- 1Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Guizhou University, Guiyang, China
- 2Guizhou Engineering Laboratory for Synthetic Drugs, School of Pharmaceutical Sciences, Guizhou University, Guiyang, China
Haedoxans are a series of sesquilignan natural products isolated from the traditional insecticidal plant Phryma leptostachya. Given their significant insecticidal activity, haedoxans and related analogs have been considered as potential agents for plant defense. Moreover, these compounds also exhibit promising antifungal, antibacterial, and anticancer activities. The present paper is a review of the structure, biological activity, and chemical synthesis of naturally occurring haedoxan-like molecules.
Introduction
Phryma leptostachya is a perennial herb that is widespread in nature (Lee et al., 2002; Park et al., 2005; Endo and Miyauchi, 2006; Li et al., 2019a,b; Xu et al., 2019). In Chinese culture, the plant has been used as a traditional Chinese medicine to treat inflammatory diseases, such as itching, allergic dermatitis, and gout (Jung et al., 2013). In East Asia, P. leptostachya has also been traditionally used as a natural insecticide (Taniguchi and Oshima, 1972a,b; Ishibashi and Taniguchi, 1998; Xiao et al., 2012a; Jung et al., 2013), for instance being used to repel mosquitos and flies in the southwest district of China (Chen et al., 2012). As a result, the secondary metabolites isolated from P. leptostachya have drawn much attention.
Previous phytochemical investigations showed that this plant is rich in lignans. Among them, (+)-haedoxan A (1, see Figure 1A), isolated in 1989 by Taniguchi, represents the major insecticidal ingredient (Taniguchi et al., 1989; Yamaguchi and Taniguchi, 1992a; Seo and Park, 2012). Structurally, this natural product is a sesquilignan, that is, a trimer of C6C3 units (n-propyl benzene). The skeleton features a furofuran core and a dioxane core with six stereogenic centers. Haedoxan D (2) and E (3) are from the same natural product family, which structurally differs from haedoxan A (1) at one of the aromatic rings. (+)-Phrymarolin I (4) and II (5) are also important ingredients of P. leptostachya extracts. As neolignans, these two compounds are phenylpropianoid dimers that share the same furofuran core with haedoxans.
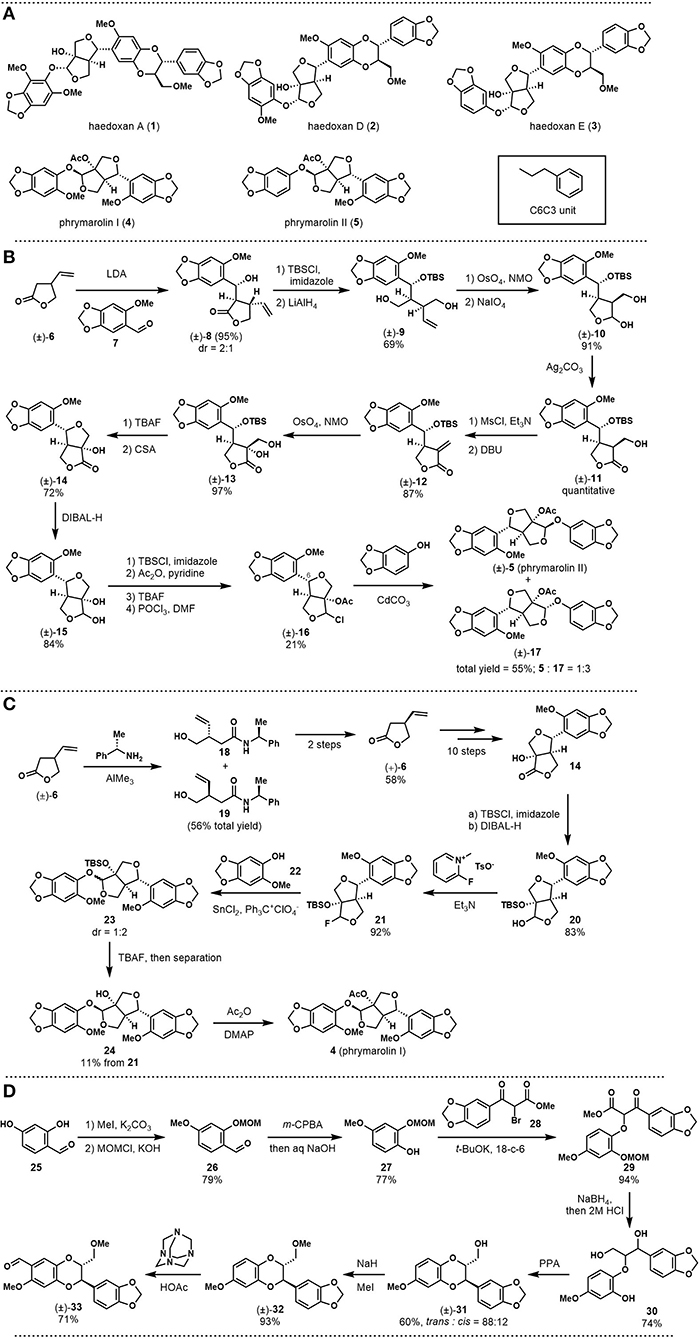
Figure 1. (A) Structures of haedoxans and phrymarolins; (B) Taniguchi's first total synthesis of (±)-phrymarolin II; (C) Taniguchi's total synthesis of (+)-phrymarolin I; (D) Taniguchi's synthesis toward (±)-haedoxan A, D, and E: fragment preparation.
Haedoxans exhibit excellent insecticidal activity against several insects, such as Musca domestica [Culex pipiens pallens (Xiao et al., 2012b)] and Mythimna separata (Xiao et al., 2012a). It is noteworthy that the insecticidal activity of Haedoxan A (1) is comparable to that of the commercial synthetic pyrethroids (Taniguchi et al., 1989; Hu et al., 2016). (+)-Phrymarolins I (4) and II (5) also show considerable synergistic activities with pyrethrin and carbamate pesticides (Park et al., 2005). Accordingly, haedoxans and phrymarolins could be used as the main insecticidal ingredients in new botanical pesticides. In addition, the potential utilities of these natural products as lead compounds in pesticide discovery are also attractive.
To date, Haedoxan A (1) has only been found in the root of P. leptostachya at very low concentration (from 0.004 to 0.009%). Although two total syntheses of headoxan A (1) have been reported by Taniguchi and Ishibashi, over 20 synthetic steps are needed to achieve a natural product with moderate selectivities (Ishibashi and Taniguchi, 1989, 1998). As a result, the availability of haedoxan A (1) is the main obstacle in its commercialization process. To address this problem, new synthetic routes for headoxans with high efficiencies and stereoselectivities are needed.
Synthetic Studies Toward Haedoxan-like Molecules
In this review, we focus on the chemical synthesis of haedoxans and some closely related natural products such as phrymarolin I (4) and II (5). While a number of synthetic studies on the lignan family have been reported, there have been limited reports on the synthesis of haedoxans. Since a phenylpropanoid trimer bears six stereogenic centers, haedoxans are the most structurally complex members in this family. It is noteworthy that besides the four contiguous stereocenters, the two chiral carbons that are remote from the furofuran core might also be a significant synthetic challenge due to stereocorrelation problems in the fragment coupling process. As a result, it would be extremely difficult to control steroselectivites in the total synthesis of haedoxans.
In 1986, Ishibashi and Taniguchi reported the synthesis of (±)-phrymarolin II (5), which represents a pioneering study on the chemical synthesis of haedoxan-like natural products (Ishibashi and Taniguchi, 1986). As shown in Figure 1B, the authors started their synthesis with an aldol reaction between lactone 6 and benzaldehyde 7 to build the left fragment of phrymarolin II (5). The adduct 8 was protected with a TBS group, and the lactone was then reduced with LiAlH4 to afford diol 9. After Upjohn dihydroxylation and oxidative cleavage with NaIO4, diol 9 was converted to semiacetal 10, which possesses one of the two tetrahydrofurans in the central fragment of the natural product. The semiacetal was then oxidized to corresponding lactone (11) with a quantitative yield. Once the lactone was established, a two-step reaction sequence was carried out to realize a β-elimination process. The resulting α, β-unsaturated lactone 12 was then oxidized with Upjohn dihydroxylation to give diol 13 at 97% yield. After deprotection of the TBS group with TBAF, the second tetrahydrofuran ring and the C6 stereocenter were established through an acid-promoted etherification reaction. The product 14 was treated with DIBAL-H to reduce the lactone moiety, and the newly formed diol was differentiated within four steps to afford chloride 16. Finally, a CdCO3 catalyzed substitution successfully introduced the right fragment to give (±)-phrymarolin II (5) with its stereoisomer 17 in a ratio of 1:3 (55% total yield).
In 1988, Ishibashi and Taniguchi improved the previous synthetic route and reported the total synthesis of (+)-phrymarolin I [4, (Ishibashi and Taniguchi, 1988)]. This asymmetric synthesis commenced with the preparation of the optically pure (+)-4. As shown in Figure 1C, aminolysis of (±)-6 with (S)-1-phenylethanamine gave two diastereoisomers that could be separated through chromatography. Then, hydrolysis of 18 followed by lactonization afforded (+)-6 at 58% yield. This chiral starting material was subjected to the above synthetic route to give 14 in an asymmetric fashion. Different from the previous synthesis, 14 was first protected by the TBS group and then reduced to lactol 20, which was then fluorinated to set the stage for the subsequent fragment coupling. In the next event, phenol 22 was introduced in the presence of SnCl2 and trityl perchlorate to provide the coupling product 23 with a diastereomeric ratio of 1:2, favoriting the undesired diastereomer. The mixed products were desilylated and separated by preparative TLC to afford 24 in 11% yield over two steps. Finally, acylation of 24 provided the desired natural product (+)-phrymarolin I (4).
One year later, Ishibashi and Taniguchi applied their developed synthetic route to the total synthesis of (±)-haedoxan A (1), D (2), and E [3, (Ishibashi and Taniguchi, 1989)]. As shown in Figure 1D, the preparation of the benzodioxane fragment 33 is not trivial. Their synthesis commenced with selective methylation and MOM protection of the benzaldehyde 25. After Dakin oxidation and hydrolysis, the resultant phenol 27 was etherificated with bromide 28 to give 29 as the coupling product. Global reduction with NaBH4 was followed with acid promoted deprotection to generate 30 at 74% yield. A PPA-mediated cyclization was then introduced to build the dioxane ring. Finally, methylation and selective formylation provided the desired aromatic fragment (±)-33.
With the above fragment in hand, the authors followed their previous synthesis to carry out an aldol reaction between lactone 6 and aldehyde 33, as shown in Figure 2A. However, although the reaction worked well, product 34 was obtained as a mixture of diastereomers due to stereochemical correlation issues. The adduct was protected with the TBS group and then purified by chromatography to afford an inseparable mixture of 35 and 36 at 55% total yield. The mixture was then submitted to the known synthetic route to give fluoride 38 within 11 steps. With this key intermediate, haedoxin A (1), D (2), E (3) were synthesized in diastereoselective fashion.
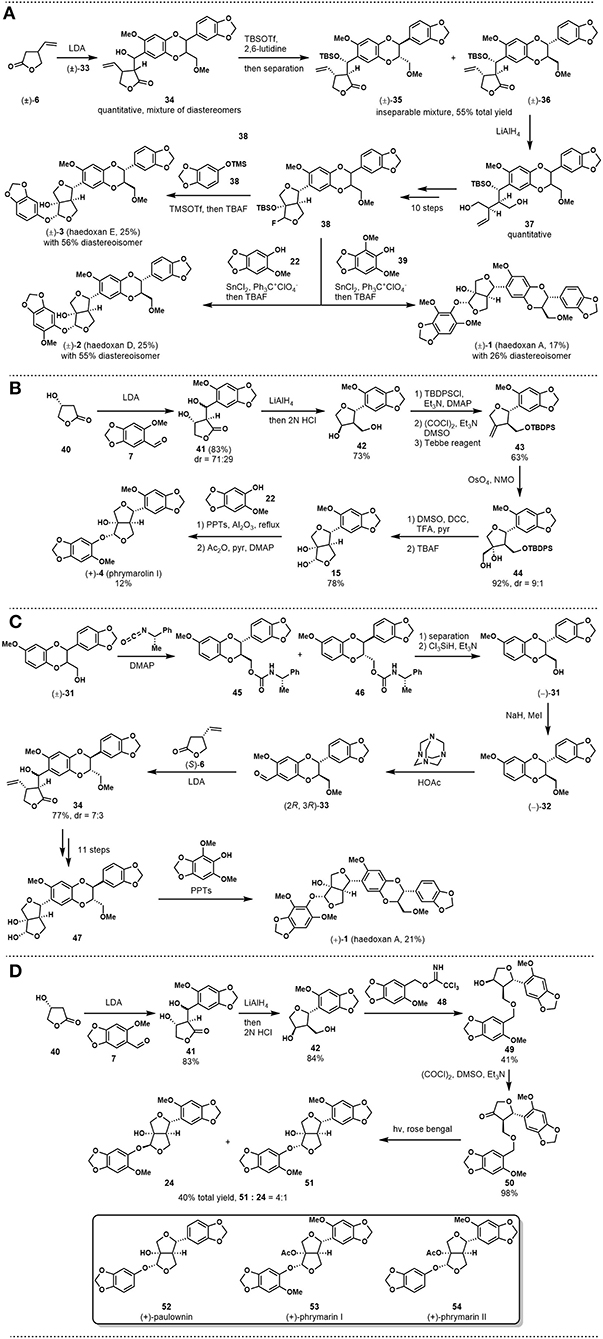
Figure 2. (A) Ishibashi and Taniguchi's total synthesis of (±)-haedoxan A, D, and E; (B) Taniguchi and Ishibashi's asymmetric synthesis of (+)-phrymarolin I; (C) Ishibashi and Taniguchi's asymmetric synthesis of (+)-haedoxan A; (D) Ishibashi's concise synthesis of (+)-paulownin, (+)-phrymarin I, and (+)-phrymarin II.
In the following decade, Taniguchi and coworkers applied their strategy to synthesizing a series of lignan analogs to explore potential insecticidal compounds (Yamaguchi and Taniguchi, 1991, 1992a,b,c; Yamaguchi et al., 1992a,b). A significant improvement of the synthetic strategy was published by Okazaki et al. (1997). In this report, the authors developed a concise synthetic route toward (+)-phrymarolin I (4). As shown in Figure 2B, the synthesis commenced with an aldol reaction using chiral lactone 40 as the nucleophile, which could be easily prepared from (R)-malate. After reductive opening of the lactone ring, the tetraol intermediate was treated with HCl solution to close the tetrahydrofuran ring and give 42. Then, a three-step reaction sequence, including alcohol protection, Swern oxidation, and Tebbe olefination, was used to prepare alkene 43. Diastereoselective dihydroxylatoin followed by Pfitzner-Moffatt oxidation and desilylation provided chiral lactol 15 at a good yield. The key intermediate that had been used in the authors' synthesis of phrymarolin II (5), 15, was subjected directly to an acid-promoted replacement reaction with phenol 22 to afford the coupling product with desired stereochemistry at 15% yield. Then, a simple acylation reaction of the above product completed the asymmetric total synthesis of (+)-phrymarolin I (4).
In 1998, Ishibashi and Taniguchi reported their asymmetric synthesis of (+)-haedoxin A (1, Ishibashi and Taniguchi, 1998). While the core strategy followed the concept of Taniguchi's previous synthesis, this new synthesis featured the use of chiral synthons to avoid stereochemical correlation problems. As shown in Figure 2C, chiral compound 33 was first prepared via an optical resolution strategy from (±)-31. This synthon was coupled with another chiral building block (S)-6 to give 34 as the adduct. Key intermediate 47 was then prepared through the known reaction sequence to set the stage for the last coupling. Instead of halogenation, the authors used the same key reaction in their synthesis of (+)-phrymarolin I (4) to install the phenol fragment directly on the lactol. This reaction provided the desired natural product, (+)-haedoxan A (1), at 21% yield.
In 2001, Ishibashi published a synthesis of (+)-paulownin, (+)-phrymarin I, and (+)-phrymarin II (Ishibashi et al., 2001). The report featured an elegant photochemical reaction that was developed by Kraus in 1990 (Kraus and Chen, 1990). As shown in Figure 2D, tetrahydrofuran intermediate 42 was synthesized through the procedures reported in Ishibashi's (+)-phrymarolin I (4) synthesis (Figure 2B). A coupling reaction between alcohol 42 and benzyl trichloroacetimidate 48, followed by a Swern oxidation, provided the key intermediate 50 at 98% yield. Ketone 50 was then submitted to the photochemical condition developed by Kraus and Chen. In this event, a new C-C bond was formed between the irradiated benzylic position and the furan carbonyl in a diastereoselective manner to give furofuran 51 and 24 at 40% total yield. Finally, 51 was transformed into the desired natural product (+)-phrymarin I through a simple acylation reaction. With this concise synthetic route, the authors also completed the synthesis of (+)-paulownin and (+)-phrymarin II. It is noteworthy that compound 24 could serve as a key intermediate in the synthesis of (+)-phrymalorin I. However, the diastereoselectivity of the key reaction did not favor this intermediate.
Summary and Further Prospects
Haedoxans and related neolignans are a family of natural insecticidal products with prominent potential applications. The main problem with the insecticide research process is the availability of sufficient samples. This review detailed the synthetic efforts toward haedoxans and phrymarolins in the past three decades. While these syntheses represent pioneering investigations on this topic, we are expecting new syntheses of haedoxans with higher efficiency, higher stereoselectivities, better step economy and redox economy, and more environmentally friendly procedures. This review may shed some light to guide future synthetic efforts on haedoxans.
Author Contributions
YC collected and organized all articles in the literature regarding haedoxans and related neolignans from Phryma Leptostachya, reviewing the abstract, introduction, details of haedoxan syntheses, and summary and further prospects of each. SX helped check all of the figures and references. JH and WX reviewed all articles in the literature and participated in significant discussions. SH reviewed the synthetic efforts toward haedoxans and phrymarolins in the past three decades and summed them up in reaction figures.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge financial support from the National Key R & D Program of China (2018YFD0200702, 2018YFD0200802, and 2018YFD0200106), the National Natural Science Foundation of China (21861010 and 21562011), the Science and Technology Foundation of Guizhou Province [2018]5781 and [2020]1Y108, and Guizhou University [2017]32.
References
Chen, C., Zhu, H., Zhao, D., and Deng, J. (2012). Lignans from Phryma leptostachya L. Helvetica Chimica Acta 95, 333–338. doi: 10.1002/hlca.201100311
Endo, Y., and Miyauchi, T. (2006). Thermonasty of young main stems of Phryma leptostachya (Phrymaceae). J. Plant Res. 119, 449–457. doi: 10.1007/s10265-006-0007-6
Hu, Z., Du, Y., Xiao, X., Dong, K., and Wu, W. (2016). Insight into the mode of action of Haedoxan A from Phryma leptostachya. Toxins 8, 53/51-53/12. doi: 10.3390/toxins8020053
Ishibashi, F., Hayashita, M., Okazaki, M., and Shuto, Y. (2001). Improved procedure for the enantiometric synthesis of 1-Hydroxy/acetoxy-2,6-diaryl-3,7-dioxabicyclo[3.3.0]octane Lignans: Total Syntheses of (+)-Paulownin, (+)-Phrymarin I and (+)-Phrymarin II. Biosci. Biotech. Biochem. 65, 29–34. doi: 10.1271/bbb.65.29
Ishibashi, F., and Taniguchi, E. (1986). Synthesis of (±)-Phrymarolin II and Its Stereoisomers. Agric. Biol. Chem. 50, 3119–3125. doi: 10.1080/00021369.1986.10867871
Ishibashi, F., and Taniguchi, E. (1988). Synthesis and absolute configuration of the acetalic lignan (+)-Phrymarolin I. Bull. Chem. Soc. Jpn. 61, 4361–4366. doi: 10.1246/bcsj.61.4361
Ishibashi, F., and Taniguchi, E. (1989). Synthesis of (±)-Haedoxan A, D, E and their stereoisomers. Agric. Biol. Chem. 53, 1565–1573. doi: 10.1271/bbb1961.53.1565
Ishibashi, F., and Taniguchi, E. (1998). Synthesis and absolute configuration of the insecticidal sesquilignan (+)-Haedoxan A. Phytochemistry 49, 613–622. doi: 10.1016/S0031-9422(98)00270-2
Jung, H., Cho, Y., Lim, H., Choi, H., Ji, D., and Lim, C. (2013). Anti-inflammatory, antioxidant, anti-angiogenic and skin whitening activities of Phryma leptostachya var. asiatica Hara extract. Biomol. Ther. 21, 72–78. doi: 10.4062/biomolther.2012.059
Kraus, G. A., and Chen, L. (1990). A total synthesis of racemic paulownin using a type II photocyclization reaction. J. Am. Chem. Soc. 112, 3464–3466. doi: 10.1021/ja00165a033
Lee, S., Min, B., and Kho, Y. (2002). Brine shrimp lethality of the compounds from Phryma leptostachya L. Arch. Pharm. Res. 25, 652–654. doi: 10.1007/BF02976939
Li, Y., Wang, S., Aioub, A. A. A., Qie, X., Wu, W., and Hu, Z. (2019b). Identification and analysis of full-length transcripts involved in the biosynthesis of insecticidal lignan (+)-haedoxan A in Phryma leptostachya. Ind. Crops Prod. 142:111868. doi: 10.1016/j.indcrop.2019.111868
Li, Y., Wei, J., Fang, J., Lv, W., Ji, Y., Aioub, A. A. A., Zhang, J., and Hu, Z. (2019a). Insecticidal activity of four lignans isolated from Phryma leptostachya. Molecules 24:1976. doi: 10.3390/molecules24101976
Okazaki, M., Ishibashi, F., Shuto, Y., and Taniguchi, E. (1997). Total synthesis of (+)-Phrymarolin I from (+)-malic acid. Biosci. Biotech. Biochem. 61, 660–663. doi: 10.1271/bbb.61.660
Park, I. I., Shin, S., Kim, C., Lee, H., Choi, W., and Ahn, Y. (2005). Larvicidal activity of lignans identified in Phryma leptostachya Var. asiatica roots against three mosquito species. J. Agric. Food Chem. 53, 969–972. doi: 10.1021/jf048208h
Seo, S., and Park, I. I. (2012). Larvicidal activity of medicinal plant extracts and lignan identified in Phryma leptostachya var. asiatica roots against housefly (Musca domestica L.). Parasitol. Res. 110, 1849–1853. doi: 10.1007/s00436-011-2709-5
Taniguchi, E., Imamura, K., Ishibashi, F., Matsui, T., and Nishio, A. (1989). Structure of the novel insecticidal sesquilignan, haedoxan A. Agric. Biol. Chem. 53, 631–643. doi: 10.1080/00021369.1989.10869338
Taniguchi, E., and Oshima, Y. (1972a). Phrymarolin-I, a Novel Lignan from Phryma leptostachya L. Agric. Biol. Chem. 36, 1013–1025. doi: 10.1271/bbb1961.36.1013
Taniguchi, E., and Oshima, Y. (1972b). Structure of Phrymarolin-II. Agric. Biol. Chem. 36, 1489–1496. doi: 10.1080/00021369.1972.10860431
Xiao, X., Hu, Z., Ji, Z., Shi, J., Zhang, J., Wei, S., et al. (2012a). Isolation, structure identification and bioactivity of active ingredients from Phryma leptostachya. Chin. J. Pestic. Sci. 14, 583–586. doi: 10.3969/j.issn.1008-7303.2012.05.19
Xiao, X., Hu, Z., Shi, B., Wei, S., and Wu, W. (2012b). Larvicidal activity of lignans from Phryma leptostachya L. against Culex pipiens pallens. Parasitol. Res. 110, 1079–1084. doi: 10.1007/s00436-011-2591-1
Xu, W., Zhao, P., Wang, M., and Liang, Q. (2019). Naturally occurring furofuran lignans: structural diversity and biological activities. Nat. Prod. Res. 33, 1357–1373. doi: 10.1080/14786419.2018.1474467
Yamaguchi, S., Ishibashi, F., and Taniguchi, E. (1992b). Insecticidal Activity of Sesquilignans with a 3-Aryl-6-mehoxy-2-mehoxymethyl-1,4-benzodioxanyl Group. Biosci. Biotech. Biochem. 56, 1760–1768. doi: 10.1271/bbb.56.1760
Yamaguchi, S., Nagata, S., and Taniguchi, E. (1992a). Effect on insecticidal activity of substituents at the 1,4-benzodioxanyl moiety of haedoxan. Biosci. Biotech. Biochem. 56, 1193–1197. doi: 10.1271/bbb.56.1193
Yamaguchi, S., and Taniguchi, E. (1991). Synthesis and insecticidal activity of lignan analogs (I). Agric. Biol. Chem. 55, 3075–3084. doi: 10.1080/00021369.1991.10867924
Yamaguchi, S., and Taniguchi, E. (1992a). Synthesis and insecticidal activity of lignan analogs (III). Biosci. Biotech. Biochem. 56, 418–422. doi: 10.1271/bbb.56.418
Yamaguchi, S., and Taniguchi, E. (1992b). Synthesis and insecticidal activity of lignan analogs (II). Biosci. Biotech. Biochem. 56, 412–417. doi: 10.1271/bbb.56.412
Keywords: haedoxans, neoligans, Phryma leptostachya, insecticidal activity, natural products
Citation: Chen Y, Xiao S, Huang J, Xue W and He S (2020) A Synthetic View on Haedoxans and Related Neolignans From Phryma leptostachya. Front. Chem. 8:460. doi: 10.3389/fchem.2020.00460
Received: 27 March 2020; Accepted: 04 May 2020;
Published: 17 June 2020.
Edited by:
Yaqiong Su, Eindhoven University of Technology, NetherlandsReviewed by:
Min Zhang, Chongqing University, ChinaYang Hua, Zhengzhou University, China
Pei Tang, Sichuan University, China
Copyright © 2020 Chen, Xiao, Huang, Xue and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Chen, eWNoZW4xQGd6dS5lZHUuY24=; Shuzhong He, c3poZUBnenUuZWR1LmNu
 Yang Chen
Yang Chen Shu Xiao1
Shu Xiao1