The role of the dopaminergic pathway in general anesthesia and its potential mechanisms are still unknown. In this study, we usedc-Fos staining combined with calcium fiber photometry recording to explore the activity of ventral tegmental area (VTA) dopaminergic neurons(VTA-DA) and nucleus accumbens (NAc) neurons during sevoflurane anesthesia. A genetically encoded dopamine (DA) sensor was used to investigate thefunction of the NAc in sevoflurane anesthesia. Chemogenetics and optogenetics were used to explore the role of the VTA-DA in sevofluraneanesthesia. Electroencephalogram (EEG) spectra, time of loss of righting reflex (LORR) and recovery of righting reflex (RORR) were recorded asassessment indicators. We found that VTA-DA and NAc neurons were inhibited during the induction period and were activated during the recoveryperiod of sevoflurane anesthesia. The fluorescence signals of dopamine decreased in the induction of and increased in the emergence from sevoflurane anesthesia.Activation of VTA-DA and the VTADA-NAc pathway delayed the induction and facilitated the emergence accompanying with thereduction of delta band and the augmentation of the gamma band. These data demonstrate that VTA-DA neurons play a critical role in modulating sevofluraneanesthesia via the VTADA-NAc pathway.
Heparan sulfate proteoglycans (HSPGs) are components of the cell surface and extracellular matrix, which bear long polysaccharides called heparan sulfate (HS) attached to the core proteins. HSPGs interact with a variety of ligand proteins through the HS chains, and mutations in HSPG-related genes influence many biological processes and cause various diseases. In particular, recent findings from vertebrate and invertebrate studies have raised the importance of glycosylphosphatidylinositol-anchored HSPGs, glypicans, as central players in the development and functions of synapses. Glypicans are important components of the synapse-organizing protein complexes and serve as ligands for leucine-rich repeat transmembrane neuronal proteins (LRRTMs), leukocyte common antigen-related (LAR) family receptor protein tyrosine phosphatases (RPTPs), and G-protein-coupled receptor 158 (GPR158), regulating synapse formation. Many of these interactions are mediated by the HS chains of glypicans. Neurexins (Nrxs) are also synthesized as HSPGs and bind to some ligands in common with glypicans through HS chains. Therefore, glypicans and Nrxs may act competitively at the synapses. Furthermore, glypicans regulate the postsynaptic expression levels of ionotropic glutamate receptors, controlling the electrophysiological properties and non-canonical BMP signaling of synapses. Dysfunctions of glypicans lead to failures in neuronal network formation, malfunction of synapses, and abnormal behaviors that are characteristic of neurodevelopmental disorders. Recent human genetics revealed that glypicans and HS are associated with autism spectrum disorder, neuroticism, and schizophrenia. In this review, we introduce the studies showing the roles of glypicans and HS in synapse formation, neural plasticity, and neurological disorders, especially focusing on the mouse and Drosophila as potential models for human diseases.
Ionotropic receptors (IRs) are a highly divergent subfamily of ionotropic glutamate receptors (iGluR) and are conserved across Protostomia, a major branch of the animal kingdom that encompasses both Ecdysozoa and Lophothrochozoa. They are broadly expressed in peripheral sensory systems, concentrated in sensory dendrites, and function in chemosensation, thermosensation, and hygrosensation. As iGluRs, four IR subunits form a functional ion channel to detect environmental stimuli. Most IR receptors comprise individual stimulus-specific tuning receptors and one or two broadly expressed coreceptors. This review summarizes the discoveries of the structure of IR complexes and the expression and function of each IR, as well as discusses the future direction for IR studies.
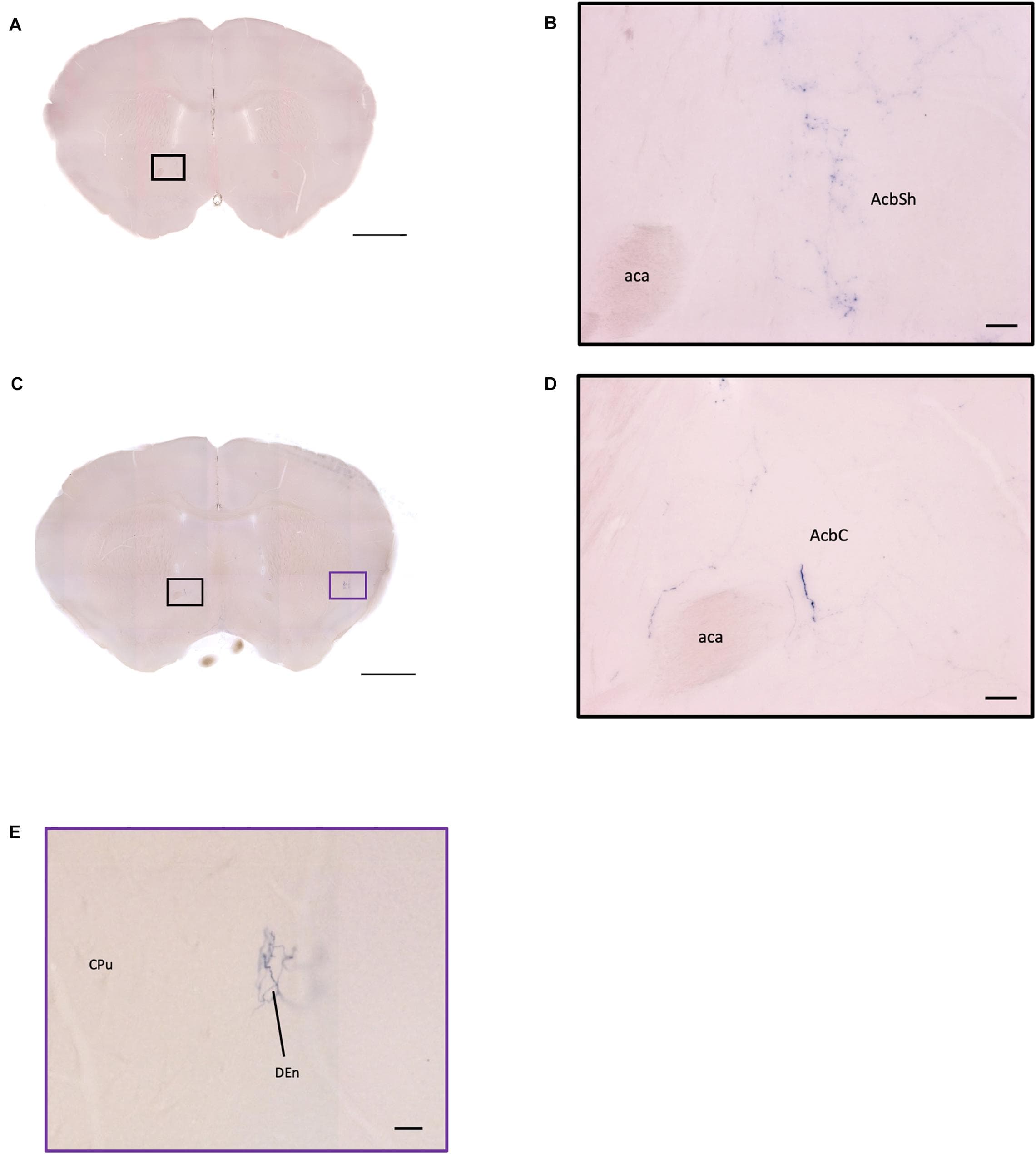
Oxytocin, a neuropeptide and peptide hormone, is produced by neurons in the hypothalamus and released by the posterior pituitary to control breastfeeding and labor. Recent studies have revealed that oxytocin in the central nervous system is also involved in modulating social interaction. To understand the potential role and innervation pattern of oxytocin neurons before sexual interaction, here we used transgenic mice which have the Cre recombinase under the control of an endogenous oxytocin promoter and Cre-dependent human placental alkaline phosphatase (AP) reporter to label the oxytocin neurons in the naive mouse brain. Since AP is located on the membrane of oxytocin neurons, AP histochemistry staining enabled us to observe the fine axonal terminals and the innervation pattern of oxytocin neurons in the thick serial coronal brain slices. Here we show that the number of AP-labeled cells varies with staining reaction time and ranges from 30% of the oxytocin immune-positive cell count to slightly higher than the oxytocin immune-positive cell count. Using AP staining with extended reaction time, which may not label all oxytocin neurons, we confirmed many innervation targets of oxytocin neurons from the anterior olfactory nucleus, some cortex regions, the limbic system, the hypothalamus, and the hindbrain, while the cell bodies were exclusively located in the hypothalamus and the bed nucleus of the stria terminalis. Finally, we observe some individual variance at the olfactory area, isocortex, striatum, paraventricular nucleus of thalamus, locus coeruleus, and Barrington’s nucleus.
![[18F]MK-9470 binds specifically to cannabinoid type 1 (CB1) receptors in the mouse brain. (A) Left: CB1 receptor knock-out mouse, right: wild-type mouse. Positron emission tomography (PET) images were summed from 40 to 60 min [injected radioactivity: 8.5 MBq (CB1−/−) and 8.0 MBq (CB1+/+), anaesthesia: 0.2 ml xylazine/ketamine, data acquisition: 60 min]. (B) Standardized volumes-of-interest (VOI) were drawn for the whole mouse brains (n = 2). Extracted radioactivity concentrations were normalized to the maximum radioactivity concentration in the wild-type mouse.](https://www.frontiersin.org/_rtmag/_next/image?url=https%3A%2F%2Fwww.frontiersin.org%2Ffiles%2FArticles%2F593793%2Ffnana-14-593793-HTML%2Fimage_m%2Ffnana-14-593793-g001.jpg&w=3840&q=75)
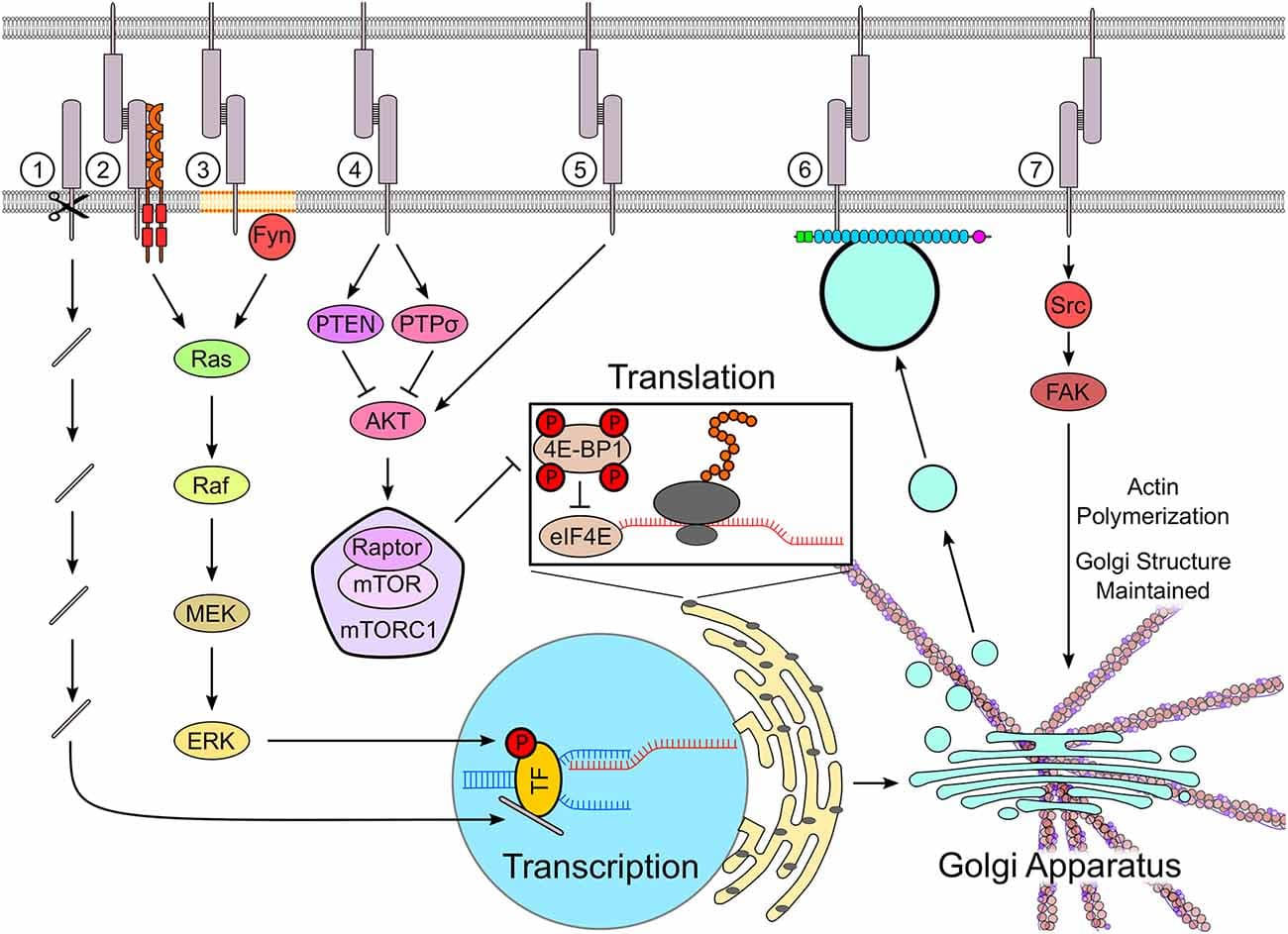
![Neurexins (NRXs) and Leukocyte common antigen-related receptor protein tyrosine phosphatases (LAR-RPTPs) serve as presynaptic hubs to orchestrate synapse organization. The trans-interaction between pre- and post-synaptic organizers generate retrograde and anterograde “synaptogenic” signals through the synaptic cleft, resulting in the presynaptic organization, including synaptic vesicles clustering, and in the postsynaptic organization, encompassing the recruitment of neurotransmitter receptors [e.g., AMPA-type and NMDA-type glutamate receptor (AMPAR and NMDAR)] and scaffold proteins (e.g., PSD-95). As presynaptic molecular hubs, NRXs and LAR-RPTPs trans-synaptically interact with multiple specific postsynaptic organizers, for instance, NRXs-neuroligins (NLGNs), NRXs-leucine-rich-repeat transmembrane neuronal proteins (LRRTMs), PTPσ-neurotrophin receptor tropomyosin-related kinase C (TrkC), PTPσ/δ-Slit and Trk-like proteins (Slitrks), along with others. Furthermore, SS4 insertion modulates NRX interactome by regulating its binding properties with its diverse ligands. Solid lines indicate protein interactions, and the dashed line indicates that the insertion of SS4 in NRXs weakens NRX-NLGN interaction.](https://www.frontiersin.org/_rtmag/_next/image?url=https%3A%2F%2Fwww.frontiersin.org%2Ffiles%2FArticles%2F567721%2Ffncel-14-00281-HTML%2Fimage_m%2Ffncel-14-00281-g001.jpg&w=3840&q=75)