- 1Department of Biochemistry, Institute of Science, Banaras Hindu University, Varanasi, India
- 2Department of Biosciences and Bioengineering, Indian Institute of Technology, Guwahati, India
- 3Department of Biotechnology, AIl India Institute of Medical Sciences, New Delhi, India
- 4Inflammation Research Center, San Diego, California, CA, United States
Increasing evidence from diverse sources during the past several years has indicated that long-term, low level, chronic inflammation mediates several chronic diseases including cancer, arthritis, obesity, diabetes, cardiovascular diseases, and neurological diseases. The inflammatory molecules and transcription factors, adhesion molecules, AP-1, chemokines, C-reactive protein (CRP), cyclooxygenase (COX)-2, interleukins (ILs), 5-lipooxygenase (5-LOX), matrix metalloproteinases (MMPs), nuclear factor (NF)-kB, signal transducer and activator of transcription 3 (STAT3), tumor necrosis factor (TNF), and vascular endothelial growth factor (VEGF) are molecular links between inflammation and chronic diseases. Thus, suppression of inflammatory molecules could be potential strategy for the prevention and therapy of chronic diseases. The currently available drugs against chronic diseases are highly expensive, minimally effective and produce several side effects when taken for long period of time. The focus of this review is to discuss the potential of nutraceuticals derived from “Mother Nature” such as apigenin, catechins, curcumin, ellagic acid, emodin, epigallocatechin gallate, escin, fisetin, flavopiridol, genistein, isoliquiritigenin, kaempferol, mangostin, morin, myricetin, naringenin, resveratrol, silymarin, vitexin, and xanthohumol in suppression of these inflammatory pathways. Thus, these nutraceuticals offer potential in preventing or delaying the onset of chronic diseases. We provide evidence for the potential of these nutraceuticals from pre-clinical and clinical studies.
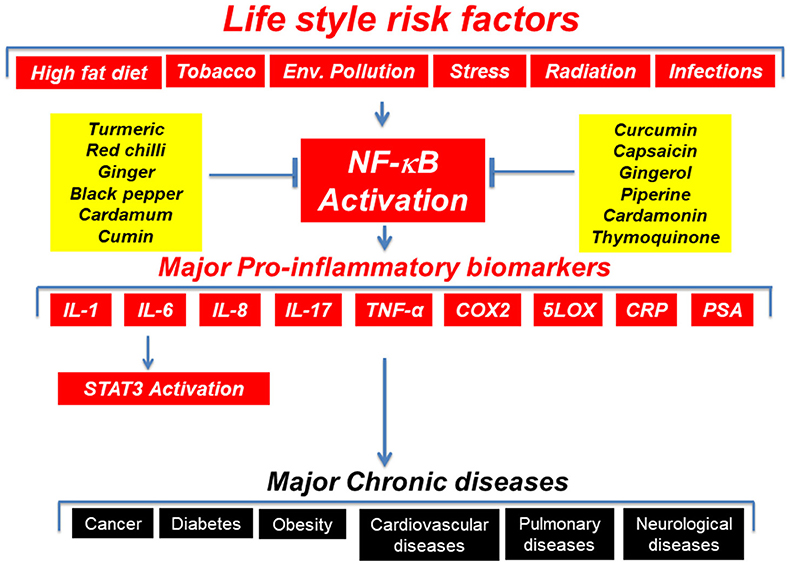
The term “inflammation” that means “to set on fire” can be both acute and chronic. Although acute inflammation is beneficial, chronic inflammation is a source for several chronic diseases including cancer, diabetes, and obesity (1). The modern science has delineated the molecular basis of inflammation. The inflammatory molecules and transcription factors such as 5-LOX, adhesion molecules, chemokines, COX-2, C-reactive protein, cytokines, MMPs, NF-κB, prostate-specific antigen (PSA), STAT3, TWIST, and vascular endothelial growth factor (VEGF) are known molecular links between inflammation and chronic diseases (Figure 1) (1). The pro-inflammatory transcription factors (NF-κB and STAT3) are the crucial regulators of inflammation (1, 2). For example, more than 500 cancer related genes are known to be regulated by NF-kB (3, 4).
The epidemiological, genetic and pharmacological studies support the association of inflammation with chronic diseases (5). For example, accumulating evidence suggest that chronic inflammation is precursor to most tumors. The gastritis (inflammation of lining of stomach) can lead to gastric cancer (6). It is estimated that almost 20% of smokers with bronchitis (inflammation of the mucous membrane in the bronchial tubes) can develop lung cancer in their lifetime (7). Similarly, colitis (inflammation of colon) is a precursor to colon cancer (8). Chronic inflammation plays a crucial role in various aspects of tumor development including cellular transformation, survival, proliferation, invasion, metastasis, and angiogenesis (5, 9). The healthy lifestyle can significantly reduce the risk of developing cancer, cardiovascular diseases, type 2 diabetes, and stroke (10).
The lifestyle factors such as alcohol, infectious agents, obesity, radiation, stress, tobacco, and toxicants are known activators of inflammatory pathways. The dietary intake of low-density lipoproteins can induce inflammation of the arteries. Omega-6 essential fatty acids commonly present in dietary vegetable oils, is known to induce inflammation. However, omega-3 fatty acids can lower inflammation. The dietary dairy protein (casein) and wheat protein (gluten) can also induce inflammation. The environmental sources of inflammation are toxicants such as adhesives, air fresheners, cleaning products, glues, latex, plastics, and synthetic fibers. The inflammation can also be induced by hormonal changes such as estrogen, progesterone, and testosterone. The lifestyle factors are known to modulate the production of inflammatory molecules (11). Lifestyle factors can also induce production of reactive oxygen species (ROS), which in turn lead to inflammation (12–15). ROS can regulate production of several inflammatory molecules such as chemokines, cyclooxygenase-2, cytokines, and pro-inflammatory transcription factors (16).
It is now well known that chronic inflammation is a cause for most chronic diseases. Thus, chronic treatment is required for most chronic diseases. In addition, dysregulation in multiple inflammatory molecules contribute to the development of chronic diseases. Yet, drugs for most of the chronic diseases are based on the modulation of more specifically a single target. Thus, these drugs are less likely to be effective. In addition, these drugs are highly expensive and are associated with numerous side effects when taken for long period of time (17–20). The implication of these facts necessitates the development of agents that are cost-effective, multi-targeted, and readily available. Because of their affordability, safety, and long-term use, agents derived from natural sources (nutraceuticals) possess enormous potential (21, 22). The sources of nutraceuticals include cereals, fruits, nuts, pulses, spices and vegetables. A recent study suggests that more than 70% of the drugs introduced over the past 25 years have been originated from nature (23).
The evidence from pre-clinical and clinical studies support the role of nutraceuticals in suppressing inflammatory pathways. Curcumin, which is derived from the golden spice turmeric, is known to modulate the production as well as activity of a number of inflammatory molecules (24). Curcumin can also directly bind to a number of inflammatory molecules. For example, the molecular docking studies have revealed that curcumin can bind at the receptor-binding sites of TNF-α by forming both noncovalent and covalent interactions (25). Curcumin can also directly bind and inhibit the activities of COX-1, COX-2, and MMP (26, 27). The potential anticancer activities of nutraceuticals by modulating NF-kB activation pathway has been documented by numerous lines of evidence. The nutraceuticals are known to suppress NF-κB activity by modulating several steps such as IKK activation, phosphorylation and degradation of IκBa, p65 nuclear translocation, phosphorylation and acetylation of p65, and p65 DNA binding. The most common nutraceuticals known to inhibit NF-κB activation include caffeic acid phenethyl ester (CAPE) (28), capsaicin (29), curcumin (30), emodin (31), epigallocatechin gallate (EGCG) (32, 33), guggulsterone (34), resveratrol (35, 36), and sanguinarine (29). Some nutraceuticals such as guggulsterone (34) and EGCG (33) act by inhibiting IKK activation. Curcumin (34, 37, 38), guggulsterone (34), capsaicin (29, 39), sanguinarine (29), emodin (31), and EGCG (33) are known to prevent phosphorylation and degradation of IκBα, which is a central point in NF-κB activation. Capsaicin (1, 29, 39) and EGCG (33) are known to inhibit nuclear translocation of NF-κB p65. Nutraceuticals can also inhibit the binding of p65 with DNA. For example, in human myeloid leukemia cells, curcumin was found to inhibit p65-DNA binding (30). Caffeic acid phenethyl ester can suppress the direct binding of the p50-p65 complex with DNA (28). In HeLa cells, emodin can oxidize the redox-sensitive site on NF-κB and thereby can prevent NF-κB-DNA binding (40). Plumbagin can inhibit NF-κB-DNA binding in breast cancer cells (41, 42). Nutraceuticals are also known to sensitize cancer cells to the chemotherapeutic agents and to induce apoptosis through modulation of NF-κB activation pathway. The most common nutraceuticals among this category are anacardic acid (43), 1'-acetoxychavicol acetate (44), noscapine (45), evodiamine (46), indirubin (47), thymoquinone (48), isodeoxyelephantopin, and withanolides (49).
Nutraceuticals are also known to inhibit STAT3 activation pathway and to suppress survival of tumor cells. For example, emodin was found to suppress STAT3 activation and to induce apoptosis in human myeloid cells (50). Similarly, suppression of STAT3 activation by capsaicin was found to induce apoptosis in multiple myeloid cells (51). Curcumin can suppress STAT3 activation pathway and tumor growth in an orthotopic murine model of ovarian cancer (52). Similarly, deguelin induced apoptosis in HTLV transformed T cells by inhibiting STAT3 phosphorylation (53). Quercetin can suppress STAT3 tyrosine phosphorylation and angiogenesis (54).
The clinical studies also support the potential of nutraceuticals in suppressing inflammatory pathways and chronic diseases. The safety, pharmacokinetics, and efficacy of nutraceuticals against numerous chronic diseases has been addressed in a number of human clinical trials. For example, EGCG, which is derived from green tea is reported to have potential against several chronic diseases (55). In prostate cancer patients, tea polyphenols are known to suppress serum levels of PSA, VEGF, and hepatocyte growth factor (HGF) (56, 57). The consumption of green tea is reported to reduce the risk of prostate adenocarcinoma (58). Similarly, black tea is known to decrease the levels of inflammatory biomarkers in colon cancer patients (59). The consumption of tea can also reduce the risk of breast cancer (60), gastric cancer (61), and lung cancer (62). Pomegranate, which is rich in isoflavonoid, such as quercetin, kaempferol, and luteolin, has been used for centuries for medicinal purposes (63). The consumption of pomegranate juice is known to significantly increase PSA doubling time in a phase II clinical trial of prostate cancer patients (64). Furthermore, pomegranate juice can decrease cell proliferation and induce apoptosis (64). The incidence of colorectal, prostate, and lung cancer can be reduced by selenium supplementation (65). The nutraceuticals have shown promise for several other chronic diseases such as acquired immunodeficiency syndrome, acute coronary syndrome, arthritis, atherosclerosis, biliary dyskinesia, cardiovascular disease, cholecystitis, chronic bacterial prostatitis, Crohn's disease, Dejerine-Sottas disease, diabetes, diabetic microangiopathy, diabetic nephropathy, gastric inflammation, gastric ulcer, idiopathic orbital inflammatory pseudotumor, irritable bowel disease, lupus nephritis, oral lichen planus, peptic ulcer, renal conditions, tropical pancreatitis, ulcerative colitis, ulcerative proctitis, uveitis, vitiligo, psoriasis, and β-thalassemia (66). In clinical trials, nutraceuticals have been used as an individual agent and also in combination with other agents. The formulations of nutraceuticals such as capsules, emulsions, liposomes, nanoparticles, powder, and tablets have been used for clinical trials.
In addition to cancer, nutraceuticals are also known to produce beneficial effects in other disease models. For example, an oral administration of curcumin at 375 mg (three times a day for 2 weeks) produced beneficial effects in patients with uveitis (67). Curcumin is also effective in patients with rheumatoid arthritis as demonstrated in clinical trials (68, 69). A short-term, double-blind, crossover study examined the efficacy of this polyphenol in 18 rheumatoid arthritis patients (68). The efficacy of curcumin was also compared with that of phenylbutazone, which is a prescription drug. The patients were administered with phenylbutazone (0.3 g/d) or curcumin (1.2 g/d) for 2 weeks. The anti-rheumatic activities of curcumin were identical with that of phenylbutazone. Furthermore, the polyphenol was very well tolerated and produced no adverse effects in patients. The polyphenol also produced anti-rheumatic activities when combined with diclofenac sodium (69). Additionally, curcumin is known to produce symptomatic relief in patients with peptic ulcers (70). One study examined the potential of curcumin against vitiligo, which is characterized by white patches over the skin on the different body parts (71). A statistically significant repigmentation was observed after 8–12 weeks of curcumin treatment. The polyphenol is known to exhibit anti-psoriatic activity possibly through modulation of phosphorylase kinase (PhK) activity (72). The efficacy of curcumin in Alzheimer's disease patients was examined in a randomized, double-blind, placebo-controlled study (73). The patients were administered with the polyphenol at 1 or 4 g doses. Although curcumin was unable to improve mental status and the serum Aβ40 levels, vitamin E level was increased in patients without any adverse effects (73). The polyphenol also reduces total cholesterol and LDL cholesterol, and increases HDL cholesterol in patients with acute coronary syndrome (74). Overall, these results suggest the beneficial effects of curcumin in patients with acute coronary syndrome. When the polyphenol was administered to 10 healthy volunteers for 7 days, reduction in serum lipid peroxides and total serum cholesterol levels, and an increase in HDL cholesterol was observed (75). In one study, the potential of curcuminoids (NCB-02) in 72 patients with type 2 diabetes (T2DM) was examined (76). The patients were randomized to receive atorvastatin (10 mg, once a day), NCB-02 (300 mg of curcumin, twice a day), or placebo for 8 weeks. The administration of curcumin was associated with an improvement in endothelial function and reduction in oxidative stress (MDA) and inflammatory markers (endothelin-1, IL-6, TNFα) suggesting the potential of curcuminoids against T2DM. However, larger, randomized clinical trials are required to confirm these observations. Like curcumin, resveratrol is also beneficial in T2DM patients (77). More specifically, administration of resveratrol at 1 g/day for 45 days suppressed fasting blood glucose, haemoglobinA1c (HbA1c), insulin and insulin resistance. Furthermore, a significant rise in high density lipoprotein cholesterol was observed after resveratrol treatment (77). In patients with non-alcoholic fatty liver disease (NAFLD), resveratrol significantly reduces the levels of glucose, cholesterol, and liver enzymes ALT and aspartate aminotransferase (78). Resveratrol also decreases the levels of ALT and hepatic steatosis in NAFLD patients (79). Conversely, resveratrol was unable to produce beneficial effects in another clinical trial of NAFLD patients (80). The post-menopausal women are at increased risk of breast cancer owing to reduced expression of sex steroid hormone binding globulin (SHBG). Furthermore, a lower ratio of 2-hydroxyestrone (2-OHE1) and 16α-hydroxyestrone (16α-OHE1) in postmenopausal cohort correlate with the higher breast cancer risk (81). An administration of resveratrol at 1 g/day for 12 weeks is known to increase SHBG levels in obese postmenopausal women (82). Resveratrol also elevates 2-OHE1/16α OHE1 ratio. Thus, it can be concluded that resveratrol has beneficial effects in postmenopausal women (82).
In conclusion, chronic inflammation is a cause for several chronic diseases. Thus, treatment of chronic diseases requires chronic treatment. Modern science has delineated the molecular links of chronic inflammation and chronic diseases. The drugs developed by pharmaceutical companies are highly expensive, produce side effects and cannot be afforded by more than 80% of world population. Nutraceuticals have also been successfully used in combination with other agents. Nutraceuticals are readily available and can modulate multiple cell signaling pathways. In addition, nutraceuticals and their sources have been consumed since ancient time. Thus, their safety is well tested. Conversely, nutraceuticals have been reported to produce undesired adverse effects by some studies. For example, oral intake of curcumin is associated with diarrhea, headache, rash, and yellow stool in some healthy volunteers. When curcumin was administered in combination with gemcitabine, abdominal pain was reported by some pancreatic cancer patients. Furthermore, nutraceuticals such as curcumin and resveratrol are associated with poor bioavailability. Overall, nutraceuticals offer promise to prevent or delay the onset of chronic diseases. However, none of the nutraceuticals have been approved for human use by regulatory entities. Moreover, nutraceuticals have been reported to produce adverse effects by some studies. More studies are required before these agents can be prescribed by clinicians for therapeutic purpose.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell (2004) 6:203–8. doi: 10.1016/j.ccr.2004.09.003
2. Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. (2005) 1056:218–33. doi: 10.1196/annals.1352.026
3. Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. (2009) 1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x
4. Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta (2010) 1799:775–87. doi: 10.1016/j.bbagrm.2010.05.004
5. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454:436–44. doi: 10.1038/nature07205
6. Peter S, Beglinger C. Helicobacter pylori and gastric cancer: the causal relationship. Digestion (2007) 75:25–35. doi: 10.1159/000101564
7. Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, et al. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. (1999) 91:675–90. doi: 10.1093/jnci/91.8.675
8. Itzkowitz SH, Yio X. Inflammation and cancer IV. colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. (2004) 287:G7–17. doi: 10.1152/ajpgi.00079.2004
10. Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Int Med. (2009) 169:1355–62. doi: 10.1001/archinternmed.2009.237
11. Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. (2006) 72:1605–21. doi: 10.1016/j.bcp.2006.06.029
12. Aggarwal BB, Sung B. The relationship between inflammation and cancer is analogous to that between fuel and fire. Oncology (Williston Park) (2011) 25:414–8.
13. Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. (2011) 7:504–11. doi: 10.1038/nchembio.607
14. Mantovani A. Cancer: inflammation by remote control. Nature (2005) 435:752–3. doi: 10.1038/435752a
16. Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer (2007) 121:2373–80. doi: 10.1002/ijc.23173
17. Atzeni F, Talotta R, Salaffi F, Cassinotti A, Varisco V, Battellino M, et al. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev. (2013) 12:703–8. doi: 10.1016/j.autrev.2012.10.021
18. Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. (2011) 2011:CD008794. doi: 10.1002/14651858.CD008794.pub2
19. Zitelli KB, Zedek D, Ranganathan P, Amerson EH. Squamous cell carcinoma of the lip associated with adalimumab therapy for ankylosing spondylitis: a case report and review of TNF-alpha inhibitors and cutaneous carcinoma risk. Cutis (2013) 92:35–9.
20. Kouklakis G, Efremidou EI, Pitiakoudis M, Liratzopoulos N, Polychronidis A. Development of primary malignant melanoma during treatment with a TNF-alpha antagonist for severe Crohn's disease: a case report and review of the hypothetical association between TNF-alpha blockers and cancer. Drug Design, Dev Ther. (2013) 7:195–9. doi: 10.2147/DDDT.S41889
21. Jensen GL. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr. (2006) 30:453–63. doi: 10.1177/0148607106030005453
22. Jensen GL, Roubenoff R. Introduction: nutrition and inflammation: Research Makes The Connection–Intersociety Research Workshop, Chicago, February 8-9:2008. JPEN J Parenter Enteral Nutr. (2008) 32:625. doi: 10.1177/0148607108325253
23. Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. (2007) 70:461–77. doi: 10.1021/np068054v
24. Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Product Rep. (2011) 28, 1937–55. doi: 10.1039/C1NP00051A
25. Wua ST, Suna JC, Leeb KJ, and Sunc YM. Docking prediction for tumor necrosis factor-α and five herbal inhibitors. Int J Eng Sci Technol. (2010) 2:4263–77.
26. Selvam C, Jachak SM, Thilagavathi R, Chakraborti AK. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg Med Chem Lett. (2005) 15:1793–7. doi: 10.1016/j.bmcl.2005.02.039
27. Girija CR, Karunakar P, Poojari CS, Begum NS, Syed AA. Molecular docking studies of curcumin derivatives with multiple protein targets for procarcinogen activating enzyme inhibition. J Proteomics Bioinform. (2010) 3:200–3. doi: 10.4172/jpb.1000140
28. Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. (1996) 93:9090–5. doi: 10.1073/pnas.93.17.9090
29. Han SS, Keum YS, Seo HJ, Chun KS, Lee SS, Surh YJ. Capsaicin suppresses phorbol ester-induced activation of NF-kappaB/Rel and AP-1 transcription factors in mouse epidermis. Cancer Lett. (2001) 164:119–26. doi: 10.1016/S0304-3835(01)00378-0
30. Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. (1995) 270:24995–5000. doi: 10.1074/jbc.270.42.24995
31. Kumar A, Dhawan S, Aggarwal BB. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene (1998) 17:913–8. doi: 10.1038/sj.onc.1201998
32. Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (-)-epigallocatechin gallate and theaflavins. Carcinogenesis (2000) 21:1885–90. doi: 10.1093/carcin/21.10.1885
33. Afaq F, Adhami VM, Ahmad N, Mukhtar H. Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea Constituent (-)-epigallocatechin-3-gallate. Oncogene (2003) 22:1035–44. doi: 10.1038/sj.onc.1206206
34. Shishodia S, Aggarwal BB. Nuclear factor-kappaB: a friend or a foe in cancer? Biochem Pharmacol. (2004) 68:1071–80. doi: 10.1016/j.bcp.2004.04.026
35. Mouria M, Gukovskaya AS, Jung Y, Buechler P, Hines OJ, Reber HA, et al. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Int J Cancer (2002) 98:761–9. doi: 10.1002/ijc.10202
36. Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. (2002) 62:4945–54.
37. Philip S, Kundu GC. Osteopontin induces nuclear factor kappa B-mediated promatrix metalloproteinase-2 activation through I kappa B alpha /IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J Biol Chem. (2003) 278:14487–97. doi: 10.1074/jbc.M207309200
38. Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood (2003) 101:1053–62. doi: 10.1182/blood-2002-05-1320
39. Park KK, Chun KS, Yook JI, Surh YJ. Lack of tumor promoting activity of capsaicin, a principal pungent ingredient of red pepper, in mouse skin carcinogenesis. Anticancer Res. (1998) 18:4201–5.
40. Jing Y, Yang J, Wang Y, Li H, Chen Y, Hu Q, et al. Alteration of subcellular redox equilibrium and the consequent oxidative modification of nuclear factor kappaB are critical for anticancer cytotoxicity by emodin, a reactive oxygen species-producing agent. Free Radic Biol Med. (2006) 40:2183–97. doi: 10.1016/j.freeradbiomed.2006.02.016
41. Ahmad A, Banerjee S, Wang Z, Kong D, Sarkar FH. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J Cell Biochem. (2008) 105:1461–71. doi: 10.1002/jcb.21966
42. Sandur SK, Ichikawa H, Sethi G, Ahn KS, Aggarwal BB. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J Biol Chem. (2006) 281:17023–33. doi: 10.1074/jbc.M601595200
43. Sung B, Pandey MK, Ahn KS, Yi T, Chaturvedi MM, Liu M, Aggarwal BB. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood (2008) 111:4880–91. doi: 10.1182/blood-2007-10-117994
44. Ichikawa H, Takada Y, Murakami A, Aggarwal BB. Identification of a novel blocker of I kappa B alpha kinase that enhances cellular apoptosis and inhibits cellular invasion through suppression of NF-kappa B-regulated gene products. J Immunol. (2005) 174:7383–92. doi: 10.4049/jimmunol.174.11.7383
45. Sung B, Ahn KS, Aggarwal BB. Noscapine, a benzylisoquinoline alkaloid, sensitizes leukemic cells to chemotherapeutic agents and cytokines by modulating the NF-kappaB signaling pathway. Cancer Res. (2010) 70:3259–68. doi: 10.1158/0008-5472.CAN-09-4230
46. Takada Y, Kobayashi Y, Aggarwal BB. Evodiamine abolishes constitutive and inducible NF-kappaB activation by inhibiting IkappaBalpha kinase activation, thereby suppressing NF-kappaB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J Biol Chem. (2005) 280:17203–12. doi: 10.1074/jbc.M500077200
47. Sethi G, Ahn KS, Sandur SK, Lin X, Chaturvedi MM, Aggarwal BB. Indirubin enhances tumor necrosis factor-induced apoptosis through modulation of nuclear factor-kappa B signaling pathway. J Biol Chem. (2006) 281:23425–35. doi: 10.1074/jbc.M602627200
48. Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol Cancer Res. (2008) 6:1059–70. doi: 10.1158/1541-7786.MCR-07-2088
49. Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. (2006) 5:1434–45. doi: 10.1158/1535-7163.MCT-06-0096
50. Muto A, Hori M, Sasaki Y, Saitoh A, Yasuda I, Maekawa T, et al. Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol Cancer Ther. (2007) 6:987–94. doi: 10.1158/1535-7163.MCT-06-0605
51. Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, et al. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. (2007) 13:3024–32. doi: 10.1158/1078-0432.CCR-06-2575
52. Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res. (2007) 13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072
53. Ito S, Oyake T, Murai K, Ishida Y. Deguelin suppresses cell proliferation via the inhibition of survivin expression and STAT3 phosphorylation in HTLV-1-transformed T cells. Leuk Res. (2010) 34:352–7. doi: 10.1016/j.leukres.2009.09.003
54. Anso E, Zuazo A, Irigoyen M, Urdaci MC, Rouzaut A, Martinez-Irujo JJ. Flavonoids inhibit hypoxia-induced vascular endothelial growth factor expression by a HIF-1 independent mechanism. Biochem Pharmacol. (2010) 79:1600–9. doi: 10.1016/j.bcp.2010.02.004
55. Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal (2008) 10:475–510. doi: 10.1089/ars.2007.1740
56. Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. (2006) 66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145
57. Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. (2003) 9:3312–9.
58. Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in southeast China. Int J Cancer (2004) 108:130–5. doi: 10.1002/ijc.11550
59. Sun CL, Yuan JM, Koh WP, Yu MC. Green tea, black tea and colorectal cancer risk: a meta-analysis of epidemiologic studies. Carcinogenesis (2006) 27:1301–9. doi: 10.1093/carcin/bgl024
60. Seely D, Mills EJ, Wu P, Verma S, Guyatt GH. The effects of green tea consumption on incidence of breast cancer and recurrence of breast cancer: a systematic review and meta-analysis. Integr Cancer Ther. (2005) 4:144–55. doi: 10.1177/1534735405276420
61. Mu LN, Lu QY, Yu SZ, Jiang QW, Cao W, You NC, et al. Green tea drinking and multigenetic index on the risk of stomach cancer in a Chinese population. Int J Cancer (2005) 116:972–83. doi: 10.1002/ijc.21137
62. Arts IC. A review of the epidemiological evidence on tea, flavonoids, and lung cancer. J Nutr. (2008) 138:1561S–6S. doi: 10.1093/jn/138.8.1561S
63. Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. (2000) 48:4581–9. doi: 10.1021/jf000404a
64. Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. (2006) 12:4018–26. doi: 10.1158/1078-0432.CCR-05-2290
65. Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. (2002) 11:630–9.
66. Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. (2013) 15:195–218. doi: 10.1208/s12248-012-9432-8
67. Lal B, Kapoor AK, Asthana OP, Agrawal PK, Prasad R, Kumar P, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. (1999) 13:318–22.
68. Deodhar SD, Sethi R, Srimal RC. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res. (1980) 71:632–4.
69. Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. (2012) 26:1719–25. doi: 10.1002/ptr.4639
70. Prucksunand C, Indrasukhsri B, Leethochawalit M, Hungspreugs K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Tropic Med Public Health (2001) 32:208–15.
71. Asawanonda P, Klahan SO. Tetrahydrocurcuminoid cream plus targeted narrowband UVB phototherapy for vitiligo: a preliminary randomized controlled study. Photomed Laser Surg. (2010) 28:679–84. doi: 10.1089/pho.2009.2637
72. Heng MC, Song MK, Harker J, Heng MK. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. (2000) 143:937–49. doi: 10.1046/j.1365-2133.2000.03767.x
73. Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. (2008) 28:110–3. doi: 10.1097/jcp.0b013e318160862c
74. Alwi I, Santoso T, Suyono S, Sutrisna B, Suyatna FD, Kresno SB, et al. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med Indonesiana (2008) 40:201–10.
75. Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian journal of physiology and pharmacology (1992) 36:273–5.
76. Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: a randomized, parallel-group, placebo-controlled, 8-week study. Drugs in R&D (2008) 9:243–50. doi: 10.2165/00126839-200809040-00004
77. Movahed A, Nabipour I, Lieben Louis X, Thandapilly SJ, Yu L, Kalantarhormozi M, et al. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid Based Complement Alter Med. (2013) 2013:851267. doi: 10.1155/2013/851267
78. Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Digest Liver Dis. (2015) 47:226–32. doi: 10.1016/j.dld.2014.11.015
79. Faghihzadeh F, Adibi P, Hekmatdoost A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. Br J Nutr. (2015) 114:796–803. doi: 10.1017/S0007114515002433
80. Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O'Moore-Sullivan TM, Lee P, et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2014) 12:2092-103.e1-6. doi: 10.1016/j.cgh.2014.02.024
81. Ruan X, Seeger H, Wallwiener D, Huober J, Mueck AO. The ratio of the estradiol metabolites 2-hydroxyestrone (2-OHE1) and 16alpha-hydroxyestrone (16-OHE1) may predict breast cancer risk in postmenopausal but not in premenopausal women: two case-control studies. Arch Gynecol Obstetrics (2015) 291:1141–6. doi: 10.1007/s00404-014-3512-1
Keywords: cancer, chronic disease, cytokine, inflammation, nutraceutical
Citation: Gupta SC, Kunnumakkara AB, Aggarwal S and Aggarwal BB (2018) Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 9:2160. doi: 10.3389/fimmu.2018.02160
Received: 02 March 2018; Accepted: 31 August 2018;
Published: 27 September 2018.
Edited by:
Mark Slevin, Manchester Metropolitan University, United KingdomReviewed by:
Maria Dos Anjos Pires, Universidade de Trás-os-Montes e Alto Douro, PortugalGarry McDowell, Manchester Metropolitan University, United Kingdom
Copyright © 2018 Gupta, Kunnumakkara, Aggarwal and Aggarwal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Subash Chandra Gupta, sgupta@bhu.ac.in
Bharat B. Aggarwal, bbaggarwal@gmail.com
 Subash Chandra Gupta
Subash Chandra Gupta