- Division of Human Nutrition and Health, Wageningen University and Research, Wageningen, Netherlands
In order to fully exploit the nutrient density concept, thorough understanding of the biological activity of single nutrients in their interaction with other nutrients and food components from whole foods is important. This review provides a narrative overview of recent insights into nutrient bioavailability from complex foods in humans, highlighting synergistic and antagonistic processes among food components for two different food groups, i.e., dairy, and vegetables and fruits. For dairy, bioavailability of vitamins A, B2, B12 and K, calcium, phosphorous, magnesium, zinc and iodine are discussed, whereas bioavailability of pro-vitamin A, folate, vitamin C and K, potassium, calcium, magnesium and iron are discussed for vegetables and fruits. Although the bioavailability of some nutrients is fairly well-understood, for other nutrients the scientific understanding of uptake, absorption, and bioavailability in humans is still at a nascent stage. Understanding the absorption and bioavailability of nutrients from whole foods in interaction with food components that influence these processes will help to come to individual diet scores that better reflect absorbable nutrient intake in epidemiologic studies that relate dietary intake to health outcomes. Moreover, such knowledge may help in the design of foods, meals, and diets that aid in the supply of bioavailable nutrients to specific target groups.
Introduction
Historically, the nutritional sciences are built on the study of single nutrients or food components in relation to health outcomes. Although this has been a useful concept when it comes to specific deficiency diseases, the picture became blurry when studying the role of nutrition in complex diseases. The notion that the sum of the parts does not necessarily explain the result of the whole has prompted a shift in focus from single nutrients to whole foods, meals, and dietary patterns. Studying the biological activity of single nutrients in their interaction with other nutrients and food components from whole foods, especially during their stay in the gastro-intestinal tract, helps to better understand the underlying positive and adverse health effects of whole foods, meals, and dietary patterns.
Bioavailability of nutrients from whole foods is a research area that has received ample attention in the past decades, although studies in humans are still limited. Over time, some consensus has been reached on the definition of bioavailability, which is the fraction of an ingested nutrient that becomes available for use and storage in the body (1). In this definition, bioavailability goes beyond mere absorption from the gut and also includes the use and storage (retention) in body tissue. The study of absorption and bioavailability of nutrients from foods in humans requires sophisticated methods that take into account endogenous nutrient losses through the enterohepatic circulation as well as incorporation of nutrients into storage tissue. Use of isotopes, both radio-isotopes and stable isotopes, have greatly improved accuracy and precision of in vivo nutrient bioavailability studies, either as a single nutrient or as part of a food, meal, or dietary pattern (2). Although in vitro methods are much cheaper and faster than in vivo methods, allowing for large numbers and experimental conditions, translation of findings to full body human conditions is still cumbersome (3, 4). Therefore, this review uses only bioavailability data from in vivo studies in humans, although the underlying mechanisms of interactions between food components are mostly based on in vitro or animal studies.
The main objective of this review is to provide an overview of insights into nutrient bioavailability from complex foods in humans, thereby highlighting the current state of knowledge on synergistic and antagonistic processes among food components. Two different food groups are put in the spotlight for this purpose, i.e., dairy, and fruits and vegetables. Both of these food groups contain a myriad of nutrients, for some of which the bioavailability is now well-understood, whereas others still require further study. Both food groups also contain many bioactive components and have a complex matrix, which affect the kinetics of nutrient release, absorption, and bioavailability. Understanding of these processes will help to better predict the true nutrient value of foods and to incorporate this information into diet scores in the future.
Milk and Dairy Foods
Dairy refers to food products that have milk—mostly cow's milk—as their main ingredient such as buttermilk, yogurt, cheese, and all closely related products. Dairy is characterized by a relatively high amount of protein and fat, and can therefore make an important contribution to calorie intake unless low-fat alternatives are consumed. Intake of dairy varies greatly between and within world regions, with an estimated average intake of milk (excluding other dairy products) of ~200–240 g per day in Western Europe and North America, ~130–300 g per day in Latin America, ~100–200 g per day in Africa, and 20–150 g in Asia1. Despite controversy around the healthfulness of dairy with respect to non-communicable disease risk, scientific evidence consistently points toward either beneficial or neutral effects (5–10). In addition, positive effects on bone mineral density have been found, as well as reduced fracture risk in some populations (9, 11). Such beneficial effects have been attributed to calcium as well as to various other nutrients and bioactive components present in milk (11).
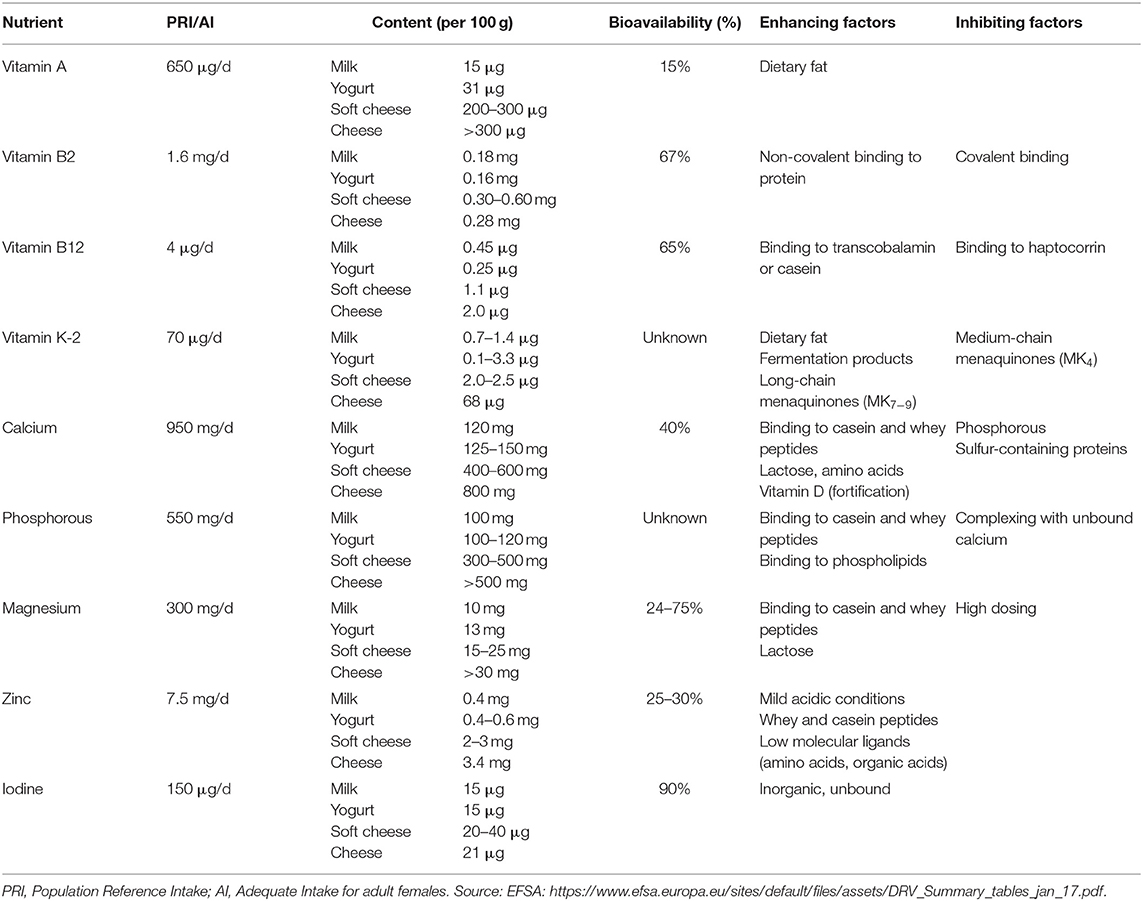
In industrialized countries, dairy stands out as source of calcium, but it also contributes for 20–40% to the intake of vitamins A, B2, B12, and K as well as of phosphorous, magnesium, zinc, and iodine (12– 23)2. In the next sections, the absorption and bioavailability of these nutrients from milk and dairy foods will be described, with special attention for calcium.
Dairy as a Source of Calcium
Dairy is by far the most important source of calcium in the human diet and it has therefore been studied most extensively among the nutrients derived from dairy. Bovine milk contains an average of 120 mg calcium per 100 mL. In Europe and North America, ~75% of dietary calcium is derived from milk and dairy products, with an additional 15% from vegetables and fruits, 5% from mineral water and the rest from other foods (24, 25). Approximately 40% of calcium from dairy sources is absorbed under normal circumstances, with higher absorption in children and lower absorption in elderly (26, 27). In the body, 99% of calcium is present in the skeleton. The efficiency of calcium storage in bone tissue is mainly determined by physiological factors (e.g., related to growth, pregnancy, and lactation), and is regulated by several hormones, such as PTH, calcitonin, calcitriol, and estrogens. Excessive absorbed calcium is excreted in urine, feces, and sweat. Adults are generally in negative calcium balance after their peak bone mass (~35 y) and loose ~10 mg of calcium each day, although in post-menopausal women the daily loss may be 40 mg per day or more (28). Bioavailability of calcium is determined by absorption in the small intestine on the one hand and by incorporation into bone tissue on the other hand. Both of these processes can be influenced by dietary factors. The bioavailability of calcium may therefore be defined as the fraction of dietary calcium that is absorbed by the intestine and is used for bone mineralization.
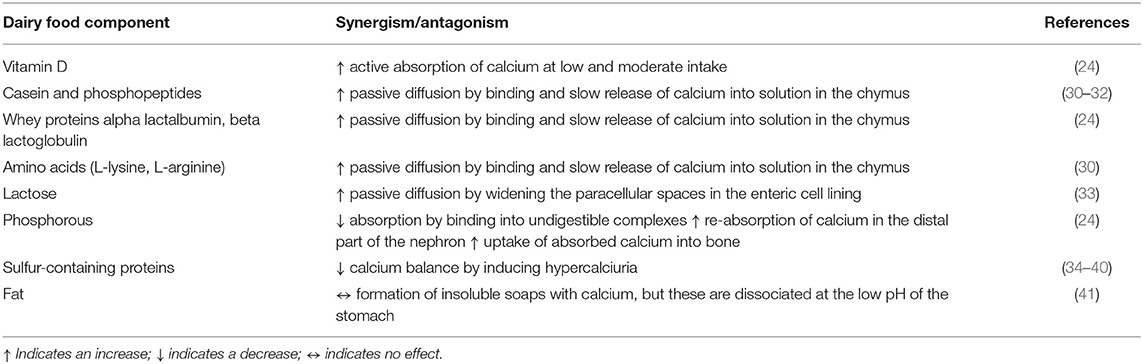
Intestinal absorption of calcium mostly happens by passive diffusion, whereas active transport at low and moderate calcium intake is under regulation of vitamin D (24). Fortification of milk with vitamin D2 was shown to enhance calcium absorption (29). A number of milk and dairy components have been found to aid in the passive absorption of calcium (and also of other divalent cations), such as phosphopeptides, casein and whey proteins, lactose and phosphorous (Table 1). Phosphopeptides, which are products of the enzymatic hydrolysis of casein, sequester calcium thereby protecting it from precipitation by anions like phosphates in the intestine (30–32). The same is true for alpha lactalbumin and beta lactoglobulin, both whey proteins (24), and for amino acids such as L-lysine and L-arginine (30). The calcium bound to these amino acids, peptides, and proteins is readily released during digestion by bringing it slowly into solution, which is an important prerequisite for passive diffusion.
Lactose also seems to enhance calcium absorption, although the mechanism has long been unclear (42). The most likely explanation is that, like other sugars, lactose widens the paracellular spaces in the enteric cell lining, thereby enhancing passive diffusion (33). However, studies have shown that this mostly happens at relatively high doses of lactose (43, 44), whereas in the amounts present in milk and dairy it is less likely to contribute much to absorption (45, 46). When lactose is hydrolyzed, such as in yogurt, or absent, such as in cheese, calcium absorption does not seem to be affected (47). Nevertheless, lactose seems to be important for calcium absorption in case of high calcium intake in combination with poor solubility, such as seen in babies and the elderly (48, 49). It may be that, similar to galactic-oligosaccharides, lactose functions as a prebiotic and stimulates calcium absorption in the cecum and colon by enhancing the growth of bifidobacteria and thereby maintaining low pH (50, 51). This hypothesis is supported by evidence from lactose-deficient patients, who do not seem to have compromised calcium absorption (52).
Apart from factors that enhance calcium absorption, several dairy components can inhibit the uptake of calcium (Table 1). Protein, especially sulfur-containing protein, has been shown to lead to a negative calcium balance through increased urinary calcium excretion (24). Nevertheless, the conclusion of a working group that more recently reviewed the current evidence linking dietary protein intake with bone health was that, although a high protein diet—either of animal or vegetable origin—is associated with increased urinary calcium excretion, this is more likely due to higher intestinal calcium absorption than to bone resorption (53, 54). Milk fats can form insoluble soaps with calcium; however, these are dissociated at the low pH of the stomach and therefore do not affect calcium bioavailability negatively (41). This explains that calcium from cheese is readily available for absorption despite the high content of saturated long chain fatty acids (24, 55).
Phosphorous plays a dual role in calcium absorption, by, on the one hand, binding calcium and inhibiting its absorption in the small intestine resulting in increased fecal excretion of calcium, and, on the other hand, after being absorbed, by increasing the reabsorption of calcium in the distal part of the nephron or by enhancing the uptake of absorbed calcium into bone (24, 54). The inhibitory properties of phosphorous on intestinal calcium absorption may partly be countered by phosphorylation of lactose, thereby keeping calcium in solution (24). However, this hypothesis has not yet been confirmed (41, 55). Also, the high phosphorous content of milk may counter the hypercalciuria induced by protein (54, 56). The recommended dietary intake ratio for calcium (mg) to phosphorous (mg) ranges from 1:1 to 1.5:1, with ratios <0.5 being associated with decreases in bone mineral density (57). Moreover, excessive intake of phosphorous has been shown to induce the secretion of fibroblast growth factor 23 (FGF-23) from bone, thereby decreasing the formation of 1,25-dihydroxyvitamin D3 and decreasing intestinal calcium absorption (57, 58). Cow's milk provides calcium and phosphorus in a reasonable balanced ratio of ~1.2:1.
Overall, although intestinal absorption of calcium from milk and dairy is very similar compared to other sources such as calcium salts, vegetables, or mineral water, its net effect on calcium retention is generally higher (59) with little difference between the various dairy products (milk, acidified milk, yogurt, skim milk, cream cheese, hard cheeses) (24). Diets including dairy products can therefore be considered as the most optimal calcium-dense option to prevent adverse health effects related to a negative calcium balance.
Dairy as a Source of Other Nutrients
Vitamins
Vitamin A
The content of vitamin A in dairy ranges between 15 and 50 μg in 100 mL of milk to over 300 μg per 100 g of full-fat cheese (Table 2). Vitamin A occurs in dairy products predominantly as retinyl palmitate (60), but small amounts of ß-carotene can also occur. Little is known about the bioavailability of vitamin A from milk and other dairy products, but one study reports that ~15% of vitamin A from milk is absorbed and this appeared not to be different for fortified milk (60). Moreover, it did not appear to be different for full fat or skim milk, despite the fact that absorption of fat-soluble vitamins is generally regarded to depend on the fat content of a meal for solubilization and stimulation of biliary secretion and for the formation of micelles (60).
Vitamin B2
With 0.18 mg of riboflavin per 100 mL of milk and 0.28 mg per 100 g of cheese, dairy forms an important source of this water soluble vitamin (Table 2). In dairy, riboflavin is mostly bound non-covalently to protein, predominantly as flavin adenine dinucleotide (FAD) and to a lesser extent as flavin mononucleotide (FMN). Milk also contains free riboflavin bound to specific binding proteins (21). Hydrolysis of FAD and FMN to riboflavin by phosphatases in the small intestine is a prerequisite for its carrier-mediated absorption (21). Riboflavin has been reported to be readily bioavailable from milk at ~67% (61).
Vitamin B12
Milk contains ~0.40–0.45 μg of vitamin B12 per 100 mL, whereas cheese can contain up to 2 μg per 100 g (Table 2). The major derivatives of vitamin B12 in bovine milk are hydroxycobalamin, adenosylcobalamin, and methylcobalamin, and it is mostly bound to the proteins haptocorrin, transcobalamin and casein depending on the cow breed (62, 63). Vitamin B12 bound to transcobalamin appeared to be better released in vitro, whereas this was cumbersome when bound to haptocorrin (mainly present in buffalo milk) and this may have implications for in vivo bioavailability (63). A study in healthy adults > 60 y old, however, revealed that ~65% of vitamin B12 from milk was absorbed (64), whereas, in comparison, absorption of vitamin B12 from animal foods is generally 50% or lower and even <5% for synthetic supplements (62, 65). Nevertheless, a study comparing cyano-B12 from a supplement with hydroxo-B12 from whey powder improved vitamin B12 status similarly (66).
Vitamin K-2
Menaquinones are primarily synthesized by bacteria, and therefore fermented dairy products such as yogurt and cheese are good sources of this vitamin (67). Milk contains ~0.7–1.4 μg of menaquinones per 100 mL, whereas full-fat hard cheese can contain up to 68 μg per 100 g (Table 2). Intake of long-chain menaquinones (MKn) in particular has been associated with decreased risk of cardiovascular disease (68, 69), in contrast to phylloquinone (vitamin K-1) derived from plant-based foods. The menaquinone content of dairy products was assessed to be highest in fermented cheeses and to be positively related to fat content (70, 71). Vitamin K is a fat soluble vitamin, and as such is absorbed through the lipid pathway. The bioavailability of menaquinones from dairy sources has not been studied in humans to date, with the exception of a study showing that MK7-fortified yogurt resulted in slightly higher plasma concentrations as compared to MK7 from a soft-gel capsule (72). The length of the isoprene chain strongly determines absorption and metabolism of menaquinones in the sense that MK7−9 are better absorbed than MK4 and have a longer half-life than vitamin K-1 (67, 73).
Minerals and Trace Elements
Phosphorous
With a content of ~100 mg of phosphorous per 100 mL milk and >500 mg per 100 g cheese, dairy is an important source of phosphorous in the diet (Table 2). Although data are still scarce, it is assumed that phosphorous derived from animal foods is more bioavailable than phosphorous derived from plant-based foods, as revealed by balance studies relating phosphorous intake from dietary sources to urinary excretion (74, 75). This may be explained by the binding of phosphorous to digestible compounds in animal foods, such as proteins and phospholipids. However, phosphorous also easily forms indigestible complexes in the gastro-intestinal tract (e.g., with calcium), and its bioavailability from dairy sources may strongly depend on interaction with other meal components (74). No studies have been done to date to directly measure the bioavailability of phosphorous from dietary sources in humans.
Zinc
With a concentration of 0.4 mg of zinc per 100 mL milk forms an important source of zinc (Table 2). Zinc is predominantly present in the protein fraction in milk, specifically in casein micelles, but is easily released under mild acidic conditions (76). Approximately 25–30% of zinc is absorbed from milk (77, 78). Apart from whey and casein peptides, also other low molecular ligands and chelators that can bind Zn, such as amino acids (histidine, methionine) and organic acids (citric, malic and lactic acid), may promote zinc absorption (79).
Magnesium
Milk contains ~10 mg of magnesium per 100 mL, but can be triple that in cheese (Table 2). Absorption of magnesium from milk was found to be strongly dose-dependent, with ~75% absorption reported from a serving of milk containing 46 mg of magnesium (80). With intake of magnesium at physiological doses, absorption seems to be predominantly due to a saturable mechanism and at higher amounts mainly by simple diffusion (80). As for other divalent metals, i.e., calcium, iron and zinc, peptides from casein or whey can bind magnesium, which may promote absorption (81). Also, lactose appeared to promote absorption of magnesium from milk in rats (81, 82), but this was not confirmed in humans (83). As for calcium, unabsorbed lactose may act as a prebiotic and stimulate magnesium uptake in the large intestine, but this needs further investigation (84, 85).
Iodine
Iodine content of milk can vary substantially with a reported range of 3.3–53.4 μg per 100 mL, depending on the way of farming, iodine intake of dairy cows, use of iodine-containing udder cleansers, season, and processing (86, 87). Iodine in milk is predominantly (>80%) present as inorganic iodide, and in line with this iodine bioavailability from milk is high (~90%) (88).
Vegetables and Fruits
Vegetables and fruits form a widely diverse food group that contains a broad range of essential nutrients. Vegetables and fruits are generally low in fat and proteins and therefore contribute relatively little to energy intake. Ample consumption of vegetables and fruits is promoted worldwide. Such recommendations are based on studies consistently showing that higher intake of vegetables and fruits is negatively associated with all-cause mortality and mortality from cardiovascular disease and cancer (89, 90). Close to 75% of the world population consumes less than the recommended 400 g of vegetables and fruits on a daily basis (91). Low consumption of vegetables and fruits is estimated to contribute 1.8% to the total global burden of disease, primarily through cardiovascular diseases and cancer (92).
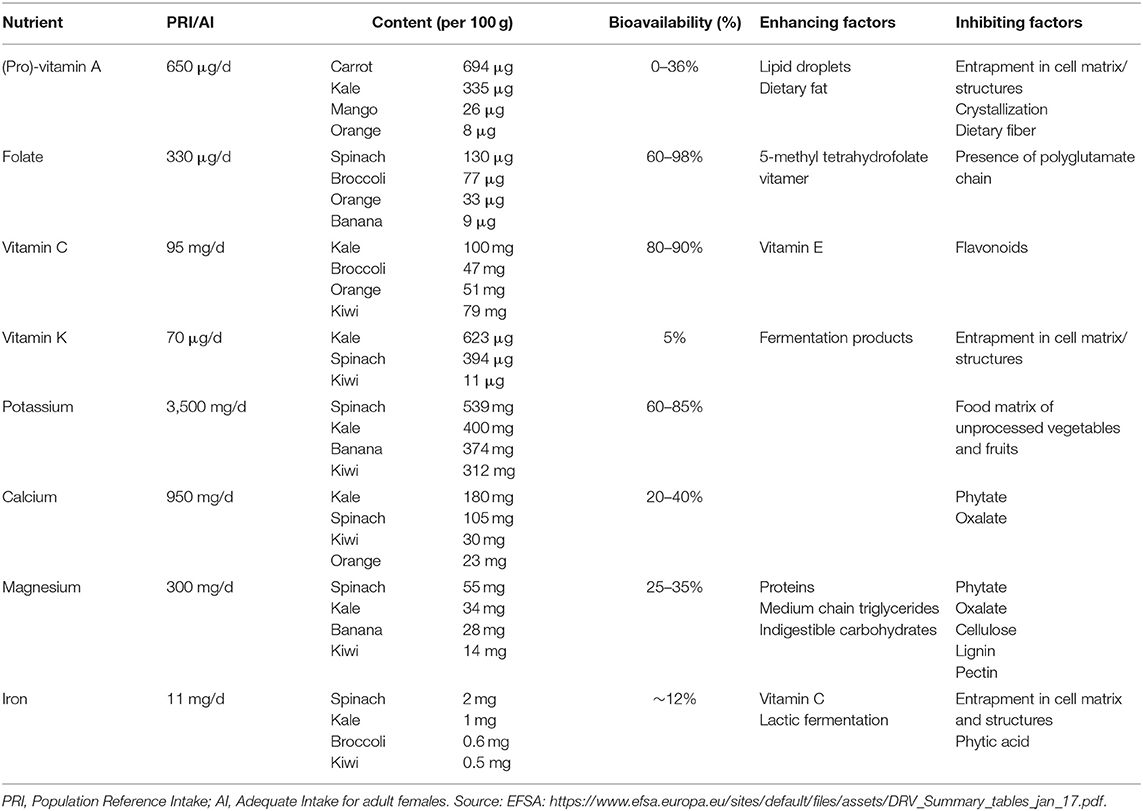
So far, studies have failed to attribute the healthful effects of vegetables and fruits to any of its isolated components. Therefore, health benefits from vegetable and fruit consumption are rather to be explained as the resultant of additive and synergistic effects of its components (63–66). They are a particular rich source of pro-vitamin A carotenoids, vitamin C, folate, vitamin K-1, potassium, calcium, magnesium, iron, and several other trace elements (14, 93)2.
Non-nutritive bioactive compounds are also present in multitude, comprising of phenolics, carotenoids, and glucosinolates. Although these bioactive compounds are regarded as non-essential for human survival, they may exert health effects such as reduced risk of non-communicable and degenerative diseases (71–76). Delivery of fiber, both digestible and indigestible, is another important nutritional aspect of vegetables and fruits. It has an important impact on satiety, gastrointestinal processing, metabolic parameters, and microbiota composition. It constitutes a group of heterogeneous polymers such as non-starch polysaccharides, cellulose, resistant starch, inulin, lignins, chitins, pectin, beta-glucans, and oligosaccharides. Dietary fiber may stimulate intestinal fermentation, thereby altering the production of microbial phenolic metabolites and enhancing mineral absorption (94, 95). However, dietary fiber can also negatively affect the absorption of nutrients because of gel formation, increased viscosity, or binding and entrapment (96–98). Other compounds present in vegetables and fruits may have negative consequences for human nutrition and health, such as alkaloids, oxalates, phytic acid, lectins, trypsin and protease inhibitors, tannins, and cyanogens. Anti-nutrients can be removed or inactivated by various food processing procedures, such as fermentation, germination, boiling, leaching, and extraction (99).
Vegetables and Fruit as Sources of Nutrients
Vitamins
Pro-Vitamin a Carotenoids
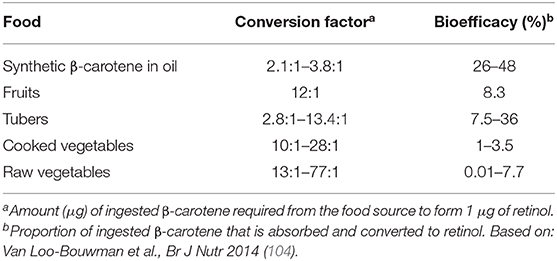
β-carotene, α-carotene and β-cryptoxanthin are the most common dietary carotenoids that can be converted to vitamin A (retinol) through central cleavage by β-carotene monooxygenase (bco1). B-carotene has the highest affinity to the cleavage enzyme, and, based on its chemical structure, can provide twice as much retinol as compared to the other two carotenoids. Therefore, and also because it is more abundant in the diet, β-carotene has received the most attention in vitamin A research. Liberation of β-carotene from the fruit or vegetable matrix is one of the main limiting steps in its bioavailability (100, 101). Green leafy vegetables, such as spinach and kale, are rich in β-carotene (Table 3), but only around 5–10% of the total content is bioavailable. In contrast, β-carotene from fruits show higher bioavailability despite their relatively lower β-carotene content (102, 103). This is explained by the digestibility of the particular plant compartment where the β-carotene is stored. Notably, green leafy vegetables store β-carotene in chloroplasts, which is not easily digestible for humans, whereas mangoes, for instance, store β-carotene in chromoplasts from which it is more readily available. Moreover, β-carotene in its crystallized form, as found in carrots, is not easily absorbed, in contrast to β-carotene present in lipid droplets as found in papaya (102, 103). The amount (μg) of β-carotene required to form 1 μg of retinol is referred to as conversion factor; this is estimated as 2.1–3.8 μg of β-carotene when it is provided as a supplement dissolved in oil (Table 4). Conversion factors for β-carotene from a wide variety of vegetables and fruits have been comprehensively summarized (104). In contrast to the earlier retinol equivalents (RE) which assumed that intake of 6 μg of β-carotene would yield 1 μg of retinol, current insights have shown that the bio-conversion efficiency is much lower for an average western diet. Therefore, new retinol activity equivalents (RAE) for β-carotene have been set at 12:1 (105). Conversion efficiency of α-carotene and β-cryptoxanthin have hardly been studied, although lately there is renewed interest in the latter (106, 107). Fat content of the diet is the most important enhancer of carotenoid absorption (108–110), whereas fiber present in the diet can reduce absorption efficiency (96).
Folate
Green leafy vegetables and citrus fruits are important dietary sources of folate (Table 3). In vegetables and fruits, folate is mostly present in its polyglutamated form. Before absorption, enzymatic cleavage of this glutamate chain by folylpoly γ-glutamyl carboxypeptidase (FGCP) is necessary. It has been shown that, as compared to supplemental folic acid, which is a monoglutamate, polyglutamated folate has a bioavailability of ~70% (111, 112). Others have shown that 5-methyl-tetrahydrofolate is the best bioavailable natural form of the vitamin (113). Folate bioavailability ranges between 60 and 98% from a diet high in vegetables and fruits (91). Whereas, the food matrix, dietary fiber, and low pH may inhibit folate bioavailability, zinc enhances FGCP activity and therefore would promote folate absorption (114). Dietary folate equivalents (DFE) have been defined as 1.7 μg of dietary folate to deliver 1 μg of folate to the body circulation (115).
Vitamin C
Certain fruits, such as kiwi and orange, but also many vegetables are rich sources of vitamin C (Table 3). Unlike some other vitamins, vitamin C derived from vegetables and fruits largely shows similar bioavailability as compared to synthetic vitamin C at 80–90% in human studies (116–118). Nevertheless, entrapment of vitamin C in the food matrix, premature degradation or inhibition by other food components may decrease its bioavailability. Vitamin C interacts with vitamin E by reducing tocopheroxyl radicals; vice versa, vitamin E might preserve vitamin C in vivo (119). Although it is uncertain if flavonoids can affect vitamin C absorption in vivo, several in vitro studies showed that flavonoids inhibit the absorption of vitamin C (120–122).
Vitamin K
Dark green leafy vegetables and herbs such as kale, parsley, spinach, and green cabbage (Table 3) are rich in phylloquinone (vitamin K1), whereas among the fruits kiwi and avocado by exception contain reasonable amounts as well (123, 124). Menaquinones (vitamin K2) are generally not found in vegetables and fruits, but an exception to this is fermented vegetables such as sauerkraut (124). Data on the bioavailability of phylloquinone from dietary sources are scarce, but some studies show <5% bioavailability from dark green leafy vegetables, while addition of fat or oils improves bioavailability markedly (124–126). Low bioavailability can be explained by binding of phylloquinone to the membranes of plant chloroplasts (127).
Minerals
Potassium
Consumption of vegetables and fruits contributes importantly to potassium intake, especially from dark green leafy vegetables and certain fruits such as banana and kiwi (Table 3). High intake of potassium has consistently been associated with reduced blood pressure and risk for hypertension (128, 129). Potassium is almost completely absorbed from dietary sources, although matrix effects may hinder potassium absorption from unprocessed vegetables and fruits to some extent. Estimates of bioavailability range between 60 and 85% from such sources (130, 131). Little is known about factors that promote or inhibit the absorption of potassium from individual dietary sources (132).
Calcium
Especially dark green leafy vegetables such as kale and spinach contribute to dietary calcium intake (Table 3). Studies have shown that calcium absorption from various vegetables is either inferior or comparable to calcium absorption from milk with bioavailability estimates ranging between 20 and 40% (133–135), although Brassica sp. vegetables showed slightly higher absorption (136). Phytate and oxalate content determine the efficiency of calcium absorption from vegetables. Phytic acid, or inositol polyphosphate, as well as oxalate, or ethanedioate, form insoluble and non-digestible complexes with divalent cations such as Fe2+, Zn2+, Ca2+, and Mg2+, which limits the bioavailability of these minerals. Oxalate is the conjugate base of oxalic acid, which is present in high amounts in certain vegetables such as spinach, cabbage, broccoli, brussels sprouts, beetroot, and rhubarb.
Magnesium
Magnesium can be derived in moderate amounts from fruits and vegetables (Table 3). Magnesium from dark green leafy vegetables was shown to have a bioavailability of 25–35% (137). Magnesium is assumed to be absorbed as the ion rather than as in the form of a complex (138). The absorption of magnesium is inhibited by oxalate (139). As explained for calcium above, oxalic acid can form indigestible complexes with divalent cations at physiological pH. It has been shown before that addition of oxalate-rich vegetables to the diet resulted in negative zinc and magnesium balances. Spinach, an oxalate rich vegetable, indeed showed lower magnesium bioavailability as compared to kale, a vegetable low in oxalate (137). Other known dietary based inhibitors of magnesium absorption are phytic acid, cellulose, lignin, and possibly pectin, whereas proteins, medium chain triglycerides, and indigestible carbohydrates are among the enhancers (139).
Iron
Green leafy vegetables are rich in iron (Table 3), but the bioavailability of iron is relatively low—around 12% (140). The low bioavailability is attributed to the indigestibility of cellular components such as chloroplasts and mitochondria where iron is stored (141). Vitamin C is well-known to aid non-heme iron bioavailability, either by enhancing iron solubility or by acting as a co-factor in the reduction of iron from the ferric to the ferrous form by duodenal cytochrome B (142, 143). Fytic acid is a strong inhibitor of iron absorption (144), whereas the inhibiting properties of oxalate are less clear. One study showed that oxalic acid did not reduce iron absorption from kale (145). A study in human volunteers showed that lactic fermentation of vegetables doubled iron absorption, which was explained by the acidic conditions that promote the presence of ferric iron, which is more stable in the gastrointestinal tract (146).
Conclusion
Both milk as well as vegetables and fruits are nutrient-dense foods that provide a myriad of nutrients which impact human metabolism and health. Bioavailability is an important explanatory step between the food source and potential health effects of its food components. Much of the health benefits of foods may be explained by additive, antagonistic and synergistic processes at the level of uptake and absorption of nutrients. As has become clear from this review, bioavailability values from whole foods have been established in humans for some nutrients, but are still lacking or need confirmation for others. Translation of this information to individual diet scores will require detailed dietary intake information, preferably at the meal level, while taking information on bioavailability of nutrients from separate foods as well as food-to-food interactions into account. This is all the more complex, since bioavailability estimates are currently already incorporated into dietary reference intakes at the population (group) level to a certain extent. Furthermore, host-related factors, e.g., nutrient status, disease state and genetics, also play an important role in nutrient uptake and bioavailability at the individual level and are often unknown. Nevertheless, accounting for nutrient bioavailability based on food intake pattern may result in better estimates of true individual absorbable nutrient intake in relation to health outcomes. Moreover, such knowledge may help in the design of foods, meals and diets that aid in the supply of nutrients to specific target groups.
Author Contributions
AM-B conducted the literature review and wrote the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with the author AM-B.
Footnotes
1. ^https://www.globaldietarydatabase.org/our-data/data-visualizations/dietary-data-region
2. ^https://wateetnederland.nl/resultaten/vitamines-en-mineralen/bronnen
References
1. Gibson R. The role of diet and host related factors in nutrient bioavailability. Food Nutr Bull. (2007) 28:S77–100. doi: 10.1177/15648265070281S108
2. Davidsson L, Tanumihardjo S. New frontiers in science and technology: nuclear techniques in nutrition. Am J Clin Nutr. (2011) 94:691S−5S. doi: 10.3945/ajcn.110.005819
3. Fairweather-Tait SJ, Lynch S, Hotz C, Hurrell RF, Abrahamse L, Beebe S, et al. The usefulness of in vitro models to assess iron and zinc bioavailability. Int J Vitam Nutr Res. (2005) 75:371–4. doi: 10.1024/0300-9831.75.6.371
4. Etcheverry P, Grusak MA, Fleige LE. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front Physiol. (2012) 3:317. doi: 10.3389/fphys.2012.00317
5. Elwood PC, Pickering JE, Ian Givens D, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. (2010) 45:925–39. doi: 10.1007/s11745-010-3412-5
6. Huth PJ, Park KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr. (2012) 3:266–85. doi: 10.3945/an.112.002030
7. de Goede J, Soedamah-Muthu SS, Pan A, Gijsbers L, Geleijnse JM. Dairy consumption and risk of stroke: a systematic review and updated dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. (2016) 5:e002787. doi: 10.1161/JAHA.115.002787
8. Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labonté M-È, Desroches S, et al. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr An Int Rev J. (2016) 7:1026–40. doi: 10.3945/an.115.011403
9. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr Res. (2016) 60:32527. doi: 10.3402/fnr.v60.32527
10. Soedamah-Muthu SS, de Goede J. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-Analyses of prospective cohort studies. Curr Nutr Rep. (2018) 7:171–82. doi: 10.1007/s13668-018-0253-y
11. Van Den Heuvel EGHM, Steijns JMJM. Dairy products and bone health: how strong is the scientific evidence? Nutr Res Rev. (2018) 31:164–78. doi: 10.1017/S095442241800001X
12. Drewnowski A. The contribution of milk and milk products to micronutrient density and affordability of the U.S. Diet J Am Coll Nutr. (2011) 30:422S−8S. doi: 10.1080/07315724.2011.10719986
13. Dugan CE, Fernandez ML. Effects of dairy on metabolic syndrome parameters: a review. J Biol Med. (2014) 87:135–47.
14. Vissers PAJ, Streppel MT, Feskens EJM, de Groot LCPGM. The contribution of dairy products to micronutrient intake in the Netherlands. J Am Coll Nutr. (2011) 30:415S−21S. doi: 10.1080/07315724.2011.10719985
15. Bath SC, Sleeth ML, McKenna M, Walter A, Taylor A, Rayman MP. Iodine intake and status of UK women of childbearing age recruited at the University of Surrey in the winter. Br J Nutr. (2014) 112:1715–23. doi: 10.1017/S0007114514002797
16. Olza J, Aranceta-Bartrina J, González-Gross M, Ortega RM, Serra-Majem L, Varela-Moreiras G, et al. Reported dietary intake and food sources of zinc, selenium, and vitamins a, e and c in the Spanish population: findings from the anibes study. Nutrients. (2017) 9:697. doi: 10.3390/nu9070697
17. Herrick KA, Perrine CG, Aoki Y, Caldwell KL. Iodine status and consumption of key iodine sources in the U.S. population with special attention to reproductive age women. Nutrients. (2018) 10:874. doi: 10.3390/nu10070874
18. Partearroyo T, De Lourdes Samaniego-Vaesken M, Ruiz E, Olza J, Aranceta-Bartrina J, Gil Á, et al. Dietary sources and intakes of folates and vitamin B12 in the Spanish population: findings from the ANIBES study. PLoS ONE. (2017) 12:e0189230. doi: 10.1371/journal.pone.0189230
19. Obeid R, Heil SG, Verhoeven MMA, van den Heuvel EGHM, de Groot LCPGM, Eussen SJPM. Vitamin B12 intake from animal foods, biomarkers, and health aspects. Front Nutr. (2019) 6:93. doi: 10.3389/fnut.2019.00093
20. Górska-Warsewicz H, Rejman K, Laskowski W, Czeczotko M. Milk and dairy products and their nutritional contribution to the average polish diet. Nutrients. (2019) 11:771. doi: 10.3390/nu11081771
21. Powers HJ. Riboflavin (vitamin B-2) and health 1, 2. Am J Clin Nutr. (2003) 77:1352–60. doi: 10.1093/ajcn/77.6.1352
22. Saedisomeolia A, Ashoori M. Riboflavin in human health: a review of current evidences. Adv Food Nutr Res. (2018) 83:57–81. doi: 10.1016/bs.afnr.2017.11.002
23. Auclair O, Han Y, Burgos SA. Consumption of milk and alternatives and their contribution to nutrient intakes among Canadian adults: evidence from the 2015. Canadian community health survey-nutrition. Nutrients. (2019) 11:1–17. doi: 10.3390/nu11081948
24. Guéguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr. (2000) 19:119S−36. doi: 10.1080/07315724.2000.10718083
25. Fishbein L. Multiple sources of dietary calcium - some aspects of its essentiality. Regul Toxicol Pharmacol. (2004) 39:67–80. doi: 10.1016/j.yrtph.2003.11.002
26. Nordin BEC, Marshall DH. Calcium in biology. In: Nordin BEC, editor. Calcium in Biology. Berlin: Springer-Verlag (1988). p. 447–71.
27. Heaney R, Recker R, Stegman M, Moy A. Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res. (1989) 4:469–75. doi: 10.1002/jbmr.5650040404
28. Avioli L. Calcium and osteoporosis. Annu Rev Nutr. (1984) 4:471–91. doi: 10.1146/annurev.nu.04.070184.002351
29. Kaushik R, Sachdeva B, Arora S, Kapila S, Wadhwa BK. Bioavailability of vitamin D2 and calcium from fortified milk. Food Chem. (2014) 147:307–11. doi: 10.1016/j.foodchem.2013.09.150
30. Lee YS, Noguchi T, Naito H. Intestinal absorption of calcium in rats given diets containing casein or amino acid mixture: the role of casein phosphopeptides. Br J Nutr. (1983) 49:67–76. doi: 10.1079/BJN19830012
31. Mykkanen H, Wasserman R. Enhanced absorption of calcium by casein phosphopeptides in rachitic and normal chicks. J Nutr. (1980) 110:2141–8. doi: 10.1093/jn/110.11.2141
32. Li Y, Tome D, Desjeux JF. Indirect effect of casein phosphopeptides on calcium absorption in rat ileum in vitro. Reprod Nutr Dev. (1989) 29:227–33. doi: 10.1051/rnd:19890210
33. Bronner F. Current concepts of calcium absorption: an overview. J Nutr. (1992) 122(Suppl. 3) :641–3. doi: 10.1093/jn/122.suppl_3.641
34. Whiting S, Draper H. The role of sulfate in the calciuria of high protein diets in adult rats. J Nutr. (1980) 110:212–22. doi: 10.1093/jn/110.2.212
35. Kerstetter J, Allen L. Protein intake and calcium homeostasis. Adv Nutr Res. (1994) 9:167–81. doi: 10.1007/978-1-4757-9092-4_10
36. Massey LK. Issues and opinions in nutrition does excess dietary protein adversely affect bone? J Nutr. (1998) 128:1054–7. doi: 10.1093/jn/128.6.1048
37. Van Beresteijn ECH, Brussaard J, Van Schaik M. Relationship between the calcium-to-protein ratio in milk and the urinary calcium excretion in healthy adults - a controlled turnover study. Am J Clin Nutr. (1990) 52:142–6. doi: 10.1093/ajcn/52.1.142
38. Linkswiler H, Zemel M, Hegsted M, Schuette S. Protein induced hypercalciuria. Fed Proc. (1981) 40:2429.
39. Hegsted M, Schuette S, Zemel M, Linkswiler H. Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake. J Nutr. (1981) 111:553–62. doi: 10.1093/jn/111.3.553
40. Allen L, Oddoye E, Margen S. Protein-induced hypercalciuria: a longer term study. Am J Clin Nutr. (1979) 32:741–9. doi: 10.1093/ajcn/32.4.741
41. Allen LH. Calcium bioavailability and absorption: a review. Am J Clin Nutr. (1982) 35:783–808. doi: 10.1093/ajcn/35.4.783
42. Miller D. Calcium in the diet; food sources, recommended intakes, and nutritional bioavailability. Adv Food Nutr Res. (1989) 33:104–55. doi: 10.1016/S1043-4526(08)60127-8
43. Pansu D, Bellaton C, Bronner F. Effect of Ca intake on saturable and nonsaturable components of duodenal Ca transport. Am J Physiol Gastrointest Liver Physiol. (1981) 3:32–7. doi: 10.1152/ajpgi.1981.240.1.G32
44. Cochet B, Jung A, Griessen M, Bartholdi P, Schaller P, Donath A. Effects of lactose on intestinal calcium absorption in normal and lactase-deficient subjects. Gastroenterology. (1983) 84:935–40. doi: 10.1016/0016-5085(83)90194-4
45. Griessen M, Cochet B, Infante F, Jung A, Bartholdi P, Donath A, et al. Calcium absorption from milk in lactase-deficient subjects. Am J Clin Nutr. (1989) 49:377–84. doi: 10.1093/ajcn/49.2.377
46. Tremaine WJ, Newcomer AD, Lawrence Riggs B, McGill DB. Calcium absorption from milk in lactase-deficient and lactase-sufficient adults. Dig Dis Sci. (1986) 31:376–8. doi: 10.1007/BF01311672
47. Nickel KP, Martin BR, Smith DL, Smith JB, Miller GD, Weaver CM. Calcium bioavailability from bovine milk and dairy products in premenopausal women using intrinsic and extrinsic labeling techniques. J Nutr. (1996) 126:1406–11. doi: 10.1093/jn/126.5.1406
48. Schuette S, Yasillo N, Thompson C. The effect of carbohydrates in milk on the absorption of calcium by postmenopausal women. J Am Coll Nutr. (1991) 10:132–9. doi: 10.1080/07315724.1991.10718137
49. Abrams SA, Griffin IJ, Davila PM. Calcium and zinc absorption from lactose-containing and lactose-free infant formulas. Am J Clin Nutr. (2002) 76:442–6. doi: 10.1093/ajcn/76.2.442
50. Szilagyi A. Review article: lactose - A potential prebiotic. Aliment Pharmacol Ther. (2002) 16:1591–602. doi: 10.1046/j.1365-2036.2002.01321.x
51. Whisner CM, Martin BR, Schoterman MHC, Nakatsu CH, McCabe LD, McCabe GP, et al. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: a double-blind cross-over trial. Br J Nutr. (2013) 110:1292–303. doi: 10.1017/S000711451300055X
52. Hodges JK, Cao S, Cladis DP, Weaver CM. Lactose intolerance and bone health: the challenge of ensuring adequate calcium intake. Nutrients. (2019) 11:718. doi: 10.3390/nu11040718
53. Rizzoli R, Biver E, Bonjour JP, Coxam V, Goltzman D, Kanis JA, et al. Benefits and safety of dietary protein for bone health—an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteopororosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos Int. (2018) 29:1933–48. doi: 10.1007/s00198-018-4534-5
54. Bonjour JP, Kraenzlin M, Levasseur R, Warren M, Whiting S. Dairy in adulthood: from foods to nutrient interactions on bone and skeletal muscle health. J Am Coll Nutr. (2013) 32:251–63. doi: 10.1080/07315724.2013.816604
55. Buchowski MS, Miller DD. Calcium bioavailability from ripening cheddar cheese. J Food Sci. (1990) 55:1293–5. doi: 10.1111/j.1365-2621.1990.tb03919.x
56. Spencer H, Kramer L, Osis D. Do protein and phosphorus cause calcium loss? J Nutr. (1988) 118:657–60. doi: 10.1093/jn/118.6.657
57. Calvo MS, Tucker KL. Is phosphorus intake that exceeds dietary requirements a risk factor in bone health? Ann N Y Acad Sci. (2013) 1301:29–35. doi: 10.1111/nyas.12300
58. Takeda E, Yamamoto H, Yamanaka-Okumura H, Taketani Y. Dietary phosphorus in bone health and quality of life. Nutr Rev. (2012) 70:311–21. doi: 10.1111/j.1753-4887.2012.00473.x
59. Recker R, Heaney R. The effect of milk supplements on calcium metabolism, bone metabolism and calcium balance. Am J Clin Nutr. (1985) 41:254–63. doi: 10.1093/ajcn/41.2.254
60. Herrero C, Granado F, Blanco I, Olmedilla B. Vitamin A and E content in dairy products: their contribution to the recommended dietary allowances (RDA) for elderly people. J Nutr Heal Aging. (2002) 6:57–9. doi: 10.1007/s00394-006-0612-0
61. Dainty JR, Bullock NR, Hart DJ, Hewson AT, Turner R, Finglas PM, et al. Quantification of the bioavailability of riboflavin from foods by use of stable-isotope labels and kinetic modeling. Am J Clin Nutr. (2007) 85:1557–64. doi: 10.1093/ajcn/85.6.1557
62. Gille D, Schmid A. Vitamin B12 in meat and dairy products. Nutr Rev. (2015) 73:106–15. doi: 10.1093/nutrit/nuu011
63. Fedosov SN, Nexo E, Heegaard CW. Vitamin B 12 and its binding proteins in milk from cow and buffalo in relation to bioavailability of B 12. J Dairy Sci. (2019) 102:4891–905. doi: 10.3168/jds.2018-15016
64. Russell RM, Baik H, Kehayias JJ. Older men and women efficiently absorb vitamin B-12 from milk and fortified bread. J Nutr. (2001) 131:291–3. doi: 10.1093/jn/131.2.291
65. Doets EL, In't Veld PH, Szczecinska A, Dhonukshe-Rutten RAM, Cavelaars AEJM, Van 't Veer P, et al. Systematic review on daily vitamin B 12 losses and bioavailability for deriving recommendations on vitamin B 12 intake with the factorial approach. Ann Nutr Metab. (2013) 62:311–22. doi: 10.1159/000346968
66. Naik S, Mahalle N, Greibe E, Ostenfeld MS, Heegaard CW, Nexo E, et al. Hydroxo-B12 for supplementation in B12 deficient lactovegetarians. (2019) 12:1–14. doi: 10.3390/nu11102382
67. Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. (2013) 4:463–73. doi: 10.3945/an.113.003855
68. Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MHJ, van der Meer IM, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam study. J Nutr. (2004) 134:3100–5. doi: 10.1093/jn/134.11.3100
69. Gast GCM, de Roos NM, Sluijs I, Bots ML, Beulens JWJ, Geleijnse JM, et al. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. (2009) 19:504–10. doi: 10.1016/j.numecd.2008.10.004
70. Fu X, Harshman SG, Shen X, Haytowitz DB, Karl JP, Wolfe BE, et al. Multiple vitamin K forms exist in dairy foods. Curr Dev Nutr. (2017) 1:e000638. doi: 10.3945/cdn.117.000638
71. Vermeer C, Raes J, van 't Hoofd C, Knapen MHJ, Xanthoulea S. Menaquinone content of cheese. Nutrients. (2018) 10:446. doi: 10.3390/nu10040446
72. Knapen MHJ, Braam LAJLM, Teunissen KJ, Van't Hoofd CM, Zwijsen RML, Van Den Heuvel EGHM, et al. Steady-state vitamin K2 (menaquinone-7) plasma concentrations after intake of dairy products and soft gel capsules. Eur J Clin Nutr. (2016) 70:831–6. doi: 10.1038/ejcn.2016.3
73. Marles RJ, Roe AL, Oketch-Rabah HA. US pharmacopeial convention safety evaluation of menaquinone-7, a form of vitamin K. Nutr Rev. (2017) 75:553–78. doi: 10.1093/nutrit/nux022
74. St-Jules DE, Jagannathan R, Gutekunst L, Kalantar-Zadeh K, Sevick MA. Examining the proportion of dietary phosphorus from plants, animals, and food additives excreted in urine. J Ren Nutr. (2017) 27:78–83. doi: 10.1053/j.jrn.2016.09.003
75. McClure ST, Rebholz CM, Phillips KM, Champagne CM, Selvin E, Appel LJ. The percentage of dietary phosphorus excreted in the urine varies by dietary pattern in a randomized feeding study in adults. J Nutr. (2019) 149:816–23. doi: 10.1093/jn/nxy318
76. Blakeborough P, Salter DN, Gurr MI. Zinc binding in cow's milk and human milk. Biochem J. (1983) 209:505–12. doi: 10.1042/bj2090505
77. Sandström B, Cederblad ALB. Zinc absorption from human milk, cow's milk, and infant formulas. Am J Dis Child. (1983) 137:726–9. doi: 10.1001/archpedi.1983.02140340010002
78. Talsma EF, Moretti D, Ly SC, Dekkers R, van den Heuvel EGHM, Fitri A, et al. Zinc absorption from milk is affected by dilution but not by thermal processing, and milk enhances absorption of zinc from high-phytate rice in young Dutch women. J Nutr. (2017) 147:1086–93. doi: 10.3945/jn.116.244426
79. Lönnerdal B. Dietary factors influencing zinc absorption 1. J Nutr. (2000) 130:1378S−83S. doi: 10.1093/jn/130.5.1378S
80. Ekmekcioglu C. Intestinal bioavailability of minerals and trace elements from milk and beverages in humans. Nahrung Food. (2000) 44:390–7. doi: 10.1002/1521-3803(20001201)44:6<390::AID-FOOD390>3.0.CO;2-Y
81. Vegarud GE, Langsrud T, Svenning C. Mineral-binding milk proteins and peptides; occurrence, biochemical and technological characteristics. Br J Nutr. (2000) 84:91–8. doi: 10.1017/S0007114500002300
82. Brink EJ, Dekker PR, Van Beresteijn ECH, Beynen AC. Bioavailability of magnesium and calcium from cow's milk and soya-bean beverage in rats. Br J Nutr. (1992) 68:271–82. doi: 10.1079/BJN19920084
83. Brink EJ, van Beresteijn ECH, Dekker PR, Beynen AC. Urinary excretion of magnesium and calcium as an index of absorption is not affected by lactose intake in healthy adults. Br J Nutr. (1993) 69:863–70. doi: 10.1079/BJN19930086
84. van den Heuvel EGHM, Muijs T, Brouns F, Hendriks HFJ. Short-chain fructo-oligosaccharides improve magnesium absorption in adolescent girls with a low calcium intake. Nutr Res. (2009) 29:229–37. doi: 10.1016/j.nutres.2009.03.005
85. Whisner CM, Castillo LF. Prebiotics, bone and mineral metabolism. Calcif Tissue Int. (2018) 102:443–79. doi: 10.1007/s00223-017-0339-3
86. van der Reijden OL, Zimmermann MB, Galetti V. Iodine in dairy milk: sources, concentrations and importance to human health. Best Pract Res Clin Endocrinol Metab. (2017) 31:385–95. doi: 10.1016/j.beem.2017.10.004
87. van de Kamp ME, Saridakis I, Verkaik-Kloosterman J. Iodine content of semi-skimmed milk available in the Netherlands depending on farming (organic versus conventional) and heat treatment (pasteurized versus UHT) and implications for the consumer. J Trace Elem Med Biol. (2019) 56:178–83. doi: 10.1016/j.jtemb.2019.08.008
88. Van Der Reijden OL, Galetti V, Bürki S, Zeder C, Krzystek A, Haldimann M, et al. Iodine bioavailability from cow milk: a randomized, crossover balance study in healthy iodine-replete adults. Am J Clin Nutr. (2019) 110:102–10. doi: 10.1093/ajcn/nqz092
89. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum NN, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-A systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. (2017) 46:1029–56. doi: 10.1093/ije/dyw319
90. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. (2014) 349:g4490. doi: 10.1136/bmj.g4490
91. Hall JN, Moore S, Harper SB, Lynch JW. Global variability in fruit and vegetable consumption. Am J Prev Med. (2009) 36:402–9.e5 doi: 10.1016/j.amepre.2009.01.029
92. Lock K, Pomerleau J, Causer L, Altmann DR, Mckee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull WHO. (2005) 83:100–8.
93. Auestad N, Hurley JS, Fulgoni VL, Schweitzer CM. Contribution of food groups to energy and nutrient intakes in five developed countries. Nutrients. (2015) 7:4593–618. doi: 10.3390/nu7064593
94. Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. (2017) 105:10–22. doi: 10.3945/ajcn.116.136051
95. Scholz-Ahrens KE, Schrezenmeir J. Inulin, oligofructose and mineral metabolism — experimental data and mechanism. Br J Nutr. (2002) 87:S179–86. doi: 10.1079/BJN/2002535
96. Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr. (1999) 129:2170–6. doi: 10.1093/jn/129.12.2170
97. Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev. (2014) 72:429–52. doi: 10.1111/nure.12114
98. Bohn T. Bioavailability of Non-Provitamin A carotenoids. Curr Nutr Food Sci. (2008) 4:240–58. doi: 10.2174/157340108786263685
99. Sandberg A. The effect of food processing on phytate hydrolysis and availability of iron and zinc. Adv Exp Med Biol. (1991) 289:499–508. doi: 10.1007/978-1-4899-2626-5_33
100. Castenmiller JJM, West CE, Linssen JPH, van het Hof KH, Voragen AGJ. The food matrix of spinach is a limiting factor in determining the bioavailability of β-Carotene and to a lesser extent of lutein in humans. J Nutr. (1999) 129:349–55. doi: 10.1093/jn/129.2.349
101. van het Hof KH, Tijburg LBM, Pietrzik K, Weststrate JA. Influence of feeding different vegetables on plasma levels of carotenoids, folate and vitamin C. Effect of disruption of the vegetable matrix. Br J Nutr. (1999) 82:203–12. doi: 10.1017/S0007114599001385
102. Schweiggert RM, Mezger D, Schimpf F, Steingass CB, Carle R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. (2012) 135:2736–42. doi: 10.1016/j.foodchem.2012.07.035
103. Schweiggert RM, Kopec RE, Villalobos-Gutierrez MG, Högel J, Quesada S, Esquivel P, et al. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: a randomised cross-over study. Br J Nutr. (2014) 111:490–8. doi: 10.1017/S0007114513002596
104. Van Loo-Bouwman CA, Naber THJ, Schaafsma G. A review of vitamin A equivalency of β-carotene in various food matrices for human consumption. Br J Nutr. (2014) 111:2153–66. doi: 10.1017/S0007114514000166
105. Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Washington DC: National Academy Press (2001).
106. Burri BJ. Beta-cryptoxanthin as a source of vitamin A. J Sci Food Agric. (2015) 95:1786–94. doi: 10.1002/jsfa.6942
107. Burri BJ, La Frano MR, Zhu C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr Rev. (2016) 74:69–82. doi: 10.1093/nutrit/nuv064
108. Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, et al. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. (2004) 80:396–403. doi: 10.1093/ajcn/80.2.396
109. Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res. (2012) 56:866–77. doi: 10.1002/mnfr.201100687
110. White WS, Zhou Y, Crane A, Dixon P, Quadt F, Flendrig LM. Modeling the dose effects of soybean oil in salad dressing on carotenoid and fat-soluble vitamin bioavailability in salad vegetables. Am J Clin Nutr. (2017) 106:1041–51. doi: 10.3945/ajcn.117.153635
111. Melse-Boonstra A, Verhoef P, West CE, Van Rhijn JA, Van Breemen RB, Lasaroms JJP, et al. A dual-isotope-labeling method of studying the bioavailability of hexaglutamyl folic acid relative to that of monoglutamyl folic acid in humans by using multiple orally administered low doses. Am J Clin Nutr. (2006) 84:1128–33. doi: 10.1093/ajcn/84.5.1128
112. Melse-Boonstra A, West CE, Katan MB, Kok FJ, Verhoef P. Bioavailability of heptaglutamyl relative to monoglutamyl folic acid in healthy adults. Am J Clin Nutr. (2004) 79:424–9. doi: 10.1093/ajcn/79.3.424
113. Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinetics. (2010) 49:535–48. doi: 10.2165/11532990-000000000-00000
114. Melse-Boonstra A. Dietary Folate: Bioavailability Studies in Humans (PhD thesis). Wageningen University (2003). p. 1–166.
115. Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington DC: National Academy Press. (1998).
116. Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C [13]. Nature. (1979) 278:737–8. doi: 10.1038/278737a0
117. Carr AC, Vissers MCM. Synthetic or food-derived vitamin C-are they equally bioavailable? Nutrients. (2013) 5:4284–304. doi: 10.3390/nu5114284
118. Padayatty SJ, Levine M. Vitamin C: the known and the unknown and goldilocks. Oral Dis. (2016) 22:463–93. doi: 10.1111/odi.12446
119. Tanaka K, Hashimoto T, Tokumaru S, Iguchi H, Kojo S. Interactions between vitamin C and vitamin E are observed in tissues of inherently scorbutic rats. J Nutr. (1997) 127:2060–4. doi: 10.1093/jn/127.10.2060
120. Song J, Kwon O, Chen S, Daruwala R, Eck P, Park JB, et al. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J Biol Chem. (2002) 277:15252–60. doi: 10.1074/jbc.M110496200
121. Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, et al. 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na +-dependent vitamin C transporter but not glucose transporter pathways. J Biol Chem. (2005) 280:5211–20. doi: 10.1074/jbc.M412925200
122. Park JB, Levine M. Intracellular accumulation of ascorbic acid is inhibited by flavonoids via blocking of dehydroascorbic acid and ascorbic acid uptakes in HL-60, U937 and jurkat cells. J Nutr. (2000) 130:1297–302. doi: 10.1093/jn/130.5.1297
123. Bolton-Smith C, Price RJG, Fenton ST, Harrington DJ, Shearer MJ. Compilation of a provisional UK database for the phylloquinone (vitamin K1) content of foods. Br J Nutr. (2000) 83:389–99.
124. Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, et al. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int J Mol Sci. (2019) 20:896. doi: 10.3390/ijms20040896
125. Gijsbers BLMG, Jie K-SG, Vermeer C. Effect of food composition on vitamin K absorption in human volunteers. Br J Nutr. (1996) 76:223–9. doi: 10.1079/BJN19960027
126. Novotny JA, Kurilich AC, Britz SJ, Baer DJ, Clevidence BA. Vitamin K absorption and kinetics in human subjects after consumption of 13C-labelled phylloquinone from kale. Br J Nutr. (2010) 104:858–62. doi: 10.1017/S0007114510001182
127. Beulens JWJ, Booth SL, Van Den Heuvel EGHM, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr. (2013) 110:1357–68. doi: 10.1017/S0007114513001013
128. Geleijnse JM, Kok FJ, Grobbee DE. Blood pressure response to changes in sodium and potassium intake: a metaregression analysis of randomised trials. J Hum Hypertens. (2003) 17:471–80. doi: 10.1038/sj.jhh.1001575
129. Binia A, Jaeger J, Hu Y, Singh A, Zimmermann D. Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. (2015) 33:1509–20. doi: 10.1097/HJH.0000000000000611
130. Naismith DJ, Braschi A. An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int J Food Sci Nutr. (2008) 59:438–50. doi: 10.1080/09637480701690519
131. MacDonald-Clarke CJ, Martin BR, McCabe LD, McCabe GP, Lachcik PJ, Wastney M, et al. Bioavailability of potassium from potatoes and potassium gluconate: a randomized dose response trial. Am J Clin Nutr. (2016) 104:346–53. doi: 10.3945/ajcn.115.127225
132. Stone MS, Martyn L, Weaver CM. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients. (2016) 8:444. doi: 10.3390/nu8070444
133. Heaney RP, Weaver CM. Calcium absorption from kale. Am J Clin Nutr. (1990) 51:656–7. doi: 10.1093/ajcn/51.4.656
134. Weaver CM, Heaney RP, Nickel KP, Packard PI. Calcium bioavailability from high oxalate vegetables: Chinese vegetables, sweet potatoes and rhubarb. J Food Sci. (1997) 62:524–5. doi: 10.1111/j.1365-2621.1997.tb04421.x
135. Charoenkiatkul S, Kriengsinyos W, Tuntipopipat S, Suthutvoravut U, Weaver CM. Calcium absorption from commonly consumed vegetables in healthy Thai women. J Food Sci. (2008) 73:H218–21. doi: 10.1111/j.1750-3841.2008.00949.x
136. Heaney RP, Weaver CM, Hinders S, Martin B, Packard PT. Absorbability of calcium from brassica vegetables: broccoli, bok choy, and kale. J Food Sci. (1993) 58:1378–80. doi: 10.1111/j.1365-2621.1993.tb06187.x
137. Bohn T, Davidsson L, Walczyk T, Hurrell RF. Fractional magnesium absorption is significantly lower in human subjects from a meal served with an oxalate-rich vegetable, spinach, as compared with a meal served with kale, a vegetable with a low oxalate content. Br J Nutr. (2004) 91:601–6. doi: 10.1079/BJN20031081
138. Vormann J. Magnesium : nutrition and metabolism. Mol Aspects Med. (2003) 24:27–37. doi: 10.1016/S0098-2997(02)00089-4
139. Schuchardt JP, Hahn A. Intestinal absorption and factors influencing bioavailability of magnesium - An update. Curr Nutr Food Sci. (2017) 13:260–78. doi: 10.2174/1573401313666170427162740
140. McMillan T, Johnston F. The absorption of iron from spinach by six young women, and the effect of beef upon the absorption. J Nutr. (1951) 44:383–98. doi: 10.1093/jn/44.3.383
141. Crispin DJ, Street G, Varey JE. Kinetics of the decomposition of [2Fe-2S] ferredoxin from spinach: implications for iron bioavailability and nutritional status. Food Chem. (2001) 72:355–62. doi: 10.1016/S0308-8146(00)00236-3
142. Hallberg L, Brune M, Rossander L. Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum Nutr Appl Nutr. (1986) 40:97–113.
143. Lane DJR, Bae DH, Merlot AM, Sahni S, Richardson DR. Duodenal cytochrome b (DCYTB) in iron metabolism: an update on function and regulation. Nutrients. (2015) 7:2274–96. doi: 10.3390/nu7042274
144. Hallberg L, Brune M, Rossander L. Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr. (1989) 49:140–4. doi: 10.1093/ajcn/49.1.140
145. Bonsmann SSG, Walczyk T, Renggli S, Hurrell RF. Oxalic acid does not influence nonhaem iron absorption in humans: a comparison of kale and spinach meals. Eur J Clin Nutr. (2008) 62:336–41. doi: 10.1038/sj.ejcn.1602721
Keywords: bioavailability, vitamins, minerals, dairy, fruits, vegetables
Citation: Melse-Boonstra A (2020) Bioavailability of Micronutrients From Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 7:101. doi: 10.3389/fnut.2020.00101
Received: 04 December 2019; Accepted: 05 June 2020;
Published: 24 July 2020.
Edited by:
Ellen G. H. M. Van Den Heuvel, FrieslandCampina, NetherlandsReviewed by:
Richard Hurrell, ETH Zürich, SwitzerlandEster Betoret, Consejo Superior de Investigaciones Científicas (CSIC), Spain
Copyright © 2020 Melse-Boonstra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alida Melse-Boonstra, alida.melse@wur.nl
 Alida Melse-Boonstra
Alida Melse-Boonstra