Cilia are ubiquitous and highly conserved extensions that endow the cell with motility and sensory functions. They were present in the first eukaryotes and conserved throughout evolution (Carvalho-Santos et al., 2011). Paramecium has around 4,000 motile cilia on its surface arranged in longitudinal rows, beating in waves to ensure movement and feeding. As with cilia in other model organisms, direction and speed of Paramecium ciliary beating is under bioelectric control of ciliary ion channels. In multiciliated cells of metazoans as well as paramecia, the cilia become physically entrained to beat in metachronal waves. This ciliated organism, Paramecium, is an attractive model for multidisciplinary approaches to dissect the location, structure and function of ciliary ion channels and other proteins involved in ciliary beating. Swimming behavior also can be a read-out of the role of cilia in sensory signal transduction. A cilium emanates from a BB, structurally equivalent to the centriole anchored at the cell surface, and elongates an axoneme composed of microtubule doublets enclosed in a ciliary membrane contiguous with the plasma membrane. The connection between the BB and the axoneme constitutes the transition zone, which serves as a diffusion barrier between the intracellular space and the cilium, defining the ciliary compartment. Human pathologies affecting cilia structure or function, are called ciliopathies, which are caused by gene mutations. For that reason, the molecular mechanisms and structural aspects of cilia assembly and function are actively studied using a variety of model systems, ranging from unicellular organisms to metazoa. In this review, we will highlight the use of Paramecium as a model to decipher ciliary beating mechanisms as well as high resolution insights into BB structure and anchoring. We will show that study of cilia in Paramecium promotes our understanding of cilia formation and function. In addition, we demonstrate that Paramecium could be a useful tool to validate candidate genes for ciliopathies.
The katanin family of microtubule-severing enzymes is critical for cytoskeletal rearrangements that affect key cellular processes like division, migration, signaling, and homeostasis. In humans, aberrant expression, or dysfunction of the katanins, is linked to developmental, proliferative, and neurodegenerative disorders. Here, we review current knowledge on the mammalian family of katanins, including an overview of evolutionary conservation, functional domain organization, and the mechanisms that regulate katanin activity. We assess the function of katanins in dividing and non-dividing cells and how their dysregulation promotes impaired ciliary signaling and defects in developmental programs (corticogenesis, gametogenesis, and neurodevelopment) and contributes to neurodegeneration and cancer. We conclude with perspectives on future katanin research that will advance our understanding of this exciting and dynamic class of disease-associated enzymes.
Frontiers in Endocrinology
Comorbidities of adrenal-related endocrine disorders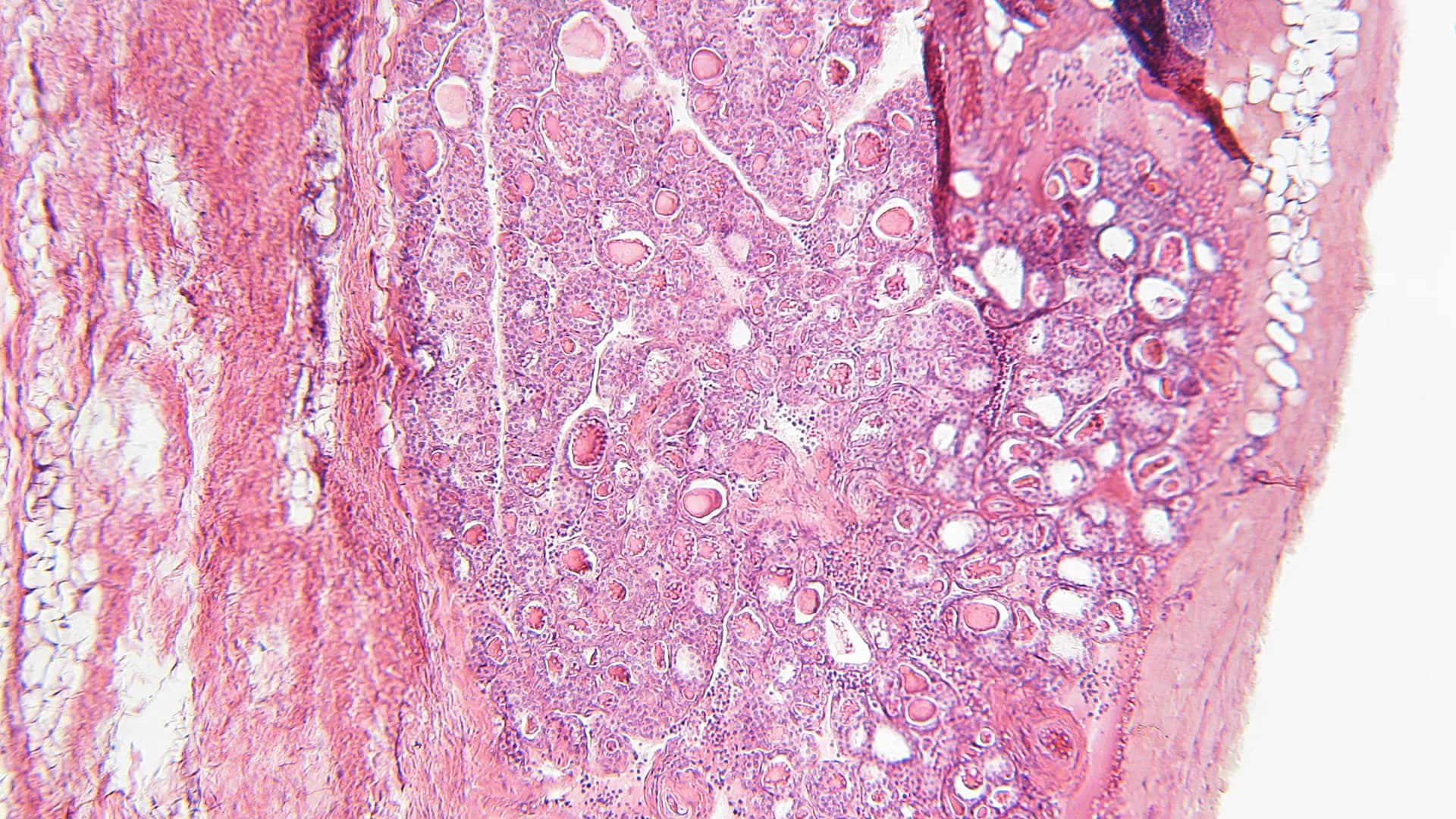
Frontiers in Endocrinology
Comorbidities of adrenal-related endocrine disorders







