Vitamin B1 (thiamin) is an essential coenzyme for all cells. Recent findings from experimental cell biology and genome surveys have shown that thiamin cycling by plankton is far more complex than was previously understood. Many plankton cells cannot produce thiamin (are auxotrophic) and obligately require an exogenous source of thiamin or one or more of 5 different thiamin-related compounds (TRCs). Despite this emerging evidence for the evolution among plankton of complex interactions related to thiamin, the influence of TRCs on plankton community structure and productivity are not understood. We report measurements of three dissolved TRCs 4-amino-5-aminomethyl-2-methylpyrimidine (AmMP), 5-(2-hydroxyethyl)-4-methyl-1,3-thiazole-2-carboxylic acid (cHET), and 4-methyl-5-thiazoleethanol (HET) that have never before been assayed in seawater. Here we characterize them alongside other TRCs that were measured previously [thiamin and 4-amino-5-hydroxymethyl-2-methylpyrimidine (HMP)], in depth profiles from a latitudinal transect in the north Atlantic in March 2018. TRC concentrations ranged from femptomolar to picomolar. Surface depletion relative to a maximum near the bottom of the euphotic zone and low concentrations at deeper depths were consistent features. Our observations suggest that when bacterial abundance and production are low, TRC concentrations approach a steady state where TRC production and consumption terms are balanced. Standing stocks of TRCs also appear to be positively correlated with bacterial production. However, near the period of peak biomass in the accumulation phase of a bloom we observed an inverse relationship between TRCs and bacterial production, coincident with an increased abundance of Flavobacteria that comparative genomics indicates could be vitamin B1 auxotrophs. While these observations suggest that the dissolved pool of TRCs is often at steady state, with TRC production and consumption balanced, our data suggests that bloom induced shifts in microbial community structure and activity may cause a decoupling between TRC production and consumption, leading to increased abundances of some populations of bacteria that are putatively vitamin B1 auxotrophs.
Mesoscale eddies play a key role in structuring open ocean ecosystems, affecting the entire trophic web from primary producers to large pelagic predators including sharks and elephant seals. Recent advances in the tracking of pelagic predators have revealed that these animals forage in the mesopelagic and the depth and duration of their foraging dives are affected by the presence of eddies. The ways in which eddies impact the distribution of mesopelagic micronekton, however, remain largely unknown. During a multi-seasonal experiment we used a shipboard scientific echosounder transmitting at 38 kHz to observe the distribution of acoustic backscattering in the energetic mesoscale eddy field of the northwestern Atlantic. Observations were collected at 24 stations with 6 located in anticyclonic and 7 in cyclonic eddies. The sampled anticyclonic eddies are characterized by intense acoustic backscattering in the mesopelagic and changes in the intensity of acoustic backscattering layers match gradients of surface properties. Furthermore, mesopelagic daytime backscattering is positively correlated with sea level anomaly. These results suggest that anticyclonic eddies in the northwestern Atlantic impact the distribution of mesopelagic micronekton and may have the potential to locally enhance or structure spatially mesopelagic communities.
The annual North Atlantic phytoplankton bloom represents a hot spot of biological activity during which a significant fraction of net community production (NCP) can be partitioned into dissolved organic carbon (DOC). The fraction of seasonal NCP that is not respired by the heterotrophic bacterial community and accumulates as seasonal surplus DOC (ΔDOC) in the surface layer represents DOC export potential to the upper mesopelagic zone, and in the North Atlantic this is facilitated by winter convective mixing that can extend to depths > 400 m. However, estimates of ΔDOC and vertical DOC export for the western North Atlantic remain ill-constrained and the influence of phytoplankton community structure on the partitioning of seasonal NCP as ΔDOC is unresolved. Here, we couple hydrographic properties from autonomous in situ sensors (ARGO floats) with biogeochemical data from two meridional transects in the late spring (∼44–56°N along ∼−41°W) and early autumn (∼42–53°N along ∼−41°W) as part of the North Atlantic Aerosols and Marine Ecosystems Study (NAAMES). We estimate that 4–35% of seasonal NCP is partitioned as ΔDOC and that annual vertical DOC export ranges between 0.34 and 1.15 mol C m–2 in the temperate and subpolar western North Atlantic. Two lines of evidence reveal that non-siliceous picophytoplankton, like Prochlorococcus, are indicator species of the conditions that control the accumulation of DOC and the partitioning of NCP as ΔDOC.
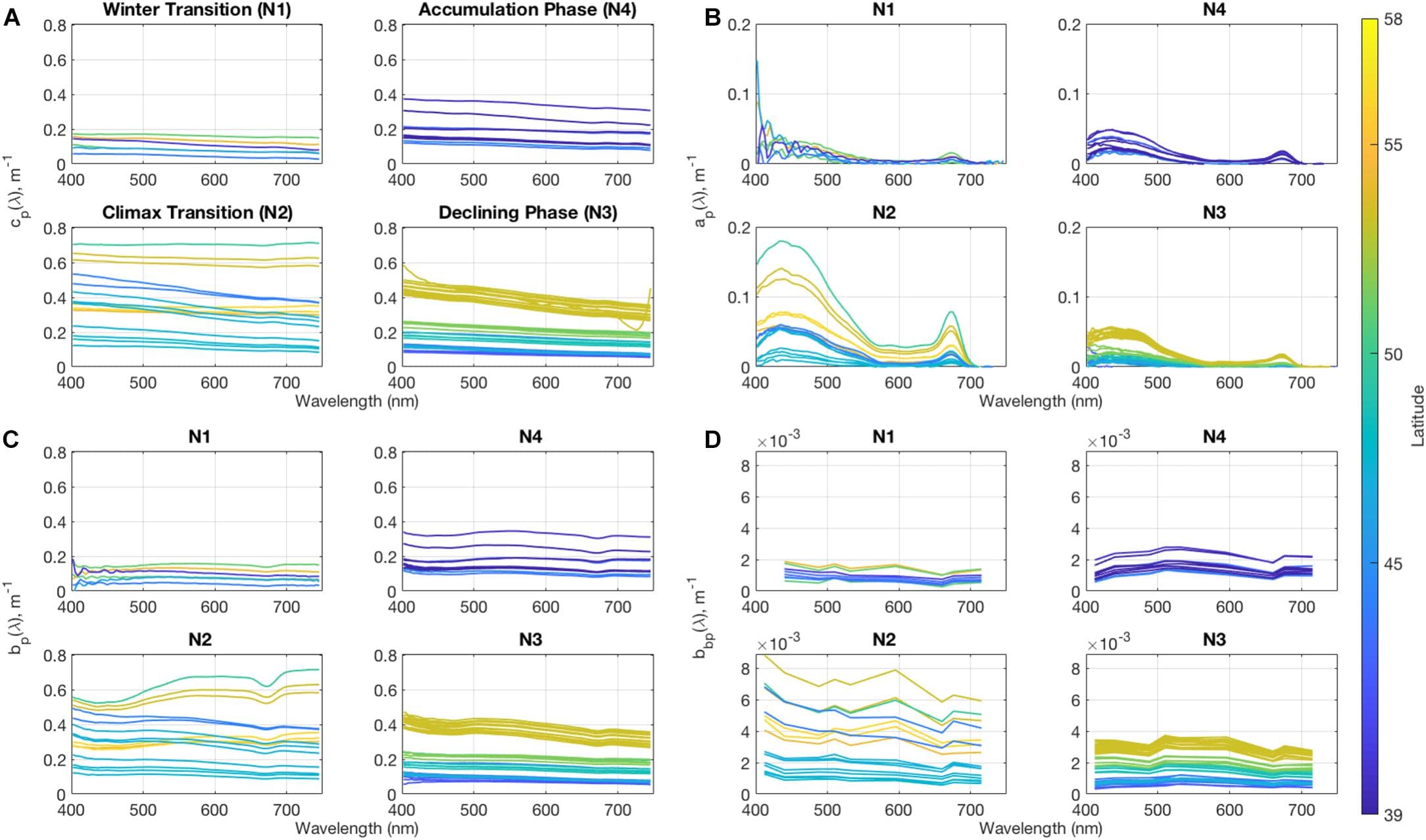
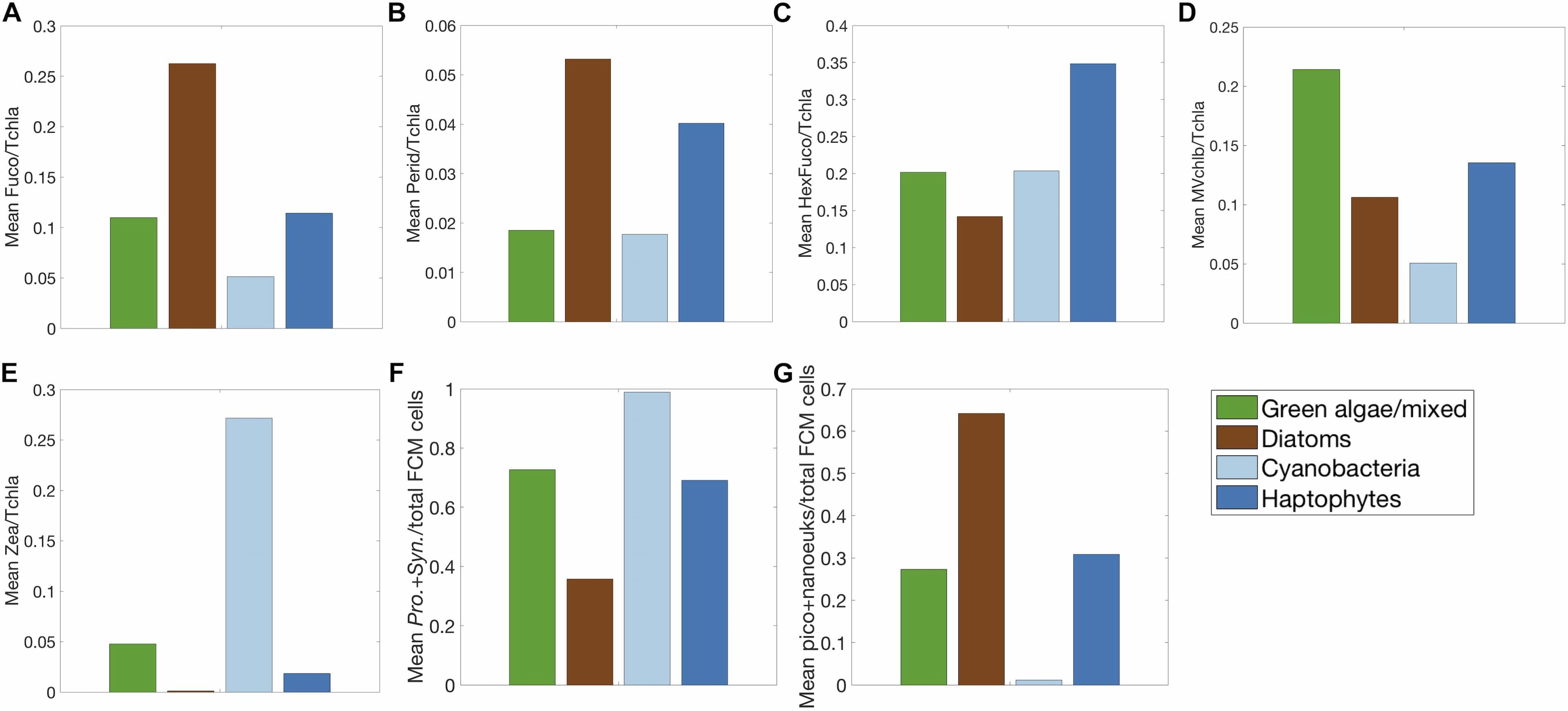
Analysis of phytoplankton chemotaxonomic markers from high performance liquid chromatography (HPLC) pigment determination is a common approach for evaluating phytoplankton community structure from ocean samples. Here, HPLC phytoplankton pigment concentrations from samples collected underway and from CTD bottle sampling on the North Atlantic Aerosols and Marine Ecosystems Study (NAAMES) are used to assess phytoplankton community composition over a range of seasons and environmental conditions. Several data-driven statistical techniques, including hierarchical clustering, Empirical Orthogonal Function, and network-based community detection analyses, are applied to examine the associations between groups of pigments and infer phytoplankton communities found in the surface ocean during the four NAAMES campaigns. From these analyses, five distinguishable phytoplankton community types emerge based on the associations of phytoplankton pigments: diatom, dinoflagellate, haptophyte, green algae, and cyanobacteria. We use this dataset, along with phytoplankton community structure metrics from flow cytometric analyses, to characterize the distributions of phytoplankton biomarker pigments over the four cruises. The physical and chemical drivers influencing the distribution and co-variability of these five dominant groups of phytoplankton are considered. Finally, the composition of the phytoplankton community across the onset, accumulation, and decline of the annual phytoplankton bloom in a changing North Atlantic Ocean is compared to historical paradigms surrounding seasonal succession.
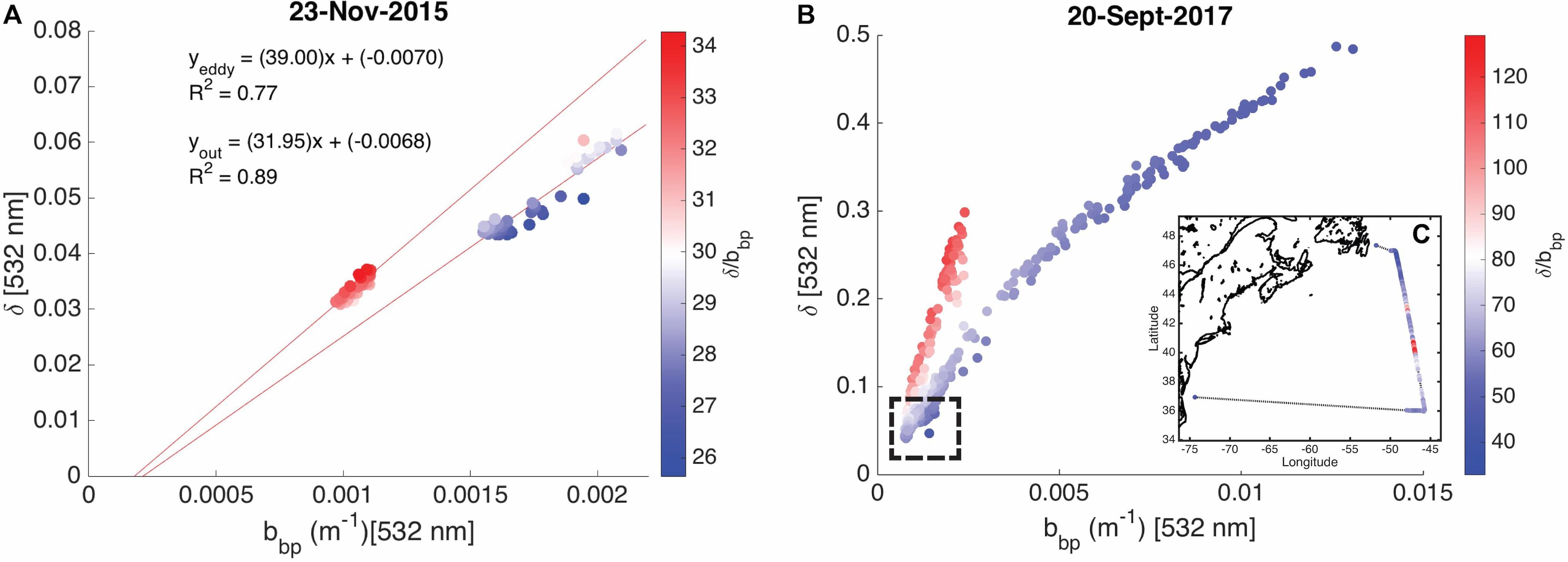
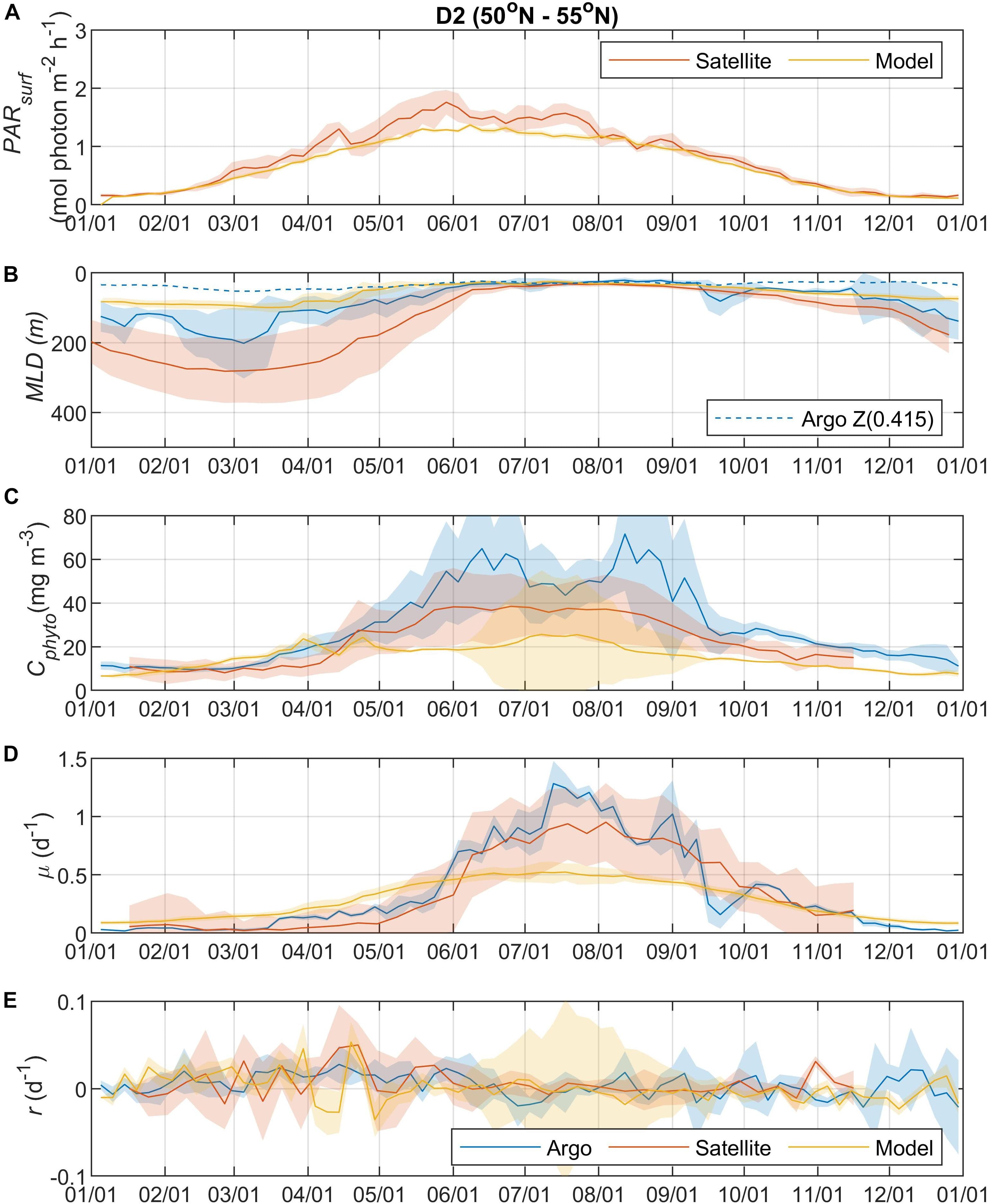
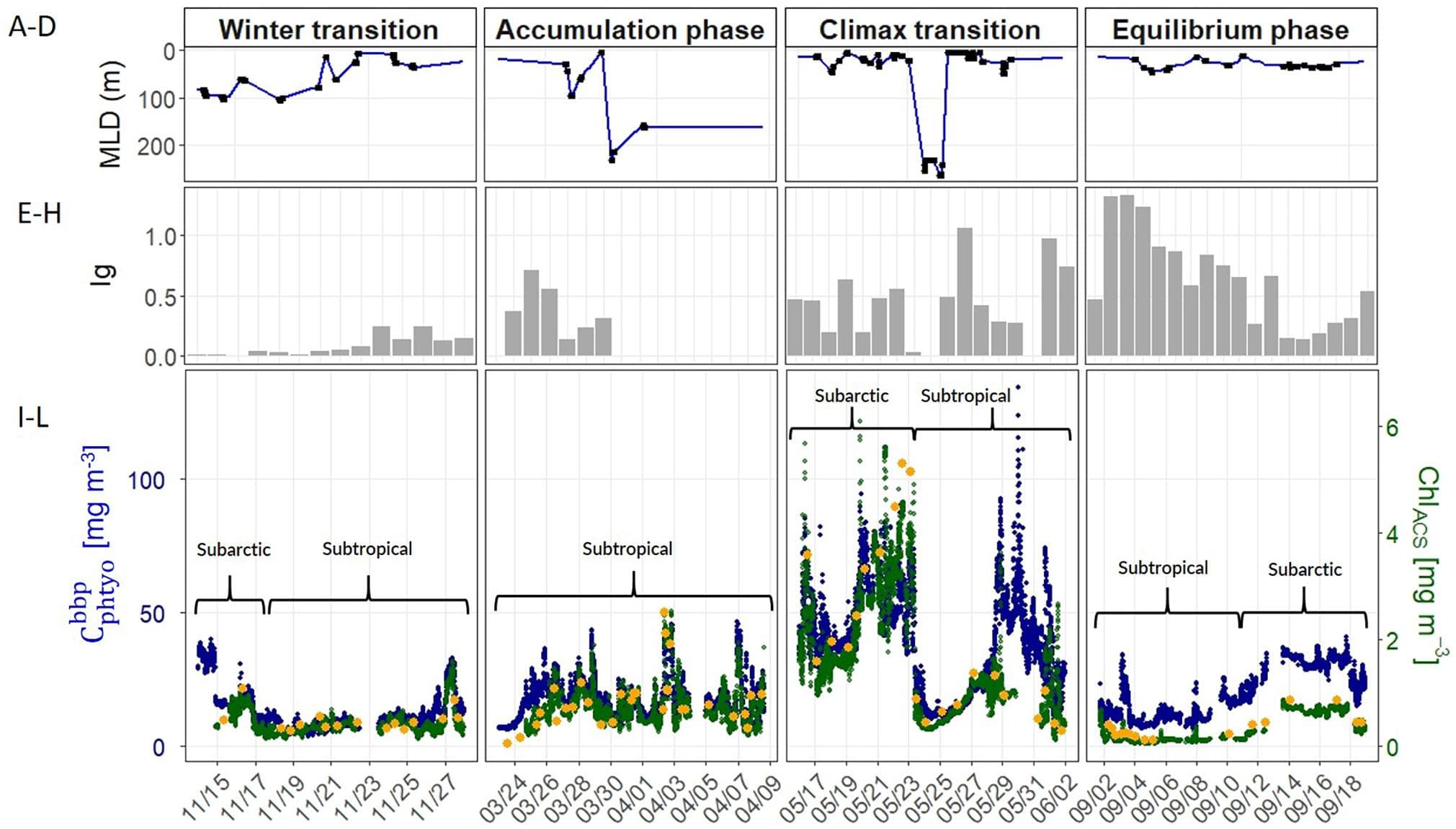
Phytoplankton division rate (μ), loss rate (l), and specific accumulation rate (r) were calculated using Chlorophyll-a (Chl) and phytoplankton carbon (Cphyto) derived from bio-optical measurements on 12 Argo profiling floats in a north-south section of the western North Atlantic Ocean (40° N to 60° N). The float results were used to quantify the seasonal phytoplankton phenology and bloom dynamics for the region. Latitudinally varying phytoplankton dynamics were observed. In the north, the CPhyto peak was higher, occurred later, and was accompanied by higher total annual CPhyto accumulation. In contrast, in the south, stronger μ-r decoupling occurred despite smaller seasonal variations in mixed layer depth (suggesting the possibility of other ecological forcing), and was accompanied by an increasing portion of winter to total annual production, consistent with relief of nutrient limitation. The float observations of phytoplankton phenology for the mixed layer were compared to ocean color satellite remote sensing observations and found to be similar. A similar comparison to an eddy-resolving ocean simulation found the model only reproduced some aspects of the observed phytoplankton phenology, indicating possible biases in the simulated physical forcing, turbulent dynamics, and bio-physical interactions. In addition to seasonal patterns in the mixed layer, the float measurements provided information on the vertical distribution of physical and biogeochemical quantities and therefore are complementary to the remote sensing measurements. Seasonal phenology patterns arise from interactions between “bottom-up” (e.g., resources for growth) and “top-down” (e.g., grazing, mortality) factors that involve both biological and physical drivers. The Argo float data are consistent with the disturbance recovery hypothesis over the full, annual seasonal cycle; for the late winter/early spring transition, the float data are also consistent with other bloom hypotheses (e.g., critical photosynthesis, critical division rate, and meso/sub-mesoscale physics) that highlight the importance of brief, episodic boundary layer shoaling for decoupling of division and grazing rates.
The ability to quantify spatio-temporal variability in phytoplankton growth and productivity is essential to improving our understanding of global carbon dynamics and trophic energy flow. Satellite-based observations offered the first opportunity to estimate depth-integrated net primary production (NPP) at a global scale, but early modeling approaches could not effectively address variability in algal physiology, particularly the effects of photoacclimation on changes in cellular chlorophyll. Here, a previously developed photoacclimation model was used to derive depth-resolved estimates of phytoplankton division rate (μ) and NPP. The new approach predicts NPP values that closely match discrete measurements of 14C-based NPP and effectively captured both spatial and temporal variability observed during the four field campaigns of the North Atlantic Aerosols and Marine Ecosystems Study (NAAMES). We observed favorable growth conditions for phytoplankton throughout the annual cycle in the subtropical western North Atlantic. As a result, high rates of μ are sustained year-round resulting in a strong coupling between growth and loss processes and a more moderate spring bloom compared to the high-latitude subarctic region. Considerable light limitation was observed in the subarctic province during the winter, which resulted in divergent growth dynamics, stronger decoupling from grazing pressure and a taxonomically distinct phytoplankton community. This study demonstrates how detailed knowledge of phytoplankton division rate furthers our understanding of global carbon cycling by providing insight into the resulting influence on phytoplankton taxonomy and the loss processes that dictate the fate of fixed carbon.