- 1State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Guiyang, China
- 2Key Laboratory of Marine Drugs, Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao, China
- 3College of Life Sciences, Shandong University of Technology, Zibo, China
- 4Department of Physiology, Faculty of Science, Mahidol University, Bangkok, Thailand
- 5Laboratory for Marine Drugs and Bioproducts of Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
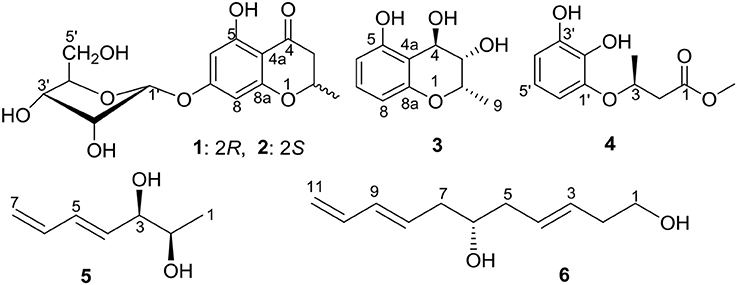
Five new polyketides (2–6) and ten known compounds (1 and 7–15) were obtained from the fermentation products of the endophytic fungus Cladosporium sp. OUCMDZ-302 with the mangrove plant, Excoecaria agallocha (Euphorbiaceae). The new structures of 2–6 were established on the basis of ECD, specific rotation and spectroscopic data as well as the chemical calculation. Compound 7 showed cytotoxicity against H1975 cell line with an IC50 value of 10.0 μM. Compounds 4 and 8–10 showed radical scavenging activity against DPPH with the IC50 values of 2.65, 0.24, 5.66, and 6.67 μM, respectively. In addition, the absolute configuration of compound 1 was solidly determined by X-ray and sugar analysis of the acidic hydrolysates for the first time as well as those of compounds 8–10 in this paper.
Introduction
Mangrove plants and endophytic fungi are two principal sources of new and bioactive natural products (Zhang et al., 2006; Wu et al., 2008). Excoecaria agallocha (Euphorbiaceae), also known as blind-your-eye, is mainly used to treat sores and stings. More than 72 cytotoxic diterpenoids have been identified from E. agallocha, structurally belonging to labdane (Konishi et al., 1999, 2003; Anjaneyulu and Rao, 2000; Annam et al., 2015), isopimarane/ent-isopimarane (Anjaneyulu et al., 2003; Wang and Guo, 2004; Kang et al., 2005; Wang et al., 2005; Gowri Ponnapalli et al., 2013), atisane/ent-atisane (Konishi et al., 2000; Wang et al., 2009), ent-kaurane (Anjaneyulu et al., 2002; Li et al., 2010), and beyerane-type (Anjaneyulu et al., 2002).
In our ongoing investigations of new and bioactive compounds from endophytes associated with mangrove plants (Lin et al., 2008; Lu et al., 2009, 2010; Wang et al., 2012; Kong et al., 2014; Zhu et al., 2018), an endogenous fungal strain OUCMDZ-302 identified as Cladosporium sp., was isolated from the surface-sterilized stems of E. agallocha. The secondary metabolites of the genus Cladosporium were mainly reported as polyketides derivatives, such as macrolides (Jadulco et al., 2001; Zhang et al., 2001; Shigemori et al., 2004), α-pyrones (Jadulco et al., 2002), α-pyridone (Ye et al., 2005), and binaphthyl derivatives (Sakagami et al., 1995). Herein we report five new polyketides (2–6) (Figure 1) isolated from the EtOAc extract of Cladosporium sp. OUCMDZ-302, along with the ten known structures (Figure S36 and Table S1), (2R)-7-O-α-D-ribofuranosyl-5-hydroxy-2-methylchroman-4-one (1) (Hu et al., 2017), 7-O-α-D-ribosyl-5-hydroxy-2-propylchromone (7) (Zhao et al., 2015), 3-(2,3-dihydroxy phenoxy)butanoic acid (8) (Dai et al., 2009), (2S,4S)-4-methoxy-2-methylchroman-5-ol (9) (Wu et al., 2010), (2S,4S)-2-methylchroman-4,5-diol (10) (Teles et al., 2005), (±)-5,7-dihydroxy-2-methyl chroman-4-one (11) (Rao et al., 1994), (±)-5-hydroxy-2-methylchroman-4-one (12) (Dai et al., 2006), 1-(2,6-dihydroxyphenyl) ethanone (13) (Dhami and Stothers, 1965), 1-(2,6-dihydroxyphenyl)-1-butanone (14) (Huang et al., 2005), and 2-butyryl-3,5-dihydroxycyclohex-2-enone (15) (Igarashi et al., 1993). Compound 7 showed inhibitory activity against H1975 cell line with an IC50 value of 10.0 μM. Compounds 4 and 8–10 exhibited radical scavenging activity against DPPH with IC50 values of 2.65, 0.24, 5.66, and 6.67 μM, respectively. In addition, the absolute configurations of compounds 8–10 were resolved and that 1 was solidified in this paper.
Materials and Methods
General Experimental Procedures
The NMR, ECD, [α]D, UV and IR spectra were recorded on JEOL JNM-ECP 600, JASCO J-810, JASCO P-1020 digital, Beckman DU® 640 and Nicolet NEXUS 470 spectrophotometers, respectively. ESI-MS, EI-MS and GC-MS were measured on Q-TOF ULTIMA GLOBAL GAA076 LC, VG Autospec-3000 and Agilent 6890/5973 spectrometers, respectively. Semipreparative HPLC and chiral separation was performed on a YMC-pack ODS-A column [10 × 250 mm, 5 μm, 4 mL/min] and a CHIRALPAK IA column [20 × 250 mm, 5 μm, 10 mL/min]. TLC was performed on plates precoated with silica gel GF254 (10–40 μm). The column chromatography (CC) was performed over silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China) and Sephadex LH-20 (Amersham Biosciences, Sweden), respectively. The seawater for the cultural medium of Cladosporium sp. OUCMDZ-302 was collected from Yellow Sea near Qingdao.
Fungal Material
The strain Cladosporium sp. OUCMDZ-302 was isolated from the surface sterilized stems of the mangrove plant E. agallocha grown in Wenchang, Hainan, China. Briefly, the stems were washed with tap water and sterile distilled water in sequence. The stems with clean surface were further sterilized in a sequence of 75% ethanol for 2 min, 0.1% of HgCl2 for 3 min, and sterile distilled water. The outer bark was removed, and the inner bark was cut into small pieces that were then placed on a potato dextrose agar (PDA) plate and cultured at 28°C for 3 days. A single colony was transferred to PDA media and was identified according to its morphological characteristics (Figure S35) by Prof. Kui Hong, Wuhan University. A voucher specimen is deposited in our laboratory at −80°C. The working strain was prepared on PDA slants and stored at 4°C.
Fermentation and Extraction
The producing fungal strain Cladosporium sp. OUCMDZ-302 was inoculated into a 500 mL cylindrical flask containing 100 mL of seawater consisting of 2% maltose, 2% mannitol, 1% glucose, 1% monosodium glutamate, 0.3% yeast extract, 0.1% corn flour, 0.05% KH2PO4, 0.03% MgSO4· 7H2O (pH 6.5) and cultured at 28°C for 48 h on a rotary shaker at 120 rpm. The seed culture was transferred into three hundred and fifty 500 mL conical flasks (200 mL/flask) containing the same medium, and performed at 28°C for 7 days on rotary shakers at 160 rpm. The whole fermentation broth (70 L) was filtered through cheese cloth to separate the mycelia from filtrate. The filtrate was concentrated to about one-quarter of the original volume under reduced pressure and then extracted three times with equal volumes of ethyl acetate (EtOAc) and concentrated to dryness. The mycelia were extracted three times with acetone and concentrated to an aqueous solution. The aqueous solution was subsequently extracted three times with equal volumes of EtOAc and concentrated. Both EtOAc extractions were combined to give 45 g of the extract.
Isolation
The extract (45 g) was separated into eight fractions (Fr.1–Fr.8) on a silica gel column (8.5 × 15 cm, 200–300 mesh) using a step gradient elution with CHCl3-petroleum ether (V/V 0:100–100:0, 4 L) and then MeOH–CHCl3 (V/V 0:100–100:0, 16 L). Fr.1 (5.4 g) was separated on a silica gel column (4.5 × 10 cm, 200–300 mesh) eluted with CHCl3-petroleum ether (V/V, 1: 1, 3L) to give 12 (1g). Fr.3 (0.3 g) was further purified by semipreparative HPLC (60% MeOH/H2O) to give 10 (7 mg, tR 4.97 min). Fr.4 (4.3 g) was separated into two subfractions by column chromatography over silica gel (RP-18) eluting with gradient H2O-MeOH (50–100%). Fr.4-1 (1.4 g) was separated by Sephadex LH-20 (3 × 75 cm, MeOH, 300 mL) to obtain three fractions (130 mL, Fr.4-1-1; 90 mL, Fr.4-1-2; 80 mL, Fr.4-1-3). Fr.4-1-2 (140 mg) was purified by semipreparative HPLC (30% MeOH/H2O) to yield 5 (1 mg, tR 8.24 min), 13 (30 mg, tR 20.20 min), and 15 (5 mg, tR 18.15 min). Fr.4-1-3 (190 mg) was purified by semipreparative HPLC (30% MeOH/H2O, 0.15% CF3CO2H) to give 4 (15 mg, tR 16.76 min) and 8 (30 mg, tR 14.41 min). Fr.4-2 (360 mg) was purified by semipreparative HPLC (50% MeOH/H2O) to give 6 (10 mg, tR 12.28 min), and 14 (24 mg, tR 18.12 min). Fr.5 (1.1 g) was separated into two subfractions by a silica gel column (2.6 × 10 cm, 200–300 mesh) eluted with MeOH–CHCl3 (V/V 1:40, 1L). Fr.5-1 (40 mg) was purified by semipreparative HPLC (25% MeOH/H2O) to give 3 (3 mg, tR 6.76 min), and Fr.5-2 (80 mg) was purified by semipreparative HPLC (50% MeOH/H2O, 0.15% CF3CO2H) to give compounds 11 (5 mg, tR 6.78 min). Fr.6 (2.8 g) was separated into two subfractions by a silica gel column (4.5 × 10 cm, 200–300 mesh) eluted with CHCl3-petroleum ether MeOH–CHCl3 (V/V 1:25, 2L). Fr.6-1 (110 mg) was purified by semipreparative HPLC (60% MeOH/H2O) to give 9 (10 mg, tR 10.28 min). Fr.6-2 (340 mg) was purified by semipreparative HPLC (50% MeOH/H2O) to give 7 (18 mg, tR 13.55 min) and the mixture of 1 and 2 (70 mg, tR 5.56 min). The mixture of 1 and 2 were further purified by a chiral column (Chiralpak IA, MeOH–MeCN–EtOH 40:40:20) to yield compound 1 (35.4 mg, tR 8.22 min) and 2 (23.7 mg, tR 4.69 min).
ECD, [α]D and Coupling Constant Calculation
Calculations for ECD and [α]D were performed in HyperChem 7.5 and Gaussian 03 (Frisch et al., 2004; Chen et al., 2016; Jin et al., 2018). Karplus formula was used to compute the coupling constant (3J) from the proton-proton torsion angle (Haasnoot et al., 1980).
Cytotoxic Assays
Cytotoxicities of compounds 1–14 against HL-60 and K562 cell lines were assayed by the MTT method (Mosmann, 1983), while those for BEL-7402, A549, HeLa, and H1975 cell lines were tested by SRB (Skehan et al., 1990) methods. Adriamycin was used as the positive control with the IC50 values of 0.02, 0.21, 0.48, 1.32, 0.32, and 0.38, respectively.
Anti-oxidant Activities
The anti-oxidant activities of compounds 1–14 were evaluated by DPPH assay in vitro (Wang et al., 2007). Vitamin C was used as the positive control with an IC50 value of 3.29 μM.
Antimicrobial Assays
The antimicrobial activities of compounds 1–14 against E. coli, E. aerogenes, P. aeruginosa, B. subtilis, and C. albicans were evaluated by an agar dilution method (Zaika, 1988). Ciprofloxacin lactate and ketoconazole was used as the positive controls for bacteria and fungi with MIC values of 4.0, 0.5, 32.0, 16.0, 4.1 μg/mL, respectively.
Results and Discussion
Identification of Compounds
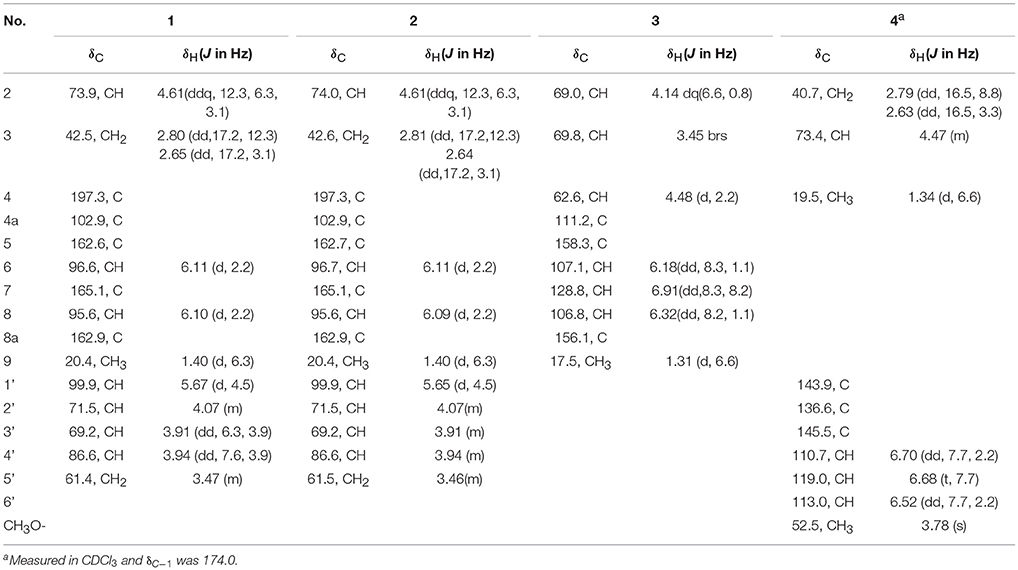
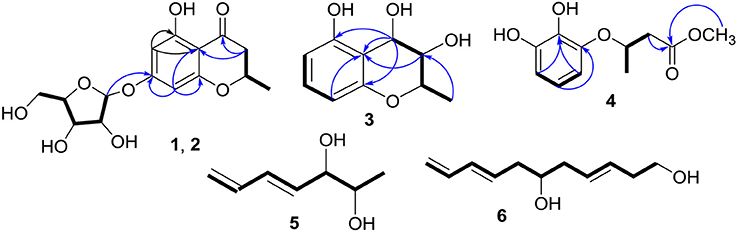
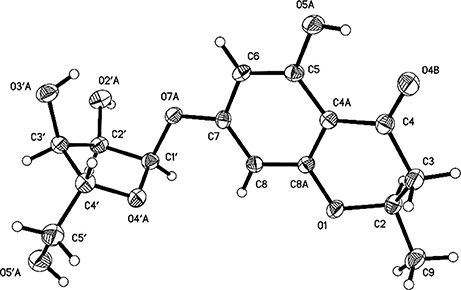
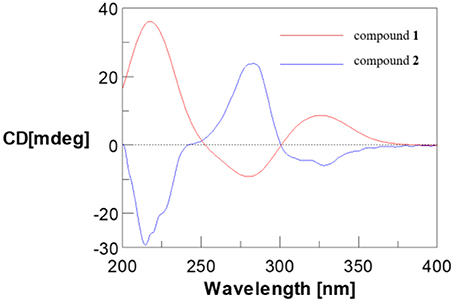
Compounds 1 and 2 were first isolated as an isomeric mixture whose molecular formula was determined to be C15H18O8 by HRESIMS at m/z 327.1068 [M+H]+ (calcd 327.1080), indicating seven degrees of unsaturation. An interpretation of the 1D (Table 1, Figures S1–S3) and 2D NMR (Figure 2 and Figures S4–S6) spectra established a pentose moiety and a benzopyrane moiety similar to those of 5,7-dihydroxy-2-methylchroman-4-one (11) (Rao et al., 1994). The upfield shift of C-7 (−1.5 ppm) and the key HMBC correlations between the anomeric proton ( 5.66/5.68) and C-7 (δ 165.1/165.0) indicated that 1 and 2 were 7-O-pentosides of 11. Acidic hydrolysis of the mixture of 1 and 2 with 2 M HCl yielded (±)-11 and D-ribose that was identified by GC-MS analysis of the reaction products with L-cysteine methyl ester and Me3SiCl (Figures S33, S34) (Deyrup et al., 2007). These data indicated that 1 and 2 are a pair of epimers at C-2. Separation of 2-epimeric mixture of 1 and 2 was achieved on a chiral column using MeOH-MeCN-EtOH as eluent. And then, NMR data of optically-pure 1 (Figures S7, S8) and 2 (Figures S9, S10) were obtained. X-ray single crystal diffraction of 1 revealed the α-glycosidic bond and 2R-configuration (Figure 3). The ECD Cotton effects of compounds 1 and 2 were opposite in sign (Figure 4), confirming the opposite configuration of C-2. Thus, the structures of 1 and 2 were unambiguously elucidated as (2R)- and (2S)-7-O-α-D- ribofuranosyl-5-hydroxy-2-methylchroman-4-one, respectively. This is the first time that to solidify the absolute configuration of compound 1, although it was reported last year (Hu et al., 2017).
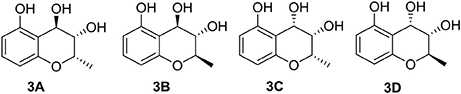
The molecular formula of compound 3 was determined to be C10H12O4 based on the HRESIMS peak at m/z 195.0659 [M–H]− (calcd 195.0657), indicating five degrees of unsaturation. The NMR data (Table 1, Figures S11–S14) was similar to those of 10 (Teles et al., 2005), except for the upfield methylene signal at δH/C 1.50 & 1.88/38.0 that was replaced by the one of an oxygenated methine at δH/C 3.45/69.8. This was further supported by the 1H-1H COSY from H-9 (δ 1.31) to H-4 (δ 4.48) through H-2 (δ 4.14) and H-3 (δ 3.45) (Figure 2 and Figure S15), and the key HMBC correlations of H-9 to C-3 (δ 69.8) and H-3 to C-4a (δ 111.2) (Figure 2 and Figure S16). In order to confirm the relative configuration, we calculated the coupling constant of H2-H3 and H3-H4 for the four possible relative configurations 3A–3D (Figure 5). The computational 3J value of 3A was most near to the measured result (Table 3). The absolute configuration was established by calculation of the specific rotation. The measured [α]D value of 3 (−53.6) is consistent with the calculated one for (2S,3S,4R)-3 (−100) and opposite to the calculated one for (2R,3R,4S)-3 (+102). Thus, the structure of 3 was identified as (2S,3S,4R)-2-methylchroman-3,4,5-triol.
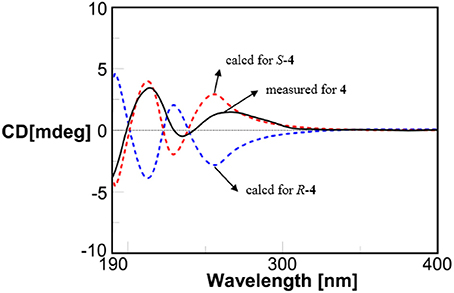
Compound 4 showed the molecular formulae of C11H14O5 based on HRESIMS peaks at m/z 225.0767 [M–H]− (calcd 225.0763), indicating five degrees of unsaturation. The 1D (Figures S17–S19) and HMQC (Figure S20) NMR spectra of 4 displayed three sp2 methines and four sp2 quaternary carbon signals, one sp3 oxygenated methine signals, one sp3 methylene signals and two methyl group (including one methoxy). The 1D NMR data (Table 1) of 4 were almost identical to those of 8 [Figure S1; (Dai et al., 2009)] except for an additional methoxy (δH/C 3.78/52.5) and the upfield shift for carbonyl carbon (−3.5 ppm), indicating that 4 is the methyl ester of 8. This was confirmed by analysis of 1H-1H COSY correlation (Figure S21) and the key HMBC between the methoxy protons at δH 3.78 and the carbonyl carbon at δC−1 174.0 (Figure 2 and Figure S22). The specific rotations of both 4 ([α]D +14.4) and 8 ([α]D +8.2) were opposite to the synthetic analog, R-3-(3-methoxyphenyloxy)butanoic acid ([α]D −31.2) (Kawasaki et al., 2008), indicating both 4 and 8 as S-configuration. The S-configuration of 4 was also backed by the coincidence of experimental and calculated ECD curves (Figure 6). Thus, the structure of compounds 4 and 8 were established as methyl (3S)-3-(2,3-dihydroxyphenyloxy) butanoate and (3S)-3-(2,3-dihydroxyphenyloxy)butanoic acid, respectively.
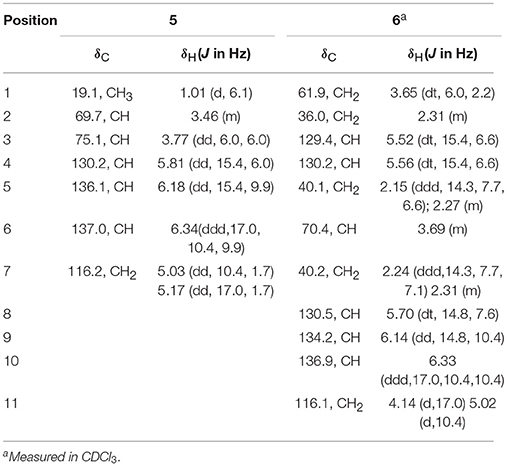
The molecular formula of compound 5 was determined as C7H12O2 based on the HREIMS peak at m/z 128.0845 [M]+ (calcd 128.0837), corresponding to two degrees of unsaturation. The IR spectrum showed hydroxy groups at 3442 cm−1 and double bonds at 3080 and 1646 cm−1. The 1D NMR spectra (Table 2, Figures S23–S25) of 5 showed two double bonds one of which is terminal, two oxygenated methines and one methyl group (Table 1). These groups were connected to the full structure of CH2 = CH–CH = CH–CH (OH)–CH (OH)–CH3 on the basis of 1H-1H COSY correlations from the methyl (δH 1.01) to the methylene (δH 5.03/5.17) through the two oxygenated methines (Figure 2 and Figure S26). The large value of 3JH−4, H−5 (15.4 Hz) corresponded to E-Δ4 double bond. The large 3JH−2, H−3 value (6.0 Hz) and the downfield methyl carbon signal (δC−1 19.1) indicated an anti-conformation (Jarvis et al., 1996; Zhang and O'doherty, 2005; Nilewski et al., 2009), corresponding to threo-configuration of 2,3-diol (Zheng et al., 2010). In order to confirm the relative configuration of compound 5, 3JH−2, H−3 of threo-5 and erythro-5 were computed. The results showed that the predicted 3JH−2, H−3 values of threo-5 (5.5 Hz) matched with the measured one (6.0 Hz) while the calculated one of erythro-5 (3.8 Hz) was inconsistent, indicating threo- configuration. The direction of the specific rotation of 5 ([α]D −6.8) were similar to the structurally related t-butyl (6S,7S)-6,7-dihydroxyocta-2,4-dienoate ([α]D −23) (Zhang and O'doherty, 2005), and opposite to t-butyl (6R,7R)-6,7-dihydroxyocta-2,4-dienoate ([α]D +22.9) (Zhang and O'doherty, 2005). The structure of 5 was thus deduced as (2S,3S,4E)-hepta-4,6-diene-2,3-diol.
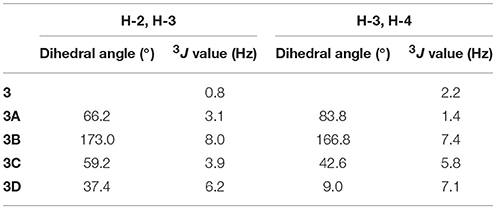
Table 3. The calculated 3JH−2,H−3 and 3JH−3,H−4 values of compound 3 for the four possible relative configurations.
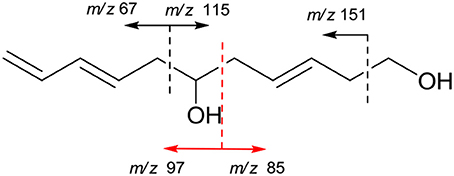
The molecular formula of compound 6 was determined to be C11H18O2 based on the HREIMS peak at m/z 182.1305 [M]+ (calcd. 182.1307), indicating three degrees of unsaturation. The EIMS of 6 illustrated in Figure 7 indicates the existence of -CH2OH, -C6H9O, and -C5H9O moieties. The 1H (Figure S27) and 13C (Figure S28) NMR spectra and DEPT (Figure S29) and HMQC (Figure S30) experiments of 6 revealed 11 signals including three double bonds one of which is terminal, one oxygenated methine, four methylenes one of which is oxygenated. The 1H-1H COSY (Figure S31) correlations from H-1 (δ 3.65) to H-11 (δ 4.14/5.02) in sequence established the structure, CH2 = CH–CH = CH–CH2-CH(OH)–CH2-CH = CH–CH2-CH2OH, which was supported by HMBC correlations (Figure S32). The large values of 3JH−3, H−4 (15.4 Hz) and 3JH−8, H−9 (14.8 Hz) suggested that both Δ3 and Δ8 double bonds were E-configurations. The direction of specific rotation of 6 ([α]D +2.0) is similar to that of (S)-dodeca-3,5-diene-1,7-diol ([α]D +56) (Zhang and Kyler, 1989), suggesting S-configuration at C-6. Thus, the structure of 6 was deduced as (3E,8E,6S)-undeca-3,8,10-triene-1,6-diol.
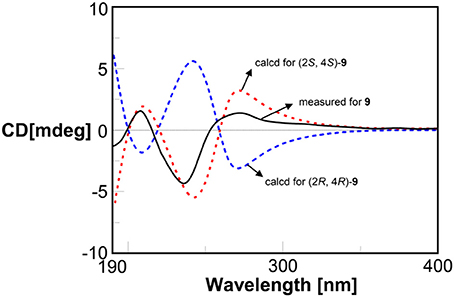
The relative configurations of compounds 9 and 10 were determined as (–)-trans-4-methoxy-2-methylchroman-5-ol (Wu et al., 2010) and (–)-trans-2-methyl chroman-4,5 -diol (Teles et al., 2005), respectively. The absolute configuration of compound 9 was determined by quantum chemical ECD calculation. The measured ECD of 9 was coincident with the calculated ECD of (2S,4S)-9 and opposite to ECD of (2R,4R)-9 (Figure 8). Thus, compound 9 was established to be (2S,4S)-4-methoxy-2-methyl chroman-5-ol. The similar sign of the specific rotations of 9 and 10 ( −2.0 vs. −6.0, MeOH) suggests the same absolute configuration. Therefore, compound 10 was determined to be (2S,4S)-2-methylchroman-4,5-diol. The absolute configurations of compounds 9 and 10 were determined for the first time in this study.
(2R)-7-O-α-D-Ribofuranosyl-5-hydroxy-2-methylchroman-4-one (1): White amorphous powder; +198.9 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (3.62), 278 (3.55), 320 (2.77) nm; ECD (MeOH) λmax (Δε) 211 (+14.21), 284 (−3.51), 327 (+3.42); IR (KBr) νmax 3,416, 1,646, 1,573, 1,354, 1,295, 1,195, 1,155, 1,076, 1,029 cm−1; 1H and 13C NMR (Table 1); HRESIMS m/z 327.1068 [M+H]+ (calcd for C15H19O8 327.1080).
(2S)-7-O-α-D-Ribofuranosyl-5-hydroxy-2-methylchroman-4-one (2): White amorphous powder; +118.6 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (3.62), 278 (3.55), 320 (2.77) nm; ECD (MeOH) λmax (Δε) 211 (−10.04), 284 (+9.40), 330 (−2.13); IR (KBr) νmax 3,416, 1,646, 1,573, 1,354, 1,295, 1,195, 1,155, 1,076, 1,029 cm−1; 1H and 13C NMR (Table 1); HRESIMS m/z 327.1068 [M+H]+ (calcd for C15H19O8 327.1080).
(2S,3S,4R)-2-Methylchroman-3,4,5-triol (3): Colorless oil; −53.6 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 200 (3.25), 270 (2.26) nm; IR (KBr) νmax 3,429, 2,356, 1,627, 1,4,01, 1,090 cm−1; 1H and 13C NMR (Table 1); HRESIMS m/z 195.0659 [M–H] − (calcd. for C10H11O4: 195.0657).
Methyl (3S)-3-(2,3-dihydroxyphenyloxy)butanoate (4): Colorless oil; +14.4 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 200 (3.32), 270 (2.19) nm; IR (KBr) νmax 3,409, 2,356, 1,706, 1,606, 1,481, 1,202, 1,063, 1,010 cm−1; 1H and 13C NMR (Table 1); HRESIMS m/z 225.0767 [M–H]−(calcd. for C11H13O5 225.0763)
(2S,3S,4E)-Hepta-4,6-diene-2,3-diol (5): Colorless oil; −6.8 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 200 (3.09), 217 (3.38) nm; IR (KBr) νmax 3442, 3080, 1646, 1540, 1023, 446 cm−1; 1H and 13C NMR (Table 2); EIMS m/z (%): 129 (45), 256 (8), 111 (26), 97 (51), 83 (69), 82 (38); HREIMS m/z 128.0845 [M]+ (calcd. for C7H12O2 128.0837).
(3E,8E,6S)-Undeca-3,8,10-trien-1,6-diol (6): Colorless oil; +2.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 200 (2.86), 218 (3.16) nm; IR (KBr) νmax 3,390, 2,927, 1,715, 1,421, 1,047, 973 cm−1; 1H and 13C NMR (Table 2); EIMS m/z (%): 181 (8), 164 (9), 151 (14), 129 (17), 115 (16), 97 (31), 85 (28), 71 (53), 67(88), 53(10); HREIMS m/z 182.1305 [M]+ (calcd. for C11H18O2 182.1307).
X-ray Crystallographic Data of 1
Compound 1 was obtained as a colorless monoclinic crystal with molecular formula of C15H18O8 from MeOH and H2O. Space group P21, a = 7.0121(7) Å, b = 10.6659(11) Å, c = 9.8560(8) Å, α = 90.00°, β = 95.3230(10)°, γ = 90.00°, V = 733.95(12) Å3, Z = 2, Dcalcd = 1.476 mg/m3, μ = 0.121 mm−1, F(000) = 344, crystal size 0.42 ×0.30 ×0.21 mm. A total of 3413 unique reflections (2θ<50°= were collected on a CCD area detector diffractometer with graphite monochromated Mo-Ka radiation (λ = 0.71073 Å). The structure was solved by direct methods (SHELXS-97) and expanded using Fourier techniques (SHELXL-97). The final cycle of full-matrix least squares refinement was based on 2053 unique reflections (2θ <50°) and 210 variable parameters and converged with unweighted and weighted agreement factors of R1 = 0.0421, Rw = 0.0981 and R = 0.0374 for I>2sigma(I) data. Crystallographic data (excluding structure factors) for structure 1 in this paper have been deposited in the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 883328 [fax: +44 (0)-1223-336033 or e-mail: ZGVwb3NpdEBjY2RjLmNhbS5hYy51aw==].
Biogenetic Origin
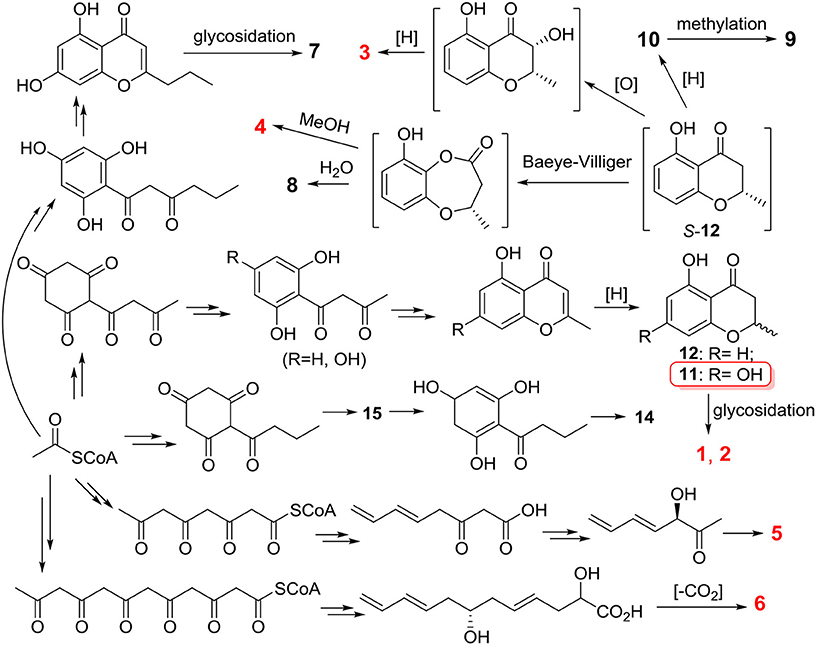
These compounds were postulated to be biosynthesized by the polyketide pathway from acetyl coenzyme A (Figure 9). The acetyl-CoA units underwent condensation, cyclization, dehydration and hydrogenation to produce compounds 11 and 12. Compound 11 formed compounds 1 and 2 by glycosidation. (S)-12 underwent oxidation and reduction to yield compound 3. The reduction of (S)-12 produced compound 10 that was transformed to compound 9 followed by methylation. (S)-12 was subjected to Baeyer-Villiger oxidation followed by methanolysis and hydrolysis to yield compounds 4 and 8, respectively. Compounds 5 and 6 were formed from different lengths of acetyl-CoA units by condensation, reduction, dehydration, and decarboxylation. The condensation of acetyl-CoA units followed by cyclization and reduction formed compound 15 that was transformed to compound 14 after enolization and dehydration.
Biological Activity
Compounds 1–14 were tested for cytotoxic effects on the HL-60, BEL-7402, K562, A549, HeLa, and H1975 cell lines, DPPH scavenging activity, and antimicrobial activities against E. coli, E. aerogenes, P. aeruginosa, B. subtilis, and C. albicans. As the results, compound 6 was cytotoxic to H1975 cell line with an IC50 values of 10.0 μM, while compounds 4 and 8–10 showed DPPH radical scavenging activity with the IC50 values of 2.65, 0.24, 5.66, and 6.67 μM, respectively. None of the compounds exhibit antimicrobial activities.
Conclusions
Five new polyketides were isolated and identified from the fermentation of the mangrove fungus Cladosporium sp. OUCMDZ-302 with Excoecaria agallocha. The new compound 4 showed DPPH radical scavenging activity with an IC50 value of 2.65 μM.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This research was financially supported by the National Natural Science Foundation of China (NSFC, Nos. 81561148012, U1501221 and 21502034), the 100 Leading Talents of Guizhou Province (fund for W Zhu), the science and technology project of Guizhou (Grant No. QKHT Z-2014-4007), the technology plan of Guizhou (Grant No. QKH YSZ-2015-4009), and by the Thailand Research Fund (TRF) through the International Research Network (IRN-58W0004). The fungus Cladosporium sp. OUCMDZ-302 was identified by Prof. Kui Hong, Wuhan University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2018.00344/full#supplementary-material
References
Anjaneyulu, A. S., and Rao, V. L. (2000). Five diterpenoids (agallochins A–E) from the mangrove plant Excoecaria agallocha Linn. Phytochemistry 55, 891–901. doi: 10.1016/S0031-9422(00)00251-X
Anjaneyulu, A. S., Rao, V. L., and Sreedhar, K. (2002). ent-Kaurane and beyerane diterpenoids from Excoecaria agallocha. J. Nat. Prod. 65, 382–385. doi: 10.1021/np010262u
Anjaneyulu, A. S., Rao, V. L., and Sreedhar, K. (2003). Agallochins J-L, new isopimarane diterpenoids from Excoecaria agallocha L. Nat. Prod. Res. 17, 27–32. doi: 10.1080/1057563021000027975
Annam, S. Ch., Ankireddy, M., Sura, M. B., Ponnapalli, M. G., Sarma, A. V., and S, JB. (2015). Epimeric excolides from the stems of Excoecaria agallocha and structural revision of rhizophorin A. Org. Lett. 17, 2840–2843. doi: 10.1021/acs.orglett.5b01257
Chen, Z. B., Hao, J. J., Wang, L. P., Wang, Y., Kong, F. D., and Zhu, W. M. (2016). New α-glucosidase inhibitors from marine algae-derived Streptomyces sp. OUCMDZ-3434. Sci. Rep. 6:20004. doi: 10.1038/srep20004
Dai, J., Krohn, K., Draeger, S., and Schulz, B. (2009). New naphthalene-chroman coupling products from the endophytic fungus, Nodulisporium sp. from Erica arborea. Eur. J. Org. Chem. 2009, 1564–1569. doi: 10.1002/ejoc.200801106
Dai, J., Krohn, K., Flörke, U., Draeger, S., Schulz, B., Kiss-Szikszai, A., et al. (2006). Metabolites from the endophytic fungus Nodulisporium sp. from Juniperus cedre. Eur. J. Org. Chem. 2006, 3498–3506. doi: 10.1002/ejoc.200600261
Deyrup, S. T., Gloer, J. B., O'donnell, K., and Wicklow, D. T. (2007). Kolokosides A–D: triterpenoid glycosides from a Hawaiian isolate of Xylaria sp. J. Nat. Prod. 70, 378–382. doi: 10.1021/np060546k
Dhami, K. S., and Stothers, J. B. (1965). 13C NMR studies: part iii. carbon-13 nmr spectra of substituted acetophenones. Can. J. Chem. 43, 479–497. doi: 10.1139/v65-064
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., et al. (2004). Gaussian 03, Revision E.01, 1st Edn. Wallingford: Gaussian.
Gowri Ponnapalli, M., Ankireddy, M., Rao Annam, S. C. V. A., Ravirala, S., Sukki, S., and Tuniki, V. R. (2013). Unusual ent-isopimarane-type diterpenoids from the wood of Excoecaria agallocha. Tetrahedron Lett. 54, 2942–2945. doi: 10.1016/j.tetlet.2013.03.105
Haasnoot, C. A. G., De Leeuw, F. A. A. M., and Altona, C. (1980). The relationship between proton-proton NMR coupling constants and substituent electronegativities–I: an empirical generalization of the karplus equation. Tetrahedron 36, 2783–2792. doi: 10.1016/0040-4020(80)80155-4
Hu, M., Yang, X. Q., Zhou, Q. Y., Li, S. Q., Wang, B. Y., Ruan, B. H., et al. (2017). Benzopyran derivatives from endophytic Daldinia eschscholzii JC-15 in Dendrobium chrysotoxum and their bioactivities. Nat. Prod. Res. 1–5. doi: 10.1080/14786419.2017.1419236
Huang, H. R., Feng, X. L., She, Z. G., Lin, Y. C., Vrijmoed, L. L. P., and Jones, E. B. G. (2005). 1-(2,6-Dihydroxyphenyl)butanone. Acta Crys. Sec. E 61, o282–o283. doi: 10.1107/S160053680500022X
Igarashi, M., Tetsuka, Y., Mimura, Y., Takahashi, A., Tamamura, T., Sato, K., et al. (1993). AB5046 A and B, novel chlorosis-inducing substances from Nodulisporium sp. J. Antibiot. 46, 1843–1848. doi: 10.7164/antibiotics.46.1843
Jadulco, R., Brauers, G., Edrada, R. A., Ebel, R., Wray, V., Sudarsono, S., et al. (2002). New metabolites from sponge-derived fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 65, 730–733. doi: 10.1021/np010390i
Jadulco, R., Proksch, P., Wray, V., Sudarsono, Berg, A., and Gräfe, U. (2001). New macrolides and furan carboxylic acid derivative from the sponge-derived fungus Cladosporium herbarum. J. Nat. Prod. 64, 527–530. doi: 10.1021/np000401s
Jarvis, B. B., Wang, S., and Ammon, H. L. (1996). Trichoverroid stereoisomers. J. Nat. Prod. 59, 254–261.doi: 10.1021/np960078m
Jin, Y., Qin, S., Gao, H., Zhu, G., Wang, W., Zhu, W., et al. (2018). An anti-HBV anthraquinone from aciduric fungus Penicillium sp. OUCMDZ-4736 under low pH stress. Extremophiles 22, 39–45. doi: 10.1007/s00792-017-0975-6
Kang, J., Chen, R. Y., and Yu, D. Q. (2005). A new isopimarane-type diterpene and a new natural atisane-type diterpene from Excoecaria agallocha. J. Asian. Nat. Prod. Res. 7, 729–734. doi: 10.1080/1028602042000324943
Kawasaki, M., Asano, Y., Katayama, K., Inoue, A., Hiraoka, C., Kakuda, H., et al. (2008). Asymmetric synthesis of 2-substituted 4-chromanones using enzyme-catalyzed reactions. J. Mol. Catal. B-Enzy. 54, 93–102. doi: 10.1016/j.molcatb.2007.12.022
Kong, F., Wang, Y., Liu, P., Dong, T., and Zhu, W. (2014). Thiodiketopiperazines from the marine-derived fungus Phoma sp. OUCMDZ-1847. J. Nat. Prod. 77, 132–137. doi: 10.1021/np400802d
Konishi, T., Konoshima, T., Fujiwara, Y., Kiyosawa, S., Miyahara, K., and Nishi, M. (1999). Stereostructures of new labdane-type diterpenes, excoecarins F, G1, and G2 from the wood of Excoecaria agallocha. Chem. Pharm. Bull. 47, 456–458. doi: 10.1248/cpb.47.456
Konishi, T., Konoshima, T., Maoka, T., and Fujiwara, Y. (2000). Novel diterpenes, excoecarins M and N from the resinous wood of Excoecaria agallocha. Tetrahedron. Lett. 41, 3419–3422. doi: 10.1016/S0040-4039(00)00391-9
Konishi, T., Yamazoe, K., Konoshima, T., and Fujiwara, Y. (2003). Seco-labdane type diterpenes from Excoecaria agallocha. Phytochemistry 64, 835–840. doi: 10.1016/j.phytochem.2003.09.001
Li, Y., Liu, J., Yu, S., Proksch, P., Gu, J., and Lin, W. (2010). TNF-α inhibitory diterpenoids from the chinese mangrove plant Excoecaria agallocha L. Phytochemistry 71, 2124–2131.doi: 10.1016/j.phytochem.2010.08.011
Lin, Z., Zhu, T., Fang, Y., Gu, Q., and Zhu, W. (2008). Polyketides from Penicillium sp. JP-1, an endophytic fungus associated with the mangrove plant Aegiceras corniculatum. Phytochemistry 69, 1273–1278. doi: 10.1016/j.phytochem.2007.10.030
Lu, Z., Wang, Y., Miao, C., Liu, P., Hong, K., and Zhu, W. (2009). Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. J. Nat. Prod. 72, 1761–1767. doi: 10.1021/np900268z
Lu, Z., Zhu, H., Fu, P., Wang, Y., Zhang, Z., Lin, H., et al. (2010). Cytotoxic polyphenols from the marine-derived fungus Penicillium expansum. J. Nat. Prod. 73, 911–914. doi: 10.1021/np100059m
Mosmann, T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65, 55–63.doi: 10.1016/0022-1759(83)90303-4
Nilewski, C., Geisser, R. W., Ebert, M. O., and Carreira, E. M. (2009). Conformational and configurational analysis in the study and synthesis of chlorinated natural products. J. Am. Chem. Soc. 131, 15866–15876. doi: 10.1021/ja906461h
Rao, A. V. R., Gaitonde, A. S., Prakash, K. R. C., and Rao, S. P. (1994). A concise synthesis of chiral 2-methyl chroman-4-ones: stereo selective build-up of the chromanol moiety of anti-HIV agent calanolide A. Tetrahedron Lett. 35, 6347–6350.doi: 10.1016/S0040-4039(00)73429-0
Sakagami, Y., Sano, A., Hara, O., Mikawa, T., and Marumo, S. (1995). Cladosporol, β-1, 3-glucan biosynthesis inhibitor, isolated from fungus, Cladosporium cladosporioides. Tetrahedron Lett. 36, 1469–1472. doi: 10.1016/0040-4039(95)00061-G
Shigemori, H., Kasai, Y., Komatsu, K., Tsuda, M., Mikami, Y., and Kobayashi, J. I. (2004). Sporiolides A and B, new cytotoxic twelve-membered macrolides from a marine-derived fungus Cladosporium species. Marine Drugs 2, 164–169. doi: 10.3390/md204164
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., et al. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer. Inst. 82, 1107–1112. doi: 10.1093/jnci/82.13.1107
Teles, H. L., Silva, G. H., Castro-Gamboa, I., Bolzani Vda S., Pereira, J. O., Costa-Neto, C. M., et al. (2005). Benzopyrans from Curvularia sp., an endophytic fungus associated with Ocotea corymbosa (Lauraceae). Phytochemistry 66, 2363–2367.doi: 10.1016/j.phytochem.2005.04.043
Wang, J. D., and Guo, Y. W. (2004). Agallochaols A and B, two new diterpenes from the Chinese mangrove Excoecaria agallocha L. Hel. Chim. Acta. 87, 2829–2833. doi: 10.1002/hlca.200490253
Wang, J. D., Li, Z. Y., and Guo, Y. W. (2005). Secoatisane- and isopimarane- type diterpenoids from the Chinese mangrove Excoecaria agallocha L. Hel. Chim. Acta. 88, 979–985. doi: 10.1002/hlca.200590092
Wang, J., Lu, Z., Liu, P., Wang, Y., Li, J., Hong, K., et al. (2012). Cytotoxic polyphenols from the fungus Penicillium expansum 091 006 endogenous with the mangrove plant Excoecaria agallocha. Planta. Med. 78, 1861–1866. doi: 10.1055/s-0032-1315395
Wang, W. L., Zhu, T. J., Tao, H. W., Lu, Z. Y., Fang, Y. C., Gu, Q. Q., et al. (2007). Three novel, structurally unique spirocyclic alkaloids from the halotolerant B-17 fungal strain of Aspergillus variecolor. Chem. Biodivers. 4, 2913–2919. doi: 10.1002/cbdv.200790240
Wang, Z. C., Lin, Y. M., Feng, D. Q., Ke, C. H., Lin, P., Yan, C. L., et al. (2009). A new atisane-type diterpene from the bark of the mangrove plant Excoecaria agallocha. Molecules 14:414. doi: 10.3390/molecules14010414
Wu, J., Xiao, Q., Xu, J., Li, M. Y., Pan, J. Y., and Yang, M. H. (2008). Natural products from true mangrove flora: source, chemistry and bioactivities. Nat. Prod. Rep. 25, 955–981. doi: 10.1039/b807365a
Wu, Z. C., Li, D. L., Chen, Y. C., and Zhang, W. M. (2010). A new isofuranonaphthalenone and benzopyrans from the endophytic fungus Nodulisporium sp. A4 from Aquilaria sinensis. Hel. Chim. Acta 93, 920–924. doi: 10.1002/hlca.200900307
Ye, Y. H., Zhu, H. L., Song, Y. C., Liu, J. Y., and Tan, R. X. (2005). Structural revision of aspernigrin A, reisolated from Cladosporium herbarum IFB-E002. J. Nat. Prod. 68, 1106–1108. doi: 10.1021/np050059p
Zaika, L. L. (1988). Spices and herbs: their antimicrobial activity and its determination1. J. Food Safety 9, 97–118. doi: 10.1111/j.1745-4565.1988.tb00511.x
Zhang, H. W., Song, Y. C., and Tan, R. X. (2006). Biology and chemistry of endophytes. Nat. Prod. Rep. 23, 753–771. doi: 10.1039/b609472b
Zhang, H., Tomoda, H., Tabata, N., Miura, H., Namikoshi, M., Yamaguchi, Y., et al. (2001). Cladospolide D, a new 12-membered macrolide antibiotic produced by Cladosporium sp. FT-0012. J. Antibiot 54, 635–641. doi: 10.7164/antibiotics.54.635
Zhang, P., and Kyler, K. S. (1989). Enzymic asymmetric hydroxylation of pentadienols using soybean lipoxygenase. J. Am. Chem. Soc. 111, 9241–9242. doi: 10.1021/ja00208a024
Zhang, Y., and O'doherty, G. A. (2005). Remote steric effect on the regioselectivity of Sharpless asymmetric dihydroxylation. Tetrahedron 61, 6337–6351.doi: 10.1016/j.tet.2005.03.119
Zhao, G. Y., Fan, J. Y., Hua, C. P., Yan, W., Chen, C. J., Lu, Y. H., et al. (2015). Resveratrol improves fungal ribosylation capacity through a unique mechanism. RSC Adv. 5, 5657–5663. doi: 10.1039/C4RA12851F
Zheng, J., Xu, Z., Wang, Y., Hong, K., Liu, P., and Zhu, W. (2010). Cyclic tripeptides from the halotolerant fungus Aspergillus sclerotiorum PT06-1. J. Nat. Prod. 73, 1133–1137. doi: 10.1021/np100198h
Keywords: Cladosporium sp., mangrove fungus, Excoecaria agallocha, polyketides, anti-oxidation
Citation: Wang L, Han X, Zhu G, Wang Y, Chairoungdua A, Piyachaturawat P and Zhu W (2018) Polyketides From the Endophytic Fungus Cladosporium sp. Isolated From the Mangrove Plant Excoecaria agallocha. Front. Chem. 6:344. doi: 10.3389/fchem.2018.00344
Received: 08 June 2018; Accepted: 23 July 2018;
Published: 14 August 2018.
Edited by:
Xian-Wen Yang, Third Institute of Oceanography, State Oceanic Administration, ChinaReviewed by:
Massuo Jorge Kato, Universidade de São Paulo, BrazilRoberta Teta, Università degli Studi di Napoli Federico II, Italy
Copyright © 2018 Wang, Han, Zhu, Wang, Chairoungdua, Piyachaturawat and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Zhu, d2VpbWluZ3podUBvdWMuZWR1LmNu
†These authors have contributed equally to this work
 Liping Wang
Liping Wang Xiuli Han
Xiuli Han Guoliang Zhu2
Guoliang Zhu2 Weiming Zhu
Weiming Zhu