
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 30 June 2020
Sec. Plant Pathogen Interactions
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.01432
 Pascal Mülner1,2
Pascal Mülner1,2 Elisa Schwarz1
Elisa Schwarz1 Kristin Dietel1
Kristin Dietel1 Helmut Junge1
Helmut Junge1 Stefanie Herfort3
Stefanie Herfort3 Max Weydmann3
Max Weydmann3 Peter Lasch3
Peter Lasch3 Tomislav Cernava2
Tomislav Cernava2 Gabriele Berg2
Gabriele Berg2 Joachim Vater1,3*
Joachim Vater1,3*Plant growth promoting rhizobacteria attain increasing importance in agriculture as biofertilizers and biocontrol agents. These properties significantly depend on the formation of bioactive compounds produced by such organisms. In our work we investigated the biosynthetic potential of 13 industrially important strains of the Bacillus subtilis complex by mass spectrometric methodology. Typing of these organisms was performed with MALDI-TOF mass spectrometry followed by comprehensive profiling of their bioactive peptide products. Volatiles were determined by gas chromatography-mass spectrometry. Representative products of the members of the B. subtilis complex investigated in detail were: the surfactin familiy (surfactins, lichenysins, pumilacidins); the iturin family (iturins, mycosubtilins and bacillomycins); plantazolicin and the dual lantibiotics lichenicidins, as well as a wide spectrum of volatiles, such as hydrocarbons (alkanes/alkenes), alcohols, ketones, sulfur-containing compounds and pyrazines. The subcomplexes of the B. subtilis organizational unit; (a) B. subtilis/Bacillus atrophaeus; (b) B. amyloliquefaciens/B. velezensis; (c) B. licheniformis, and (d) B. pumilus are equipped with specific sets of these compounds which are the basis for the evaluation of their biotechnological and agricultural usage. The 13 test strains were evaluated in field trials for growth promotion of potato and maize plants. All of the implemented strains showed efficient growth stimulation of these plants. The highest effects were obtained with B. velezensis, B. subtilis, and B. atrophaeus strains.
Plant growth promoting rhizobacteria (PGPR) and endophytes attain increasing importance as efficient biofertilizers, biostimulants and biocontrol organisms for a sustainable and enviromentally friendly alternative to conventional agriculture (Saharan and Nehra, 2011; Hardoim et al., 2015). PGPR establish an mutualistic symbiosis by interacting with plant roots in the rhizosphere or colonizing plant vessels and organs as endophytes and are enhancing plant growth through production of secondary metabolites, phytohormones and facilitation of nutrient uptake (Barea et al., 2005; Drogue et al., 2012).
In contrast to agrochemicals, these biological compounds are biodegradable and only produced at site (Lugtenberg and Kamilova, 2009). Among other plant associated microorga-nisms, many PGPR are capable of triggering plant defense mechanisms (van Loon and Glick, 2004). This elicitation of innate immunity responses of plants is called induced systemic resistance (ISR) (van Loon et al., 1998). ISR is induced by dynamic chemical communication between plants and bacteria (van Loon, 2007; Farag et al., 2013). Numerous PGPR produce a variety of antimicrobial compounds to inhibit phytopathogens (Ongena and Jacques, 2008; Pérez-García et al., 2011). Such compounds are frequently produced non-ribosomally by specific multienzyme systems. In addition, also a wide spectrum of bioactive peptides of ribosomal origin, such as lantibiotics and bacteriocines are recruited for plant protection. Prominent microorganisms with such abilities include members of the B. subtilis complex, Paenibacillus as well as Pseudomonas strains (Lim et al., 1991; Hong and Meng, 2003; Peighami-Ashnaei et al., 2009; Asari et al., 2016; Rybakova et al., 2016).
Two specific effects of such organisms are in focus for future agricultural developments, (a) their ability to stimulate plant growth and performance, and (b) their biocontrol effects to protect their plant hosts against deleterious effects of phytopathogenic competitors as well as against abiotic stress (Berg, 2015). PGPR organisms are qualified to increase plant harvest significantly and to protect plants against harmful bacteria, fungi, nematodes and insects. For both effects their biosynthetic potential to produce a broad spectrum of secondary metabolites, such as bioactive peptides, polyketides and hybrids thereof as well as small volatile organic compounds (VOCs) (Audrain et al., 2015; Kanchiswamy et al., 2015; Panpatte et al., 2017) are of outmost importance. VOCs produced by PGPR exhibit a high chemical diversity and play a significant role in the stimulation of plant growth, control of plant pathogens, regulation of phytohormone synthesis and metabolism as well as induction of systemic resistance against plant diseases (Kloepper et al., 2004; Werner et al., 2016; Bailly and Weisskopf, 2017; Wu et al., 2018). Therefore, they are of high value for plant productivity and health.
The plant-growth enhancing activity of members of the B. subtilis complex are of great industrial relevance. The four industrially important species Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus licheniformis, and Bacillus pumilus are part of the Bacillus subtilis complex (Fritze, 2004; Fan et al., 2017). They represent a family of genetically closely related organisms. Since a few decades the exploration of the biosynthetic potential of members of the B. subtilis complex for the production of secondary metabolites is under current investigation (Kowall et al., 1998; Bonmatin et al., 2003; Vater et al., 2003; Koumoutsi et al., 2004; Argeulles-Arias et al., 2009; Monaci et al., 2016; Zhao and Kuipers, 2016; Tahir et al., 2017a). Studies on the nature and application of lipopeptides and volatiles formed by such rhizobacteria are of particular interest for biocontrol as well as productivity and health of plants. This research field is presently under intensive reviewing (Caulier et al., 2019; Dunlap et al., 2019; Olishevska et al., 2019; Rabbee et al., 2019; Keswani et al., 2020) with a focus on the biosynthetic equipment of the members of the B. subtilis complex for bioactive compounds.
Both phenotypic and phylogenetic approaches, such as 16S rRNA sequencing, led to difficulties in distinguishing different species of the Bacillus subtilis group (Rooney et al., 2009). Recently, it was demonstrated by phylogenomic analysis that the B. subtilis complex can be organized into four subcomplexes (Fan et al., 2017; Dunlap et al., 2019). Subcomplex I (subtilis) diverges into two main branches comprising the closely related B. subtilis and B. atrophaeus strains. Subcomplex II (amyloliquefaciens) is divided into B. amyloliquefaciens, subspecies amyloliquefaciens and B. amyloliqufaciens, subspecies plantarum which recently has been renamed as B. velezensis. Subcomplexes III and IV are represented by B. licheniformis and B. pumilus strains, respectively.
Our research was focussed on the investigation of the biosynthetic capacity of 13 industrially important PGPR-strains of the B. subtilis complex using modern mass spectrometric techniques of high resolution and sensitivity. In particular, MALDI-TOF MS and GC-MS are qualified for this task as the methods of choice, both for rapid typing and characterization of the investigated organisms as well as for efficient exploration of their biosynthetic potential. Here we present a comprehensive mass spectrometric study of the bioactive peptides and volatile compounds produced by these strains which play an important role in their plant growth promoting abilities. The increase of the produce in the harvest of potatoes and maize induced by them was studied in field trials.
The matrices α-cyanohydroxycinnamic acid (CCA) and dihydroxy-benzoic acid (DHB) used for MALDI-TOF MS were obtained from Bruker (Bremen, Germany). Acetonitrile (ACN, HPLC grade) was purchased from Merck (Darmstadt, Germany), trifluoroacetic acid (TFA) from Sigma-Aldrich (Deisenhofen, Germany). Type strains of the B. subtilis complex were available from DSM (Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany).
For the preparation of surface extracts the investigated organisms of the B. subtilis complex were grown on Agar plates using three cultivation media: (a) Lysogeny Broth (LB) medium; (b) Landy medium (Landy et al., 1948) and (c) TSA-medium [composition per L: 15 g casein peptone (pancreatic), 5 g soya peptone, 5 g sodium chloride, 15 g agar per L deionized water, final pH = 7.3] solidified with 1.5% agar in petri dishes for 24; 48, and 72 h at 30°C. In addition, liquid fermentations were carried out in 100 ml Erlenmeyer flasks at 30°C and 200 rpm in an orbital shaker (Buchler, Germany) to detect products released by the strains into the culture medium. Cells were harvested after 8; 10; 12; 24; 48; and 72 h of incubation. The PGPR were cultivated both in Landy and TSA medium.
In order to obtain complete profiles of bioactive peptides produced by the investigated PGPR of the B. subtilis complex, their products were detected by MALDI-TOF MS (a) in surface extracts of cells picked either from agar plates or harvested from liquid cultures by centrifugation for 10–20 min at 15000 rpm, (b) in culture supernatants after growth for 8; 10; 12; 24; 48; and 72 h and (c) after disintegration by solubilization with 80% acetonitrile.
For (a) a wire loop of cell material was picked, suspended in 50 μl 50% acetonitrile/0.1% TFA and extracted for 15 min by vigorous vortexing. Finally, cells were spun down at 15000 rpm for 10 min.
PGPR strains were identified by MALDI-TOF MS according to the procedure developed by Lasch et al. (2008). A wire loop of cell material was picked from Agar plates and transferred into 20 μl of water and mixed with 80 μl of pure TFA (Uvasol grade; Merck, Darmstadt, Germany). The suspension of bacteria was incubated under gentle mixing for 30 min at room temperature until cells were completely solubilized. Then 10 μl of the suspension were diluted 1:10 with bidistilled water adjusting a final TFA-concentration of 8%. Now the samples get turbid, because a part of the solubilized material becomes insoluble and precipitates. Two μl of the turbid suspension were mixed with 2 μl of a saturated solution of the CCA matrix for MALDI-TOF mass spectrometric analysis. Mass spectra were generated in linear mode in the mass range of 1–10 kDa. The effects of 3 × 3000 laser shots were accumulated. Data acquisition and analysis were carried out using the MALDI-Biotyper software package provided by Bruker (Bremen, Germany) and a Matlab-based software (Microbe MS). For MALDI-TOF MS identification analysis, samples were processed, as outlined in Lasch et al. (2008, 2015). For evaluation the obtained mass spectra were smoothed and baseline-corrected.
Bioactive peptides of the tested PGPR of the B. subtilis complex were detected and identified by MALDI-TOF MS, as outlined previously (Vater et al., 2017, 2018). A Bruker Autoflex Speed TOF/TOF mass spectrometer (Bruker Daltonik, Bremen, Germany) was used with smartbeam laser technology using a 1 kHz frequency-tripled Nd-YAG laser (λex = 355 nm). Samples (2 μl) of surface extracts and culture supernatants were mixed with 2 μl matrix solution (a saturated solution of α-hydroxy-cinnamic acid in 50% aqueous ACN containing 0.1% TFA), spotted on the target, air dried and measured. Mass spectra were obtained by positive-ion detection in reflector mode. Monoisotopic masses were obtained. Parent ions were detected with a resolution of 10,000.
Sequence analysis of the lipopeptide products was performed by MALDI-LIFT-TOF/TOF mass spectrometry in laser induced decay (LID) mode (Suckau et al., 2003). The product ions in the LIFT-TOF/TOF fragment spectra were obtained with a resolution of 1000.
The mass spectrometry results did not allow optical isomers to be distinguished. Therefore, the configurations of the amino acid components were not indicated.
The PGPR strains were grown in 20 ml headspace vials (75.5 × 22.5 mm; Chromtech, Idstein, Germany) filled with 8 ml of nutrient agar (Sifin Diagnostic GmbH, Berlin, Germany) and mVOCs measured according to the protocol of Cernava et al. (2015). Equal distribution was assured by parallel application of cell material in three vials per organism. After 24 h of incubation at 30°C, the volatiles accumulating inside the vials were measured by headspace-solid phase microextraction (HS-SPME) GC-MS. Compound separation and detection was performed on a system combining a gas chromatograph 7890A with a quadrupol mass spectrometer 5975C (Agilent Technologies, Waldbronn, Germany). A solid phase microelution (SPME) fiber consisting of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) was used for sampling (Sigma-Aldrich, St. Louis, United States). Head space (HS) samples entered a (5%-phenyl)methylpolysiloxane column (60 m × 0.25 mm) i.d.; 0.25 μm film thickness (DB-5MS; Agilent Technologies, Waldbronn, Germany). Subsequently, electron ionization with 70 eV and detection in the mass range 25–350 Da were performed. The fiber was desorbed at 200°C for 8 min to eliminate potential residues before initial measurements. 270°C was chosen for the inlet temperature. The temperature gradient of the column was maintained at 70°C for 1.5 min, raised to 200°C at a rate of 16°C/min and finally kept at 200°C for 0.5 min. The helium flow rate was adjusted to 1.2 ml/min. For identification of the volatiles the received spectra were matched to the NIST (National Institute of Standards and Technology) Mass Spectral Database 14 via MS Search 2.2. Additionally, the Kovats Indices of all compounds were calculated and verified with corresponding entries in the online database Chemspider.1
The field trials were carried out in field at D-18190 Gross Lüsewitz, Mecklenburg-Vorpommern (Germany) (54°04′44.7′′N 12°21′45.0′′E) with slightly loamy sand (total N 21, P 9.6, K 1.0 and Mg 4.0 mg/100 g soil, pH 5.2), the typical soil type of the area. The experimental field was divided into plots with four replicates per treatment in a randomized block design. For potato the plots were 21 square meter (7 m × 3 m) and for maize 18 square meter (3 m × 6 m). The distance between the rows were 75 cm with 4 rows per plot. Tubers of Solanum tuberosum cv. Goldmarie (Norica Nordring-Kartoffelzucht- und Vermehrungs-GmbH) were treated with the microbial inoculum (0.5 l/ha or 1.0 l/ha in 300 l water via in-furrow spray at planting using a Parzellenspritze PL1, AirMix 110-03, 3.2 kp/cm2). Seeds of Zea mays cv. Colisee (KWS Saat SE & Co. KG) were treated with the microbial inoculum (0.5 l/ha or 1.0 l/ha) in 300 l water via in-furrow spray at sowing (Parzellenspritze PLL1, type of nozzle: AirMix 110-03; operating pressure 3.2 kp/cm2). The microbial inoculum of each strain contained 2.5E+10 cfu/ml. Mineral fertilizer was added based on a chemical analsis of the soil and was applied before planting (N 121.5 kg/ha, P 37.5 kg/ha, K 127.5 kg/ha). The potatos were planted on 16th of May and harvested on 20th of September (BBCH99). The maize was sown on 4th of June and harvested on 18th of October (BBCH86). For yield determination only they were harvested and weight was measured. The effect of microbial inoculum on potato or maize growth was calculated based on assessed weight of the individual tubers of all plants of the two middle rows of each plot. The mean of the four replicates was calculated and the standard deviation was determined.
In our work we investigated 13 bacterial test strains of industrial relevance. In the first step these organisms were classified by MALDI-TOF mass spectrometry. MALDI-TOF disintegration mass spectra were generated and evaluated using the Bruker Daltonik MALDI Biotyper (Bruker Daltonik, Bremen, Germany) and a Matlab-based software (Microbe MS) comprising mass spectra of a wide spectrum of bacterial strains including highly pathogenic organisms (Lasch et al., 2015). In this way, typing of the investigated PGPR strains was achieved. As demonstrated in Table 1 all of them were distributed among the four subcomplexes of the B. subtilis species complex. The MALDI-TOF mass spectra of these strains were correlated with those of the corresponding type strains.
Bacillus subtilis 168T is the type strain for B. subtilis; DSM 7264T for B. atrophaeus; DSM 7T for B. amyloliquefaciens, subsp. amyloliquefaciens; DSM 23117T (FZB42) for B. amyloliquefaciens, subsp. plantarum, now designated as B. velezensis; DSM 13T for B. licheniformis and DSM 27Tfor B. pumilus. Prominent mass peaks showing high intensities were taken as biomarkers for strain identification from the MALDI-TOF disintegration mass spectra of the different types of organisms (Figure 1 and Supplementary Figures S1, S2). They were related to the corresponding peaks in the mass spectra of the reference strains. In each subcomplex at least six of such biomarkers were selected for rapid typing of members of the B. subtilis complex. The obtained biomarkers are summarized in Table 2.
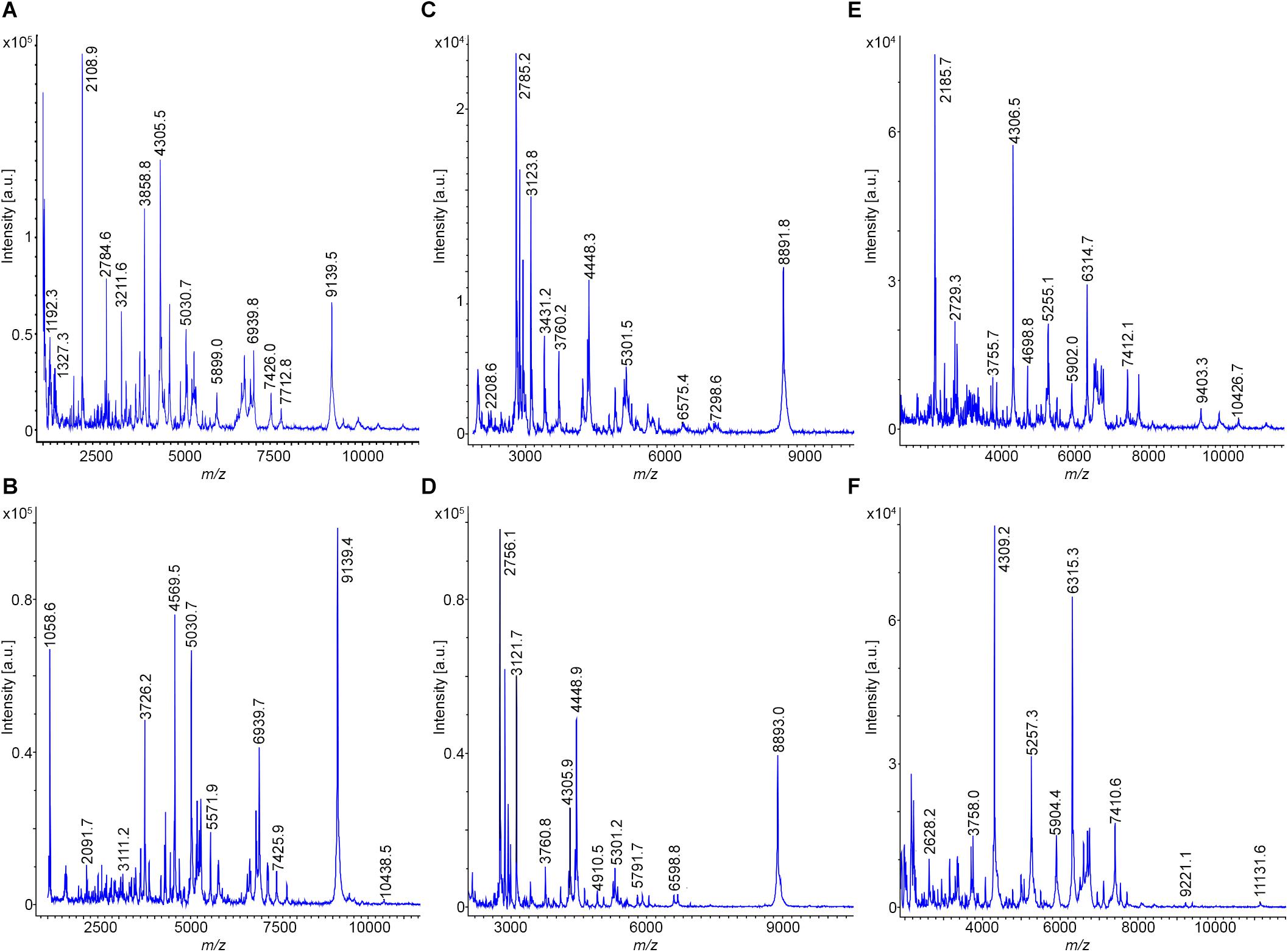
Figure 1. MALDI-TOF mass spectra for typing of B. subtilis 168T (A) and DSM 32873 (B); B. atrophaeus DSM 32285 (C) and DSM 29418 (D) as well as B. amyloliquefaciens DSM 7T (E) and DSM 32875 (F). Cell material was picked from agar plates, solubilized with 80% trifluoroacetic acid and processed as described under Methods. Mass spectra were taken in the mass range from 1–10 kDa.
By evaluation of the MALDI-TOF mass spectra taken after complete disintegration of the bacterial cells by the Bruker and RKI data bases the following assignments of the investigated strains were obtained (see Table 1): DSM 32873 and Sc-S7 were classified as B. subtilis strains. DSM 32285 and 29418 were identified as B. atrophaeus. DSM 32875; DSM 23117T (FZB42); DSM 29399 (FZB45); Rs-MS87 and Sc-K143 are members of the subcomplex amyloliquefaciens. Here a clear differentiation between subspecies amyloliquefaciens and subspecies plantarum (B. velezensis) was accomplished. The mass spectrum of DSM 32875 is different from the other strains and corresponds well with that of DSM 7T, the type strain of B. amyloliquefaciens, subsp. amyloliquefaciens, while the mass spectra of DSM 29399 (FZB45); Rs-MS87 and Sc-K143 correlate nicely with that of DSM 23117T (FZB42) which is the type strain for B. velezensis. DSM 32874 and M18 fit into the subcomplex B. licheniformis. Their mass spectra correspond well with the B. licheniformis type strain DSM13T. Abi11 and Rs-Ts276 were identified as B. pumilus strains and related to the B. pumilus type strain DSM 27T. Our mass spectrometric classification of the investigated PGPR strains agrees with previous biochemical and genetic data.
After having classified our test strains as members of the B. subtilis complex the main aim of our work was focused on the exploration of their biosynthetic potential for the production of bioactive peptides and volatiles which play an important role in plant growth promoting and biocontrol activities of PGPR strains. The obtained profiles were related to those of commonly used type strains available from DSM. For this purpose product formation of both test and type strains was studied in a space- and time fashioned manner.
We initiated a comprehensive study of the bioactive peptides produced by our strains attributed to the B. subtilis complex in the mass range from 400 to 3500 Da.
In order to determine the complete production of bioactive compounds, three series of mass spectrometric tests were performed: MALDI-TOF mass spectra were taken (a) from surface extracts and (b) after complete disintegration of cells picked from agar plates as well as (c) from culture supernatants to detect products that were released into the culture medium. For tests a and b cells were grown on agar plates at 30°C for 24; 48; and 72 h using three cultivation media (Landy, LB and TSA). Surface extracts were obtained by extraction of cell material picked from the plates with 50% acetonitrile/0.1% TFA. For test b to detect products within cells and attached to their surface, cell material was disintegrated with 80% TFA and processed as indicated above. In addition, for test c the isolates were cultivated for 8–72 h either in the Landy- or TSA- medium at 30°C. Released products were detected by MALDI-TOF MS. Thus, product formation of the investigated PGPR strains of the B. subtilis complex was obtained in a space- and time fashioned manner. The obtained results were summarized in Table 1. All investigated strains produced the siderophore bacillibactin with mass numbers of [M + H,Na,K]+ = 883.4/905.4/921.4 which was preferentially detected in culture supernatants.
Structure elucidation of bioactive peptides was performed by fragment analysis using LIFT-MALDI-TOF/TOF MS (Suckau et al., 2003). Polyketide formation will be the subject of a forthcoming publication.
Two B. subtilis strains were investigated, DSM 32873 and Sc-S7. Bioactive peptides produced by strain DSM 32873 are demonstrated in Figures 2A–D. Figure 2A shows the MALDI-TOF mass spectrum of a surface extract of this organism using TSA as cultivation medium. It produces the surfactin and fengycin lipopeptide families which are formed non-ribosomally at multimodular enzyme complexes (Menkhaus et al., 1993; Steller et al., 1998). Prominent species were found for surfactin at m/z = 1058.8 and m/z = 1522.1 for fengycin, resp. The mass peak at m/z = 1336.9 can be attributed to plantazolicin, a highly condensed thiazole/oxazole modified microcin (TOMM) of ribosomal origin (Kalyon et al., 2011; Scholz et al., 2011). Plantazolicin was not found in the culture supernatant (Figure 2B). Surfactins and fengycins together with the siderophore bacillibactin were released into the culture medium, as is apparent from the mass spectrum of the culture supernatant, when DSM 32873 was grown for 24 h at 30°C in the Landy medium (Figure 2B). The mass spectra in Figures 2C,D display the surfactin and fengycin complexes in their full complexity. The observed mass peaks for surfactin can be assigned to the protonated forms and the alkali adducts of C13-C15 surfactins, resp. Fengycins represent the protonated species and alkali adducts of C14-C17 fengycins A and B, which differ in the amino acid component at position 6. Ala in the case of fengycin A is replaced by Val for fengycin B. Similar results were obtained for B. subtilis strain Sc-S7, but in contrast to DSM 32873 it did not produce plantazolicin. A specific feature of B. subtilis strains is the complete absence of iturin compounds. The product spectrum determined mass spectrometrically is in accordance with the product pattern derived from genome sequencing of B. subtilis strains (Fan et al., 2017; Harwood et al., 2018). Surfactin was identified mass spectrometrically by LIFT-MALDI-TOF/TOF fragment analysis. In Figure 3 the fragment pattern of C14-surfactin is demonstrated. The parent ions were [M + H,Na]+ = 1022.7 and 1044.5, respectively. Applying MALDI-TOF MS the alkali adducts of surfactin dominate in the mass spectra. The same feature was found for the fragment ions, particularly for the larger ones. Therefore, for sequence determination of surfactins both the protonated and sodiated product ions were available. From the product ions derived from the LIFT-MALDI-TOF/TOF fragment spectrum, the surfactin sequence was approached in several steps. In the low mass range m/z < 160 Da the immonium ions allow detection of the amino acid components of surfactin. From the observed di- and tripeptide fragments the complete lipopeptide sequence can be modeled. Finally, the protonated bn and yn – ions as well as their sodium adducts were collected and compared with the calculated values, from which in the upper part of Figure 3 the complete sequence of C14-surfactin was derived. In general, it was not possible to discriminate between Leu and Ile in our MALDI-LIFT-TOF/TOF studies for sequence elucidation of surfactin and other investigated bioactive peptides. Here, the assignment was performed according to literature data.
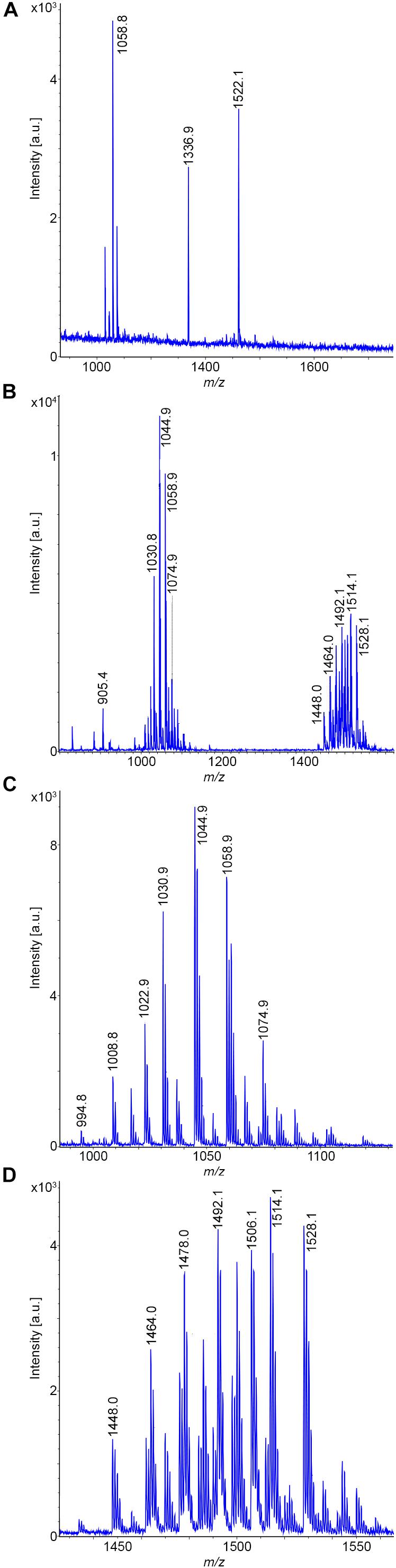
Figure 2. Detection of bioactive peptides produced by B. subtilis DSM 32873: (A) MALDI-TOF mass spectrum of a surface extract of this organism grown on TSA-agar. (B) MALDI-TOF mass spectrum of the culture supernatant of DSM 32873 grown in the Landy medium for 24 h at 30°C. (C,D) MALDI-TOF mass spectra of the surfactin- (C) and fengycin (D)-complex. C13–C15-surfactins and C14–C17-fengycins A and B were detected.
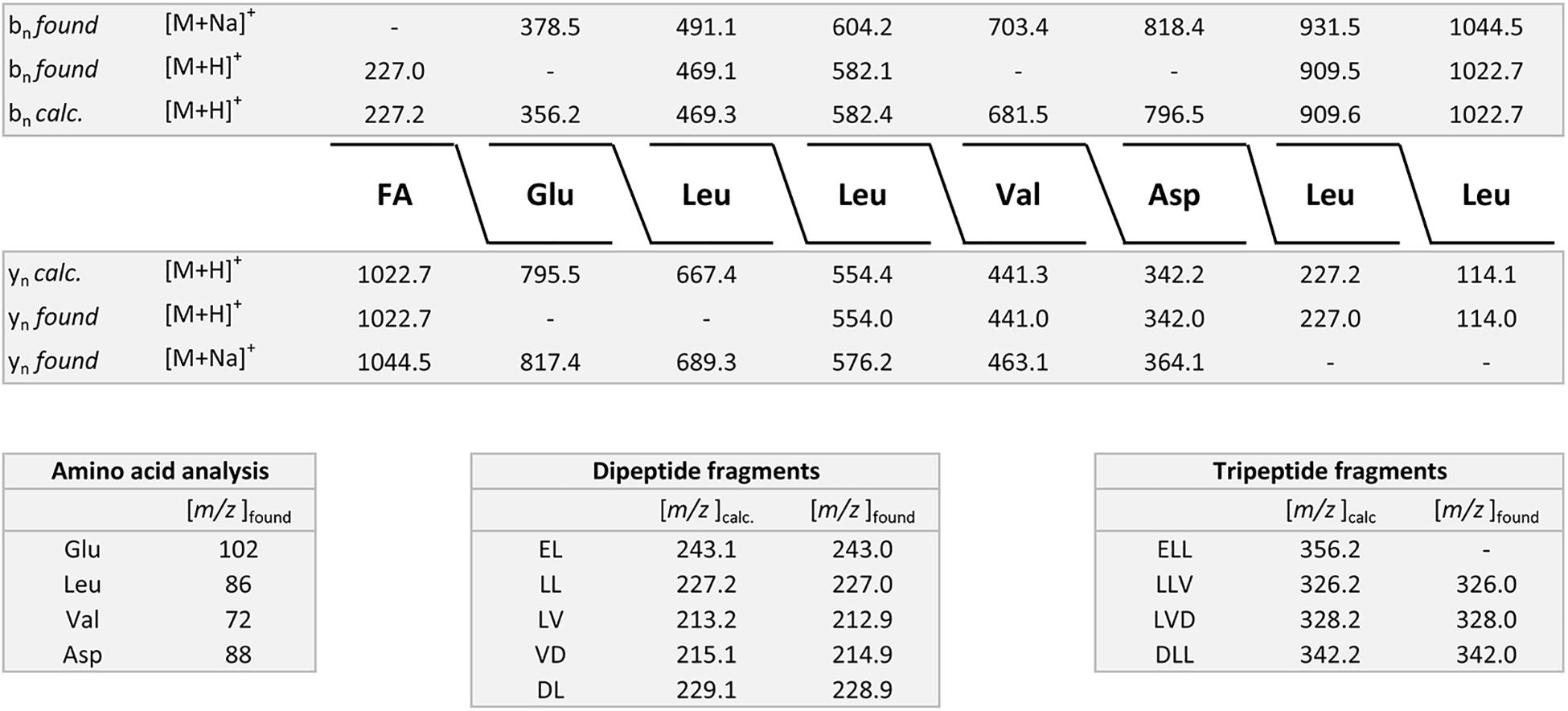
Figure 3. Mass spectrometric sequence determination of a C14-surfactin produced by B. subtilis DSM 32873 with parent ions [M + H,Na]+ = 1022.7 and 1044.5 Da derived from product ion patterns obtained by LIFT-MALDI-TOF/TOF fragment spectra. FA: CH3-(CH2)10-CHOH-CH2-CO-.
The results obtained for B. atrophaeus are exhibited in Figures 4A–F and compared with those determined for the type strain B. atrophaeus DSM 7264T. Two test strains DSM 32285 and DSM 29418 were investigated. Figures 4A,B show the MALDI-TOF mass spectra of surface extracts from these bacteria. In contrast to B. subtilis, the B. atrophaeus strains produce iturin compounds (Bonmatin et al., 2003; Vater et al., 2003). DSM 32285 produces C16-C19 iturins C (Vater et al., 2002, 2003), while DSM 29418 forms C15–C17 mycosubtilins (Bonmatin et al., 2003), which dominate strongly in the mass spectra. In addition, minor amounts of surfactins and fengycins were found at the surface of these organisms. Remarkably, surface extracts of DSM 29418 contain a product with high intensity at m/z = 2754.6 which still needs to be identified. This metabolite is specific for B. atrophaeus. DSM 32285 revealed a similar product at m/z = 2783.5, but with much lower intensity. In Figures 4C,D mass spectra of the culture supernatants of these strains are presented. Also here the iturin compounds dominate, but the fengycin peaks appeared with appreciably higher intensities than in the surface extracts. Obviously, a major part of the fengycins was released into the culture medium. In both spectra the siderophore bacillibactin was visible ([M + H,Na,K]+ = 883.4; 905.4 and 921.4). The highest intensity was found for its sodium adduct at m/z = 905.4. The product at m/z = 2754.6 appeared also in the culture supernatant of strain DSM 29418 in high amount. Figure 4E shows the mass spectra of the surfactin and iturin C clusters produced by DSM 32285, while the mycosubtilin family formed by DSM 29418 is exhibited in Figure 4F. The product patterns of these strains were compared with that of the B. atrophaeus type strain DSM 7264T. Its products essentially correspond to those found for strain DSM 29418 (surfactin; mycosubtilin; fengycin and the compound with m/z = 2754.6), but a characteristic feature of the type strain is the high amount of fengycins released into the culture medium. The mass spectrometric sequence determination of a C16-fengycin A by LIFT-MALDI-TOF/TOF MS is shown in Figure 5. In the lower part of this figure the immonium ions for the amino acid components and the mass peaks for the di- and tripeptide fragments are listed. In the upper part, the complete sequence determination of the C16-fengycin is demonstrated for a ring opening between Tyr(3) and a-Thr(4). In addition, a partial sequence segment ranging from Pro(7) via the lactone linkage between Ile(10) and Tyr(3) to Ala(6) is shown. It corresponds to the sequence of the peptide ring with loss of the side chain attached to L-Tyr (3). Fengycin contains proline in position 7. In the mass spectrometric fragmentation of such compounds the peptide ring preferentially is cleaved N-terminally of proline, frequently with the consequence that proline-directed product ions pn dominate in the fragment spectra.
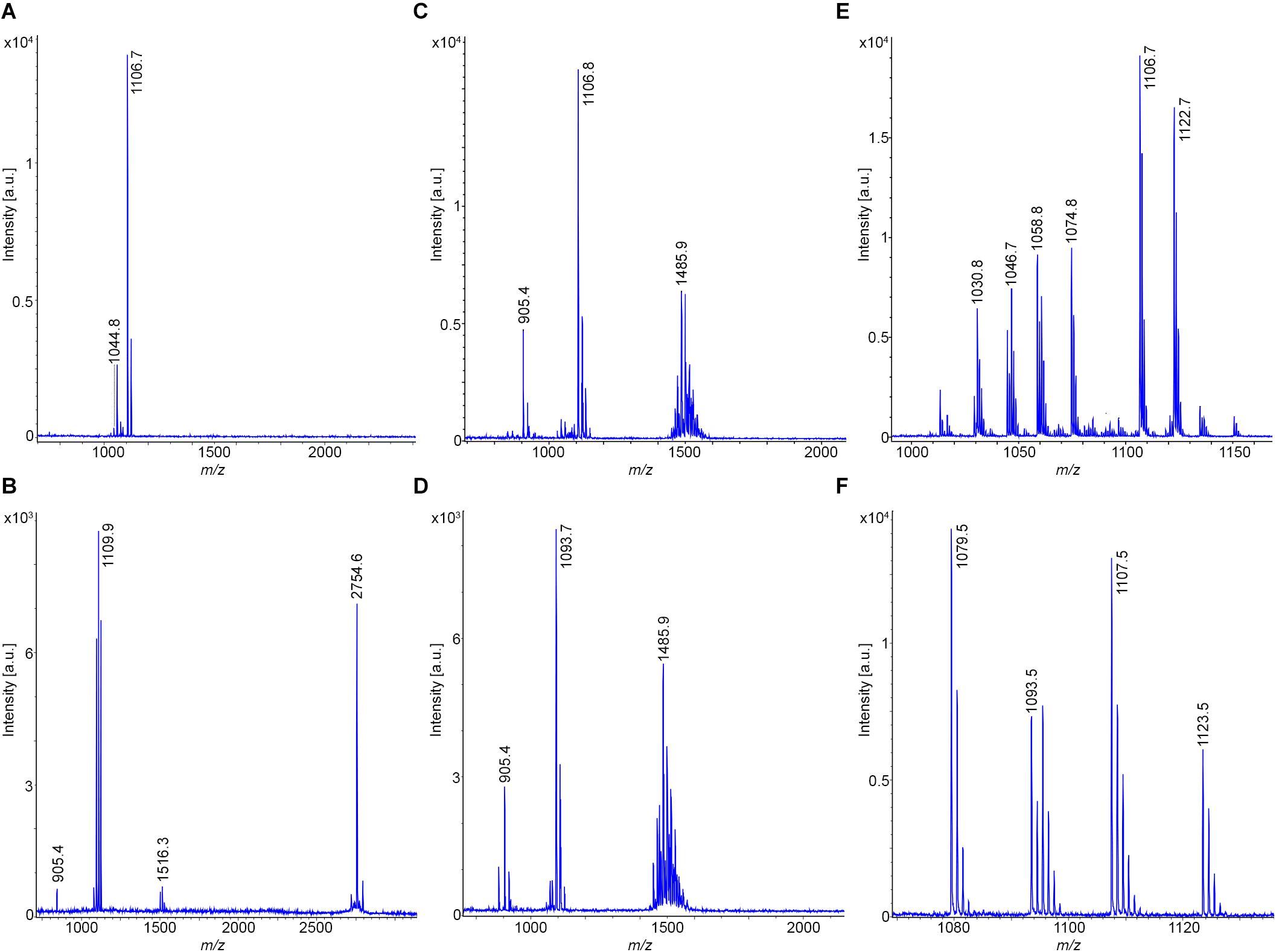
Figure 4. Detection of bioactive peptides produced by B. atrophaeus DSM 32285 and DSM 29418: (A,B) MALDI-TOF mass spectra of surface extracts of these strains. (C,D) MALDI-TOF mass spectra of culture supernatants of these strains grown for 24 h at 30°C in the Landy medium. (E) MALDI-TOF mass spectrum of the surfactin and iturin C complexes produced by strain DSM 32285. C13–C15 surfactins and C16–C19 iturins C were detected. (F) MALDI-TOF mass spectrum of the mycosubtilin complex produced by strain DSM 29418. C15-C17 mycosubtilins were detected.
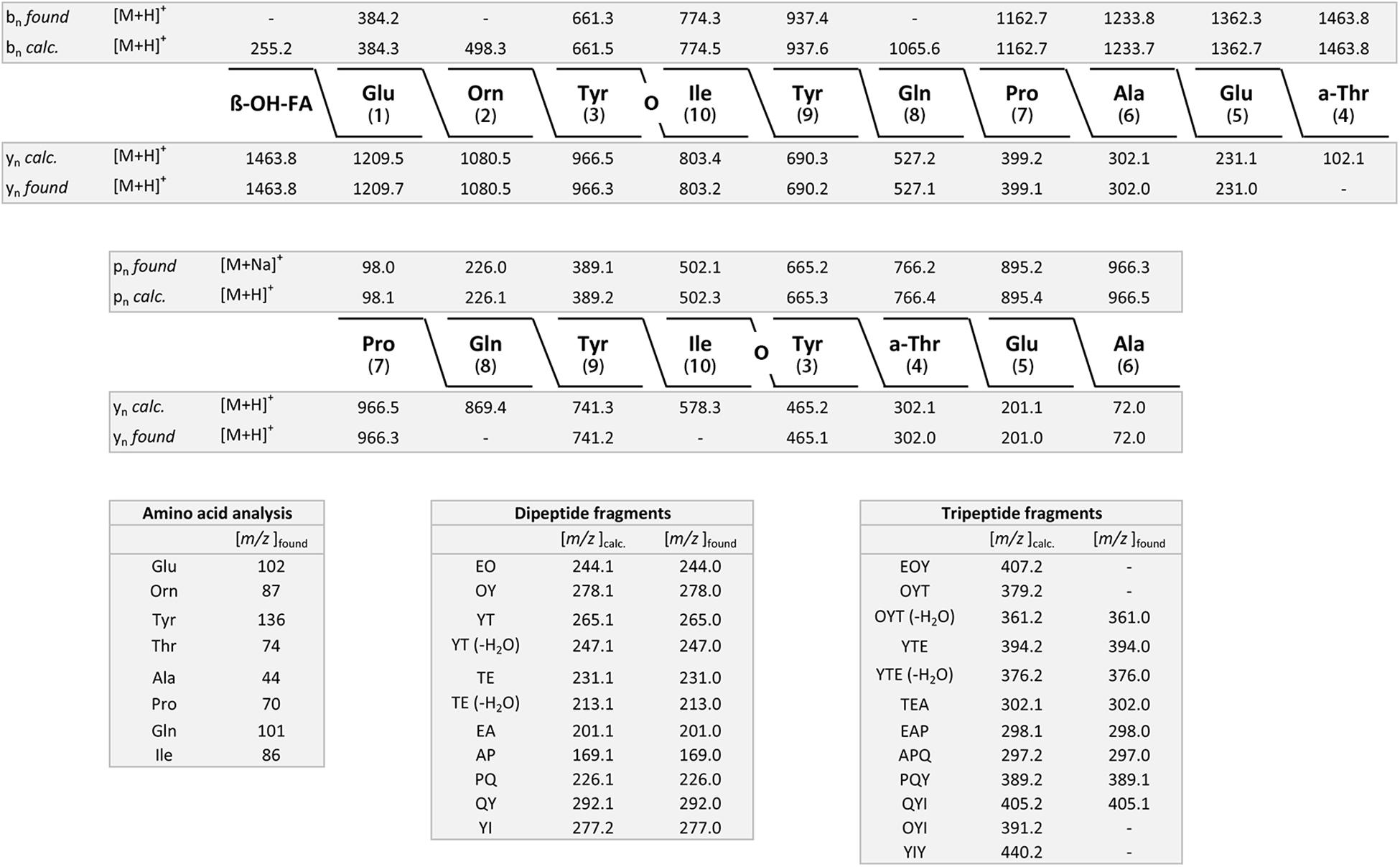
Figure 5. Mass spectrometric sequence determination of a C16-fengycin produced by DSM 7264T with a parent ion [M + H]+ = 1463.8 derived from product ion patterns obtained by LIFT-MALDI-TOF/TOF fragment spectra. pn means proline-directed ions. FA: CH3-(CH2)12-CHOH-CH2-CO-.
The structures of mycosubtilin and iturin C produced by the test strains DSM 32285 and DSM 29418 were also determined by LIFT-MALDI-TOF/TOF fragment analysis. In Supplementary Figure S3 the fragment pattern of C17-iturin C produced by strain DSM 32285 is demonstrated with a parent ion [M + H]+ = 1084.6. Proline-directed pn and yn – product ions were used for mass spectrometric sequence determination. The fragment pattern for structure elucidation of C15-mycosubtilin formed by B. atrophaeus DSM 29418 with parent ions [M + H,Na]+ = 1057.6 and 1079.6 is shown in Supplementary Figure S4.
Figure 6A shows the MALDI-TOF mass spectrum of a surface extract of the type strain DSM 7T of B. amyloliquefaciens, subsp. amyloliquefaciens, which was grown on Landy-agar for 24 h at 30°C. The dominating lipopeptide product is iturin A. C14-C16-iturin A variants were detected. Surfactins were found at a lower amount, while fengycins were completely missing. Similar results were obtained for strain DSM 32875 (see Figure 6B), which clearly belongs to same subspecies as DSM 7T. Here C14-C17 iturins A were found.
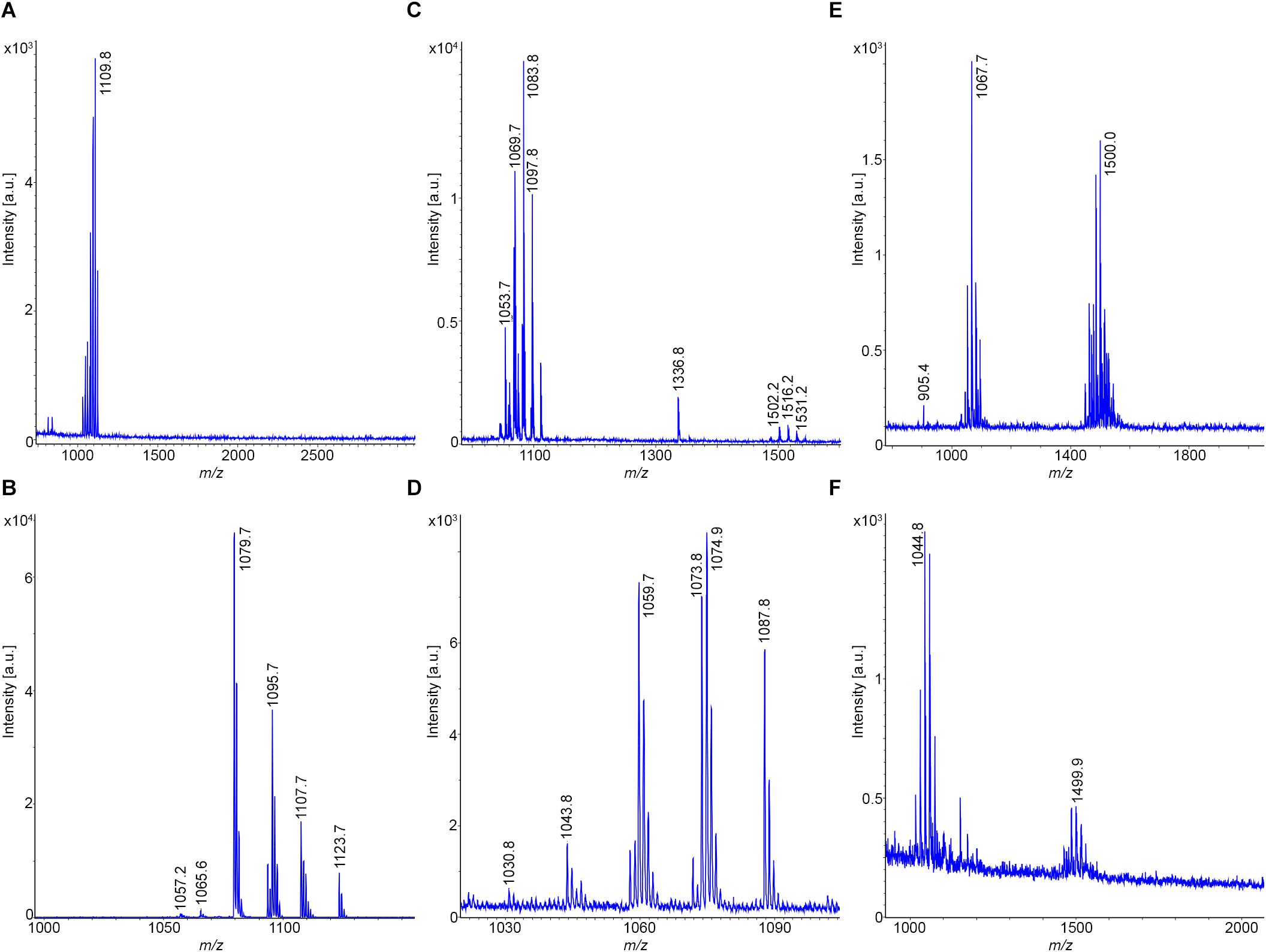
Figure 6. Detection of bioactive peptides produced by B. amyloliquefaciens, subsp. amyloliquefaciens DSM 7T (type strain) and DSM 32875 as well as B. velezensis DSM 23117T (FZB42) and DSM 29399 (FZB45). (A,B) MALDI-TOF mass spectra of surface extracts of B. amyloliquefaciens DSM 7T and DSM 32875 grown on Landy-agar. (C,D) MALDI-TOF mass spectra of surface extracts of B. velezensis DSM 23117T (FZB42; type strain) and DSM 29399 (FZB45) grown on Landy-agar. (E,F) MALDI-TOF mass spectra of culture supernatants of these strains cultivated for 24 h at 30°C in the Landy medium.
In contrast, the type strain for B. amyloliquefaciens, subsp. plantarum, B. velezensis DSM 23117T (FZB42) exhibits a particularly rich product pattern (Chen et al., 2007; Fan et al., 2018). A mass spectrum of this bacterium, which was grown under the same conditions as DSM 7T, is shown in Figure 6C. It produces all three families of lipopeptides, the surfactins, bacillomycins D as iturin compounds and fengycins, together with the TOMM microcin plantazolicin. Bacillomycins D were the main compounds in the surface extract. In Figure 6E the mass spectrum of a sample of the culture supernatant of DSM 23117T (FZB42) is shown, which was cultivated in the Landy medium for 24 h at 30°C. Here bacillibactin appeared. Again, the intensity of the fengycin mass peaks was higher than in the mass spectra of the surface extracts, which was also observed for the tested B. atrophaeus strains. Similar results have been obtained for strains Rs-MS87 and Sc-K143, which can be classified as B. velezensis. A variation was observed for DSM 29399 (FZB45), which has also been identified as a B. velezensis strain. In contrast to the type strain DSM 23117 (FZB42), it produces bacillomycin L instead of the D-variant as main product (see Figures 6D,F). Additionally, unlike the other examined strains here plantazolicin is completely missing. The MALDI-TOF mass spectra of the bacillomycin D and L- complexes are displayed in Figures 7A,B. In the surface extracts of strains FZB42 (DSM 23117T), Rs-MS87 and Sc-K143 C14-C17 bacillomycins D were detected. DSM 29399 (FZB45) produced C14–C16 bacillomycins L. Mass spectrometric structure analysis was performed for C14-bacillomycin D (parent ions for the protonated and sodiated forms: m/z = 1031.5 and 1053.5) from FZB42 and for C14-bacillomycin L (parent ions for the protonated and sodiated forms: m/z = 1021.5 and 1043.5) from FZB45 by LIFT-MALDI-TOF/TOF fragment analysis. The corresponding fragment patterns for their sequence determination are shown in Supplementary Figures S5, S6.
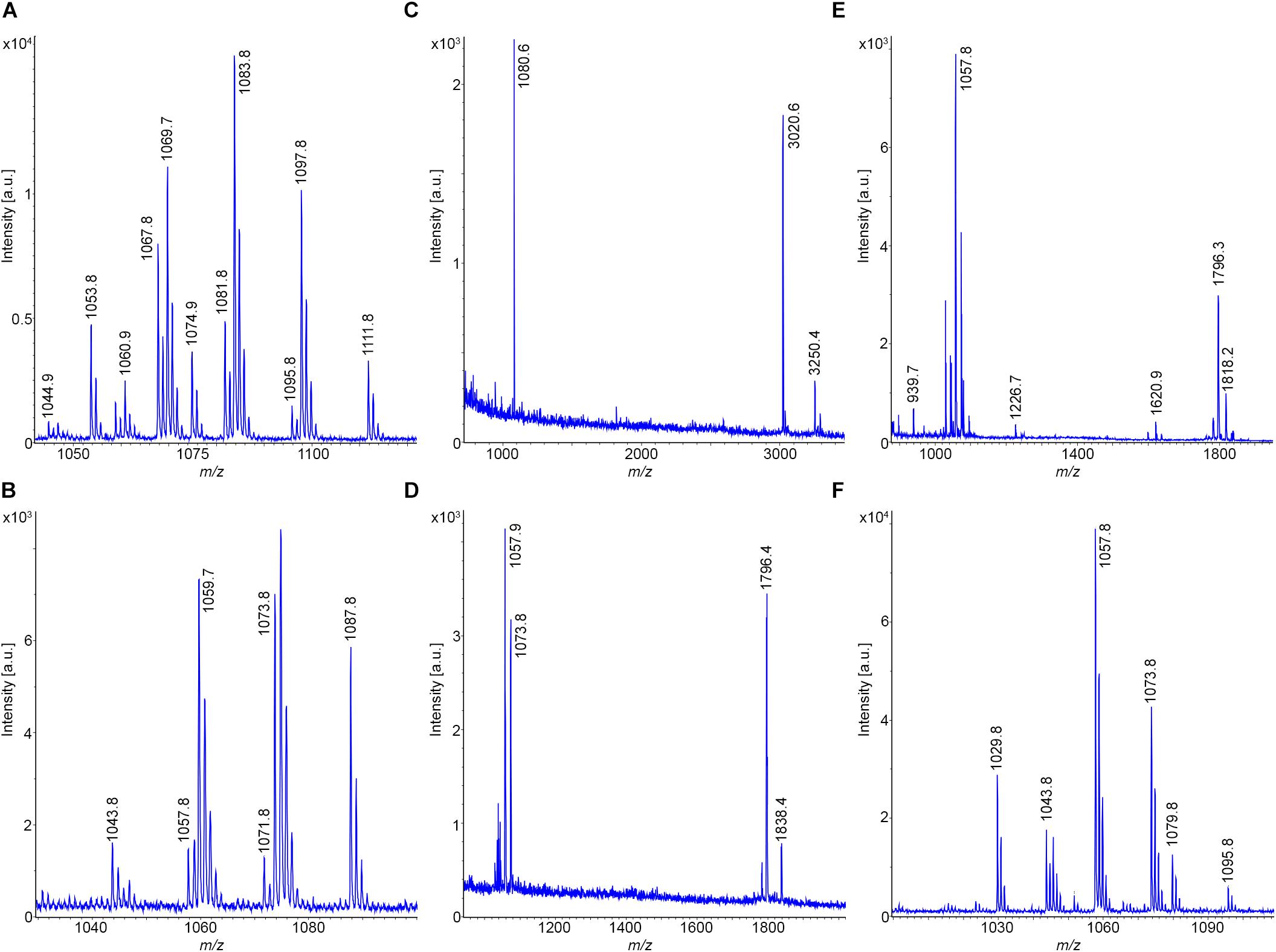
Figure 7. Detection of bioactive peptides produced by B. velezensis and B. licheniformis. (A,B) MALDI-TOF mass spectra of the bacillomycin D and L complexes produced by strains DSM 23117T (FZB42) and DSM 29399 (FZB45), respectively. C14-C17 bacillomycins D and C14-C16 bacillomycins L were detected. (C–E) MALDI-TOF mass spectra of surface extracts of B. licheniformis DSM 13T (type strain) (C); DSM 32874 (D); and M18 (E). (F) MALDI-TOF mass spectrum of the lichenysin-complex produced by strain DSM 32874. C13–C15 lichenysins were detected.
Two B. licheniformis strains, DSM 32874 and M18, were investigated for their production of bioactive peptides and compared with the data obtained for the B. licheniformis type strain DSM 13T. MALDI-TOF mass spectra of surface extracts of DSM13T; DSM32874 and M18 are shown in Figures 7C–E. The test strains DSM 32874 and M18 produced lichenysins which are lead metabolites of B. licheniformis (Pecci et al., 2010; Madslien et al., 2013; Favaro et al., 2016). The type strain DSM13T, however, is deficient in lichenysin production. Here, such compounds were neither found at the surface of this organism nor were they released into the culture medium. Lichenysins are closely related to surfactins by amino acid replacements. Frequently, L-Glu in position 1 of the surfactin sequence is mutated to L-Gln with the consequence that the mass numbers of lichenysins are by one mass lower than the corresponding surfactins. The lichenysin complex produced by DSM 32874 is exhibited in Figure 7F. C13–C15-lichenysins were detected.
Another characteristic product of B. licheniformis is lichenicidin (Begley et al., 2009; Dischinger et al., 2009; Caetano et al., 2011), a two component lantibiotic with mass numbers of m/z = 3020.6 and 3250.4, respectively. These lantipeptides specifically were found to be attached to the surface of such bacteria. They were not released into the culture medium. Lichenicidins dominate in the mass spectra of surface extracts of the type strain DSM 13T, which is a potent producer of these lantibiotics. They were visible in a growth period between 12 and 30 h. In the corresponding mass spectra of the test strains DSM 32874 and M18 they were found in only rather low amount.
Interestingly, in the mass spectra of the tested B. licheniformis strains several not yet identified compounds were detected. A prominent mass peak was found for all investigated test strains at m/z = 1796.4. This mass number refers to the protonated form of this compound. In addition, also peaks for its alkali adducts were monitored together with mass peaks at m/z = 1838.4 and 1880.2 which compared with the main species show a difference of 42 and 84 mass units, respectively. Presumably, these peaks represent the mono- and bi-acetylated forms of this compound. In the mass spectra of the type strain DSM 13T which is lacking m/z = 1796.4 another compound was found with a mass number of m/z = 1080.6 that remains to be identified. Lichenicidins were selectively found in surface extracts, when DSM 13T was grown on TSA-agar, while the compound with m/z = 1080.6 appeared specifically in samples cultivated on Landy-agar.
MALDI-TOF mass spectra of the culture supernatants of DSM 32874 and M18 also revealed the presence of lichenysins and the compound at m/z = 1796.4. The structure of a C15-lichenysin (parent ions for the protonated and sodiated forms: m/z = 1035.7 and 1057.4) from strain DSM 32874 was determined by LIFT-MALDI-TOF/TOF fragment analysis as shown in Supplementary Figure S7.
Two B. pumilus strains, Abi11 and strain Rs-Ts276, were investigated for their biosynthetic potential and compared with that of the type strain DSM 27T. In Figures 8A–C MALDI-TOF, mass spectra of surface extracts of these strains are displayed. The test strains Abi11 and Rs-Ts276 produce pumilacidins, which are also closely related to surfactins. Pumilacidins (Naruse et al., 1990), prominent lipopeptides of B. pumilus, differ from surfactins by longer fatty acid side chains (C13-C15–ß-OH-fatty acids for surfactins and C15-C19 – species for pumilacidins).
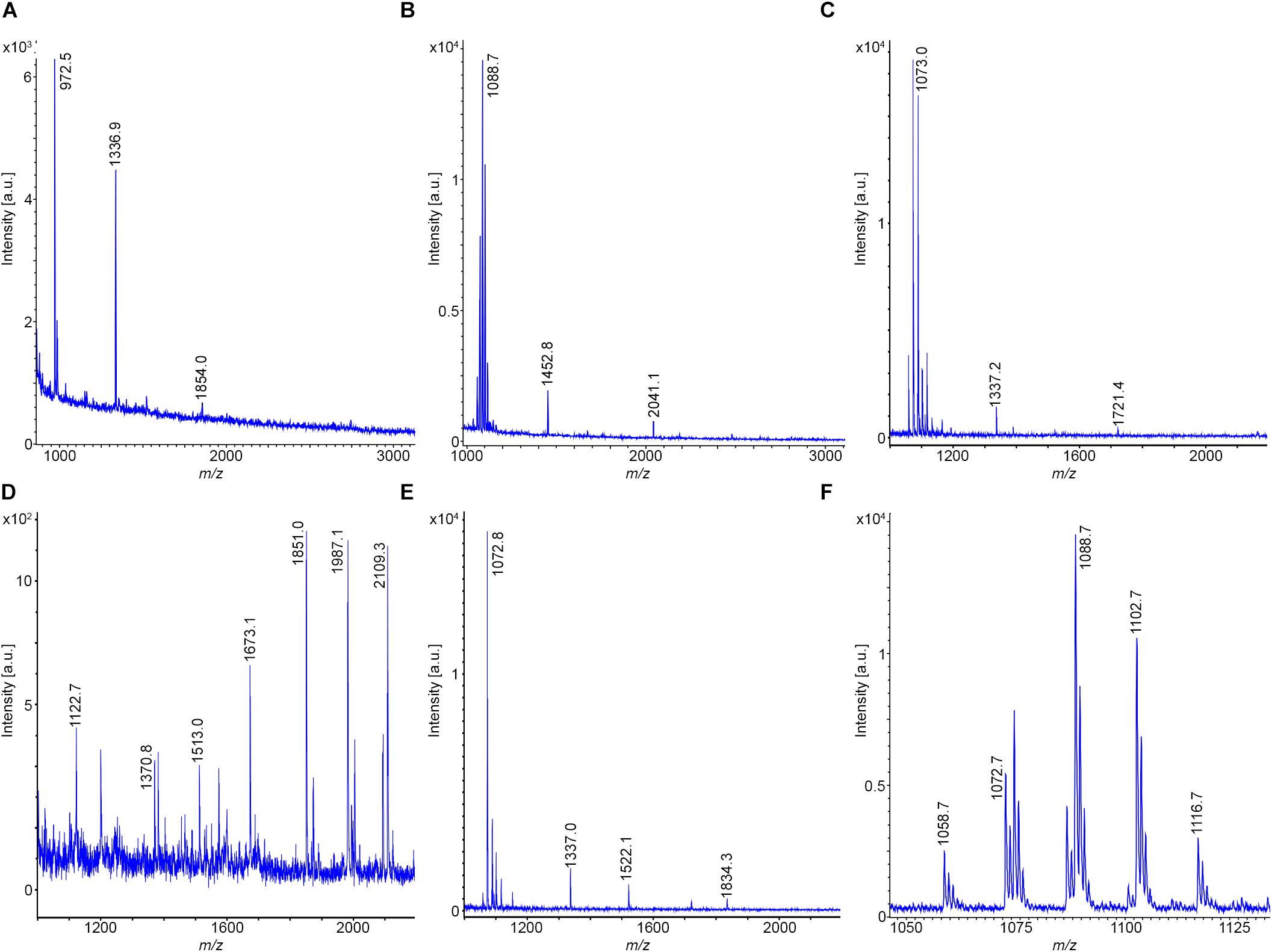
Figure 8. Detection of bioactive peptides produced by B. pumilus. (A–C) MALDI-TOF mass spectra of surface extracts of B. pumilus DSM 27T (type strain) (A); Abi11 (B) and Rs-Ts276 (C). MALDI-TOF mass spectra of the culture supernatant of B. pumilus Rs-Ts276 grown in the Landy-medium for 24 h at 30°C (D) and of a surface extract of the cell pellet obtained after 72 h (E). (F) MALDI-TOF mass spectrum of the pumilacidin-complex produced by Abi11. C15–C19 pumilacidins were detected.
Remarkably, the type strain DSM 27T is deficient in pumilacidin production, while the test strains Abi11 and Rs-Ts 276 are good pumilacidin producers. Obviously, these lipopeptides are attached to the outer cell wall. Therefore, they were predominantly found in surface extracts of B. pumilus cells grown on agar plates or were obtained from cell pellets sedimented from liquid cultures. On the other hand, the yield of pumilacidins was low in culture filtrates. They were found over the complete range of cultivation times from 10 to 72 h.
Similar to B. subtilis and B. velezensis, most of the B. pumilus strains form plantazolicin, which specifically was found in surface extracts. For example, good yields of this compound was obtained from DSM 27T cells picked from LB and TSA- agar plates (Figure 8A). Plantazolicin was also formed by Rs-Ts 276 (Figure 8C), but not by Abi 11 (Figure 8B). It was not observed in culture filtrates of the investigated B. pumilus strains. Strain Rs-Ts276 produces compounds with mass numbers of m/z = 1851.0; 1987.1; and 2109.3 which have still to be identified (Figure 8D). They appeared in the growth period between 8 and 24 h and were released into the culture medium. They dominate in MALDI-TOF mass spectra, when TSA is used as growth medium. They were not found in surface extracts of cell pellets sedimented by centrifugation from liquid cultures (Figure 8E). Figure 8F shows a MALDI-TOF spectrum of the pumilacidin-complex produced by B. pumilus Abi 11. C15-C19 pumilacidins were detected.
The volatilomes of the selected test- and type strains have been investigated. Volatiles spectra were established by using HS SPME GC-MS, compared to the NIST MS database 14 and confirmed via Kovats indices. Organic bacterial volatiles can be clustered in six different groups: hydrocarbons, ketones and alcohols, acids, sulfur and nitrogen-containing compounds and terpenes (Audrain et al., 2015; Kanchiswamy et al., 2015). Volatile profiles frequently are highly overlapping and difficult to detect due to their low concentration. The investigated rhizobacteria revealed mainly volatile hydrocarbons (alkanes and alkenes), alcohols, ketones, few sulfur-containing compounds and nitrogen-containing pyrazines. The number of volatiles produced by these isolates range from four up to seventeen (Table 3).
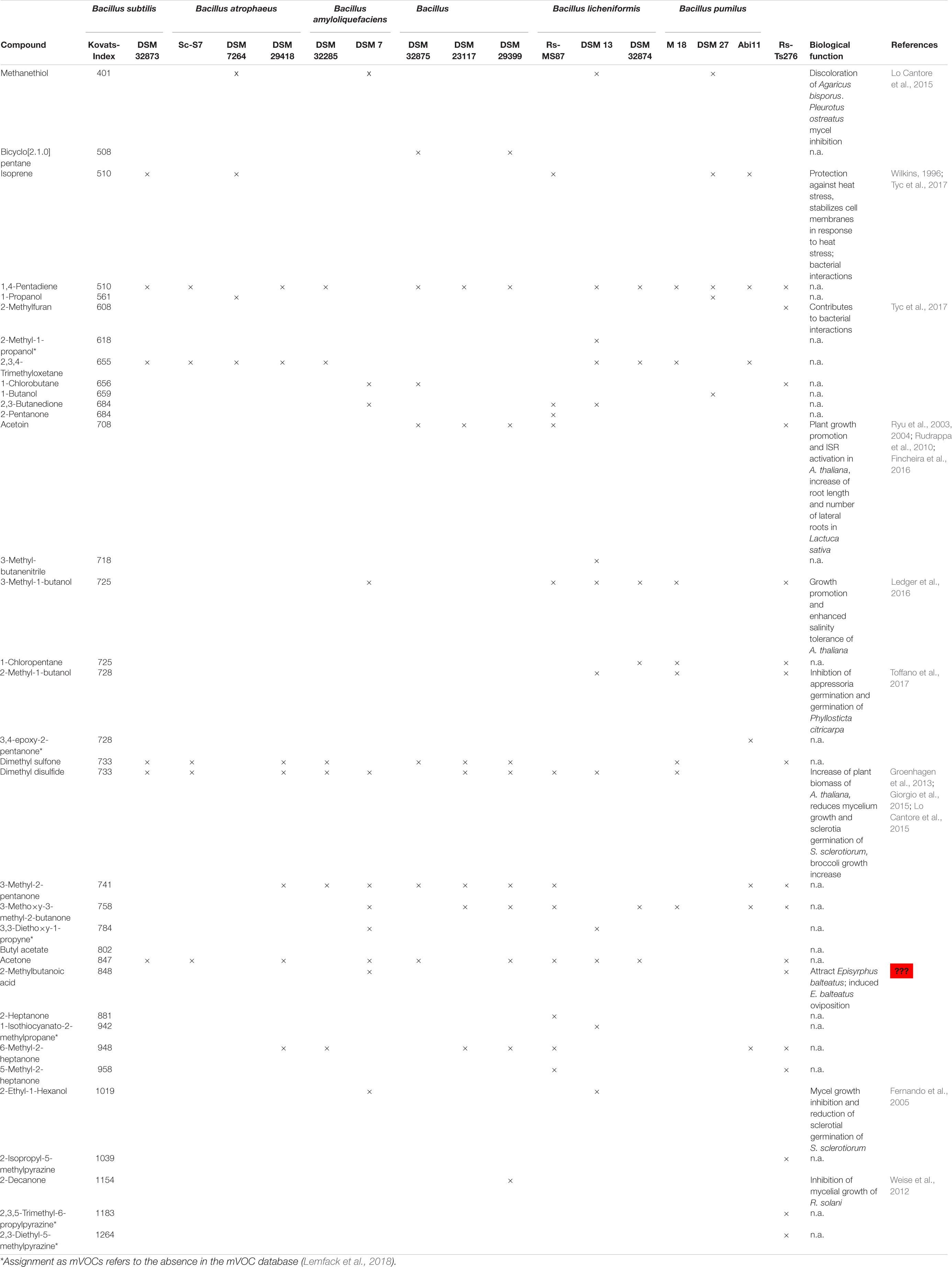
Table 3. Volatile organic compounds produced by different members of the Bacillus subtilis species complex. Identification was performed via HS-SPME GC-MS, matching to the NIST Mass Spectral Database and confirmation by the Kovats-Index.
Similar to the bioactive peptides the members of the B. subtilis complex can be classified according to their volatile production. Some of the detected VOCs, such as hydrocarbons, like 1,4 pentadiene and isoprene or sulfur containing compounds, like dimethyl sulfone and dimethyl disulfide were found wide-spread among the investigated strains, but other classes of VOCs can be specifically attributed to definite subcomplexes. For, example, acetoine was specifically produced by B. amyloliquefaciens and B. velezensis strains. 2,3,4-trimethyl-methoxetane is characteristic for B. subtilis, B. atrophaeus and B. licheniformis strains. The production of keto-compounds, such as 3-methyl-2-pentanone, 5- and 6-methyl-2-heptanone or 3-methoxy-3-methyl-2-butanone was observed for B. atrophaeus, B. amyloliquefaciens, B. velezensis and B. pumilus strains. While especially organisms belonging to B. velezensis (B. amyloliquefaciens ssp. plantarum) revealed a high variety of produced ketones, 5-methyl-2-heptanone and 6-methyl-2-heptanone could not be identified as volatile secondary metabolites of B. subtilis and B. licheniformis strains. 3-Methoxy-3-methyl-2-butanone was found in isolates belonging to the B. amyloliquefaciens subcomplex as well as to B. licheniformis and B. pumilus strains, but not for B. subtilis and B. atrophaeus. Alcohol-compounds, such as 2-methyl-1-propanol or 2- and 3-methyl- 1-butanol were predominantly formed by B. licheniformis. Alcohols, contributing with 16% to the overall diversity of microbial volatiles (Schenkel et al., 2015), did not appear in the volatile spectra of B. subtilis and B. atrophaeus. Dimethyl disulfide, reported for both growth promotion and reduction in Arabidopsis thaliana (Kai et al., 2010; Meldau et al., 2013), was not observed in the chromatograms of B. pumilus. Chlorobutane was specifically exhibited by B. amyloliquefaciens, subsp. amyloliquefaciens, whereas chloropentane was produced by B. licheniformis. The tested B. pumilus strains show a relatively high variety in volatile production. In particular, B. pumilus Rs-Ts276 is distinguished by an exceptional pattern of VOC-production, comprising hydrocarbons, alcohols, ketones as well as chlorobutane and chloropentane. A specific feature of this organism is the occurrence of pyrazine compounds. Alkylpyrazines show antifungal activity and inhibit sclerotia germination ability (Haidar et al., 2016; Mülner et al., 2019).
Furthermore, several mVOCs appeared in the volatilome of only one strain: 2-methylfuran in B. pumilus Rs-Ts276, 3-methylbutanenitrile and 1-isothiocyanato-2-methylpropane in B. licheniformis DSM13T, 2-pentanone in B. velezensis Rs-MS87, 1-butanol in B. pumilus DSM27T and 3,4-epoxy-2-pentanone in B. pumilus Abi11. Moreover, there was not a single volatile compound that could be detected in all investigated members of the B. subtilis species complex. The most abundant substance revealed to be 1,4-pentadiene that was identified in all strains apart from Rs-MS87, DSM 7264T, DSM 7T, and DSM 27T.
To evaluate their practical usage field trial experiments were performed with the investigated PGPR organisms using potato and maize as target plants. The achieved results were presented in Figures 9, 10. The relative additional yield in comparison to control in percent for potato is demonstrated for all tested rhizobacteria in Figure 9. The highest growth stimulation of approx. 25% was obtained for DSM 23117T (FZB42), the type strain for B. velezensis. Good growth promotion was also achieved by B. atrophaeus DSM 29418; B. subtilis DSM 32873 and B. velezensis DSM 29399 (FZB45), while the other organisms showed growth stimulation effects of at least 10%. In general, an increased concentration (1 l spore suspension) of the PGPR strain led to an increase in potato yield, which is especially visible for DSM 23117T (FZB42).
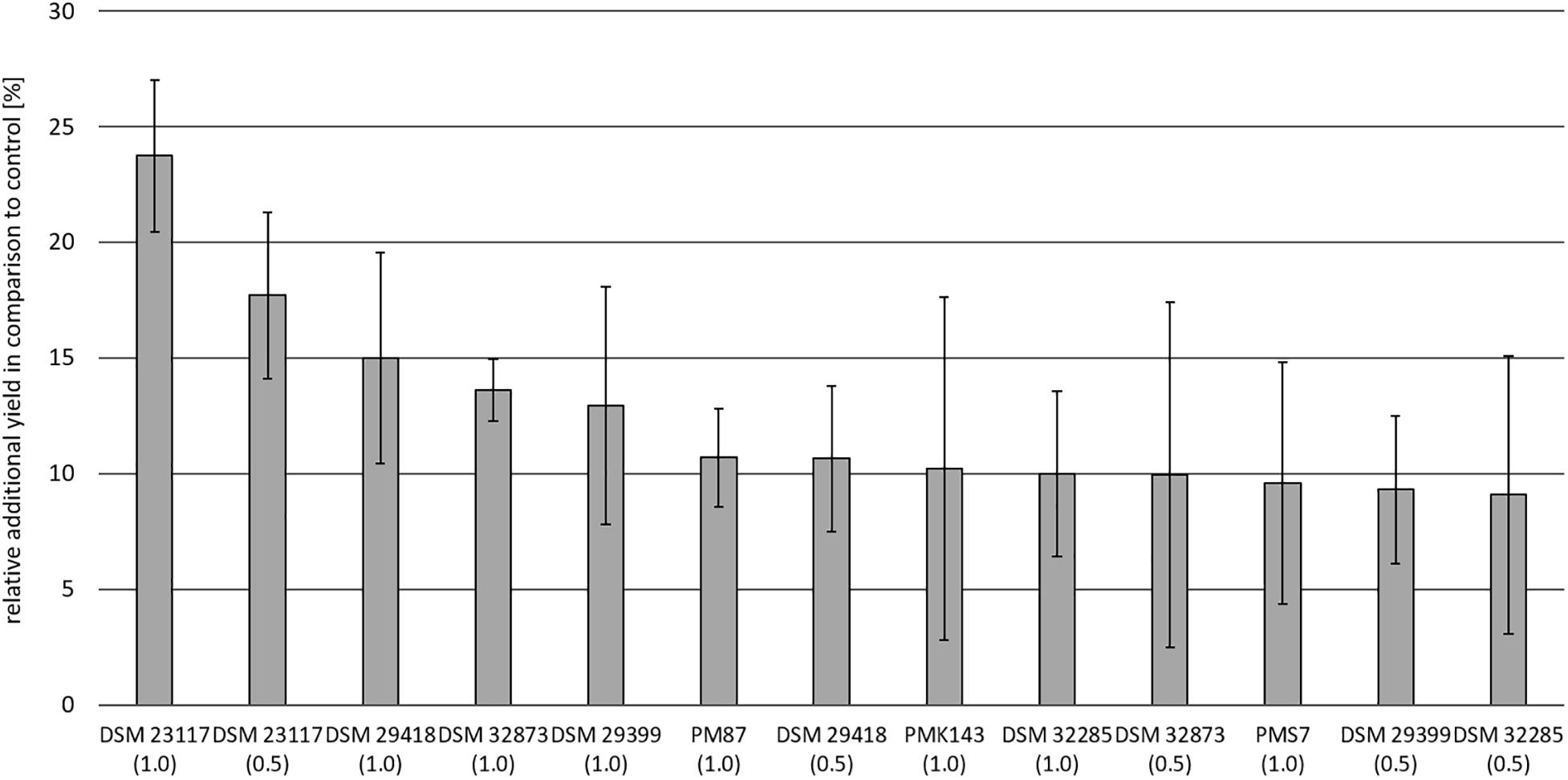
Figure 9. Field trial experiments with the investigated PGPR-strains of the B. subtilis complex with potato as target plant. Difference annual yield over control (without treatment with a PGPR strain) in percent demonstrating the growth stimulating effects of the investigated rhizobacteria. 0.5, l, or 1 l spore suspension of the PGPR strains in 300 l water were applied per hectare. Cultivar: “Goldmarie”; soil-type: slightly loamy sand; plot = 21 m2. The data are the mean of four randomized repetitions. Bars indicate standard errors between the replicates.
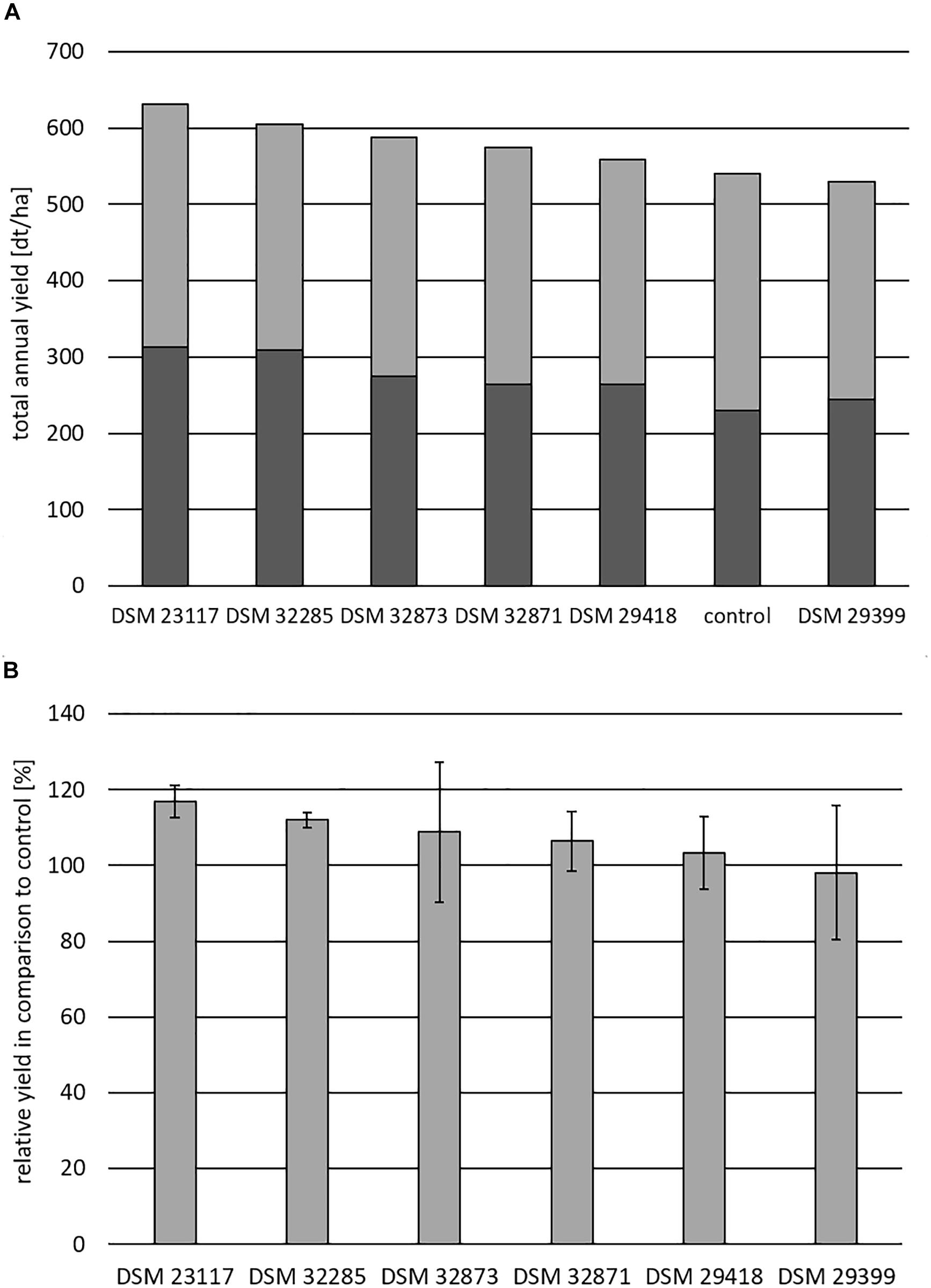
Figure 10. Field trial experiments with the investigated PGPR-strains of the B. subtilis complex with maize as target plant. (A) Shows the total annual yield in dt/ha obtained in the presence of the investigated rhizobacteria in comparison to the control without PGPR strains. The yield is differentiated in plant biomass (gray) and ear (black). (B) The relative yield in percent in the presence of the investigated PGPR strains compared with the control is exhibited. Cultivar: “Colisee”; soil-type: slightly loamy sand; plot = 18 m2. The data are the mean of four randomized repetitions. Bars indicate standard errors between the replicates.
The results for the maize field trials are presented in Figures 10A,B. Figure 10A shows the total annual yield in dt/ha. The control in the absence of the tested rhizobacteria amounted to approximately 530 dt/ha, dividing into approximately 200 dt/ha for the plant biomass and approximately 300 dt/ha for the ears. The relative yield in comparison to control in percent for maize is exhibited in Figure 10B. The production of the control was set to 100%. Again, B. velezensis DSM 23117T (FZB42) showed the highest growth stimulation effect of approximately 90 dt/ha (approximately 17%) followed by B. atrophaeus DSM 32285 (12%) and B. subtilis DSM 32873 (8.5%).
The members of the B. subtilis group are of high importance as industrially relevant Bacillus strains. Recent applications are focused on the enhancement of crop productivity and disease resistance as biostimulants and biopesticides. Most of the characterized members of the B. subtilis complex are PGPR. Some of them, as for example DSM 23117T (FZB42), the type strain of B. velezensis, are commercially applied to increase the productivity of numerous useful plants. The exploitable properties of these strains are essentially conferred by a wide spectrum of metabolites that are naturally produced by these microorganisms. Of particular importance for the activities and industrial utilization of such strains is their equipment with bioactive peptides and volatiles. In this study we performed a comprehensive screening of 13 industrially relevant test strains of the B. subtilis group for the production of such compounds, which are of high importance for their abilities as biofertilizers and biocontrol agents. For the rapid, reliable and sensitive detection of such compounds, mass spectrometric techniques are the tools of choice. In particular, MALDI-TOF MS is excellently suited to monitor metabolites, such as (lipo)peptides, polyketides and hybrids thereof, lantibiotics and biocines directly in cellular extracts and culture supernatants. On the other hand volatiles can be efficiently analyzed by GC-MS. Using such techniques, we identified and characterized the test organisms and determined their profiles for bioactive peptides and volatiles.
In the first step, the test organisms were identified and attributed to the four subcomplexes of the B. subtilis complex by MALDI-TOF MS. Thereafter, we determined their profiles for the production of bioactive peptides and volatiles. The obtained results were summarized in Tables 1, 3. They demonstrate that each subgroup is distinguished by specific sets of such compounds. As far as bioactive peptides are concerned the following features can be derived:
The members of all subcomplexes ubiquitously produce surfactins or related lipopeptides, such as lichenysins (B. licheniformis) and pumilacidins (B. pumilus) as well as the siderophore bacillibactin. Iturin compounds (iturins A and C; bacillomycins D, F and L; mycosubtilins) were produced specifically by B. atrophaeus, B. amyloliquefaciens, and B. velezensis, but not by B. subtilis, B. licheniformis, and B. pumilus. Plantazolicin is formed by B. subtilis, B. velezensis, and B. pumilus. Fengycin, an antifungal lipotridecapeptide and efficient biocontrol agent against filamentous fungi, was found for B. subtilis, B. atrophaeus, B. amyloliquefaciens, and B. velezensis, but not for B. licheniformis and B. pumilus. The structure of all these bioactive compounds is demonstrated in Figure 11.
Combining these features with specific biomarkers of members of the different subgroups of the B. subtilis complex (see Table 2) enables a rapid and reliable mass spectrometric identification of organisms that belong to the B. subtilis group without the need to consult an expensive database. For this procedure merely a disintegration mass spectrum of a strain has to be combined with a MALDI-TOF mass spectrum of a surface extract of this organism, which both can be afforded in a minimum of time. This approach yields specific information about cellular components and bioactive products, which can be used to identify an unknown bacterial strain. Results obtained with this simple approach assorts well with the identification on the basis of data base evaluation.
The profiles for bioactive peptides derived from mass spectrometric evaluation of the test strains essentially are compatible with the wealth of knowledge obtained on the genetic level from whole genome sequencing (Fan et al., 2017; Harwood et al., 2018; Dunlap et al., 2019) Table 4 shows the presence of the corresponding gene clusters in the genomes for the production of the bioactive peptides of the investigated PGPR-strains. For the detection of the gene clusters the complete genome sequences from the test strains Sc-S7, Rs-MS87 andSc-K143 as well as the type strains 168T, DSM7T, DSM13T, and DSM27T were available. For these organisms the gene cluster information was obtained by antiSMASH-4.0-analysis (Blin et al., 2017). For the other strains the relevant gene clusters were derived from the detected products.
Mass spectrometric and chemical analysis are the ultimate criteria which metabolites are really produced. This situation is particularly evident from the comparison of the production of bioactive peptides by the investigated type- and test-strains. While the test strains frequently exhibited a widely complete product spectrum, most of the type strains revealed deficiencies concerning the production of essential metabolites. The most prominent example is the type strain 168T for B. subtilis, which does not produce any non-ribosomally formed peptide or polyketide. Though the biosynthetic genes for the biosynthesis of surfactin and fengycin, for example, were found intact in the genome of this strain, both lipopeptides were not produced because of a deficient Sfp-protein preventing the loading of the 4′-phosphopantetheinyl moiety to the T-domains of the corresponding synthetases (Nakano et al., 1992; Cosmina et al., 1993; Kunst et al., 1997). DSM 7T, the well known type strain for B. amyloliquefaciens, does not produce fengycins. In the genome of DSM 7T fengycin biosynthetic genes fenA-C are missing, while only fenD-E are left (Borriss et al., 2011). Therefore, fengycin production is omitted.
Other candidates are B. licheniformis DSM13T and B. pumilus DSM 27T which in their genomes harbor the gene clusters for the production of lichenysins (Veith et al., 2004), the lead metabolite of B. licheniformis, and pumilacidins, characteristic for the genus B. pumilus. Both type strains are deficient in the formation of these compounds which obviously are not expressed. However, DSM13T is a potent producer of the dual lantibiotics lichenicidins (Begley et al., 2009; Dischinger et al., 2009; Caetano et al., 2011), while DSM27T forms plantazolicin.
Subcomplex a of the B. subtilis species complex comprises B. subtilis and the closely related B. atrophaeus strains, which though being closely related show characteristic differences in their product spectra. In contrast to B. subtilis, B. atrophaeus strains produce iturin compounds, but are lacking plantazolicin. B. atrophaeus strains revealed a compound with a mass number of m/z = 2754.6 Da, which still has to be identified. This metabolite is specific for the B. atrophaeus strains studied.
Bacillus amyloliquefaciens (subcomplex b) can be classified into two subspecies B. amyloliquefaciens, subspecies amyloliquefaciens and B. amyloliquefaciens, subspecies plantarum, now designated as B. velezensis (Fan et al., 2018). B. velezensis strains are able to colonize plant roots and organs and to live with plants in a symbiosis as endophytes. These two subspecies can clearly be distinguished mass spectrometrically both as far as their disintegration mass spectrum and their product pattern are concerned. The prototype of subspecies amyloliquefaciens is DSM 7T, which in contrast to B. velezensis strains produces iturins instead of bacillomycins and does not form fengycins and plantazolicin. From all members of the B. subtilis complex, B. velezensis strains are the most productive organisms as far as secondary metabolites are concerned. A prominent example is the type strain DSM 23117T (FZB42). Approximately 8 kDa of its genome is devoted for the production of such compounds (Chen et al., 2007), among them three lipopeptide families, the surfactins, bacillomycins D and fengycins, the siderophore bacillibactin, plantazolicin, the dipeptide bacilysin and a wide spectrum of volatiles. In addition, it forms amylocyclicin, a ring-shaped biocine with a molecular mass of m/z = 6.8 kDa showing antimicrobial activities (Scholz et al., 2014).
Bacillus licheniformis strains representing subcomplex c exhibit two specific products, the lichenysins, a lipopeptide family which is related to surfactin and the dual lantibiotics, the lichenicidins. In addition, we detected by MALDI-TOF MS another yet unknown compound with a mass number of m/z = 1796.2 Da, which is produced in appreciable extent. It has to be identified by future work.
Subcomplex d is formed by B. pumilus strains. Here, the main products are pumilacidins, which are also closely related to surfactins sharing longer fatty acid side chains with similar amino acid sequences. B. pumilus does not produce iturins and fengycins, but forms plantazolicin.
Microorganisms produce a huge number of low mass volatile organic compounds, which attain increasing attention for agricultural and biotechnological applications. Nowadays, databases are available, which comprise more than 2000 of such substances (Lemfack et al., 2014, 2018). Also, the members of the B. subtilis complex produce an extensive pattern of these volatile metabolites which are listed in Table 3. Their nature and action gain increasing attention concerning plant growth promotion (Ryu et al., 2003; Asari et al., 2016; Wu et al., 2018), biocontrol against phytopathogenic bacteria (Rajer et al., 2017; Tahir et al., 2017a; Xie et al., 2018) and fungi (Fernando et al., 2005; Zhang et al., 2013; Chaves-Lopez et al., 2015; Asari et al., 2016; Massawe et al., 2018; Hassan et al., 2019), induction of systemic resistance against phytopathogens (Ryu et al., 2004; Farag et al., 2006, 2013) as well as steering of gene expression and metabolic processes in plants (Tahir et al., 2017b). Similar to bioactive peptides the members of the B. subtilis complex show characteristic specificities concerning the produced volatiles. For example, among the different classes of such compounds ketones were frequently produced by B. atrophaeus, B. amyloliquefaciens, B. velezensis and B. pumilus strains. In particular, acetoine which is well known for its growth promoting and systemic resistance inducing effects (Ryu et al., 2003) could specifically attributed to B. amyloliquefaciens and B. velezensis strains forming subcomplex 2, while alcohols, such as 2- and 3-methyl-1-butanol were predominantly produced by B. licheniformis (subcomplex 3).
The volatilomes, a term first used by Maffei et al. (2011), of the investigated plant growth promoting bacteria were highly overlapping, an effect that is potentially influenced by media, GC MS and the fiber used in the data acquisition process. An omnipresent dilemma in profiling of microbial volatile spectra is that the concentration of many mVOCs is often merely above the threshold. Only two of the so far detected, but not yet described volatiles were unique for one of the investigated bacteria: 3,4-epoxy-2-pentanone in B. pumilus Abi11 and 2,3,5-trimethyl-6-propylpyrazine in B. pumilus Rs-Ts276. Other bacterial volatiles which were solely measured in one of the investigated strains, were previously characterized in other studies and can be found in the mVOC database (Lemfack et al., 2014, 2018). Overall, 33 volatile substances could be identified including six novel microbial volatiles according to the mVOC database (Table 3). The biological function of the majority of mVOCs has yet to be ascertained. Only 17 out of 40 volatiles have a known function with only three of them associated to plant growth promotion.
All the investigated industrial test strains show promising growth stimulation effects in field trials with potato and maize as model plants demonstrated in Figures 9, 10. The highest effects were always achieved with DSM 23117T (FZB42), the type strain for B. velezensis which gains progressive industrial attention. The knowledge of the potential of the investigated strains for the production of bioactive peptides and volatiles presented in this paper will provide the basis for future work to improve their efficacy in plant growth promotion and biocontrol activities to protect plants from infection by phytopathogens.
Thirteen industrially relevant strains were identified by MALDI-TOF MS as members of the B. subtilis community and were attributed to the corresponding subcomplexes. In our work we present a comprehensive mass spectrometric exploration of the bioactive peptides and volatiles produced by the members of the B. subtilis complex in a time- and space directed manner for the first time. Each of the four subcomplexes of the B. subtilis species complex is equipped with a specific set of such compounds. Their profiles can be used for rapid mass spectrometric typing of their producer organisms. The impact of the tested rhizobacteria on the yield in growing potato and maize was investigated in field trials. Growth stimulation effects between 10 and 25% were obtained. Future research will be focused on the mechanisms of the detected metabolites in plant growth stimulation and plant health improvement. Especially the understanding of the modes of action of mVOCs on plant growth is widely unknown, apart from a few volatiles. Deeper insight and further knowledge will help to identify ideal plant growth promoting bacteria for specific applications in agriculture.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
KD, ES, PM, and JV designed and developed the experimental strategy and the concept of the manuscript. ES, PM, KD, and SH did the microbiological work (strain management and sample preparation). JV, SH, and MW performed the generation, evaluation and presentation of the mass spectrometric data. PM studied the volatilomes of the tested strains. KD and HJ initiated and supervised the field trial tests. JV, TC, and PM wrote the manuscript. HJ, PL, TC, and GB were involved in preparing the manuscript. All authors contributed to the article and approved the submitted version.
This project is part of the BestPass International Network funded by the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie grant agreement no. 676480. Financial support by the Bundesministerium für Bildung und Forschung (Projekt: ENDOBICA) is gratefully acknowledged.
PM, ES, KD, HJ, and JV were employed by the company ABiTEP GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Angelika Schaefer (Graz) for her assistance in volatile analytics. The engagement of Thomas Kirsch in the evaluation of the field trial experiments is gratefully acknowledged.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01432/full#supplementary-material
Argeulles-Arias, A., Ongena, M., Halimi, B., Lara, Y., Brans, A., Joris, B., et al. (2009). Bacillus amyloliquefaciens GAI as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Fact. 8:63. doi: 10.1186/1475-2859-8-63
Asari, S., Matzén, S., Petersen, M. A., Bejai, S., and Meijer, J. (2016). Multiple effects of Bacillus amyloliquefaciens volatile compounds: plant growth promotion and growth inhibition of phytopathogens. FEMS Microbiol. Ecol. 92:fiw070. doi: 10.1093/femsec/fiw070
Audrain, B., Farag, M. A., Ryu, C. M., and Ghigo, J. M. (2015). Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 39, 222–233. doi: 10.1093/femsre/fuu013
Bailly, A., and Weisskopf, L. (2017). Mining the volatilomes of plant-associated microbiota for new biocontrol solutions. Front. Microbiol. 8:1638. doi: 10.3389/fmicb.2017.01638
Barea, J. M., Pozo, M. J., Azcón, R., and Azcón-Aguilar, C. (2005). Microbial co-operation in the rhizosphere. J. Exp. Bot. 56, 1761–1778. doi: 10.1093/jxb/eri197
Begley, M., Cotter, P. D., Hill, C., and Ross, R. P. (2009). Identification of a novel two-peptide lantibiotic lichenicidin, following rational genome mining for LanM proteins. Appl. Environ. Microbiol. 75, 5451–5460. doi: 10.1128/AEM.00730-09
Berg, G. (2015). Beyond borders: investigating microbiome interactivity and diversity for advanced biocontrol technologies. Microb. Biotechnol. 8, 5–7. doi: 10.1111/1751-7915.12235
Blin, K., Wolf, T., Chevrette, M. G., Lu, X., Schwalen, C. J., Kautsar, S. A., et al. (2017). antiSMASH 4.0 – improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 45, W36–W41. doi: 10.1093/nar/gkx319
Bonmatin, J.-M., Laprevote, O., and Peypoux, F. (2003). Diversity among microbial cyclic lipopeptides: iturins and surfactins. Activity-structure relationships to design new bioactive agents. Comb. Chem. High Throughput Screen. 56, 541–556. doi: 10.2174/138620703106298716
Borriss, R., Chen, X.-H., Rueckert, C., Blom, J., Becker, A., Baumgarth, B., et al. (2011). Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: a proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int. J. Syst. Evol. Microbiol. 61, 1786–1801. doi: 10.1099/ijs.0.023267-0
Caetano, T., Krawczyk, J. M., Mösker, E., Süssmuth, R. D., and Mendo, S. (2011). Heterologous expression, biosynthesis, and mutagenesis of type II lantibiotics from Bacillus licheniformis in Escherichia coli. Chem. Biol. 18, 90–100. doi: 10.1016/j.chembiol.2010.11.010
Caulier, S., Nannan, C., Gillis, A., Licciardi, F., Bragard, C., and Mahillon, J. (2019). Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 10:302. doi: 10.3389/fmicb.2019.00302
Cernava, T., Aschenbrenner, I. A., Grube, M., Liebminger, S., and Berg, G. (2015). A novel assay for the detection of bioactive volatiles evaluated by screening of lichen-associated bacteria. Front. Microbiol. 6:398. doi: 10.3389/fmicb.2015.00398
Chaves-Lopez, C., Serio, A., Gianotti, A., Sacchetti, G., Ndagijimana, M., Ciccarone, C., et al. (2015). Diversity of food-borne Bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 119, 487–499. doi: 10.1111/jam.12847
Chen, X. H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., et al. (2007). Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 25, 1007–1014. doi: 10.1038/nbt1325
Cosmina, P., Rodriguez, F., de Ferra, F., Grandi, G., Perego, M., Venema, G., et al. (1993). Sequence and analysis of the genetic locus responsible for surfactin synthesis in Bacillus subtilis. Mol. Microbiol. 8, 821–831. doi: 10.1111/j.1365-2958.tb01629.x
Dischinger, J., Josten, M., Szekat, C., Sahl, H.-G., and Bierbaum, G. (2009). Production of the novel two-peptide lantibiotic lichenicidin by Bacillus licheniformis DSM 13. PLoS One 4:e6788. doi: 10.1371/journal.pone.0006788
Drogue, B., Doré, H., Borland, S., Wisniewski-Dyé, F., and Prigent-Combaret, C. (2012). Which specificity in cooperation between phytostimulating rhizobacteria and plants? Res. Microbiol. 163, 500–510. doi: 10.1016/j.resmic.2012.08.006
Dunlap, C. A., Bowman, M. J., and Rooney, A. P. (2019). Iturinic lipopeptide diversity in the Bacillus subtilis group-important antifungals for plant disease biocontrol applications. Front. Microbiol. 10:1794. doi: 10.3389/fmicb.2019.01794
Fan, B., Blom, J., Klenk, H.-P., and Borriss, R. (2017). Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 8:22. doi: 10.3389/fmicb.2017.00022
Fan, B., Wang, C., Song, X. F., Ding, X. L., Wu, L., Wu, H., et al. (2018). Bacillus velezensis FZB42 in 2018: the Gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 9:2491. doi: 10.3389/fmicb.2018.02491
Farag, M. A., Ryu, C.-M., Sumner, L. W., and Pare, P. W. (2006). GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67, 2262–2268. doi: 10.1016/j.phytochem.2006.07.021
Farag, M. A., Zhang, H., and Ryu, C.-M. (2013). Dynamic chemical communication between plants and bacteria through air borne signals: induced resistance by bacterial volatiles. J. Chem. Ecol. 39, 1007–1018. doi: 10.1007/s10886-013-0317-9
Favaro, G., Bogialli, S., di Gangi, I. M., Nigris, S., Baldan, E., Squartini, A., et al. (2016). Characterization of lipopeptides produced by Bacillus licheniformis using chromatography with accurate tandem mass spectrometry. Rapid Commun. Mass Spectrom. 30, 2237–2252. doi: 10.1002/rcm.7705
Fernando, W. G. D., Ramarathnam, R., Krishnamoorthy, A. S., and Savchuk, S. C. (2005). Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 37, 955–964. doi: 10.1016/J.SOILBIO.2004.10.021
Fincheira, P., Venthur, H., Mutis, A., Parada, M., and Quiroz, A. (2016). Growth promotion of Lactuca sativa in response to volatile organic compounds emitted from diverse bacterial species. Microbiol. Res. 193, 39–47. doi: 10.1016/j.micres.2016.09.008
Fritze, D. (2004). Taxonomy of the genus Bacillus and related genera: the aerobic endospore-forming bacteria. Phytopathology 94, 1245–1248. doi: 10.1094/PHYTO.2004.94.11.1245
Giorgio, A., De Stradis, A., Lo Cantore, P., and Iacobellis, N. S. (2015). Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front. Microbiol. 6:1056. doi: 10.3389/fmicb.2015.01056
Groenhagen, U., Baumgartner, R., Bailly, A., Gardiner, A., Eberl, L., Schulz, S., et al. (2013). Production of bioactive volatiles by different Burkholderia ambifaria strains. J. Chem. Ecol. 39, 892–906. doi: 10.1007/s10886-013-0315-y
Haidar, R., Roudet, J., Bonnard, O., Dufour, M. C., Corio-Costet, M. F., Fert, M., et al. (2016). Screening and modes of action of antagonistic bacteria to control the fungal pathogen Phaeomoniella chlamydospora involved in grapevine trunk diseases. Microbiol. Res. 192, 172–184. doi: 10.1016/j.micres.2016.07.003
Hardoim, P. R., van Overbeck, L. S., Berg, G., Pirttilä, A. M., Compant, S., Campisano, A., et al. (2015). The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 79, 293–320. doi: 10.1128/MMBR.00050-14
Harwood, C. R., Mouillon, J.-M., Pohl, S., and Arnau, J. (2018). Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. Rev. 42, 721–738. doi: 10.1093/femsre/fuy028
Hassan, Z. U., Thani, R. A., Alnaimi, H., Migheli, Q., and Jaoua, S. (2019). Investigation and application of Bacillus licheniformis volatile compounds for the biological control of toxigenic Aspergillus and Penicillium spp. ACS Omega 4, 17186–17193. doi: 10.1021/acsomega.9b01638
Hong, T.-Y., and Meng, M. (2003). Biochemical characterization and antifungal activity of an endo- 1,3-β-glucanase of Paenibacillus sp. isolated from garden soil. Appl. Microbiol. Biotechnol. 61, 472–478. doi: 10.1007/s00253-003-1249-z
Kai, M., Crespo, E., Cristescu, S. M., Harren, F. J. M., Francke, W., and Piechulla, B. (2010). Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl. Microbiol. Biotechnol. 88, 965–976. doi: 10.1007/s00253-010-2810-1
Kalyon, B., Helaly, S. E., Scholz, R., Nachtigall, J., Vater, J., Borriss, R., et al. (2011). Plantazolicin A and B: structure elucidation of ribosomally synthesized thiazole/oxazole peptides from Bacillus amyloliquefaciens FZB42. Org. Lett. 20, 2996–2999. doi: 10.1021/ol200809m
Kanchiswamy, C. N., Malnoy, M., and Maffei, M. E. (2015). Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 6:151. doi: 10.3389/fpls.2015.00151
Keswani, C., Singh, H. B., Garcia-Estrada, C., Caradus, J., He, Y.-W., Mezaache-Aichour, S., et al. (2020). Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 104, 1013–1034. doi: 10.1007/s00253-019-10300-8
Kloepper, J. W., Ryu, C.-M., and Zhang, S. (2004). Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94, 1259–1266. doi: 10.1094/phyto.2004.94.11.1259
Koumoutsi, A., Chen, X.-H., Henne, A., Liesegang, H., Hitzeroth, G., Franke, P., et al. (2004). Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J. Bacteriol. 186, 1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004
Kowall, M., Vater, J., Kluge, B., Stein, T., Franke, P., and Ziessow, D. (1998). Separation and characterization of surfactin isoforms produced by Bacillus subtilis OKB 105. J. Colloid Inteface Sci. 204, 1–8. doi: 10.1006/jcis.1998.5538
Kunst, F., Ogasawara, N., Moszer, I., Albertini, A. M., Alloni, G., Bertero, M. G., et al. (1997). The complete genome sequence of the gram positive bacterium Bacillus subtilis. Nature 390, 249–256. doi: 10.1038/36886
Landy, M., Warren, G. H., Rosenman, S. B., and Colio, L. G. (1948). Bacillomycin; an antibiotic from Bacillus subtilis active against pathogenic fungi. Proc. Soc. Exp. Biol. Med. 67, 539–541. doi: 10.3181/00379727-67-16367
Lasch, P., Nattermann, H., Erhard, M., Stämmler, M., Grunow, R., Bannert, N., et al. (2008). A MALDI-ToF mass spectrometry compatible inactivation method for highly pathogenic microbial cells and spores. Anal. Chem. 80, 2026–2034. doi: 10.1021/ac701822
Lasch, P., Wahab, T., Weil, S., Palyi, B., Tomaso, H., Zange, S., et al. (2015). Identification of highly pathogenic microorganisms by matrix-assisted laser desorption ionization-time of flight mass spectrometry: results of an interlaboratory ring trial. J. Clin. Microbiol. 53, 2632–2640. doi: 10.1128/JCM.00813-15
Ledger, T., Rojas, S., Timmermann, T., Pinedo, I., Poupin, M. J., Garrido, T., et al. (2016). Volatile-mediated effects predominate in Paraburkholderia phytofirmans growth promotion and salt stress tolerance of Arabidopsis thaliana. Front. Microbiol. 7:1838. doi: 10.3389/fmicb.2016.01838
Lemfack, M. C., Gohlke, B.-O., Toguem, S. M., Preissner, S., Piechulla, B., and Preissner, R. (2018). mVOC 2.0: a database of microbial volatiles. Nucleic Acids Res. 46, D1261–D1265. doi: 10.1093/narlgkx1016
Lemfack, M. C., Nickel, J., Dunkel, M., Preissner, R., and Piechulla, B. (2014). MVOC: a database of microbial volatiles. Nucleic Acids Res. 42, D744–D748. doi: 10.1093/nar/gkt1250
Leroy, P. D., Sabri, A., Heuskin, S., Thonart, P., Lognay, G., Verheggen, F. J., et al. (2011). Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nat. Commun. 2:348. doi: 10.1038/ncomms1347
Lim, H.-S., Kim, Y.-S., and Kim, S.-D. (1991). Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl. Environ. Microbiol. 57, 510–516. doi: 10.1128/aem.57.2.510-516.1991
Lo Cantore, P., Giorgio, A., and Iacobellis, N. S. (2015). Bioactivity of volatile organic compounds produced by Pseudomonas tolaasii. Front. Microbiol. 6:1082. doi: 10.3389/fmicb.2015.01082
Lugtenberg, B., and Kamilova, F. (2009). Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 63, 541–556. doi: 10.1146/annurev.micro.62.081307.162918
Madslien, E. H., Ronning, H. T., Lindback, T., Hassel, B., Andersson, M. A., and Granum, P. E. (2013). Lichenysin is produced by most Bacillus licheniformis strains. J. Appl. Microbiol. 115, 1068–1080. doi: 10.1111/jam.12299
Maffei, M. E., Gertsch, J., and Appendino, G. (2011). Plant volatiles: production, function and pharmacology. Nat. Prod. Rep. 28, 1359–1380. doi: 10.1039/c1np00021g
Massawe, V. C., Hanif, A., Farzand, A., Mburu, D. K., Ochola, S. O., Wu, L., et al. (2018). Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytophatology 108, 1373–1385.
Meldau, D. G., Meldau, S., Hoang, L. H., Underberg, S., Wunsche, H., and Baldwin, I. T. (2013). Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 2013, 2731–2747. doi: 10.1105/tpc113.114744
Menkhaus, M., Ullrich, U., Kluge, B., Vater, J., Vollenbroich, D., and Kamp, R. M. (1993). Structural and functional organization of the surfactin synthetase multienzyme system. J. Biol. Chem. 268, 7678–7684.
Monaci, L., Quintieri, L., Caputo, L., Visconti, A., and Baruzi, F. (2016). Rapid profiling of antimicrobial compounds characterising B. subilis TR50 cell-free filtrate by high-performance liquid chromatography coupled to high-resolution OrbitrapTM mass spectrometry. Rapid Commun. Mass Spectrom. 30, 45–53. doi: 10.1002/rcm.7408
Mülner, P., Bergna, A., Wagner, P., Sarajliæ, D., Dietel, K., Grosch, R., et al. (2019). Microbiota associated with sclerotia of soilborne fungal pathogens – a novel source of biocontrol agents producing bioactive volatiles. Phytobiomes J. 3, 125–136. doi: 10.1094/PBIOMES-11-18-0051-R
Nakano, M. M., Corbell, N., Besson, J., and Zuber, P. (1992). Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 232, 313–321. doi: 10.1007/bf00280011
Naruse, N., Tenmyo, O., Kobaru, S., Kamei, H., Miyaki, T., Konishi, M., et al. (1990). Pumilacidin, a complex of new antiviral antibiotics. Production, isolation, chemical properties, structure and biological activity. J. Antibiot. 43, 267–280. doi: 10.7164/antibiotics.43.267
Olishevska, S., Nickzad, A., and Deziel, E. (2019). Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl. Microbiol. Biotechnol. 103, 1189–1215. doi: 10.1007/s00253-018-9541-0
Ongena, M., and Jacques, P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16, 115–125. doi: 10.1016/j.tim.2007.12.009
Panpatte, D. G., Shukla, Y. M., Shelat, H. N., Vyas, R. V., and Jhala, Y. K. (2017). “Bacterial volatile organic compounds: a new insight for sustainable agriculture,” in Microorganisms for Green Revolution, ed. D. G. Panpatte (Berlin: Springer). doi: 10.1007/978-981-10-6241-4-8
Pecci, Y., Rivardo, F., Martinotti, M. G., and Allegrone, G. (2010). LC/ESI-MS/MS characterization of lipopeptide biosurfactants produced by the Bacillus licheniformis V9T14 strain. J. Mass Spectrom. 45, 772–778. doi: 10.1002/jms.1767
Peighami-Ashnaei, S., Sharifi-Tehrani, A., Ahmadzadeh, M., and Behboudi, K. (2009). Interaction of different media on production and biocontrol efficacy of Pseudomonas fluorescens P-35 and Bacillus subtilis B-3 against grey mould of apple. J. Plant Pathol. 91, 65–70. doi: 10.4454/jpp.v91i1.625
Pérez-García, A., Romero, D., and de Vicente, A. (2011). Plant protection and growth stimulation by microorganisms: biotechnological applications of bacilli in agriculture. Curr. Opin. Biotechnol. 22, 187–193. doi: 10.1016/j.copbio.2010.12.003
Rabbee, M. F., Ali, S., Choi, J., Hwang, B. S., Jeong, S. C., and Baek, K.-H. (2019). Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules 24:1046. doi: 10.3390/molecules24061046
Rajer, F. U., Wu, H., Xie, Y., Xie, S., Raza, W., Tahir, H. A. S., et al. (2017). Volatile organic compounds produced by a soil-isolate, Bacillus subtilis FA26 induce adverse ultra-structural changes to the cells of Clavibacter michiganensis ssp. sepedonicus, the causal agent of bacterial ring rot of potato. Microbiology 163, 523–530. doi: 10.1099/mic.0.000451
Rooney, A. P., Price, N. P. J., Ehrhardt, C., James, L., Sewzey, J. L., and Bannan, J. D. (2009). Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Int. J. Syst. Evol. Microbiol. 59, 2429–2436. doi: 10.1099/ijs.0.009126-0
Rudrappa, T., Biedrzycki, M. L., Kunjeti, S. G., Donofrio, N. M., Czymmek, K. J., Paul, W. P., et al. (2010). The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun. Integr. Biol. 3, 130–138. doi: 10.4161/cib.3.2.10584
Rybakova, D., Cernava, T., Köberl, M., Liebminger, S., Etemadi, M., and Berg, G. (2016). Endophytes-assisted biocontrol: novel insights in ecology and the mode of action of Paenibacillus. Plant Soil 405, 125–140. doi: 10.1007/s11104-015-2526-1
Ryu, C.-M., Farag, M. A., Hu, C.-H., Reddy, M. S., Klöpper, J. W., and Pare, P. W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134, 1017–1026. doi: 10.1104/pp.103.026583
Ryu, C.-M., Farag, M. A., Hu, C.-H., Reddy, M. S., Wei, H.-X., Pare, P. W., et al. (2003). Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100, 4927–4932. doi: 10.1073/pnas.0730845100
Saharan, B. S., and Nehra, V. (2011). Plant growth promoting rhizobacteria: a critical review. Life Sci. Med. Res. 21, 1–30.
Schenkel, D., Lemfack, M. C., Piechulla, B., and Splivallo, R. (2015). A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front. Plant Sci. 6:707. doi: 10.3389/fpls.2015.00707
Scholz, R., Molohon, K. J., Nachtigall, J., Vater, J., Markley, A. L., Süssmuth, R. D., et al. (2011). Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J. Bacteriol. 193, 215–224. doi: 10.1128/JB.00784-10
Scholz, R., Vater, J., Budiharjo, A., Wang, Z., He, Y., Dietel, K., et al. (2014). Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 196, 1842–1852. doi: 10.1128/JB.01474-14
Steller, S., Vollenbroich, D., Leenders, F., Stein, T., Conrad, B., Hofemeister, J., et al. (1998). Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem. Biol. 6, 31–41. doi: 10.1016/S1074-5521(99)80018-0
Suckau, D., Resemann, A., Schuerenberg, M., Hufnagel, P., Franzen, J., and Holle, A. (2003). A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Anal. Bioanal. Chem. 376, 952–956. doi: 10.1007/s00126-003-2057-0
Tahir, H. A. S., Gu, Q., Niu, Y., Huo, R., and Gao, X. (2017a). Bacillus volatiles adversely affect the physiology and ultra-structure of Ralstonia solanacearum and induce systemic resistance in tabacco against bacterial wilt. Sci. Rep. 7:40481. doi: 10.1038/srep40481
Tahir, H. A. S., Gu, Q., Wu, H., Raza, W., Hanif, A., Wu, L., et al. (2017b). Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 8:171. doi: 10.3389/fmicb.2017.00171
Toffano, L., Fialho, M. B., and Pascholati, S. F. (2017). Potential of fumigation of orange fruits with volatile organic compounds produced by Saccharomyces cerevisiae to control citrus black spot disease at postharvest. Biol. Control 108, 77–82. doi: 10.1016/j.biocontrol.2017.02.009
Tyc, O., de Jager, V. C. L., van den Berg, M., Gerards, S., Janssens, T. K. S., Zaagmann, N., et al. (2017). Exploring bacterial interspecific interactions for discovery of novel antimicrobial compounds. Microb. Biotechnol. 10, 910–925. doi: 10.1111/1751-7915.12735
van Loon, L. C. (2007). Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 119, 243–254. doi: 10.1007/978-1-4020-6776-1_2
van Loon, L. C., Bakker, P. A. H. M., and Pieterse, C. M. J. (1998). Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36, 453–483. doi: 10.1146/annurev.phyto.36.1.453
van Loon, L. C., and Glick, B. R. (2004). “Increased plant fitness by rhizobacteria,” in Molecular Ecotoxicology of Plants, ed. H. Sandermann (Berlin: Springer), 177–205. doi: 10.1007/978-3-662-08818-0_7
Vater, J., Gao, X., Hitzeroth, G., Wilde, C., and Franke, P. (2003). Whole cell “– matrix-assisted laser desorption ionization-time of flight – mass spectrometry, an emerging technique for efficient screening of biocombinatorial libraries of natural compounds – present state of research. Comb. Chem. High Throughput Screen. 56, 557–567. doi: 10.2174/138620703106298725
Vater, J., Herfort, S., Doellinger, J., Weydmann, M., Borriss, R., and Lasch, P. (2018). Genome mining of the lipopeptide biosynthesis of Paenibacillus polymyxa E681 in combination with mass spectrometry: discovery of the lipoheptapeptide paenilipoheptin. ChemBioChem 19, 744–753. doi: 10.1002/cbic.201700615
Vater, J., Herfort, S., Doellinger, J., Weydmann, M., Dietel, K., Faetke, S., et al. (2017). Fusaricidins from Paenibacillus polymyxa M-1, a family of lipohexapeptides of unusual complexity - a mass spectrometric study. J. Mass Spectrom. 52, 7–15. doi: 10.1002/jms.3891
Vater, J., Kablitz, B., Wilde, C., Franke, P., Mehta, N., and Cameotra, S. S. (2002). Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl. Environ. Microbiol. 68, 6210–6219. doi: 10.1128/AEM.68.12.6210-6219.2002
Veith, B., Herzberg, C., Steckel, S., Feesche, J., Maurer, K. H., Ehrenreich, P., et al. (2004). The complete genome sequence of Bacillus licheniformis DSM13, an organism with great industrial potential. J. Mol. Biotechnol. 7, 204–211. doi: 10.1159/000079829
Weise, T., Kai, M., Gummesson, A., Troeger, A., von Reuß, S., Piepenborn, S., et al. (2012). Volatile organic compounds produced by the phytopathogenic bacterium Xanthomonas campestris pv. vesicatoria 85-10. Beilstein J. Org. Chem. 8, 579–596. doi: 10.3762/bjoc.8.65
Werner, S., Polle, A., and Brinkmann, N. (2016). Belowground communication: impacts of volatile organic compounds (VOCs) from soil fungi on other soil-inhabiting organisms. Appl. Microbiol. Biotechnol. 100, 8651–8665. doi: 10.1007/s00253-016-7792-1
Wilkins, K. (1996). Volatile metabolites from actinomycetes. Chemosphere 32, 1427–1434. doi: 10.1016/0045-6535(96)00051-3
Wu, Y., Zhou, J., Li, C., and Ma, Y. (2018). Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. Microbiol. Open 8:e813. doi: 10.1002/mbo3.813
Xie, S., Zang, H., Wu, H., Rajer, F. U., and Gao, X. (2018). Antibacterial effects of volatiles produced by Bacillus strain D13 against Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 19, 49–58. doi: 10.1111/mpp.12494
Zhang, X., Li, B., Wang, Y., Guo, Q., Lu, X., Li, S., et al. (2013). Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Appl. Microbiol. Biotechnol. 97, 9525–9534. doi: 10.1007/s00253-013-5198-x
Keywords: plant growth promoting rhizobacteria, bioactive peptides, volatiles, MALDI-TOF mass spectrometry, GC-MS, field trials
Citation: Mülner P, Schwarz E, Dietel K, Junge H, Herfort S, Weydmann M, Lasch P, Cernava T, Berg G and Vater J (2020) Profiling for Bioactive Peptides and Volatiles of Plant Growth Promoting Strains of the Bacillus subtilis Complex of Industrial Relevance. Front. Microbiol. 11:1432. doi: 10.3389/fmicb.2020.01432
Received: 21 March 2020; Accepted: 02 June 2020;
Published: 30 June 2020.
Edited by:
Suha Jabaji, McGill University, CanadaReviewed by:
Fabian M. Commichau, Brandenburg University of Technology Cottbus-Senftenberg, GermanyCopyright © 2020 Mülner, Schwarz, Dietel, Junge, Herfort, Weydmann, Lasch, Cernava, Berg and Vater. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joachim Vater, dmF0ZXJtakB3ZWIuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.