- 1Department of Neurology, the First Hospital of Hebei Medical University, Shijiazhuang, China
- 2Brain Aging and Cognitive Neuroscience Key Laboratory of Hebei, Shijiazhuang, China
- 3Department of Neurology, the First Hospital of Shijiazhuang, Shijiazhuang, China
- 4Emergency Department, CNPC Central Hospital, Langfang, China
Normal aging is accompanied by hippocampus-dependent cognitive impairment, which is a risk factor of Alzheimer’s disease. This study aims to investigate the effect of high frequency-repetitive transcranial magnetic stimulation (HF-rTMS) on hippocampus-dependent learning and memory in aged mice and explore its underlying mechanisms. Forty-five male Kunming mice (15 months old) were randomly divided into three groups: aged sham, 5 Hz rTMS, and 25 Hz rTMS. Two sessions of 5 Hz or 25 Hz rTMS comprising 1,000 pulses in 10 trains were delivered once a day for 14 consecutive days. The aged sham group was treated by the reverse side of the coil. In the adult sham group, 15 male Kunming mice (3 months old) were treated the same way as the aged sham group. A Morris water maze (MWM) was conducted following the stimulation, and synaptic ultrastructure was observed through a transmission electron microscope. HF-rTMS improved spatial learning and memory impairment in the aged mice, and 5 Hz was more significant than 25 Hz. Synaptic plasticity-associated gene profiles were modified by HF-rTMS, especially neurotrophin signaling pathways and cyclic adenosine monophosphate response element binding protein (CREB) cofactors. Compared to the aged sham group, synaptic plasticity-associated proteins, i.e., synaptophysin (SYN) and postsynaptic density (PSD)-95 were increased; brain-derived neurotrophic factor (BDNF) and phosphorylated CREB (pCREB) significantly increased after the 5 Hz HF-rTMS treatment. Collectively, our results suggest that HF-rTMS ameliorated cognitive deficits in naturally aged mice. The 5 Hz rTMS treatment significantly enhanced synaptic structural plasticity and activated the BDNF/CREB pathway in the hippocampus.
Introduction
Increased human longevity has magnified the negative impact that aging can have on cognitive integrity in older individuals (Foster et al., 2017). Normal aging is associated with declining cognitive function, which may result from changes in hippocampus circuits (Vanguilder and Freeman, 2011). Hippocampal-functional dependent learning and memory capabilities are vulnerable during brain aging (Gray and Barnes, 2015), and these involve attention, working memory, and episodic memory (Rosenzweig and Barnes, 2003).
Synaptic plasticity is crucial for the involvement of hippocampal neurocircuitry in spatial cognition (Torii et al., 2012). It is well accepted that synaptic plasticity in the normal aging process is still reserved in the hippocampus and provides opportunities for potential interventions in prodromal stages before dementia.
Repetitive transcranial magnetic stimulation (rTMS) is a safe, inexpensive, noninvasive extracranial stimulation method. Delivering electrical stimuli to excite or inhibit the brain can affect neuron activity so as to play different roles in neural modulation by adjusting stimulation parameters such as frequency and intensity (Houdayer et al., 2008). Generally, high-frequency stimulation (HF-rTMS) is considered to evoke neural excitability (Medina and Túnez, 2013). At present, rTMS is widely used in clinical treatment mainly for neurological and psychological diseases (Kim et al., 2010); for example, by ameliorating the degree of depression, reducing the motor symptoms associated with Parkinson’s disease, and regulating cognition and mood in stroke patients (Guse et al., 2010; Liao et al., 2015). Some neuroscientific studies have shown that HF-rTMS (e.g., 10 Hz, 15 Hz, or 20 Hz) applied over the left dorsolateral prefrontal cortex (DLPFC) with a motor threshold within a range of 80%–110% was likely to bring out significant cognitive improvement in adult patients or healthy volunteers (Cattaneo and Silvanto, 2008).
Since rTMS is known as state-dependent (Kim et al., 2012), whether it plays the same role in an “aged” context that it does in adults is being considered. Additionally, a five-daily 10 Hz HF-rTMS was reported to improve attentional control in normally aging individuals (Cui et al., 2013). To establish a mouse model that mimics human aging and use for general cognitive changes, an outbred stock of Kunming (KM) mice (Chen et al., 2004), derived from the Swiss albino mouse, is widely employed in studies on neuroscience. Because of the high heterogeneous background, these mice are more similar than inbred stock are to the human population. Age-related cognitive impairment in KM mice has been well confirmed (Hoogendam et al., 2010; Wang et al., 2015). Previous work has demonstrated that 25 Hz HF-rTMS improved non-spatial memory performance in aged KM mice, regulated neuronal excitability, and modified voltage-dependent Ca2+ channels compared to age-matched sham mice (Hoogendam et al., 2010). However, whether spatial learning and memory are influenced by HF-rTMS is to be confirmed in this study.
It has been reported that the therapeutic effects of rTMS are coordinated by several signaling pathways (e.g., brain-derived neurotrophic factor, BDNF pathway) and networks involved in the brain’s structural and functional regulation (Kim et al., 2010; Pell et al., 2011; Chang et al., 2016). rTMS-induced excitability modulation shares common characteristics with long-term potentiation (LTP)/long-term depression (LTD)-induced synaptic plasticity (Ma et al., 2014). Whether the conditioning effect of HF-rTMS on the aged brain recruits the mechanism of synaptic plasticity remains largely unclear. Ma et al. (2014) reported that the neuronal structural plasticity-related gene and protein (e.g., synaptophysin, SYN and PSD95) that were involved in low-frequency rTMS were found to reduce the improvement of cognitive function in aged mice (Vorhees and Williams, 2006). However, the mechanisms regarding synaptic plasticity for HF-rTMS in modifying age-related cognitive impairment remain to be discussed.
Different TMS frequencies have different effects on cognition in animal experiments. Zhang et al. (2015) showed that a 5 Hz frequency stimulation could improve spatial learning and memory ability in rats with vascular dementia. Other studies have shown that low-frequency magnetic stimulation can significantly improve learning and memory function in rats (Trebbastoni et al., 2016). Previous studies have also shown that low-frequency rTMS can improve learning and memory ability in aged mice; even at 1 Hz rTMS, they still showed cognitive improvement (Huang et al., 2017). However, preliminary studies from our laboratory suggest that high-frequency magnetic stimulation may be more effective than low-frequency magnetic stimulation for cognitive improvement (data not shown). Therefore, 5 Hz and 25 Hz stimulation frequencies were selected for observation in this study as the commonly used high-frequency range (5–25 Hz).
Here, we aim to identify the role and possible mechanisms of HF-rTMS on hippocampus-dependent cognitive impairment in aged mice.
Materials and Methods
Animals
Forty-five aged (15 months old) and 15 adult (3 months old) male KM mice were purchased from the Experimental Animal Center of Hebei Medical University (Shijiazhuang, China) and raised under temperature conditions of 20–22°C and a 12-h light-dark cycle. Mice had access to food and water ad libitum. All animal experiments were performed under an animal study protocol approved by the ethics committee of Hebei Medical University.
HF-rTMS Treatment
The detailed stimulation procedures, including parameters and patterns, were described in previous research (Hoogendam et al., 2010). Briefly, HF-rTMS was performed using an MCF-B65 butterfly coil (outer diameter 90 mm) connected to a MagProX100 magnetic stimulator (active pulse width 280 μs, maximum output 4.2 T, MagVenture, Denmark). Aged mice (15 months old) were randomly divided into three groups (n = 15 for each group): aged sham, 5 Hz rTMS, and 25 Hz rTMS. In the rTMS groups, mice were exposed to HF-rTMS (5 Hz or 25 Hz) with the coil placed 1 cm above the mouse’s head for 14 consecutive days, 100 pulses per train, with a 30 s interval at 20% maximum output (0.84T) and 10 trains daily. In the aged sham group, mice were treated in a similar way to the aged rTMS mice using the reverse side of the coil without the rTMS effect. In the adult sham group (3 months old, n = 15), mice were treated in the same way as the aged sham group with the reverse side of the coil. During the procession of rTMS or sham rTMS, mice were fixed calmly with a flexible plastic tube with holes at both ends. The small hole on the head end was kept to enable them to breathe, and the bigger hole was suitable for the mice to probe into the tube; a sponge was used to fix the mice gently at the tail end.
MWM for Spatial Learning and Memory Test
The Morris water maze (MWM) test was carried out according to the protocols modified from Morris (1984) to assess the spatial-related form of learning and memory (Han et al., 2016). The water maze was a circular pool (with a diameter of 120 cm and a height of 60 cm). The pool was divided into four quadrants (I, II, III, and IV) by two imaginary lines. The water depth in the pool was 45 cm, just 1 cm above the platform, with a temperature in the range of 20–22°C. Before the test, a non-toxic black pigment was added to make the water opaque so that the platform became invisible to the mice. The platform (8 cm in diameter) was placed in the middle of the target quadrant (e.g., quadrant I) and maintained during spatial navigation. The surroundings of the pool were shaded by curtains, and the indoor light brightness was adjusted to avoid the reflection of the water surface in the video capture system, which was 2 m above the water maze. Quiet was kept throughout training and testing. The start positions of the MWM in each trial were randomized. In the place navigation test (representing learning ability), all mice were released once at each of the four start positions on each day for five consecutive days. Mice were allowed to search for the platform for a maximum of 60 s. The time started when the rats were put into the water and ended when they found and climbed onto the platform; this was recorded as “escape latency.” Mice that could not find the platform within 60 s were led to the platform and allowed to stay for 10 s to become familiar with the environment and the platform position. In this case, escape latency was considered as 60 s. After navigation, a spatial probe trial was performed on day six. The platform was removed, and the mice were released at an additional probe position. Mice were allowed to search for the platform for 60 s. The ratio of swimming distance in the original quadrant to the total distance (distance ratio) and the time of platform crossing in the original quadrant were recorded and analyzed by the ANY-maze video-tracking analysis system (Stoelting, USA).
Transmission Electron Microscope Analysis for Synapses
Following the procedure in our former research (Annoni et al., 2016), mice (n = 3/group) from the three aged groups were anesthetized and perfused with perfusion fluid containing 3% paraformaldehyde and 1% glutaraldehyde in 0.1 M PB (pH 7.4). Hippocampi were separated and stored in 4% glutaraldehyde. Hippocampi were then trimmed to approximately 1 mm3 and cut into sections (100 μm). The sections were fixed using osmium acid, dehydrated in ethanol, embedded in araldite, and stained with toluidine blue. The cell layer was located in the field of view of the light microscope. The sections at a thickness of 70 nm were prepared from the middle third of the CA1 stratum radiatum using an ultramicrotome and collected on Formvar-coated single-slot grids, followed by a double-stain with uranyl acetate and lead citrate. Images were captured with iTEM software for Hitachi 7500 transmission electron microscopy (Electron Microscopy Experimental Center of Hebei Medical University, Shijiazhuang, China). The average thickness of postsynaptic density (PSD), the width of synaptic cleft, and the ratio of perforated synapse and synaptic curvature were quantified and analyzed with NIS-Elements BR software.
Real-Time Polymerase Chain Reaction Gene Array
At 7 days after rTMS, i.e., 1 day after the MWM test, mice (n = 6/group) in the three aged groups were sacrificed. Total RNA was isolated with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) from the hippocampus tissue. An equal amount of RNA was then converted into cDNA using SABiosciences’ RT2 First Strand Kit (Qiagen, Valencia, CA, USA) as per the manufacturer’s protocol. Afterward, complementary DNA was amplified by polymerase chain reaction (PCR) using RT2 SYBR® Green qPCR Mastermix (Qiagen, Valencia, CA, USA). Synaptic plasticity gene profiling was done using a 96-well format RT2 Profile PCR Array Mouse Synaptic Plasticity kit (SABiosciences, Qiagen, Valencia, CA, USA) with an ABI7500 qRT-PCR instrument (Applied Biosystems, Foster City, CA, USA; Zhang et al., 2012). This array analyzed 84 genes involved in mouse synaptic plasticity, including 30 immediate-early response and two late response genes, 28 genes involved in LTP, 21 LTD genes, 19 neuronal receptors, 15 PSD genes, 10 cyclic adenosine monophosphate response element binding protein (CREB) cofactors, nine cell adhesion genes, five extracellular matrix and proteolytic processing genes as well as two other genes involved in synaptic plasticity. Data were normalized for GAPDH levels by the ΔΔCt method. The relative abundance of each mRNA species was assessed and analyzed using programs provided by the manufacturer, and individual genes were analyzed using SDS software. A gene was considered differentially regulated if the difference was ≥1.5-fold and P-values were <0.05 compared to the aged sham group.
Western Blotting for Synapse Plasticity-Related Proteins
At 7 days after rTMS, the aged mice (n = 6/group) were sacrificed, and the hippocampus tissues were homogenized in a lysis buffer containing 20 mM Tris–HCl (pH 7.4), 0.5% Triton X-100, and HaltTM protease inhibitors (87785, Thermo Scientific). Protein concentrations were determined by the Bradford method. Samples were denatured after boiling for 10 min. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in 5% skimmed milk in Tris-HCl containing 0.1% Tween-20 and then probed overnight with primary antibodies, respectively, i.e., anti-SYN (1:2,000, Abcam, Cambridge, MA, USA), anti-PSD95 (1:2,000, Abcam, Cambridge, MA, USA), anti-CREB (1:1,000, Abcam, Cambridge, MA, USA), anti-phospho-CREB (anti-pCREB; 1:2,000, Abcam, Cambridge, MA, USA), anti-brain derived neurotrophic factor (anti-BDNF, 1:2,000, Abcam, Cambridge, MA, USA), anti-β-actin (1:2,000, Abcam, Cambridge, MA, USA), and anti-GAPDH (1:2,000, Abcam, Cambridge, MA, USA). Membranes were then washed in TBST [150 mmol/L of NaCl, 50 mmol/L of Tris (pH 7.4), and 0.1% Tween-20] and incubated with anti-rabbit immunoglobulin G (IgG) horseradish-peroxidase-conjugated secondary antibodies for 2 h. The bands were immunodetected using an enhanced chemiluminescence kit (ECL, Millipore, and Temecula, CA, USA), detected using the Odyssey IR fluorescence scanning imaging system, and quantified by densitometric analysis using the ALPHA analytical system. β-actin or GAPDH was used as an internal control. The ratio of OD value was calculated for relative protein expression level (Zhang et al., 2013).
Statistical Analysis
Data is presented as the mean ± standard error of the mean (SEM) and analyzed with SPSS 22.0 statistical software (IBM, Chicago, IL, USA). Student’s t-test was used for the spatial navigation test and gene differential expression. Before the t-test, an F-test was conducted to determine the equality of the variances of the two normal populations. A P-value < 0.05 was considered statistically significant.
Results
HF-rTMS Improved Spatial Learning and Memory Performance in Aged Mice
Spatial navigation: the mean escape latency in the aged groups (aged sham, 5 Hz, and 25 Hz rTMS) was significantly increased compared to the adult sham group (P < 0.05). Compared to the aged sham group, the mean escape latency decreased significantly in the 5 Hz (P < 0.01) and 25 Hz rTMS groups (P < 0.05, Figure 1A). Spatial probe: compared to the adult sham group, the distance ratio of the aged sham and 25 Hz groups were significantly increased (P < 0.05, Figure 1B), and the time of platform crossing was decreased in the aged sham group (P < 0.05, Figure 1C). Compared to the aged sham group, the distance ratio was significantly increased in the 5 Hz rTMS group (P < 0.05, Figure 1B), and the time of platform crossing was significantly increased in the 5 Hz and 25 Hz rTMS groups (P < 0.05, Figure 1C). In summary, spatial learning and memory were impaired in aged mice compared to adult mice. HF-rTMS improved spatial learning and memory ability in aged mice, and 5 Hz showed a more significant difference than did 25 Hz.
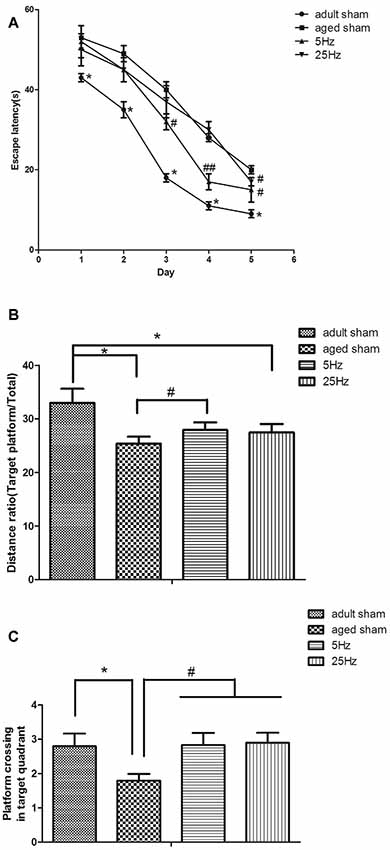
Figure 1. Repetitive transcranial magnetic stimulation (rTMS) is beneficial for spatial learning and memory in mice, as tested by the Morris water maze (MWM). (A) The mean escape latency in the spatial learning test. *P < 0.05 aged sham group vs. adult sham group; #P < 0.05 and ##P < 0.01 compared to the aged sham group. (B) The ratio of swimming distance in the original quadrant to the total distance (distance ratio). *P < 0.05 vs. adult sham group; #P < 0.05 vs. aged sham group. (C) The time of platform crossing in the original quadrant. *p < 0.05 vs. adult sham group; #P < 0.05 vs. aged sham group. Data are shown as mean ± standard error of the mean (SEM); n = 15 for each group.
Synaptic Ultrastructural Parameters Were Significantly Modulated by HF-rTMS
This study focused only on excitatory synapses. PSD thickness (nm), synaptic cleft width (nm), synaptic curvature, and the number of perforated synapses were counted in typical Gray I synapses (Figure 2). Compared to the aged sham group, PSD thickness was increased, and the width of the synaptic cleft was decreased significantly in the 5 Hz rTMS group (p < 0.05, Table 1); no significant difference of all the parameters was found in the 25 Hz rTMS group.
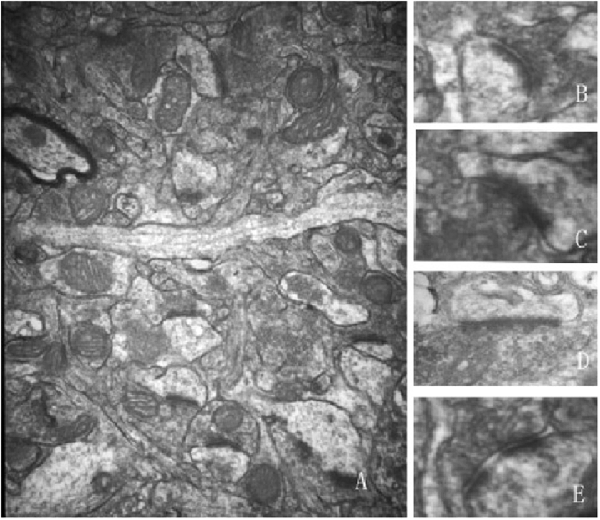
Figure 2. Ultrastructure alterations of the Gray I synapse were modulated and normalized by rTMS (40,000×). (A) A typical electron microscopic view for calculating and measuring synapses. Typical synaptic morphology is shown as: (B) convex shape; (C) concave shape; (D) plain shape; (E) perforated synapse. Synaptic parameters were calculated and analyzed after rTMS according to the microphotographs. Data are shown in Table 1.
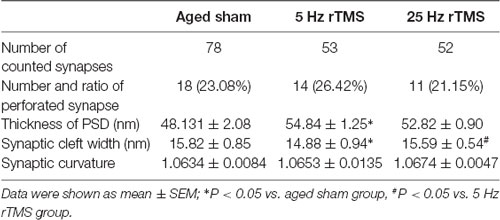
Table 1. Comparison of synaptic parameters in aged high frequency-repetitive transcranial magnetic stimulation (HF-rTMS) groups.
Synaptic Plasticity-Associated Gene Profiles Were Modified by HF-rTMS
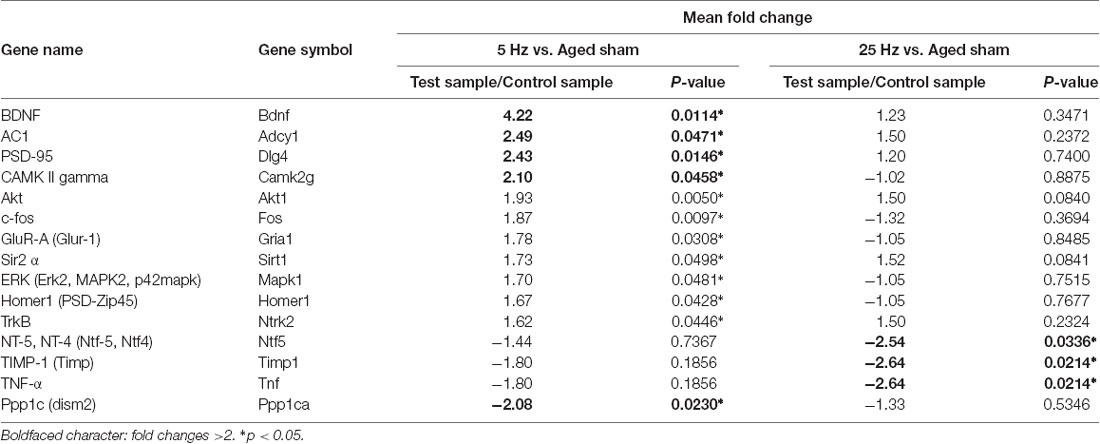
A different gene expression pattern was shown after a different HF-rTMS treatment. A more than 1.5-fold up-regulated or down-regulated change was considered as a differential expression (Table 2). Compared to the aged sham group, 12 genes were significantly regulated more than 1.5-fold in the 5 Hz rTMS group, and 4 of them up-regulated more than 2-fold: BDNF, AC1, PSD95, and CAMK II. The maximum up-regulated gene was BDNF at 4.22-fold. PP1 was the only significant down-regulated gene, at 2.8-fold (Table 3). As for the 25 Hz rTMS group vs. the aged sham group, three genes were found to be significantly changed, and all were down-regulated, i.e., Timp1, Tnf-α, and Nt-4/5, with a maximum of 2.64-fold for Timp1 (Table 3). CREB cofactors, IEGs, LTP, and PSD-related genes were significant based on their classification of differential gene expression. Compared with the KEGG pathway database, neurotrophin signaling pathways (in which BDNF, TrkB, Akt, CaMK, and CREB act as signaling molecules) were matched most for the 5 Hz rTMS group compared to the aged sham group; there was no significant matching for the 25 Hz rTMS group vs. the aged sham group.
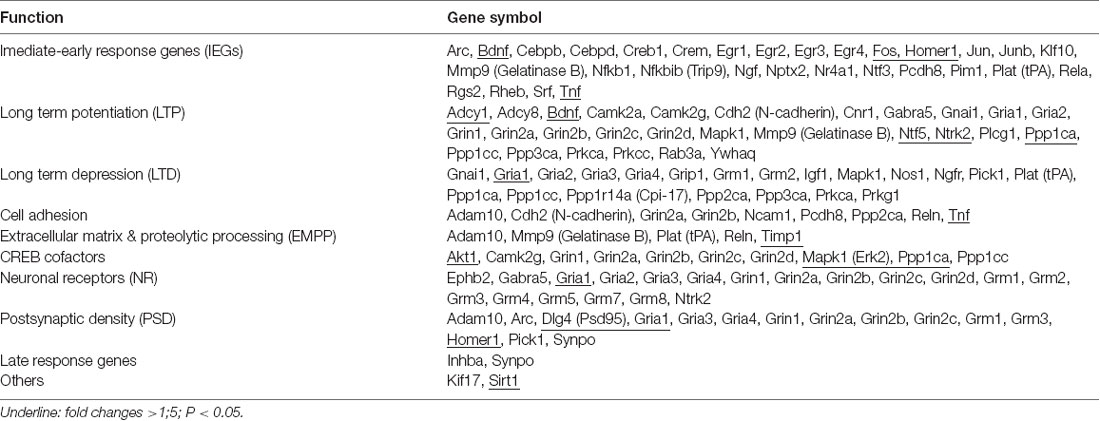
Table 2. Total 84 synaptic plasticity associated genes were classified as 10 groups according to the functions.
Effect of HF-rTMS on Synaptic Plasticity-Associated Proteins: SYN, PSD95, BDNF, and CREB
The pre- and post-synaptic markers assessed with western blotting are shown in Figure 3. Compared to the aged sham group, SYN and PSD95 both increased significantly in the rTMS groups. Compared to the 5 Hz rTMS group, no significant difference was found for either SYN or PSD95 in the 25 Hz group.
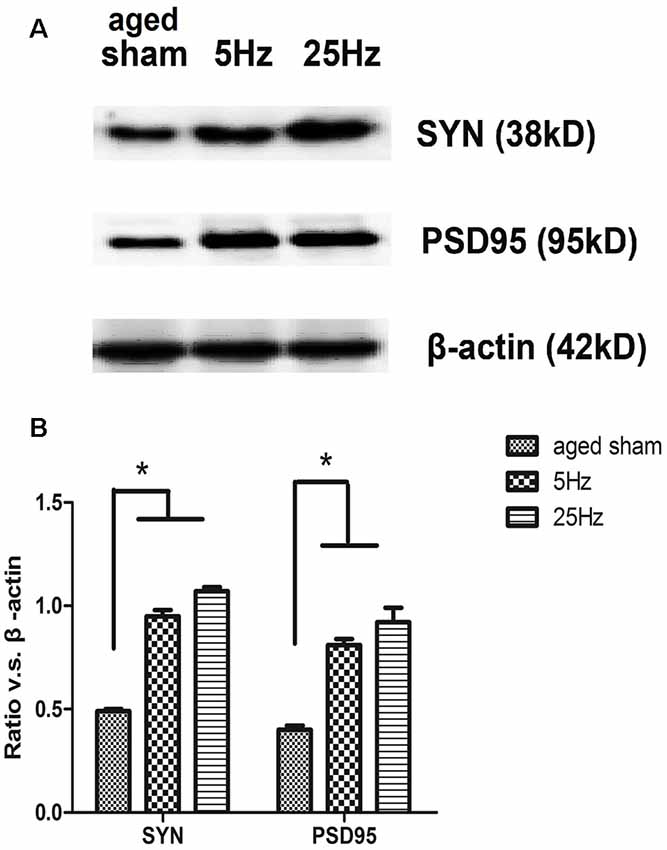
Figure 3. The expression of the synaptic marker in the hippocampus of aged mice after rTMS. (A) Western blotting bands for 7 days after rTMS. synaptophysin (SYN) and postsynaptic density (PSD)-95 levels were increased in both the 5 Hz rTMS and 25 Hz rTMS groups. (B) Statistic analysis of relative protein expression of SYN and PSD95. Ratio of OD value vs. β-actin is shown as mean ± SEM (n = 6 for each group). *p < 0.05 vs. sham group.
BDNF/CREB signaling cascade-associated proteins were additionally assessed by western blotting (Figure 4). Compared to the aged sham group, BDNF and pCREB increased significantly in the 5 Hz rTMS group (p < 0.05) but not in the 25 Hz group. No significant difference in total CREB was found among the groups.
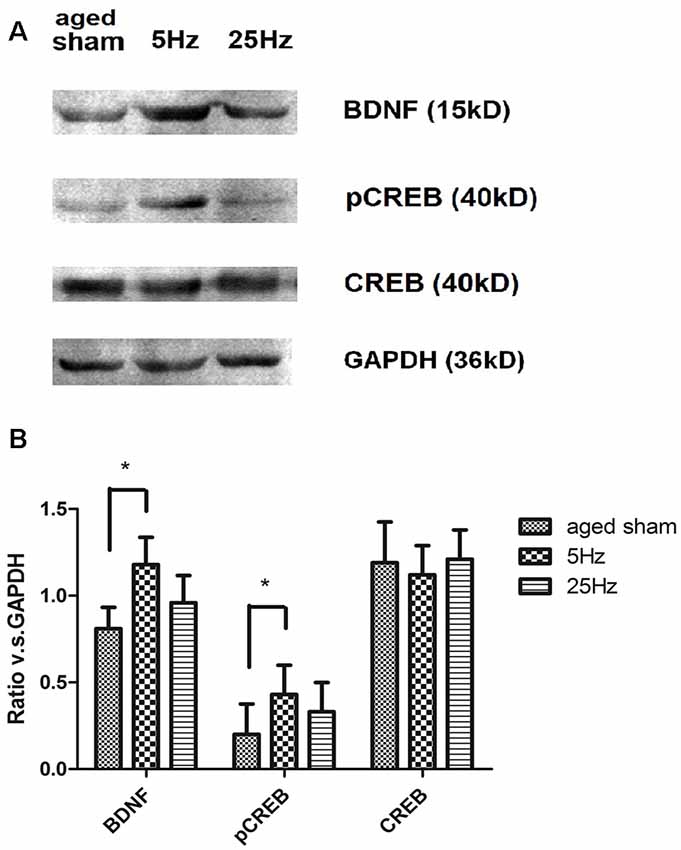
Figure 4. The effect of rTMS on brain-derived neurotrophic factor (BDNF), pCREB, and total CREB levels in the hippocampus 7 days after rTMS. (A) Western blotting bands for BDNF, total CREB, and pCREB in the hippocampus. (B) Statistic analysis of relative protein expression level of BDNF, total CREB, and pCREB. Ratio of OD value vs. GAPDH is shown as mean ± SEM; n = 6 for each group. *P < 0.05 vs. sham group.
Discussion
Age-related cognitive impairment is considered a pre-symptomatic phase of dementia, which may take decades to evolve (Allison et al., 2016). Aspects of spatial navigation may be particularly sensitive in detecting the earliest cognitive impairments of preclinical Alzheimer’s disease (Miniussi and Ruzzoli, 2013). Nowadays, rTMS has been demonstrated to be a key tool in the study of complex brain processes (Luber and Lisanby, 2014; Mok et al., 2016). In the present study, aged KM mice were used to study the effect of two frequencies of rTMS (5 Hz and 25 Hz) on spatial cognitive impairment via the MWM test. Identified with considerable evidence for the deleterious effects of aging on cognition (Zhang et al., 2015; Fabbri et al., 2016; Korthauer et al., 2016; Beason-Held et al., 2017), our behavioral data in the MWM test showed that spatial learning and memory were decreased in aged KM mice when compared to adult mice. However, coinciding with the report that 5 Hz rTMS improved the cognition of adult rats and vascular dementia (Olvera-Cortés et al., 2012; Shang et al., 2016), we found that, over 14 days, both the 5 Hz and 25 Hz HF-rTMS treatments can significantly improve spatial learning and memory ability in aged mice. Although both the 5 Hz and 25 Hz HF-rTMS stimulation groups had significant improvements in spatial learning, the extent of improvement in spatial memory appeared to be greater in the 5 Hz HF-rTMS group than in the 25 Hz HF-rTMS group. We speculated that the effects under a different frequency might delegate a different cognitive domain (Bouchard and Villeda, 2015). The 25 Hz HF-rTMS treatment might play a part in the acquisition of new information but not in memory retrieval. Despite the variability in targeted cognitive domains and outcomes, the results generally show an enhancement or uniform benefit for HF-rTMS.
At present, the effect of TMS on cognitive function is still controversial, and there are differing opinions on parameter setting, treatment duration, and efficacy evaluation methods. In recent years, most studies on the cognitive effects of TMS on healthy people tend to show that high-frequency TMS can significantly improve cognitive ability in normal subjects. Several scholars have reported that low-frequency TMS can improve the performance of working memory. Clinical experiments have found that different stimulation frequencies have different effects, sometimes even opposite effects. It was found that stimulating the left DLPFC for 2 weeks was more likely to improve cognitive function with frequencies of 5 Hz, 10 Hz, or 15 Hz, an intensity of 80%–110% motor threshold, and 10–15 consecutive sequences. Adult Patients were more likely to improve in cognitive function than were healthy volunteers. Other studies have confirmed that the cognitive improvement effect of 20 Hz TMS on Alzheimer’s patients can last for three to 6 months (Gorsler et al., 2003; Lenz et al., 2016).
Behavioral results indicated that 5 Hz and 25 Hz HR-rTMS could improve the spatial learning and non-spatial memory of aged mice. Overall, 5 Hz HF-rTMS might have a better effect on improving learning and memory than 25 Hz will.
It is becoming increasingly evident that latent plasticity, dormant in the aged CNS, could be reactivated as a means to rejuvenate cognitive functions late in life (Brehmer et al., 2014; Shen et al., 2015). Since functional plasticity has been reported in previous literature (Hoogendam et al., 2010), we focused on assessing the effects of HF-rTMS on synaptic structural plasticity. SYN and PSD95, which are markers of pre- and post-synapse, were increased by 5 Hz and 25 Hz rTMS on the protein level, suggesting a structure modification role of rTMS in the aged brain, in accordance with many studies (Vorhees and Williams, 2006; Cooperrider et al., 2014; Xiao et al., 2014; Wang et al., 2016). The increase in PSD thickness and decrease in cleft width might be the major morphological change during LTP’s induction, while the increase in the curvature of the synaptic interface and the number of perforated synapses might be responsible for its maintenance (Antonenko et al., 2016). The present result of synaptic ultrastructural parameters showed that PSD thickness increased and the cleft width decreased after HF-rTMS, especially significant in the 5 Hz group, while the synaptic curvature and percentage of perforated synapse did not significantly change. It is speculated that 5 Hz rTMS might play a role mainly in LTP induction; however, 25 Hz rTMS might not behave in the same way. The limitation of the study is that we only emphasize excitatory synapses and do not investigate inhibitory synapses on rTMS affecting GABAergic action in the hippocampus, which deserves further study.
Therefore, it is of great interest to study the putative neural mechanisms underlying synaptic plasticity changes after HF-rTMS in the particular context of the aged brain. Hippocampal pathway plasticity is associated with the ability to form novel memories in older adults (Roy et al., 2014). To investigate which plasticity-associated genes are affected by HF-rTMS, we performed an mRNA-based PCR array analysis of plasticity-related genes in the hippocampal tissues of three aged groups (Panja and Bramham, 2014; Verma et al., 2016). Results demonstrated that synaptic plasticity-associated gene profiles were modified by HF-rTMS. The genetic factor highlighted CREB cofactors (but not the CREB gene per se) and neurotrophin signaling pathways after the 5 Hz treatment. BDNF is a molecular neurotrophic factor that plays a key role in neuronal survival and plasticity. Decreased levels of BDNF are associated with neurodegenerative diseases with neuronal loss, such as Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and Huntington’s disease. BDNF has emerged as a regulator of stable, late-phase LTP at excitatory glutamatergic synapses in the adult brain (Ma et al., 2013; Kim et al., 2016). One of the downstream cascades activated by BDNF is the cascade linked to the transcription factor CREB pathway. Activated CREB (pCREB, the phosphorylation form of CREB) can lead to synaptic restructuring to support LTP (Paramanik and Thakur, 2013). The pCREB protein acts as a molecular switch for neuronal plasticity (Bramham and Messaoudi, 2005; Zhang et al., 2016) and is well known for its role in long-term memory. On the other hand, BDNF is also a downstream gene regulated by CREB (Carlezon et al., 2005; Ehrlich and Josselyn, 2016). Significant increases of pCREB and BDNF in the dentate gyrus of adult and aged mice were connected with better learning and spatial memory (Nam et al., 2014). BDNF acts in concert with pCREB for positive feedback regulation of structural and morphological plasticity (Hernandez and Abel, 2008).
The formation and storage of long-term memory require transcription and synthesis of new proteins (Rosa and Fahnestock, 2015). Because real-time PCR arrays hinted at noteworthy changes in BDNF and CREB cofactors in mRNA levels, which may not always correlate with protein expression (Alijanpour et al., 2015), we additionally assessed the differentially expressed genes for the BDNF, total, and phosphorylated CREB by western blotting. The pCREB/CREB ratio in the hippocampus increased in the mice that showed successful memory retrieval (Hellmann et al., 2012). In an in vitro system, repetitive magnetic stimulation of SH-SY5Y cells resulted in increased intracellular cAMP levels and increased pCREB. Similar results were obtained in this study that pCREB, but not CREB, was increased in vivo after HF-rTMS intervention. The increase in the BDNF-pCREB level may act as a modulator of synaptic plasticity, suggesting a possible mechanism for the protective role of 5 Hz HF-rTMS on memory impairment occurring during the normal aging process. Strategies that exploit upstream factors or that target specific CREB-regulated genes, rather than CREB itself, could make a promising contribution to the treatment of age-related cognition impairment. In regard to 25 Hz rTMS, mechanisms such as neurogenesis or anti-inflammation might play some role.
Because of the limitations in electrophysiological techniques for the aged brain and the limited number of aged animals, the functional change on LTP/LTD and related ion channels were not studied in this research. Furthermore, to elucidate the exact cascades of the signaling pathway involved in the process, biochemical and molecular changes will be detected with a special inhibitor and stimulator in our future work. Finally, another important consideration for future application of HF-rTMS as a new rehabilitation therapy is to investigate the intensity and coil location of HF-rTMS that improves elderly cognitive dysfunction.
Although further investigations are required to validate the results and fully understand the underlying mechanisms, in the present study, we investigated the effect of HF-rTMS on hippocampus-dependent cognitive impairment in aged mice. We reported that: (1) 5 Hz and 25 Hz HF-rTMS reverse hippocampus-dependent cognitive impairment, which (2) is accompanied by hippocampal structural plasticity. (3) These changes, especially under the 5 Hz rTMS treatment, depend at least in part on the activation of BDNF/CREB pathway.
Ethics Statement
All animal experiments were performed under an animal study protocol approved by the ethics committee of Hebei Medical University.
Author Contributions
The work presented here was carried out in collaboration between all authors. MW and YG defined the research theme. YG and HW carried out the laboratory experiments. BH and YW analyzed the data. XL and LW co-worked on associated data collection and interpretation.
Funding
The study was supported by the medical applicable technology tracking project of Hebei province (G201641), Directive Subject of Hebei Provincial Health Planning Commission (ZL20140184); Major Medical Research Projects of Hebei Provincial Finance Department (ZD2013077) and Health care and biomedicine special project (18277705D).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We appreciate the “Editage” company for its help to improve the English writing.
References
Alijanpour, S., Rezayof, A., Sepehri, H., and Delphi, L. (2015). Alterations in the hippocampal phosphorylated CREB expression in drug state-dependent learning. Behav. Brain Res. 292, 109–115. doi: 10.1016/j.bbr.2015.06.003
Allison, S. L., Fagan, A. M., Morris, J. C., and Head, D. (2016). Spatial navigation in preclinical Alzheimer’s disease. J. Alzheimers Dis. 52, 77–90. doi: 10.3233/JAD-150855
Annoni, J. M., Chouiter, L., and Demonet, J. F. (2016). [Age-related cognitive impairment: conceptual changes and diagnostic strategies]. Rev. Med. Suisse 12, 774–779.
Antonenko, D., Külzow, N., Cesarz, M. E., Schindler, K., Grittner, U., and Flöel, A. (2016). Hippocampal pathway plasticity is associated with the ability to form novel memories in older adults. Front. Aging Neurosci. 8:61. doi: 10.3389/fnagi.2016.00061
Beason-Held, L. L., Hohman, T. J., Venkatraman, V., An, Y., and Resnick, S. M. (2017). Brain network changes and memory decline in aging. Brain Imaging Behav. 11, 859–873. doi: 10.1007/s11682-016-9560-3
Bouchard, J., and Villeda, S. A. (2015). Aging and brain rejuvenation as systemic events. J. Neurochem. 132, 5–19. doi: 10.1111/jnc.12969
Bramham, C. R., and Messaoudi, E. (2005). BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 76, 99–125. doi: 10.1016/j.pneurobio.2005.06.003
Brehmer, Y., Kalpouzos, G., Wenger, E., and Lövdén, M. (2014). Plasticity of brain and cognition in older adults. Psychol. Res. 78, 790–802. doi: 10.1007/s00426-014-0587-z
Carlezon, W. A. Jr., Duman, R. S., and Nestler, E. J. (2005). The many faces of CREB. Trends Neurosci. 28, 436–445. doi: 10.1016/j.tins.2005.06.005
Cattaneo, Z., and Silvanto, J. (2008). Time course of the state-dependent effect of transcranial magnetic stimulation in the TMS-adaptation paradigm. Neurosci. Lett. 443, 82–85. doi: 10.1016/j.neulet.2008.07.051
Chang, W. H., Uhm, K. E., Shin, Y. I., Pascual-Leone, A., and Kim, Y. H. (2016). Factors influencing the response to high-frequency repetitive transcranial magnetic stimulation in patients with subacute stroke. Restor. Neurol. Neurosci. 34, 747–755. doi: 10.3233/rnn-150634
Chen, G. H., Wang, Y. J., Zhang, L. Q., and Zhou, J. N. (2004). Age- and sex-related disturbance in a battery of sensorimotor and cognitive tasks in Kunming mice. Physiol. Behav. 83, 531–541. doi: 10.1016/j.physbeh.2004.09.012
Cooperrider, J., Furmaga, H., Plow, E., Park, H. J., Chen, Z., Kidd, G., et al. (2014). Chronic deep cerebellar stimulation promotes long-term potentiation, microstructural plasticity, and reorganization of perilesional cortical representation in a rodent model. J. Neurosci. 34, 9040–9050. doi: 10.1523/JNEUROSCI.0953-14.2014
Cui, L. B., Zhou, X. Y., Zhao, Z. J., Li, Q., Huang, X. Y., and Sun, F. Z. (2013). The Kunming mouse: as a model for age-related decline in female fertility in human. Zygote 21, 367–376. doi: 10.1017/s0967199412000123
Ehrlich, D. E., and Josselyn, S. A. (2016). Plasticity-related genes in brain development and amygdala-dependent learning. Genes Brain Behav. 15, 125–143. doi: 10.1111/gbb.12255
Fabbri, E., An, Y., Zoli, M., Tanaka, T., Simonsick, E. M., Kitner-Triolo, M. H., et al. (2016). Association between accelerated multimorbidity and age-related cognitive decline in older baltimore longitudinal study of aging participants without dementia. J. Am. Geriatr. Soc. 64, 965–972. doi: 10.1111/jgs.14092
Foster, T. C., Kyritsopoulos, C., and Kumar, A. (2017). Central role for NMDA receptors in redox mediated impairment of synaptic function during aging and Alzheimer’s disease. Behav. Brain Res. 322, 223–232. doi: 10.1016/j.bbr.2016.05.012
Gorsler, A., Bäumer, T., Weiller, C., Münchau, A., and Liepert, J. (2003). Interhemispheric effects of high and low frequency rTMS in healthy humans. Clin. Neurophysiol. 114, 1800–1807. doi: 10.1016/s1388-2457(03)00157-3
Gray, D. T., and Barnes, C. A. (2015). Distinguishing adaptive plasticity from vulnerability in the aging hippocampus. Neuroscience 309, 17–28. doi: 10.1016/j.neuroscience.2015.08.001
Guse, B., Falkai, P., and Wobrock, T. (2010). Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J. Neural Transm. 117, 105–122. doi: 10.1007/s00702-009-0333-7
Han, B., Yu, L., Geng, Y., Shen, L., Wang, H., Wang, Y., et al. (2016). Chronic stress aggravates cognitive impairment and suppresses insulin associated signaling pathway in APP/PS1 mice. J. Alzheimers Dis. 53, 1539–1552. doi: 10.3233/jad-160189
Hellmann, J., Jüttner, R., Roth, C., Bajbouj, M., Kirste, I., Heuser, I., et al. (2012). Repetitive magnetic stimulation of human-derived neuron-like cells activates cAMP-CREB pathway. Eur. Arch. Psychiatry Clin. Neurosci. 262, 87–91. doi: 10.1007/s00406-011-0217-3
Hernandez, P. J., and Abel, T. (2008). The role of protein synthesis in memory consolidation: progress amid decades of debate. Neurobiol. Learn. Mem. 89, 293–311. doi: 10.1016/j.nlm.2007.09.010
Hoogendam, J. M., Ramakers, G. M., and Di Lazzaro, V. (2010). Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3, 95–118. doi: 10.1016/j.brs.2009.10.005
Houdayer, E., Degardin, A., Cassim, F., Bocquillon, P., Derambure, P., and Devanne, H. (2008). The effects of low- and high-frequency repetitive TMS on the input/output properties of the human corticospinal pathway. Exp. Brain Res. 187, 207–217. doi: 10.1007/s00221-008-1294-z
Huang, Z., Tan, T., Du, Y., Chen, L., Fu, M., Yu, Y., et al. (2017). Low-frequency repetitive transcranial magnetic stimulation ameliorates cognitive function and synaptic plasticity in APP23/PS45 mouse model of Alzheimer’s disease. Front. Aging Neurosci. 9:292. doi: 10.3389/fnagi.2017.00292
Kim, S. H., Han, H. J., Ahn, H. M., Kim, S. A., and Kim, S. E. (2012). Effects of five daily high-frequency rTMS on Stroop task performance in aging individuals. Neurosci. Res. 74, 256–260. doi: 10.1016/j.neures.2012.08.008
Kim, B. R., Kim, D. Y., Chun, M. H., Yi, J. H., and Kwon, J. S. (2010). Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am. J. Phys. Med. Rehabil. 89, 362–368. doi: 10.1097/phm.0b013e3181d8a5b1
Kim, S., Kim, M. S., Park, K., Kim, H. J., Jung, S. W., Nah, S. Y., et al. (2016). Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J. Ginseng. Res. 40, 55–61. doi: 10.1016/j.jgr.2015.05.001
Korthauer, L. E., Nowak, N. T., Moffat, S. D., An, Y., Rowland, L. M., Barker, P. B., et al. (2016). Correlates of virtual navigation performance in older adults. Neurobiol. Aging 39, 118–127. doi: 10.1016/j.neurobiolaging.2015.12.003
Lenz, M., Galanis, C., Müller-Dahlhaus, F., Opitz, A., Wierenga, C. J., Szabo, G., et al. (2016). Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat. Commun. 7:10020. doi: 10.1038/ncomms10020
Liao, X., Li, G., Wang, A., Liu, T., Feng, S., Guo, Z., et al. (2015). Repetitive transcranial magnetic stimulation as an alternative therapy for cognitive impairment in Alzheimer’s disease: a meta-analysis. J. Alzheimers Dis. 48, 463–472. doi: 10.3233/jad-150346
Luber, B., and Lisanby, S. H. (2014). Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 85, 961–970. doi: 10.1016/j.neuroimage.2013.06.007
Ma, J., Zhang, Z., Kang, L., Geng, D., Wang, Y., Wang, M., et al. (2014). Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp. Gerontol. 58, 256–268. doi: 10.1016/j.exger.2014.08.011
Ma, J., Zhang, Z., Su, Y., Kang, L., Geng, D., Wang, Y., et al. (2013). Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neurons. Neurochem. Int. 62, 84–91. doi: 10.1016/j.neuint.2012.11.010
Medina, F. J., and Túnez, I. (2013). Mechanisms and pathways underlying the therapeutic effect of transcranial magnetic stimulation. Rev. Neurosci. 24, 507–525. doi: 10.1515/revneuro-2013-0024
Miniussi, C., and Ruzzoli, M. (2013). Transcranial stimulation and cognition. Handb. Clin. Neurol. 116, 739–750. doi: 10.1016/B978-0-444-53497-2.00056-5
Mok, R. M., Myers, N. E., Wallis, G., and Nobre, A. C. (2016). Behavioral and neural markers of flexible attention over working memory in aging. Cereb. Cortex 26, 1831–1842. doi: 10.1093/cercor/bhw011
Morris, R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60. doi: 10.1016/0165-0270(84)90007-4
Nam, S. M., Choi, J. H., Yoo, D. Y., Kim, W., Jung, H. Y., Kim, J. W., et al. (2014). Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J. Med. Food 17, 641–649. doi: 10.1089/jmf.2013.2965
Olvera-Cortés, M. E., García-Alcántar, I., Gutiérrez-Guzmán, B., Hernández-Pérez, J. J., López-Vázquez, M. Á., and Cervantes, M. (2012). Differential learning-related changes in theta activity during place learning in young and old rats. Behav. Brain Res. 226, 555–562. doi: 10.1016/j.bbr.2011.10.019
Panja, D., and Bramham, C. R. (2014). BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology 76, 664–676. doi: 10.1016/j.neuropharm.2013.06.024
Paramanik, V., and Thakur, M. K. (2013). Role of CREB signaling in aging brain. Arch. Ital. Biol. 151, 33–42. doi: 10.4449/aib.v151i1.1461
Pell, G. S., Roth, Y., and Zangen, A. (2011). Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 93, 59–98. doi: 10.1016/j.pneurobio.2010.10.003
Rosa, E., and Fahnestock, M. (2015). CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiol. Aging 36, 2406–2413. doi: 10.1016/j.neurobiolaging.2015.04.014
Rosenzweig, E. S., and Barnes, C. A. (2003). Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 69, 143–179. doi: 10.1016/s0301-0082(02)00126-0
Roy, A., Modi, K. K., Khasnavis, S., Ghosh, S., Watson, R., and Pahan, K. (2014). Enhancement of morphological plasticity in hippocampal neurons by a physically modified saline via phosphatidylinositol-3 kinase. PLoS One 9:e101883. doi: 10.1371/journal.pone.0101883
Shang, Y., Wang, X., Shang, X., Zhang, H., Liu, Z., Yin, T., et al. (2016). Repetitive transcranial magnetic stimulation effectively facilitates spatial cognition and synaptic plasticity associated with increasing the levels of BDNF and synaptic proteins in Wistar rats. Neurobiol. Learn. Mem. 134, 369–378. doi: 10.1016/j.nlm.2016.08.016
Shen, C., Sun, F. L., Zhang, R. Y., Zhang, L., Li, Y. L., Zhang, L., et al. (2015). Tetrahydroxystilbene glucoside ameliorates memory and movement functions, protects synapses and inhibits α-synuclein aggregation in hippocampus and striatum in aged mice. Restor. Neurol. Neurosci. 33, 531–541. doi: 10.3233/rnn-150514
Torii, T., Sato, A., Nakahara, Y., Iwahashi, M., Itoh, Y., and Iramina, K. (2012). Frequency-dependent effects of repetitive transcranial magnetic stimulation on the human brain. Neuroreport 23, 1065–1070. doi: 10.1097/wnr.0b013e32835afaf0
Trebbastoni, A., Pichiorri, F., D’Antonio, F., Campanelli, A., Onesti, E., Ceccanti, M., et al. (2016). Altered cortical synaptic plasticity in response to 5-Hz repetitive transcranial magnetic stimulation as a new electrophysiological finding in amnestic mild cognitive impairment converting to Alzheimer’s disease: results from a 4-year prospective cohort study. Front. Aging Neurosci. 7:253. doi: 10.3389/fnagi.2015.00253
Vanguilder, H. D., and Freeman, W. M. (2011). The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front. Aging Neurosci. 3:8. doi: 10.3389/fnagi.2011.00008
Verma, R., Harris, N. M., Friedler, B. D., Crapser, J., Patel, A. R., Venna, V., et al. (2016). Reversal of the detrimental effects of post-stroke social isolation by pair-housing is mediated by activation of BDNF-MAPK/ERK in aged mice. Sci. Rep. 6:25176. doi: 10.1038/srep25176
Vorhees, C. V., and Williams, M. T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858. doi: 10.1038/nprot.2006.116
Wang, H. L., Xian, X. H., Wang, Y. Y., Geng, Y., Han, B., Wang, M. W., et al. (2015). Chronic high-frequency repetitive transcranial magnetic stimulation improves age-related cognitive impairment in parallel with alterations in neuronal excitability and the voltage-dependent Ca2+ current in female mice. Neurobiol. Learn. Mem. 118, 1–7. doi: 10.1016/j.nlm.2014.11.002
Wang, J., Yuan, J., Pang, J., Ma, J., Han, B., Geng, Y., et al. (2016). Effects of chronic stress on cognition in male SAMP8 mice. Cell. Physiol. Biochem. 39, 1078–1086. doi: 10.1159/000447816
Xiao, Y., Fu, H., Han, X., Hu, X., Gu, H., Chen, Y., et al. (2014). Role of synaptic structural plasticity in impairments of spatial learning and memory induced by developmental lead exposure in Wistar rats. PLoS One 9:e115556. doi: 10.1371/journal.pone.0115556
Zhang, X. Q., Mu, J. W., Wang, H. B., Jolkkonen, J., Liu, T. T., Xiao, T., et al. (2016). Increased protein expression levels of pCREB, BDNF and SDF-1/CXCR4 in the hippocampus may be associated with enhanced neurogenesis induced by environmental enrichment. Mol. Med. Rep. 14, 2231–2237. doi: 10.3892/mmr.2016.5470
Zhang, S., Xin, H., Li, Y., Zhang, D., Shi, J., Yang, J., et al. (2013). Skimmin, a coumarin from hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evid. Based Complement. Alternat. Med. 2013:819296. doi: 10.1155/2013/819296
Zhang, N., Xing, M., Wang, Y., Tao, H., and Cheng, Y. (2015). Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF-NMDAR pathways in a rat model of vascular dementia. Neuroscience 311, 284–291. doi: 10.1016/j.neuroscience.2015.10.038
Keywords: repetitive transcranial magnetic stimulation, high frequency, aged, cognitive impairment, synaptic plasticity
Citation: Ma Q, Geng Y, Wang H, Han B, Wang Y, Li X, Wang L and Wang M (2019) High Frequency Repetitive Transcranial Magnetic Stimulation Alleviates Cognitive Impairment and Modulates Hippocampal Synaptic Structural Plasticity in Aged Mice. Front. Aging Neurosci. 11:235. doi: 10.3389/fnagi.2019.00235
Received: 06 September 2018; Accepted: 14 August 2019;
Published: 18 September 2019.
Edited by:
Fannie Onen, Hôpital Bichat-Claude-Bernard, FranceReviewed by:
Cathy W. Levenson, Florida State University, United StatesChris Baeken, Ghent University, Belgium
Arjun Vijay Masurkar, Langone Medical Center, New York University, United States
Copyright © 2019 Ma, Geng, Wang, Han, Wang, Li, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-wei Wang, d2FuZ213MzM5QDE2My5jb20=
† These authors have contributed equally to this work
 Qinying Ma1,2†
Qinying Ma1,2† Ming-wei Wang
Ming-wei Wang