- 1Instituto de Investigación en Ciencias de la Alimentación, CIAL (CSIC-UAM), Campus de la Universidad Autónoma de Madrid, Madrid, Spain
- 2Department of Food and Nutritional Sciences, The University of Reading, Reading, United Kingdom
Background and Limitations of the Conventional in vitro Methods to Assess Digestibility of Dietary Carbohydrates
Scientific evidence of human digestion of foods gathered during the last decades has strengthened the relationship between digestibility and the possible effects of foods on human health (1). The high complexity of the gastrointestinal (GI) environment and the lack of an easy direct access to most of the parts of the GI tract (2) with the exception of the oral cavity, prevent the broad implementation of in vivo animal, or human trials for assessing the digestion, absorption, and metabolism of dietary food ingredients. Therefore, in vitro testing has a central place in the current investigations of food's digestive fate.
Non- or slowly digestible carbohydrates may have appealing properties that are associated with a series of beneficial physiological effects, such as low-calorie (important in preventing obesity), low-glycemic (helpful in managing diabetes and cardiovascular disease), and low-digestible (helpful in reducing the intestinal transit time and in positively modulating the gut microbiota composition and activity). These beneficial effects have sparked the interest, from both academic and industry perspectives, in carbohydrates showing resistance to digestion (or slow digestibility) and absorption in the small intestine and thus being available in the large intestine as substrates for fermentation by gut microbiota.
Since Southgate published, in the late 1960s, two methods for measuring available (3) and unavailable (4) carbohydrates in foods by hydrolyzing starch with amyloglucosidase and pullulanase of fungal origin, a plethora of models ensued with the common rationale of using amylolytic enzymes that, in some cases, may be combined with a microbial invertase (5). Remarkably, the substrate specificity, hydrolytic mechanism and influence in physiologic responses of amylolytic enzymes from fungal or bacterial sources commonly used in in vitro starch digestion assays has been demonstrated to be noticeably different from their equivalent in mammals (6, 7).
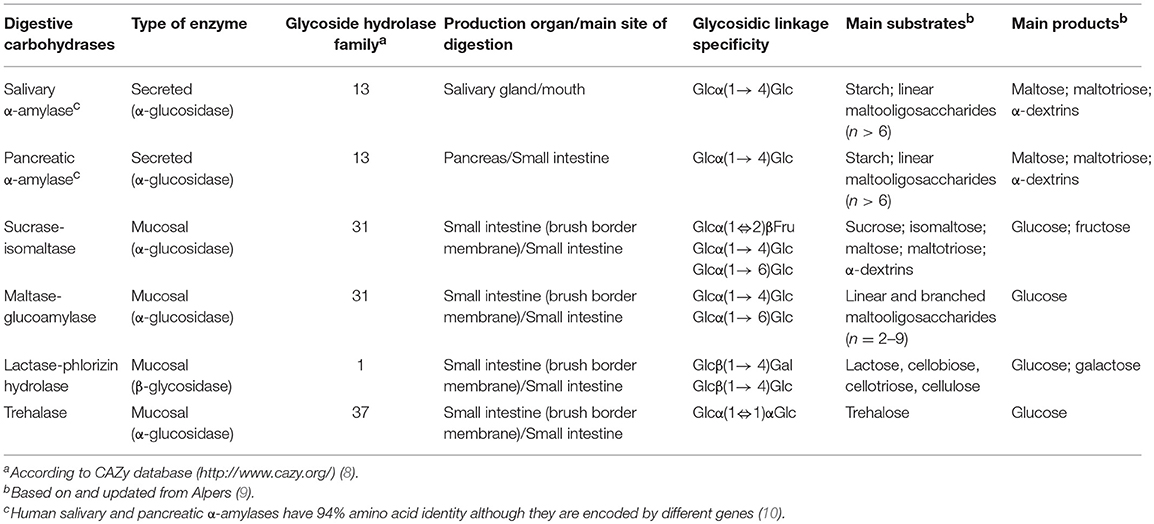
The current state-of-the-art reveals that human gastrointestinal digestion of dietary carbohydrates is a multistage process, involving up to six different carbohydrases produced by three different organs (Table 1). The main function of the oral phase is the conversion of food into a homogenous mass during mastication, whilst the starchy carbohydrates undergo a very limited salivary α-amylolysis. The main site for carbohydrate digestion is the small intestine, where the chyme is mixed with α-amylase containing secretions of the pancreas. In addition, the key role played by the mucosal disaccharidases embedded in the small intestinal brush border membrane vesicles that are responsible for the final stage of luminal digestion prior to absorption needs to be recognized (11, 12) (Table 1). Strikingly, the inclusion of small intestine mucosal carbohydrases has been ignored in the vast majority of in vitro models developed to date. This issue is particularly critical in the assessment of not only non-starchy carbohydrates, but also in the quality of starch digestion since the small intestine mucosal α-glucosidases have been reported to play an important role by influencing the rate, location and extent of glucose release which is associated with high glycaemia-related health issues such as diabetes and other metabolic syndromes (7). Nevertheless, recent research is addressing this caveat by developing alternative in vitro methodology aiming to better model the interaction of the carbohydrate with small intestine mucosal carbohydrases as explained below.
Development of Alternative in vitro Models to Assess Carbohydrate Digestibility Using Mammalian Small Intestinal Brush Border Carbohydrases
TIMcarbo: A Dynamic GI Tract Model Mimicking the in vivo Physical Processing and Temporal Changes in Luminal Conditions
This method has been built on the well-known TNO Gastro-Intestinal Model (TIM), which is a multi-compartmental dynamic model that was developed by TNO in The Netherlands in the early 1990s in response to industrial demand to study food products under more physiologically relevant conditions as compared to contemporary digestion models (13, 14). The TIMcarbo model includes an oral digestion phase by using a model of artificial mastication with a bacterial salivary α-amylase. After the luminal gastrointestinal digestion, which is simulated by adding pancreatic juice containing proteases, lipases and amylase, among other factors, the TIMcarbo mimics the carbohydrate uptake by the epithelium. This is achieved by dialysis of the products of digestion by a commercial rat brush border enzyme extract complemented with a bacterial β-galactosidase (15). The use of a microbial lactase is justified by the fact that the rat brush border extract contains a much lower activity than that found in humans. Finally, the in vitro methodology is combined with an in silico kinetic modeling that allows the prediction of glycemic response curves in humans.
This model is highly relevant to studying the digestion of whole foods and meals where the physical condition of the digesta changes over time, e.g., viscosity or particle size reduction that are key determinants of the rate of carbohydrate digestion and absorption in vivo (5, 16, 17). However, this refined method is not so suitable for screening studies or for testing the digestibility of carbohydrates available at low amounts, which is usually a limiting factor when the digestibility of novel carbohydrates produced at laboratory scale needs to be assessed.
Batch in vitro Digestion Assays Using Rat or Pig Small Intestine Mucosal Carbohydrases
Batch models do not pretend to mimic the physical and mechanical processes that occur in vivo. Consequently, in this type of digestion model the products of digestion are not removed and therefore, they do not model the absorption process. However, this type of model is especially suited for assessing the digestibility of isolated carbohydrates or single foods and can provide valuable information on mechanistic studies.
In line with the brush border enzyme digestion included in the TIMCarbo model, the use of rat small intestinal extract has been successfully applied for the assessment of digestibility of several prebiotic carbohydrates. These are non-digestible carbohydrates with the ability to selectively modulate the composition and/or activity of the gut microbiota, thus conferring benefit(s) upon host health (18), such as fructooligosaccharides (19), galactooligosaccharides (20, 21), or isomaltooligosaccharides (22).
In addition to the use of the rat intestinal extract, Lee et al. (23) investigated the contribution of the four main individual recombinant mucosal α-glucosidases (that is, glucoamylase, maltase, sucrase and isomaltase) on a range of unusual α-linked glycemic disaccharides with two glucose units. These authors provided intriguing differences in hydrolytic properties of the individual rat mucosal α-glucosidases to disaccharide substrates differing in glycosidic linkages. Furthermore, they concluded that mammalian mucosal carbohydrases must be used in in vitro assessment of digestion of glycemic carbohydrates instead of microbial digestive enzymes. More recently, a newly developed in vitro digestion model, based on the use of a commercial rat small intestinal extract under physiological conditions of temperature and pH, was successful for evaluating the digestibility of dietary oligosaccharides of degree of polymerization up to four with a requirement for a minimum of 0.5 mg of carbohydrate (24). This method demonstrated its capacity to distinguish between well-recognized digestible and non-digestible carbohydrates; the tested digestible carbohydrates were readily hydrolyzed whereas the oligosaccharides classified as non-digestible were barely hydrolyzed. Remarkably, a good correlation was found between this in vitro method and in vivo data collected on growing (25) or neonatal (26) rats and from aspiration of the gut content at the terminal ileum of healthy humans (27) on galactooligosaccharides and fructooligosaccharides digestion.
The high physiological and anatomical similarity of the pig and human digestive tracts (28) may provide additional advantages of using pig small intestinal material instead of that from rats. Thus, alternative methods based on the use of mammalian intestinal enzymes derived from pigs (6) or weaning piglets (29) have recently been proposed. Moreover, Tanabe et al. (30) successfully applied an improved AOAC 2009.01 method by using porcine small intestinal enzyme instead of fungal amyloglucosidase to accurately determine non-digestible oligosaccharides in marketed food products. In this context, the use of small intestinal brush border membrane vesicles conveniently isolated and purified from post-weaned pigs has recently shown to be a reliable and straightforward strategy to evaluate prebiotic carbohydrate digestibility, under physiological conditions of pH, temperature and time. These prebiotics included lactulose, different mixtures of commercial galactooligosaccharides, and an emerging prebiotic as novel galactooligosaccharides derived from lactulose (31). Recently, a pioneering approach, based on the study of the trans-β-galactosylation activity of the pig β-galactosidase embedded in the brush border membrane vesicles, has provided significant insights into the reaction mechanisms involved in the human digestion of dietary carbohydrates and has informed the development of non- or slowly-digestible carbohydrates (32). Lastly, a novel optimized in vivo and in vitro ileal fermentation assay, based on growing pigs as an animal model for simulating digestion in human adults, has been reported last year (33). Interestingly, the predicted values for ileal organic matter digestibility (calculated on the basis of the in vivo/in vitro ileal fermentation) were quite similar to the values measured in vivo, warranting the potential use of appropriate, and refined in vitro approaches using intestinal material from growing pigs to replace in vivo studies, in relation to ileal digestibility.
The Need for Harmonization and Standardization of an in vitro Test Methodology for Assessing Digestibility of Dietary Carbohydrates
Despite recent advances in developing in vitro methods to assess carbohydrate digestibility, there is an obvious need to design a standardized batch gastrointestinal digestion method based on physiologically relevant conditions. By considering the state-of-art, it is clear that the mucosal disaccharidases embedded in the small intestinal brush border membrane vesicles must be considered in addition to α-amylases, since they play a key role in the digestion of carbohydrates and in the subsequent uptake of monosaccharides by the intestinal mucosa (Table 1). Additionally, the use of mammalian digestive enzymes should be prioritized over the use of microbial enzymes since the former better reflect the carbohydrase activities of enzymes of the human gastrointestinal tract. However, there are still important uncertainties that prevent the development of a standardized methodology in the short-term. These include (but not exclusively) the following aspects:
(i) A large variety of enzymes from different sources have been used to date, also differing in the assays used to determine the enzyme activity. This clearly impairs an appropriate characterization of the carbohydrases used as well as the comparison of outcomes from different studies.
(ii) The regular supply of commercial pig mucosal small intestinal enzymes is nonexistent whilst that of rats is restricted.
(iii) The expression of mucosal carbohydrases greatly varies among the different segments of the small intestine and is also driven by the type of diet, as well as the host genetics, health status and age, among other factors (34).
Possible solutions to overcome these drawbacks could be the production of individual recombinant mucosal carbohydrases that should provide an easier handling, as well as a more predictable and controllable enzyme activity (23). Likewise, estimations of the relative amount of the different mammalian mucosal carbohydrases have been already reported (35). These values could be useful to establish a physiologically relevant ratio among the different mucosal carbohydrases. Thus, the sucrase-isomaltase complex seems to be the major mucosal carbohydrase, followed by the maltase-glucoamylase complex, whereas the trehalase is the less abundant enzyme (36). Finally, a key aspect that could help to drive harmonization and allow more effective comparison of different in vitro digestibility conditions would be to include appropriate suits of control digestible carbohydrates (for instance, maltose, sucrose, starch-derived oligosaccharides, lactose, etc.) and well-recognized non-digestible comparator carbohydrates (such as lactulose or fructooligosaccharides).
Although there is still a considerable way to go in the full understanding of the physical and chemical dynamics of mammalian intestinal mucosal carbohydrases, the growing relevance, based on their beneficial impact of human health, of non-digestible carbohydrates emphasizes the necessity of developing a cost-efficient, fit-for-purpose, easily implemented, and physiologically relevant in vitro methodology which considers all human carbohydrases involved in dietary carbohydrate digestion.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work has been financed by the Spanish Ministry of Economy, Industry and Competitiveness (Project AGL2017-84614-C2-1-R).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bohn T, Carriere F, Day L, Deglaire A, Egger L, Freitas D, et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit Rev Food Sci Nutr. (2018) 58:2239–61. doi: 10.1080/10408398.2017.1315362
2. Sousa T, Paterson R, Moore V, Carlsson A, Abrahamsson B, Basit AW. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. (2008) 363:1–25. doi: 10.1016/j.ijpharm.2008.07.009
3. Southgate DAT. Determination of carbohydrates in foods I. – Available carbohydrate. J Sci Food Agri. (1969) 20:326–30.
4. Southgate DAT. Determination of carbohydrates in foods II. – Unavailable carbohydrates. J Sci Food Agric. (1969) 20:331–5.
5. Woolnough JW, Monro JA, Brennan CS, Bird AR. Simulating human carbohydrate digestion in vitro: a review of methods and the need for standardization. Int J Food Sci Technol. (2008) 43:2245–56. doi: 10.1111/j.1365-2621.2008.01862.x
6. Tanabe K, Nakamura S, Oku T. Inaccuracy of AOAC method 2009.01 with amyloglucosidase for measuring non-digestible oligosaccharides and proposal for an improvement of the method. Food Chem. (2014) 151:539–46. doi: 10.1016/j.foodchem.2013.11.121
7. Lin AH-M, Lee B-H, Chang W-J. Small intestine mucosal α-glucosidase: a missing feature of in vitro starch digestibility. Food Hydrocolloids. (2016) 53:163–71. doi: 10.1016/j.foodhyd.2015.03.002
8. Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucl Acids Res. (2014) 42:D490–5. doi: 10.1093/nar/gkt1178
9. Alpers DH. Carbohydrates / Digestion, absorption, and metabolism. In: Caballero, B editor. Encyclopedia of Food Sciences and Nutrition, 2nd ed. Cambridge, MA: Elsevier (2003). p. 881–87.
10. Meisenberg G, Simmons WH. Digestive enzymes. In: Meisenberg, G and Simmons, WH editor. Principles of Medical Biochemistry, 4th ed. Amsterdam: Elsevier (2016). p. 342–50.
12. Picariello G, Ferranti P, Addeo F. Use of brush border membrane vesicles to simulate the human intestinal digestion. Food Res Int. (2016) 88:327–35. doi: 10.1016/j.foodres.2015.11.002
13. Minekus M, Marteau P, Havenaar R, Huis in't Veld J. A multi-compartmental dynamic computer-controlled model simulating the stomach and small intestine. ATLA. (1995) 23:197–209.
14. Minekus M. The TNO Gastro-Intestinal Model (TIM). In: Verhoeckx, K et al., editors. The Impact of Food BioActives on Health. Heidelberg: Springer (2015). p. 37–46. doi: 10.1007/978-3-319-16104-4
15. Bellmann S, Minekus M, Sanders P, Bosgra S, Havenaar R. Human glycemic response curves after intake of carbohydrate foods are accurately predicted by combining in vitro gastrointestinal digestion with in silico kinetic modeling. Clin Nutr Exp. (2018) 17:8–22. doi: 10.1016/j.yclnex.2017.10.003
16. Lifschitz CH, Grusak MA, Butte NF. Carbohydrate digestion in humans from a β-glucan-enriched barley is reduced. J Nutr. (2002) 132:2593–6. doi: 10.1093/jn/132.9.2593
17. Turnbull CM, Baxter AL, Johnson SK. Water-binding capacity and viscosity of Australian sweet lupin kernel fibre under in vitro conditions simulating the human upper gastrointestinal tract. Int J Food Sci Nutr. (2005) 56:87–94. doi: 10.1080/09637480500081080
18. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
19. Oku T, Tokunaga T, Hosoya N. Nondigestibility of a new sweetener, “Neosugar,” in the rat. J Nutr. (1984) 114:1574–81.
20. Ohtsuka K, Tsuji K, Nakagawa Y, Ueda H, Ozawa O, Uchida T, et al. Availability of 4′galactosyllactose (O-β-D-Galactopyranosyl-(1→ 4)-O-β-D-Galactopyranosyl-(1→ 4)-D-Glucopyranose) in rat. J Nutr Sci Vitaminol. (1990) 36:265–76.
21. Ferreira-Lazarte A, Montilla A, Mulet-Cabero A-I, Rigby N, Olano A, Mackie A, et al. Study on the digestion of milk with prebiotic carbohydrates in a simulated gastrointestinal model. J Funct Foods. (2017) 33:149–54. doi: 10.1016/j.jff.2017.03.031
22. Kaulpiboon J, Rudeekulthamrong P, Watanasatitarpa S, Ito K, Pongsawasdi P. Synthesis of long-chain isomaltooligosaccharides from tapioca starch and an in vitro investigation of their prebiotic properties. J Mol Catal B. (2015) 120:127–35. doi: 10.1016/j.molcatb.2015.07.004
23. Lee B-H, Rose DR, Lin AH-M, Quezada-Calvillo R, Nichols BL, Hamaker BR. Contribution of the individual small intestinal α-glucosidases to digestion of unusual α-linked glycemic disaccharides. J Agric Food Chem. (2016) 64:6487–94. doi: 10.1021/acs.jafc.6b01816
24. Ferreira-Lazarte A, Olano A, Villamiel M, Moreno FJ. Assessment of in vitro digestibility of dietary carbohydrates using rat small intestinal extract. J Agric Food Chem. (2017) 65:8046–53. doi: 10.1021/acs.jafc.7b01809
25. Hernández-Hernández O, Marín-Manzano MC, Rubio LA, Moreno FJ, Sanz ML, Clemente A. Monomer and linkage type of galacto-oligosaccharides affect their resistance to ileal digestion and prebiotic properties in rats. J Nutr. (2012) 142:1232–9. doi: 10.3945/jn.111.15576223
26. Jantscher-Krenn E, Marx C, Bode, L. Human milk oligosaccharides are differentially metabolized in neonatal rats. Br J Nutr. (2013) 110:640–50. doi: 10.1017/S0007114512005727
27. Molis C, Flourié B, Ouarne F, Gailing MF, Lartigue S, Guibert A et al. Digestion, excretion, and energy value of fructooligosaccharides in healthy humans. Am J Clin Nutr. (1996) 64:324–8. doi: 10.1093/ajcn/64.3.324
28. Miller ER, Ullrey DE. The pig as a model for human nutrition. Annu Rev Nutr. (1987) 7:361–82. doi: 10.1146/annurev.nu.07.070187.002045
29. Strube ML, Ravn HC, Ingerslev H-C, Meyer AS, Boye M. In situ prebiotics for weaning piglets: in vitro production and fermentation of potato galacto-rhamnogalacturonan. Appl Environ Microbiol. (2015) 81:1668–78. doi: 10.1128/AEM.03582-14
30. Tanabe K, Nakamura S, Omagari K, Oku T. Determination trial of nondigestible oligosaccharide in processed foods by improved AOAC method 2009.01 using porcine small intestinal enzyme. J Agric Food Chem. (2015) 63:5747–52. doi: 10.1021/jf505844y
31. Ferreira-Lazarte A, Gallego-Lobillo P, Moreno FJ, Villamiel M, Hernández-Hernández O. In vitro digestibility of galactooligosaccharides: effect of the structural features on their intestinal degradation. J Agric Food Chem. (2019) 67:4662–70. doi: 10.1021/acs.jafc.9b00417
32. Julio-Gonzalez LC, Hernandez-Hernandez O, Moreno FJ, Olano A, Jimeno ML, Corzo N. Trans-β-galactosidase activity of pig enzymes embedded in the small intestinal brush border membrane vesicles. Sci Rep. (2019) 9:960. doi: 10.1038/s41598-018-37582-8
33. Montoya CA, de Haas ES, Moughan PJ. Development of an in vivo and in vitro ileal fermentation method in a growing pig model. J Nutr. (2018) 148:298–305. doi: 10.1093/jn/nxx038
34. Hooton D, Lentle R, Monro J, Wickham M, Simpson R. The secretion and action of brush border enzymes in the mammalian small intestine. Rev Physiol Biochem Pharmacol. (2015) 168:59–118. doi: 10.1007/112_2015_24
35. Semenza G, Auricchio S, Mantei N. Small intestinal disaccharidases. In:. Scriver, CR, Sly, ALS, Beaudet, W, and David, V editors. Metabolic Basis of Inherited Disease, Vol. II, 8th ed. New York, NY: McGraw-Hill (2001) 1623–50.
Keywords: non-digestible carbohydrates, slowly digestible carbohydrates, brush border enzymes, carbohydrases, non-starch oligosaccharides, small intestinal brush border membrane vesicles
Citation: Hernandez-Hernandez O, Olano A, Rastall RA and Moreno FJ (2019) In vitro Digestibility of Dietary Carbohydrates: Toward a Standardized Methodology Beyond Amylolytic and Microbial Enzymes. Front. Nutr. 6:61. doi: 10.3389/fnut.2019.00061
Received: 25 February 2019; Accepted: 17 April 2019;
Published: 07 May 2019.
Edited by:
José M. Alvarez-Suarez, University of the Americas, EcuadorReviewed by:
Jie Yin, Institute of Subtropical Agriculture (CAS), ChinaAndrea Gomez-Zavaglia, National University of La Plata, Argentina
Copyright © 2019 Hernandez-Hernandez, Olano, Rastall and Moreno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Javier Moreno, amF2aWVyLm1vcmVub0Bjc2ljLmVz
 Oswaldo Hernandez-Hernandez
Oswaldo Hernandez-Hernandez Agustín Olano1
Agustín Olano1 Robert A. Rastall
Robert A. Rastall F. Javier Moreno
F. Javier Moreno