
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr. , 08 May 2019
Sec. Clinical Nutrition
Volume 6 - 2019 | https://doi.org/10.3389/fnut.2019.00066
This article is part of the Research Topic Vegetarian Dietary Patterns in the Prevention and Treatment of Disease View all 11 articles
Background: The energy content of whole, fresh fruit derives primarily from simple sugars, which are currently under heightened scrutiny for their potential contribution to obesity and chronic disease risk. Yet fruit also has a relatively low energy density, moderate palatability/reward value, and high fiber content, which together may limit energy intake. Although reasoned arguments can be made that fruit is fattening or slimming, the question is best resolved empirically.
Methods: Methods were preregistered with PROSPERO (CRD42018111830). The primary outcome is the impact of whole, fresh fruit consumption on measures of adiposity including body weight in randomized controlled trials (RCTs). Secondary outcomes are the impact of whole, fresh fruit consumption on energy intake in RCTs, and the association between whole, fresh fruit consumption and changes in measures of adiposity in prospective observational studies. CENTRAL and PubMed databases were searched through October 2018. Cochrane risk of bias tool was used to assess risk of bias in RCTs, and the GRADE method was used to judge and convey the certainty of conclusions. Reporting follows PRISMA guidelines.
Results: RCTs, and particularly those of higher quality, suggest that increasing whole, fresh fruit consumption promotes weight maintenance or modest weight loss over periods of 3–24 weeks (moderate certainty), with limited evidence suggesting that a high intake of fruit favors weight loss among people with overweight or obesity. Consistent with this, single-meal RCTs suggest that consuming whole, fresh fruit tends to decrease energy intake, particularly when consumed prior to a meal or when displacing more energy-dense foods (moderate certainty). Prospective observational studies suggest that habitually higher fruit intake is not associated with weight change, or is associated with modest protection against weight gain, over five or more years.
Conclusions: Current evidence suggests that whole, fresh fruit consumption is unlikely to contribute to excess energy intake and adiposity, but rather has little effect on these outcomes or constrains them modestly. Single-meal RCTs, RCTs lasting 3–24 weeks, and long-term observational studies are relatively consistent in supporting this conclusion. Whole, fresh fruit probably does not contribute to obesity and may have a place in the prevention and management of excess adiposity.
Worldwide, the total per capita burden of disease continues to decline, but it does not do so uniformly. Technological and economic progress have substantially relieved the ancient burdens of starvation, infectious disease, and accidents, yet they have ushered in a new era of non-communicable disorders such as obesity, diabetes, and coronary heart disease (1, 2). A key contributor to these disorders is the overconsumption of energy and accumulation of excess adipose tissue (3–6).
Sucrose and other simple sugars with a sweet taste, henceforth “sugar,” have long been suspected as a culprit in energy overconsumption and excess adiposity. In 1980, the United States Department of Agriculture Dietary Guidelines advised the public to “avoid too much sugar” and, as part of a four-point plan for reducing weight, “eat less sugar and sweets” (7). Yet scrutiny of sugar has intensified recently, both within the scientific community and outside it, with certain researchers and popular writers arguing that sugar is a particularly potent driver of obesity and non-communicable disease risk (8–10). Observational and experimental findings indeed suggest that in sufficient quantity, refined sugar can increase energy intake and adiposity (11, 12). The energy content of sweet fruits is primarily in the form of sugar. If sugar is a particularly potent driver of obesity and non-communicable disease risk, this raises the possibility that even whole, fresh fruit may have similar effects, and that conventional advice to increase fruit consumption may be misguided.
On the other hand, fruit differs from refined sugar-containing foods in important respects, such as its lower energy density, lower palatability/reward value, higher fiber content, and higher concentration of essential and non-essential micronutrients. Some of these properties are expected to limit energy intake and adiposity, and together they may render fruit slimming relative to other commonly-eaten foods. Furthermore, the human evolutionary lineage has likely consumed substantial quantities of fruit for tens of millions of years prior to the emergence of obesity and cardiometabolic disease as common health problems, suggesting that it is unlikely to be a major contributor to these conditions (13).
Although reasoned arguments can be made that fruit is fattening or slimming, the question is best resolved empirically. Previous reviews have addressed similar topics (14–17), concluding that fruit aids in the prevention of excess energy intake, is unlikely to increase adiposity, and may reduce adiposity. However, the current review is the most recent to comprehensively review the randomized controlled trial (RCT) and prospective observational literature on whole, fresh fruit intake specifically. Further, it employs best-practice systematic review methods including detailed preregistration of methods with a well-defined search strategy, adherence to PRISMA reporting guidelines, assessment of study bias using the Cochrane risk of bias tool, and assessment of certainty of conclusions using the GRADE method.
The objective of this review is to systematically review the randomized controlled trial and prospective observational research literature on the impact of whole, fresh fruit consumption on energy intake and measures of adiposity, and synthesize available studies to form overall conclusions. All studies involving human subjects are considered, and RCTs must report between-group comparisons that isolate differences in whole, fresh fruit consumption as a variable.
What is the impact of whole, fresh fruit consumption on energy intake and adiposity?
A protocol for this review was preregistered in the PROSPERO systematic review registry prior to initiating literature searches (CRD42018111830)1, with the exception of brief preliminary searches used to develop the search method. The primary outcome is the impact of whole, fresh fruit consumption on measures of adiposity, as measured by RCTs. A secondary outcome is the impact of whole, fresh fruit consumption on energy intake, as measured by RCTs. An additional secondary outcome is the association of whole, fresh fruit intake with changes of measures of adiposity, as measured by prospective observational studies.
“Fruit” is defined in the common/culinary sense of a sweet, seed-bearing plant tissue. Fruit varieties that lack seeds are included. “Whole” and “fresh” denote fruit that has not been significantly processed, i.e., raw and whole rather than cooked, pureed, dried, juiced, or powdered. Raw fruit that has been peeled or cut into bite-sized pieces is included. “Change in adiposity” is defined as change in body weight and/or other direct or indirect measures of body fatness, including but not limited to body fat percentage, body mass index, and waist circumference. “RCT” is defined as a study that assigns subjects to different intervention conditions using randomization or pseudorandomization, such that outcomes can be compared between randomized groups. “Prospective observational study” is defined as a non-intervention study that collects data on exposure variables at an earlier time point, and outcomes at a later time point, and reports the association between the two.
Studies were required to be (1) RCTs on the impact of whole, fresh fruit consumption on measures of adiposity including body weight, (2) RCTs on the impact of whole, fresh fruit consumption on energy intake, or (3) prospective observational studies on the association between fruit consumption and body weight and/or adiposity. Only published studies were considered. No language restrictions were applied. All RCTs and prospective observational studies conducted in humans and published through October 2018 were considered.
To be eligible, RCTs must include a between-group difference in fruit intake. The experimental design must isolate between-group differences in fruit intake as a variable, without substantial concurrent between-group differences in other variables such as vegetable intake. An equivalent non-fruit intervention in a comparison group, such as increased nut intake, is permitted. Energy intake of diets must not be strictly controlled to permit differences in energy intake and adiposity to arise. RCTs must report differences in measures of adiposity, or energy intake, between groups, or such differences must be calculable from data available in the manuscript. Changes in weight and/or adiposity must be measured with a minimum of 2 weeks between baseline and end line to allow meaningful differences in adiposity to emerge, while energy intake RCTs can represent any duration.
For observational outcomes, studies must be prospective observational studies that report the association between the consumption of fresh, whole fruit consumption and subsequent changes in measures of adiposity including body weight. During the course of study selection, the author felt it was appropriate to add one exclusion criterion that was not prespecified: observational studies were excluded if they were potentially confounded by a concurrent intervention. For example, an observational study that reports the association between fruit intake and weight change may be confounded if it is conducted in subjects that received advice to increase fruit intake as part of a weight loss intervention. This criterion excluded four studies, whose findings are broadly similar to those that met inclusion criteria (18–21).
The search strategy was designed in collaboration with Ben Harnke, Education and Reference Librarian, the University of Colorado Health Sciences Library. RCT searches were conducted in the Cochrane controlled register of trials (CENTRAL), and prospective observational study searches were conducted in PubMed. CENTRAL compiles RCTs from multiple sources including PubMed, Embase, Clinicaltrials.gov, handsearches, and other biomedical resources. Brief preliminary searches were used to develop the search method by verifying that studies identified in previous review papers were present (14–16). Formal searches were conducted in November 2018, following preregistration.
The adiposity RCT search employed the following search terms in CENTRAL: ((Fruit OR fruits):ti,ab OR [mh Fruit]) AND ((weight OR “body mass index” OR BMI OR “waist circumference” OR “body fat” OR adiposity OR overweight OR obes* OR leanness OR Overnutrition OR “over nutrition”):ti,ab OR [mh ”Body Weight”] OR [mh “Body Weight Changes”] OR [mh “Overweight”] OR [mh “Thinness”] OR [mh “Body Fat Distribution”]).
The energy intake RCT search employed the following search terms in CENTRAL: ((Fruit OR fruits):ti,ab OR [mh Fruit]) AND ((“energy” OR calorie* OR caloric OR kilocalorie* OR kcal OR “joule” OR kilojoule OR kJ):tiab) AND ((intake OR consum* OR ingest* OR ate OR eat OR eating):ti,ab OR [mh “Energy Intake”] OR [mh Eating]).
The prospective observational study search employed the following search terms in PubMed, in addition to the “observational study” publication type filter: (Fruit[tiab] OR fruits[tiab] OR “Fruit”[Mesh:NoExp]) AND (weight[tiab] OR “body mass index”[tiab] OR BMI[tiab] OR “waist circumference”[tiab] OR “body fat”[tiab] OR adiposity[tiab] OR overweight[tiab] OR obes*[tiab] OR leanness[tiab] OR Overnutrition[tiab] OR “over nutrition”[tiab] OR “Body Weight”[Mesh:NoExp] OR “Body Weight Changes”[Mesh] OR “Overweight”[Mesh] OR “Thinness”[Mesh] OR “Body Fat Distribution”[Mesh]) AND (prospective*[tiab] OR cohort[tiab] OR longitudinal[tiab] OR follow up stud*[tiab] OR followup stud*[tiab] OR incidence stud*[tiab] OR “Cohort Studies”[Mesh]).
In addition, previous review papers on fruit, energy intake, and adiposity were hand searched for relevant studies (14–16).
After search records were identified, SG examined titles and abstracts for studies that met inclusion criteria. Potential candidates were compiled in Excel spreadsheets for examination of full text. SG then examined the full text of each study to verify that inclusion criteria were satisfied, resulting in the exclusion of some studies.
Data were extracted from studies that met inclusion criteria into Excel spreadsheets and Cochrane Review Manager 5.3. Data presented in manuscripts were taken at face value and authors were not contacted for additional information. For RCTs, the following data were extracted: first author, year of publication, number and characteristics of subjects, summary of intervention, duration of intervention, between-group difference in weight with statistical significance, between-group difference in other measures of adiposity with statistical significance, Cochrane risk of bias assessment (based on random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting), additional noteworthy study features. When not directly reported, between-group differences in measures of adiposity and energy intake were calculated based on available data whenever possible.
For observational studies, the following data were extracted: first author, year, number and characteristics of subjects, measure of fruit intake, length of follow-up, covariates accounted for, association between fruit consumption and weight change with statistical significance, association between fruit consumption and other measures of adiposity with statistical significance, additional noteworthy study features. Data from the most adjusted model that does not include energy intake as a covariate were generally preferred, since energy intake is a mechanism by which fruit intake may impact body weight. If an unadjusted or minimally adjusted model was the only one available without energy intake as a covariate, the most adjusted model was selected.
Body mass index measures were only collected if body weight was not reported, since the two measures are redundant in adults who have achieved their final height.
Risk of bas was estimated using the Cochrane risk of bias tool for individual studies, according to Cochrane guidelines2 and using Cochrane Review Manager 5.3, and GRADE was used to judge and communicate the certainty of conclusions for each outcome3 Risk of bias score was used as a criterion to weight the informativeness of each study when synthesizing conclusions, and GRADE was used to judge and communicate the certainty of each conclusion. Risk of bias graphs and summaries were created using Cochrane Review Manager 5.3.
This review employed a narrative synthesis method whereby study quality was evaluated and conclusions were informally weighted according to study quality (informal, i.e., a quantitative method was not applied to weight the informativeness of individual studies). Among the three outcomes considered, the preregistered primary outcome was assigned the highest weight. Reporting follows PRISMA guidelines (22). Quantitative meta-analysis of RCTs was judged to be suboptimal in this context, given the limited number of studies identified, the fact that the study pool would have been further narrowed by applying more stringent meta-analysis inclusion criteria, and widely varying study methods and quality. Although meta-analyses are commonly performed on fewer than ten studies, typical methods for accomplishing this do not adequately control the false positive rate, particularly in the context of high heterogeneity (23). Finally, given the high risk of bias of most studies identified, the author believes it is more informative to focus on high-quality trials than to pool their findings with less informative trials.
Figure 1 summarizes the search and study selection process using the PRISMA flow diagram. 4,264 records were identified in database searches, 1,671 for body weight RCTs, 848 for energy intake RCTs, and 1,745 for prospective observational studies. 4,201 records were excluded on the basis of title and abstract contents, leaving 63 potentially eligible studies; 16 body weight RCTs, 10 energy intake RCTs, and 37 observational studies. Many studies were excluded on first pass because they did not isolate whole, fresh fruit intake as a variable; for example, they reported associations between combined fruit/vegetable intake and weight outcomes. Upon inspection of full-text manuscripts, 41 studies met inclusion criteria; 11 body weight RCTs, 5 energy intake RCTs, and 25 observational studies. Reasons for exclusion were that studies were duplicates (n = 3), interventions were not long enough to meet inclusion criteria (n = 1), interventions involved processed rather than whole, fresh fruit (n = 2), studies did not report data that are pertinent to this review (n = 8), observational studies were potentially confounded by a concurrent intervention (n = 4), and observational studies reported data in a cross-sectional rather than prospective manner (n = 4).
RCTs reporting the impact of whole, fresh fruit consumption on body weight and adiposity were published between 1992 and 2016 and are summarized in Table 1. Of the 11 RCTs identified, two included fewer than 20 subjects, four included 21–50 subjects, two included 51–100 subjects, and three included more than 100 subjects. Interventions represented a variety of fruit types, but guava (n = 3), apple (n = 2), and grapefruit (n = 2) were named more commonly than other fruit. The quantity of fruit varied widely, with the highest intake representing a goal of 15 percent of energy intake from fruit-derived fructose, which implies ~30 percent of total energy intake from fruit. Interventions lasted 3–24 weeks, with 6 and 12 weeks being most common. All RCTs reported weight changes, and six also reported changes in other measures of adiposity. Four trials reported performing a power calculation as part of trial design, and three trials were preregistered, of which two were preregistered for an adiposity-related outcome.
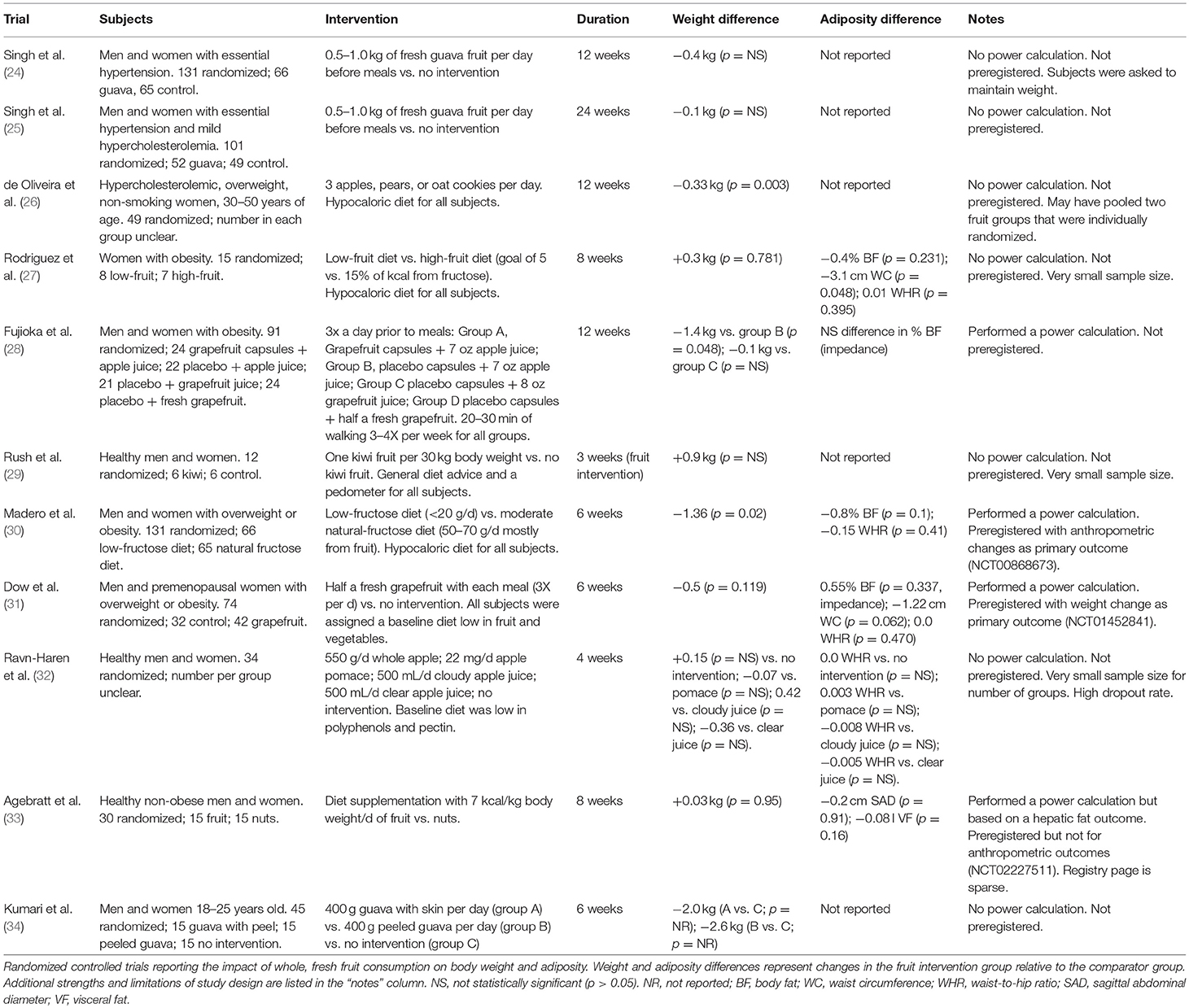
Table 1. Primary outcome: impact of whole, fresh fruit consumption on measures of adiposity in RCTs.
RCTs reporting the impact of whole, fresh fruit consumption on energy intake were published between 2003 and 2016 and are summarized in Table 2. Of the 5 RCTs identified, two included fewer than 20 subjects, two included 21–50 subjects, and one included more than 50 subjects. Interventions represented several fruit types, but apple (n = 2) was named more commonly than other fruit. As in the body weight RCTs (some of which also met inclusion criteria for energy intake), the quantity of fruit varied widely, with the highest intake representing a goal of 15 percent of calorie intake from fruit-derived fructose, which implies ~30 percent of total energy intake from fruit. Interventions lasted between one meal and 12 weeks, with single-meal and 8-week trials being the most common. Three trials used self-report methods to measure energy intake, two of which used a 3-day weighed food record; food intake in the other two trials was directly measured by investigators. The latter two trials were single-meal studies. Three trials reported performing a power calculation as part of study design, although one was powered for a hepatic fat outcome. Only one trial was preregistered, also for a hepatic fat outcome.
Prospective observational studies reporting the impact of whole, fresh fruit consumption on body weight and adiposity change were published between 2002 and 2018 and are summarized in Table 3. Of the 25 studies identified, seven included fewer than 1,000 subjects, six included 1,000–10,000 subjects, seven included 10,001–50,000 subjects, and five included more than 50,000 subjects. Follow-up length ranged from 6 months to 24 years. Eleven studies reported weight changes, and 18 reported changes in other measures of adiposity. None were preregistered, and only one applied an adjustment for multiple comparisons (Bonferroni correction) to control family-wise error rate when testing several hypotheses using a single data set.
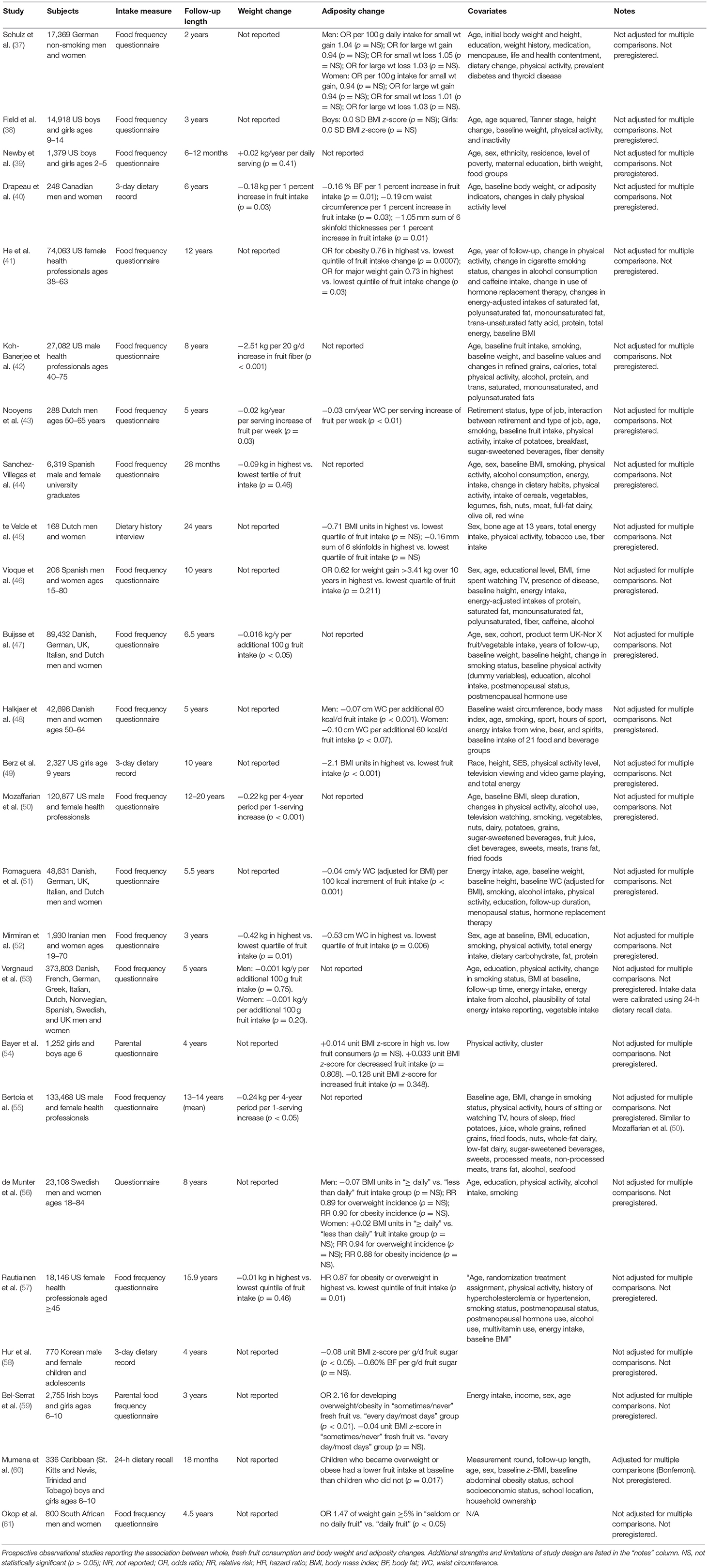
Table 3. Secondary outcome: association of whole, fresh fruit intake with changes in measures of adiposity in prospective observational studies.
Figure 2 presents the Cochrane risk of bias graph, and Figure 3 presents the Cochrane risk of bias summary for RCTs reporting the impact of whole, fresh fruit consumption on body weight and other measures of adiposity. Six of 11 included trials are at an unclear risk of bias from random sequence generation and allocation concealment due to incomplete description of the randomization process, while the other five are at a low risk of bias. All 11 trials are at a high risk of bias due to lack of blinding of participants, an unavoidable consequence of including whole, fresh fruit consumption as an intervention. This leaves open the possibility that part of the impact of fruit observed in these trials is attributable to placebo effects. The risk of bias due to blinding of outcome assessment is low in all 11 trials, not because investigators were consistently blinded to treatment assignment, but because there is a low risk of bias in measuring body weight due to its simple and objective nature. Nine of 11 trials had a low risk of attrition bias due to low dropout rates, while the other two were at high risk due to high dropout rates. Nine of 11 trials were at an unclear risk of selective reporting bias due to insufficient information about initial study design resulting from a lack of detailed preregistration information, while the other two were at low risk due to detailed preregistration. The risk of other bias was fairly evenly split between low (n = 3), unclear (n = 4), and high (n = 4) risk of bias. The two studies with a low risk of other bias were scored as such because they offered detailed preregistration information with primary outcomes relevant to adiposity.
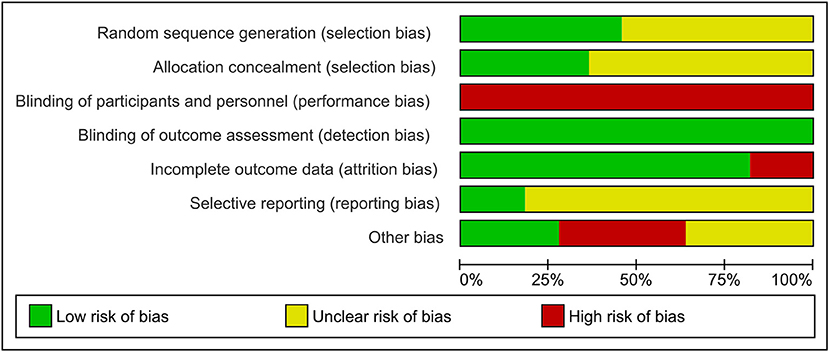
Figure 2. Risk of bias graph for the primary outcome: the impact of whole, fresh fruit consumption on measures of body weight and adiposity in RCTs. Bars illustrate the proportion of trials that received a particular risk of bias score in each risk of bias domain.
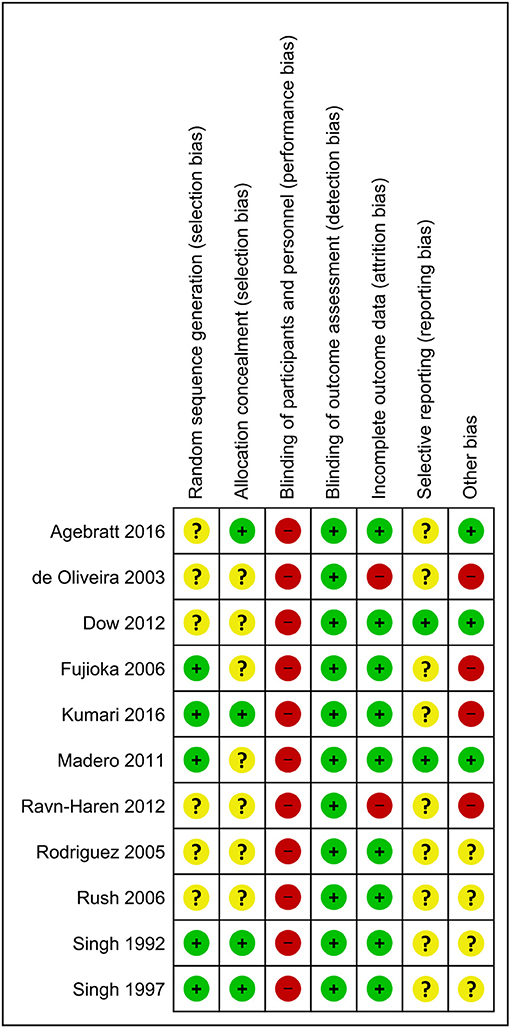
Figure 3. Risk of bias summary for individual trials contributing to the primary outcome: the impact of whole, fresh fruit consumption on measures of body weight and adiposity in RCTs. Colored dots represent low risk (green), unclear risk (yellow), and high risk (red) in each risk of bias domain for each trial.
Body weight RCTs that scored most favorably on the Cochrane risk of bias tool are Madero et al. (30), Dow et al. (31), Agebratt et al. (33), Singh (25), Singh et al. (24), and Kumari et al. (34) (Figure 3). Each received a score of “low risk” in four or five of seven domains.
Figure 4 presents the Cochrane risk of bias graph, and Figure 5 presents the Cochrane risk of bias summary for RCTs reporting the impact of whole, fresh fruit consumption on energy intake. This literature fared more poorly in risk of bias scoring than the body weight RCT literature. All energy intake RCTs are at an unclear risk of bias from random sequence generation, and four of five are at an unclear risk of bias from allocation concealment. This is due to incomplete description of the randomization process. For the same reason as the body weight RCTs, all energy intake RCTs are at a high risk of bias due to lack of blinding of participants, leaving open the possibility of placebo effects. The risk of bias due to blinding of outcome assessment is high in three of five energy intake RCTs due to reliance on self-reported measures of energy intake, and low in two of five due to directly measured energy intake. Four of five trials had a low risk of attrition bias due to low dropout rates. All trials were at an unclear risk of selective reporting bias due to insufficient information about initial study design resulting from a lack of detailed preregistration information. The risk of other bias was unclear in four of five trials due to insufficient information.
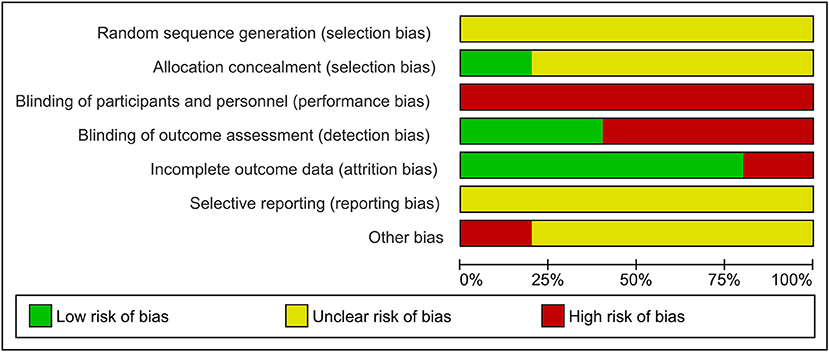
Figure 4. Risk of bias graph for a secondary outcome: the impact of whole, fresh fruit consumption on energy intake in RCTs. Bars illustrate the proportion of trials that received a particular risk of bias score in each risk of bias domain.
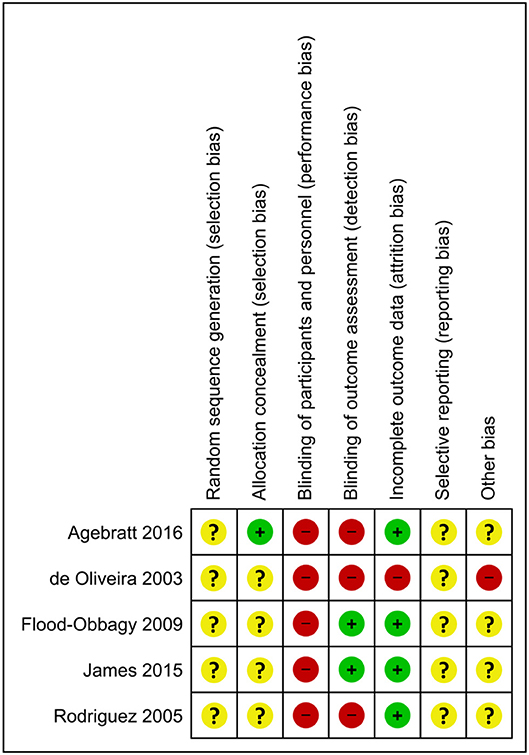
Figure 5. Risk of bias summary for individual trials contributing to a secondary outcome: the impact of whole, fresh fruit consumption on energy intake in RCTs. Colored dots represent low risk (green), unclear risk (yellow), and high risk (red) in each risk of bias domain for each trial.
Energy intake RCTs that scored most favorably on the Cochrane risk of bias tool are Flood-Obbagy and Rolls (35), James et al. (36), and Agebratt et al. (33) (Figure 5), although none received a score of “low risk” in more than two of seven domains.
Although observational studies were not assessed using the Cochrane risk of bias tool, features that are informative of bias risk will nevertheless be considered here. All observational studies included in this review used self-report methods to measure fruit intake, most commonly food frequency questionnaires (Table 3). This introduces a substantial source of error that may also introduce an unknown degree of bias. Validation studies suggest that the Pearson correlation coefficient between fruit intake measured by food frequency questionnaires and 7-day weighed food record is 0.50–0.67, implying that 65–75 percent (R2) of the variability in fruit intake identified by 7-day weighed food record is not captured by food frequency questionnaire (62). Although it has been argued that self-report error is randomly distributed and should not bias associations, the author is uncertain to what extent this argument is correct, and in which contexts.
Observational studies are inherently more limited than RCTs as tools for causal inference because relationships between exposure and outcome variables may be confounded by other variables. For example, between-person variation in fruit and vegetable intake has a genetic component and “individuals genetically predisposed to low fruit and vegetable consumption may be predisposed to higher [body mass index],” suggesting a possible source of bias that could be both important and difficult to correct (63). For this reason among others, observational relationships between fruit intake and body weight changes can be difficult to interpret. Most of the studies represented in Table 3 adjusted extensively for confounding variables in an attempt to limit confounding bias. However, it is difficult to be certain that the most impactful confounding variables were measured and appropriately incorporated into multivariate models.
In addition, it is difficult to be certain that observational relationships were not overadjusted, attenuating the measured association between fruit intake and body weight/adiposity outcomes. In this regard, it is notable that many studies adjusted for energy intake, which seems suboptimal for the purposes of this review since modified energy intake may be a key intermediate variable between fruit intake and body weight/adiposity outcomes.
An additional potential source of bias is that none of the observational studies reported preregistration, leaving open the possibility that outcome selection and data analysis methods were (perhaps inadvertently) guided toward preferred outcomes. The great diversity of analytic methods represented in these studies amplifies this concern because it demonstrates that the possible space of analytic methods is vast (64). Preregistration narrows this space a priori.
Finally, only one observational study reported adjusting significance tests for multiple comparisons, e.g., Bonferroni correction. The more hypothesis tests that are performed on a single data set, the higher the likelihood of a false positive finding. Since the commonly accepted false positive rate in the biomedical research community is 5 percent, testing ten hypotheses yields a 40 percent risk of that at least one of the ten tests will return a false positive finding. Many of the observational studies identified here tested ten or more hypotheses, and most represent datasets that have been analyzed using many statistical tests in other contexts (64). Overall, the risk of bias in the observational literature considered here appears quite high and must limit the strength of causal inferences drawn from it.
The primary outcome of this review is the impact of whole, fresh fruit consumption on measures of adiposity including body weight, as measured by RCTs. Given the limited number of studies identified, which would be further reduced by more stringent meta-analysis inclusion criteria, and large variation of methodology and study quality, qualitative synthesis appears most appropriate.
Of the 11 RCTs included, seven reported numerical reductions of body weight as a result of increased fruit consumption relative to a comparison group, while four reported numerical increases of weight (Table 1). Among trials that reported numerical reductions of body weight, three comparisons were statistically significant (26, 28, 30), while none of the comparisons suggesting weight increases achieved statistical significance. This is consistent with the possibility that the weight increases result from random chance. In support of this possibility, studies that reported non-significant weight increases from fruit consumption tended to be statistically underpowered, with as few as six subjects per group (Table 1).
Among the two trials that reported performing a power calculation a priori and were preregistered with measures of adiposity as the primary outcome, Madero et al. (30) reported a significant weight loss of −1.36 kg over 6 weeks of substantially increased fruit consumption (30), while Dow et al. (31) reported a non-significant weight loss of −0.5 kg over 6 weeks of modestly increased fruit consumption (31). In addition, Madero et al. (30) received the lowest risk of bias score of all studies considered, and Dow et al. (31) tied for the second lowest risk of bias. In contrast, three of the four trials reporting non-significant increases of body weight from increased fruit consumption were among those with the highest risk of bias, while the fourth, which reported a non-significant and negligible weight gain of 0.03 kg over 8 weeks, was among those with relatively lower risk of bias (Figure 3). It is evident that the trials reporting weight reductions tend to have higher methodological quality than those reporting weight increases, suggesting that the former should be viewed as more informative.
Six trials reported measures of adiposity other than body weight, including body fat percentage, waist circumference, waist-to-hip ratio, sagittal abdominal diameter, and visceral fat volume (Table 1). Only one measure achieved statistical significance, a waist circumference reduction of −3.1 cm over 8 weeks of a high-fruit diet (27). However, this finding is questionable due to the study's small sample size, high risk of bias, and apparent lack of correction for multiple comparisons.
Since Madero et al. (30) and Dow et al. (31) appear to surpass the others in methodological quality and relevance to adiposity modification, these will be discussed in detail and will contribute disproportionately to the overall conclusions of this review (30, 31). These two trials are particularly relevant to adiposity modification because all subjects had overweight or obesity. Madero et al. (30) was preregistered with anthropometric changes as the primary outcome, and sample size was selected using a power calculation. Sample size was larger than all but one other trial, which had the same number of subjects. This trial also received the lowest risk of bias score among the 11 trials identified, with low risk in all categories except participant blinding, in which high risk is unavoidable due to the impossibility of blinding subjects, and allocation concealment, which was judged as unclear because it was not described in the manuscript.
One hundred and thirty one men and women with overweight or obesity were randomly assigned to eat a low-fructose diet (<20 g/d) vs. a natural-fructose diet (50–70 g/d) in which most of the fructose was from whole, fresh fruit, for 6 weeks. The latter is approximately equivalent to five to eight whole medium apples per day, or eleven to fifteen whole oranges per day. After 6 weeks, the natural-fructose group had lost 1.36 kg more weight than the low-fructose group (p = 0.02). The trial also reported non-significant reductions of body fat percentage and waist-to-hip ratio in the natural-fructose group relative to the low-fructose group.
Dow et al. (31) was preregistered with body weight change as the primary outcome, and sample size was selected using a power calculation (31). This trial tied for the second-lowest risk of bias score among the 11 trials identified, with low risk of bias in four of seven domains (Figure 3). Its risk of bias in participant blinding was judged as high, which is unavoidable. Its risk of bias due to randomization and allocation concealment are unclear due to a lack of information in the manuscript.
Seventy-four men and premenopausal women with overweight or obesity were randomly assigned to eat half a fresh grapefruit three times per day prior to each meal, vs. no intervention, for 6 weeks. In addition, all subjects were assigned to a baseline diet low in fruit and vegetables. After 6 weeks, the grapefruit group had lost 0.5 kg more weight than the control group, but this difference was not statistically significant (p = 0.119). Between-group differences in body fat percentage (+0.55 percent), waist circumference (−1.22 cm), and waist-to-hip ratio (0.0) were also non-significant.
Informally weighting the strength of findings according to study quality and risk of bias, the overall RCT literature suggests that increasing whole, fresh fruit consumption promotes weight maintenance or modest weight loss over periods of 3–24 weeks, with limited evidence suggesting that high intakes of fruit lead to weight loss among people with overweight or obesity. The overall quality of evidence according to the GRADE method is moderate, indicating that the true effect of fruit consumption on measures of adiposity is probably close to the effect estimated in the RCTs considered here, particularly those of higher quality.
A secondary outcome of this review is the impact of whole, fresh fruit consumption on measures of energy intake, as measured by RCTs. Given the small number of studies identified, and large variation of methodology and study quality, qualitative synthesis appears most appropriate.
Of the five RCTs included, four reported numerical reductions of energy intake as a result of increased fruit consumption relative to a comparison group, while one reported a numerical increase of energy intake (Table 2). Among trials that reported numerical reductions of energy intake, two comparisons were statistically significant (35, 36), while the comparison suggesting numerically increased energy intake reported a small-magnitude effect (+47 kcal/d) that did not achieve statistical significance (27). This is consistent with the possibility that the finding of increased energy intake resulted from random chance. In support of this possibility, this study was likely statistically underpowered, with seven and eight subjects per group (Table 1). It did not report performing an a priori power calculation and was also at high risk of bias due to its use of a self-reported measure of energy intake, and not blinding subjects (unavoidable) or investigators (avoidable) (Figure 5).
Only one energy intake trial was preregistered, and its primary outcome was a change of hepatic fat content rather than energy intake (33). Among the three trials that reported performing a power calculation a priori, two reported statistically significant single-meal reductions of energy intake of 134–187 kcal from meals including a fruit preload relative to no preload or a confectionary snack (35, 36), and the third reported a non-significant 216-kcal reduction of daily energy intake from a high-fruit diet relative to a high-nut diet (33). The former two trials were the only two to employ direct measurement of energy intake by investigators rather than self-reported intake (Table 2). The three trials that performed power calculations received the most favorable risk of bias scores among the five trials identified, although none of the five trials received a low risk score in more than two of seven domains (Figure 5).
Consistent with the adiposity RCTs, it is evident that the trials reporting energy intake reductions, and particularly statistically significant ones, tend to have higher methodological quality than the trial reporting energy intake increase, suggesting that the former should be viewed as more informative. However, it is notable that the three trials reporting non-significant effects, while relying on self-reported data, were the only three with follow-up periods longer than a single meal (Table 2).
Flood-Obbagy and Rolls (35) and James et al. (36) appear to surpass the other trials in methodological quality due to direct measurement of energy intake, lower risk of bias score than the other three, and sufficient statistical power supported by a priori power calculations (35, 36). Flood-Obbagy and Rolls (35) also has the largest sample size of the five trials identified, and its effective sample size is amplified by its crossover design. These two trials will be discussed in detail and will contribute disproportionately to the overall conclusions of this review. Although these trials may provide a higher level of certainty than the others, they are less relevant to energy intake control in the context of obesity because their subjects were either lean (35) or mildly overweight (36) on average.
Flood-Obbagy and Rolls (35) received one of the lowest risk of bias scores among the five trials identified, although none of the trials were at a low risk of bias. It received a low risk of bias score in blinding of outcome assessment due to directly measured energy intake, and low risk of attrition bias due to low attrition (Figure 5). It received an unclear risk of bias score for random sequence generation, allocation concealment, selective reporting, and other bias due to insufficient information in the manuscript and the absence of preregistration. It received a high risk of bias score in participant blinding, in which high risk is unavoidable.
Fifty-nine men and women 18–45 years old, with body mass index of 23.7 kg/m2 (M) and 24.3 kg/m2 (W), received five food preloads in random order on different days, followed after 15 min by an ad libitum test meal of cheese tortellini, tomato sauce, and water (35). Preload conditions were whole apple, apple sauce, apple juice with added soluble fiber (pectin), apple juice, or no preload. All preloads were adjusted to contain equal energy (125 kcal) and weight (266 g) except the no-preload control. Total meal energy intake as directly measured by investigators, including preload, was lowest in the whole apple condition and highest in the no-preload condition, with a highly statistically significant difference of −187 kcal (Table 2). Energy intake in the whole apple condition was also significantly lower than all other conditions. Total meal energy intake increased with each processing step away from whole fresh apples, in the following order: whole apples < apple sauce < apple juice with fiber < apple juice.
James et al. (36) received a risk of bias score identical to Flood-Obbagy and Rolls (35) for similar reasons (Figure 5) (36). Twelve healthy pre-menopausal women, with body mass index of 26.6 kg/m2, received two preloads in random order on different days, followed after 60 min by an ad libitum test meal of pasta with Bolognese sauce and olive oil. Preload conditions were fresh mixed berries vs. soft berry-flavored candies and were matched for energy content (65 kcal). The two preloads also contained a similar amount of sugar (12.1 vs. 15.5 g). Energy intake at the test meal, as directly measured by investigators, was 134 kcal lower in the mixed berry condition than in the candy condition (p < 0.001).
Informally weighting the strength of findings according to study quality and risk of bias, the overall RCT literature suggests that increasing whole, fresh fruit consumption reduces energy intake, particularly when consumed prior to a meal or instead of more energy-dense foods. However, these findings have uncertain relevance to energy intake control in obesity because the most informative trials were conducted in subjects who were lean or modesty overweight. In addition, the most informative trials used single-meal designs, limiting conclusions about the impact of fruit consumption on long-term energy intake. The overall quality of evidence according to the GRADE method is moderate, indicating that the true effect of pre-meal fruit consumption on short-term energy intake in people without obesity is probably close to the effect estimated in the RCTs considered here. Longer-term effects, and those in people with obesity, are less certain. Nevertheless, the energy intake RCT literature is broadly consistent with findings from the body weight RCT literature.
A secondary outcome of this review is the association between whole, fresh fruit consumption and measures of adiposity including body weight, as measured by prospective observational studies. Of the 25 studies identified, 11 reported weight changes over time, and of those, ten reported that people who consumed larger amounts of fruit gained numerically less weight over time (or lost more weight) than people who consumed less fruit (Table 3). Seven of these associations were statistically significant, all suggesting that higher fruit intake is associated with superior weight control over time.
Eighteen studies reported changes in measures of adiposity other than weight over time, 12 of which reported statistically significant differences between higher and lower consumers of fruit (Table 3). Among these 12, all reported that markers of adiposity in people who consume larger amounts of fruit tend to increase less over time (or decline more rapidly) than in people who consume less fruit. Although the Cochrane risk of bias tool was not applied to these studies, as discussed previously they all appear to be at a high risk of bias due to a combination of unavoidable and potentially avoidable design features.
Nevertheless, some studies appear more informative than others. Bertoia et al. (55) [similar to Mozaffarian et al. (50)] and Vergnaud et al. (53) will be discussed further due to the fact that they have the largest sample sizes of the 11 studies identified, they rely on contextually-validated food frequency questionnaires, they have relatively long follow-up periods, and together they represent men and women of 11 nations (35, 36).
Bertoia et al. (55) compiled data from three cohorts representing 133,468 US male and female health professionals (55). Diet assessment was performed using food frequency questionnaires administered at 4-year intervals for a mean of 13–14 years. Analyses controlled for a wide variety of diet and lifestyle factors (Table 3), and although they did not control for demographic variables such as income and education, included cohorts were fairly homogeneous in these respects. Notably, analyses did not control for energy intake, which is preferable because energy intake is likely a mediating variable between whole, fresh fruit intake and adiposity.
In contrast to most other studies identified, Bertoia et al. (55) examined the association between changes in self-reported fruit intake and changes in measures of adiposity. In other words, if a person reported increasing fruit intake over the course of the follow-up period, were they also less likely to gain weight over time? Although this “change-on-change” method remains fundamentally observational, it may avoid some of the confounding potential of traditional nutritional epidemiology study designs (65). The study reported that a one-serving increase of daily fruit intake was associated with a highly statistically significant 0.24 kg reduction of body weight per 4-year period, and did not report associations with other measures of adiposity.
Vergnaud et al. (53) represents 373,803 Danish, French, German, Greek, Italian, Dutch, Norwegian, Spanish, Swedish, and UK men and women, making it the largest cohort of the 11 studies identified (53). Diet assessment was performed using food frequency questionnaires and the duration of follow-up was 5 years. In contrast to Bertoia et al. (55) but similar to most other nutritional epidemiology studies, Vergnaud et al. (53) reports the association between baseline self-reported fruit intake and weight change over a 5-year period. Analyses controlled for several basic diet, lifestyle, and demographic factors, including energy intake (Table 3). Systematic underestimation or overestimation of dietary intakes between study centers was addressed using a dietary calibration study. The study reported that 100 g higher daily fruit intake was associated with a non-significantly lower rate of weight gain of −0.001 kg per year in men and women, and did not report associations with other measures of adiposity.
Informally weighting the strength of findings according to study quality, the overall prospective observational literature suggests that habitually higher fruit intake is associated with no effect on weight, or modest protection against weight gain. Although these findings must be interpreted cautiously due to limitations of study design, they are broadly consistent with the findings of energy intake and body weight RCTs discussed previously and may suggest that the short- to medium-term effects observed in RCTs persist in the long term.
The primary outcome of this review is the impact of whole, fresh fruit consumption on measures of adiposity including body weight, as measured by RCTs. Overall, these RCTs suggest that increasing intake of whole, fresh fruit promotes weight maintenance or modest weight loss over periods of 3–24 weeks. High intakes of whole, fresh fruit in people with obesity may promote some degree of weight loss. RCTs provide little information about more direct measures of adiposity such as body fat percentage. The strength of evidence supporting this conclusion is moderate, indicating that the true effect of fruit consumption on measures of adiposity is probably close to the effect estimated here.
Secondary outcomes of RCTs reporting the impact of fruit consumption on energy intake, and prospective observational studies reporting associations between fruit intake and measures of adiposity, were broadly consistent with the primary outcome. The strength of evidence supporting conclusions regarding energy intake RCTs is moderate, while the prospective observational findings did not receive a GRADE assessment but are likely at high risk of bias. As with the primary outcome, RCTs and prospective observational studies of higher quality tend to support the hypothesis that higher intakes of whole, fresh fruit either do not impact weight or modestly attenuate weight gain over time.
Limitations of this review relate both to the review itself, and to the studies that underlie it. Although quantitative pooling of RCT data appeared suboptimal in this context due to the limited number of studies and considerable heterogeneity in study methods and quality, narrative synthesis is inherently more subjective than quantitative meta-analysis. The author endeavored to limit the potential for bias by preregistering a detailed research plan and adhering to widely accepted defined methods for assessing and reporting evidence, such as the Cochrane risk of bias tool, the GRADE method, and PRISMA guidelines. The author attempted to be transparent in methods and reasoning so the reader may form his or her own views. In addition, the greater subjectivity of narrative reviews may be counterbalanced in some instances by a superior ability to focus on high-quality studies rather than diluting their evidence value by pooling them with lower-quality studies. Finally, the author was not funded for this work and has no connection with Big Fruit, eliminating this potential source of real or perceived bias.
An additional limitation of this review is that due to resource constraints, study selection, data extraction, risk of bias scoring, and GRADE evaluation were performed by one person. The Cochrane handbook for Systematic Reviews of Interventions recommends that systematic reviews be conducted by at least two people to reduce the risk of errors4.
The conclusions of this review are also limited by the underlying evidence. Although 11 RCTs were available for the primary outcome of adiposity, most had serious limitations of sample size, lack of preregistration, and/or risk of bias. Conclusions of the primary outcome of this review rest disproportionately on two high-quality trials. Energy intake RCTs were fewer in number and tended to be lower-quality than adiposity RCTs. Prospective observational studies typically had serious limitations including lack of preregistration, lack of correction for multiple comparisons, and potential confounding and overcorrection, which together raise substantial concerns of bias. Some of these limitations are inherent to observational methods, while others are potentially avoidable. Nevertheless, the consistency of findings across the three primary and secondary outcomes is somewhat reassuring.
Consistent with earlier reviews on this topic (14, 17), available evidence suggests that increasing intake of whole, fresh fruit probably does not increase adiposity, but instead has either no impact on adiposity or constrains it modestly. Findings consistent with this hypothesis are observed in studies representing single meals, 3–24 week periods, and periods of five or more years. Although some uncertainty remains, these findings support increasing the consumption of whole, fresh fruit as part of a multi-factor approach to controlling excess energy intake and adiposity. These findings also suggest that if the sugar content of fruit favors increased energy intake and adiposity, this is outweighed by its other properties, such as lower calorie density, moderate palatability/reward value, higher fiber content, and micronutrient content, at least when consumed as part of typical diet patterns.
These findings support existing recommendations by organization such as the US Department of Agriculture and the World Health Organization to increase fruit consumption as a public health measure (66, 67). Although increasing consumption of whole, fresh fruit is unlikely to have a large impact on population obesity rates on its own, it may make a positive contribution as part of a broader public health strategy for obesity control. Similarly, healthcare providers should not expect large changes in adiposity as a result of increasing whole, fresh fruit consumption alone, but it is reasonable to include it as part of a broader package of slimming diet and lifestyle behaviors. Furthermore, it is unlikely to cause adiposity gain despite its sugar content, at least as part of a typical mixed diet. Increasing intake of whole, fresh fruit may be more effective as an adiposity control measure when presented as a replacement for calorie-dense dessert foods.
Several opportunities for reducing the uncertainty of conclusions on this topic are apparent. Additional high-quality RCTs with changes in adiposity as the preregistered primary endpoint would be useful, particularly if they report an accurate measure of total and regional adiposity such as dual-energy X-ray absorptiometry. High-quality energy intake RCTs, preregistered and with complete description of randomization processes, involving direct measurement of energy intake in people with obesity over longer periods of time would also contribute substantially. Additional energy intake RCTs could also compare the impact of fruit intake in different contexts, such as pre-meal vs. intra-meal vs. after-meal intake, to identify the most effective strategy for energy intake control. Finally, prospective observational studies that use accurate measurement instruments, are conducted according to a preregistered research plan, and adjust for multiple comparisons would reduce uncertainty.
SG is the sole contributor to this manuscript, aside from its search strategy, which was designed in collaboration with Ben Harnke.
This research was not conducted in association with any public or private organization. SG is the author of a general-audience book on the neurobiology of overeating, The Hungry Brain, which does not currently yield royalties but may in the future. This book mentions fruit but does not place a special emphasis on it. SG is the co-creator of a weight management program, the Ideal Weight Program, from which he receives revenue. This program permits the consumption of fruit but does not place a special emphasis on it.
Many thanks to Ben Harnke, Education and Reference Librarian at the University of Colorado Health Sciences Library, for his generous help in designing the review's search strategy.
1. ^https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=111830
2. ^https://handbook-5-1.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm
3. ^https://handbook-5-1.cochrane.org/chapter_12/12_2_1_the_grade_approach.htm.
4. ^https://handbook-5-1.cochrane.org/chapter_2/2_3_4_1_the_importance_of_a_team.htm
1. Murray, CJL, Vos, T, Lozano, R, Naghavi, M, Flaxman, AD, Michaud, C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61690-0
2. Trowell, HC, and Burkitt, DP Western Diseases: Their Emergence and Prevention. Harvard University Press (1981). 474 p.
3. Knowler, WC, Barrett-Connor, E, Fowler, SE, Hamman, RF, Lachin, JM, Walker, EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
4. Hall, KD, and Guo, J Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. (2017) 152:1718–27.e3. doi: 10.1053/j.gastro.2017.01.052
5. Fontana, L, Meyer, TE, Klein, S, and Holloszy, JO Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. (2004) 101:6659–63. doi: 10.1073/pnas.0308291101
6. Chan, JM, Rimm, EB, Colditz, GA, Stampfer, MJ, and Willett, WC Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. (1994) 17:961–9. doi: 10.2337/diacare.17.9.961
7. Nutrition and Your Health. Dietary Guidelines for Americans. US Department of Agriculture and US Department of Health and Human Services (1980).
8. Lustig, RH, Schmidt, LA, and Brindis, CD Public health: the toxic truth about sugar. Nature. (2012) 482:27–9. doi: 10.1038/482027a
9. Johnson, RJ, Perez-Pozo, SE, Sautin, YY, Manitius, J, Sanchez-Lozada, LG, Feig, DI, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. (2009) 30:96–116. doi: 10.1210/er.2008-0033
11. Morenga, LT, Mallard, S, and Mann, J Dietary sugars and body weight: systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ. (2013) 346:e7492. doi: 10.1136/bmj.e7492
12. Sievenpiper, JL, de Souza, RJ, Mirrahimi, A, Yu, ME, Carleton, AJ, Beyene, J, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med. (2012) 156:291–304. doi: 10.7326/0003-4819-156-4-201202210-00007
13. Klein, RG The Human Career: Human Biological and Cultural Origins, 3rd ed. Chicago, IL: University Of Chicago Press (2009). 1024 p.
14. Alinia, S, Hels, O, and Tetens, I The potential association between fruit intake and body weight–a review. Obes Rev Off J Int Assoc Study Obes. (2009) 10:639–47. doi: 10.1111/j.1467-789X.2009.00582.x
15. Ledoux, TA, Hingle, MD, and Baranowski, T Relationship of fruit and vegetable intake with adiposity: a systematic review. Obes Rev Off J Int Assoc Study Obes. (2011) 12:e143–50. doi: 10.1111/j.1467-789X.2010.00786.x
16. Sharma, SP, Chung, HJ, Kim, HJ, and Hong, ST Paradoxical effects of fruit on obesity. Nutrients. (2016) 8:633. doi: 10.3390/nu8100633
17. Hebden, L, O'Leary, F, Rangan, A, Singgih Lie, E, Hirani, V, and Allman-Farinelli, M Fruit consumption and adiposity status in adults: a systematic review of current evidence. Crit Rev Food Sci Nutr. (2017) 57:2526–40. doi: 10.1080/10408398.2015.1012290
18. Sartorelli, DS, Franco, LJ, and Cardoso, MA High intake of fruits and vegetables predicts weight loss in Brazilian overweight adults. Nutr Res. (2008) 28:233–8. doi: 10.1016/j.nutres.2008.02.004
19. Lamb, MJE, Griffin, SJ, Sharp, SJ, and Cooper, AJM Fruit and vegetable intake and cardiovascular risk factors in people with newly diagnosed type 2 diabetes. Eur J Clin Nutr. (2017) 71:115–21. doi: 10.1038/ejcn.2016.180
20. Linde, JA, Utter, J, Jeffery, RW, Sherwood, NE, Pronk, NP, and Boyle, RG Specific food intake, fat and fiber intake, and behavioral correlates of BMI among overweight and obese members of a managed care organization. Int J Behav Nutr Phys Act. (2006) 3:42. doi: 10.1186/1479-5868-3-42
21. Garden, FL, Marks, GB, Almqvist, C, Simpson, JM, and Webb, KL Infant and early childhood dietary predictors of overweight at age 8 years in the CAPS population. Eur J Clin Nutr. (2011) 65:454–62. doi: 10.1038/ejcn.2011.7
22. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
23. IntHout, J, Ioannidis, JP, and Borm, GF The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. (2014) 14:25. doi: 10.1186/1471-2288-14-25
24. Singh, RB, Rastogi, SS, Singh, R, Ghosh, S, and Niaz, MA Effects of guava intake on serum total and high-density lipoprotein cholesterol levels and on systemic blood pressure. Am J Cardiol. (1992) 70:1287–91. doi: 10.1016/0002-9149(92)90763-O
25. Singh, RB, Rastogi, SS, Singh, R, Niaz, MA, Singh, NK, and Madhu, SV Effects on plasma ascorbic acid and coronary risk factors of adding guava fruit to the usual diet in hypertensives with mild to moderate. J Nutr Environ Med. (1997) 7:5–14. doi: 10.1080/13590849762754
26. Conceição de Oliveira, M, Sichieri, R, and Sanchez Moura, A Weight loss associated with a daily intake of three apples or three pears among overweight women. Nutrition. (2003) 19:253–6. doi: 10.1016/S0899-9007(02)00850-X
27. Rodríguez, MC, Parra, MD, Marques-Lopes, I, De Morentin, BEM, González, A, and Martínez, JA Effects of two energy-restricted diets containing different fruit amounts on body weight loss and macronutrient oxidation. Plant Foods Hum Nutr. (2005) 60:219–24. doi: 10.1007/s11130-005-8622-2
28. Fujioka, K, Greenway, F, Sheard, J, and Ying, Y The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. J Med Food. (2006) 9:49–54. doi: 10.1089/jmf.2006.9.49
29. Rush, E, Ferguson, LR, Cumin, M, Thakur, V, Karunasinghe, N, and Plank, L Kiwifruit consumption reduces DNA fragility: a randomized controlled pilot study in volunteers. Nutr Res. (2006) 26:197–201. doi: 10.1016/j.nutres.2006.05.002
30. Madero, M, Arriaga, JC, Jalal, D, Rivard, C, McFann, K, Pérez-Méndez, O, et al. The effect of two energy-restricted diets, a low-fructose diet versus a moderate natural fructose diet, on weight loss and metabolic syndrome parameters: a randomized controlled trial. Metab Clin Exp. (2011) 60:1551–9. doi: 10.1016/j.metabol.2011.04.001
31. Dow, CA, Going, SB, Chow, HHS, Patil, BS, and Thomson, CA The effects of daily consumption of grapefruit on body weight, lipids, and blood pressure in healthy, overweight adults. Metab Clin Exp. (2012) 61:1026–35. doi: 10.1016/j.metabol.2011.12.004
32. Ravn-Haren, G, Dragsted, LO, Buch-Andersen, T, Jensen, EN, Jensen, RI, Németh-Balogh, M, et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur J Nutr. (2013) 52:1875–89. doi: 10.1007/s00394-012-0489-z
33. Agebratt, C, Ström, E, Romu, T, Dahlqvist-Leinhard, O, Borga, M, Leandersson, P, et al. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PLoS ONE. (2016) 11:e0147149. doi: 10.1371/journal.pone.0147149
34. Kumari, S, Rakavi, R, and Mangaraj, M Effect of guava in blood glucose and lipid profile in healthy human subjects: a randomized controlled study. J Clin Diagn Res. (2016) 10:BC04–7. doi: 10.7860/JCDR/2016/21291.8425
35. Flood-Obbagy, JE, and Rolls, BJ The effect of fruit in different forms on energy intake and satiety at a meal. Appetite. (2009) 52:416–22. doi: 10.1016/j.appet.2008.12.001
36. James, LJ, Funnell, MP, and Milner, S An afternoon snack of berries reduces subsequent energy intake compared to an isoenergetic confectionary snack. Appetite. (2015) 95:132–7. doi: 10.1016/j.appet.2015.07.005
37. Schulz, M, Kroke, A, Liese, AD, Hoffmann, K, Bergmann, MM, and Boeing, H Food groups as predictors for short-term weight changes in men and women of the EPIC-potsdam cohort. J Nutr. (2002) 132:1335–40. doi: 10.1093/jn/132.6.1335
38. Field, AE, Gillman, MW, Rosner, B, Rockett, HR, and Colditz, GA Association between fruit and vegetable intake and change in body mass index among a large sample of children and adolescents in the United States. Int J Obes. (2003) 27:821–6. doi: 10.1038/sj.ijo.0802297
39. Newby, PK, Peterson, KE, Berkey, CS, Leppert, J, Willett, WC, and Colditz, GA Dietary composition and weight change among low-income preschool children. Arch Pediatr Adolesc Med. (2003) 157:759–64. doi: 10.1001/archpedi.157.8.759
40. Drapeau, V, Després, JP, Bouchard, C, Allard, L, Fournier, G, Leblanc, C, et al. Modifications in food-group consumption are related to long-term body-weight changes. Am J Clin Nutr. (2004) 80:29–37. doi: 10.1093/ajcn/80.1.29
41. He, K, Hu, FB, Colditz, GA, Manson, JE, Willett, WC, and Liu, S Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes. (2004) 28:1569–74. doi: 10.1038/sj.ijo.0802795
42. Koh-Banerjee, P, Franz, M, Sampson, L, Liu, S, Jacobs, DR, Spiegelman, D, et al. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am J Clin Nutr. (2004) 80:1237–45. doi: 10.1093/ajcn/80.5.1237
43. Nooyens, AC, Visscher, TL, Schuit, AJ, van Rossum, CT, Verschuren, WM, van Mechelen, W, et al. Effects of retirement on lifestyle in relation to changes in weight and waist circumference in Dutch men: a prospective study. Public Health Nutr. (2005) 8:1266–74. doi: 10.1079/PHN2005756
44. Sánchez-Villegas, A, Bes-Rastrollo, M, Martínez-González, MA, and Serra-Majem, L Adherence to a mediterranean dietary pattern and weight gain in a follow-up study: the SUN cohort. Int J Obes. (2006) 30:350–8. doi: 10.1038/sj.ijo.0803118
45. te Velde, SJ, Twisk, JWR, and Brug, J Tracking of fruit and vegetable consumption from adolescence into adulthood and its longitudinal association with overweight. Br J Nutr. (2007) 98:431–8. doi: 10.1017/S0007114507721451
46. Vioque, J, Weinbrenner, T, Castelló, A, Asensio, L, and Hera, MG de la Intake of fruits and vegetables in relation to 10-year weight gain among spanish adults. Obesity. (2008) 16:664–70. doi: 10.1038/oby.2007.121
47. Buijsse, B, Feskens, EJ, Schulze, MB, Forouhi, NG, Wareham, NJ, Sharp, S, et al. Fruit and vegetable intakes and subsequent changes in body weight in European populations: results from the project on Diet, Obesity, and Genes (DiOGenes). Am J Clin Nutr. (2009) 90:202–9. doi: 10.3945/ajcn.2008.27394
48. Halkjær, J, Tjønneland, A, Overvad, K, and Sørensen, TIA Dietary predictors of 5-year changes in waist circumference. J Acad Nutr Diet. (2009) 109:1356–66. doi: 10.1016/j.jada.2009.05.015
49. Berz, JPB, Singer, MR, Guo, X, Daniels, SR, and Moore, LL Use of a DASH food group score to predict excess weight gain in adolescent girls in the national growth and health study. Arch Pediatr Adolesc Med. (2011) 165:540–6. doi: 10.1001/archpediatrics.2011.71
50. Mozaffarian, D, Hao, T, Rimm, EB, Willett, WC, and Hu, FB Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. (2011) 364:2392–404. doi: 10.1056/NEJMoa1014296
51. Romaguera, D, Ängquist, L, Du, H, Jakobsen, MU, Forouhi, NG, Halkjær, J, et al. Food Composition of the diet in relation to changes in waist circumference adjusted for body mass index. PLoS ONE. (2011) 6:e23384. doi: 10.1371/journal.pone.0023384
52. Mirmiran, P, Bahadoran, Z, Golzarand, M, Shiva, N, and Azizi, F Association between dietary phytochemical index and 3-year changes in weight, waist circumference and body adiposity index in adults: tehran Lipid and Glucose study. Nutr Metab. (2012) 9:108. doi: 10.1186/1743-7075-9-108
53. Vergnaud, AC, Norat, T, Romaguera, D, Mouw, T, May, AM, Romieu, I, et al. Fruit and vegetable consumption and prospective weight change in participants of the European prospective investigation into cancer and nutrition-physical activity, nutrition, alcohol, cessation of smoking, eating out of home, and obesity study. Am J Clin Nutr. (2012) 95:184–93. doi: 10.3945/ajcn.111.019968
54. Bayer, O, Nehring, I, Bolte, G, and von Kries, R Fruit and vegetable consumption and BMI change in primary school-age children: a cohort study. Eur J Clin Nutr. (2014) 68:265–70. doi: 10.1038/ejcn.2013.139
55. Bertoia, ML, Mukamal, KJ, Cahill, LE, Hou, T, Ludwig, DS, Mozaffarian, D, et al. Changes in intake of fruits and vegetables and weight change in united states men and women followed for Up to 24 Years: analysis from three prospective cohort studies. PLoS Med. (2015) 12:e1001878. doi: 10.1371/journal.pmed.1001878
56. de Munter, JS, Tynelius, P, Magnusson, C, and Rasmussen, F Longitudinal analysis of lifestyle habits in relation to body mass index, onset of overweight and obesity: results from a large population-based cohort in Sweden. Scand J Public Health. (2015) 43:236–45. doi: 10.1177/1403494815569865
57. Rautiainen, S, Wang, L, Lee, IM, Manson, JE, Buring, JE, and Sesso, HD Higher intake of fruit, but not vegetables or fiber, at baseline is associated with lower risk of becoming overweight or obese in middle-aged and older women of normal BMI at baseline. J Nutr. (2015) 145:960–8. doi: 10.3945/jn.114.199158
58. Hur, YI, Park, H, Kang, JH, Lee, HA, Song, HJ, Lee, HJ, et al. Associations between sugar intake from different food sources and adiposity or cardio-metabolic risk in childhood and adolescence: the korean child-adolescent cohort study. Nutrients. (2016) 8:20. doi: 10.3390/nu8010020
59. Bel-Serrat, S, Heinen, MM, Mehegan, J, O'Brien, S, Eldin, N, Murrin, CM, et al. Predictors of weight status in school-aged children: a prospective cohort study. Eur J Clin Nutr. (2018) 1. doi: 10.1038/s41430-018-0359-8. [Epub ahead of print].
60. Mumena, WA, Francis-Granderson, I, Phillip, LE, and Gray-Donald, K Rapid increase of overweight and obesity among primary school-aged children in the Caribbean; high initial BMI is the most significant predictor. BMC Obes. (2018) 5:4. doi: 10.1186/s40608-018-0182-8
61. Okop, KJ, Lambert, EV, Alaba, O, Levitt, NS, Luke, A, Dugas, L, et al. Sugar-sweetened beverage intake and relative weight gain among South African adults living in resource-poor communities: longitudinal data from the STOP-SA study. Int J Obes. (2018) 43:603–14. doi: 10.1038/s41366-018-0216-9
62. Hu, FB, Rimm, E, Smith-Warner, SA, Feskanich, D, Stampfer, MJ, Ascherio, A, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. (1999) 69:243–9. doi: 10.1093/ajcn/69.2.243
63. Martin, LJ, Lee, SY, Couch, SC, Morrison, J, and Woo, JG Shared genetic contributions of fruit and vegetable consumption with BMI in families 20 y after sharing a household. Am J Clin Nutr. (2011) 94:1138–43. doi: 10.3945/ajcn.111.015461
64. Ioannidis, JPA The challenge of reforming nutritional epidemiologic research. JAMA. (2018) 320:969–70. doi: 10.1001/jama.2018.11025
65. Pan, A, Malik, VS, Hao, T, Willett, WC, Mozaffarian, D, and Hu, FB Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes. (2013) 37:1378–85. doi: 10.1038/ijo.2012.22538
66. 2015–2020, Dietary Guidelines - Health.gov. Available online at: https://health.gov/dietaryguidelines/2015/guidelines/ (accessed March 29, 2019)
Keywords: fruit, adiposity, body weight, obesity, energy intake, sugar
Citation: Guyenet SJ (2019) Impact of Whole, Fresh Fruit Consumption on Energy Intake and Adiposity: A Systematic Review. Front. Nutr. 6:66. doi: 10.3389/fnut.2019.00066
Received: 17 December 2018; Accepted: 23 April 2019;
Published: 08 May 2019.
Edited by:
David Katz, Yale Griffin Prevention Research Center, United StatesReviewed by:
Nada Benajiba, Princess Nourah bint Abdulrahman University, Saudi ArabiaCopyright © 2019 Guyenet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephan J. Guyenet, cy5ndXllbmV0QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.