- 1Department of Crop and Soil Sciences, North Carolina State University, Raleigh, NC, United States
- 2Center for Integrated Fungal Research, North Carolina State University, Raleigh, NC, United States
- 3Department of Plant and Soil Sciences, University of Delaware, Newark, DE, United States
Environmental science is a topic that lends itself to innovative teaching methods in secondary education. Many aspects of environmental science have macroscopically observable components, creating myriad opportunities to link classroom lessons and practical, outdoor exercises to both pique the students' curiosity about the world around them, and reinforce the fundamental knowledge imparted through books and lectures via active learning. Linking university research teams and local science teachers is a key way to incorporate cutting edge science into the classroom and to provide students with the experience of being a “scientist for the day.” Through the Soil and Water Iron Microbes in North Carolina (SWIMNC) project, local science teachers were recruited to participate in a university outreach program to bring current biogeochemical science to both students and teachers. A team of faculty, postdoctoral research associates, and graduate students delivered a classroom presentation on the importance of metals and iron oxidizing bacteria, emphasizing the role microbes play in affecting the availability and transport of metals in the environment. The group then traveled to a nearby site where iron oxides (bright orange deposits common in shallow water) form to collect samples and other field data. Students worked in small teams with a member of the university to collect and label samples, take measurements of pH and temperature, and make and record observations. Pre- and post- activity surveys assessed the impact of the combined classroom and field event on student's knowledge and attitudes relating to environmental science. Survey results indicate that the combination of classroom activities and hands-on field sampling and analysis had a positive influence on students' knowledge of environmental science, as well as their views of environmental science. This impact was most pronounced on middle school students, but was still significant for high school students. University outreach programs provide a clear opportunity to integrate current topics into secondary school classrooms. Here, research about how bacteria and metals interact in the environment provides an avenue to incorporate current research data and demonstrations into high school classroom activities, giving teachers and students the opportunity to use these approaches in lessons and projects to gain a better understanding of environmental science.
Introduction
Teaching environmental science in secondary schools provides an opportunity to combine cutting edge scientific research with a range of evidence-based teaching methods to generate enthusiasm and engagement in middle and high school students. Environmental science education is just one part of the recognized need for effective science education to increase scientific literacy in students and broaden the capabilities of the future STEM workforce, but it has great potential to offset the boredom and lack of perceived relevance that is a source of student disengagement from science (Barnett et al., 2006; McWilliam et al., 2008) by pairing basic scientific knowledge with tangible phenomena in nature. There is also evidence that environmental education has positive outcomes related to overall academic achievement and civic engagement, as well as scientific knowledge, attitudes, and skills (Ardoin et al., 2018), emphasizing the need for effective teaching in this particular area.
There are challenges in introducing current research into K-12 schools, but these can be overcome through efforts to align the scientific activity with mandated curriculum or content standards and use evidence-based teaching methods (McKeown, 2003). Teaching to engage multiple learning styles (Samples et al., 1985; Dunn and Griggs, 1995) and embracing the proven effectiveness of active learning strategies (Gabel, 2003) are important aspects of creating impactful science outreach experiences. There are many learning styles, but auditory (hear and say), visual, and kinesthetic-tactile (touch, physically involved) may be important to consider for K-12 education (Guild and Garger, 1985) although there is some disagreement in the educational literature as to the value of using learning styles to develop specific activities (Willingham et al., 2015; An and Carr, 2017). It is also important to note that multimodal engagement increases the instructor's ability to reach a broader group of students (McKeown, 2003).
According to Kolb's (2014) theory of experiential learning, experience plays a central role in the learning process. This provides a basis for using a suite of teaching methods including active learning strategies to insure the student's experience fits within existing learning theory. Active or interactive techniques include introducing student activity into traditional lectures, promoting student engagement, and facilitating collaborative, cooperative, and problem-based group efforts (Prince, 2004). Educational research (Bonwell and Eison, 1991) and meta-analyses (Springer et al., 1999; Ruiz-Primo et al., 2011; Freeman et al., 2014) indicate that active learning leads to better student attitudes and increases acquisition and retention of knowledge compared to lecturing alone, making active learning a highly recommended teaching method (Felder et al., 2000). Promoting student engagement through cooperative, collaborative, or problem-based learning is effective (Prince, 2004). In the sciences, active learning has improved attitudes and performance in introductory biology (Armbruster et al., 2009), chemistry (Towns and Grant, 1997), physics (Burrowes, 2003; Michael, 2006), and environmental education (Ardoin et al., 2018). Frequently used interactive techniques include dividing the class into smaller groups, encouraging students to ask questions, and giving case studies (Steinert and Snell, 1999). Working in teams is a common form of active pedagogy shown to have a positive relationship with both achievement and attitudes (Johnson and Johnson, 1989; Blumenfeld et al., 1996; Bossert, 1998) and prior investigations indicate that individuals learn more when interacting with the environment and their peers (Adams and Hamm, 1994; Johnson et al., 2007).
One of the common models for short-term science outreach is the “scientist-in-the-classroom” approach (Laursen et al., 2007; Houseal et al., 2014), which infuses the enthusiasm and expertise of a scientist into secondary education. This model allows students to experience scientific methodology first hand, increasing their understanding of science and how it is relevant to their everyday life in a tangible way (McKeown, 2003; Laursen et al., 2007). A majority of the existing literature is descriptive and expresses enthusiasm for this model of engagement, but little research has been performed to document the effectiveness of this approach (Laursen et al., 2007). Existing studies of an established outreach program (Laursen et al., 2007) and student-teacher-scientist partnerships (Houseal et al., 2014) reported enhanced student interest and engagement, with concurrent positive knowledge gains and attitude shifts. Environmental science lends itself well to a combination of indoor lecture and outdoor “scientist for a day” activities that can directly link basic science knowledge to observable phenomena in the world around them. There may be added benefits to conducting educational sessions outdoors, including increased retention of information (Kuo et al., 2018), improved attitudes (Cheng and Monroe, 2010), and better ability to recognize science as a tool to understand the world surrounding them (Barnett et al., 2006).
To this end, we conducted a set of combined classroom and field outreach activities designed to integrate current research data and provide a hands-on experience for students and teachers at an active field site. Secondary school biology and environmental science teachers were recruited through the Soil and Water Iron Microbes in North Carolina (SWIMNC) program, part of a National Science Foundation grant that involves investigation of the way that microbes affect the availability and transport of metals in the environment. Herein, we will describe the paired classroom and field outreach activities conducted by a team of university researchers associated with the Soil Biogeochemistry lab at North Carolina State University and discuss impacts on student scientific knowledge and attitude as assessed by pre- and post- activity surveys.
Materials and Methods
Ethics Statement
The North Carolina State University institutional review board (IRB # 5813) approved this study. Signed parental or guardian consent forms were obtained prior to the activity, and surveys were conducted anonymously to protect student data and privacy.
Combined Classroom and Field Activity Overview
This outreach activity provided an exciting opportunity for biology and environmental science teachers to integrate current environmental research topics into their classrooms. Three classroom groups from North Carolina, two from Wake County and one from Guilford County, participated in the activity, summarized in Table 1: Group A consisted of 13 female high school Honors and AP Biology juniors and seniors; Group B was made up of 14 middle school Honors Environmental Science students of both genders; Group C consisted of 9 female senior Honors and AP Biology students. Each group participated in a separate 1-day activity, with two groups participating in 2015, and one group in 2016. Due to the small sample size, all three groups were treated the same and the impact of the outreach activity was assessed by comparing pre- and post-event survey results. The teachers also participated in the outreach activity, but the sample size (two) was too small to provide significant results.
The format of the activity consisted of two parts: an hour-long classroom lecture and a 2-h field-sampling event. A university research team traveled to the teachers' classroom to deliver a presentation on the importance of metals and iron oxidizing bacteria in the environment. Immediately following the presentation, the team then traveled with the participating class to a nearby site where iron oxides (bright orange deposits common in shallow water) form to work with the students to collect samples and other field data. Pre-event and exit surveys were given anonymously to assess shifts in mean knowledge and attitudes about environmental science.
Classroom Presentation Description
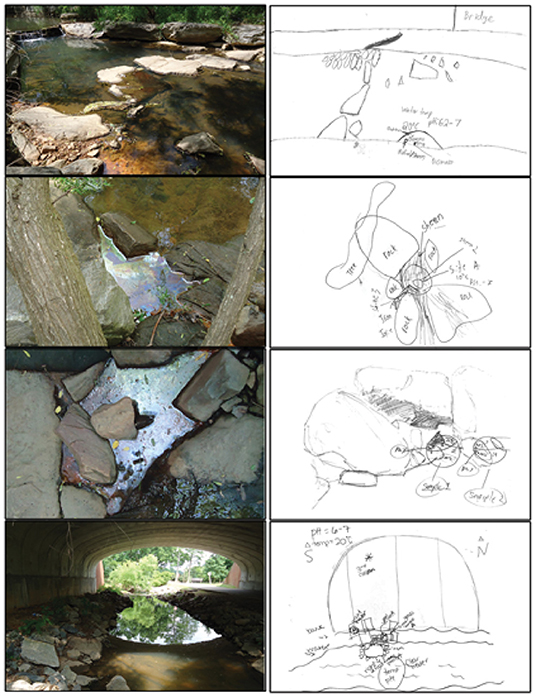
A team of faculty, postdocs, and graduate students from the NC State University Soil Biogeochemistry group presented a lecture covering general soil science, iron-specific chemistry, iron oxidizing bacteria, and field site activity specific information. The team members first introduced themselves and briefly discussed how they got interested in soil biogeochemistry and what educational and experience paths brought them to their current positions at NC State University. The professor began the presentation with a broad introduction to soil science and the major processes in soil biogeochemistry to give context. The bulk of the presentation was jointly given by graduate students whose thesis and dissertation research involved the field site used for the outreach activity. Both the role of iron in biology and basic iron redox chemistry were described. With this foundation established, the role of iron oxidizing bacteria in the environment was discussed, including what environments they live in, why and how they oxidize iron, and what they look like in streams (Figure 1). The masters and doctoral students showed examples of iron biomineral formations and presented fact sheets describing the two major morphologies of circumneutral iron biomineral formations, orange slimy deposits or an oily appearing mineral sheen on the surface of the water. SEM images of the common species of iron oxidizing bacteria and their associated iron biominerals were included. The description of the formation of iron oxidizing communities in near neutral pH streams emphasized the role of oxygenated stream water and anaerobic groundwater seeps in forming a thin layer where the water had only small amounts of oxygen, creating a zone where iron oxidizing bacteria can live. A brief overview of the differences between iron minerals and iron biominerals and why they are of interest was also given by the graduate student presenters.
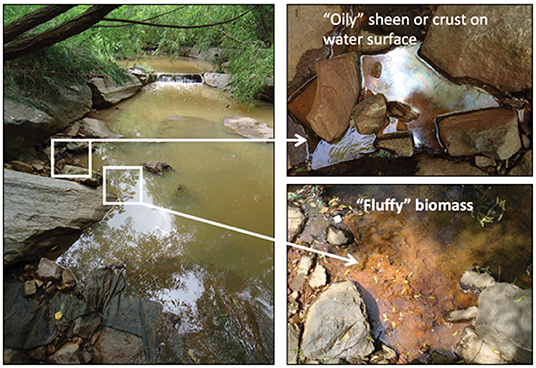
Figure 1. Images from the Rocky Branch Creek sampling site show the “oily” sheen or crust and orange “fluffy” appearance of the biogenic iron oxide and biomass material present, illustrating the macroscopic nature of these deposits.
The postdoctoral researcher involved in the project presented the final third of the presentation focused on an overview of what can be observed and measured in the field, including water temperature and pH, and size and morphology of the iron oxide formations (Figure 2). This was complemented by a description of what can be subsequently analyzed in the lab to give the students a clearer image of how the field-sampling event fits into the bigger scientific study. Methods of growing the iron oxidizing bacteria collected from the stream were shown and different laboratory systems to study the formation of biominerals and how they interact with other chemicals in water were disclosed. The final slides encompassed an overview of the protocol the students would be following at the field site and the proper personal protective equipment (PPE) needed for this type of sample collecting activity.
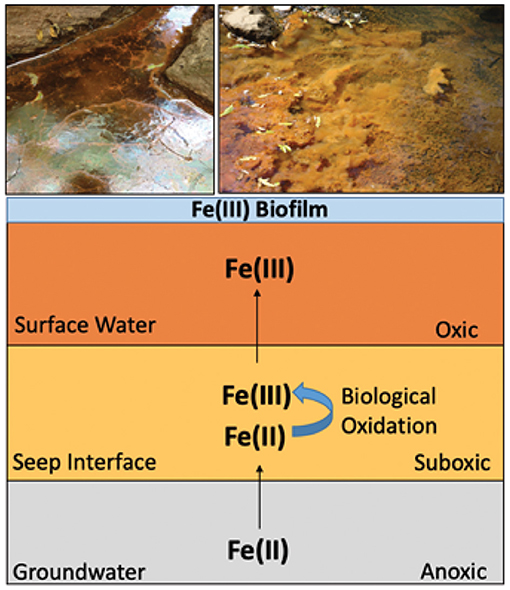
Figure 2. Illustrates the biogeochemical anatomy of a groundwater-stream seep interface. Anoxic groundwater rich in Fe(II) seeps into oxygenated surface stream water, creating an interface where biological iron oxidation can occur in the suboxic zone (Sowers, 2016).
Field Sampling Description
Participants were taken to a site along the Rocky Branch Creek Greenway, conveniently located near the NC State campus. The greenway is a wide, paved path immediately adjacent to the Rocky Branch Creek stream, providing both easy access to the site and a safe place to assemble. This site has been sampled frequently as part of an ongoing study investigating the formation of iron oxides by iron oxidizing bacteria in circumneutral streams in North Carolina (Andrews and Duckworth, 2016; Sowers, 2016; Almaraz et al., 2017; Sowers et al., 2017; Whitaker and Duckworth, 2018; Whitaker et al., 2018).
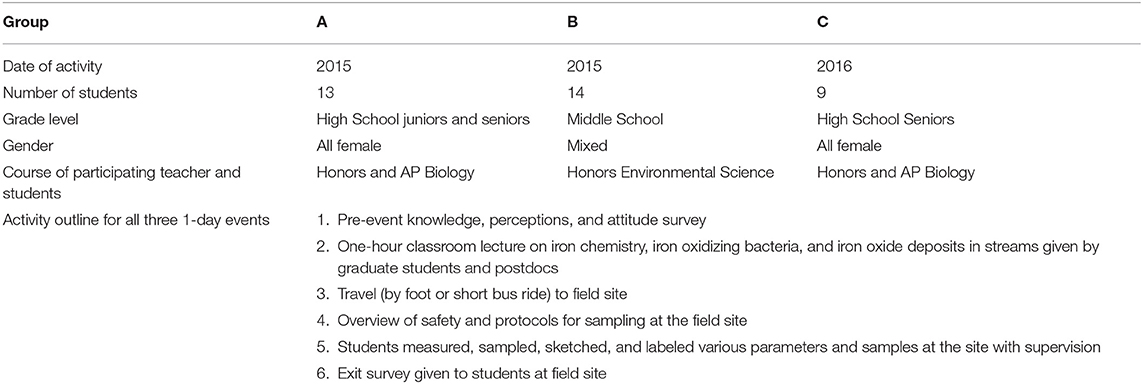
Upon arrival, students were first given a PPE and safety orientation by the postdoctoral researcher. Safety glasses and nitrile gloves were required, and hazards posed by potentially slippery rocks near the stream edge were emphasized. Participants were divided into groups of three to five students and each group was assigned a member of the research team as Group Leader. Group Leaders included postdocs, masters and doctoral students, and faculty familiar with both iron biogeochmistry and the field site. The Group Leaders assisted students as they followed the sampling protocol (Figure 3) as well as answering questions. Once participants had divided into groups and become familiar with the PPE, the research team gave an overview of the site, pointing out areas within the site that had either persistent or intermittent biogenic iron oxide formations and discussing the impact of storm events. Examples of both the fluffy orange biomass morphology and the iron sheen morphology were highlighted (Figure 1). The postdoctoral researcher demonstrated the different steps to the research protocol again and then each group chose one of the indicated areas and proceeded to sketch the field site and sub-site where they would be sampling (Figure 4). Students used a thermometer to measure the stream water temperature and litmus paper to determine the stream pH. To collect water and iron oxide samples, students first labeled 15 and 50 mL plastic screw-cap falcon tubes with the appropriate site name and letter or number indicating which specific spot was sampled. Two different sampling methods were used. First, students used the pre-labeled tubes to scoop up either minerals or orange biomass (“whole samples”). Second, students used a large syringe and attached 0.2 μm syringe filter to collect samples and separate water and solids. The filtered water was placed in a 50 mL tube and the filter that collected the solids from the water was placed in a separate vial. Participants were instructed to create a consistent labeling plan that connected the whole, water, and filter samples to each other and to the place from which they were collected, as indicated on the site sketches.

Figure 3. The field sampling protocol for the outreach event outlines the sample notation and collection steps used by the university team when collecting iron oxide and stream water samples.
Outreach Event Evaluation (Surveys)
Students anonymously filled out scantron surveys immediately prior to the in-class lecture presentation by the university research team, and exit surveys immediately after the conclusion of the field-sampling activity. These surveys consisted of two types of questions to evaluate knowledge, perception, and attitudes relating to the iron biogeochemistry at the center of this activity (Tables 2, 3). The first 10 questions (Figure 5, Table 2) assessed student knowledge of the scientific subject presented, including iron chemistry, biomineral formation, and the easily visible macroscopic characteristics of iron oxides in streams. The remaining 10 questions (Table 3) used a 5-point Likert scale to gauge participants' perceptions and attitudes toward environmental science, their comprehension of specific components of science relating to the activity, and whether this activity improved their knowledge. Surveys were based on prior outreach surveys developed for biogeochemical outreach workshops (Harrington et al., 2013; Unfried et al., 2015). The surveys were anonymous to protect student data and privacy, so individual pre- and exit surveys could not be paired. Instead, a t-test was performed comparing the means of the pre- and exit surveys by setting the null hypothesis to the pre-survey mean and evaluating whether a significant shift between the pre-survey and exit survey means had occurred. Also, because the surveys were anonymous, it was not possible to separate results by gender in Group B.
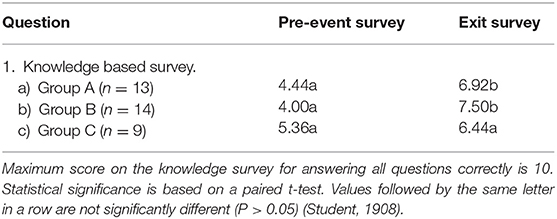
Table 2. Average responses to knowledge survey questions (Figure 5) for the groups A–C.
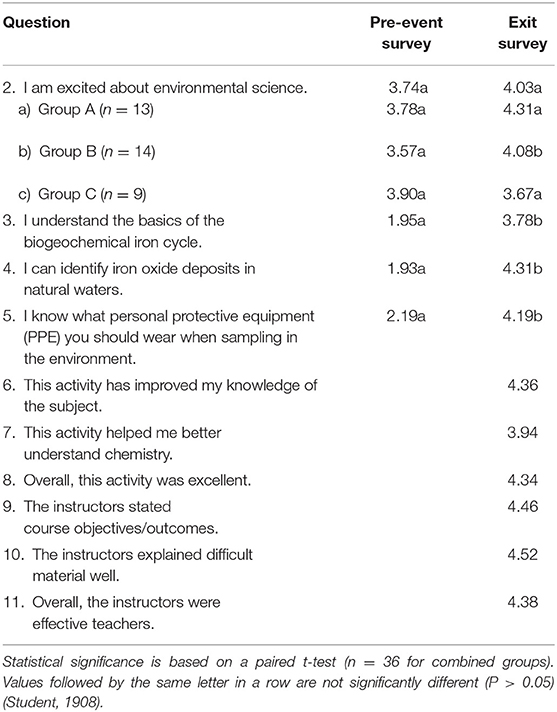
Table 3. Average responses to perception and attitude survey questions for the combined groups A–C based on a modified Likert 5-point scale (5 = strongly agree) (Likert, 1932).
Results
Knowledge Questions
The knowledge question results for each of the three student groups are shown in Table 2. Ten questions assessed knowledge of iron chemistry and iron biogeochemistry (Figure 5) and a score of 10 would indicate all questions were answered correctly. Mean scores for the aggregated knowledge questions are shown for each group, with different letters in the same row indicating statistically significant differences in the pre- and post- scores. Groups A and B had lower initial mean scores on the pre-survey knowledge questions than Group C. For both Group A and Group B, the mean scores improve significantly between the pre- and post- surveys with Group B showing the largest increase in correct answers. Group C had the highest initial scores on the pre-survey knowledge questions and a small increase in mean scores in the exit survey, but it was not statistically significant.
Perception and Attitude Questions
To assess the impact of the outreach activity on student's perception of their ability to conduct iron biogeochemical field sampling, and attitudes toward environmental science and the outreach activity as a whole, the survey included 10 questions using a Likert 5-point scale (5 = strongly agree) (Likert, 1932). The first four questions appraise changes in participant response from pre- to exit survey, while the remaining six questions only record participant perceptions and attitudes at the conclusion of the activity. For all but one question, the three groups responded similarly and their pooled responses are reported. For the “I am excited about environmental science” question, the results for each group are reported separately, as well as pooled (Table 3).
When asked about their excitement regarding environmental science, only Group B had a significant increase in positive response in the exit survey compared to the pre-event survey. Groups A and C did not show a significantly different response in the pre- and post- surveys. There was a significant increase in the reported ability of all three groups to understand the basics of the iron biogeochemical cycle, identify iron oxides in streams, and know what PPE to wear for environmental sampling over the course of the combined classroom and field outreach event.
Questions 6 and 7 regarding the impact of the activity on improving their awareness of the subject of iron biogeochemistry and improving their understanding of chemistry revealed that participants had a positive perception of the ability of the activity to improve their knowledge. Similarly, based on questions 9, 10, and 11, participants had strong positive perceptions of the ability of the instructors to state activity objectives and outcomes, to explain difficult material well, and their overall effectiveness as teachers. In addition, responses to question 8 indicated that the students liked the overall activity.
Discussion
The SWIMNC outreach activity had a positive outcome with regard to increasing student subject knowledge and attitudes, in addition to providing opportunities to train graduate students and postdoctoral researchers in outreach provision. Although there are several limitations to this study, including small sample size, lack of a true control group, and gender and age imbalances, the results (Tables 2, 3) are consistent with literature indicating that hands-on science and learning through inquiry-based approaches are effective, motivating, and stimulate interest in science (Bredderman, 1983; Blosser, 1985; Trowbridge and Bybee, 1990; Ebenezer and Haggerty, 1999; Houseal et al., 2014). Houseal et al. (2014) suggests that scientific inquiry can best be taught through experiential authentic experiences, where student practices mirror practitioners. This notion was incorporated into the outreach program discussed here using published (Sowers, 2016; Almaraz et al., 2017; Sowers et al., 2017; Whitaker and Duckworth, 2018; Whitaker et al., 2018) sampling protocols (Figure 3) at an active research site. This authenticity exposes students to broader views of what being a scientist actually entails, while simultaneously inspiring interest in becoming a scientist through these field activities (Barnett et al., 2006).
Combining the classroom lecture presentation and field sampling activity provided a framework to use many different learning styles (Felder and Silverman, 1988) to deliver a balanced teaching approach with a greater chance of reaching all students (Felder and Spurlin, 2005). The classroom presentation included spoken descriptions (verbal), pictures and diagrams (visual), facts (sensing), and underlying theory (intuitive) related to microbial iron oxidation in natural waters. It also gives students a chance to process the information individually (reflective) before working in groups (active) in the field. This combination of approaches to conveying the same information positively impacts student knowledge and attitudes (Stevenson et al., 2013). Additionally, there is evidence to suggest that incorporating an outdoor session into the event may be especially effective for engaging diverse students (Laursen et al., 2007; Stevenson et al., 2013).
Impact on Knowledge
Group C, composed of senior females, had the highest initial knowledge scores and did not show a significant increase at the end of the activity. Group A contained a mix of female juniors and seniors and did show a significant improvement in scores. However, Group B had the largest increase in knowledge from the activity which suggests middle school may be the target age group for such activities, in agreement with Stevenson et al. (2013) who noted that middle school is a pivotal time for influencing environmental literacy. We should note that Group B was the youngest, but also the most diverse (gender, ethnicity, and socioeconomic diversity) which may also account for the larger increase of knowledge due to the outreach activity (Stevenson et al., 2013). Differences in students' assumed knowledge based on school courses could explain the differences in initial survey scores between the groups, but more work needs to be done to better assess the variables that affect the increases in scores by the end of the activity.
During the course of the field activity, the need to be able to connect samples to the collection site with sketches (Figure 4) and notes of sufficient detail to be able to return in a week or a month or a year and sample the same spot was emphasized. This concept of sampling over time underscored the importance of consistent labeling schemes. Students appreciated the ability to be creative in their labeling schemes and group names. The main point emphasized was that the schemes needed to be clear and make sense and be something that you could refer back to and use going forward. But the details of the labeling (numbers, letters, descriptions) were left completely up to the participants. This encouraged group discussion about how best to identify sites, sketch markers, and logically set up a labeling system, increasing awareness of practical aspects of scientific methodology.
Impact on Attitudes
No significant impact on the view of environmental science among high school groups was reported. This may be because the participating classes were in advanced biology and environmental science classes, and therefore potentially already had a favorable view of environmental science, although the surveys did not collect data to assess this possibility. However, the data does indicate a positive impact on the middle school group's excitement about environmental science. This was also easily observable in the energy levels of the students while in the field, and again supports the notion that middle school may be a pivotal time to get students excited about environmental science (Stevenson et al., 2013).
For all three groups, there was a significant increase in students' assessment of their understanding of the iron biogeochemical cycle and even more so for their ability to identify iron oxide deposits in streams. More confidence in ability to identify iron oxides may be due to the very visible, distinctive color, and morphology. Classroom lectures on the biogeochemical cycle of iron are less tangible than seeing the stream water biogenic iron formations in person. There was also a marked increase in knowing what PPE to wear. Students likely had some experience with wearing gloves in class labs, but less of an idea of what was needed in the field to keep both samples uncontaminated and people safe.
Although the surveys were not designed to capture information about the outreach impact on student perceptions of “stereotypical” scientists, previous research by Miller et al. (2018) suggests that increased exposure to diversity in scientists has a positive effect in broadening the stereotype of a scientist. Multiple lines of evidence indicate that student attitudes about science and scientific careers impact their success (Schinske et al., 2015). The university research team included female and minority scientists, providing students with a variety of role models (Laursen et al., 2007). An added benefit of involving a diverse university research team in these outreach activities is the chance to showcase the real people who become scientists and their different pathways to achieving that goal.
The SWIMNC outreach activity aimed to increase student knowledge and excitement about environmental science by combining classroom learning, a hands-on field sampling activity, and a highly visible macroscopic phenomenon. Providing students with a “scientist-for-a-day” experience at a site being used in an ongoing scientific study had a positive impact on both environmental science knowledge and attitudes, particularly with younger groups of students. This suggests that these types of outreach activities may be more effective in imparting knowledge and excitement when participants are middle school or early high school students. Based on the survey results and anecdotal evidence from the three groups to participate in this activity to date, this approach is a promising way to educate and excite students.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by The North Carolina State University institutional review board (IRB # 5813). Signed parental or guardian consent forms were obtained prior to the activity, and surveys were conducted anonymously to protect student data and privacy. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MA, AW, TS, and OD produced classroom activities and lead activities. MA conceived and designed the surveys and assessments, analyzed the data, and wrote the paper with guidance from OD.
Funding
We are grateful for support received from the National Science Foundation Geobiology and Low-Temperature Geochemistry Program (award EAR-125515). This work was supported by the USDA National Institute of Food and Agriculture, Hatch project NC02440 and NC02713.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jason Painter, Joe Thompson, Ben Uster, and Wayne Roper for assistance.
References
Adams, D. M., and Hamm, M. (1994). New Designs for Teaching and Learning. San Francisco, CA: Jossey-Bass Inc.
Almaraz, N., Whitaker, A. H., Andrews, M. Y., and Duckworth, O. W. (2017). Assessing biomineral formation by iron-oxidizing bacteria in a circumneutral creek. J. Contemp. Water Res. Educ. 160, 60–71. doi: 10.1111/j.1936-704X.2017.03240.x
An, D., and Carr, M. (2017). Learning styles theory fails to explain learning and achievement: recommendations for alternative approaches. Personal. Individual Differ. 116, 410–416 doi: 10.1016/j.paid.2017.04.050
Andrews, M. Y., and Duckworth, O. (2016). A universal assay for the detection of siderophore activity in natural waters. BioMetals 29, 1085–1095. doi: 10.1007/s10534-016-9979-4
Ardoin, N. M., Bowers, A. W., Roth, N. W., and Holthuis, N. (2018). Environmental education and K-12 student outcomes: a review and analysis of research. J. Environ. Educ. 49, 1–17. doi: 10.1080/00958964.2017.1366155
Armbruster, P., Patel, M., Johnson, E., and Weiss, M. (2009). Active learning and student-centered padagogy improve student attitudes and performance in instriductory biology. CBE Life Sci. Educ. 8, 203–213. doi: 10.1187/cbe.09-03-0025
Barnett, M., Lord, C., Strauss, E., Rosca, C., Langford, H., and Chavez, D. (2006). Using the urban environment to engage youths in urban ecology field studies. J. Environ. Educ. 37, 3–11. doi: 10.3200/JOEE.37.2.3-11
Blosser, P. E. (1985). Improving science education Information Bulletin No. 3. Columbus, OH: ERIC (Educational Resources Information Center), Clearinghouse for Science, Mathematics, and Environmental Education.
Blumenfeld, P., Marx, R., Solloway, E., and Krajcik, J. (1996). Learning with peers: from small group cooperation to collaborative communities. Educ. Res. 25, 37–40. doi: 10.2307/1176492
Bonwell, C. C., and Eison, J. A. (1991). Active Learning: Creating Excitement in the Classroom. Washington, DC: George Washington University.
Bredderman, T. (1983). Effects of activity-based elementary science on student outcomes: a qualitative analysis. Rev. Educ. Res. 53, 499–518. doi: 10.3102/00346543053004499
Burrowes, P. (2003). Lord's constructivist model put to a test. Am. Biol. Teacher 65, 491–502. doi: 10.1662/0002-7685(2003)065[0491:ASATTG]2.0.CO;2
Cheng, J.-H., and Monroe, M. (2010). Connection to nature: children's affective attitude toward nature. Environ. Behav. 44, 31–49. doi: 10.1177/0013916510385082
Dunn, R., and Griggs, S. A. (1995). Multiculturalism and Learning Style: Teaching and Counseling Adolescents. Westport, CT: Praeger.
Ebenezer, J., and Haggerty, S. (1999). Becoming a Secondary School Science Teacher. Upper Saddle River, NJ: Merrill/Prentice Hall.
Felder, R., Woods, D., Stice, J., and Rugarcia, A. (2000). The future of engineering education II: teaching methods that work. Chem. Eng. Educ. 34, 26–39.
Felder, R. M., and Silverman, L. K. (1988). Learning and teaching styles in engineering education. Eng. Educ. 78, 674–681.
Felder, R. M., and Spurlin, J. (2005). Applications, reliability and validity of the index of learning styles. Int. J. Eng. Educ. 21, 103–112. doi: 10.1037/t43782-000
Freeman, S., Eddy, S. L., McDonough, M., Smith, M. K., Okoroafor, N., Jordt, H., et al. (2014). Active learning increases student performance in science, engineering, and mathematics. PNAS 111, 8410–8415. doi: 10.1073/pnas.1319030111
Guild, P., and Garger, S. (1985). Marching to Different Drummers. Alexandria, VA: Association for Supervison and Curriculum Development.
Harrington, J. M., Gardner, T. G., Amoozegar, A., Andrews, M. Y., Rivera, N. A., and Duckworth, O. W. (2013). A workshop for developing learning modules for science classes based on biogeochemical research. Nat. Sci. Educ. 42, 75–84. doi: 10.4195/nse.2013.0004
Houseal, A. K., Abd-El-Khalick, F., and Destefano, L. (2014). Impact of a student-teacher-scientist partnership on students' and teachers' content knowledge, attitudes toward science, and pedagogical practices. J. Res. Sci. Teach. 51, 84–115. doi: 10.1002/tea.21126
Johnson, D. W., and Johnson, R. (1989). Cooperation and Competition: Theory and Research. Edina, MN: Interaction Book Company.
Johnson, D. W., Johnson, R. T., and Smith, K. (2007). The state of cooperative learning in postsecondary and professional settings. Educ. Psychol. Rev. 19, 15–29. doi: 10.1007/s10648-006-9038-8
Kolb, D. A. (2014). Experiential Learning: Experience as the Source of Learning and Development. Upper Saddle River, NJ: Prentice-Hall Inc.
Kuo, M., Browning, M. H. E. M., and Penner, M. L. (2018). Do lessons in nature boost subsequent classroom engagement? Refueling students in flight. Front. Psychol. 8:2253. doi: 10.3389/fpsyg.2017.02253
Laursen, S., Liston, C., Thury, H., and Graf, J. (2007). What good is a scientist in the classroom? Participant outcomes and program design features for a short-duration science outreach intervention in K-12 classrooms. CBE Life Sci. Educ. 6, 49–64. doi: 10.1187/cbe.06-05-0165
McKeown, R. (2003). Working with K-12 schools: insights for scientists. BioScience 53, 870–875. doi: 10.1641/0006-3568(2003)053[0870:WWKSIF]2.0.CO;2
McWilliam, E., Poronnik, P., and Taylor, P. G. (2008). Re-designing science pedagogy: reversing the flight from science. J. Sci. Educ. Technol. 17, 226–235. doi: 10.1007/s10956-008-9092-8
Michael, J. (2006). Where's the evidence that active learning works? Adv. Physiol. Educ. 30, 159–167. doi: 10.1152/advan.00053.2006
Miller, D. I., Nolla, K. M., Eagly, A. H., and Uttal, D. H. (2018). The development of children's gender-science stereotypes: a meta-analysis of 5 decades of U.S. draw-a-scientist studies. Child Dev. 89, 1943–1955. doi: 10.1111/cdev.13039
Prince, M. (2004). Does active learning work? A review of the research. J. Eng. Educ. 93, 223–231. doi: 10.1002/j.2168-9830.2004.tb00809.x
Ruiz-Primo, M., Briggs, D., Iverson, H., Talbot, R., and Shepard, L. (2011). (2011) Impact of undergraduate science course innovations on learning. Science 331, 1269–1270. doi: 10.1126/science.1198976
Samples, B., Hammond, B., and McCarthy, B. (1985). 4MAT and Science: Toward Wholeness in Science Education. Barrington, IL: Excel.
Schinske, J., Cardenas, M., and Kaliangara, J. (2015). Uncovering scientist stereotypes and their relationships with student race and student success in a diverse community college setting. CBE Life Sci. Educ. 14, 1–16. doi: 10.1187/cbe.14-12-0231
Sowers, T. D. (2016). Arsenate and Arsenite Sorption to Abiogenic and Biogenic Iron (Oxyhydr)oxides Formed in Natural and Laboratory Environments. Raleigh, NC: North Carolina State University.
Sowers, T. D., Harrington, J. M., Polizzotto, M. L., and Duckworth, O. W. (2017). Sorption of arsenic to biogenic iron (oxyhydr)oxides produced in circumneutral environments. Geochim. Cosmochim. Acta 198, 194–207. doi: 10.1016/j.gca.2016.10.049
Springer, L., Stanne, M., and Donovan, S. (1999). Effects of small-group learning on undergraduates in science, mathematics, engineering, and technology. Rev. Educ. Res. 69, 21–51. doi: 10.3102/00346543069001021
Steinert, Y., and Snell, L. S. (1999). Interactive lecturing: strategies for increasing participation in large group presentations. Med. Teacher 21, 37–42. doi: 10.1080/01421599980011
Stevenson, K. T., Peterson, M. N., Bondell, H. D., Mertig, A. G., and Moore, S. E. (2013). Environmental, institutional, and demographic predictors of environmental literacy among middle school children. PLoS ONE 8:9519. doi: 10.1371/journal.pone.0059519
Towns, M., and Grant, E. (1997). “I believe I will go out of this class actually knowing something”: cooperative learning activities in physical chemistry. J. Res. Sci. Teach. 34, 819–835. doi: 10.1002/(SICI)1098-2736(199710)34:8<819::AID-TEA5>3.0.CO;2-Y
Trowbridge, L., and Bybee, R. (1990). Becoming a Secondary School Science Teacher. Columbus, OH: Merrill.
Unfried, A., Faber, M., Stanhope, D. S., and Wiebe, E. (2015). The development and validation of a measure of student attitudes toward science, technology, engineering, and math (S-STEM). J. Psychoeduc. Assessment 33, 622–639. doi: 10.1177/0734282915571160
Whitaker, A. H., and Duckworth, O. W. (2018). Cu, Pb, and Zn sorption to biogenic iron (Oxyhydr)oxides formed in circumneutral environments. Soil Syst. 2:18. doi: 10.3390/soilsystems2020018
Whitaker, A. H., Peña, J., Amor, M., and Duckworth, O. W. (2018). Cr(vi) uptake and reduction by biogenic iron (oxyhydr)oxides. Environ. Sci. Processes Impacts 20, 1056–1068. doi: 10.1039/C8EM00149A
Keywords: iron oxides, biogeochemistry, environmental science, secondary education, outreach, field sampling
Citation: Andrews MY, Whitaker AH, Sowers TD and Duckworth OW (2020) Soil and Water Iron Microbes in North Carolina (SWIMNC) Outreach: Positive Impact of Combining Classroom and Field Experiences to Promote Learning and Shift Attitudes. Front. Educ. 4:151. doi: 10.3389/feduc.2019.00151
Received: 12 August 2019; Accepted: 06 December 2019;
Published: 08 January 2020.
Edited by:
Philippe C. Baveye, AgroParisTech Institut des Sciences et Industries du Vivant et de L'environnement, FranceReviewed by:
Adrian Sin Loy Loh, National Junior College, SingaporeThomas K. Karikari, University of Warwick, United Kingdom
Copyright © 2020 Andrews, Whitaker, Sowers and Duckworth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Owen W. Duckworth, b3dlbl9kdWNrd29ydGhAbmNzdS5lZHU=
 Megan Y. Andrews
Megan Y. Andrews Andrew H. Whitaker
Andrew H. Whitaker Tyler D. Sowers1,3
Tyler D. Sowers1,3 Owen W. Duckworth
Owen W. Duckworth