- 1European Space Agency (ESA), European Astronaut Center (EAC), Space Medicine Team (HRE-OM), Cologne, Germany
- 2Faculty of Medicine Paris VI, Sorbonne University, Paris, France
- 3Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada
- 4Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne, United Kingdom
- 5KBR GmbH, Cologne, Germany
- 6Center of Human & Applied Physiological Sciences (CHAPS), King's College London, London, United Kingdom
- 7Faculty of Medicine, Utrecht University, Utrecht, Netherlands
Detrimental health effects from ionizing radiation to living organisms is one of the key concerns identified and addressed by Radiation Protection institutions, nationally and internationally on Earth and for human spaceflight. Thus, new methods for mitigating the adverse effects of ionizing radiation are urgently needed for terrestrial health and deep space exploration. Caloric restriction and (intermittent-) fasting have been reported to elicit a variety of immediate and long-term physiological effects. The rapidly growing body of evidence of research studies investigating the effects of caloric restriction and dietary fasting points toward a multitude of benefits affecting numerous physiological systems. Therefore, a systematic review was performed to evaluate the evidence of caloric restriction and dietary fasting on the physiological response to ionizing radiation in humans and animals. All experimental studies of humans, animals, and eukaryotic cell lines available in PubMed, Cochrane library, and specialized databases were searched comparing irradiation post-caloric restriction or fasting to a non-nutritionally restricted control group on a broad range of outcomes from molecular to clinical responses. The initial search yielded 2,653 records. The final analysis included 11 studies. Most studies investigated survival rate or cancer occurrence in animals. Included studies did not reveal any benefit from pre exposure caloric restriction, except when performed with post radiation caloric restriction. However, the effects of pre-exposure fasting suggest increased resilience to ionizing radiation.
Introduction
Ionizing radiation has numerous negative biological effects on almost all living organisms (1). Humans may be challenged not only by classical background radiation and nuclear events/disasters but also via exposure to radiotherapy, air travel or even space travel. Over the past few decades, the field of radiation protection has made significant progress in devising methodologies to protect humans from ionizing radiation including development of regulations relating to the exposure to radiation use in medical, civilian, and military contexts (2).
However, greater knowledge of the mechanisms that determine the biological damage resulting from ionizing radiation is required in order to inform the development of more effective radiotherapy delivery methodologies and protective countermeasures. Terrestrially, this is of critical importance in radiotherapy in order to reduce secondary tissue damage, while maximizing radiation delivery to cancerous tissues. Furthermore, mitigating the negative effects of ionizing radiation is arguably the most significant challenge that must be addressed to facilitate human space exploration, beyond the relative protection provided by the Earth's magnetic field in low earth orbit (3).
Ionizing radiation is known to induce significant damage to cells. For instance, ionizing radiation exposure is reported to induce pathological states including acute radiation syndrome (4), (solid and non-solid) cancer (5), and organ dysfunction (e.g., radiation pneumonitis, radiation enteritis) (6, 7). The occurrence of some pathological states are directly dependent on the total received radiation dose (deterministic effects) (8), while other pathological states such as cancer appear to have probabilistic increases with dose (stochastic effects) (9).
The physiological effects of ionizing radiation exposure can be categorized as either direct or indirect (10). Direct effects refer to the immediate damage ionizing radiation induces on DNA within cellular nuclei such as single or double strand breakages in DNA. Indirect effects characterize the interaction between ionizing radiation and other molecules, such as water. In contrast, the so-called “bystander effect” is defined as the biological response observed in cells without direct exposure to radiation. For instance, indirect effects generate free radicals and reactive oxygen species that create an environment of increased genetic instability leading to indirect damage to DNA and other cellular components (10). In normal physiologic states, cellular activity generating oxidative stress via production of free radicals and ROS is mitigated by the antioxidant system (11) via the donation of electrons by antioxidants. Thus, the balance between free radical production and antioxidant activity determines the cellular oxidative stress. Thus, stimulation of increased antioxidant activity is likely to reduce oxidative stress, and resultant DNA damage, thereby potentially mitigating at least some of the damage induced by ionizing radiation.
Interestingly, research has shown that fasting (defined as the complete absence of food for >12 h) can extend rodent lifespans (12, 13). Furthermore, caloric restriction—defined as reduced caloric intake for more than 12 h—can also extend rodent (14, 15) and primates lifespans (16). Whilst several physiological mechanisms have been proposed over the years, more recently, there is increasing evidence that fasting and caloric restriction can directly modulate and reduce cellular oxidative stress, which may underpin lifespan extension (17, 18). Such mechanisms may also potentially reduce oxidative stress and thus represent a biological countermeasure to ionizing radiation. Considering the above, in addition to reducing ionizing radiation one possibility to reduce the overall risk of developing dose-dependent or stochastic radiation-induced medical conditions could be to reduce the radiation-sensitivity of cells, thus increasing their radiation resilience.
Human research investigating the effects of fasting and caloric restriction on radio-protective mechanisms seems to be scarce. An in vitro pilot study utilizing human serum showed an increased oxidative stress resistance after caloric restriction (19). Other studies have tried to describe cellular mechanisms responsible for the link between food intake and oxidative stress and have provided potential explanations through the interaction with energy sensing pathways (20, 21). Proteins such as Sirtuins (22–27), FOXO (28), TOR (29), AMPK (30), or NRF2 (31) seem to be key players in the mediation of stress resistance and anti-oxidant response to fasting and caloric restriction. Based on these findings, it could be suggested that caloric restriction or fasting might mitigate biological damage induced by secondary effects of ionizing radiation, through positive interaction with the cellular antioxidant system while also decreasing incidence of diseases like cancer (32). Thus, the aim of this review was to evaluate the evidence for fasting and/or caloric restriction as an approach to radioprotection. For terrestrial applications, there are various implications of exploring the protection that could be provided to patients undergoing radiation therapy or medical imaging, to those exposed to higher than baseline radiation from occupational sources, and to accidental radiation exposure incidents.
Materials and Methods
Terminology
The terms caloric restriction and fasting are sometimes conflated. Fasting is typically defined in the literature as no, or minimal, caloric intake for at least 12 h (33). Moreover, fasting and caloric restriction can be applied in a number of ways (e.g., intermittent fasting, periodic fasting, continuous fasting, continuous caloric restriction, or intermittent caloric restriction). This review defines “fasting” as a complete absence of food for longer than 12 h, while the term “caloric restriction” refers to reduced caloric intake (compared to normal) for longer than 12 h. Such term do not include “starvation” which relates to a chronic nutritional insufficiency and thus is beyond the scope of the present study.
Search Strategy
The guidelines in the Cochrane handbook (www.cochrane.com; version 5.1) were followed using tools created by the Aerospace Medicine Systematic Review Group (AMSRG: http://aerospacemed.rehab/systematic-review-group) for data extraction, quality assessment of studies, and effect size calculations. Furthermore, this review followed the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA). PubMed, Embase, Cochrane Library and space agencies' local databases (NASA, ESA, and DLR) were searched for eligible studies published before November 30th, 2018. The detailed search strategy using Boolean logic is shown in Supplementary Table 1. Due to the lack of an advanced research tool using Boolean logic in NASA's, ESA's, and DLR's internal archives, the search strategy was adapted to employ simple keywords (Supplementary Table 2). Accepted languages were English, German, Russian, Polish, Italian, Dutch, and French. All non-English articles were translated by the authors except for Italian articles which required the assistance of a collaborator (who is mentioned in the Acknowledgments).
Eligibility Criteria (PICOs)
The following PICOS (Population, Intervention, Control, Outcomes, and Study design) eligibility criteria were applied:
P—Humans, animals, and eukaryotic cell lines.
I—Fasting (> or equal to 12 h) or caloric restriction (reduction of at least 30% of normal intake for > or equal to 12 h) prior to a partial or whole-body exposure to ionizing radiation
C—Same population as in the intervention group, with the same ionizing radiation exposure but without any dietary restriction.
O—Molecular, biochemical (short or long term) or clinical responses (short or long term) to ionizing radiation exposure.
S—Controlled trials
A complete list of all included outcomes is shown in Supplementary Table 3.
Study Selection
Two reviewers independently assessed the eligibility of the studies based on the PICOs criteria via screening performed using the Rayyan web application (34). After duplicates were removed, the initial screening was performed using titles and abstracts. Articles were excluded if the title or abstract did not reveal a direct link to the current eligibility criteria (see PICOs). All remaining articles were then screened as full text. A third, and independent experienced reviewer resolved any disagreements.
Data Extraction
Data from the included studies were extracted using an adapted version of the Cochrane Collaboration's Data collection form for RCTs and non-RCTs (RCT: randomized controlled trial, version 3, April 2014, https://dplp.cochrane.org/data-extraction-forms). The extracted information included characteristics of the study (authors, design, and publication year), population (age, sex, and species/breed if available), radiation (type, intensity, duration, target, circumstances, and control group), fasting and caloric restriction (duration, intensity, control group, chronology with the irradiation), statistical methods and outcomes (parameters, values, time points).
Assessment of Study Quality
The quality of included studies was appraised and described by the two reviewers using the Cochrane Collaboration's risk of bias analysis tool (https://www.ncbi.nlm.nih.gov/books/NBK132494/bin/appf-fm1.pdf). Uncertainties or discrepancies were discussed with an independent experienced third reviewer. Risks were scored as “low,” “high,” or “unclear.”
Data Analysis and Statistics
Effect sizes were calculated for all studies that presented their results as means and standard deviations/standard error of the mean. Presented effect sizes were calculated and bias corrected for potential small sample sizes using the Hedge's g method (35) and they refer for any given effect to be either in favor of the control group, or in favor of the intervention group. For ease of interpretation all outcomes were presented to show a positive effect as being “beneficial,” therefore any original outcomes that have negative “beneficial” effects were inverted by multiplying by −1 for presentation in the overall results (e.g., a fall in resting heart rate results in a negative effect size but it is associated with better general physical fitness and thus is considered a beneficial outcome). Thresholds for effect sizes were defined as 0.1 (small), 0.3 (moderate), 0.5 (large), 0.7 (very large), and 0.9 (extremely large) for comparisons between intervention and control groups (35). Cappelli et al. (36), reported only raw data from which to determine mean time of survival after exposure and its standard deviation. Yoshida et al. (37) and Bonilla et al. (38) reported standard error that was converted to standard deviation via: SD = SE x √N. In addition, when results were only presented as figures (38–40), a plot reader was used to extract the data (https://automeris.io/WebPlotDigitizer).
Not all studies provided data that allowed effect size calculations using the Hedge's g method. Although these extracted data are inconsistent with the standard practice of Cochrane systematic reviews, they are reported as they provide valuable information in the context of this review. For data extracted from studies that could not be used in the effect size analysis, the statistical significance (P < 0.05) of individual studies was reported. Some of the older studies did not report any significance values and thus were highlighted by “significance not reported.” To meet the criteria for Cochrane systematic reviews this data is not presented in the results section and has been moved to the beginning of the discussion (see section “other noteworthy findings not able to be included in the analysis”). In order to illustrate the potential of the studied interventions, both types of data were merged and analyzed in Excel (Professional plus 2016 edition, Microsoft, US) and the meta data was presented using the SankeyMATIC tool (http://sankeymatic.com/build/).
Results
Study Selection
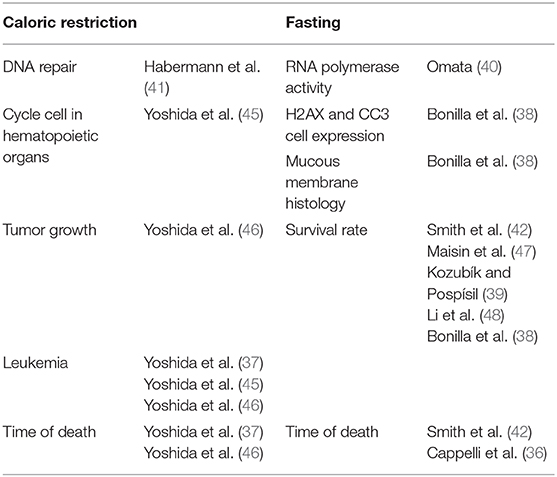
The initial search identified 2,441 studies after duplicates were removed. Abstract and title screening excluded 2,309 studies that did not meet the eligibility criteria. This left 132 studies for which attempts to obtain full text versions were made. Since the present selection contained many old studies unavailable electronically, Sorbonne University library network and King's College London Library's support were requested for obtaining physical copies. Additionally, article authors were contacted through ResearchGate (https://www.researchgate.net) and email solicitations to request access to full text articles. After two rounds of full text screening, a further 121 studies were excluded for various reasons, including 26 studies for which full text could not be obtained (listed in Figure 1). Following the screening process, 11 studies, including one RCT and ten controlled trials (CT) were included for analysis (Table 1). Of the 11 included studies five were eligible to calculate effect sizes (Figures 2–4). Studies that did not allow for effect size calculations are presented in the Supplementary Material together with the meta data of all included studies. Included studies mostly reported using animal samples (seven mouse, two rat and one with Guinea Pig). Habermann et al. (41) was the only human study included. All included studies administered either a single high dose of γ-rays (three studies) or X rays (eight studies). There was a high degree of variability on the dosage of radiation delivered to the study population ranging from 1.23 to 12 Gy. Of the 11 studies included, seven enforced fasting and four caloric restriction as an intervention. The outcome parameters varied from clinical (survival rate, leukemia or cancer occurrence) to histological (number of regenerating crypts, number of crypts, villi height in the intestinal mucosa) and biochemical (RNA polymerase activity, DNA repair activity, hematopoietic cell cycle size, and DNA damage markers). A full meta-analysis was not performed due to high heterogeneity in both intervention and reported outcomes across included studies. All the metadata, together with the calculated effect sizes for is available in Supplementary Table 1 (caloric restriction) and Supplementary Table 2 (fasting).
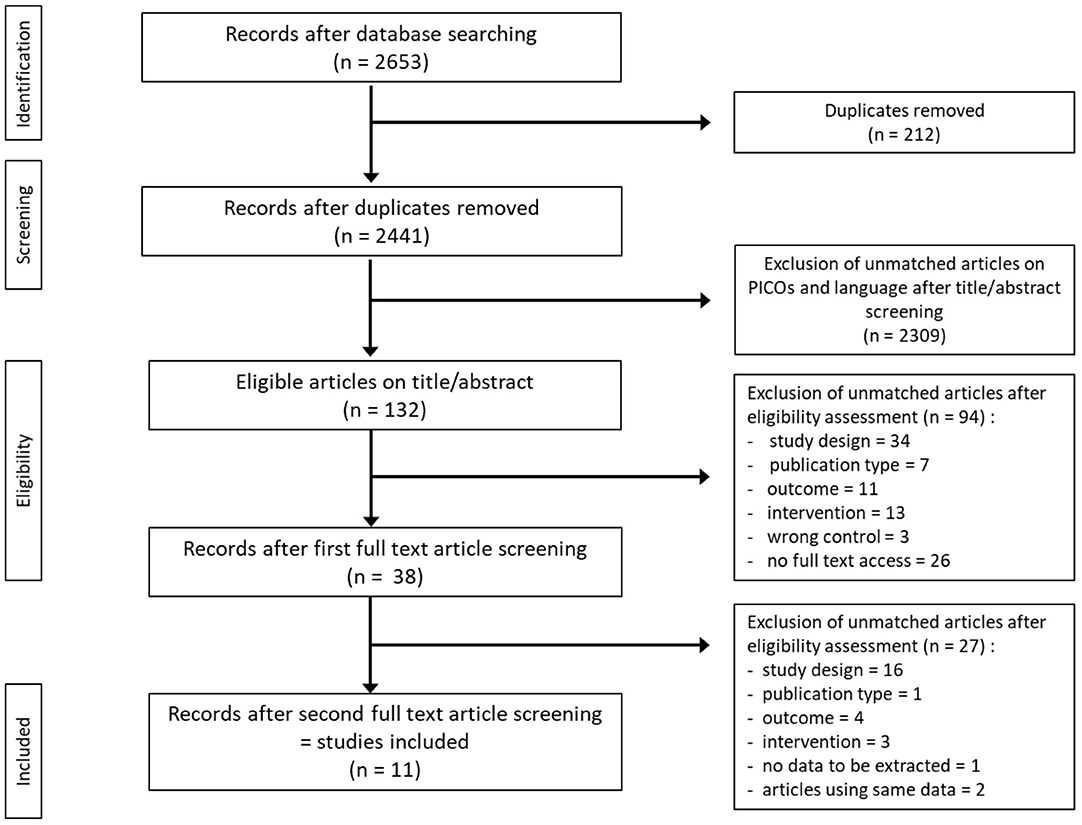
Figure 1. Flow diagram of search and screening methodology. The flow of search results numbers and author's eligibility screening assessment is represented here.
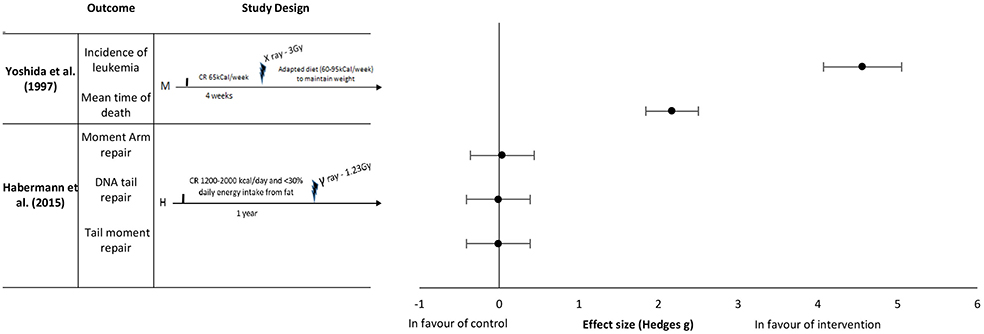
Figure 2. Effect sizes for caloric restriction. Outcomes are plotted with the Hedges' G calculated for each outcome extracted and bias corrected for sample size. Confidence intervals of 95% are represented by the error bars. Effect size values that are in the positive rightward direction indicate a beneficial effect in favor of the intervention group compared to the control group. M, Mice; H, Humans.
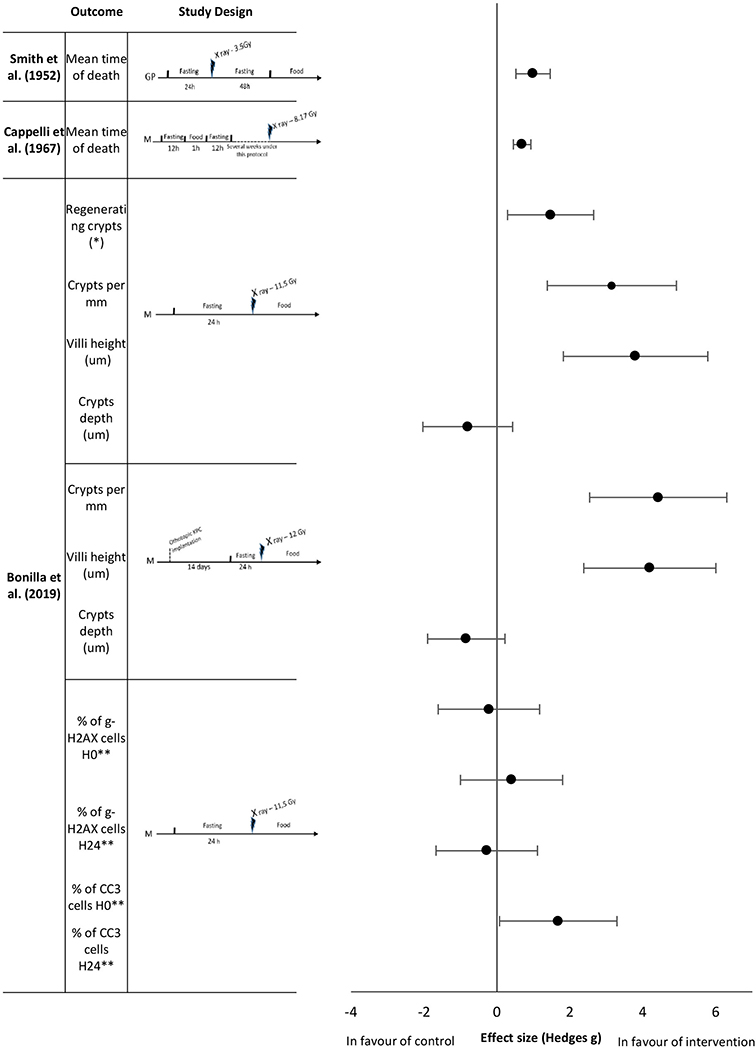
Figure 3. Effect sizes for fasting. Outcomes are plotted with the Hedges' G calculated for each outcome extracted and bias corrected for sample size. Confidence intervals of 95% are represented by the error bars. Effect size values that are in the positive rightward direction indicate a beneficial effect in favor of the intervention group compared to the control group. GP, Guinea pigs; M, mice. *per circumference. ** effect size multiplied by −1.
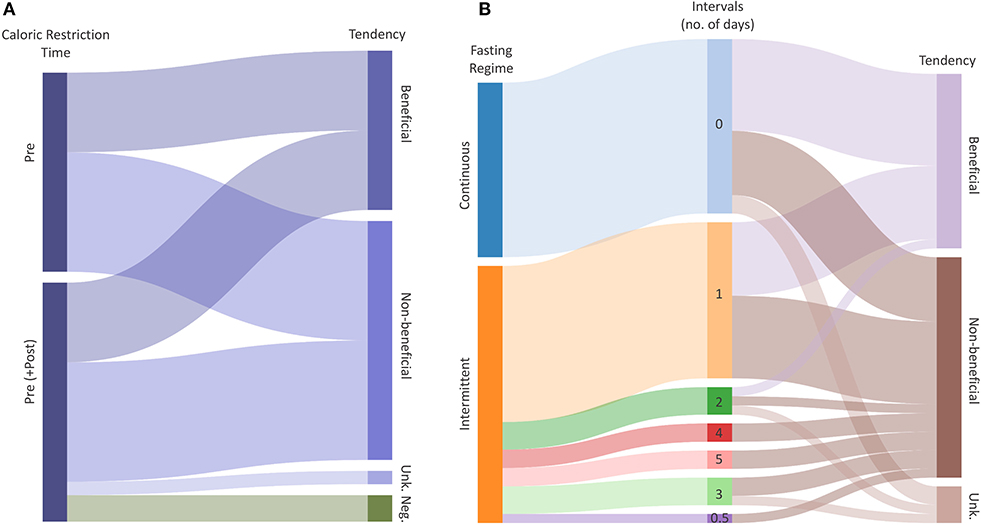
Figure 4. Radio-protective potential of dietary restrictions. (A) Caloric Restriction (CR) explained by the time and Tendency of the intervention. Flows represent the total number of outcomes with a certain potential for effectiveness. (B) Fasting explained by the regime (Intermittent, Continuous), Intervals and radio-protective effectiveness of the intervention. The middle column represents the duration of one interval in days. Flows represent the total number of outcomes given intervals and tendency, respectively. Tendencies indicate whether the CR or Fasting have radio-protective potential (Beneficial), potentially increase radio-sensitivity (Negative) or have no effect (Non-beneficial). “Unk.” represents an unknown tendency.
Methodological Quality
Several studies failed to provide sufficient details to permit a complete assessment of their potential risk of bias (e.g., selection, performance, and bias evaluation). Therefore, these studies were identified as possessing unclear overall risk of bias. All results for the assessment of the methodological quality of the included studies are presented in Supplementary Table 4.
Effect Sizes of Interventions
Caloric Restriction
Yoshida et al. (37) reported an effect in favor of pre-exposure caloric restriction of 65 kCal per week for 4 weeks on the incidence of leukemia with an extremely large effect size of 4.56 and a mean time of death with an extremely large effect size of 2.17 (Figure 2). The mice in this study were kept under a restrictive diet before irradiation, as well as given a moderate caloric restriction after irradiation (reduced caloric intake to maintain weight of 60–95 kCal per week). Habermann et al. (41) studied the repair capacity of radiation damaged DNA in vitro following chronic caloric restriction in humans. The intake restriction implemented in this study was 1,200–2,000 kCal/day caloric intake for 1 year before radiation exposure. In this study, no difference was found between the control and intervention groups on the various types of DNA damage (Moment arm repair, DNA tail repair, and tail moment repair) with effect sizes of 0.04, −0.01 and −0.01, respectively (Figure 2).
Fasting
To date, literature has focused on short and unique fasting sequences as an intervention prior to irradiation. Smith et al. (42) analyzed the mean time of death of guinea pigs exposed to ionizing radiation after 24 h of fasting, followed by 48 h of fasting after exposure. Fasting improved the mean survival time with an extremely large effect size of 0.99 (Figure 3). Longer fasting sequences were also studied. Cappelli et al. (36) showed that prolonged intermittent fasting with short fasting periods for several weeks increased the mean survival time of mice with a large effect size of 0.69 (Figure 3). Bonilla et al. (38) studied the effects of short term fasting in mice before abdominal irradiation upon a range of histological and biological outcomes. At the intestinal level, all measured histological parameters (number of regenerating crypts, number of crypts, villi height in the intestinal mucosa) showed an extremely large effect size (1.47, 3.15, and 3.80, respectively,) in favor of the fasting group; except for crypts depth analysis which had a large effect size of −0.8 in favor of the control group (Figure 3). The same authors reported no differences in γ-H2AX levels, but they observed that the number of CC3 positive cells in fasted animals was higher 24 h after radiation exposure, with an extreme large effect size of 1.68 (Figure 3). They also studied the same histological parameters (regenerating crypts not studied) with an analogous study protocol that exposed mice to orthotopic pancreatic cancer cell implantation 2 weeks before the fasting period followed by the irradiation. Here, the effect sizes of number of crypts and villi height were extremely large, 4.42 and 4.19, respectively; crypts depth analysis remained showed an effect size of −0.84.
Discussion
Out of 46 outcomes on fasting and 18 outcomes on caloric restriction, only 13 and 5, respectively, had sufficient data to calculate effect sizes. Furthermore, a high degree of heterogeneity was found in the outcomes, interventions and populations, preventing result pooling and meta-analysis performance. There were large to extremely large effects in favor of pre-exposure fasting in animals for clinical (incidence of leukemia, mean time of death), histological (crypts density, villi height, regenerating crypts) and biological parameters [% of CC3 cells 24 h after irradiation with a 24 h fasting period before irradiation (H24)]. In contrast, effect sizes for crypts depth, % of γ-H2AX at H0 (no fasting)/H24 and % of CC3 cells at H0 were not significant. Caloric restriction results revealed extremely large positive effect sizes for incidence of leukemia and mean time of death in mice, but in association with post exposure weight control. No effect on DNA damage was found in the only human study included in this review between a pre exposure caloric restriction and its control group.
Evidence Based on Effect Size Calculation
Caloric Restriction
Caloric restriction prior to irradiation did not improve the resistance to radiation induced damage in mice or humans, unless it was combined with caloric restriction post-exposure to ionizing radiation. A reduction in the occurrence of leukemia and survival rates in mice if the caloric restriction intervention was given after irradiation (43, 44). According to the authors, a reduction in the oncogenesis pathway rather than the initiation of cancer may be the potential mechanism of cellular adaptation observed with a reduced caloric intake. The evidence outlined above indicates that longitudinal caloric restriction before, during and after irradiation might be needed to observe any significant beneficial results. Thus, the timing and continuity of reduced caloric intake after irradiation seem to be important factors to consider. The studies included in this systematic review employed various reduced caloric intake definitions, both in terms of duration and number of calories provided to the study population. This variability likely had an influence in the outcomes that were assessed. For example, Habermann et al. (41) restricted calories to study participants for 1 year before radiation while Yoshida et al. (37) employed a short-term caloric restriction regime for only 4 weeks before radiation. Despite substantial study design heterogeneity and the data included in this review, the presented evidence suggests that both pre and post ionizing radiation exposure caloric intake restriction may improve median time to death and reduce cancer incidence. However, further studies are needed evaluating duration, timing, and extent of reduced caloric intake in ameliorating biological damage induced by ionizing radiation.
Fasting
The main outcomes reported in the studies that employed fasting as an intervention were mean time to death and survival parameters. The studies included in this systematic review suggest that fasting may improve (rat) survival rates and time to death when exposed to ionizing radiation (see Figure 3). These results are consistent with those generally observed with prolonged intermittent fasting in mice although there is variability, depending on mouse strain. In addition responses are likely to be highly dependent upon radiation type e.g., X-Rays and γ-Rays and dosage. However, there is insufficient data, to evaluate these differences. Analogous to the included caloric restriction studies, there was a high degree of experimental (timing, duration, and type of fasting method) heterogeneity. In general the main beneficial outcomes were observed with different types of fasting (continuous, intermittent) as well as timing relative to the radiation (fasting before, fasting before and after), although they may influence outcomes and it has been hypothesized that reduced oxidative stress combined with increased antioxidant activity could explain the observed beneficial outcomes through energy sensing pathways involving Sirtuins, FOXO or TOR (22, 28, 29). However, all fasting studies were in animal models. Thus, further research (including in humans) is required to determine the exact permutation of the duration, type, and timing of fasting that is required to maximize the radioprotective effect, and the underlying mechanisms.
Other Noteworthy Findings Not Able to Be Included in the Effect Size Analysis
Caloric Restriction
Some of the included studies did not report sufficient data to calculate effect sizes. Yoshida et al. (45) investigated a group of mice given only reduced caloric intake pre-irradiation followed by normal food access after irradiation. In this study no difference in the incidence of leukemia was reported. However, when associated with a continued reduced caloric intake after irradiation (weight maintenance), the incidence of leukemia in mice was significantly reduced in comparison to the control group. Thus, in Yoshida et al. (46), only a post-exposure adapted diet of 60–95 kCal/week to maintain weight vs. normal food access after irradiation appeared to have the effect of reduced myeloid leukemia incidence, increased number of tumor free mice and median time of death if the same pre-irradiation caloric restriction protocol of 65 kCal/week for 4 weeks was used (Supplementary Figure 1, Supplementary Table 5). However, no differences were observed for the other tumors.
Fasting
Maisin et al. (47) studied the effect of fasting in rats that were irradiated while wearing liver shielding. Fasting significantly improved the survival rate (from 25 to 50%) in liver shielded animals compared to non-fasted animals (Supplementary Figure 2, Supplementary Table 6). No difference was found without liver protection (0% survival in both groups). Omata et al. (40) investigated the activity of Mn2+ (Manganese) and Mg2+ (Magnesium) dependant RNA polymerase in the liver of mice that were fasted and the non-fasted control group following irradiation. They reported a decrease (significance not reported) in the activity of RNA polymerase in the fasting group compared to the control group when irradiated. A similar result was reported in the non-irradiated groups (fasted and non-fasted), indicating a link between fasting itself and these enzymes (significance not reported). In all these studies, the animals were fasted until 24 h after irradiation with 6.5 Gy of X-ray radiation.
Kozubík and Pospísil (39) investigated the survival rate of several strains of mice exposed to different protocols of intermittent fasting. Fasting improved the survival rate in each group of mice, but the effects varied depending on fasting duration. Survival benefit was seen when the intermittent fasting intervention was longer than 1 week. Additionally, short periods of food access between fasting periods appeared to reinforce the survival effect although no dose effect relationship was highlighted (significance not reported). A more recent study by Li and co-workers (48) investigated short fasting periods in mice (12, 48, and 72 h with eight mice per group) before radiation, which showed a survival rate of 0, 12.5, and 50%, respectively, in comparison to 0% survival in the control group following 7.5 Gy of γ-rays significance not reported. Additionally, Bonilla et al. (38) reported a survival rate of 100% at day 30 in the fasted intervention group and a 0% survival rate in the non-fasted control group (significance not reported).
Can Caloric Restriction and Fasting Help to Mitigate Radiation Damage?
The present systematic literature review was hampered by the somewhat limited information on the biological effect of ionizing radiation under caloric restrictions and fasting conditions. An attempt was also made to also analyze tendencies in biological responses to ionizing radiation (Figure 4). In doing so, we have tried to identify potential links between the time and duration of the (caloric restriction/fasting-) interventions, and their effectiveness to mitigate radiation damage. We have merged all extracted data including available meta-data (Supplementary Tables 1, 2), and we assigned a “tendency” to each outcome, based on calculated effect sizes or by reported significance levels of the included studies. The collected data of all included studies did not allow to perform a comprehensive meta-analysis, but the analyzed data suggest tendencies toward beneficial effects of caloric restriction and fasting in response to ionizing radiation. For caloric restriction (Supplementary Table 1), the reported tendencies were equally distributed between “beneficial” and “non-beneficial.” Two “negative” tendencies were reported relating to the loss of the circulating and spleen hematopoietic stem cell fractions (45). Such effects may result from differences in control mice at baseline followed by slower rates of proliferation after irradiation. However, these levels did not appear to influence the overall beneficial effect of reduced rates of leukemia in restricted mice. For studies using caloric restriction as an intervention, all reported beneficial tendencies referred to clinical outcomes (Supplementary Table 1).
The included fasting studies reporting biological responses to ionizing radiation revealed in total 47 reported outcomes, with 19 defined as “beneficial,” and 24 being non-beneficial. The included fasting studies employed either intermittent (28 studies) or continuous fasting (19 studies; Figure 3, Supplementary Table 6). Interestingly, our analysis revealed that the majority of outcomes demonstrated a radio-protective effect during the first 3 days of fasting, and then gradually decreasing with each additional fasting day. The data suggests the radio-protective potential of fasting is less dependent on the type, but rather the duration of fasting although the quality of evidence is insufficient to draw conclusions and thus further research is needed to identify the optimal fasting regimes.
Quality of Evidence
The overall risk of bias was difficult to estimate due the lack of information. This might have exposed this review to selection bias since the majority of these articles was selected by title only since abstracts were not available. An overview of the Cochrane risk of bias evaluation of all included studies is presented in Supplementary Table 4. Use of a systematic approach minimized the risk of missing studies. However, since it was not possible to use Boolean logic in screening the literature in Space Agency's local databases, searches in these databases had to be limited to single keyword searches, exposing a risk of missing relevant studies. Moreover, the search yielded a significant number of non-English papers that was addressed through translation (with the help of native speakers) of articles in several languages (French, German, Russian, and Italian). Lastly, despite the authors' endeavor to recover full text articles, 26 articles, mostly old, could not be obtained.
Transferability of Results
Although scientific communities have been paying more attention to studying radiation effects on living organisms, there are serious limitations in transferring findings from in-vitro, animal and human occupational and clinical radiotherapy studies to healthy populations, like the population of astronauts (3). Those imperfect analogs differ in radiation qualities, energies, doses and dose rates from the conditions that astronauts will experience during spaceflight. In case of human studies, which mainly comprise of radiotherapy patients, drawing conclusions from populations with greater health risks and subjected to additional treatments may introduce additional bias. Thus, relying on those surrogates restricts the ability to translate radiation knowledge to spaceflight scenarios (3).
This review has several limitations that should be considered when interpreting its findings to humans from the animal (rodent) models used in all but one of the included studies. In animal studies, generally single, whole-body and short radiation exposure is the norm in contrast to short, partial-body and repeated irradiation of fractionated radiotherapy in humans. A plethora of studies has been published on nutrition in patients receiving radiotherapy, however, including radiotherapy patients would include clinical patient populations that would introduce unacceptable levels of confounding factors such as clinical physiological changes, co-morbidities, and pharmacological effects and these confounding factors would substantially decrease or potentially prevent any transferability to the population that we address in the present study. Therefore, most radiotherapy studies did not meet the inclusion criteria for the present study. No data were also available on continuous exposure to whole body ionizing radiation in humans in this systematic review. Nonetheless radiation qualities (x rays and γ-rays) and doses reported in this review are consistent with partial radiotherapy, with cancer occurrence and survival rate being two of the most commonly observed outcomes. However, the present study included a significant number of older studies that lacked complete reporting of methodological details leading to the inability to define risk of bias. Moreover, incomplete or inappropriate (by modern standards) data reporting was a recurrent issue preventing data extraction and effect size analysis.
Terrestrial Implications
The findings in this review could assist with the goal of preserving healthy tissues while also reducing secondary cancers in clinical radiotherapy. The potentially synergistic positive mechanisms of cellular protection that appear to be provided by fasting and reduced caloric intake may assist with the goal of preserving healthy tissues while also reducing secondary cancers in clinical radiotherapy (49). There is growing evidence that suggests the benefit of fasting in cancer care. Studies have investigated the capacity of fasting to enhance the radiation sensitivity of cancer cells like glioma or breast cancer in mice (50, 51). Thus, a positive effect of fasting on cancer treatment was observed in animals, limiting the damage of surrounding healthy cells and increasing the cancer sensitivity to radiotherapy. If those effects are verified in humans, this could lead to practical considerations to support cancer therapy. Furthermore, similar results with chemotherapy were found in mouse models; short-term fasting has shown to improve chemotherapy efficiency with etoposide (52), mitoxantrone, oxaliplatin (53), doxorubicin, cyclophosphamide and cisplatin (54) in various cancers and protected mice from etoposide-induced lethal DNA Damage (55). Since chemotherapy can cause similar damage to ionizing radiation on cellular level (56), the cross-talk between fasting and stress response needs to be further investigated. Even though no links between fasting and DNA damage were found in the present study, synergies between responses to ionizing radiation and chemotherapy and influence of fasting call for further research. One of the hypotheses to explain the protective effect of fasting would involve better autophagy activity of the immune system (57). Chemo-tolerance seems also to be enhanced in pre-fasted mice with neuroblastoma exposed to etoposide (52). Furthermore, they could potentially be applied in other clinical contexts given the apparent reduction of oxidative stress and upregulation of antioxidant activity that play a part in numerous pathological states (58) such as cardiovascular (59) and neurodegenerative diseases (60).
However, caution should also be taken when applying the findings to potentially vulnerable or frail populations such as oncology patients receiving various modalities of cancer therapies. There are conflicting studies showing detrimental effects of fasting or caloric restriction in frail populations as the risk of cachexia and nutrient deficient states can have negative impacts on wound healing, therapeutic responses, and downstream complications (61–63). Thus, such caloric restrictive or fasting strategies to high risk patients due to possible adverse effects should be applied with caution. Nonetheless, this is an area that requires much more studies before it is implemented to current patients and safety should be the first priority in considering novel adjunctive methods to improve patient outcomes. However, maintaining a long-term caloric restriction can be a difficult task due to issues of compliance. The adherence rates appear greater for intermittent fasting than other traditional nutritional methods like chronic caloric restriction (64). Prolonged intermittent fasting has also been performed over time by various religious cohorts with a relatively high safety profile if exemptions like pregnancy or diabetes are respected (65). Recent studies have also shown that prolonged fasting is safe if done under medical supervision and guidance in selected patients (66). If proven to be effective, diet modification as a concurrent therapy in cancer patients must be implemented with patient safety as a priority in patients who may already be frail. Several studies have already indicated a good profile of tolerance of short-term fasting during chemotherapy in humans (67, 68). Whereas, a short term fasting seems possible to implement, chronic restriction could raise other concerns. Since cancer patients are often exposed to great weight loss, cachexia and sarcopenia due to the cancer itself or as a side effect of treatment (69), a chronic restrictive diet can worsen those parameters and thus clinicians should consider the entire patient's clinical profile, quality of life, and desired outcomes in future trials to assess its efficacy and safety. The current aims of cancer patients' nutrition recommend the optimization of caloric intake quality and quantity (70). Increasing the caloric intake during the allotted eating periods of intermittent fasting schedules to achieve overall recommended daily caloric intake ranges remains a possibility. However, further research is needed to determine the best possible combination of fasting modalities, timing, and duration to achieve maximal beneficial effect while maintaining patient safety. For now, this systematic review points toward a possible link between fasting or reduced caloric intake with improved clinical outcomes. It is hoped that further investigations into the mechanisms and extent of the benefit, such diet modifications, would improve clinical care to achieve better patient outcomes.
Implications for Human Spaceflight
A number of space agencies have committed to “returning” to the Moon and venturing into deep space. The European Space Agency (representing the ESA member states) together with NASA (the United States), Roscosmos (Russia), CSA (Canada), and JAXA (Japan), have recently announced plans to build the lunar gateway—an orbiting space station around the Moon, that will be a base to support both robots and astronauts exploring the lunar surface (https://www.esa.int/Science_Exploration/Human_and_Robotic_Exploration/Gateway_to_the_Moon). Today astronauts on missions to the International Space Station (ISS) are partially isolated from space radiation environment by the protective shield of the Earth's magnetosphere and atmosphere (71). Leaving these “natural” shields on the way to the Moon would expose astronauts to significantly higher ionizing radiation that differs in regard to its “quality” (composition and energies) as well as its “quantity” (dose) which pose serious threats to human health (3). The risks could be reduced by effective shielding capabilities combined with limited time spent in space, however current shielding materials seem to be insufficient and additionally, they can be sources of ionizing radiation due to spallation events (3, 72). Therefore, there is a need for additional protective radio-protective means such as pharmaceutical and biological countermeasures, as beyond low Earth orbit, astronauts will be exposed to significant doses of primary (solar radiation and galactic cosmic radiation) and secondary ionizing radiation (73). Based on the evidence in this review, prolonged intermittent fasting with short breaks may be a potential biological candidate as a countermeasure mitigating the effects of ionizing radiation. Implementing dietary fasting would be relatively feasible since it is possible to maintain the overall caloric intake of current recommendations by adapting the period of eating (74). Mission planning could also consider the possibility of short term fasting during periods of high solar activity at solar maximums. However, one must also consider the impact of such a strategy on other countermeasures such as physical exercise to prevent musculoskeletal and aerobic astronaut deconditioning (75). However, Winnard et al. (76) recently showed that breaks in exercise of up to 7 days are unlikely to have a major impact on musculoskeletal deconditioning. Thus, with greater understanding of the biological responses to the complex space radiation environment and how to protect against it, dietary restrictions could potentially play an important role in mission planning as a biological countermeasure to radiation damage to ensure the best protection possible for astronauts.
Conclusion
Whilst the data is incomplete there is some evidence to suggest that fasting and caloric restriction might play a protective role with respect to ionizing radiation in rodents. The radio-protective effect (i.e., lower cancer incidence and greater survival) with caloric restriction was only seen if implemented before and after irradiation whereas the benefits of fasting were seen regardless of timing. The potential application and mechanisms of radioprotection provided by dietary changes could have various applications in both terrestrial and space medicine. However, the transferability of knowledge from animal (rodent) models to humans is questionable and given the paucity of research in the field, these observations are only hypothesis generating. Future research should focus on the role of fasting and reduced caloric intake in humans, in particular examining clinical outcomes in patients undergoing radiotherapy. Furthermore, included studies employed a range of radiation types, doses, and locations which require evaluation and further optimized standardization in future research for the potential benefit in radiotherapy patients and astronauts.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
SV and DK: data curation, formal analysis, investigation, methodology, software, and writing—original draft. AF and TW: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing, review, and editing. US and NC: supervision, writing, review, and editing. AW and DG: supervision, writing, formal analysis, data curation, review, and editing. All authors: contributed to the article and approved the submitted version.
Funding
This work was supported by the Space Medicine Team (HRE-OM) of the European Space Agency (ESA) under the leadership of Dr. Guillaume Weerts.
Conflict of Interest
The project was funded by the Space Medicine Team of the European Space Agency (ESA HRE-OM) and KBR GmbH. The funder KBR GmbH provided support in the form of salaries for the authors DG and TW but did not have any role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The specific roles of all authors are articulated in the author contributions section.
Acknowledgments
The authors would like to thank Denis Sebart for his thorough dedication to publication retrieval, as well as Arnaud Viateau and Pierre-Antony Vialatte for their assistance. Special thanks should be given to Vittorio Manfredi for his Italian translations. Advice given by Quinlan Buchlak and Daniel Robson have been a great help in the realization of this project. The authors would also like to express gratitude to Dr. Bernd Johannes for his expertise in biostatistics.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2020.584543/full#supplementary-material
References
1. Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules–mechanisms of damage and emerging methods of detection. Antioxid Redox Signal. (2014) 21:260–92. doi: 10.1089/ars.2013.5489
2. IAEA. Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards General Safety: No GSR Part 3. Vienna: IAEA Safety Standards (2014). p. 436.
3. Chancellor JC, Blue RS, Cengel KA, Auñón-Chancellor SM, Rubins KH, Katzgraber HG, et al. Limitations in predicting the space radiation health risk for exploration astronauts. npj Micrograv. (2018) 4:8. doi: 10.1038/s41526-018-0043-2
4. Goans RE, Flynn DF. Acute radiation syndrome in humans. Medical Consequences of Radiological Nuclear Weapons. (2012) p. 17–38. Available online at: http://www.cs.amedd.army.mil/borden/FileDownloadpublic.aspx?docid=423f63d0-dc11-4ab1-99f2-31a5b47cc5ca
5. Gilbert ES. Ionizing radiation and cancer risks: what have we learned from epidemiology? Int J Radiat Biol. (2009) 85:467. doi: 10.1080/09553000902883836
6. Bledsoe TJ, Nath SK, Decker RH. Radiation pneumonitis. Clin Chest Med. (2017) 38:201–8. doi: 10.1016/j.ccm.2016.12.004
7. Harb AH, Abou Fadel C, Sharara AI. Radiation enteritis. Curr Gastroenterol Rep. (2014) 16:383. doi: 10.1007/s11894-014-0383-3
8. Fry RJ. Deterministic effects. Health Phys. (2001) 80:338–43. doi: 10.1097/00004032-200104000-00009
9. Hamada N, Fujimichi Y. Classification of radiation effects for dose limitation purposes: history, current situation and future prospects. J Radiat Res. (2014) 55:629. doi: 10.1093/jrr/rru019
11. Benzie IF. Evolution of antioxidant defence mechanisms. Eur J Nutr. (2000) 39:53–61. doi: 10.1007/s003940070030
12. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. (2014) 19:181–92. doi: 10.1016/j.cmet.2013.12.008
13. Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. (1990) 55:69–87. doi: 10.1016/0047-6374(90)90107-Q
14. Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. (1986) 116:641–54. doi: 10.1093/jn/116.4.641
15. Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. (1982) 215:1415–8. doi: 10.1126/science.7063854
16. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. (2009) 325:201–4. doi: 10.1126/science.1173635
17. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. (1996) 273:59–63. doi: 10.1126/science.273.5271.59
18. Song J, Ke S-F, Zhou C-C, Zhang S-L, Guan Y-F, Xu T-Y, et al. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J Gerontol Ser A Biol Sci Med Sci. (2014) 69:44–57. doi: 10.1093/gerona/glt122
19. Allard JS, Heilbronn LK, Smith C, Hunt ND, Ingram DK, Ravussin E, et al. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS ONE. (2008) 3:e3211. doi: 10.1371/journal.pone.0003211
20. Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. (2015) 161:106–18. doi: 10.1016/j.cell.2015.02.020
21. Martens CR, Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol. (2016) 594:7177–95. doi: 10.1113/JP272348
22. Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. (2009) 20:325–31. doi: 10.1016/j.tem.2009.03.008
23. Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. (2013) 27:2072–85. doi: 10.1101/gad.227439.113
24. Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging. (2013) 5:144–50. doi: 10.18632/aging.100544
25. Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. (2010) 12:662–7. doi: 10.1016/j.cmet.2010.11.015
26. Traba J, Geiger SS, Kwarteng-Siaw M, Han K, Ra OH, Siegel RM, et al. Prolonged fasting suppresses mitochondrial NLRP3 inflammasome assembly and activation via SIRT3-mediated activation of superoxide dismutase 2. J Biol Chem. (2017) 292:12153–64. doi: 10.1074/jbc.M117.791715
27. Vassilopoulos A, Fritz KS, Petersen DR, Gius D. The human sirtuin family: evolutionary divergences and functions. Hum Genomics. (2011) 5:485–96. doi: 10.1186/1479-7364-5-5-485
28. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. (2004) 303:2011–5. doi: 10.1126/science.1094637
29. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. (2013) 493:338–45. doi: 10.1038/nature11861
30. Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. (2007) 17:1646–56. doi: 10.1016/j.cub.2007.08.047
31. Martín-Montalvo A, Villalba JM, Navas P, de Cabo R. NRF2, cancer and calorie restriction. Oncogene. (2011) 30:505–20. doi: 10.1038/onc.2010.492
32. De Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. (2019) 381:2541–51. doi: 10.1056/NEJMra1905136
33. Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG, et al. Flipping the metabolic switch: understanding and applying the health benefits of fasting. Obesity. (2018) 26:254–68. doi: 10.1002/oby.22065
34. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
35. Ellis PD. Effect sizes and the interpretation of research results in international business. J Int Bus Stud. (2010) 41:1581–8. doi: 10.1057/jibs.2010.39
36. Cappelli B, Cittadini G, Romano E. Radiosensitivity after panirradiation at different hours of the day. Atti Accad Fisiocrit Siena Med Fis. (1967) 16:858–68.
37. Yoshida Inoue T, Nojima K, Hirabayashi Y, Sado T. Calorie restriction reduces the incidence of myeloid leukemia induced by a single whole-body radiation in C3H/He mice. Proc Natl Acad Sci USA. (1997) 94:2615–9. doi: 10.1073/pnas.94.6.2615
38. Bonilla M, de la C, Stemler KM, Jeter-Jones S, Fujimoto TN, Molkentine J, Torres GMA, et al. Fasting reduces intestinal radiotoxicity, enabling dose-escalated radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. (2019) 105:537–47. doi: 10.1016/j.ijrobp.2019.06.2533
39. Kozubík A, Pospísil M. Protective effect of intermittent fasting on the mortality of gamma-irradiated mice. Strahlentherapie. (1982) 158:734–8.
40. Omata S, Ichii S, Yago N. Effects of whole-body x-irradiation on nuclear RNA polymerase activities in rat tissues. J Biochem. (1968) 63:695–700. doi: 10.1093/oxfordjournals.jbchem.a128833
41. Habermann N, Makar KW, Abbenhardt C, Xiao L, Wang C-Y, Utsugi HK, et al. No effect of caloric restriction or exercise on radiation repair capacity. Med Sci Sport Exerc. (2015) 47:896–904. doi: 10.1249/MSS.0000000000000480
42. Smith WW, Ackermann IB, Smith F. Body weight, fasting and forced feeding after whole body X-irradiation. Am J Physiol Content. (1952) 168:382–90. doi: 10.1152/ajplegacy.1952.168.2.382
43. Gross L, Dreyfuss Y. Reduction in the incidence of radiation-induced tumors in rats after restriction of food intake. Proc Natl Acad Sci USA. (1984) 81:7596–8. doi: 10.1073/pnas.81.23.7596
44. Gross L, Dreyfuss Y. Prevention of spontaneous and radiation-induced tumors in rats by reduction of food intake. Proc Natl Acad Sci USA. (1990) 87:6795. doi: 10.1073/pnas.87.17.6795
45. Yoshida Hirabayashi Y, Inoue T. Calorie restriction reduces the incidence of radiation-induced myeloid leukaemia. IARC Sci Publ. (2002) 156:553–5. Availabe online at: https://pubmed.ncbi.nlm.nih.gov/12484259/
46. Yoshida Hirabayashi Y, Watanabe F, Sado T, Inoue T. Caloric restriction prevents radiation-induced myeloid leukemia in C3H/HeMs mice and inversely increases incidence of tumor-free death: implications in changes in number of hemopoietic progenitor cells. Exp Hematol. (2006) 34:274–83. doi: 10.1016/j.exphem.2005.11.016
47. Maisin J, Van Lancker J, Dunjic A, Lambert G, Passeau L. Influence of fasting on the survival of animals irradiated in toto with the liver protected. C R Seances Soc Biol Fil. (1953) 147:1517–20.
48. Li JX, Tong J, Zhu SN, Gu XL, Song L, Zhong HL, et al. Fasting attenuates γ-ray radiation and drug toxicity in mice. Toxicol Lett. (2010) 196:S173. doi: 10.1016/j.toxlet.2010.03.591
49. Taylor CW, Kirby AM. Cardiac side-effects from breast cancer radiotherapy. Clin Oncol. (2015) 27:621–9. doi: 10.1016/j.clon.2015.06.007
50. Saleh A, Simone B, Palazzo J, Savage JE, Sano Y, Dan T, et al. Caloric restriction augments radiation efficacy in breast cancer. Cell Cycle. (2013) 12:1955–63. doi: 10.4161/cc.25016
51. Safdie F, Brandhorst S, Wei M, Wang W, Lee C, Hwang S, et al. Fasting enhances the response of glioma to chemo- and radiotherapy. PLoS ONE. (2012) 7:e44603. doi: 10.1371/journal.pone.0044603
52. Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, et al. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci USA. (2008) 105:8215–20. doi: 10.1073/pnas.0708100105
53. Pietrocola F, Pol J, Vacchelli E, Rao S, Enot DP, Baracco EE, et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell. (2016) 30:147–60. doi: 10.1016/j.ccell.2016.05.016
54. Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. (2012) 4:124ra27. doi: 10.1126/scitranslmed.3003293
55. Tinkum KL, Stemler KM, White LS, Loza AJ, Jeter-Jones S, Michalski BM, et al. Fasting protects mice from lethal DNA damage by promoting small intestinal epithelial stem cell survival. Proc Natl Acad Sci USA. (2015) 112:E7148–54. doi: 10.1073/pnas.1509249112
56. Hauer-Jensen M, Denham JW, Andreyev HJN. Radiation enteropathy-pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. (2014) 11:470–9. doi: 10.1038/nrgastro.2014.46
57. van Niekerk G, Hattingh SM, Engelbrecht A-M. Enhanced therapeutic efficacy in cancer patients by short-term fasting: the autophagy connection. Front Oncol. (2016) 6:242. doi: 10.3389/fonc.2016.00242
58. Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, et al. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci. (2019) 20:4472. doi: 10.3390/ijms20184472
59. Pignatelli P, Menichelli D, Pastori D, Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. (2018) 76:713–22. doi: 10.5603/KP.a2018.0071
60. Kim GH, Kim JE, Rhie SJ, Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol. (2015) 24:325–40. doi: 10.5607/en.2015.24.4.325
61. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Prim. (2018) 4:1–18. doi: 10.1038/nrdp.2017.105
62. Marceca GP, Londhe P, Calore F. Management of cancer cachexia: attempting to develop new pharmacological agents for new effective therapeutic options. Front Oncol. (2020) 10:298. doi: 10.3389/fonc.2020.00298
63. Biswas AK, Acharyya S. Understanding cachexia in the context of metastatic progression. Nat Rev Cancer. (2020) 20:274–84. doi: 10.1038/s41568-020-0251-4
64. Ravussin E, Gilmore LA, Redman LM. Calorie restriction in humans: impact on human health. Mol Basis Nutr Aging. (2016) 677–92. doi: 10.1016/B978-0-12-801816-3.00048-0
65. Trepanowski JF, Bloomer RJ. The impact of religious fasting on human health. Nutr J. (2010) 9:57. doi: 10.1186/1475-2891-9-57
66. Finnell JS, Saul BC, Goldhamer AC, Myers TR. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC Complement Altern Med. (2018) 18:67. doi: 10.1186/s12906-018-2136-6
67. Dorff TB, Groshen S, Garcia A, Shah M, Tsao-Wei D, Pham H, et al. Safety and feasibility of fasting in combination with platinum-based chemotherapy. BMC Cancer. (2016) 16:360. doi: 10.1186/s12885-016-2370-6
68. Safdie Dorff T, Quinn D, Fontana L, Wei M, Lee C, et al. Fasting and cancer treatment in humans: a case series report. Aging. (2009) 1:988–1007. doi: 10.18632/aging.100114
69. Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer cachexia: beyond weight loss. J Oncol Pract. (2016) 12:1163–71. doi: 10.1200/JOP.2016.016832
70. Dev R, Wong A, Hui D, Bruera E. The evolving approach to management of cancer cachexia. Oncology. (2017) 31:23–32. Availabe online at: https://pubmed.ncbi.nlm.nih.gov/28090619/
71. Letaw JR, Silberberg R, Tsao CH. Radiation hazards on space missions outside the magnetosphere. Adv Sp Res. (1989) 9:285–91. doi: 10.1016/0273-1177(89)90451-1
72. Cucinotta FA, Kim M-HY, Ren L. Evaluating shielding effectiveness for reducing space radiation cancer risks. Radiat Meas. (2006) 41:1173–85. doi: 10.1016/j.radmeas.2006.03.011
73. Maalouf M, Durante M, Foray N. Biological effects of space radiation on human cells: history, advances and outcomes. J Radiat Res. (2011) 52:126–46. doi: 10.1269/jrr.10128
74. Wilhelmi de Toledo F, Buchinger A, Burggrabe H, Hölz G, Kuhn C, Lischka E, et al. Fasting therapy - an expert panel update of the 2002 consensus guidelines. Forschende Komplementärmedizin Res Complement Med. (2013) 20:434–43. doi: 10.1159/000357602
75. Gaffney CJ, Fomina E, Babich D, Kitov V, Uskov K, Green DA. The effect of long-term confinement and the efficacy of exercise countermeasures on muscle strength during a simulated mission to Mars: data from the Mars500 study. Sport Med Open. (2017) 3:40. doi: 10.1186/s40798-017-0107-y
Keywords: fasting, caloric restriction, radio-protection, SIRTUIN, irradation, space flight, deep space, radiology
Citation: Valayer S, Kim D, Fogtman A, Straube U, Winnard A, Caplan N, Green DA, van Leeuwen FHP and Weber T (2020) The Potential of Fasting and Caloric Restriction to Mitigate Radiation Damage—A Systematic Review. Front. Nutr. 7:584543. doi: 10.3389/fnut.2020.584543
Received: 17 July 2020; Accepted: 25 August 2020;
Published: 18 September 2020.
Edited by:
Jasminka Z. Ilich, Florida State University, United StatesReviewed by:
Nathaniel J. Szewczyk, University of Nottingham, United KingdomPhilippe G. Frank, Université de Tours, France
Copyright © 2020 Valayer, Kim, Fogtman, Straube, Winnard, Caplan, Green, van Leeuwen and Weber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tobias Weber, dG9iaWFzLndlYmVyQGVzYS5pbnQ=
 Simon Valayer
Simon Valayer David Kim
David Kim Anna Fogtman
Anna Fogtman Ulrich Straube
Ulrich Straube Andrew Winnard
Andrew Winnard Nick Caplan
Nick Caplan David A. Green
David A. Green Flora H. P. van Leeuwen1,7
Flora H. P. van Leeuwen1,7 Tobias Weber
Tobias Weber