- 1Department of Parasitology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
- 2Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
- 3Tropical Projects, Hitchin, United Kingdom
Treatment for toxoplasmosis is not completely successful because of their unwanted side effects, and new treatments are needed. Imiquimod has ability to moderate immune response and used to treat a wide variety of infections and tumors. The aim of the present study was to evaluate the effect of imiquimod on the tachyzoites of T. gondii and infected macrophages in vitro and in BALB/c mice. The viability of T. gondii was assessed in the presence of various concentrations of imiquimod by direct counting after 6 and 24 h. The MTT assay was used to identify the viability of uninfected macrophages. The apoptotic effects were determined with flow cytometry on the tachyzoites and infected macrophages. For evaluation of parasite load in pre-treatment or post-treatment of macrophages Quantitative real time PCR (qPCR) was performed. For in vivo experiments, BALB/c mice received imiquimod before and after challenge with parasites. The mortality rate of mice, parasite numbers in spleen, and the INF-γ and IL-4 cytokine levels in spleen lymphocytes were evaluated. Imiquimod demonstrated anti-Toxoplasma effects by reducing the number of tachyzoites. The results of flow cytometry for drug-treated tachyzoites showed that apoptosis did not rise significantly relative to the control group (p < 0.05). Moreover, apoptosis was enhanced in infected macrophages as the concentration of imiquimod was reduced. The parasitic burden in imiquimod pretreated macrophages was significantly lower than those treated after infection (p < 0.01). A marked reduction was observed in survival rate, parasite load and INF-γ level in BALB/c mice that received imiquimod before parasitic challenge relative to those received drug after parasitic challenge (p < 0.01). Overall, imiquimod in the pretreated group had greater anti-Toxoplasma effects than imiquimod in posttreated group in vitro and in vivo. imiquimod may be considered as a candidate for use against Toxoplasmosis both therapeutically and prophylactically.
Introduction
Toxoplasma gondii, a ubiquitous apicomplexan parasite, causes a neglected parasitic disease known as toxoplasmosis which affects a significant portion of the human and animal population worldwide (Dubey et al., 2020). It is a major public health concern and it is estimated that more than 30 percent of people in the world are infected with T. gondii.
The infection can be contracted in a number of different ways, including consumption of undercooked meat containing tissue cysts, ingestion of contaminated water or food containing mature oocytes, and congenitally by transfer from a pregnant women to her fetus (Mendez and Koshy, 2017; Fallahi et al., 2018). It may also occur in individuals who received blood transfusions or organ transplantation from infected individuals.
In humans, toxoplasmosis is usually causes asymptomatic in immunocompetent persons, although it can lead to mild clinical manifestations such as swollen lymph nodes and flu-like symptoms (Gharavi et al., 2011; Gigley, 2016). In those with a weakened immune system (patients with malignancies, those with HIV/AIDS and organ transplant recipients), toxoplasmosis can cause widespread signs of differing severity such as encephalitis, seizures, vision disorders, poor coordination or even can contribute to the cause of death if untreated (Gigley, 2016). In addition, in pregnant mothers that acquire the infection during pregnancy, toxoplasmosis may cause miscarriage or neuropsychological manifestations, including hydrocephalus, blindness, mental retardation, encephalitis, etc. in newborns with congenital infection (Fishman, 2013; Wang et al., 2017).
Few effective control strategies are available for prevention and control of toxoplasmosis and unfortunately treatments are not completely satisfactory. The recommended treatment for toxoplasmosis is a combination of pyrimethamine (PYR) and sulfadiazine (SDZ) which is associated with unwanted side effects including significant toxicity, prolonged treatment, high cost and potential parasite resistance. Alternative agents such as clarithromycin, atovaquone, azithromycin, dapsone, co-trimoxazole (trimethoprim-sulfamethoxazole) are also used. However, treatment is unable to eradicate bradyzoites in tissue cysts (Montazeri et al., 2016; Montoya and Gomez, 2016; Foroutan et al., 2019). Therefore, it is of great importance for researchers to identify novel anti-Toxoplasma drugs with specific activity that could eliminate both tachyzoites and tissue cysts.
Although humoral immune plays a role against T. gondii infection, cellular immunity plays a crucial role in controlling and restricting growth of the parasite in both acute and chronic infection stages. Protection against toxoplasmosis generally is developed through both CD4+ and CD8 + T cells and production of cytokines from immune system cells (Sasai et al., 2018).
As a member of non-nucleoside isocyclic imidazoquinoline amines, imiquimod has the ability to moderate immune response and therefore it is used to treat a wide variety of viral infections and tumors (Sidky et al., 1992). Although the mechanism of action of imiquimod is not well defined (Miller et al., 1999; Stanley, 2002), it can stimulate the immune system by helping to activate monocytes, macrophages and dendritic cells to produce chemokines and proinflammatory cytokines such as IL-12, TNF-α, IL-6, IL-1a and IFN-α (Buates and Matlashewski, 1999; Dockrell and Kinghorn, 2001; Khamesipour, 2014). It is possible that this compound also has a role in treating some autoimmune diseases in human such as Bechet's disease, multiple sclerosis and optic neuritis (Geisse et al., 2004; Jabari et al., 2019). The therapeutic effects of imiquimod on cutaneous leishmaniasis have been reported previously (Buates and Matlashewski, 1999, 2001; Mehravaran et al., 2020). No studies have been conducted to investigate the toxoplasmacidal activity of imiquimod. Accordingly, the current study was planned to evaluate the effect of imiquimod on T. gondii and infected macrophages in vitro and in BALB/c mice.
Materials and Methods
In vitro Assays
Parasite Culture and Harvesting
Tachyzoites of the virulent strain RH of T. gondii were obtained from the Parasitology Department of Tarbiat Modares University. Initially, tachyzoites of the virulent RH strain of T. gondii were maintained by serial passage in Vero cells in 50 cm2 flasks.
Tachyzoites were collected and washed three times with cold phosphate-buffered saline (PBS; pH 7.4) following centrifugation at 1,000 g for 10 min at 4°C. Parasite concentrations were determined by trypan blue exclusion in a hemocytometer (Neubauer) chamber and were used immediately in the experiments (Mikaeiloo et al., 2016).
Preparation of Drugs
Imiquimod powder was obtained from Toulouse France (Invivogen, Toulouse, France, Lot No: IMQ-37-01A) and 4 mg was dissolved in 1 ml of dimethyl sulfoxide (DMSO) (Daejung Korea, Lot No: D0096QJ5) as a stock solution and then 10, 1, 0.1, and 0.01 μg/ml dilutions were prepared with Dulbecco's Modified Eagle Medium (DMEM). Sulfadiazine and pyrimethamine (Sigma–Aldrich, St. Louis, USA) were diluted in DMSO and used as positive controls at concentrations of 40 and 1 μg/ml, respectively.
Cytotoxicity Evaluation on Tachyzoites
The anti-tachyzoite activity of imiquimod was evaluated by light microscopic examination to determine the optimum concentrations for further experiments., Tachyzoites (2 × 106 cells/well, 100 μl) supplemented with complete RPMI 1640 medium in the presence of various concentrations (10, 1, 0.1, and 0.01 μg/ml) of imiquimod were seeded as triplicates in a 96-well microtiter plate. Parasites plus complete RPMI 1640 medium (without drug) were used as an untreated control while SDZ + PYR was used as the positive control. After 6 and 24 h incubation at 37°C, tachyzoites were directly counted in the hemocytometer chamber using a phase contrast microscope and the results from the experimental groups compared with those of the control groups.
Macrophage Culture
In the present work, the Raw.264.7 macrophage cell line, derived from mouse BALB/c monocyte macrophages, was obtained from Department of Medical Parasitology in Tarbiat Modares University of Tehran, Iran. These cells were first grown and propagated in cell culture flasks containing RPMI 1640 medium (Gibco, Germany) supplemented with 10% heat-inactivated FBS and 100 IU ml−1 of Penicillin and 100 μg ml−1 of streptomycin incubated at 37°C (5% CO2). Growth was observed every day by inverted microscope and when cells reached about 80–90% confluence, they were carefully trypsinized and sub-cultured into a new flask containing fresh culture medium.
Treatment and Collection of Infected Macrophage Cells
The study was conducted to investigate both therapeutic effects and preventive effects of imiquimod. For assessment of therapeutic effects, macrophages were seeded into 24-well microplates (1 ml per well with 1 × 105 cells/well in 10% FCS RPMI 1640 medium) and incubated at a temperature of 37°C with 5% CO2 for 24 h. After adherence of the cells, they were infected using T. gondii in a ratio of 2:1 (parasites: macrophages) and the plates maintained under the previous conditions. Six hours after infection, the cells were washed twice with cold phosphate buffer saline (PBS) to remove unadhered macrophages from the wells and fresh culture medium was supplemented with the different concentrations of drugs. Next, the samples were collected and transferred in to a 1.5 mm DNase/RNase-free tubes and kept at a temperature of −70°C before estimating the parasite load.
To evaluate the preventive effects of imiquimod, the cultured macrophages were treated with the different concentrations of imiquimod before infection with tachyzoites. The remaining stages were accomplished as described above.We harvested the tachyzoites for pre and post treatment in the same condition.
Quantitative Real Time PCR (qPCR) for Assessment of Parasite Load in Infected Macrophages
For evaluation of parasite load in both the above in vitro models, RNA was extracted using the Qiagen RNA isolation kit (RNeasy Mini Kit, Qiagen). A nanodrop device (Roche-Germany) was used to determine the quantity and purity of the extracted RNA. Following this, cDNA was generated using a cDNA Synthesis Kit (Quanti Tect Reverse Transcription Kit, Qiagen) from RNA samples. To create the standard curve, samples of the real-time PCR assay, 6-fold-dilutions ranging from 2 × 101 to 2 × 106 parasites (total DNA extract from a sample containing 107 tachyzoites of strain RH per ml) were prepared and then threshold cycle (Ct) values were calculated for these standard curves. SYBR-green real-time PCR using repetitive element (RE) gene primers were taken to calculate the number of parasites in the treated and control samples. Each sample was amplified in final volumes of twenty-five microliters containing 12.5 μl of SYBR Green PCR Master Mix (without ROX), 2 μl of template cDNA, 8.5 μl of injected distilled water, 1 μl of the forward primer (5′−AGG GAC AGA AGT CGA AGG GG−3′) and 1 μl of the reverse primer (5′−GCA GCC AAG CCG GAA ACA TC−3′) (Montazeri et al., 2016). Amplification was accomplished under the following conditions: 95°C for 15 min (initial step), 40 cycles at 95°C for 15 s (denaturation step), 60°C for 15 s (annealing step), and 72°C for 15 s (amplification step).
Toxicity of Imiquimod in Uninfected Macrophages
The MTT assay was used to determine the viability of macrophages exposed to imiquimod. Briefly, 1 mL of Raw.264.7 macrophage cells, suspended in RPMI 1640 medium enriched with 10 % FBS, were seeded at a concentration of 1 × 105cells/well in 96-well microtiter plates and incubated in 5% CO2 and 95% humidity for 24 h. After adhering the cells, they were exposed to different concentrations of drug (from 10 to 0.01 μg/ml) and incubated for a further 24 h under the same conditions. After this step, 20 μL of solution of MTT (0.5 μg/ml) was added in to each well and incubated for 4 h. The plates were centrifuged for 10 min at 3,000 g and then the contents of each well was discharged slowly and replaced with 100 μL of dimethyl sulfoxide (DMSO) to distinguish between the viable cells (formazan formation) and decayed cells. Finally, absorbance at 570 nm of each well, using an ELISA reader, was evaluated within 30 min. The following formula was used to calculation the percentage of cell viability in comparison to control groups, including both un-treated and treated with sulfadiazine (40 μg/mL) plus pyrimethamine (1 μg/mL): % viable cells = (drug well absorption – blank well absorption / control well absorption – blank well absorption) ×100.
Evaluation of Apoptosis by Flow Cytometry
Evaluation of necrosis and apoptosis in cells were undertaken using Annexin V-FITC Apoptosis Detection Kit (Bio Vision, Palo Alto, USA). In brief, in 12-well plates, 5 × 105 tachyzoites and infected macrophages were cultured separately and were exposed to 0.01 and 0.1 μg/ mL concentrations of imiquimod. Cells not exposed to drug were used as the control. Tachyzoites were incubated for 3 h, while infected macrophages were incubated for 24 h. After incubation the cells were washed in cold PBS and centrifuged at 1,000 g for 5 min. The supernatant was then drained off and replaced with 500 μL binding buffer, followed by 5 μL of annexin V and 5 μL of propidium iodide (PI). Samples were kept in the dark at a temperature of 24 ± 2°C for 5 min and were analyzed by FACSCaliber flow cytometer (BD Biosciences) with FlowJo software (Ebrahimisadr et al., 2018).
In vivo Assay
Animal Design
Sixty BALB/C mice (female, 6–8 weeks old) were purchased from the Royan Institute (Tehran, Iran) and housed in a colony room at an ambient temperature of 20–25°C and relative humidity ranging from 55 ± 65% under a 12/12 h light/dark cycle and free access of food and water. All mouse experiments were carried out in accordance with approved protocols by the Tarbiat Modares University of Medical Sciences Ethical Laws Committee's Institutional Animal Care and Use Committee. The mice were distributed into six groups (ten animals in each cage). Mice in 5 of the groups were injected intraperitoneally with 1 × 104 T. gondii tachyzoites, with the remaining group uninfected as a healthy control. Four hours later all groups were treated as follows: One group before challenge was treated with imiquimod (1 mg/kg, once a week, intraperitoneal injection) for 21 days and the other group received imiquimod at the same dose (1 mg/kg, once a week, intraperitoneal injection) after infection. The third group received sulfadiazine (40 mg/kg/day, orally) plus pyrimethamine (1 mg/kg/day, orally) (SDZ+PYR) and fourth group received sulfadiazine (10 mg/kg/day, orally) plus pyrimethamine (0.5 mg/kg/day, orally) in combination with imiquimod (0.5 mg/kg, once a week, intraperitoneal injection) (SDZ+PYR+IQ). The two remaining groups were healthy and infection controls and only received PBS (intraperitoneal injection) over the study period.
Survival Assay
To evaluate the efficacy of imiquimod against T. gondii infection five mice per group were randomly selected after treatment as mentioned above. Survival was checked daily and the mortality rate was documented for each group until all mice had died.
Quantitative Real Time PCR (qPCR) for Evaluation of Parasite Load in Spleen Tissues
Three days after treatment five mice in each group were sacrificed and their spleens were removed and immersed in 5 ml of cold PBS (pH 7.4). Subsequently, a piece of spleen tissue from each of the study groups was cut, weighed and placed in to a 1.5 mm DNase/RNase-free tubes and then homogenized with a tissue grinder. The rest of spleens were kept for cytokine assessment. The parasite numbers in spleen tissues of the mice were measured by quantitative real time PCR (qPCR) as above.
Cytokine Measurements
Lymphocytes were prepared by crushing spleen tissues and culturing (5 × 106 cells/ml) in 24-well plates in the RPMI1640 medium (Gibco, Germany) supplemented with 10% FCS (Gibco-BRL, France), penicillin (100 unit ml−1), and streptomycin (100 μgml−1) (Sigma, Germany) under sterile conditions. For preparing Toxoplasma Lyzate Antigen (TLA), 2 × 109 tachyzoites of T. gondii RH strainwere passed through 3 μm pore size filter membranes and then centrifuged at 4°C, 3 times for 15 min to separate the supernatant. Subsequently, 1 ml of protease inhibitor (phenylmethylsulphonyl fluoride) was added to the supernatant and then T. gondii tachyzoites were lysed through 5 freeze–thaw cycles alternately in liquid nitrogen and a water bath at 37°C. The protein concentration of TLA was obtained by Bradford method. In order to stimulate the lymphocyte cells, TLA (50 μg/ml) was added to each well followed by incubation at 37°C under 5% CO2 for 3 days. After incubation, the supernatant was harvested and stored frozen at −70°C until used for serological tests. Levels of both IL4 and IFN-γ in culture supernatants were assessed using an ELISA kit (Mabtag, Germany) following the manufacturer's recommended procedure (Zaki et al., 2020).
Date Analysis and Statistics
Statistical analyses were accomplished using IBM SPSS Statistics Version 21 and graphical presentation of data are performed using Graph Pad Prism version 8.0.1 and Microsoft Office Excel 2013. Data were presented as the mean, standard deviation (SD), and 95% confidence interval (CI). In order to determine statistical significance, analysis of variance (One-Way-ANOVA) was employed. The Kaplan-Meier estimate was done for evaluation of survival rate. In this work parametric tests such as unpaired samples t-test were used for the comparison of the results between the treatment and control groups. Differences were presumed significant compared to control at 5% (p < 0.05).
Results
Tachyzoite Assay by Microscopic Observation
As shown in Figure 1, the number of tachyzoites declined significantly in the presence of all concentrations of imiquimod compared to those in control group after 24 h (p < 0.05). The maximum effect was observed after 24 h with imiquimod 0.01 μg/ml, while the least effect was seen with imiquimod 10 μg/ml. Toxic effects of imiquimod on tachyzoites were found to be both dose- and time dependent. Additionally, it was shown that the anti-Toxoplasma effects of imiquimod in combination with sulfadiazine plus pyrimethamine (IQ+SDZ+PYR) were similar to positive control group (SDZ+PYR).
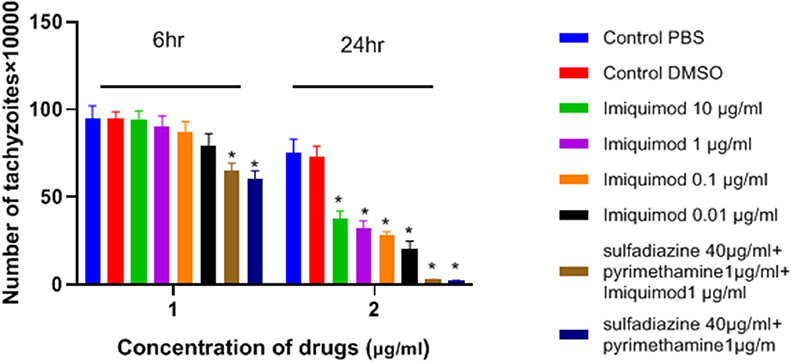
Figure 1. Mean and standard deviation of the number of T. gondii tachyzoites exposed different concentrations of imiquimod for 6 and 24 h compared to control groups (sulfadiazine plus pyrimethamine and untreated). There is statistical difference between control groups and treated groups (*P < 0.05 compared to controls).
Uninfected Macrophage Viability Test
The effects of imiquimod on uninfected macrophages were investigated by measurement of optical density (OD) following MTT assay. Lower concentrations of drug (e.g., 0.01 and 0.1 μg/ mL) were associated with greater viability after 24 h (Figure 2). Additionally, viability of both sulfadiazine 40 μg/mL plus pyrimethamine 1 μg/mL (SDZ+PYR) alone and in combination with imiquimod 1 μg/mL (SDZ+PYR+IQ) was good (93 and 90.9%, respectively).
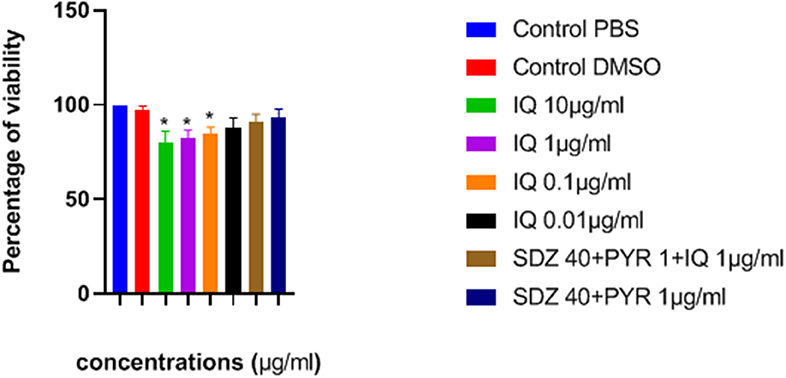
Figure 2. Mean and SD of percentage of macrophage viability exposed to different concentrations of drugs after 24 h. There is statistical difference with control group (*P < 0.05). IQ, Imiquimod; SDZ, Sulfadiazine; PYR, pyrimethamine.
Flow Cytometry Analysis
Flow cytometric analysis showed that apoptosis after 3 h exposure of tachyzoites to 0.01 and 0.1 μg/mL imiquimod (7.3 and 5.7%, respectively) did not differ significantly from the control group (P < 0.05) (Figure 3). Sulfadiazine plus pyrimethamine (SDZ+PYR) alone and in combination with imiquimod (SDZ+PYR+IQ) induced more apoptosis but was not significantly different from control. After 24 h, treatment of infected macrophages with 0.01 or 0.1 μg/mL imiquimod induced apoptosis in 18.66 and 18.00% respectively, in comparison to 1.8% in control (untreated) macrophages. Most apoptosis occurred in sulfadiazine plus pyrimethamine (SDZ+PYR) alone and in combination with imiquimod (SDZ+PYR+IQ) groups (Figure 4).
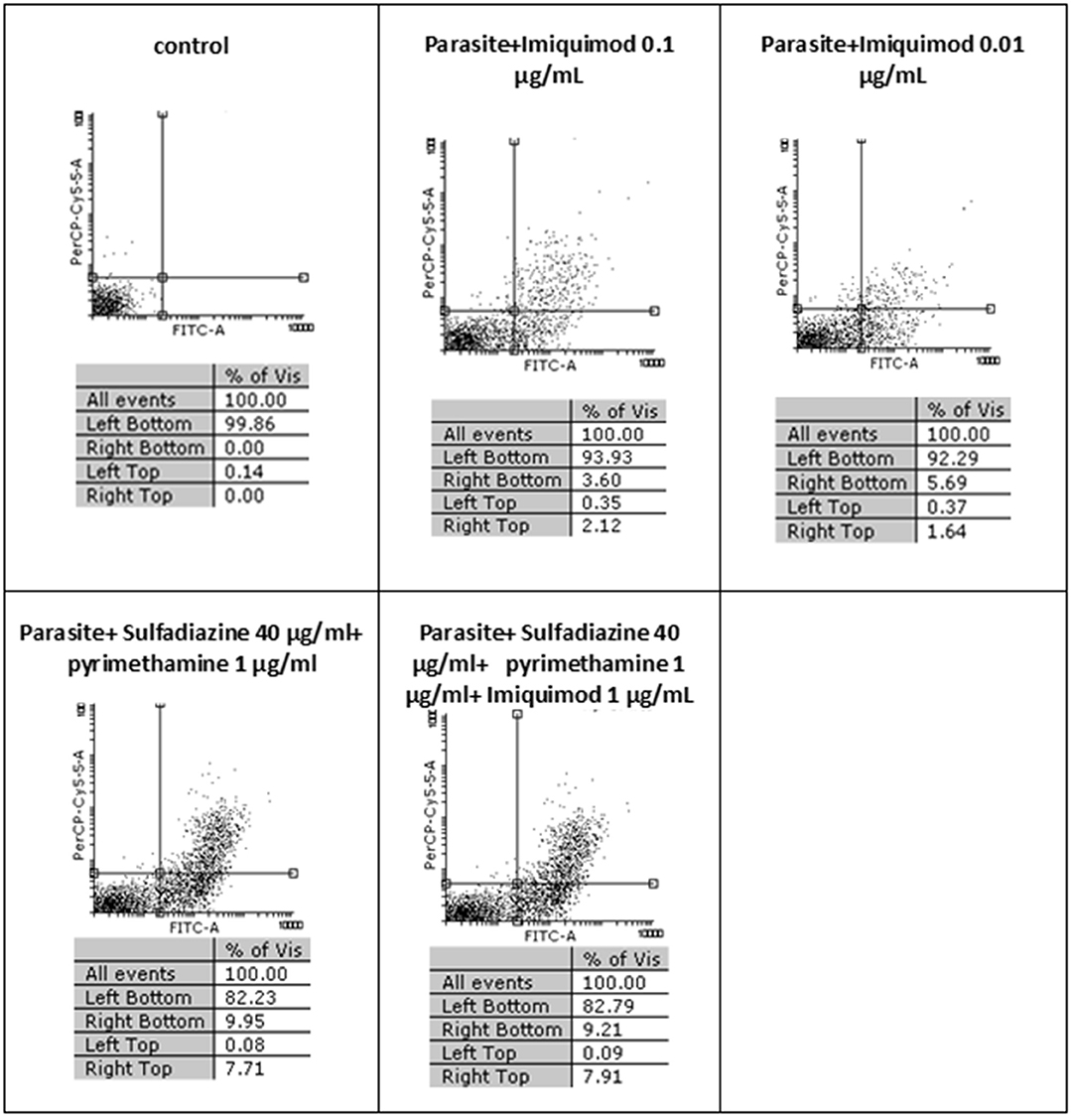
Figure 3. The effect of different concentrations of imiquimod on T. gondii tachyzoites viability and comparing them with the control group (untreated) after 3 h. Regions of quadrat shows: necrosis cells (propidium iodide positive) in left top, late apoptosis in right top, apoptotic cells (annexin positive) in right bottom and live cells in left bottom.
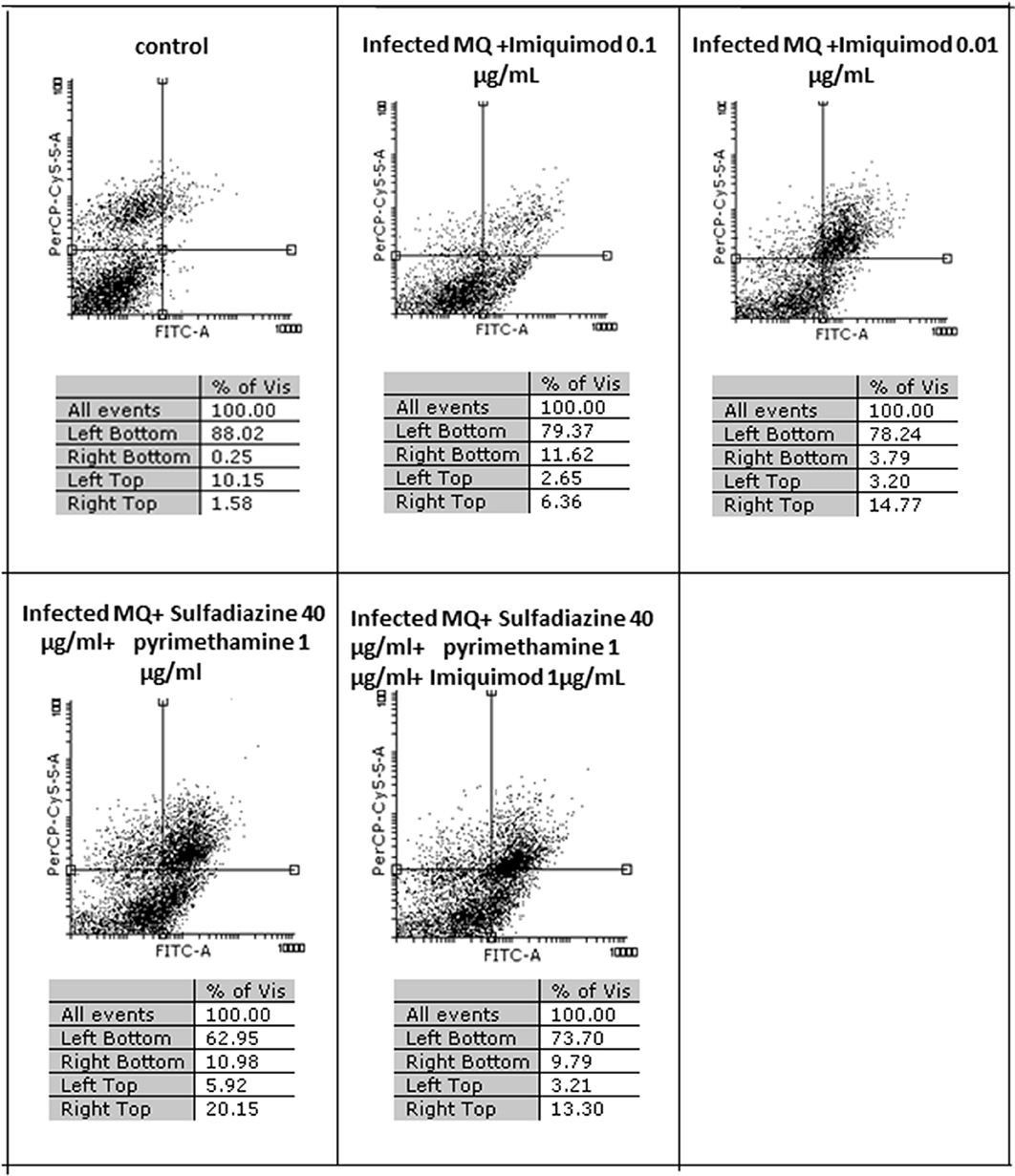
Figure 4. The effect with 2 concentrations (1 and 0.01 μg/ml) of imiquimod and sulfadiazine/pyrimethamine with and without imiquimod on infected macrophage viability compared to untreated control after 24 h. Regions of quadrat shows: necrosis cells (propidium iodide positive) in left top, late apoptosis in right top, apoptotic cells (annexin positive) in right bottom and live cells in left bottom.
Measurement of Parasite Load of Macrophage Cells Using Quantitative Real Time PCR (qPCR)
Table 1 and Figure 5 show the results of qPCR analysis for macrophages treated pre- and post-infection after 24 h incubation. The results showed that parasite loads in both groups treated with all concentrations of drugs were reduced in comparison with untreated macrophages (negative control). Additionally, a very large decrease was observed after 24 h in parasite load in macrophages treated with imiquimod before infection (p < 0.001).
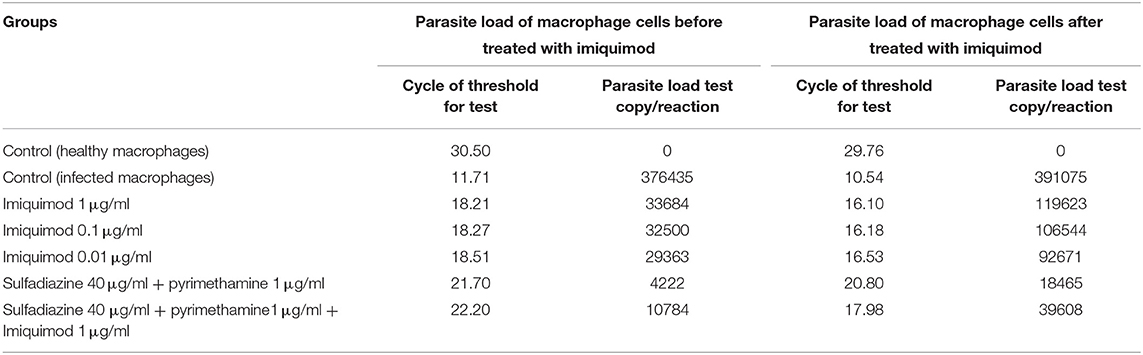
Table 1. Cycle of Threshold (CT) and parasite load test copy/reaction according to Real Time PCR method for macrophages before and after infection with 1 × 104 tachyzoite forms of T. gondii RH strain and control groups.
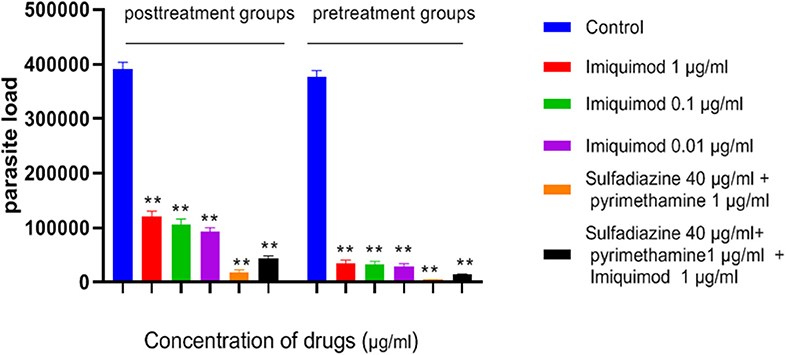
Figure 5. Parasite load of macrophage cells before and after treated with drugs. Values are mean ± standard deviation of two independent experiments. **P < 0.01 compared with untreated group.
Quantification of Parasite Load of Spleen Tissues Using Quantitative Real Time PCR (qPCR)
The spleen parasite burden for all groups was evaluated by comparison with the standard curve and quantitative real-time PCR (qPCR) analysis. Animals receiving imiquimod before being challenged with tachyzoites (pre-treatment group) showed lower parasite loads than those receiving drug after infection (post-treatment groups). Reduced parasite load was found in all treated mice in both groups (pre-treatment and post-treatment groups), and therefore statistically significant difference was seen between drug and control groups (p < 0.001). The parasite load decreased significantly in groups treated with sulfadiazine plus pyrimethamine (SDZ+PYR) alone and in combination with imiquimod (SDZ+PYR+IQ) compared with the groups receiving PBS (p < 0.001) (Table 2).
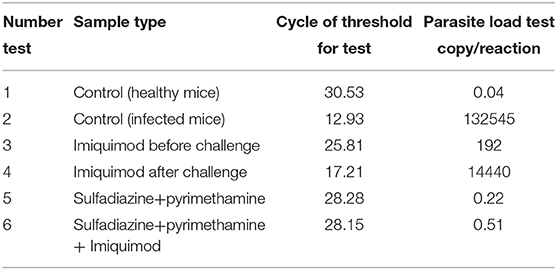
Table 2. Cycle of Threshold (CT) and parasite load of Spleen Tissues according to Quantitative real time PCR (qPCR) method for all treated and control groups.
Determination of Cytokine Secretion Levels
ELISA results showed an increasing trend in the IFNγ cytokine levels among all treated groups in comparison with the untreated groups (P < 0.05) (Figure 6). It was found that the mean IFN-γ level in animals treated with imiquimod before challenge with parasite was significantly higher than the values obtained for other groups (P < 0.05). Also, the highest mean IFN-γ levels after 3 days of exposure was related to the mice treated with sulfadiazine plus pyrimethamine (SDZ+PYR) alone and in combination with imiquimod (SDZ+PYR+IQ) in comparison with other groups (P < 0.05). A marked decrease in IL4 production was detected in treated animals compared to control mice.
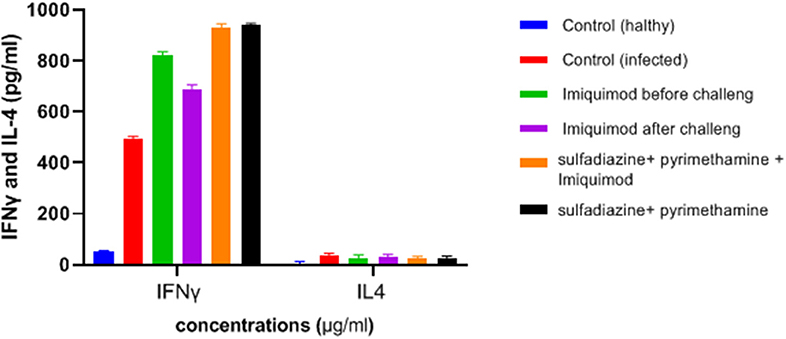
Figure 6. Levels of IFNγ and IL-4 (pg/ml) cytokines in spleen lymphocyte culture in test and control groups after 72 h stimulation with Toxoplasma Lyzate Antigen. SDZ, Sulfadiazine (40 mg/kg/day); PYR, pyrimethamine (1 mg/kg/day); IQ, Imiquimod (1 mg/kg, once a week).
Survival Rate Measurement
Evaluation of survival rate in mice (5 animals/group) was undertaken over 4–12 days after the infection. Death in the untreated mice occurred from day 5 after infection and all had died by day 6 of the study. Mice treated with imiquimod before infection showed prolonged survival time compared with post-treated and control groups (P < 0.05). Moreover, all mice were alive following post infection treatment with sulfadiazine plus pyrimethamine (SDZ+PYR) alone and in combination with imiquimod (SDZ+PYR+IQ) (Figure 7).
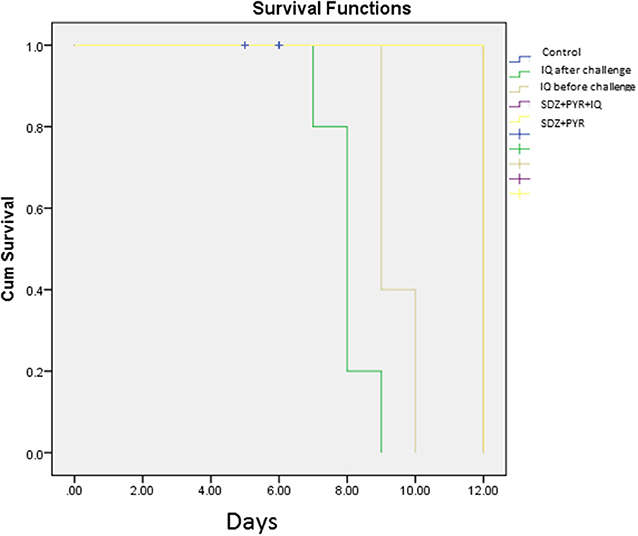
Figure 7. Survival rates of treated and control BALB/c mice after and before challenge with 1 × 104 tachyzoite forms of T. gondii RH strain (5 mice per group).
Discussion
Severe and fatal complications of toxoplasmosis remains a problem that lacks a global solution. Current treatment for T. gondii infection fails to achieve therapeutic goals owing to poor tolerability, limited efficacy and cost (Maubon et al., 2010; Pinzan et al., 2015). As there are no effective therapeutic strategies available to improve early treatment T. gondii infection, the development of new anti-Toxoplasma drugs should have high priority to tackle toxoplasmosis and reduce infection.
Imidazoquinoline compounds such as imiquimod work as immune response modifiers with antiproliferative, antiparasite, antifungal, and antiviral activities that have been examined in several previous studies (van der Fits et al., 2009; de Sousa et al., 2014; Tio et al., 2018; Jabari et al., 2019). The cytotoxic effect of imiquimod against various cancers has been investigated, and it was shown that it induces apoptosis as well as increasing levels of the opioid growth factor receptor (OGFr) (Wybran and Plotnikoff, 1991; Zagon et al., 2008). It is also well established that imiquimod contributes to both innate and cell-mediated immunity through activation of toll-like receptor 7 (TLR7) macrophages, monocytes and dendritic cells (Hemmi et al., 2002; Jabari et al., 2019). Consequently, stimulation of immune cells by imiquimod leads to the production of cytokines such as interferon-α (IFN-α), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), essential to elicit immune responses (Buates and Matlashewski, 1999; Khamesipour, 2014). Although a number of studies have demonstrated the anti-parasitic properties of imiquimod (Mehravaran et al., 2020), there have been no investigations of its effect on Toxoplasma infections. In this regard, imiquimod alone or in combination with other compounds reduced proliferation of promastigotes and amastigotes of L. major in vitro and in-vivo. It was concluded that this drug could be useful to treatment of leishmaniasis by modulating the immune reaction preferentially toward a Th1-predominant state (Buates and Matlashewski, 1999, 2001; Mehravaran et al., 2020).
Jabari et al. (2019) investigated the in-vitro effect of imiquimod against L. major promastigotes and amastigotes. Imiquimod either singly or in combination had a pronounced effect in reduction and prevention of macrophage infection with amastigotes (Jabari et al., 2019). Ebrahimisadr et al. (2019) assessed antileishmanial effects of imiquimod on L. major using Real-Time PCR and reported a significant reduction in the mean lesion size in BALB/c mice. Additionally, their results showed a significant increase in expression of TLR2 and TLR4 genes in lesion RNA extracted from infected mice (Ebrahimisadr et al., 2018). The anti-leishmanial activity of monophosphoryl lipid A and imiquimod adjuvants and soluble Leishmania antigen in a nano-liposome carrier was evaluated by Emami et al. (2018), who reported that this combination could be an appropriate delivery system to induce the cellular immune pathway against L. major infection in BALB/c mice (Emami et al., 2018).
In the present study, the effectiveness of different concentrations of the imiquimod against T. gondii tachyzoites was investigated and showed imiquimod reduced the number of tachyzoites in a concentration- and time-dependent manner. Notably, the maximum tachyzoites death occurred with a imiquimod concentration of 0.01 μg/ml, after 24 h. Considerable toxicity was observed for T. gondii tachyzoites of treated with sulfadiazine plus pyrimethamine alone or in combination with imiquimod (SDZ+PYR+IQ). Toxicity of imiquimod in uninfected macrophages was dose-dependent; The MTT assay showed that as concentrations of imiquimod were decreased, viability of uninfected macrophages increased. It had previously been demonstrated that imiquimod could induce apoptosis in uninfected macrophages as well as parasites (Ebrahimisadr et al., 2018; Jabari et al., 2019). Induction of programed cell death occurred in both tachyzoites and in infected macrophages when treated with imiquimod, although these apoptotic effects were not remarkable. Also, necrosis was low in infected macrophages treated with imiquimod compared to healthy macrophages without treatment. Therefore, this drug may have positive effects against toxoplasmosis infection through augmentation of the immune response as well as expression of cell level receptors. Under in vitro conditions our experiment demonstrated that adjuvant and/or preventive effects of imiquimod can decrease the burden of parasites in macrophages. In this regard, when treatment of macrophages with imiquimod was done before infection, a remarked reduction in parasite burden was observed. Interestingly, the lowest parasite burden occurred with a concentration of 0.01 μg /mL imiquimod.
Macrophages as antigen-presenting cells are the primary site of action for imiquimod and have a pivotal role to recognize T. gondii-infected cells (Buates and Matlashewski, 2001; Innes et al., 2019). Therefore, it can be concluded that imiquimod has the ability to limit toxoplasmosis by generating a powerful immune response following induction of macrophages. In the murine model, the parasitic burden and mortality rate in BALB/c mice that received imiquimod before parasitic challenge was lower than those received drug after parasitic challenge. Overall, the lowest parasite burden and mortality rate was seen in mice treated with sulfadiazine plus pyrimethamine in combination with imiquimod (SDZ+PYR+IQ).
Both humoral and cellular immune responses must be stimulated in order to generate protection against T. gondii infection (Foroutan et al., 2019). In the mouse model, production of from immune system cells is required for both innate and adaptive immune responses. IFN-γ plays a fundamental role in limiting toxoplasmosis by enhancing the defensive function of macrophages and inducing a strong Th1 type immune response (Suzuki et al., 1988; Zhang et al., 2015; Innes et al., 2019). The present study indicated that a TH1-type cellular immune response was induced in all treated mice with production of IFN-γ and prolonged survival time compared to the untreated control group. In addition, the imiquimod treated mice before infection also displayed greater levels of IFN-γ compared to the control group. However, levels of IL-4 production were low among treated mice.
These results demonstrate a significant anti-Toxoplasma activity for imiquimod against T. gondii tachyzoites and infected macrophages. Imiquimod in the pretreated group had greater anti-Toxoplasma effects than imiquimod in post-treated group in vitro and in vivo. The in vivo experiments showed that it can trigger a strong immune response by enhancing IFN-γ level and survival time and by decreasing the parasite load in BALB/c mice. Therefore, it can be inferred that imiquimod could be used as an adjunctive treatment for the treatment of disease due to T. gondii. Imiquimod should therefore be considered for further study either alone or along with other anti-Toxoplasma products in future studies.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Ethical Committee of Tarbiat Modares University approved this study.
Author Contributions
FG was supervisor of this research, conceived the study, and designed the study protocol. LZ performed the experiments, analyzed the data, and drafted the manuscript. JH the advisor of project, designed the study protocol and critically revised the manuscript. ZS and JS were advisors of the research. All authors read and approved the final version of the manuscript.
Funding
This project was financially supported by Tarbiat Modares University, Iran.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all staff of Department of Parasitology of Tarbiat Modares University, Iran. This paper is issued from thesis of LZ, Ph.D. student of Medical Parasitology.
References
Buates, S., and Matlashewski, G. (1999). Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: efficacy and mode of action. J. Infect. Dis. 179, 1485–1494. doi: 10.1086/314782
Buates, S., and Matlashewski, G. (2001). Identification of genes induced by a macrophage activator, S-28463, using gene expression array analysis. Antimicrob Agents Chemother. 45, 1137–1142. doi: 10.1128/AAC.45.4.1137-1142.2001
de Sousa, M. D. G. T., Belda, W. Jr., Spina, R., Lota, P. R., Valente, N. S., Brown, G. D., et al. (2014). Topical application of imiquimod as a treatment for chromoblastomycosis. Clin. Infect. Dis. 58, 1734–1737. doi: 10.1093/cid/ciu168
Dockrell, D., and Kinghorn, G. (2001). Imiquimod and resiquimod as novel immunomodulators. J. Antimicrob. Chemother. 48, 751–755. doi: 10.1093/jac/48.6.751
Dubey, J. P., Cerqueira-Cézar, C. K., Murata, F. H. A., Verma, S. K., Kwok, O. C. H., Pedersen, K., et al. (2020). White-tailed deer (Odocoileus virginianus) are a reservoir of a diversity of Toxoplasma gondii strains in the USA and pose a risk to consumers of undercooked venison. Parasitology 147, 775–781. doi: 10.1017/S0031182020000451
Ebrahimisadr, P., Ghaffarifar, F., Horton, J., Dalimi, A., and Sharifi, Z. (2018). Apoptotic effect of morphine, imiquimod and nalmefene on promastigote, infected and uninfected macrophages with amastigote of Leishmania major by flow cytometry. Iran J. Pharm. Res. 17, 986–994.
Ebrahimisadr, P., Ghaffarifar, F., Horton, J., Dalimi, A., and Sharifi, Z. (2019). Evaluation of the effect of morphine and imiquimodon expression of TLR2 and TLR4 from lesion RNA extracted from BALB/c mice infected with leishmania major. Avicenna J. Med. Biotechnol. 11, 202–205.
Emami, T., Rezayat, S. M., Khamesipour, A., Madani, R., Habibi, G., and Hojatizade, M. (2018). The role of MPL and imiquimod adjuvants in enhancement of immune response and protection in BALB/c mice immunized with soluble Leishmania antigen (SLA) encapsulated in nanoliposome. Artif Cells Nanomed. Biotechnol. 46, 324–333. doi: 10.1080/21691401.2018.1457042
Fallahi, S., Rostami, A., Shiadeh, M. N., Behniafar, H., and Paktinat, S. (2018). An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J. Gynecol. Obstet. Hum. Reprod. 47, 133–140. doi: 10.1016/j.jogoh.2017.12.003
Fishman, J. A. (2013). Opportunistic infections—coming to the limits of immunosuppression? Cold Spring Harb. Perspect. Med. 3:a015669. doi: 10.1101/cshperspect.a015669
Foroutan, M., Zaki, L., Tavakoli, S., Soltani, S., Taghipour, A., and Ghaffarifar, F. (2019). Rhomboid antigens are promising targets in the vaccine development against Toxoplasma gondii. EXCLI J. 18:259. doi: 10.17179/excli2018-1993
Foroutan, M., Zaki, L., Tavakoli, S., Soltani, S., Taghipour, A., and Ghaffarifar, F. (2019). Rhomboid antigens are promising targets in the vaccine development against Toxoplasma gondii. EXCLI J. 18:259.
Geisse, J., Caro, I., Lindholm, J., Golitz, L., Stampone, P., and Owens, M. (2004). Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J. Am. Acad. Dermatol. 50, 722–733. doi: 10.1016/j.jaad.2003.11.066
Gharavi, M., Jalali, S., Khademvatan, S., and Heydari, S. (2011). Detection of IgM and IgG anti-Toxoplasma antibodies in renal transplant recipients using ELFA, ELISA and ISAGA methods: comparison of pre-and post-transplantation status. Ann. Trop. Med. Parasitol. 105, 367–371. doi: 10.1179/1364859411Y.0000000022
Gigley, J. P. (2016). The diverse role of NK cells in immunity to Toxoplasma gondii infection. PLoS Pathog. 12:e1005396. doi: 10.1371/journal.ppat.1005396
Hemmi, H., Kaisho, T., Takeuchi, O., Sato, S., Sanjo, H., Hoshino, K., et al. (2002). Small anti-viral compounds activate immune cells via the TLR7 MyD88–dependent signaling pathway. Nat. Immunol. 3, 196–200. doi: 10.1038/ni758
Innes, E. A., Hamilton, C., Garcia, J. L., Chryssafidis, A., and Smith, D. (2019). A one health approach to vaccines against Toxoplasma gondii. Food Waterborne Parasitol. 15:e00053. doi: 10.1016/j.fawpar.2019.e00053
Jabari, J., Ghaffarifar, F., Horton, J., Dalimi, A., and Sharifi, Z. (2019). Evaluation of morphine with imiquimod as opioid growth factor receptor or nalmefene as opioid blocking drug on leishmaniasis caused by leishmania major in vitro. Iran J. Parasitol. 14, 394–403. doi: 10.18502/ijpa.v14i3.1478
Khamesipour, A. (2014). Therapeutic vaccines for leishmaniasis. Expert Opin. Biol. Ther. 14, 1641–1649. doi: 10.1517/14712598.2014.945415
Maubon, D., Bougdour, A., Wong, Y.-S., Brenier-Pinchart, M.-P., Curt, A., Hakimi, M.-A., et al. (2010). Activity of the histone deacetylase inhibitor FR235222 on Toxoplasma gondii: inhibition of stage conversion of the parasite cyst form and study of new derivative compounds. Antimicrob. Agents Chemother. 54, 4843–4850. doi: 10.1128/AAC.00462-10
Mehravaran, A., Mirahmadi, H., and Akhtari, J. (2020). Liposomes containing the imiquimod adjuvant as a vaccine in the cutaneous leishmaniasis model. Nanomed. J. 7, 29–39. doi: 10.22038/nmj.2020.07.04
Mendez, O. A., and Koshy, A. A. (2017). Toxoplasma gondii: entry, association, and physiological influence on the central nervous system. PLoS Pathog. 13:351. doi: 10.1371/journal.ppat.1006351
Mikaeiloo, H., Ghaffarifar, F., Dalimi, A., Sharifi, Z., and Hassan, Z. M. (2016). Apoptotic activity and anti-Toxoplasma effects of artemether on the tachyzoites and experimental infected Vero and J774 cell lines by Toxoplasma gondii. Indian J. Pharmacol. 48,179–185. doi: 10.4103/0253-7613.178838
Miller, R., Gerster, J., Owens, M., Slade, H., and Tomai, M. (1999). Review article imiquimod applied topically: a novel immune response modifier and new class of drug. Int. J. Immunopharmacol. 21, 1–14. doi: 10.1016/S0192-0561(98)00068-X
Montazeri, M., Ebrahimzadeh, M. A., Ahmadpour, E., Sharif, M., Sarvi, S., and Daryani, A. (2016). Evaluation of propranolol effect on experimental acute and chronic toxoplasmosis using quantitative PCR. Antimicrobial. Agents Chemotherapy. 60, 7128–7133. doi: 10.1128/AAC.01323-16
Montoya, J. G., and Gomez, C. A. (2016). “Toxoplasmosis after solid organ transplantation,” in Transplant Infections. 4th Edn, eds D. S. Per Ljungman and M. Boeckh (Springer), 781–793. doi: 10.1007/978-3-319-28797-3_43
Pinzan, C. F., Sardinha-Silva, A., Almeida, F., Lai, L., Lopes, C. D., Lourenço, E. V., et al. (2015). Vaccination with recombinant microneme proteins confers protection against experimental toxoplasmosis in mice. PLoS ONE 10:e0143087. doi: 10.1371/journal.pone.0143087
Sasai, M., Pradipta, A., and Yamamoto, M. (2018). Host immune responses to Toxoplasma gondii. Int. Immunol. 30, 113–119. doi: 10.1093/intimm/dxy004
Sidky, Y. A., Borden, E. C., Weeks, C. E., Reiter, M. J., Hatcher, J. F., and Bryan, G. T. (1992). Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 52, 3528–3533.
Stanley, M. (2002). Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 27, 571–577. doi: 10.1046/j.1365-2230.2002.01151.x
Suzuki, Y., Orellana, M. A., Schreiber, R. D., and Remington, J. S. (1988). Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240, 516–518. doi: 10.1126/science.3128869
Tio, D., Kirtschig, G., Hoekzema, R., and Van Montfrans, C. (2018). Lymphoedema in patients with lentigo maligna treated with imiquimod: a long-term adverse effect. Br. J. Dermatol. 178, 1441–1442. doi: 10.1111/bjd.16267
van der Fits, L., Mourits, S., Voerman, J. S., Kant, M., Boon, L., Laman, J. D., et al. (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845. doi: 10.4049/jimmunol.0802999
Wang, Z.-D., Liu, H.-H., Ma, Z.-X., Ma, H.-Y., Li, Z.-Y., Yang, Z.-B., et al. (2017). Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta-analysis. Front. Microbiol. 8:389. doi: 10.3389/fmicb.2017.00389
Wybran, J., and Plotnikoff, N. (1991). Methionine Enkephalin, A New Lymphokine for the Treatment of ARC Patients. Stress and Immunity. (Boca Raton, FL: CRC Press).
Zagon, I. S., Donahue, R. N., Rogosnitzky, M., and Mclaughlin, P. J. (2008). Imiquimod upregulates the opioid growth factor receptor to inhibit cell proliferation independent of immune function. Exp. Biol. Med. (Maywood). 233, 968–979. doi: 10.3181/0802-RM-58
Zaki, L., Ghaffarifar, F., Sharifi, Z., Horton, J., and Sadraei, J. (2020). Toxoplasma gondii: preventive and therapeutic effects of morphine and evaluation of treatment parameters of tachyzoites and infected macrophages in vitro and in a murine model. EXCLI J. 19, 514–527. doi: 10.17179/excli2019-1961
Keywords: Toxoplasma gondii, imiquimod, macrophages, in vivo, in vitro
Citation: Zaki L, Ghaffarifar F, Sharifi Z, Horton J and Sadraei J (2020) Effect of Imiquimod on Tachyzoites of Toxoplasma gondii and Infected Macrophages in vitro and in BALB/c Mice. Front. Cell. Infect. Microbiol. 10:387. doi: 10.3389/fcimb.2020.00387
Received: 30 March 2020; Accepted: 24 June 2020;
Published: 31 July 2020.
Edited by:
Shuai Wang, University of Pennsylvania, United StatesReviewed by:
Bellisa Freitas Barbosa, Federal University of Uberlandia, BrazilRenato Augusto DaMatta, State University of the North Fluminense Darcy Ribeiro, Brazil
Mahbobeh Montazeri, Mazandaran University of Medical Sciences, Iran
Copyright © 2020 Zaki, Ghaffarifar, Sharifi, Horton and Sadraei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatemeh Ghaffarifar, ghafarif@modares.ac.ir
 Leila Zaki
Leila Zaki