
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Genet. , 02 April 2019
Sec. Computational Genomics
Volume 10 - 2019 | https://doi.org/10.3389/fgene.2019.00262
This article is part of the Research Topic Intelligent Systems for Genome Functional Annotations View all 9 articles
FeatSNP is an online tool and a curated database for exploring 81 million common SNPs’ potential functional impact on the human brain. FeatSNP uses the brain transcriptomes of the human population to improve functional annotation of human SNPs by integrating transcription factor binding prediction, public eQTL information, and brain specific epigenetic landscape, as well as information of Topologically Associating Domains (TADs). FeatSNP supports both single and batched SNP searching, and its interactive user interface enables users to explore the functional annotations and generate publication-quality visualization results. FeatSNP is freely available on the internet at FeatSNP.org with all major web browsers supported.
Genome-wide association studies (GWAS) and expression quantitative trait loci (eQTL) analyses have identified thousands of genetic variants that are associated with a wide range of human phenotypes, shedding lights on the understanding of the genetic effect to human diseases. However, a key challenge for scientists in the human genetics community is to understand the molecular mechanism connecting significant genetic variant and specific phenotype. More than 90% of SNPs associated with human phenotypes are located in non-protein-coding regions, and cannot be explained by alteration of amino acid sequence of proteins (Welter et al., 2014). Recently, mounting evidence suggests that disease-associated non-coding SNPs are highly enriched in tissue-specific regulatory elements including enhancers, which can be detected and defined by specific chromatin modifications (Carey et al., 2015; Zhou et al., 2015; Agrawal et al., 2018). Moreover, some non-coding SNPs are found to be located within transcription factor (TF) binding motifs, which affect the TF binding affinity and result in allele switching and/or allele-specific regulation of target genes (Andersson et al., 2014; Roadmap Epigenomics et al., 2015; Nelson et al., 2016). These evidences underscore the potential causal role of non-coding genetic variants in affecting human diseases and phenotypes through regulation of gene expression (Claussnitzer et al., 2015).
Here we introduce FeatSNP, an online tool and database which provides an interactive user interface (UI) for inquiring brain-specific functional and epigenetic annotation of human SNPs. Unlike traditional SNP functional annotation databases, such as RegulomeDB (Boyle et al., 2012) and HaploReg (Ward and Kellis, 2012), FeatSNP focuses on the collection and curation of brain-specific functional genomics data, including epigenomes, transcriptomes, and eQTL data, to better annotate the regulatory potential of single SNP. Specifically, FeatSNP supplies a series of new features to facilitate research understanding the functional annotation of SNP on human brain (Supplementary Table S1). FeatSNP uses human brain transcriptomes to improve and refine the prediction of allele-specific TF binding motifs. The expression correlation between SNP-associated gene and predicted SNP-associated TFs was used to determine the best allele-associated TF candidate. The interactive UI allows the users easily to browse functional annotation and generate analysis results and high quality figures.
FeatSNP consists of a front end UI implemented with HTML/PHP/JavaScript, and a backend NoSQL database implemented with MongoDB (v3.2.7) as shown in Figure 1. The current SNP dataset contains 81,144,876 bi-allelic SNPs from dbSNP (V144), with SNP accession number as unique identifier in the database. Human dbSNP build 144 was downloaded from ftp.ncbi.nih.gov/snp, which includes 84,435,229 SNPs records, 1,591,294 insertions records, 2,595,517 deletions records, 33,234 indel records, and 110 Multiple Nucleotide Polymorphisms (MNPs) records. After filtering redundant records, 81,144,876 of 84,435,229 biallelic SNPs were used to generate functional annotations and were curated by the FeatSNP database. The genome coordinates (hg19) of 81,144,876 SNPs were used to associate the SNPs with their nearest genes based on 56,642 records of GENOCDE gene annotation Release 19 (GRCh37.p13).
To predict impact of allele-specific TF binding affinity by SNPs, the Position Weight Matrix (PWM) of 519 vertebrate TFs were collected from JASPAR (Core Vertebrate 2016) (Mathelier et al., 2016). After evaluating the motif weight PWM of 519 TFs at base-pair resolution (Supplementary Figure S2), the reference and alternate alleles for every SNP with flanking 10 bp of genomic sequences both upstream and downstream were obtained from the UCSC Genome Browser. FIMO (Grant et al., 2011) was used to scan the 21 bp sequence to identify binding motifs matching any of the 519 TF PWMs, and calculate the TFBS motif scores. Only instances where a motif in the sequence (i) passed the threshold of P < 1e-2 in either the reference or the alternate allele, and (ii) contained the SNP location and (iii) the difference of motif scores between the reference and the alternate allele was greater than 2, were recorded in the database.
1,259 transcriptome datasets of 13 brain tissues generated by the GTEx consortium (Gibson, 2015) were used to calculate the Pearson correlation between each SNP associated gene and predicted binding TFs. The lowly expressed gene and TFs (expression of all samples in one tissue less than 0.2RPKM) were removed. The correlation and gene expression in 13 brain tissues were visualized by using JavaScript package Highcharts (v5.0.2).eQTL data of 10 brain tissues generated by GTEx consortium were negative-log10 transformed and further visualized by using Highcharts (v5.0.2).
Histone modification ChIP-seq data of 10 brain tissues were downloaded from NIH Roadmap Epigenomics data portal. Bedtools was used to identify SNPs residing in peaks of 7 histone modification marks (H3K4me3, H3K36me3, H3K27me3, H3K4me1, H3K27ac, H3K9me3, and H3K9ac) that were identified by macs2 (Zhang et al., 2008) with default parameters. To enhance the user experience, the WashU epigenome browser (Zhou et al., 2015) was embedded in the UI to display epigenetic landscape in a 200 bp region surrounding each SNP. The browser also displays DNA methylation data (Whole Genome Bisulfite Sequencing) of 4 neuronal progenitor and brain tissues generated by Roadmap Epigenomics Project, enhanced epilogos visualization1 of all 127 epigenomes, and topologically associating domains (TAD) data of GM12878, IMR90, and Hap1 cell lines (Rao et al., 2014; Sanborn et al., 2015). eQTL data of 10 brain tissues generated by GTEx consortium were also visualized on the embedded WashU epigenome browser.
The association records of SNP and human disease/traits (V1.0.2) were downloaded from GWAS Catalog. 33,894 associations with p-value smaller then 5E-8 were kept and classified based on 1,374 human disease/traits categories. The functional annotations of these 33,894 SNPs were reported on FeatSNP.org/html_file/disease_classification.html (Supplementary Figure S3).
To illustrate the use of FeatSNP, we performed the analysis using rs8070723 as an example. rs8070723 is an intronic A/G SNP (major allele A frequency 0.881, minor allele G frequency 0.119) in MAPT, the gene that encodes the microtubule-associated protein tau, and is associated with Progressive Supranuclear Palsy (Hoglinger et al., 2011) and with Parkinson’s Disease (UK Parkinson’s Disease Consortium et al., 2011). To better understand the regulatory potential of this human disease-associated SNP, we inquired the epigenetic annotation of rs8070723 in FeatSNP through Single SNP ID Searching function on SNP Query Page (Supplementary Figure S4). The database first reported the basic information of SNP rs8070723, including genomic location, allelic frequency, surrounding DNA sequence, and associated gene (Figures 2A–C). Users can further access the genetic information and associated human disease or traits of inquired SNPs on dbSNP and GWAS Catalog through external links.
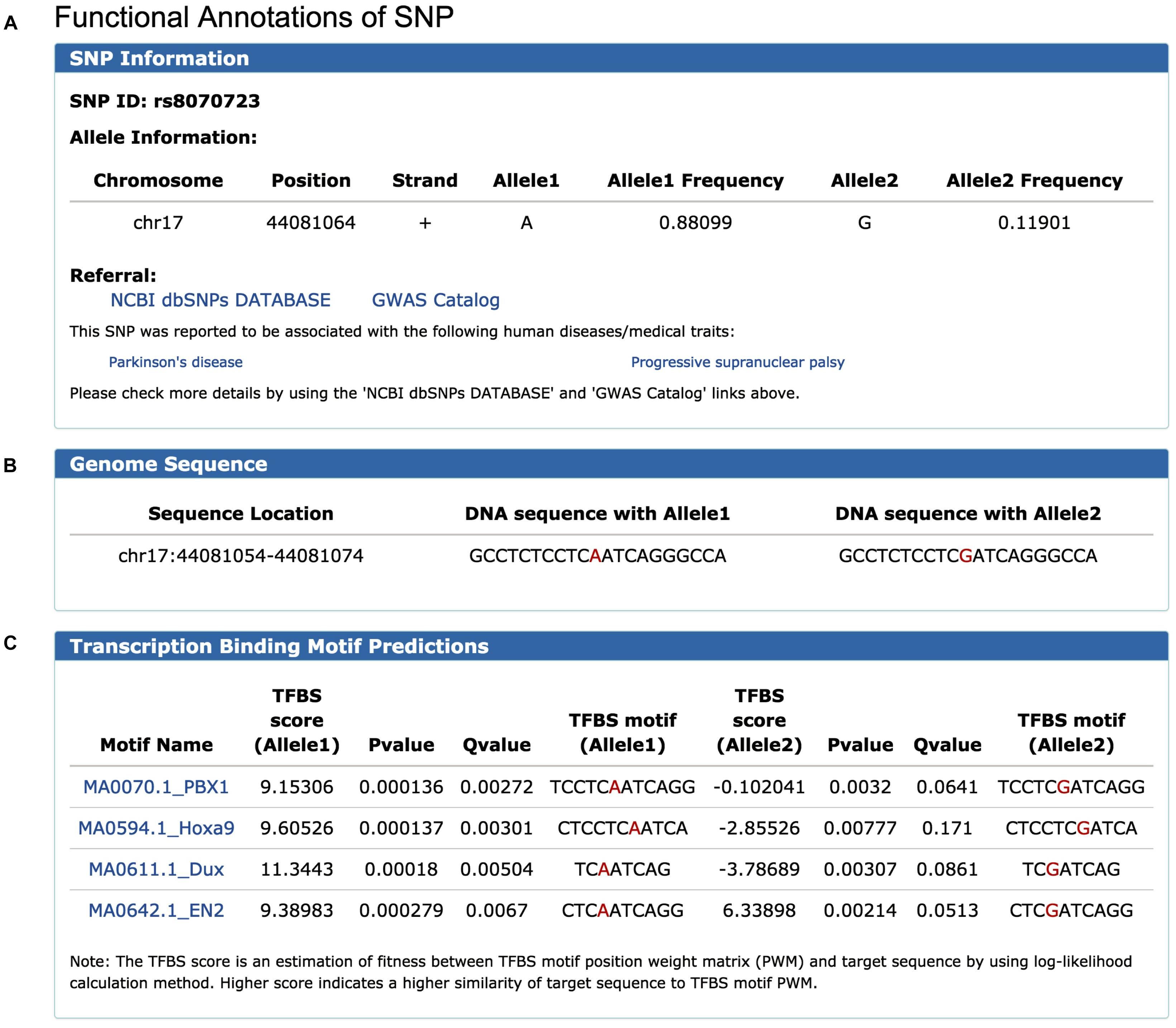
Figure 2. Information of SNP rs8070723 retrieved from FeatSNP. (A) Genomic information of rs8070723, with external links to NCBI dbSNPs and GWAS Catalog. (B) DNA sequence around rs8070723. (C) Predicted TFs binding motifs containing rs8070723 with A/G alleles.
FeatSNP found four potential TF binding motifs harboring rs8070723 with A allele, including PBX1, Hoxa9, Dux, and EN2. All four TF binding motifs had high TFBS scores in A allele, and the TFBS motifs were destroyed with G allele with low TFBS scores (Figure 2C). PBX1 encodes a nuclear protein that belongs to the PBX homeobox family of transcriptional factors, and studies suggested PBX1 regulates the patterning of the cerebral cortex (Golonzhka et al., 2015) and its transcriptional network controls dopaminergic neuron development in Parkinson’s disease (Villaescusa et al., 2016). EN2 encodes homeodomain-containing proteins and has been implicated in the control of pattern formation during development of the central nervous system (Genestine et al., 2015). Hoxa9 is an important homeobox transcription factor and plays important roles in myeloid leukemogenesis (Siriboonpiputtana et al., 2017). Dux-family transcription factors were recently identified to regulate zygotic genome activation in placental mammals (De Iaco et al., 2017). Thus, PBX1 and EN2 could be the potential master TFs affected by the SNP rs8070723.
Since FeatSNP curated 1,259 transcriptome data of 13 brain tissues generated by the GTEx consortium (Gibson, 2015), we were able to further check the expression level of PBX1 and EN2 in multiple brain regions in FeatSNP database. EN2 was only expressed in the cerebellum of the brain (Supplementary Figure S1A) and did not correlate with expression level of MAPT (Figure 3A). We found that PBX1 highly expressed in different brain regions (Supplementary Figure S1B), and we also found the expression of MAPT had strong and specific correlation with PBX1 in multiple brain regions (Figure 3A), especially in anterior cingulate cortex (r = 0.808), nucleus accumbens (r = 0.768), and frontal cortex (r = 0.768) (Figure 3B), which were considered as major affected regions of Progressive Supranuclear Palsy (Salmon et al., 1997).
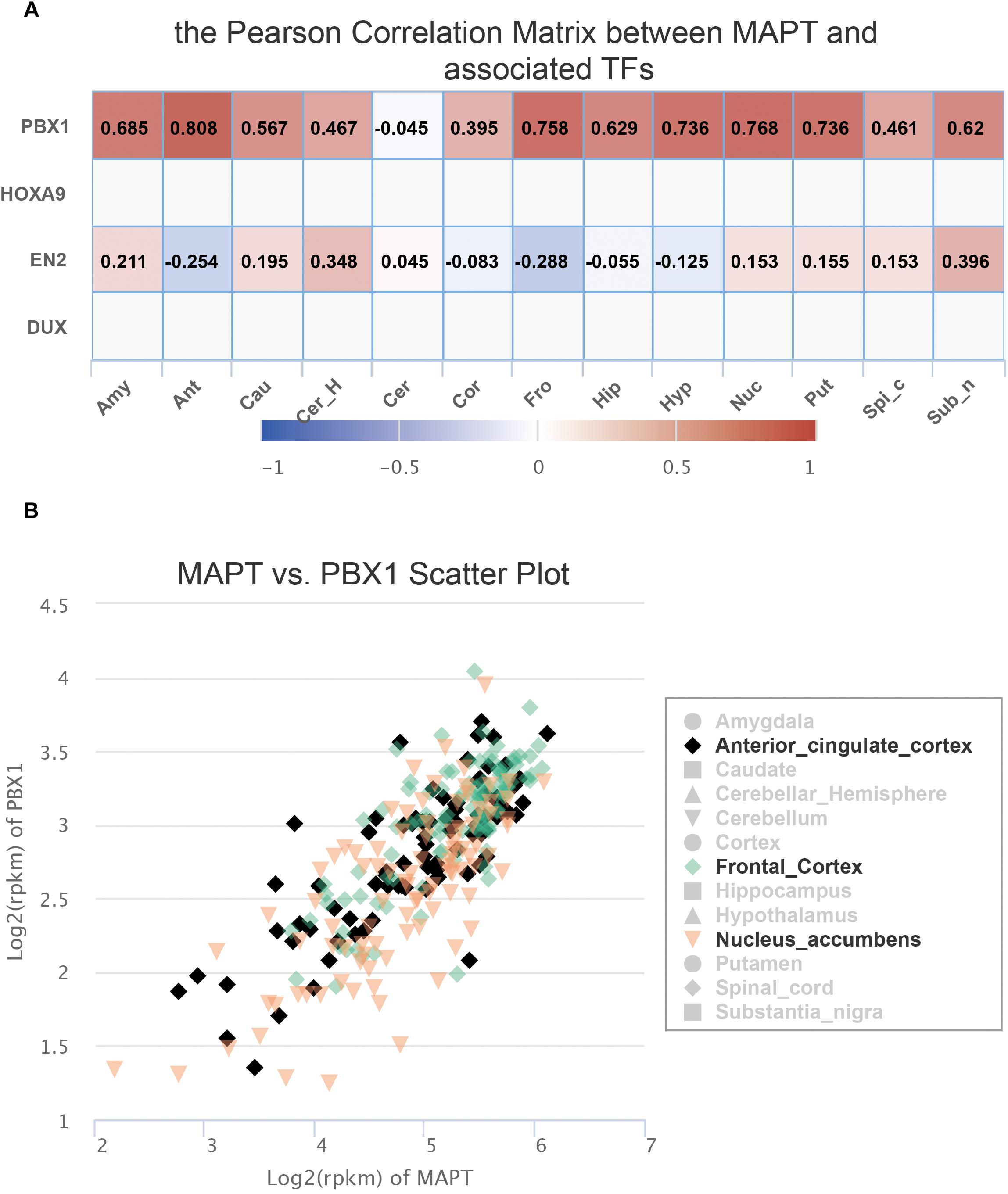
Figure 3. Expression information of SNP rs8070723 tagged gene retrieved from FeatSNP. (A). Pearson Correlation Matrix between rs8070723 tagged MAPT and potential bound TFs. (B). Scatter plot of gene expression of MAPT and PBX1 in anterior cingulate cortex, frontal cortex, and nucleus accumbens.
We further explored the epigenetic annotation of the genomic regions tagged by rs8070723 in 10 brain regions by using epigenome data generated from Roadmap Consortium, which were also curated in FeatSNP database. We found the regions tagged by SNP rs8070723 enriched for strong active histone modification signals including H3K4me1, H3K9ac, and H3K27ac in 8 brain tissues (Figure 4A). Such active histone modifications were generally associated with active enhancer and promoter functions. Chromatin epigenetic status prediction based on chromHMM (Ernst and Kellis, 2012) suggested that the regions tagged by SNP rs8070723 could be considered as strong enhancers (Figure 4B). Finally, we explored the eQTL data in 13 brain tissues, and found rs8070723 was associated with several genes’ expression, including MAPT (Figures 4B,C). MAPT gene mutations have been associated with several neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease. Our result suggests that rs8070723 G allele might influence MAPT expression level by reducing the binding affinity of upstream regulatory protein PBX1, therefore providing a mechanistic association with neurodegenerative diseases including Progressive Supranuclear Palsy and Parkinson’s Disease.
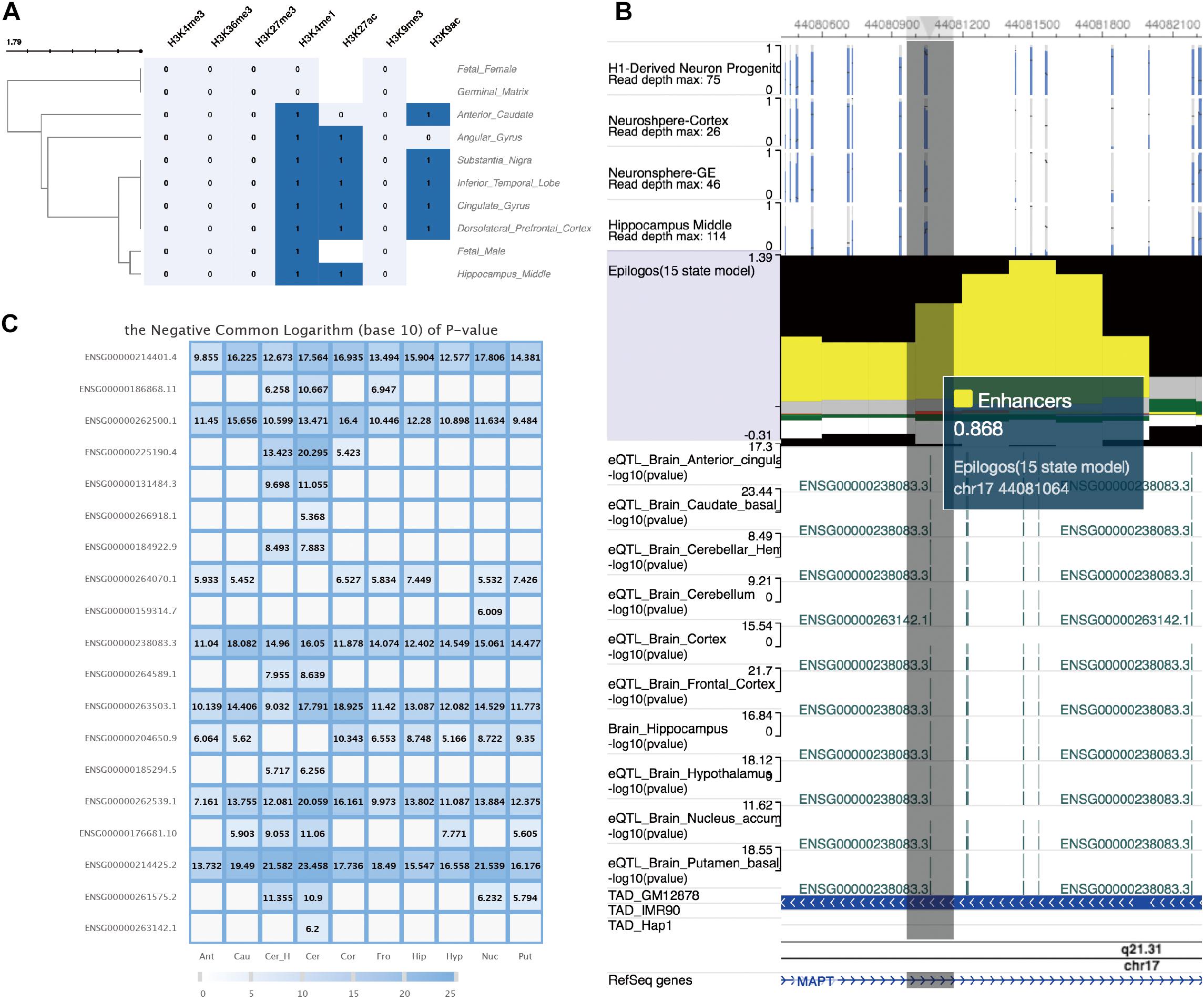
Figure 4. Epigenetic annotation and eQTL information of SNP rs8070723 tagged genomic loci retrieved from FeatSNP. (A) Clustering visualization of epigenetic annotation to the genomic loci tagged by SNP rs8070723. (B) WashU EpiGenome Brower view of genomic loci tagged by rs8070723. Top: DNA methylation level of CpG sites in four neuronal cells. Middle track: Epilogos visualization of chromHMM predicted chromatin status. Followed eQTL tracks: log10 transformed p-value of eQTL in 10 brain regions. TAD track: Topological Associated Domain tracks of GM12878, IMR90, and Hap1. Bottom: RefSeq gene annotation track. (C) Complete eQTL information of SNP rs8070723 in 10 brain regions.
In summary, FeatSNP is an interactive database providing brain-specific functional genomics resources to investigate the regulatory potential of human SNPs. This database provides a multitude types of functional annotations, including TF binding motif prediction, epigenetic landscape, expression correlation and eQTL information. We anticipate that this database will facilitate scientists to investigate the functional impact of their candidate genetic variants in a more streamlined, rapid, and efficient fashion.
Publicly available datasets were analyzed in this study. This data can be found here: http://www.roadmapepigenomics.org/.
C-yM and BZ performed the data analysis, C-yM and PG developed the database and website. PM, TW, and BZ designed and supervised the study.
This work was supported by National Institutes of Health grant DA027995, HG007175, HG007354, and Goldman Sachs Philanthropy Fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00262/full#supplementary-material
Agrawal, A., Chou, Y. L., Carey, C. E., Baranger, D. A. A., Zhang, B., Sherva, R., et al. (2018). Nabis dependence. Mol. Psychiatry 23, 1293–1302. doi: 10.1111/den.13366
Andersson, R., Gebhard, C., Miguel-Escalada, I., Hoof, I., Bornholdt, J., Boyd, M., et al. (2014). An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461. doi: 10.1038/nature12787
Boyle, A. P., Hong, E. L., Hariharan, M., Cheng, Y., Schaub, M. A., Kasowski, M., et al. (2012). Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 22, 1790–1797. doi: 10.1101/gr.137323.112
Carey, C. E., Agrawal, A., Zhang, B., Conley, E. D., Degenhardt, L., Heath, A. C., et al. (2015). Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: evidence from an endocannabinoid system-level analysis. J. Abnorm. Psychol. 124, 860–877. doi: 10.1037/abn0000079
Claussnitzer, M., Dankel, S. N., Kim, K. H., Quon, G., Meuleman, W., Haugen, C., et al. (2015). FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373, 895–907. doi: 10.1056/NEJMoa1502214
De Iaco, A., Planet, E., Coluccio, A., Verp, S., Duc, J., and Trono, D. (2017). DUX-family transcription factors regulate zygotic genome activation in placental mammals. Nat. Genet. 49, 941–945. doi: 10.1038/ng.3858
Ernst, J., and Kellis, M. (2012). ChromHMM: automating chromatin-state discovery and characterization. Nat. Methods 9, 215–216. doi: 10.1038/nmeth.1906
Genestine, M., Lin, L., Durens, M., Yan, Y., Jiang, Y., Prem, S., et al. (2015). Engrailed-2 (En2) deletion produces multiple neurodevelopmental defects in monoamine systems, forebrain structures and neurogenesis and behavior. Hum. Mol. Genet. 24, 5805–5827. doi: 10.1093/hmg/ddv301
Gibson, G. (2015). Human genetics. GTEx detects genetic effects. Science 348, 640–641. doi: 10.1126/science.aab3002
Golonzhka, O., Nord, A., Tang, P. L. F., Lindtner, S., Ypsilanti, A. R., Ferretti, E., et al. (2015). Pbx regulates patterning of the cerebral cortex in progenitors and postmitotic neurons. Neuron 88, 1192–1207. doi: 10.1016/j.neuron.2015.10.045
Grant, C. E., Bailey, T. L., and Noble, W. S. (2011). FIMO: scanning for occurrences of a given motif. Bioinformatics 27, 1017–1018. doi: 10.1093/bioinformatics/btr064
Hoglinger, G. U., Melhem, N. M., Dickson, D. W., Sleiman, P. M., Wang, L. S., Klei, L., et al. (2011). Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699–705. doi: 10.1038/ng.859
Mathelier, A., Fornes, O., Arenillas, D. J., Chen, C. Y., Denay, G., Lee, J., et al. (2016). JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 44, D110–D115. doi: 10.1093/nar/gkv1176
Nelson, E. C., Agrawal, A., Heath, A. C., Bogdan, R., Sherva, R., Zhang, B., et al. (2016). Evidence of CNIH3 involvement in opioid dependence. Mol. Psychiatry 21, 608–614. doi: 10.1038/mp.2015.102
Rao, S. S., Huntley, M. H., Durand, N. C., Stamenova, E. K., Bochkov, I. D., and Robinson, J. T. (2014). A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680. doi: 10.1016/j.cell.2014.11.021
Roadmap Epigenomics, C., Kundaje, A., Meuleman, W., Ernst, J., Bilenky, M., Yen, A., et al. (2015). Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330. doi: 10.1038/nature14248
Salmon, E., Van der Linden, M. V., and Franck, G. (1997). Anterior cingulate and motor network metabolic impairment in progressive supranuclear palsy. Neuroimage 5, 173–178. doi: 10.1006/nimg.1997.0262
Sanborn, A. L., Rao, S. S., Huang, S. C., Durand, N. C., Huntley, M. H., Jewett, A. I., et al. (2015). Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl. Acad. Sci. U.S.A. 112, E6456–E6465. doi: 10.1073/pnas.1518552112
Siriboonpiputtana, T., Zeisig, B. B., Zarowiecki, M., Fung, T. K., Mallardo, M., Tsai, C. T., et al. (2017). Transcriptional memory of cells of origin overrides beta-catenin requirement of MLL cancer stem cells. EMBO J. 36, 3139–3155. doi: 10.15252/embj.201797994
UK Parkinson’s Disease Consortium, Wellcome Trust Case Control Consortium, Spencer, C. C., Plagnol, V., Strange, A., Gardner, M., et al. (2011). Dissection of the genetics of Parkinson’s disease identifies an additional association 5’ of SNCA and multiple associated haplotypes at 17q21. Hum. Mol. Genet. 20, 345–353. doi: 10.1093/hmg/ddq469
Villaescusa, J. C., Li, B., Toledo, E. M., Rivetti di Val Cervo, P., Yang, S., Stott, S. R., et al. (2016). A PBX1 transcriptional network controls dopaminergic neuron development and is impaired in Parkinson’s disease. EMBO J. 35, 1963–1978. doi: 10.15252/embj.201593725
Ward, L. D., and Kellis, M. (2012). HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 40, D930–D934. doi: 10.1093/nar/gkr917
Welter, D., MacArthur, J., Morales, J., Burdett, T., Hall, P., Junkins, H., et al. (2014). The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 42, D1001–D1006. doi: 10.1093/nar/gkt1229
Zhang, Y., Liu, T., Meyer, C. A., Eeckhoute, J., Johnson, D. S., Bernstein, B. E., et al. (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9:R137. doi: 10.1186/gb-2008-9-9-r137
Keywords: SNP, database, epigenetics, brain, transcription factor
Citation: Ma C-y, Madden P, Gontarz P, Wang T and Zhang B (2019) FeatSNP: An Interactive Database for Brain-Specific Epigenetic Annotation of Human SNPs. Front. Genet. 10:262. doi: 10.3389/fgene.2019.00262
Received: 19 November 2018; Accepted: 08 March 2019;
Published: 02 April 2019.
Edited by:
Shandar Ahmad, Jawaharlal Nehru University, IndiaReviewed by:
Wei-Hua Chen, Huazhong University of Science and Technology, ChinaCopyright © 2019 Ma, Madden, Gontarz, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Wang, dHdhbmdAd3VzdGwuZWR1 Bo Zhang, YnpoYW5nMjlAd3VzdGwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.