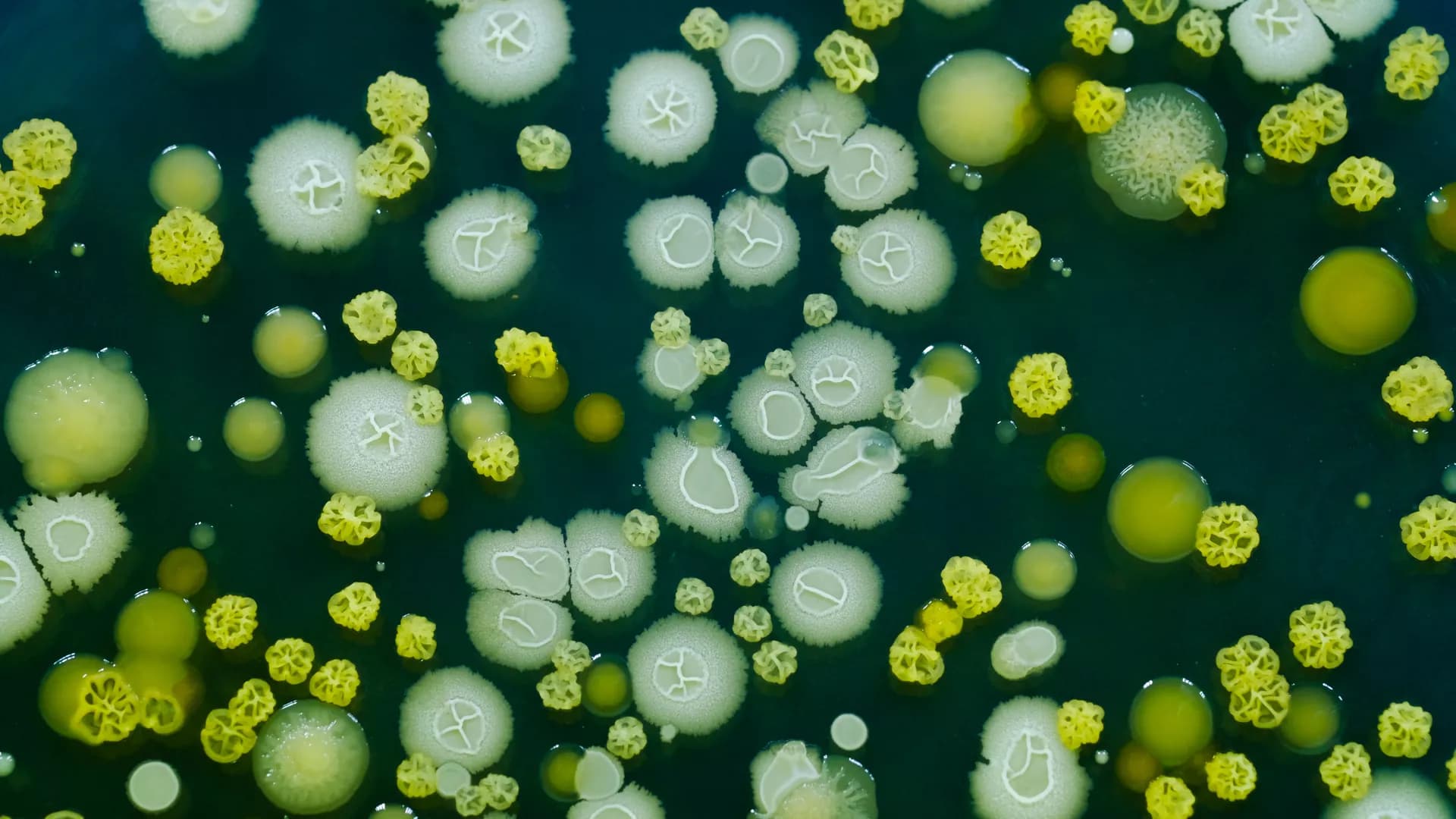
As one of the most common oral diseases in kids, early childhood caries affects the health of children throughout the world. Clinical investigations show the copresence of Candida albicans and Streptococcus mutans in ECC lesions, and mechanistic studies reveal co-existence of C. albicans and S. mutans affects both of their cariogenicity. Clearly a comprehensive understanding of the interkingdom interaction between these two microorganisms has important implications for ECC treatment and prevention. To this end, this review summarizes advances in our understanding of the virulence of both C. albicans and S. mutans. More importantly, the synergistic and antagonistic interactions between these two microbes are discussed.
Candida albicans (C. albicans) is the most frequent strain associated with cross-kingdom infections in the oral cavity. Clinical evidence shows the co-existence of Streptococcus mutans (S. mutans) and C. albicans in the carious lesions especially in children with early childhood caries (ECC) and demonstrates the close interaction between them. During the interaction, both S. mutans and C. albicans have evolved a complex network of regulatory mechanisms to boost cariogenic virulence and modulate tolerance upon stress changes in the external environment. The intricate relationship and unpredictable consequences pose great therapeutic challenges in clinics, which indicate the demand for de novo emergence of potential antimicrobial therapy with multi-targets or combinatorial therapies. In this article, we present an overview of the clinical significance, and cooperative network of the cross-kingdom interaction between S. mutans and C. albicans. Furthermore, we also summarize the current strategies for targeting cross-kingdom biofilm.
Mesoporous silica nanoparticles (MSNs) hold promise as safer and more effective medication delivery vehicles for treating oral disorders. As the drug’s delivery system, MSNs adapt to effectively combine with a variety of medications to get over systemic toxicity and low solubility issues. MSNs, which operate as a common nanoplatform for the co-delivery of several compounds, increase therapy effectiveness and show promise in the fight against antibiotic resistance. MSNs offer a noninvasive and biocompatible platform for delivery that produces long-acting release by responding to minute stimuli in the cellular environmen. MSN-based drug delivery systems for the treatment of periodontitis, cancer, dentin hypersensitivity, and dental cavities have recently been developed as a result of recent unparalleled advancements. The applications of MSNs to be embellished by oral therapeutic agents in stomatology are discussed in this paper.