, inflammation and wound-healing involve macrophage cell influx, increased levels TGF-β1 and β3, MMPs-2, -3, and -9, and presence of fibronectin and laminin; these are linked with tumor progression and result in metastasis (14, 105–107). The interaction between cells and fibronectin via β1 integrins results in the onset of human breast cancer (108). Upregulated expression of TGF-β triggers matrix deposition and growth of fibroblasts in the healing wound, thus, accelerating tumor growth (107, 109). Indeed, MMPs are essential for the process of angiogenesis and lymphangiogenesis; both processes are essential in wound healing and tumor initiation and progression (110, 111). These findings further support the hypothesis that inflammation could contribute to the development of TNBC after pregnancy.
Interestingly, Azim et al. reported that the receptor activator of the nuclear factor κB ligand (RANKL) is found to be repressed in TNBC compared with other types of breast cancer, while the receptor activator for nuclear factor κB (RANK) was found to be highly expressed in TNBC (112). However, the link between these TNBC patients and pregnancy was not been found (113). Table 2 summarizes function of the identified genes in normal cells and PABC.
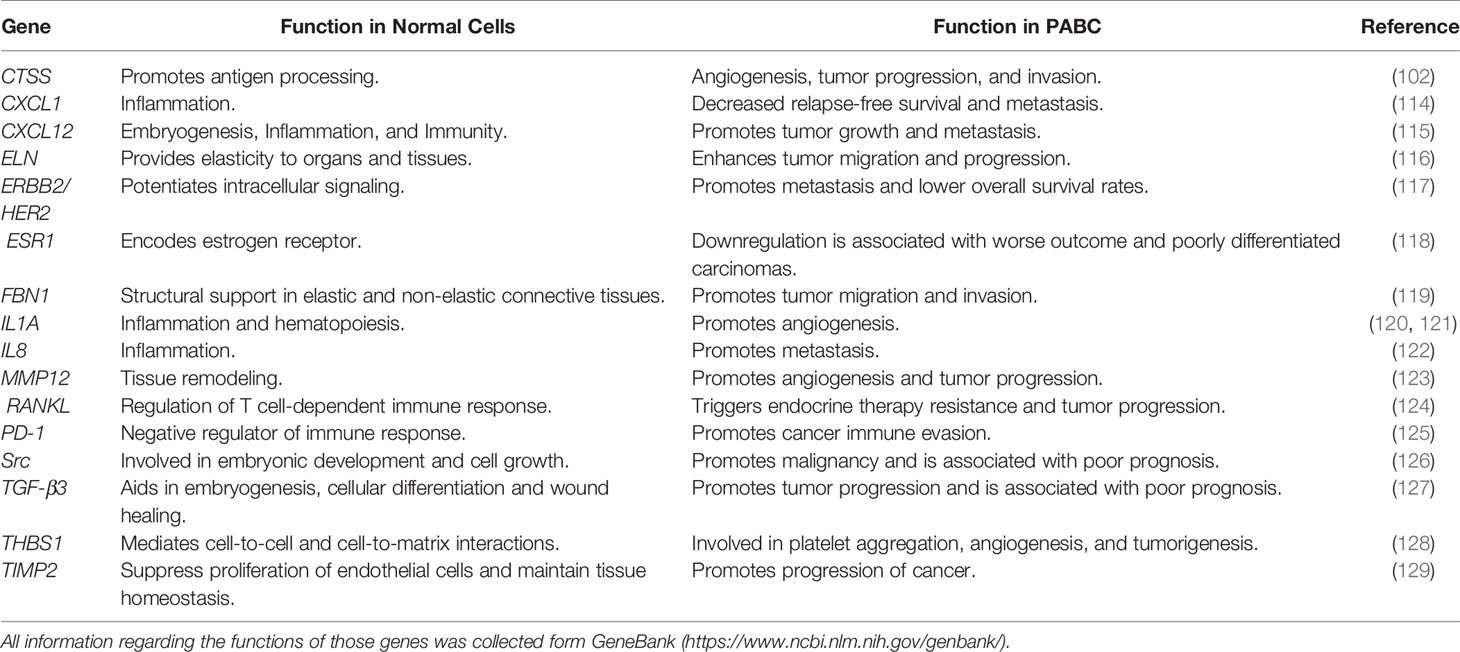
Table 2 Genes reported to having unique pattern of expression in triple-negative breast cancers (TNBC) patients and their associated functions.
Tumor suppressors, BRCA1/2 are involved in DNA damage repair, cell cycle control, transcription and ER type alpha activity (130). Mutations in BRCA1/2 are considered as risk factors for the onset of breast cancer (131); Atchley et al. reported a significant association between mutations in breast cancer susceptibility gene 1 (BRCA1) and TNBC, with more than 2/3 of BRCA1 mutations cases being of TNBC phenotype (132). Earlier studies have indicated that BRCA1/2 mutation carriers can be at a higher risk for PABC (133, 134). A study by Johannsson et al. analyzed the incidence of PABC in carriers of BRCA1 and BRCA2 mutations in comparison with premenopausal Swedish women aged ≤ 40 with sporadic PABC (133). The study showed that BRCA1/2 carriers are at an increased risk for PABC and hence should be monitored carefully during pregnancy and in the postpartum period (133). Another study revealed a significantly higher (25%) PABC frequency among BRCA1/2 mutation carriers compared with non-PABC cases (135). Although deleterious BRCA1 mutations are frequently encountered on both sporadic and hereditary TNBC (136), no link between pregnancy and these mutations has been found. Similarly, no association between TNBC and mutations in BRCA2 gene were reported (137). Based on the previous discussion, we underline here that the exact link between pregnancy and TNBC remains to be elucidated.
Signaling Networks in Pregnancy-Associated Breast Cancer
Earlier investigations suggested various underlying molecular mechanisms underpinning the onset of PABC. In an in-vivo study by Wagner et al. (138), using WAP-Cre/Rosa-LacZ transgenic mice, the authors identified a mixed population of alveolar cells called parity-induced mammary epithelial cells (PI-MECs) in the mammary gland of parous, non-pregnant female mice; these cells were not present in nulliparous females. PI-MECs rely on the transcription factor p63 for survival (138–142); one-time pregnant mice (MMTV–Her2/Neu mouse model) lacking p63 have lower tumors, thus indicating a tumor-promoter role for PI-MECs (138). Furthermore, another study showed that increased expression of p63 inhibits the p53 and STAT3 pathways; however, p63 enhances the expression of the pro-survival signaling STAT5 pathway, thus, initiating PI-MEC-induced tumorigenesis (142) (Figure 1). On the other hand, another study found loss of p63 during pregnancy reduced cyclin D1 levels, thus, suggesting a role of p63 in inducing PI-MEC survival post-partum (143). As shown in Figure 1, p63 levels increases cyclin D1which in turn inhibits estrogen receptor-alpha (ER-a) transactivation, further inhibiting the expression of BRCA1 (144). Following BRCA1 inhibition, PTEN is inactivated thereby activating mdm2 and blocking p53 (145) (Figure 1), which leads to genomic instability. Alternatively, pregnancy aids premalignant MECs evasion of apoptotic signaling through the activation of the JAK-STAT5 axis (146–148). In addition, receptor tyrosine kinases initiate downstream oncogenic signaling pathways such as PI3K/Akt which further activate either glycogen synthase kinase 3-beta (GSK3-β) or mdm2 (Figure 1).
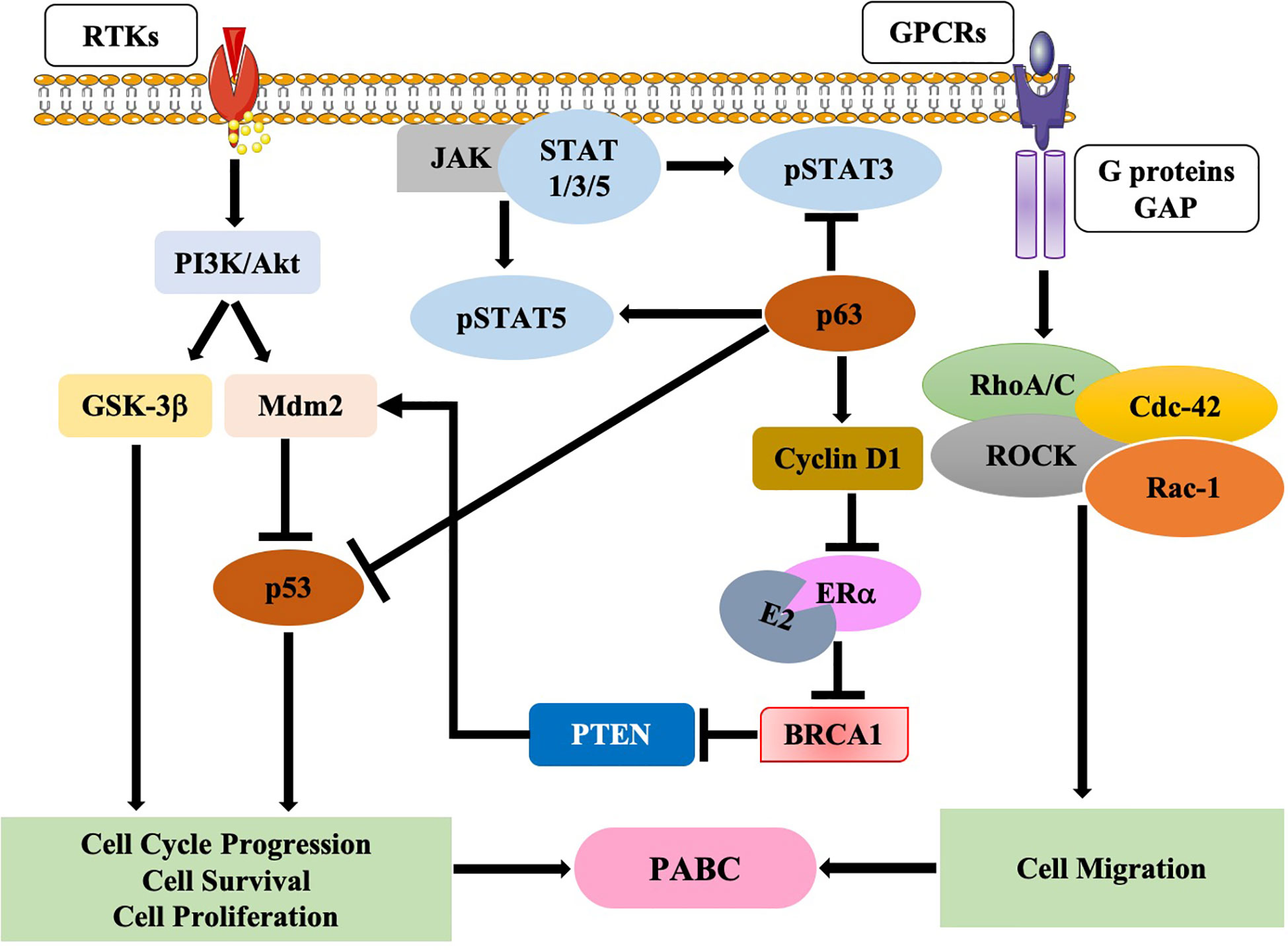
Figure 1 Molecular pathways in pregnancy-associated breast cancer (PABC). Schematic diagram showing various pathways that are involved in the onset and progression of PABC.
On the other hand, in pregnant women, GPCRs are activated (84), these growth factors enhance focal adhesion kinase (FAK) which in turn activates RhoA/Rac1/cdc42/ROCK complex, thereby initiating cell migration (149) (Figure 1).
However, further understanding of the underlying molecular mechanisms of PABC is needed to help pave the way for the development of possible therapeutic strategies.
Diagnosis of Pregnancy-Associated Breast Cancer
Since pregnancy associated TNBCs have a poor prognosis and diagnostic delays may occur in pregnancy due to effects of pregnancy related hormones, increased awareness can help in paving the way for appropriate treatment.
Vis a vis PABC diagnosis, assessing breast symptoms during pregnancy and postpartum period can be perplexing due to hormonally induced changes in breast tissue that can result in augmented firmness and nodularity (150). Furthermore, postpartum lactational mastitis symptoms may mimic locally advanced or inflammatory breast cancer. The majority of PABCs are diagnosed after presenting with a palpable mass (151). To determine the scope of disease is critical in treatment decision-making.
Breast imaging includes mammography, ultrasound, and magnetic resonance imaging (MRI). While ultrasound is proven to be safe and commonly used in pregnancy (152), mammography confers minimal dose to the fetus with abdominal shielding (153). Contrast-enhanced breast MRI can be a useful diagnostic tool in non-PABC, however, in pregnancy, the safety of gadolinium still remains controversial. A free gadolinium is considered toxic as it can cross the placenta and stay in the amniotic fluid, which can be taken in by the fetus re-entering the fetal circulation (154). In addition, prone positioning required during breast MRI can apply a sustained pressure on the gravid uterus, disrupting uterine blood flow (154). In case of metastatic PABC, diagnostic workup prior to the delivery is required to enable therapeutic interventions. Generally, the pregnant patient is subjected to either chest X-ray with abdominal shielding, liver ultrasound or non-contrast supine MRI to check for lung, liver or bone metastasis, respectively (153).
Based on the imaging results, biopsy [fine needle aspiration (FNAC) or core needle biopsy (CNB)] is done for definite diagnosis of a breast mass (155). Although, FNAC is less traumatic with a low complication rate than CNB and generally does not require local anesthesia, FNAC provides inadequate information about the histopathological type, grade, steroid receptors, HER2 expression, and intrinsic behavior of the tumor (155, 156). Hence, CNB is considered as a more reliable method of pathological diagnosis of breast cancer (156). The tissue obtained from a biopsy is tested to determine the status of the hormone receptors (ER, PR) as well as Her2 and proliferation index (Ki-67) (157). Biopsies are performed either under ultrasound or stereotactic guidance (158). In addition, during the first and second trimesters of pregnancy, incisional or excisional biopsy can be safely done (159).
Once diagnosis of breast cancer has been completed by imaging methods and histopathology, it is essential not to postpone the treatment. It can be given post-delivery if the patient is in near term. If the patient is close to term, the treatment must commence (160).
Treatment of Pregnancy-Associated Breast Cancer
Treatment options for PABC remain challenging and may require special considerations. Surgery (e.g., modified radical mastectomy) is usually considered as the primary line of treatment in breast cancer during pregnancy but neoadjuvant chemotherapy has been widely used as a primary treatment option for advanced HER2-positive and TNBC (161). There are several concerns regarding the use of neoadjuvant chemotherapy that pertain to the potential peripartum complications and the impact on the fetal outcome (162). However, studies have shown that during the first trimester, chemotherapeutic agents are not advised as they may be potentially teratogenic (161). On the other hand, after completion of the first trimester, chemotherapeutic agents may be safely administered without the risk of fetal malformations (161). Multiple studies that explored the use of chemotherapeutic drugs for the treatment of breast cancer during pregnancy showed that the majority of drugs (taxanes and vinorelbine) are non-toxic during the second and third trimesters of pregnancy. However, these drugs may increase the risk of intrauterine growth restriction and preterm labor. Cytotoxic drugs may also induce both maternal and infant leukopenia, hence, chemotherapy after 35 weeks of gestation is contraindicated to avoid delivery of a leukopenic infant (161). Other drugs including methotrexate, trastuzumab and tamoxifen should also be avoided during pregnancy due to their effect on the central nervous system, cardiac, gastrointestinal and skeletal malformations, oligohydramnios (low levels of amniotic fluid), preterm labor, and spontaneous abortions (163–165). All these considerations should be taken into account when optimizing treatment options of breast cancer during pregnancy. A recent 4th ESO-ESMO guideline also emphasizes the need of an individual basis approach following the international guidelines and an expanded multidisciplinary team that will involve gynecologists/obstetricians as well as perinatologists, in addition to patients’ own preferences (166).
Adjuvant chemotherapy is helpful and encouraged in patients with high-risk breast cancer including those with PABC. High-risk prognostic factors include estrogen and progesterone receptor negative status, HER2 status, high tumor grade, high TNM, and younger age of the patient (167). Patients that are treated with neoadjuvant chemotherapy and extensive residual disease (burden) are also strong candidates for adjuvant chemotherapy.
Radiation therapy is not advised during pregnancy as it can pose a high risk for fetal toxicity and malformations, childhood cancers and delays in neurocognitive development (168, 169). However, adjuvant radiotherapy (postpartum) can be safely used as in other breast cancer cases and following strict indications for adjuvant radiotherapy.
Breast cancer in young women (age < 40 years) tend to recur and therefore younger age of diagnosis, and hence longer lifespan places these patients at a statistically increased risk of recurrence and distant metastasis over time (170). Van Nes and van de Velde recommended mastectomy in younger patients over breast-conserving treatment (170). Any delay in treatment due to fallacies regarding risk of local and systemic therapy may worsens oncologic outcomes. Ambiguities regarding the safety of diagnostic modalities and treatment of PABC may lead to worse outcomes in the group of younger pregnant women with breast cancer. However, recent studies provide robust data on the safety of diagnostic procedures that can enable a successful treatment of patients with this challenging malignancy.
Sentinel lymph node biopsy (SLNB) is a part of routine management of breast cancer and has been widely used in clinical practice. SLNB recommendation is proposed for patients with clinically node negative breast cancers, those with or without 1–2 suspicious lymph nodes on imaging, and for patients that were not treated with neoadjuvant systemic therapy (171). In contrast to the American Society of Clinical Oncology (ASCO) guidelines, the National Comprehensive Cancer Network (NCCN) guideline indicates lack of scientific evidence regarding the use of SLNB in pregnant women. While, the NCCN also advises that SLNB use should be an individualized decision, but not directly offered to pregnant women < 30 weeks’ gestation. Of note, NCCN does not recommend the use of isosulfan or methylene blue dyes for SLNB in pregnancy while use of radioactive tracer (e.g., technetium 99m sulfur colloid) is also supported by limited scientific data regarding the fetal radiation dose (171).
Conclusions And Future Directions
PABC incidence increases as women choose delayed childbirth, and while it is a rare form of breast cancer with a significant propensity for triple-negative phenotype; Nevertheless, PABC is a diagnostically and therapeutically challenging disease bearing various risks for affected woman and fetus. Although immunotherapy with immune checkpoint inhibitors may be a promising therapeutic approach for patients with PABC, it can trigger various autoimmune side effects or immune-related adverse events (irAEs); there are several endocrine-related irAEs (172). The most common endocrinopathies reported from clinical trials include hypothyroidism and hypophysitis in patients treated with anti-PD-1/PD-L1 antibodies and anti-CTLA4, respectively (173–178). In addition, hypopituitarism, type 1 diabetes mellitus and primary adrenal insufficiency have also been reported (172). On the other hand, treatment with Dasatinib alone or combined with immune checkpoint inhibitors could be another therapeutic rationale given that PABC frequently overexpress the corresponding receptors Src and PD-L1. Nevertheless, despite the overall poor outcome, we believe that the complete gene and miRNA profiles of PABC can aid in identifying novel therapeutic targets and biomarkers to manage this rare, but fatal disease. In conclusion, the etiology of PABC remains largely unknown, thus, further cellular and animal models in addition to preclinical and clinical studies in the field are necessary.
Author Contributions
AA conceptualized the study. SA, IS, SM, HFA, SV and AA wrote, reviewed, and edited the manuscript. AA, HFA and SV acquired the funding. All authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Our lab is supported by grants from Qatar University: # QUCP-CMED-2019-1, QUHI-CMED-19/20-1, and QUCG-CMED-20/21-2.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Mrs. A. Kassab for her critical reading of the manuscript.
Abbreviations
AMBER, African American breast cancer epidemiology and Risk Consortium; ASCO, American Society of Clinical Oncology; AURKA, Aurora Kinase A; BIRC5, Baculoviral IAP Repeat Containing 5; BRCA, Breast cancer susceptibility gene; Ca2+, Calcium ions; cdc, Cell division control protein; CNB, Core needle biopsy; CTLA4, Cytotoxic T-Lymphocyte Associated Protein 4; CXCL, C-X-C Motif Chemokine Ligand; CXCR, C-X-C Motif Chemokine Receptor 4; ELN, Elastin; ER/ESR, Estrogen receptor; ERBB2, Erb-B2 Receptor Tyrosine Kinase 2; FAK, Focal adhesion kinase; FBN, Fibrillin; FNAC, Fine needle aspiration; GPCR, G protein-coupled receptor; GSK3-β, glycogen synthase kinase 3-beta; HER2, human epidermal growth factor receptor 2; IGF1, Insulin Like Growth Factor 1; IL, Interleukin; ILC, Invasive lobular carcinoma; irAEs, Immune-related adverse events; JAK, Janus Kinase; LAR, Luminal androgen receptor; mdm2, Mouse double minute 2 homolog; MMP, Matrix metallopeptidase; MMTV, Mouse mammary tumor virus; MRI, Magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; NST, No-special-type; p53, Tumor protein 53; p63, Tumor protein 63; PABC, Pregnancy-associated breast cancer; PD-1, Programmed cell death protein 1; PD-L1, Programmed cell death-ligand 1; PGR/PR, Progesterone receptor; PI-MECs, Parity-induced mammary epithelial cells; PTEN, Phosphatase And Tensin Homolog; Rac1, Rac Family Small GTPase 1; RANKL, Receptor activator of the nuclear factor κB ligand; RhoA, Ras Homolog Family Member A; ROCK, Rho Associated Coiled-Coil Containing Protein Kinase; SLNB, Sentinel lymph node biopsy; src, SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase; STAT, Signal Transducer And Activator Of Transcription; TDO2, Tryptophan-2,3-dioxygenase; TGF, Transforming growth factor; TILs, Tumor-infiltrating lymphocytes; TNBC, Triple-negative breast cancer.
References
2. Tao Z, Shi A, Lu C, Song T, Zhang Z, Zhao J. Breast Cancer: Epidemiology and Etiology. Cell Biochem Biophys (2015) 72(2):333–8. doi: 10.1007/s12013-014-0459-6
3. Tamimi RM, Colditz GA, Hazra A, Baer HJ, Hankinson SE, Rosner B, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat (2012) 131(1):159–67. doi: 10.1007/s10549-011-1702-0
4. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature (2000) 406(6797):747–52. doi: 10.1038/35021093
5. Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature (2012) 490(7418):61–70. doi: 10.1038/nature11412
6. Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci (2003) 100(14):8418. doi: 10.1073/pnas.0932692100
7. Lachapelle J, Foulkes WD. Triple-negative and basal-like breast cancer: implications for oncologists. Curr Oncol (Toronto Ont) (2011) 18(4):161–4. doi: 10.3747/co.v18i4.824
8. Foulkes WD, Smith IE, Reis-Filho JS. Triple-Negative Breast Cancer. N Engl J Med (2010) 363(20):1938–48. doi: 10.1056/NEJMra1001389
9. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest (2011) 121(7):2750–67. doi: 10.1172/JCI45014
10. Jézéquel P, Loussouarn D, Guérin-Charbonnel C, Campion L, Vanier A, Gouraud W, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res (2015) 17:43. doi: 10.1186/s13058-015-0550-y
11. Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist (2011) 16 Suppl 1:1–11. doi: 10.1634/theoncologist.2011-S1-01
12. Dent R, Trudeau M, Pritchard K II, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res (2007) 13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.Ccr-06-3045
13. Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat (2009) 115(2):423–8. doi: 10.1007/s10549-008-0086-2
14. Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer (2006) 6(4):281–91. doi: 10.1038/nrc1839
15. Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med (1994) 331(1):5–9. doi: 10.1056/nejm199407073310102
16. Asztalos S, Pham TN, Gann PH, Hayes MK, Deaton R, Wiley EL, et al. High incidence of triple negative breast cancers following pregnancy and an associated gene expression signature. SpringerPlus (2015) 4:710–0. doi: 10.1186/s40064-015-1512-7
17. Antonelli NM, Dotters DJ, Katz VL, Kuller JA. Cancer in pregnancy: a review of the literature. Part I. Obstet Gynecol Surv (1996) 51(2):125–34. doi: 10.1097/00006254-199602000-00022
18. Ji Y II, Kim KT. Gynecologic malignancy in pregnancy. Obstet Gynecol Sci (2013) 56(5):289–300. doi: 10.5468/ogs.2013.56.5.289
19. Pavlidis NA. Coexistence of Pregnancy and Malignancy. Oncologist (2002) 7(4):279–87. doi: 10.1634/theoncologist.2002-0279
20. Litton JK, Theriault RL. Breast cancer and pregnancy: current concepts in diagnosis and treatment. Oncologist (2010) 15(12):1238–47. doi: 10.1634/theoncologist.2010-0262
21. Keyser EA, Staat BC, Fausett MB, Shields AD. Pregnancy-associated breast cancer. Rev Obstet Gynecol (2012) 5(2):94–9. doi: 10.1136/ijgc-00009577-200303001-00301
22. Ishida T, Yokoe T, Kasumi F, Sakamoto G, Makita M, Tominaga T, et al. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case-control study in Japan. Jpn J Cancer Res (1992) 83(11):1143–9. doi: 10.1111/j.1349-7006.1992.tb02737.x
23. Middleton LP, Amin M, Gwyn K, Theriault R, Sahin A. Breast carcinoma in pregnant women. Cancer (2003) 98(5):1055–60. doi: 10.1002/cncr.11614
24. Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat (2013) 138(2):549–59. doi: 10.1007/s10549-013-2437-x
25. Wang B, Yang Y, Jiang Z, Zhao J, Mao Y, Liu J, et al. Clinicopathological characteristics, diagnosis, and prognosis of pregnancy-associated breast cancer. Thoracic Cancer (2019) 10(5):1060–8. doi: 10.1111/1759-7714.13045
26. Michieletto S, Saibene T, Evangelista L, Barbazza F, Grigoletto R, Rossi G, et al. Preliminary monocentric results of biological characteristics of pregnancy associated breast cancer. Breast (2014) 23(1):19–25. doi: 10.1016/j.breast.2013.10.001
27. Parente JT, Amsel M, Lerner R, Chinea F. Breast cancer associated with pregnancy. Obstet Gynecol 71 (1988) (6 Pt 1):861–4:(1988).
29. Fisher CJ, Egan MK, Smith P, Wicks K, Millis RR, Fentiman IS. Histopathology of breast cancer in relation to age. Br J Cancer (1997) 75(4):593–6. doi: 10.1038/bjc.1997.103
30. Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: when they collide. J Mammary Gland Biol Neoplasia (2009) 14(2):87–98. doi: 10.1007/s10911-009-9119-7
31. Ali SA, Gupta S, Sehgal R, Vogel V. Survival outcomes in pregnancy associated breast cancer: a retrospective case control study. Breast J (2012) 18(2):139–44. doi: 10.1111/j.1524-4741.2011.01201.x
32. Shousha S. Breast carcinoma presenting during or shortly after pregnancy and lactation. Arch Pathol Lab Med (2000) 124(7):1053–60. doi: 10.1043/0003-9985(2000)124<1053:BCPDOS>2.0.CO;2
33. Kollias J, Elston CW, Ellis IO, Robertson JF, Blamey RW. Early-onset breast cancer–histopathological and prognostic considerations. Br J Cancer (1997) 75(9):1318–23. doi: 10.1038/bjc.1997.223
34. Bertheau P, Steinberg SM, Cowan K, Merino MJ. Breast cancer in young women: clinicopathologic correlation. Semin Diagn Pathol (1999) 16(3):248–56.
35. McCready J, Arendt LM, Glover E, Iyer V, Briendel JL, Lyle SR, et al. Pregnancy-associated breast cancers are driven by differences in adipose stromal cells present during lactation. Breast Cancer Res (2014) 16(1):R2. doi: 10.1186/bcr3594
36. Petrek JA. Breast cancer during pregnancy. Cancer (1994) 74(S1):518–27. doi: 10.1002/cncr.2820741341
37. Guinee VF, Olsson H, Möller T, Hess KR, Taylor SH, Fahey T, et al. Effect of pregnancy on prognosis for young women with breast cancer. Lancet (1994) 343(8913):1587–9. doi: 10.1016/s0140-6736(94)93054-6
38. Ribeiro G, Jones DA, Jones M. Carcinoma of the breast associated with pregnancy. Br J Surg (1986) 73(8):607–9. doi: 10.1002/bjs.1800730805
39. Joshi S, Dialani V, Marotti J, Mehta TS, Slanetz PJ. Breast disease in the pregnant and lactating patient: radiological-pathological correlation. Insights Into Imaging (2013) 4(5):527–38. doi: 10.1007/s13244-012-0211-y
40. Usmani K, Moran EM, Haider W, Afzal H, Ahmad N. Breast cancer in pregnant and lactating women. J Environ Pathol Toxicol Oncol (1995) 14(3-4):227–34:(1995).
41. Amant F, Deckers S, Van Calsteren K, Loibl S, Halaska M, Brepoels L, et al. Breast cancer in pregnancy: recommendations of an international consensus meeting. Eur J Cancer (2010) 46(18):3158–68. doi: 10.1016/j.ejca.2010.09.010
42. Mathelin C, Annane K, Treisser A, Chenard MP, Tomasetto C, Bellocq JP, et al. Pregnancy and post-partum breast cancer: a prospective study. Anticancer Res (2008) 28(4c):2447–52.
43. Johansson ALV, Andersson TM-L, Hsieh C-C, Jirström K, Cnattingius S, Fredriksson I, et al. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int J Cancer (2018) 142(7):1343–54. doi: 10.1002/ijc.31174
44. Sullivan M, Patel AS, Wang J, Helenowski IB, Khan SA. Molecular Subtype Distribution of Pregnancy-Associated Breast Cancer. Am J Clin Pathol (2013) 140(suppl_1):A091–1. doi: 10.1093/ajcp/140.suppl1.091
45. Genin AS, Antoine M, Aractingi S, Rouzier R. Pregnancy stimulates tumor angiogenesis in breast carcinoma. Anticancer Res (2014) 34(1):125–31.
46. Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res (1982) 42(8):3232–9.
47. Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol (1988) 24(1):29–43. doi: 10.1016/0277-5379(88)90173-3
48. Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev (1993) 15(1):48–65. doi: 10.1093/oxfordjournals.epirev.a036116
49. Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res (2002) 4(5):197–201. doi: 10.1186/bcr452
50. Dickson RB, Stancel GM. Chapter 8: Estrogen Receptor-Mediated Processes in Normal and Cancer Cells. JNCI Monogr (2000) 2000(27):135–45. doi: 10.1093/oxfordjournals.jncimonographs.a024237
51. Elledge RM, Ciocca DR, Langone G, McGuire WL. Estrogen receptor, progesterone receptor, and HER-2/neu protein in breast cancers from pregnant patients. Cancer (1993) 71(8):2499–506. doi: 10.1002/1097-0142(19930415)71:8<2499::aid-cncr2820710812>3.0.co;2-s
52. Madaras L, Kovacs KA, Szasz AM, Kenessey I, Tokes AM, Szekely B, et al. Clinicopathological features and prognosis of pregnancy associated breast cancer - a matched case control study. Pathol Oncol Res (2014) 20(3):581–90. doi: 10.1007/s12253-013-9735-9
53. Chuang S-C, Lin C-H, Lu Y-S, Hsiung CA. Association of pregnancy and mortality in women diagnosed with breast cancer: A Nationwide Population Based Study in Taiwan. Int J Cancer (2018) 143(10):2416–24. doi: 10.1002/ijc.31777
54. Bonnier P, Romain S, Dilhuydy JM, Bonichon F, Julien JP, Charpin C, et al. Influence of pregnancy on the outcome of breast cancer: a case-control study. Societe Francaise de Senologie et de Pathologie Mammaire Study Group. Int J Cancer (1997) 72(5):720–7. doi: 10.1002/(sici)1097-0215(19970904)72:5<720::aid-ijc3>3.0.co;2-u
55. Nugent P, O’Connell TX. Breast Cancer and Pregnancy. Arch Surg (1985), 20(11):1221–4. doi: 10.1001/archsurg.1985.01390350007001
56. Merkel DE. Pregnancy and breast cancer. Semin Surg Oncol (1996) 12(5):370–5. doi: 10.1002/(sici)1098-2388(199609/10)12:5<370::Aid-ssu13>3.0.Co;2-t
57. Work ME, John EM, Andrulis IL, Knight JA, Liao Y, Mulligan AM, et al. Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer (2014) 110(5):1367–77. doi: 10.1038/bjc.2013.807
58. Harvell DME, Kim J, O’Brien J, Tan A-C, Borges VF, Schedin P, et al. Genomic signatures of pregnancy-associated breast cancer epithelia and stroma and their regulation by estrogens and progesterone. Hormones Cancer (2013) 4(3):140–53. doi: 10.1007/s12672-013-0136-z
59. Bae SY, Kim SJ, Lee J, Lee ES, Kim E-K, Park HY, et al. Clinical subtypes and prognosis of pregnancy-associated breast cancer: results from the Korean Breast Cancer Society Registry database. Breast Cancer Res Treat (2018) 172(1):113–21. doi: 10.1007/s10549-018-4908-6
60. Bae SY, Jung SP, Jung ES, Park SM, Lee SK, Yu JH, et al. Clinical Characteristics and Prognosis of Pregnancy-Associated Breast Cancer: Poor Survival of Luminal B Subtype. Oncology (2018) 95(3):163–9. doi: 10.1159/000488944
61. Han B-y, Li X-g, H.-y. Zhao X. Hu and H. Ling: Clinical features and survival of pregnancy-associated breast cancer: a retrospective study of 203 cases in China. BMC Cancer (2020) 20(1):244. doi: 10.1186/s12885-020-06724-5
62. Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy M-A, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res BCR (2004) 6(2):R75–91. doi: 10.1186/bcr753
63. Jindal S, Gao D, Bell P, Albrektsen G, Edgerton SM, Ambrosone CB, et al. Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling. Breast Cancer Res BCR (2014) 16(2):R31–1. doi: 10.1186/bcr3633
64. O’Brien J, Schedin P. Macrophages in breast cancer: do involution macrophages account for the poor prognosis of pregnancy-associated breast cancer? J Mammary Gland Biol Neoplasia (2009) 14(2):145–57. doi: 10.1007/s10911-009-9118-8
65. Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med (2011) 17(9):1109–15. doi: 10.1038/nm.2416
66. McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol (2006) 168(2):608–20. doi: 10.2353/ajpath.2006.050677
67. Russo J, Mailo D, Hu YF, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res (2005) 11(2 Pt 2):931s–6s.
68. Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst (2011) 103(3):250–63. doi: 10.1093/jnci/djq526
69. Ma H, Ursin G, Xu X, Lee E, Togawa K, Duan L, et al. Reproductive factors and the risk of triple-negative breast cancer in white women and African-American women: a pooled analysis. Breast Cancer Res (2017) 19(1):6. doi: 10.1186/s13058-016-0799-9
70. Yang XR, Sherman ME, Rimm DL, Lissowska J, Brinton LA, Peplonska B, et al. Differences in Risk Factors for Breast Cancer Molecular Subtypes in a Population-Based Study. Cancer Epidemiol Biomarkers Prev (2007) 16(3):439–43. doi: 10.1158/1055-9965.Epi-06-0806
71. Trivers KF, Lund MJ, Porter PL, Liff JM, Flagg EW, Coates RJ, et al. The epidemiology of triple-negative breast cancer, including race. Cancer Causes Control (2009) 20(7):1071–82. doi: 10.1007/s10552-009-9331-1
72. Dolle JM, Daling JR, White E, Brinton LA, Doody DR, Porter PL, et al. Risk Factors for Triple-Negative Breast Cancer in Women Under the Age of 45 Years. Cancer Epidemiol Biomarkers Prev (2009) 18(4):1157–66. doi: 10.1158/1055-9965.Epi-08-1005
73. Islam T, Matsuo K, Ito H, Hosono S, Watanabe M, Iwata H, et al. Reproductive and hormonal risk factors for luminal, HER2-overexpressing, and triple-negative breast cancer in Japanese women. Ann Oncol (2012) 23(9):2435–41. doi: 10.1093/annonc/mdr613
74. Ambrosone CB, Zirpoli G, Hong CC, Yao S, Troester MA, Bandera EV, et al. Important Role of Menarche in Development of Estrogen Receptor-Negative Breast Cancer in African American Women. J Natl Cancer Inst (2015) 107(9). doi: 10.1093/jnci/djv172
75. Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat (2009) 113(2):357–70. doi: 10.1007/s10549-008-9926-3
76. Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women : Implications for breast cancer screening recommendations. Cancer (2011) 117(12):2747–53. doi: 10.1002/cncr.25862
77. Palmer JR, Viscidi E, Troester MA, Hong CC, Schedin P, Bethea TN, et al. Parity, lactation, and breast cancer subtypes in African American women: results from the AMBER Consortium. J Natl Cancer Inst (2014) 106(10). doi: 10.1093/jnci/dju237
78. Basaran D, Turgal M, Beksac K, Ozyuncu O, Aran O, Beksac MS. Pregnancy-associated breast cancer: clinicopathological characteristics of 20 cases with a focus on identifiable causes of diagnostic delay. Breast Care (Basel Switzerland) (2014) 9(5):355–9. doi: 10.1159/000366436
79. Langer A, Mohallem M, Stevens D, Rouzier R, Lerebours F, Chérel P. A single-institution study of 117 pregnancy-associated breast cancers (PABC): Presentation, imaging, clinicopathological data and outcome. Diagn Interv Imaging (2014) 95(4):435–41. doi: 10.1016/j.diii.2013.12.021
80. Murphy CG, Mallam D, Stein S, Patil S, Howard J, Sklarin N, et al. Current or recent pregnancy is associated with adverse pathologic features but not impaired survival in early breast cancer. Cancer (2012) 118(13):3254–9. doi: 10.1002/cncr.26654
81. Pilewskie M, Gorodinsky P, Fought A, Hansen N, Bethke K, Jeruss J, et al. Association between recency of last pregnancy and biologic subtype of breast cancer. Ann Surg Oncol (2012) 19(4):1167–73. doi: 10.1245/s10434-011-2104-6
82. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N Engl J Med (2004) 351(27):2817–26. doi: 10.1056/NEJMoa041588
83. Hsiao Y-H, Su YA, Tsai H-D, Mason JT, Chou M-C, Man Y-g. Increased invasiveness and aggressiveness in breast epithelia with cytoplasmic p63 expression. Int J Biol Sci (2010) 6(5):428–42. doi: 10.7150/ijbs.6.428
84. Azim H Jr, Brohée S, Peccatori FA, Desmedt C, Loi S, Lambrechts D, et al. Biology of breast cancer during pregnancy using genomic profiling. Endocrine Rel Cancer (2014) 21:545–54. doi: 10.1530/ERC-14-0111
85. D’Addio F, Riella LV, Mfarrej BG, Chabtini L, Adams LT, Yeung M, et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol (2011) 187(9):4530–41. doi: 10.4049/jimmunol.1002031
86. Habicht A, Dada S, Jurewicz M, Fife BT, Yagita H, Azuma M, et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J Immunol (2007) 179(8):5211–9. doi: 10.4049/jimmunol.179.8.5211
87. Blanco L Jr, Pincus J, Siziopikou K. Abstract PD6-07: PD-L1 is highly expressed in tumor infiltrating lymphocytes in pregnancy associated breast cancer. Cancer Res (2017) 77(4 Supplement):PD6-07-PD6-07. doi: 10.1158/1538-7445.Sabcs16-pd6-07
88. Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget (2015) 6(7):5449–64. doi: 10.18632/oncotarget.3216
89. Ács B, Madaras L, Tőkés A-M, Kovács AK, Kovács E, Ozsvári-Vidákovich M, et al. PD-1, PD-L1 and CTLA-4 in pregnancy-related - and in early-onset breast cancer: A comparative study. Breast (Edinburgh Scotland) (2017) 35:69–77. doi: 10.1016/j.breast.2017.06.013
90. Maurice P, Guillaume J-L, Benleulmi-Chaachoua A, Daulat AM, Kamal M, Jockers R. GPCR-interacting proteins, major players of GPCR function. Adv Pharmacol (2011) 62:349–80. doi: 10.1016/B978-0-12-385952-5.00001-4
91. De Francesco EM, Sotgia F, Clarke RB, Lisanti MP, Maggiolini M. G protein-coupled receptors at the crossroad between physiologic and pathologic angiogenesis: old paradigms and emerging concepts. Int J Mol Sci (2017) 18(12):2713. doi: 10.3390/ijms18122713
92. De Francesco EM, Sotgia F, Clarke RB, Lisanti MP, Maggiolini M. G Protein-Coupled Receptors at the Crossroad between Physiologic and Pathologic Angiogenesis: Old Paradigms and Emerging Concepts. Int J Mol Sci (2017) 18(12). doi: 10.3390/ijms18122713
93. Maggiolini M, Santolla MF, Avino S, Aiello F, Rosano C, Garofalo A, et al. Identification of two benzopyrroloxazines acting as selective GPER antagonists in breast cancer cells and cancer-associated fibroblasts. Future Med Chem (2015) 7(4):437–48. doi: 10.4155/fmc.15.3
95. Ruiz R, Herrero C, Strasser-Weippl K, Touya D, St Louis J, Bukowski A, et al. Epidemiology and pathophysiology of pregnancy-associated breast cancer: A review. Breast (2017) 35:136–41. doi: 10.1016/j.breast.2017.07.008
96. Case AS. Pregnancy-associated Breast Cancer. Clin Obstet Gynecol (2016) 59(4):779–88. doi: 10.1097/grf.0000000000000235
97. Schedin PJ, Thackray LB, Malone P, Fontaine SC, Friis RR, Strange R. Programmed cell death and mammary neoplasia. Cancer Treat Res (1996) 83:3–22. doi: 10.1007/978-1-4613-1259-8_1
98. Asztalos S, Gann PH, Hayes MK, Nonn L, Beam CA, Dai Y, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) (2010) 3(3):301–11. doi: 10.1158/1940-6207.capr-09-0069
99. Barton M, Filardo EJ, Lolait SJ, Thomas P, Maggiolini M, Prossnitz ER. Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. J Steroid Biochem Mol Biol (2018) 176:4–15. doi: 10.1016/j.jsbmb.2017.03.021
100. Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res (2009) 11(6):R81. doi: 10.1186/bcr2448
101. Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle (2007) 6(1):60–4. doi: 10.4161/cc.6.1.3669
102. Gautam J, Bae YK, Kim JA. Up-regulation of cathepsin S expression by HSP90 and 5-HT7 receptor-dependent serotonin signaling correlates with triple negativity of human breast cancer. Breast Cancer Res Treat (2017) 161(1):29–40. doi: 10.1007/s10549-016-4027-1
103. Rogers TJ, Christenson JL, Greene L II, O’Neill K II, Williams MM, Gordon MA, et al. Reversal of Triple-Negative Breast Cancer EMT by miR-200c Decreases Tryptophan Catabolism and a Program of Immunosuppression. Mol Cancer Res (2019) 17(1):30–41. doi: 10.1158/1541-7786.mcr-18-0246
104. Asztalos S, Pham TN, Gann PH, Hayes MK, Deaton R, Wiley EL, et al. High incidence of triple negative breast cancers following pregnancy and an associated gene expression signature. Springerplus (2015) 4:710(2015). doi: 10.1186/s40064-015-1512-7
105. Schedin P, O’Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J Mammary Gland Biol Neoplasia (2007) 12(1):71–82. doi: 10.1007/s10911-007-9039-3
106. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
107. Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol (2008) 9(8):628–38. doi: 10.1038/nrm2455
108. White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, et al. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell (2004) 6(2):159–70. doi: 10.1016/j.ccr.2004.06.025
109. Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev (2002) 12(1):22–9. doi: 10.1016/s0959-437x(01)00259-3
110. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature (2005) 438(7070):967–74. doi: 10.1038/nature04483
111. Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol (2004) 25(7):387–95. doi: 10.1016/j.it.2004.05.003
112. Azim HA, Peccatori FA, Brohée S, Branstetter D, Loi S, Viale G, et al. RANK-ligand (RANKL) expression in young breast cancer patients and during pregnancy. Breast Cancer Res (2015) 17(1):24. doi: 10.1186/s13058-015-0538-7
113. Lee E, McKean-Cowdin R, Ma H, Spicer DV, Van Den Berg D, Bernstein L, et al. Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young women. J Clin Oncol (2011) 29(33):4373. doi: 10.1200/JCO.2010.33.6446
114. Bièche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, et al. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer (2007) 14(4):1039–52. doi: 10.1677/erc.1.01301
115. Sun Y, Mao X, Fan C, Liu C, Guo A, Guan S, et al. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol (2014) 35(8):7765–73. doi: 10.1007/s13277-014-1816-1
116. Krishnan R, Cleary EG. Elastin gene expression in elastotic human breast cancers and epithelial cell lines. Cancer Res (1990) 50(7):2164–71.
117. Tan M, Yu D. “Molecular Mechanisms of ErbB2-Mediated Breast Cancer Chemoresistance”. In: Madame Curie Bioscience Database [Internet]. Austin, TX: Landes Bioscience (2013).
118. Chen J-Q, Russo J. ERalpha-negative and triple negative breast cancer: molecular features and potential therapeutic approaches. Biochim Biophys Acta (2009) 1796(2):162–75. doi: 10.1016/j.bbcan.2009.06.003
119. Lien H-C, Lee Y-H, Juang Y-L, Lu Y-T. Fibrillin-1, a novel TGF-beta-induced factor, is preferentially expressed in metaplastic carcinoma with spindle sarcomatous metaplasia. Pathology (2019) 51(4):375–83. doi: 10.1016/j.pathol.2019.02.001
120. Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA (2003) 100(5):2645–50. doi: 10.1073/pnas.0437939100
121. Singer CF, Kronsteiner N, Hudelist G, Marton E, Walter I, Kubista M, et al. Interleukin 1 system and sex steroid receptor expression in human breast cancer: interleukin 1alpha protein secretion is correlated with malignant phenotype. Clin Cancer Res (2003) 9(13):4877–83. doi: 10.1136/ijgc-00009577-200303001-00301
122. Bendre MS, Gaddy-Kurten D, Mon-Foote T, Akel NS, Skinner RA, Nicholas RW, et al. Expression of interleukin 8 and not parathyroid hormone-related protein by human breast cancer cells correlates with bone metastasis in vivo. Cancer Res (2002) 62(19):5571–9.
123. Decock J, Thirkettle S, Wagstaff L, Edwards DR. Matrix metalloproteinases: protective roles in cancer. J Cell Mol Med (2011) 15(6):1254–65. doi: 10.1111/j.1582-4934.2011.01302.x
124. Phungern K, Antonia KR, Joanne E. The NF-KB pathway and endocrine therapy resistance in breast cancer. Endocrine Rel Cancer (2019) 26(6):R369–80. doi: 10.1530/ERC-19-0087
125. Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 Pathway from Discovery to Clinical Implementation. Front Immunol (2016) 7:550. doi: 10.3389/fimmu.2016.00550
126. Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist (2009) 14(7):667–78. doi: 10.1634/theoncologist.2009-0009
127. Ghellal A, Li C, Hayes M, Byrne G, Bundred N, Kumar S. Prognostic significance of TGF beta 1 and TGF beta 3 in human breast carcinoma. Anticancer Res (2000) 20(6b):4413–8.
128. Huang T, Sun L, Yuan X, Qiu H. Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget (2017) 8(48):84546–58. doi: 10.18632/oncotarget.19165
129. Remacle A, McCarthy K, Noël A, Maguire T, McDermott E, O’Higgins N, et al. High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int J Cancer (2000) 89(2):118–21. doi: 10.1002/(sici)1097-0215(20000320)89:2<118::aid-ijc3>3.0.co;2-8
130. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science (2003) 302(5645):643–6. doi: 10.1126/science.1088759
131. Chan JL, Johnson LNC, Sammel MD, DiGiovanni L, Voong C, Domchek SM, et al. Reproductive Decision-Making in Women with BRCA1/2 Mutations. J Genet Couns (2017) 26(3):594–603. doi: 10.1007/s10897-016-0035-x
132. Atchley DP, Albarracin CT, Lopez A, Valero V, Amos C II, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol (2008) 26(26):4282–8. doi: 10.1200/jco.2008.16.6231
133. Johannsson O, Loman N, Borg A, Olsson H. Pregnancy-associated breast cancer in BRCA1 and BRCA2 germline mutation carriers. Lancet (1998) 352(9137):1359–60. doi: 10.1016/s0140-6736(05)60750-7
134. Cullinane CA, Lubinski J, Neuhausen SL, Ghadirian P, Lynch HT, Isaacs C, et al. Effect of pregnancy as a risk factor for breast cancer in BRCA1/BRCA2 mutation carriers. Int J Cancer (2005) 117(6):988–91. doi: 10.1002/ijc.21273
135. Hou N, Ogundiran T, Ojengbede O, Morhason-Bello I, Zheng Y, Fackenthal J, et al. Risk factors for pregnancy-associated breast cancer: a report from the Nigerian Breast Cancer Study. Ann Epidemiol (2013) 23(9):551–7. doi: 10.1016/j.annepidem.2013.06.008
136. Silva E, Gatalica Z, Snyder C, Vranic S, Lynch JF, Lynch HT. Hereditary breast cancer: part II. Management of hereditary breast cancer: implications of molecular genetics and pathology. Breast J (2008) 14(1):14–24. doi: 10.1111/j.1524-4741.2007.00516.x
137. Riahi A, Gourabi ME, Chabouni-Bouhamed H. Dissimilarity between sporadic, non-BRCA1/2 families and hereditary breast cancer, linked to BRCA genes, in the Tunisian population. Breast Cancer (2016) 23(5):807–12. doi: 10.1007/s12282-015-0648-1
138. Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development (2002) 129(6):1377–86.
139. Matulka LA, Triplett AA, Wagner K-U. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol (2007) 303(1):29–44. doi: 10.1016/j.ydbio.2006.12.017
140. Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol (2007) 212(3):729–36. doi: 10.1002/jcp.21071
141. Chang THT, Kunasegaran K, Tarulli GA, De Silva D, Voorhoeve PM, Pietersen AM. New insights into lineage restriction of mammary gland epithelium using parity-identified mammary epithelial cells. Breast Cancer Res (2014) 16(1):R1. doi: 10.1186/bcr3593
142. Yallowitz AR, Alexandrova EM, Talos F, Xu S, Marchenko ND, Moll UM. p63 is a prosurvival factor in the adult mammary gland during post-lactational involution, affecting PI-MECs and ErbB2 tumorigenesis. Cell Death Differ (2014) 21(4):645–54. doi: 10.1038/cdd.2013.199
143. Forster N, Saladi SV, van Bragt M, Sfondouris ME, Jones FE, Li Z, et al. Basal Cell Signaling by p63 Controls Luminal Progenitor Function and Lactation via NRG1. Dev Cell (2014) 28(2):147–60. doi: 10.1016/j.devcel.2013.11.019
144. Wang C, Fan S, Li Z, Fu M, Rao M, Ma Y, et al. Cyclin D1 Antagonizes BRCA1 Repression of Estrogen Receptor α Activity. Cancer Res (2005) 65(15):6557–67. doi: 10.1158/0008-5472.Can-05-0486
145. Lee C, Kim J-S, Waldman T. Activated PI3K signaling as an endogenous inducer of p53 in human cancer. Cell Cycle (Georgetown Tex.) (2007) 6(4):394–6. doi: 10.4161/cc.6.4.3810
146. Haricharan S, Dong J, Hein S, Reddy JP, Du Z, Toneff M, et al. Mechanism and preclinical prevention of increased breast cancer risk caused by pregnancy. Elife (2013) 2:e00996. doi: 10.7554/eLife.00996
147. Du Z, Li Y. RCAS-TVA in the mammary gland: an in vivo oncogene screen and a high fidelity model for breast transformation? Cell Cycle (2007) 6(7):823–6. doi: 10.4161/cc.6.7.4074
148. Du Z, Podsypanina K, Huang S, McGrath A, Toneff MJ, Bogoslovskaia E, et al. Introduction of oncogenes into mammary glands in vivo with an avian retroviral vector initiates and promotes carcinogenesis in mouse models. Proc Natl Acad Sci USA (2006) 103(46):17396–401. doi: 10.1073/pnas.0608607103
149. Gupta SK, Malhotra SS, Malik A, Verma S, Chaudhary P. Cell Signaling Pathways Involved During Invasion and Syncytialization of Trophoblast Cells. Am J Reprod Immunol (2016) 75(3):361–71. doi: 10.1111/aji.12436
150. Rojas K, Bilbro N, Manasseh D-M, Borgen PI. A Review of Pregnancy-Associated Breast Cancer: Diagnosis, Local and Systemic Treatment, and Prognosis. J Women’s Health (2019) 28(6):778–84. doi: 10.1089/jwh.2018.7264
151. Al-Amri AM. Clinical presentation and causes of the delayed diagnosis of breast cancer in patients with pregnancy associated breast cancer. J Family Community Med (2015) 22(2):96–100. doi: 10.4103/2230-8229.155383
152. Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database System Rev (2015) 2015(7):CD007058. doi: 10.1002/14651858.CD007058.pub3
153. Committee Opinion No. 723. Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol (2017) 130(4):e210–6. doi: 10.1097/aog.0000000000002355
154. Wagner L, Applegate KACR practice guideline for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation. American College of Radiology (2008). 1–15.
155. Mitra S, Dey P. Fine-needle aspiration and core biopsy in the diagnosis of breast lesions: A comparison and review of the literature. CytoJournal (2016) 13:18–8. doi: 10.4103/1742-6413.189637
156. Saha A, Mukhopadhyay M, Das C, Sarkar K, Saha AK, Sarkar DK. FNAC Versus Core Needle Biopsy: A Comparative Study in Evaluation of Palpable Breast Lump. J Clin Diagn Res JCDR (2016) 10(2):EC05–EC8. doi: 10.7860/JCDR/2016/15889.7185
157. Penault-Llorca F, Viale G. Pathological and molecular diagnosis of triple-negative breast cancer: a clinical perspective. Ann Oncol (2012) 23:vi19–22. doi: 10.1093/annonc/mds190
158. Langer A, Mohallem M, Berment H, Ferreira F, Gog A, Khalifa D, et al. Breast lumps in pregnant women. Diagn Intervent Imaging (2015) 96(10):1077–87. doi: 10.1016/j.diii.2015.07.005
159. Collins JC, Liao S, Wile AG. Surgical management of breast masses in pregnant women. J Reprod Med (1995) 40(11):785–8.
160. Lenhard MS, Bauerfeind I, Untch M. Breast cancer and pregnancy: challenges of chemotherapy. Crit Rev Oncol Hematol (2008) 67(3):196–203. doi: 10.1016/j.critrevonc.2008.02.007
161. Molckovsky A, Madarnas Y. Breast cancer in pregnancy: a literature review. Breast Cancer Res Treat (2008) 108(3):333–8. doi: 10.1007/s10549-007-9616-6
162. Ring AE, Smith IE, Jones A, Shannon C, Galani E, Ellis PA. Chemotherapy for breast cancer during pregnancy: an 18-year experience from five London teaching hospitals. J Clin Oncol (2005) 23(18):4192–7. doi: 10.1200/jco.2005.03.038
163. Loibl S, Schmidt A, Gentilini O, Kaufman B, Kuhl C, Denkert C, et al. Breast Cancer Diagnosed During Pregnancy: Adapting Recent Advances in Breast Cancer Care for Pregnant Patients. JAMA Oncol (2015) 1(8):1145–53. doi: 10.1001/jamaoncol.2015.2413
164. Sekar R, Stone PR. Trastuzumab use for metastatic breast cancer in pregnancy. Obstet Gynecol (2007) 110(2 Pt 2):507–10. doi: 10.1097/01.Aog.0000267133.65430.44
165. Loibl S, von Minckwitz G, Gwyn K, Ellis P, Blohmer JU, Schlegelberger B, et al. Breast carcinoma during pregnancy. International recommendations from an expert meeting. Cancer (2006) 106(2):237–46. doi: 10.1002/cncr.21610
166. Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim Jr HA, Bianchi-Micheli G, et al. ESO–ESMO 4th International Consensus Guidelines for Breast Cancer in Young Women (BCY4). Ann Oncol (2020) 31(6):674–96. doi: 10.1016/j.annonc.2020.03.284
167. Kuerer HM, Gwyn K, Ames FC, Theriault RL. Conservative surgery and chemotherapy for breast carcinoma during pregnancy. Surgery (2002) 131(1):108–10. doi: 10.1067/msy.2002.115357
168. Greskovich Jr JF, Macklis RM. Radiation therapy in pregnancy: risk calculation and risk minimization. Semin Oncol (2000) 27(6):633–45.
169. Otake M, Schull WJ, Lee S. Threshold for radiation-related severe mental retardation in prenatally exposed A-bomb survivors: a re-analysis. Int J Radiat Biol (1996) 70(6):755–63. doi: 10.1080/095530096144644
170. van Nes JG, van de Velde CJ. The preferred treatment for young women with breast cancer–mastectomy versus breast conservation. Breast (2006) 15 Suppl 2:S3–10. doi: 10.1016/s0960-9776(07)70009-7
171. National Comprehensive Cancer Network. NCCN Guidelines. Plymouth Meeting, PA 19462. Harborside Press, LLC (2020).
172. Cukier P, Santini FC, Scaranti M, Hoff AO. Endocrine side effects of cancer immunotherapy. Endocrine Rel Cancer (2017) 24(12):T331. doi: 10.1530/erc-17-0358
173. Corsello S, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab (2013) 98:1361–75. doi: 10.1210/jc.2012-4075
174. Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol (2017) 13(4):195–207. doi: 10.1038/nrendo.2016.205
175. Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med (2016) 375(19):1845–55. doi: 10.1056/NEJMoa1611299
176. González-Rodríguez E, Rodríguez-Abreu D. Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist (2016) 21(7):804–16. doi: 10.1634/theoncologist.2015-0509
177. O’Donnell PH, Plimack ER, Bellmunt J, Berger R, Montgomery RB, Heath K, et al. Pembrolizumab (Pembro; MK-3475) for advanced urothelial cancer: Results of a phase IB study. J Clin Oncol (2015) 33(7_suppl):296–6. doi: 10.1200/jco.2015.33.7_suppl.296
Keywords: breast cancer, pregnancy, gene deregulation, delayed childbearing, triple-negative
Citation: Allouch S, Gupta I, Malik S, Al Farsi HF, Vranic S and Al Moustafa A-E (2020) Breast Cancer During Pregnancy: A Marked Propensity to Triple-Negative Phenotype. Front. Oncol. 10:580345. doi: 10.3389/fonc.2020.580345
Received: 05 July 2020; Accepted: 20 November 2020;
Published: 23 December 2020.
Edited by:
Hee Jeong Kim, Asan Medical Center, South KoreaReviewed by:
Chantal Reyna, University of Cincinnati, United StatesPierre-Yves Desprez, California Pacific Medical Center, United States
Juan Palazzo, Baptist Hospital of Miami, United States
Copyright © 2020 Allouch, Gupta, Malik, Al Farsi, Vranic and Al Moustafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ala-Eddin Al Moustafa, YWFsbW91c3RhZmFAcXUuZWR1LnFh; YWxhLWVkZGluLmFsbW91c3RhZmFAbWNnaWxsLmNh; Semir Vranic, c3ZyYW5pY0BxdS5lZHUucWE=
†These authors have contributed equally to this work
‡This work is dedicated to the memory of my beloved ex-wife “Naima Bachnou” who passed away because of breast cancer during pregnancy
 Soumaya Allouch
Soumaya Allouch Ishita Gupta
Ishita Gupta Shaza Malik
Shaza Malik Halema F. Al Farsi
Halema F. Al Farsi Semir Vranic
Semir Vranic Ala-Eddin Al Moustafa
Ala-Eddin Al Moustafa