Background: The mechanism responsible for the FLASH effect remains undetermined yet critical to the clinical translation of FLASH radiotherapy. The potential role of intertrack interactions in the FLASH effect, arising from the high spatio-temporal concentrations of particle tracks at UHDRs, has been widely discussed but its influence is unknown.
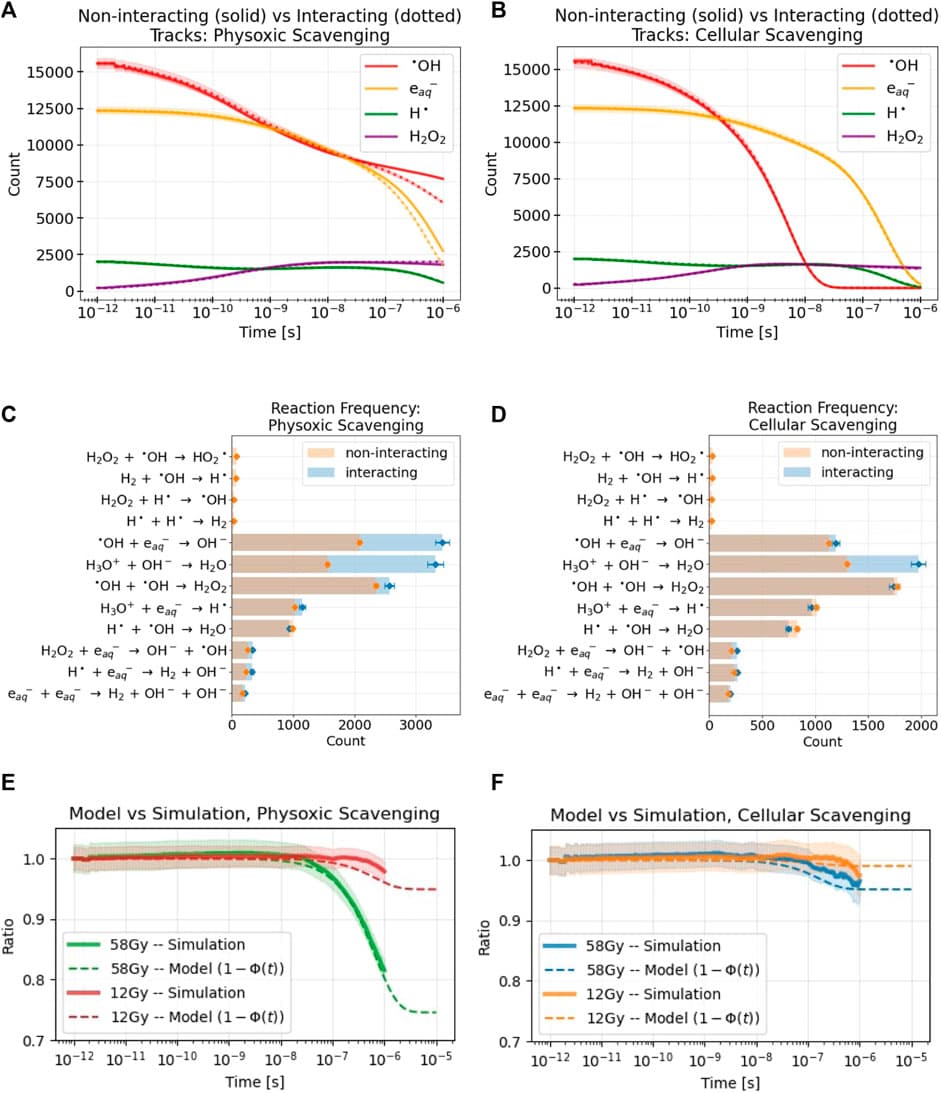
Methods: We construct an analytical model of the distribution, diffusive evolution, and chemical interaction of particle tracks in an irradiated target. We fit parameters of the model to Monte Carlo (MC) simulations of electron tracks, and include the effects of scavenging capacities of different target media. We compare the model’s predictions to MC simulations of many interacting electron tracks, and use the comparison to predict the prevalence of intertrack interactions in the parameter space where the FLASH effect is observed in vivo, and where differential reactive species (RS) yields have been observed in aqua.
Results: MC simulations of interacting electron tracks demonstrate negligible changes in RS yields at 12 Gy both in oxygenated water and in cellular scavenging conditions, but significant changes at 58 Gy in oxygenated water. The model fits well to the simulation data, and predicts that pulse doses delivered in 0.5 μs would be necessary for intertrack interactions to affect RS yields in cellular scavenging conditions, and in 0.5 μs for water at 4% O2. The model defines optimal beam parameters (e.g., dose, pulse width, LET) to maximize intertrack interactions, and indicates that decreasing the pulse width of electron pulses further below ≈0.5 μs has no effect on intertrack interactions.
Conclusion: The results of the MC simulations indicate that intertrack interactions do not play a role in the parameters space where the FLASH effect is observed. However, potentially critical limitations in the simulations performed provide the possibility that intertrack interactions occur much more readily than predicted. More accurate simulations, as well as experimental characterization of RS yields across the pulse parameter space, are necessary to more confidently confirm or deny the role of intertrack interactions in the FLASH effect.
The treatment of deep-seated tumours with electrons of very high energies (VHEE, 70–150 MeV) has already been explored in the past, suggesting that a dosimetric coverage comparable with state-of-the-art proton (PT) or photon radiotherapy (RT) could be achieved with a large ( 10) number of fields and high electron energy. The technical and economical challenges posed by the deployment of such beams in treatment centres, together with the expected small therapeutic gain, prevented the development of such technique. This scenario could radically change in the light of recent developments that occurred in the compact, high-gradient, electron acceleration technology and, additionally, of the experimental evidence of the sparing of organs at risk achieved in ultra-high dose rate irradiation, also referred to as FLASH. Electrons with the energy required to treat intracranial lesions could be provided, at dose rates compatible with what is needed to trigger the FLASH effect, by accelerators that are a few metres long, and the organ sparing could be exploited to significantly simplify the irradiation geometry, decreasing the number of fields needed to treat a patient. In this paper, the case of two patients affected by a chordoma and a meningioma, respectively, treated with protons in Trento (IT) is presented. The proton plans have been compared with VHEE plans and X-ray intensity-modulated radiotherapy (IMRT) plans. The VHEE plans were first evaluated in terms of physical dose distribution and then assuming that the FLASH regimen can be achieved. VHEE beams demonstrated their potential in obtaining plans that have comparable tumour coverage and organs at risk sparing when benchmarked against current state-of-the-art IMRT and PT. These results were obtained with a number of explored fields that was in the range between 3 and 7, consistent with what is routinely performed in IMRT and PT conventional irradiations. The FLASH regimen, in all cases, showed its potential in reducing damage to the organs placed nearby the target volume, allowing, particularly in the chordoma case where the irradiation geometry is more challenging, a better tumour coverage with respect to the conventional treatments.
Frontiers in Physics
Multi-Modality Based AI in Biomedical Physics for Disease Diagnosis and Treatment