
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Chem. , 03 August 2020
Sec. Organic Chemistry
Volume 8 - 2020 | https://doi.org/10.3389/fchem.2020.00582
This article is part of the Research Topic New Hypervalent Iodine Reagents for Oxidative Coupling View all 10 articles
The modification of quinoxalin-2(1H)-ones via direct C-H bond functionalization has begun to receive widespread attention, due to quinoxalin-2(1H)-one derivatives' various biological activities and pharmaceutical properties. This mini review concentrates on the accomplishments of arylation, trifluoromethylation, alkylation, and alkoxylation of quinoxalin-2(1H)-ones with hypervalent iodine(III) reagents as reaction partners or oxidants. The reaction conditions and mechanisms are compared and discussed in detail.
In recent years, direct C-H functionalization has become one of the most popular topics due to its advantages of having a high bonding efficiency and good atomic economy, and some remarkable achievements having been accomplished in this field (Ackermann et al., 2009; Yang et al., 2017; Yi et al., 2017). Among these significant works, hypervalent iodine reagents have been widely used as ideal and highly efficient oxidants or reaction partners (Kita et al., 1994; Nasrallah et al., 2019) due to their superior bench stability, high reactivity, low toxicity, environmental friendliness, ease of operation, and ready availability (Dohi et al., 2009; Sun and Shi, 2014). For instance, diverse iodine(III) reagents were invented to introduce fluorinated group into organic molecules (Yang et al., 2013; Matsuzaki et al., 2014; Suzuki et al., 2014; Das and Shibata, 2017; Das et al., 2017; Wang et al., 2017). Because of the large size of iodine atoms, a linear three-center, four-electron (3c-4e) bond (L–I–L) which uses a non-hybridized 5p orbital of iodine atom is formed. This 3c-4e bond, termed a “hypervalent bond,” is highly polarized, longer, and weaker than normal covalent bonds, so the hypervalent iodine compounds have high electrophilic reactivity (Zhdankin and Stang, 2008). The distinctive reactivities of hypervalent iodine compounds are similar to those of heavy metals such as leadIV, mercuryII, cadmiumIV, and thalliumIII. However, compared with heavy metals, iodine is greener and cheaper (Yoshimura and Zhdankin, 2016), and the annual production of iodine reagents is 30,000 tons (Yusubov and Zhdankin, 2015). It is promising that hypervalent iodine compounds can be an environmentally sustainable alternative to heavy metals. The different hypervalent iodine reagents vary in properties. For instances, iodosobenzene and its derivatives have strong oxidability and can replace many toxic oxidants in various oxidation reactions; iodonium salts have no significant oxidative capacity but can react in various ways due to the special leaving ability of the -IAr fragment. Iodonium ylides and imides are excellent carbene and nitrene precursors, respectively, while heterocyclic iodanes have a higher stability than their acyclic analogs, which makes it possible to separate them and makes them a good alternative to several unstable iodine derivatives (Zhdankin and Stang, 2002; Stang, 2003; Wirth, 2005; Küpper et al., 2011).
On the other hand, quinoxalin-2(1H)-one is a privileged structural motif found in various natural active products and drug molecules (Liu et al., 2011; Galal et al., 2014; Pereira et al., 2015). 3-substituted quinoxalinone derivatives specifically have attracted much attention because of their distinctive biological and pharmacological activities. For instance, CFTRact-J027 is a safe and efficient CFTR (cystic fibrosis transmembrane conductance regulator) activator which increases intestinal fluid secretion (Cil et al., 2016), and ML281 is a nanomolar STK33 inhibitor that selectively kills KRAS cancers (Weïwer et al., 2012). Some bioactive molecules containing quinoxalin-2(1H)-one skeleton, such as Compounds 1-3, also show potential applications in medicinal chemistry fields (Meyer et al., 2006; Khattab et al., 2015; Qin et al., 2015) (Figure 1A). Because of their synthetic usefulness and potential biological importance, the introduction of functional groups into the C3-position of the quinoxalin-2(1H)-ones has already become a research hotspot, and various protocols for the direct C3-H functionalization of quinoxalin-2(1H)-ones have been reported (Ebersol et al., 2019; Gu et al., 2019; Hong et al., 2019; Li et al., 2019; Peng et al., 2019; Rostoll-Berenguer et al., 2019; Teng et al., 2019; Wang et al., 2019a, 2020; Xie et al., 2019b; Zhao et al., 2019; Zheng and Studer, 2019; Tian et al., 2020; Yuan et al., 2020). In particular, the C3-H functionalization of quinoxalin-2(1H)-ones involving hypervalent iodine reagents has drawn wide attention for the aforementioned advantages of hypervalent iodine reagents, mainly including arylation (Paul et al., 2017; Yin and Zhang, 2017), trifluoromethylation (Wang et al., 2018; Xue et al., 2019a), alkylation (Wang et al., 2019b; Xie et al., 2019a; Xue et al., 2019b; Shen et al., 2020), and alkoxylation (Xu et al., 2019; Yang et al., 2019) of quinoxalin-2(1H)-ones, which provide convenient and environmentally friendly means for the synthesis of 3-substituted quinoxalinone derivatives. In this mini review, we will focus on the progress being made in the direct C3-H functionalization of quinoxalin-2(1H)-ones involving the hypervalent iodine reagents and discuss their mechanisms, in order to inspire more applications of hypervalent iodine reagents in related reactions.
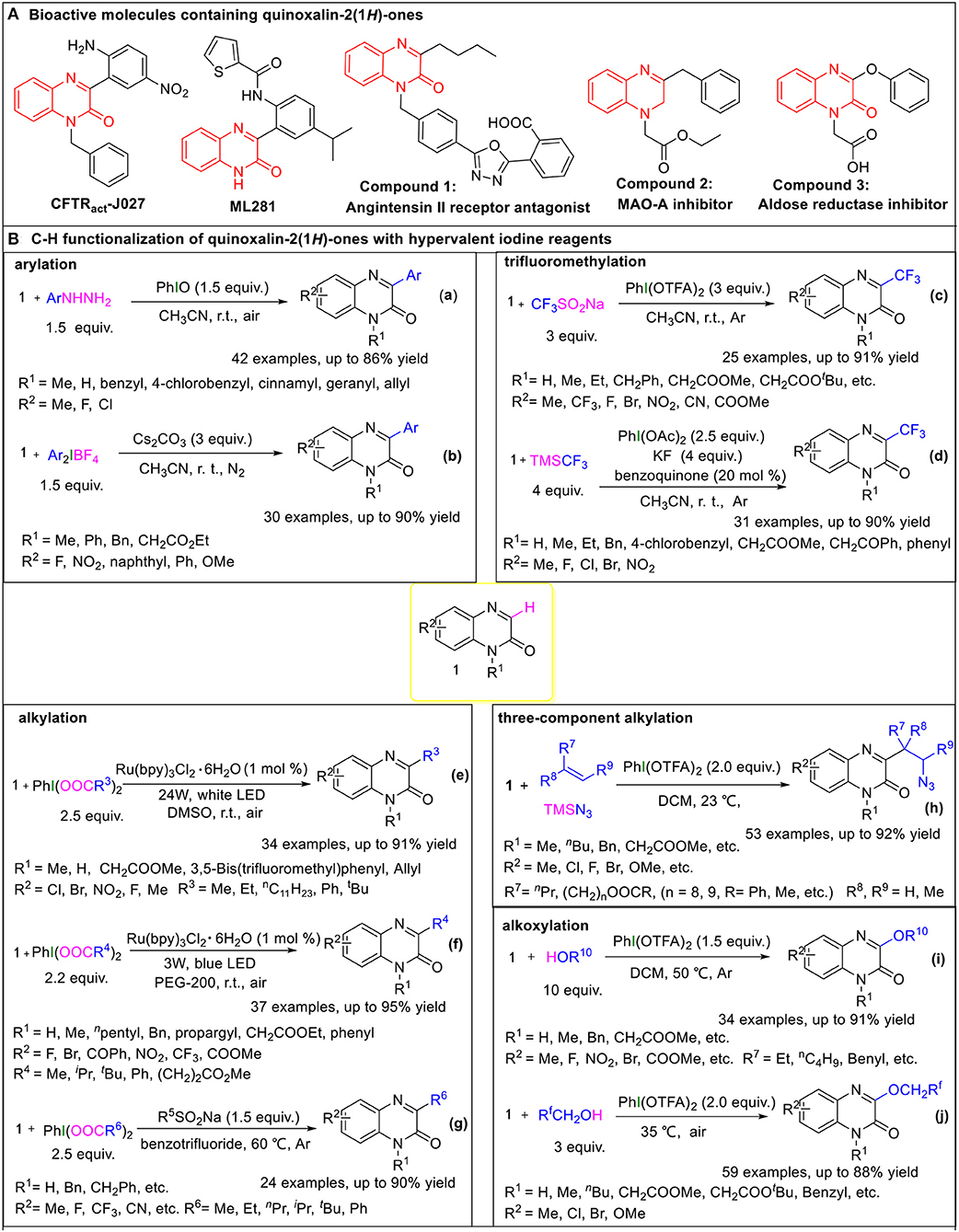
Figure 1. (A) Bioactive molecules containing quinoxalin-2(1H)-ones. (B) C-H functionalization of quinoxalin-2(1H)-ones with hypervalent iodine reagents.
Due to the distinctive pharmaceutical and electrical activities of 3-arylquinoxalin-2(1H)-ones, various methods for the direct C3-H arylation of quinoxalin-2(1H)-ones have been reported, involving two ones taking advantage of hypervalent iodine reagents.
In March 2017, Paul et al. reported on iodosobenzene-promoted oxidative C3-arylation of quinoxalin-2(1H)-ones with arylhydrazines, which is a widely used aryl radical source (Ravi et al., 2015; Rossi et al., 2015) (Figure 1B, Equation a) (Paul et al., 2017). The transformation afforded a variety of 3-arylquinoxalin-2(H)-one derivatives in moderate to good yields with a broad substrate scope, and the reaction conditions were mild, using only iodosobenzene as the oxidant. This protocol developed a new system for the synthesis of 3-arylquinoxalin-2(1H)-one derivatives. The aryl-TEMPO adduct was detected in the presence of the radical trapping reagent (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO) under the standard conditions, indicating that an aryl radical was involved in the reaction. Based on the experimental results, a plausible mechanism was proposed by the authors (Figure 2A). First, arylhydrazine adds to PhIO to form the iodine(III) active species 2, which then eliminates the H2O and PhI to give aryldiazene 3. Subsequently, the intermediate 3 is oxidized by oxygen to afford the diazenyl radical 4. The radical 4 releases molecular nitrogen to generate aryl radical 5, which reacts with quinoxalin-2(H)-one 1 to form the intermediate 6. Eventually, the intermediate 6 is oxidized by the hydroperoxyl radicals generated in the process of the formation of 4 to provide the final product 7.
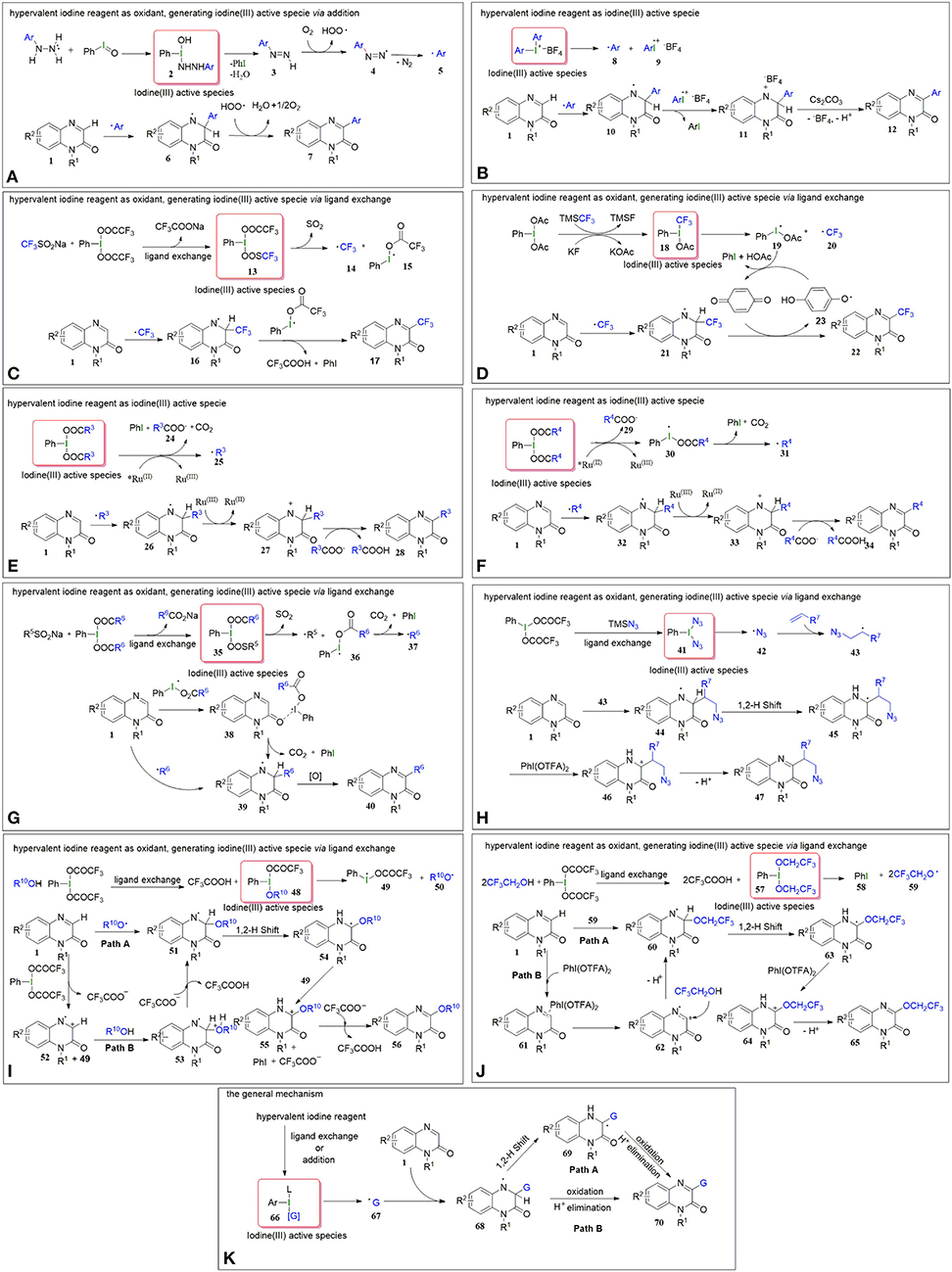
Figure 2. (A) The possible mechanism of C-H arylation (a) of Quinoxalin-2(1H)-ones. (B) The possible mechanism of C-H arylation (b) of Quinoxalin-2(1H)-ones. (C) The possible mechanism of C-H trifluoromethylationn (c) of Quinoxalin-2(1H)-one. (D) The possible mechanism of C-H trifluoromethylationn. (d) of Quinoxalin-2(1H)-ones. (E) The possible mechanism of C-H alkylation (e) of Quinoxalin-2(1H)-ones. (F) The possible mechanism of C-H alkylation (f) of Quinoxalin-2(1H)-ones. (G) The possible mechanism of C-H alkylation (g) of Quinoxalin-2(1H)-ones. (H) The possible mechanism of C-H alkylation (h) of Quinoxalin-2(1H)-ones. (I) The possible mechanism of C-H alkoxylation (i) of Quinoxalin-2(1H)-ones. (J) The possible mechanism of C-H alkoxylation (j) of Quinoxalin-2(1H)-ones. (K) A general mechanism for these reactions.
In the same year, Zhang group reported a method for C3-H arylation of quinoxalin-2(1H)-ones to give 3-arylquinoxalin-2(1H)-ones with diaryliodonium salts (Figure 1B, Equation b) (Yin and Zhang, 2017). Diaryliodonium salts are generally used in organic synthesis as aryl radical sources due to their easy availability (Yamaoka et al., 2013; Wang D. et al., 2014). This protocol could tolerate a series of readily available diaryliodonium salts and quinoxalin-2(1H)-ones, indicating its broad substrate scope. A possible mechanism was proposed for the coupling reaction based on the results of radical trapping with TEMPO (Figure 2B). Initially, aryl radical 8 is generated via the decomposition of diaryliodonium salt, and then immediately adds to quinoxalin-2(1H)-one to form the nitrogen radical 10. The nitrogen radical 10 is then oxidized by high-valence iodonium salt 9 to produce the intermediate 11, which eliminates the H+ and in the presence of Cs2CO3 to give the final product 12.
Because of the special biological and drug activities of 3-trifluoromethylquinoxalin-2(1H)-one derivatives (Patel et al., 2000; Carta et al., 2006), it is necessary to explore efficient, simple, and mild methods to synthesize the 3-trifluoromethylquinoxalin-2(H)-ones. In 2018, we employed sodium trifluoromethanesulfinate (Langlois reagent) as a trifluoromethyl source and successfully realized the C3-H trifluoromethylation of quinoxalin-2(H)-ones under mild and transition metal-free conditions (Figure 1B, Equation c) (Wang et al., 2018). Due to the low-cost of sodium trifluoromethanesulfinate and easy availability of hypervalent iodine reagents, this protocol offers simple, efficient, and cheap access to 3-trifluoromethylquinoxalin-2(H)-ones. When the reaction was performed in the presence of the radical trapping reagent 1,1-diphenylethylene (DPE), the target product (3-trifluoromethylquinoxalin-2(H)-one) was isolated only in a 46% yield, and a DPE-CF3 coupled byproduct (3,3,3-trifluoroprop-1-ene-1,1-diyl)dibenzene was observed, which suggested that the reaction followed a radical mechanism (Figure 2C). First, [bis(trifluoroacetoxy)iodo]benzene (PhI(OTFA)2) undergoes anion exchange with sodium trifluoromethanesulfinate to afford iodine(III) active species 13, which then releases sulfur dioxide to generate trifluoromethyl radical 14 and I-radical 15. Thereafter, trifluoromethyl radical 14 reacts with quinoxalin-2(1H)-ones to afford the intermediate 16, which eventually goes through oxidization and H-elimination to form the final product 17.
Soon afterwards, in March 2019, Xue et al. reported a method to synthesize 3-trifluoromethylquinoxalin-2(1H)-ones with (trifluoromethyl)trimethylsilane (Ruppert-Prakash reagent) as a trifluoromethyl source under transition metal-free conditions (Figure 1B, Equation d) (Xue et al., 2019a). This protocol has the advantages of using cheaper (diacetoxyiodo)benzene(PhI(OAc)2)compared with PhI(OTFA)2 as an oxidant and stable (trifluoromethyl)trimethylsilane as the trifluoromethyl source. When TEMPO was added into the model reaction as a radical scavenger, the desired transformation was completely suppressed, while the TEMPO-CF3 adduct was obtained in a 70% yield. Based on this experiment's results, the following possible mechanism was proposed (Figure 2D). Initially, the PhI(OAc)2 reacts with KF and TMSCF3 to generate the iodine(III) active species 18, which then decomposes to I-radical 19 and trifluoromethyl radical 20. Subsequently, trifluoromethyl radical 20 attacks quinoxalin-2(1H)-one to form intermediate 21, which then oxidizes by 1,4-benzoquinone to form the final product 22, with the 1,4-benzoquinone converting to phenoxy radical 23. Finally, the phenoxy radical 23 recovers to 1,4-benzoquinone via oxidation of the I-radical 19.
As we know, phenyliodine(III) dicarboxylates have been regarded as effective reagents for the introduction of alkyl groups into organic molecules through a radical decarboxylation procedure (Togo and Katohgi, 2001; Lu et al., 2018). Besides, phenyliodine(III) dicarboxylate reagents could be easily prepared and stored (Stang et al., 1988; Mocci et al., 2007). So, several strategies for the synthesis of 3-alkylquinoxalin-2(1H)-ones with phenyliodine(III) dicarboxylates as the alkyl sources have been developed.
Very recently, Xue et al. developed a method to synthesize 3-alkylquinoxalin-2(1H)-ones with phenyliodine(III) dicarboxylates under visible-light conditions (Figure 1B, Equation e) (Xue et al., 2019b). The use of cheap and readily available phenyliodine(III) dicarboxylates as the alkylation reagents and mild reaction conditions make this protocol convenient and efficient in the synthesis of 3-alkylquinoxalin-2(1H)-ones. Two control experiments were carried out to probe into the mechanism. One was to use DMSO-d6 instead of DMSO as solvent. In this case the product was 3-methylquinoxalin-2(1H)-one but not the deuterated methyl substituted quinoxalin-2(1H)-one, which illustrated that the methyl group came from PhI(OAc)2 rather than solvent DMSO. The other one was to run the reaction in the presence of TEMPO under the standard conditions. The TEMPO-CH3 adduct was detected, indicating that the reaction was a radical process. Based on these control experiments, a plausible mechanism for this alkylation was proposed, as shown in Figure 2E. First, the catalyst Ru(II) is irradiated by visible light to produce the excited state *Ru(II), which promotes the decarboxylation of PhI(OOCR3)2 to generate alkyl radical 25. Subsequently, the alkyl radical 25 regio-selectively attacks the quinoxalin-2(1H)-ones to provide intermediate 26, which oxidizes by Ru(III) to transform into cation 27, regenerating catalyst Ru(II). Eventually, the cation 27 undergoes dehydrogenation with the assistance of carboxylate anion 24 to furnish the final product 28.
In the same year, He and coworkers also reported a method for the synthesis of 3-alkylquinoxalin-2(1H)-ones utilizing phenyliodine(III) dicarboxylates as the alkyl sources under visible-light conditions (Figure 1B, Equation f) (Xie et al., 2019a). And, more remarkable, this protocol uses eco-friendly PEG-200 as a reaction solvent. Based on the radical trapping results with dibutylhydroxytoluene (BHT) or TEMPO, a probable mechanism similar to the former was proposed (Figure 2F).
Alternatively, we accomplished the synthesis of 3-alkylquinoxalin-2(1H)-ones with phenyliodine(III) dicarboxylates as alkyl agents mediated by sodium alkylsulfinates under mild conditions (Figure 1B, Equation g) (Wang et al., 2019b). This protocol does not need any expensive metal catalysts, endowing it with an efficient process for the synthesis of 3-alkylquinoxalin-2(1H)-ones without metallic residues. Based on radical trapping experimental results with TEMPO and previous studies, a plausible mechanism was proposed, as given in Figure 2G. Initially, the anion exchange of PhI(O2CR6)2 with R5SO2Na generates the iodine(III) active species 35, which then turns into radical R5 and I-radical 36, excluding SO2 at the same time. Then I-radical 36 decomposes into alkyl radical 37 and PhI, along with the release of CO2. Next, in the presence of I-radical 36, quinoxalin-2(1H)-one transforms into intermediate 38, which undergoes decarboxylation to generate intermediate 39. Meanwhile, the attack of alkyl radical 37 on the quinoxalin-2(1H)-one can also produce intermediate 39, which is another path to producing intermediate 39. Finally, intermediate 39 transforms to final product 40 via oxidization and dehydrogenation.
In short, the above three protocols for the alkylation of quinoxalin-2(1H)-ones showed superiority in functional group tolerance under mild reaction conditions. And yet the first two featured the efficient and sustainable visible-light-induced reaction systems, while the third one displayed the character of transition metal-free conditions.
In 2020, Zhang's group developed a hypervalent iodine(III)-promoted three-component alkylation of quinoxalin-2(1H)-ones with unactivated alkenes and TMSN3 (Figure 1B, Equation h) (Shen et al., 2020). This method provides a step-economical solution for the introduction of β-azido alkyl groups into the quinoxalin-2(1H)-ones to rapidly synthesize bioactive organoazides. The various substituted quinoxalin-2(1H)-ones bearing electron-rich or electron-deficient groups, and olefins with functional groups including ester or hydroxyl substituents, were well-compatible in this transformation. A radical mechanism is proposed in Figure 2H. A double ligand exchange between PhI(OTFA)2 and TMSN3 furnishes iodine(III) active species, which provides an azide radical (42) via the homolytic cleavage of I-N bond (Matcha et al., 2013). Then, 42 chemoselectively attacks olefins to generate alkyl radical intermediate 43, which is trapped by substrate 1 to afford nitrogen radical intermediate 44. Afterwards, 44 undergoes a 1,2-H shift process to afford carbon radical 45. Finally, the target product 47 was obtained via the sequential procedures of oxidation and deprotonation from 45.
Alkoxy groups are widely present in various natural active products and drug molecules (Han et al., 2018; Zhang et al., 2019), but the methods for the introduction of alkoxy groups in quinoxalin-2(1H)-ones have been rarely reported. In 2019, we disclosed a method to synthesize 3-alkoxyquinoxalin-2(1H)-ones with alcohols and PhI(OTFA)2 under mild conditions (Figure 1B, Equation i) (Yang et al., 2019). This protocol provides easy access to 3-alkoxyquinoxalin-2(1H)-ones by using readily available and low cost alcohols as the alkoxy sources, and cheap PhI(OTFA)2 as the oxidant, and shows good functional-group tolerance. Similarly, either BHT or TEMPO was employed as the radical trapping agent to explore information on the reaction mechanism. The results indicated that a radical mechanism could be involved in this alkoxylation process (Figure 2I). First, the ligand exchange of PhI(OTFA)2 with alcohol takes place to afford the iodine(III) active species 48, which then decomposes into I-radical 49 and alkoxy radical 50. Subsequently, alkoxy radical 50 adds to quinoxalin-2(1H)-ones to generate the nitrogen radical 51. There is also another way to form the intermediate 51, namely, quinoxalin-2(1H)-one reacts with PhI(OTFA)2 to generate radical cation 52, and radical cation 52 is captured by nucleophilic alcohols to form oxonium intermediate 53 which undergoes deprotonation to afford the intermediate 51 with the assistance of trifluoroacetate. The nitrogen radical 51 converts to carbon radical 54 via 1,2-H shift process, which goes through oxidation and deprotonation successively to transform into final product 56.
In addition, fluorine-containing units have been found in various drugs and nature products and are widely used in the pharmaceutical industry and in material science (Liang et al., 2013; Wang J. et al., 2014). Fluoroalkoxyl aryl ethers have also become a research hotspot because of their special pharmaceutical and biological activities. In June 2019, Zhang group reported a method to synthesize 3-fluoroalkoxylquinoxalin-2(1H)-ones with fluoroalkyl-alcohols and PhI(OTFA)2 under catalyst-free and solvent-free conditions (Figure 1B, Equation j) (Xu et al., 2019). The use of commercially available fluoroalkyl alcohols as the fluoroalkoxyl sources, and convenient PhI(OTFA)2 as the oxidant in the absence of a catalyst and solvent, endows this novel strategy with environmental friendliness and efficiency for the direct C3-H fluoroalkoxylation of quinoxalin-2(1H)-ones. The observation of TEMPO-OCH2CF3 or DPE-OCH2CF3 adducts in the radical trapping experiments revealed that a radical pathway may be involved in the reaction. The probable mechanism of the fluoroalkoxylation is similar to the aforementioned alkoxylation of quinoxalin-2(1H)-ones (Figure 2J).
In this mini review, we summarized recent efforts on direct C3-H functionalization of quinoxalin-2(1H)-ones with the commercially available and environmentally benign hypervalent iodine reagents, mainly including arylation, trifluoromethylation, alkylation, and alkoxylation. The accomplishments have provided us with simple, mild, efficient, and eco-friendly methods for the synthesis of various C3-substituted quinoxalin-2(1H)-ones. Herein, the hypervalent iodine reagents play two different roles, reaction partners (equations b, e, f, and g), and oxidants (equations a, c, d, h, Io, and j). A general mechanism for these reactions could be given (Figure 2K). For all the reactions, iodine(III) active species are initial key intermediates. In the case of hypervalent iodine reagents as oxidants, they must initially transform to the iodine(III) active species 66 via ligand exchange (equations c, d, h, i, and j) or addition (equation a); in the case of hypervalent iodine reagents as reaction partners, they are the iodine(III) active species 66 themselves (equations b, e, f, and g). Once the formation or introduction of iodine(III) active species occurs, they decompose to afford the radical 67 (G = aryl, trifluoromethyl, alkyl, azide and alkoxyl radical), which regio-selectively adds on to the N=C bond of quinoxalin-2(1H)-one to provide the carbon radical intermediate 68 (not including the azide radical). In some situations, the intermediate 68 transforms into nitrogen radical intermediate 69 via a 1,2-H shift process. Finally, the target product 70 is obtained via the successive oxidation and H+-elimination of the carbon radical intermediate 68 or nitrogen radical intermediate 69.
There are still some limitations in the reactions involving hypervalent iodine reagents. For instance, superstoichiometric hypervalent iodine reagents or noble transition-metal-catalysts are required in some cases. Also, the application scope of hypervalent iodine reagents is slightly narrow; they have only been successful in the arylation, trifluoromethylation, alkylation, and alkoxylation of quinoxalin-2(1H)-ones up till now. It is desirable to develop efficient reaction systems and expand on more reaction models such as the acylation, alkoxycarbonylation, amination, sulfonation, and phosphonation of quinoxalin-2(1H)-ones involving hypervalent iodine reagents.
YT, JW, and H-YZ collected the related references and prepared the manuscript. YZ and JZ directed the preparation of this manuscript. All authors critically reviewed the text and figures prior to submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21776056) and the Natural Science Foundation of Hebei Province (CN) (Grant No. B2018202253).
Ackermann, L., Vicente, R., and Kapdi, A. R. (2009). Transition-metal-catalyzed direct arylation of (hetero)arenes by C-H bond cleavage. Angew. Chem. Int. Ed. 48, 9792–9826. doi: 10.1002/anie.200902996
Carta, A., Piras, S., Loriga, G., and Paglietti, G. (2006). Chemistry, biological properties and SAR analysis of quinoxalinones. Mini-Rev. Med. Chem. 6, 1179–1200. doi: 10.2174/138955706778742713
Cil, O., Phuan, P.-W., Lee, S., Tan, J., Haggie, P. M., Levin, M. H., et al. (2016). CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell. Mol. Gastroenterol. Hepatol. 2, 317–327. doi: 10.1016/j.jcmgh.2015.12.010
Das, P., and Shibata, N. (2017). Electrophilic triflyl-arylation and triflyl-pyridylation by unsymmetrical aryl/pyridyl-λ3-iodonium salts: synthesis of aryl and pyridyl triflones. J. Org. Chem. 82, 11915–11924. doi: 10.1021/acs.joc.7b01690
Das, P., Takada, M., Matsuzaki, K., Saitob, N, and Shibata, N. (2017). SF5-pyridylaryl-λ3-iodonium salts and their utility as electrophilic reagents to access SF5?pyridine derivatives in the late-stage of synthesis. Chem. Commun. 53, 3850–3853. doi: 10.1039/C7CC01043E
Dohi, T., Ito, M., Yamaoka, N., Morimoto, K., Fujioka, H., and Kita, Y. (2009). Hypervalent iodine(III), selective and efficient single-electron-transfer (SET) oxidizing agent. Tetrahedron 65, 10797–10815. doi: 10.1016/j.tet.2009.10.040
Ebersol, C., Rocha, N., Penteado, F., Silva, M. S., Hartwig, D., Lenardão, E. J., et al. (2019). A niobium-catalyzed coupling reaction of α-keto acids with ortho-phenylenediamines: synthesis of 3-arylquinoxalin-2(1H)-ones. Green Chem. 21, 6154–6160. doi: 10.1039/C9GC02662B
Galal, S. A., Khairat, S. H. M., Ragab, F. A. F., Abdelsamie, A. S., Ali, M. M., Soliman, S. M., et al. (2014). Design, synthesis and molecular docking study of novel quinoxalin-2(1H)-ones as anti-tumor active agents with inhibition of tyrosine kinase receptor and studying their cyclooxygenase-2 activity. Eur. J. Med. Chem. 86, 122–132. doi: 10.1016/j.ejmech.2014.08.048
Gu, Y.-R., Duan, X.-H., Chen, L., Ma, Z.-Y., Gao, P., and Guo, L.-N. (2019). Iminyl radical-triggered intermolecular distal C(sp3)–H heteroarylation via 1,5-hydrogen-atom transfer (HAT) cascade. Org. Lett. 21, 917–920. doi: 10.1021/acs.orglett.8b03865
Han, B., Li, K., Wang, Q., Zhang, L., Shi, J., Wang, Z., et al. (2018). Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 4, 1569–1575. doi: 10.1001/jamaoncol.2018.3039
Hong, G., Yuan, J., Fu, J., Pan, G., Wang, Z., Yang, L., et al. (2019). Transition-metal-free decarboxylative C3-difluoroarylmethylation of quinoxalin-2(1H)-ones with α,α-difluoroarylacetic acids. Org. Chem. Front. 6, 1173–1182. doi: 10.1039/C9QO00105K
Khattab, S. N., Abdel Moneim, S. A. H., Bekhit, A. A., El Massry, A. M., Hassan, S. Y., El-Faham, A., et al. (2015). Exploring new selective 3-benzylquinoxaline-based MAO-A inhibitors: design, synthesis, biological evaluation and docking studies. Eur. J. Med. Chem. 93, 308–320. doi: 10.1016/j.ejmech.2015.02.020
Kita, Y., Tohma, H., Hatanaka, K., Takada, T., Fujita, S., Mitoh, S., et al. (1994). Hypervalent iodine-induced nucleophilic substitution of para-substituted phenol ethers. Generation of cation radicals as reactive intermediates. J. Am. Chem. Soc. 116, 3684–3691. doi: 10.1021/ja00088a003
Küpper, F. C., Feiters, M. C., Olofsson, B., Kaiho, T., Yanagida, S., Zimmermann, M. B., et al. (2011). Commemorating two centuries of iodine research: An interdisciplinary overview of current research. Angew. Chem. Int. Ed. 50, 11598–11620. doi: 10.1002/anie.201100028
Li, K.-J., Jiang, Y.-Y., Xu, K., Zeng, C.-C., and Sun, B.-G. (2019). Electrochemically dehydrogenative C–H/P–H cross-coupling: effective synthesis of phosphonated quinoxalin-2(1H)-ones and xanthenes. Green Chem. 21, 4412–4421. doi: 10.1039/C9GC01474H
Liang, T., Neumann, C. N., and Ritter, T. (2013). Introduction of fluorine and fuorine-containing functional groups. Angew. Chem. Int. Ed. 52, 8214–8264. doi: 10.1002/anie.201206566
Liu, R., Huang, Z., Murray, M. G., Guo, X., and Liu, G. (2011). Quinoxalin-2(1H)-one derivatives as inhibitors against hepatitis C virus. J. Med. Chem. 54, 5747–5768. doi: 10.1021/jm200394x
Lu, S.-C., Li, H.-S., Gong, Y.-L., Zhang, S.-P., Zhang, J.-G., and Xu, S. (2018). Combination of PhI(OAc)2 and 2-nitropropane as the source of methyl radical in room-temperature metal-free oxidative decarboxylation/cyclization, Construction of 6-methyl phenanthridines and 1-methyl isoquinolines. J. Org. Chem. 83, 15415–15425. doi: 10.1021/acs.joc.8b02701
Matcha, K., Narayan, R., and Antonchick, A. P. (2013). Metal-free radical azidoarylation of alkenes: rapid access to oxindoles by cascade C-N and C-C bond-forming reactions. Angew. Chem. Int. Ed. 52, 7985–7989. doi: 10.1002/anie.201303550
Matsuzaki, K., Okuyama, K., Tokunaga, E., Shiro, M., and Shibata, N. (2014). Sterically demanding unsymmetrical diaryl-λ3-iodanes for electrophilic pentafluorophenylation and an approach to α-pentafluorophenyl carbonyl compounds with an all-carbon stereocenter. Chem. Open 3, 233–237. doi: 10.1002/open.201402045
Meyer, E., Joussef, A. C., and de Souza, L. B. P. (2006). Synthesis of new 1,2,4- and 1,3,4-oxadiazole derivatives as potential nonpeptide angiotensin II receptor antagonists. Synthetic Commun. 36, 729–741. doi: 10.1080/00397910500447066
Mocci, F., Uccheddu, G., Frongia, A., and Cerioni, G. (2007). Solution structure of some λ3 iodanes: An 17O NMR and DFT study. J. Org. Chem. 72, 4163–4168. doi: 10.1021/jo070111h
Nasrallah, A., Lazib, Y., Boquet, V., Darses, B., and Dauban, P. (2019). Catalytic intermolecular C(sp3)-H amination with sulfamates for the asymmetric synthesis of amines. Org. Process Res. Dev. 24, 724–728. doi: 10.1021/acs.oprd.9b00424
Patel, M., McHugh, R. J., Cordova, B. C., Klabe, R. M., Erickson-Viitanen, S., Trainor, G. L., et al. (2000). Synthesis and evaluation of quinoxalinones as HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. Lett. 10, 1729–1731. doi: 10.1016/S0960-894X(00)00321-8
Paul, S., Ha, J. H., Park, G. E., and Lee, Y. R. (2017). Transition metal-free iodosobenzene-promoted direct oxidative 3-arylation of quinoxalin-2(H)-ones with arylhydrazines. Adv. Synth. Catal. 359, 1515–1521. doi: 10.1002/adsc.201700070
Peng, S., Hu, D., Hu, J.-L., Lin, Y.-W., Tang, S.-S., Tang, H.-S., et al. (2019). Metal-free C3 hydroxylation of quinoxalin-2(1H)-ones in water. Adv. Synth. Catal. 361, 5721–5726. doi: 10.1002/adsc.201901163
Pereira, J. A., Pessoa, A. M, Cordeiro, M. N. D. S., Fernandes, R., Prudêncio, C., Noronha, J. P., et al. (2015). Quinoxaline, its derivatives and applications: A State of the Art review. Eur. J. Med. Chem. 97, 664–672. doi: 10.1016/j.ejmech.2014.06.058
Qin, X., Hao, X., Han, H., Zhu, S., Yang, Y., Wu, B., et al. (2015). Design and synthesis of potent and multifunctional aldose reductase inhibitors based on quinoxalinones. J. Med. Chem. 58, 1254–1267. doi: 10.1021/jm501484b
Ravi, M., Chauhan, P., Kant, R., Shukla, S. K., and Yadav, P. P. (2015). Transition-metal-free C-3 arylation of quinoline-4-ones with arylhydrazines. J. Org. Chem. 80, 5369–5376. doi: 10.1021/acs.joc.5b00739
Rossi, R., Lessi, M., Manzini, C., Marianetti, G., and Bellina, F. (2015). Transition metal-free direct C-H (hetero)arylation of heteroarenes: A sustainable methodology to access (hetero)aryl-substituted heteroarenes. Adv. Synth. Catal. 357, 3777–3814. doi: 10.1002/adsc.201500799
Rostoll-Berenguer, J., Blay, G., Muñoz, M. C., Pedro, J. R., and Vila, C. (2019). A combination of visible-light organophotoredox catalysis and asymmetric organocatalysis for the enantioselective mannich reaction of dihydroquinoxalinones with ketones. Org. Lett. 21, 6011–6015. doi: 10.1021/acs.orglett.9b02157
Shen, J., Xu, J., Huang, L., Zhu, Q., and Zhang, P. (2020). Hypervalent iodine(III)-promoted rapid cascade reaction of quinoxalinones with unactivated alkenes and TMSN3. Adv. Synth. Catal. 362, 230–241. doi: 10.1002/adsc.201901314
Stang, P. J. (2003). Polyvalent iodine in organic chemistry. J. Org. Chem. 68, 2997–3008. doi: 10.1021/jo030022e
Stang, P. J., Boehshar, M., Wingert, H., and Kitamura, T. (1988). Acetylenic esters. Preparation and characterization of alkynyl carboxylates via polyvalent iodonium species. J. Am. Chem. Soc. 110, 3272–3278. doi: 10.1021/ja00218a043
Sun, C.-L., and Shi, Z.-J. (2014). Transition-metal-free coupling reactions. Chem. Rev. 114, 9219–9280. doi: 10.1021/cr400274j
Suzuki, S., Kamo, T., Fukushi, K., Hiramatsu, T., Tokunaga, E., Dohi, T., et al. (2014). Iodoarene-catalyzed fluorination and aminofluorination by an Ar-I/HF pyridine/mCPBA system. Chem. Sci. 5, 2754–2760. doi: 10.1039/c3sc53107d
Teng, Q.-H., Yao, Y., Wei, W.-X., Tang, H.-T., Li, J.-R., and Pan, Y.-M. (2019). Direct C-H sulfenylation of quinoxalinones with thiols under visible-light-induced photocatalyst-free conditions. Green Chem. 21, 6241–6245. doi: 10.1039/C9GC03045J
Tian, M., Liu, S., Bu, X., Yu, J., and Yang, X. (2020). Covalent Organic Frameworks: A sustainable photocatalyst toward visible-light-accelerated C3 arylation and alkylation of quinoxalin-2(1H)-ones. Chem. Eur. J. 26, 369–373. doi: 10.1002/chem.201903523
Togo, H., and Katohgi, M. (2001). Synthetic uses of organohypervalent iodine compounds through radical pathways. Synlett. 2001, 0565–0581. doi: 10.1055/s-2001-13349
Wang, D., Ge, B., Li, L., Shan, J., and Ding, Y. (2014). Transition metal-free direct C-H functionalization of quinones and naphthoquinones with diaryliodonium salts, Synthesis of aryl naphthoquinones as β-secretase inhibitors. J. Org. Chem. 79, 8607–8613. doi: 10.1021/jo501467v
Wang, J., Jia, S., Okuyama, K., Huang, Z., Tokunaga, E., Sumii, Y., et al. (2017). Synthesis of sulfur perfluorophenyl compounds using a pentafluorobenzenesulfonyl hypervalent iodonium ylide. J. Org. Chem. 82, 11939–11945. doi: 10.1021/acs.joc.7b01908
Wang, J., Sánchez-Roselló, M., Aceña, J. L., del Pozo, C., Sorochinsky, A. E., Fustero, S., et al. (2014). Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506. doi: 10.1021/cr4002879
Wang, J., Sun, B., Zhang, L., Xu, T., Xie, Y., and Jin, C. (2020). Transition-metal-free direct C-3 cyanation of quinoxalin-2(1H)-ones with ammonium thiocyanate as the “CN” source. Org. Chem. Front. 7, 113–118. doi: 10.1039/C9QO01055F
Wang, L., Liu, H., Li, F., Zhao, J., Zhang, H.-Y., and Zhang, Y. (2019a). Copper-catalyzed C3-H difluoroacetylation of quinoxalinones with ethyl bromodifluoroacetate. Adv. Synth. Catal. 361, 2354–2359. doi: 10.1002/adsc.201900066
Wang, L., Zhang, Y., Li, F., Hao, X., Zhang, H.-Y., and Zhao, J. (2018). Direct C-H trifluoromethylation of quinoxalin-2(1H)-ones under transition-metal-free conditions. Adv. Synth. Catal. 360, 3969–3977. doi: 10.1002/adsc.201800863
Wang, L., Zhao, J., Sun, Y., Zhang, H.-Y., and Zhang, Y. (2019b). A catalyst-free minisci-type reaction: the C-H alkylation of quinoxalinones with sodium alkylsulfinates and phenyliodine(III) dicarboxylates. Eur. J. Org. Chem. 2019, 6935–6944. doi: 10.1002/ejoc.201901266
Weïwer, M., Spoonamore, J., Wei, J., Guichard, B., Ross, N. T., Masson, K., et al. (2012). A potent and selective quinoxalinone-based STK33 inhibitor does not show synthetic lethality in KRAS-dependent cells. ACS Med. Chem. Lett. 3, 1034–1038. doi: 10.1021/ml300246r
Wirth, T. (2005). Hypervalent iodine chemistry in synthesis: Scope and new directions. Angew. Chem. Int. Ed. 44, 3656–3665. doi: 10.1002/anie.200500115
Xie, L.-Y., Jiang, L.-L., Tan, J.-X., Wang, Y., Xu, X.-Q., Zhang, B., et al. (2019a). Visible-light-initiated decarboxylative alkylation of quinoxalin-2(1H)-ones with phenyliodine(III) dicarboxylates in recyclable ruthenium(II) catalytic system. ACS Sustain. Chem. Eng. 7, 14153–14160. doi: 10.1021/acssuschemeng.9b02822
Xie, L.-Y., Peng, S., Fan, T.-G., Liu, Y.-F., Sun, M., Jiang, L.-L., et al. (2019b). Metal-free C3-alkoxycarbonylation of quinoxalin-2(1H)-ones with carbazates as ecofriendly ester sources. Sci. China Chem. 62, 460–464. doi: 10.1007/s11426-018-9446-1
Xu, J., Yang, H., Cai, H., Bao, H., Li, W., and Zhang, P. (2019). Transition-metal and solvent-free oxidative C-H fluoroalkoxylation of quinoxalinones with fluoroalkyl alcohols. Org. Lett. 21, 4698–4702. doi: 10.1021/acs.orglett.9b01578
Xue, W., Su, Y., Wang, K.-H., Cao, L., Feng, Y., Zhang, W., et al. (2019a). Phenyliodonium diacetate mediated carbotrifluoromethylation of quinoxalin-2(1H)-ones. Asian J. Org. Chem. 8, 887–892. doi: 10.1002/ajoc.201900118
Xue, W., Su, Y., Wang, K.-H., Zhang, R., Feng, Y., Cao, L., et al. (2019b). Visible-light induced decarboxylative alkylation of quinoxalin-2(1H)-ones at the C3-position. Org. Biomol. Chem. 17, 6654–6661. doi: 10.1039/C9OB01169B
Yamaoka, N., Sumida, K., Itani, I., Kubo, H., Ohnishi, Y., Sekiguchi, S., et al. (2013). Single-electron-transfer (SET)-induced oxidative biaryl coupling by polyalkoxybenzene-derived diaryliodonium(III) salts. Chem. Eur. J. 19, 15004–15011. doi: 10.1002/chem.201301148
Yang, Q., Han, X., Zhao, J., Zhang, H.-Y., and Zhang, Y. (2019). Direct C3 alkoxylation of quinoxalin-2(1H)-ones with alcohols via cross-dehydrogenative coupling under catalyst-free conditions. J. Org. Chem. 84, 11417–11424. doi: 10.1021/acs.joc.9b01181
Yang, Y., Lan, J., and You, J. (2017). Oxidative C-H/C-H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863. doi: 10.1021/acs.chemrev.6b00567
Yang, Y-D., Azuma, A., Tokunaga, E., Yamasaki, M., Shiro, M., and Shibata, N. (2013). Trifluoromethanesulfonyl hypervalent iodonium ylide for copper-catalyzed trifluoromethylthiolation of enamines, indoles, and β-ketoEsters. J. Am. Chem. Soc. 135, 8782–8785. doi: 10.1021/ja402455f
Yi, H., Zhang, G., Wang, H., Huang, Z., Wang, J., Singh, A. K., et al. (2017). Recent advances in radical C-H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085. doi: 10.1021/acs.chemrev.6b00620
Yin, K., and Zhang, R. (2017). Transition-metal-free direct C-H arylation of quinoxalin-2(1H)-ones with diaryliodonium salts at room temperature. Org. Lett. 19, 1530–1533. doi: 10.1021/acs.orglett.7b00310
Yoshimura, A., and Zhdankin, V. V. (2016). Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 116, 3328–3435. doi: 10.1021/acs.chemrev.5b00547
Yuan, J.-W., Zhu, J.-L., Zhu, H.-L., Peng, F., Yang, L.-Y., Mao, P., et al. (2020). Transition-metal free direct C–H functionalization of quinoxalin-2(1H)-ones with oxamic acids leading to 3-carbamoyl quinoxalin-2(1H)-ones. Org. Chem. Front. 7, 273–285. doi: 10.1039/C9QO01322A
Yusubov, M. S., and Zhdankin, V. V. (2015). Iodine catalysis, A green alternative to transition metals in organic chemistry and technology. Res-Effic Technol. 1, 49–67. doi: 10.1016/j.reffit.2015.06.001
Zhang, K., Hong, R., Kaping, L., Xu, F., Xia, W., Qin, G., et al. (2019). CDK4/6 inhibitor palbociclib enhances the effect of pyrotinib in HER2-positive breast cancer. Cancer Lett. 447, 130–140. doi: 10.1016/j.canlet.2019.01.005
Zhao, L., Wang, L., Gao, Y., Wang, Z., and Li, P. (2019). Visible-light-induced alkoxylation of quinoxalin-2(1H)-ones with alcohols for the synthesis of heteroaryl ethers. Adv. Synth. Catal. 361, 5363–5370. doi: 10.1002/adsc.201900732
Zhdankin, V. V., and Stang, P. J. (2002). Recent developments in the chemistry of polyvalent iodine compounds. Chem. Rev. 102, 2523–2584. doi: 10.1021/cr010003+
Zhdankin, V. V., and Stang, P. J. (2008). Chemistry of polyvalent iodine. Chem. Rev. 108, 5299–5358. doi: 10.1021/cr800332c
Keywords: hypervalent Iodine(III) reagent, quinoxalin-2(1H)-one, C-H functionalization, arylation, trifluoromethylation, alkylation, alkoxylation
Citation: Tan Y, Wang J, Zhang H-Y, Zhang Y and Zhao J (2020) The C3-H Bond Functionalization of Quinoxalin-2(1H)-Ones With Hypervalent Iodine(III) Reagents. Front. Chem. 8:582. doi: 10.3389/fchem.2020.00582
Received: 01 April 2020; Accepted: 05 June 2020;
Published: 03 August 2020.
Edited by:
Jian-Wei Han, East China University of Science and Technology, ChinaReviewed by:
Cheng-Pan Zhang, Wuhan University of Technology, ChinaCopyright © 2020 Tan, Wang, Zhang, Zhang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Yu Zhang, emhhbmdoeUBoZWJ1dC5lZHUuY24=; Yuecheng Zhang, eWN6aGFuZ0BoZWJ1dC5lZHUuY24=; Jiquan Zhao, emhhb2pxQGhlYnV0LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.