
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Water , 03 March 2022
Sec. Water and Human Systems
Volume 4 - 2022 | https://doi.org/10.3389/frwa.2022.779860
This article is part of the Research Topic Water Security and Sustainable Development in an Uncertain World View all 12 articles
 Jia Yee Ho1
Jia Yee Ho1 Amanda Anne Lavinya1
Amanda Anne Lavinya1 Dominic Shuen Wei Kay1
Dominic Shuen Wei Kay1 Cindy Ik Sing Lee2
Cindy Ik Sing Lee2 Ahmad Haikal Razmi1
Ahmad Haikal Razmi1 Claire L. Walsh3
Claire L. Walsh3 Michaela L. Goodson1*
Michaela L. Goodson1* Jeyanthy Eswaran1,4*
Jeyanthy Eswaran1,4*As in many low- and middle-income countries around the world, thousands of local communities in Southeast Asia rely on river water to sustain their livelihoods. However, poor water quality threatens the health of both humans and ecosystems. The aim of this review was to examine the available literature to investigate how health outcomes in Malaysia have been studied and reported as directly attributable to human infections from river water. Computer-aided searches from 10 electronic databases were undertaken, with searches limited to the English language and publication dates since January 2010. The literature search revealed that the predominant river water infections identified in Malaysia were bacterial (coliforms, Salmonella spp., typhoid, leptospirosis, melioidosis), viral (including dengue, hepatitis, enterovirus), parasitic infections including amoebiasis, giardiasis and cryptosporidiosis, helminth infections, Blastocystis infections and sarcocystosis. No studies were found that have attempted to evaluate the impact of water related infection on human health longitudinally. Moreover, the possibility of integrated water governance systems that could reduce infection and improve water quality, particularly for marginalized groups have not been discussed or studied. Several cross-sectional studies identified infections at a point in time, but large longitudinal data sets of water infection parameters and how they influence human health outcomes have not been reported. Using Malaysia as a demonstration case study, we suggest a number of recommendations based on using a systems approach to tackle the challenges involved in data collection and integration, which is central to the understanding, strategic planning and management of water-borne infections.
In Southeast Asia (SEA), thousands of local communities rely on river water for sustainable livelihoods; this may be through operating fisheries, coastal farming or tourism. These economic activities form an important water-food nexus, which has driven regional socioeconomic development for more than 40 years (Blancas and El-Hifnawi, 2014; Pangare et al., 2014). According to The World Health Organization (WHO), almost 10% of the Global Disease Burden could be prevented by improving water access, sanitation, hygiene and management. The WHO and United Nations International Children's Emergency Fund's (UNICEF) Joint Monitoring Programme (JMP) has reported that ~785 million people worldwide lack access to safe water in 2019 with the majority living in rural areas of low and middle income countries (LMICs) (World Health Organization, 2017, 2019; Gomez et al., 2019).
Global burden of disease assessments over the last 25 years have allowed the influence of water accessibility, sanitation and hygiene (WASH) practices on health outcomes to be estimated (Prüss-Ustün et al., 2014). They are commonly reported in the context of communicable diseases such as diarrhoeal illnesses, nematode infections, lymphatic filariasis, trachoma, schistosomiasis, malaria and dengue (Prüss-Ustün et al., 2014; Troeger et al., 2017). However, there are several challenges involved in identifying and evaluating the direct impact of poor water sanitation and hygiene on human health, as exposure at the individual and household level is difficult and expensive to monitor. Hence, most water quality assessments are undertaken at the point of distribution. Diarrhoeal diseases are often non-specific and describe symptoms such as stomach pain and stool features rather than identifying a pathogen in every case due to difficulties in accessing the appropriate tests and laboratory services in resource limited settings. As a consequence, the burden of disease at the country level is often by inference and association with water sources, such as river water, rather than a well-documented pathway from exposure at the river to disease development.
A fundamental goal of applied epidemiology is to determine a relationship between the two factors (in this case exposure to unclean water causing diarrhea illness) removing confounders, mediators or modifying factors. Unfortunately, most of the time, because data is collected based on a historic event, it is prone to recall and interviewer bias, along with sample and detection bias as only severe cases tend to get reported. When symptoms rather than objective evidence of a specific microorganism are used as surrogate markers of an infection, data will likely be skewed showing a large number of false positives (Aiello and Larson, 2002). The United Nations Sustainable Development Goals (SDGs); SDG3 (Good health and wellbeing), SDG6 (Clean water and sanitation), SDG12 (Responsible consumption and production), SDG13 (Climate action), SDG14 (Life below water) and SDG15 (Life on land), all directly affect or are influenced by water accessibility and quality. There is a huge disparity in provision of WASH between countries and LMICs are beginning to develop national policies to implement the WASH requirements warranting an evaluation of the impact of water on health in emerging countries.
Here, in this review, we initially evaluate the impact of water sanitation and hygiene on human health in an upper middle-income country, Malaysia. In 2017, Malaysia had a population of 30.6 million with a life expectancy for women of 77.3 years and men, 72.4 years with the greatest number of deaths, from ischaemic heart disease, lower respiratory tract infections, stroke and road traffic injuries. Drowning comprised 0.52% deaths in <5-year-olds, but 8.6% total deaths in 5- to 14-year-olds. Diarrhoeal illnesses caused 1.46% total deaths in <5-year-olds and 0.98% total deaths in 5–14-year-olds. In Malaysia, residential, agricultural and industrial wastes are the three main sources of river pollution. In the 1920's, Malaysia introduced pollution-related legislation to control river pollution through the “Waters Act 1920.” This has been amended and improved several times over the years to regulate environmental issues, pertaining to drinking water, household water and water used for industrial purposes and recreation. The impact of water-borne infections on human health in Malaysia has been reported and they are sporadically reviewed. However, there is currently no systematic review that has comprehensively evaluated the evidence that connects water-borne infections to health. The aims of this review were firstly to examine river water and health related articles from 2010 in Malaysia, assessing the types of water-borne infection and the challenges involved in their management, and secondarily to use this data to make recommendations for future data collection in order to facilitate the development of integrated, water-borne infection management strategies using systems approach in Malaysia for the future.
In this study, computer-aided searches of the following electronic databases were undertaken, limiting searches to English Language and publication since January 2010: PubMed, Medline, Ovid, ProQuest databases, Scopus, Web of Science, JSTOR, EBSCO, Compendex and Google Scholar. The keywords and combinations of keywords are listed in Supplementary Table 1. The types of studies included in the review comprised: randomized (including cluster randomized) controlled trials, quasi randomized and non-randomized controlled trials, case control and cohort studies related to an event or intervention, observational studies and time series or interrupted time series design studies related only to humans. Titles and abstracts were screened by a single reviewer, and data extraction were carried out by two independent reviewers, using a structured and piloted form. Differences between reviewers over data extraction and quality assessment were reconciled with the intervention of a third assessor, where required.
A second search was performed using various database as listed in Supplementary Table 2, as these diseases were either endemic, neglected tropical diseases, or having occasional outbreak that involved not only local citizens but also overseas travelers. The same inclusion and exclusion criteria listed in Table 1 were followed for this second search. Reference lists of articles were also screened to supplement the above searches. Searches were cross-checked to avoid duplication.
The following data from selected papers were extracted from each paper for data analysis: (i) study authors; (ii) study design; (iii) sample size; (iv) sample demographics; (v) location: urban or rural; (vi) sample demographics; (vii) time of exposure or intervention and length of monitoring period; and (viii) measurement outcomes. Data were analyzed descriptively due to the limited number of searches within each section.
Most water pollution in Malaysia is caused by human activities such as surface water pollution from point sources such as industrial effluents, leachate from unsanitary landfills, sub-standard sewage effluents and pollution at non-point sources from pesticides and herbicides used in agriculture activities. In a monsoon climate, pollution from pluvial flooding causing flash floods and surface water flooding is common but relatively predictable and preventable at least in part with some basic preparatory measures; for example, to ensure adequate drainage and run off from fields and in urban areas, ensure clean drains, separate storm drainage from sewer systems, landscaping to store or direct pluvial flood water to drains (Huang et al., 2020). This review focuses on understanding the prevalence and the impact of infections on humans related to water in various parts of Malaysia in the last 10 years. The number of articles that met our minimum inclusion criteria mentioned in the methods are screened manually and incorporated in the review (Figure 1). The results section was separated into bacterial, viral and parasitic infections based on major water-borne diseases reported in Malaysia and the number of articles included in the review from each of the states in Malaysia were also reported (Figure 2). Rare water-borne human infections in Malaysia were not covered in this review.
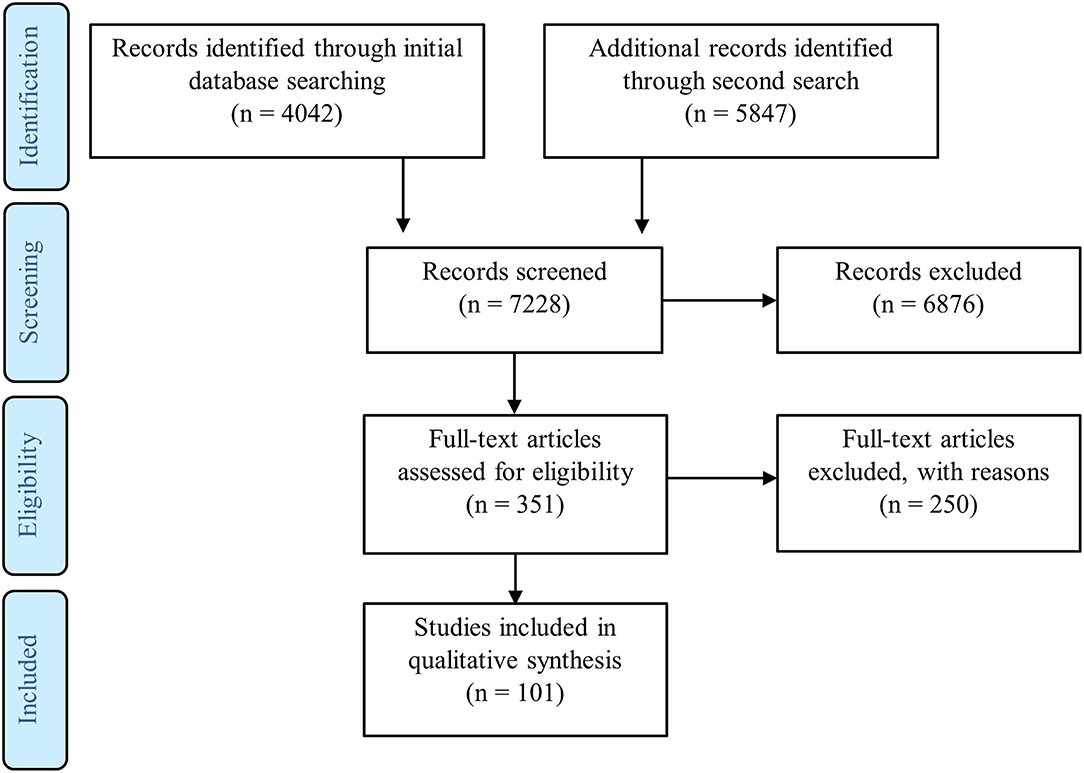
Figure 1. PRISMA statement showing flow of information through the different phases of a systematic review (Moher et al., 2009). This PRISMA diagram contains public sector information licensed under the Open Government Licence v3.0. Adapted from Moher et al. (2009).
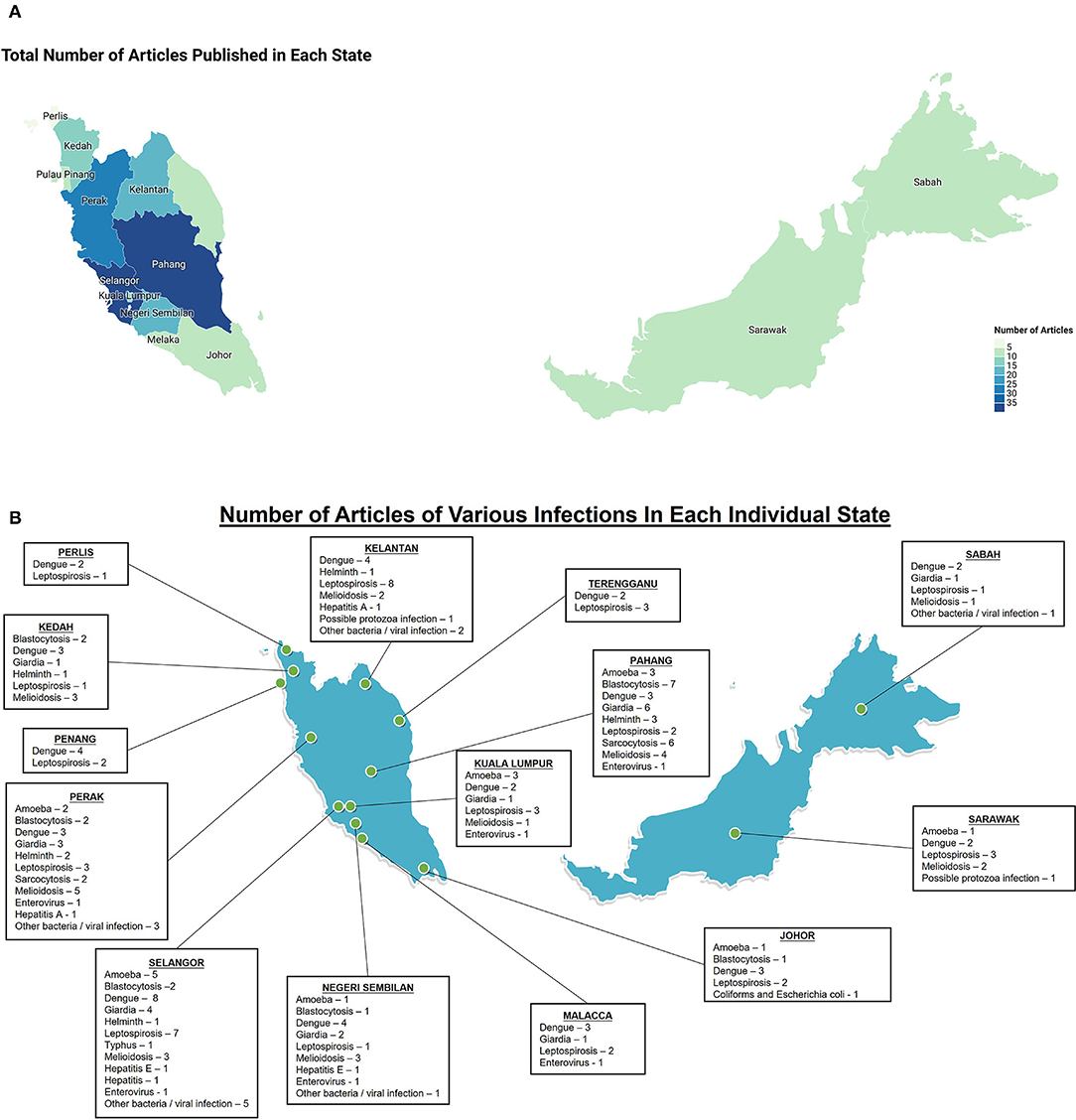
Figure 2. Elucidation of number of articles included in this review in Malaysia, mainly focus on infectious diseases related to human, however if the disease is related to zoonosis, studies on reservoir animals may be included. (A) Total number of articles that were included in each state, the color intensities are proportional to the number of articles; (B) Number of articles for each disease that were included in each state.
In this section, we discuss various studies that report major water-borne bacterial infectious diseases such as melioidosis, leptospirosis, typhoid, Enterococcus, Aeromonas spp., Helicobacter pylori, coliforms and Escherichia coli reported in humans in Malaysia.
Melioidosis, a life-threatening infection is caused by Gram negative environmental bacteria, Burkholderia pseudomallei found in contaminated water and soil. The recent modeling studies suggest that 165,000 cases of melioidosis result in 89,000 deaths worldwide per year. Around 47% (42,000 cases of 89,000 cases worldwide) of deaths were estimated to be caused by melioidosis in South Asia indicating the endemic status of the disease (Limmathurotsakul et al., 2016). Melioidosis is not, however, considered a neglected tropical disease (NTD) in the WHO's list of NTD (World Health Organization, 2020), this should be re-considered as the disease burden in the Southeast Asian tropical and subtropical regions is high (Wiersinga et al., 2018; Birnie et al., 2019).
Melioidosis can have a wide array of atypical clinical presentations, such as deep organ abscess, bacteraemia, septic shock, and multiple organ involvement, with different severity and chronicity. Lungs were generally considered as the most frequently infected organs (Wu et al., 2012; Zueter et al., 2016; Mohan et al., 2017; Yazid et al., 2017), which could be misdiagnosed as tuberculosis (Mohapatra et al., 2019). Melioidosis can also cause fetal loss although the underlying cause remains unclear (Chang et al., 2020). Other less common symptoms include endophthalmitis and seizures (Feng et al., 2018).
A study by Musa et al. (2016) showed the detection of B. pseudomallei in soil from 32 out of 60 ruminant farms spread throughout Negeri Sembilan, Pahang, Perak and Selangor (Musa et al., 2016). In this unmatched case-control study, the presence of river, stream, as well as flooding or water logging were cited as significant risk factors that contributed to the occurrence of melioidosis in the ruminant farms (Musa et al., 2015). Later in 2018, a separate study also detected B. pseudomallei in water samples, from boreholes, river, tap water and well water, in the ruminant farms (Musa et al., 2018).
Very few retrospective studies have been undertaken on patients admitted to hospital in Malaysia with melioidosis although the majority of individuals who were infected by melioidosis worked in agricultural, farming and fishing sectors (Hassan et al., 2010; Abu Hassan et al., 2019; Tang et al., 2019). Diabetes mellitus in several studies appears to be a major underlying risk factor in diagnosed melioidosis cases (Hassan et al., 2010; Zueter et al., 2016; Yazid et al., 2017; Abu Hassan et al., 2019). However, it should be noted that the related immune suppression in poorly controlled diabetics may have accelerated disease development, producing symptoms that forced infected individuals to seek medical help. Detection bias may underestimate prevalence statistics in affected communities where people with minor symptoms fail to seek medical help and achieve an objective, formal diagnosis of melioidosis. There was also a report of a diabetic patient who worked as a palm oil worker in Perak, being infected with melioidosis, tuberculosis and Salmonella at the same time, suggesting diabetes as a predisposing factor to multiple infections (Sulaiman et al., 2013). Recorded risk factors for melioidosis include co-morbidities, immune-compromission, cystic fibrosis (Mariappan et al., 2018), thalassemia, kwashiorkor, albinism and patent ductus arteriosus (Fong et al., 2015). Residing areas also play an important role in acquiring melioidosis, Abu Hassan et al. (2019) showed that living in areas with large-scale irrigation-based agriculture and mixed agriculture/pastoral environments had a higher prevalence rate of melioidosis (Abu Hassan et al., 2019).
In 2010, there was an outbreak of melioidosis and leptospirosis co-infection after a rescue operation in Pahang, Malaysia (Sapian et al., 2012) where 153 people were exposed to the outbreak. Overall, the fatality rate in this outbreak was 38.1% (8 out of 21), where all eight individuals with diabetes mellitus, four had melioidosis alone and three had a co-infection of melioidosis and leptospirosis. In this study also, the water samples were collected from the riverbank at the outbreak site, which confirmed the presence of Leptospira as well as B. pseudomallei indicating a likely environmental source of the infectious agent.
Pediatric melioidosis accounts for 5–15% of all melioidosis cases worldwide (Sanderson and Currie, 2014), however, there are not many childhood melioidosis studies reported from Malaysia. In 2017, a study from Sarawak reported an overall average annual incidence rate of 4.1 per 100,000 children (<15-years-old) (Mohan et al., 2017). In this region, most of the children (80%) resided in rural areas and had used untreated water sources for recreation and consumption; some of the children (32%) also had poor nutritional status. It is interesting to note that the B. pseudomallei isolates in this study were susceptible to gentamicin (Mohan et al., 2017), a finding which is similar to a previous study in Sarawak (Podin et al., 2014). Of note, B. pseudomallei is known to be intrinsically resistant to aminoglycosides (McEniry et al., 1988). In summary, there were several sporadic melioidosis outbreaks reported from various states of Malaysia (Sapian et al., 2012; Mohan et al., 2017) (Figure 2). However, it is difficult to assess the real disease burden as the source of infection in each study were not established. Furthermore, there is a lack of systematic disease monitoring using specific nation-wide diagnosis for melioidosis.
Leptospirosis is a bacterial disease that causes high levels of morbidity and mortalities in tropical climates. In such countries, flooding is a common cause of leptospirosis outbreak, as incidences often increase significantly during floods. Leptospirosis outbreaks are generally associated with rises in temperature, humidity, rainfall, flooding and raised river levels (Mohd Radi et al., 2018). Rodents and domestic animals serve as a reservoir and host for infection Leptospira as they transmit the disease via urine (World Health Organization, 2003). While the worldwide prevalence is not known, incidences are higher in humid tropical regions, like Southeast Asia (World Health Organization, 2003; Pappas et al., 2008).
The clinical manifestations of leptospirosis infection ranges from a mild, self-limited febrile illness to life-threatening illness; patients usually present with sudden onset of fever, chills, headache, cough and gastrointestinal symptoms. Since leptospirosis shares a number of non-specific symptoms with conditions like influenza or dengue and co-infection is not uncommon, therefore the actual prevalence rate may be significantly higher than reported (Kishimoto et al., 2004; Leung et al., 2011). Indeed, there were several reports that include leptospirosis co-infection with dengue (Suppiah et al., 2017; Philip et al., 2020), where males had a higher preponderance for co-infection and shock was a common symptom in those patients (Suppiah et al., 2017).
In Malaysia, a study reported a total of 3,604 cases and 47 cases of death in Ministry of Health hospitals in Malaysia in 2012 (Benacer et al., 2016b). Several cross-sectional studies reported the seroprevalence of leptospirosis from various states. When the seroprevalence of leptospirosis in municipal service workers from Kelantan (2012) and Selangor (2015) were investigated, they found an overall seroprevalence of 4.7 and 34.8%, respectively (Shafei et al., 2012; Samsudin et al., 2015). However, a lower seroprevalence of 9.4% was found in urban service workers from Sabah in Borneo (Atil et al., 2020). Rahman et al. (2018) screened 232 local wet market workers in Kelantan and found 78 of the respondents (33.6%) were seropositive for leptospirosis with the highest seroprevalence detected in those who sold processed food, fresh meat and fish, fruits and vegetables (Rahman et al., 2018). A screening study on a community near Rejang river basin, Sarawak in 2016 (Suut et al., 2016) revealed that the community with 86% Iban ethnicity had 37.4% seroprevalence for leptospirosis, but not all individuals were symptomatic as is often the case despite the detection of seropositive antibodies. In the same year, an outbreak of leptospirosis occurred among military reserve recruits in Kuala Lumpur following a survival exercise (Neela et al., 2019). Since not all recruits were interviewed, the study was prone to selection bias in terms of identifying risk factors. In an effort to establish a source of infection, water and soil samples were analyzed along with rat urine. This study identified L. kmetyi and L. wolffii in the environmental samples and L. interrogans and L. borgpetersenii in some of the kidneys of rodents. This study clearly suggested that exposure to an environment contaminated from rodents' excrements were a likely risk factor human leptospirosis infection.
Other studies reported detection of Leptospira in the environment (using water and soil samples) particularly from the National Service Training Center (Ridzlan et al., 2010), and residential areas with leptospirosis patients (Mohd Ali et al., 2018). The water and soil samples with Leptospira were (Benacer et al., 2013; Azali et al., 2016) identified, but it has not caused symptomatic disease in all exposed individuals. Other studies have identified Leptospira in rat populations in Peninsular Malaysia (Benacer et al., 2013, 2016a; Mohamad Ikbal et al., 2019) and Sarawak (Pui et al., 2017; Blasdell et al., 2019) as rats serve as a reservoir for the disease. A cross-sectional study by Daud et al. (2018) showed that, among cattle farmers, the seroprevalence of leptospiral antibodies was 72.5% and pathogenic Leptospira originated from the waste dump at the farm (Daud et al., 2018). In short, studies that reported leptospirosis from Malaysia mostly identify the contaminated sources that cause the outbreaks, but it is difficult to assess the prevalence rates and to understand the significance of variations in the seroprevalence reported from various communities.
Typhoid fever is a human systemic infection caused by Salmonella enterica serovar Typhimurium (S. typhi). It is a highly adapted human pathogen that is transmitted via the fecal-oral route (Yap et al., 2014). In the year 2010, there were 21.7 million typhoid infection cases and about 217,000 deaths reported worldwide (Crump and Mintz, 2010). The main reservoir of S. typhi transmission is from human carriers and food. It is spread mainly by contaminated food, water, and close contact to infected patients (Antillón et al., 2017). Among the 13 states of Malaysia, Kelantan has the highest incidence of typhoid fever (Yap et al., 2014). Typhoid fever is common among children aged more than 2 years old and teenagers in Malaysia (Ministry of Health Malaysia, 2017a). Other symptoms, such as headache, malaise, coughing and loss of appetite, were reported as early signs but the most common symptom for this disease is prolonged fever (Laishram and Singh, 2016; Roy et al., 2016; Ministry of Health Malaysia, 2017a; Singh and Sundar, 2019). Abdominal symptoms ranging from discomfort, pain, constipations, and diarrhea have been reported as well (Rasul et al., 2017; N'Cho et al., 2019; Muhammad et al., 2020). Rose spots are usually observed on the body of patients with fair skin (Rasul et al., 2017; Muhammad et al., 2020).
A case-control study was undertaken by Anita et al. (2012) to identify the risk factors for an outbreak of typhoid infection in Selangor. In this study eleven water samples were taken from different sampling sites of Congkak River (Sungai) in Selangor for microbiological analyses. No S. typhi was isolated from the water samples indicating that the river water was not the vehicle for transmission but there was evidence for sewage contamination in the river (Anita et al., 2012).
There are two types of typhoid vaccination available in Malaysia, which are Typhim Vi (Vi CPS) and Ty21a (Ministry of Health Malaysia, 2017a). Both type of vaccinations requires a booster dose every 3 years. For the general public, typhoid vaccination is voluntary. Only certain groups of people, such as food handlers, health officers, sewage and sewerage sanitation workers, workers involved in water supply operation and maintenance, and travelers to places identified as high-risk exposure toward S. typhi are advised to take the vaccination.
Several reports have described water-borne bacteria that can cause gastrointestinal diseases, including Helicobacter pylori and Enterococcus spp. Common symptom of H. pylori infection is burning pain or discomfort ranging from abdomen to the chest, also known as epigastric pain (Ministry of Health Malaysia, 2016). The discomfort, usually caused by ulcers, may last from a few minutes to hours, and usually occurs at night, which may lead to sleep disturbance. Other symptoms may include nausea or vomiting, bloody or blackish stools, bloating and weight loss. Known for its carcinogenic properties, H. pylori infection also increases the risk of gastric cancer by 6 times if untreated.
Aeromanas spp. (Batra et al., 2016) and Enterococcus faecalis (Golob et al., 2019) both recognized as nosocomial pathogens, are known for their role in contributing toward urinary tract infections. Both infections share similar symptoms like: abdominal cramps, nausea, vomiting and fever (Drancourt, 2010). Aeromonas infection may also cause acute diarrhea (Drancourt, 2010). E. faecalis infection can be exceptionally difficult to treat due to its resistance toward many drugs (Kau et al., 2005). There are some reports describing detection and characterization of these pathogenic bacteria in various water bodies in Malaysia. Khor et al. (2015) reported the presence of Aeromonas spp. that carried virulence genes in five recreational lakes in Selangor (Khor et al., 2015). In Selangor, isolates harboring enterotoxin gene from Clostridium perfringens, a gastrointestinal commensal bacteria known to cause food poisoning and antibiotic-associated diarrhea (Florence et al., 2011), were also reported from the rivers.
Two case-control studies have described the prevalence and predictors of H. pylori infection among Malays (Lee et al., 2012) and indigenous (Rahim et al., 2010) ethnic groups residing in Kelantan, northeast of Peninsular Malaysia. Both reports stated a remarkably low prevalence of H. pylori in those living in the north-eastern region, particularly the Malays. Rahim et al. (2010) mentioned that the increased prevalence of H. pylori infection among the Malays might be associated with the use of well water and pit latrines, less frequent boiling of drinking water, and infrequent hand washing practice, but it is difficult to directly attribute these factors to H. pylori infections when so many confounders were not controlled for, it is purely anecdotal and an observational finding.
Enterococcus faecalis can be found in a variety of environments including soil and water. It is usually gastrointestinal tract commensal bacteria but can become an opportunistic pathogen in immunocompromised humans. E. faecalis is also known to acquire antibiotic resistance and is prevalent in nosocomial infections. As far as environmental sources are concerned, Daniel et al. (2017) was able to isolate virulent and multidrug resistant forms of E. faecalis from animal farms, patient samples, and also wastewater and river water samples (Daniel et al., 2017) but there is no data presented to demonstrate the impact of these bacteria on humans. Similarly, Dada et al. (2013) also reported high frequencies of E. faecalis, E. faecium as well as multi-antibiotic resistant isolates at two recreational beaches, indicating fecal contamination of sea water (Dada et al., 2013).
Escherichia coli and fecal coliforms levels are the standard indicators of microbial contamination in water and food. Total coliforms and E. coli levels were studied in Semenyih River, Selangor (Al-Badaii and Shuhaimi-Othman, 2015), Matang mangrove estuary, Perak (Ghaderpour et al., 2014) and Melayu River, Johor (Ho et al., 2021). Total coliforms, E. coli (Al-Badaii and Shuhaimi-Othman, 2015), specifically Klebsiella pneumoniae (Barati et al., 2016) were found to have high resistance to multiple antibiotics in Semenyih as well as Matang mangrove estuary. At Melayu River, extended spectrum β-lactamase (ESBL)-producing and multidrug-resistant Enterobacteriaceae (Enterobacter cloacae, E. coli, K. pneumoniae) were detected (Ho et al., 2021). Data on the prevalence of human infections due to declining river water quality in Malaysia is scarce. Nevertheless, the presence of such pathogenic and antibiotic resistant bacterial strains in areas of high human activity should prompt further action particularly in monitoring water quality and educating the general public.
In conclusion, the occasional outbreaks of melioidosis, and leptospirosis and the water sources that causing these infections have been reported in Malaysia. While S. typhi, Enterococcus spp., Aeromonas spp., H. pylori, coliforms and E. coli mostly detected in rivers were reported, the systematic investigations that define the disease prevalence or the impact of such infections on human health remain unknown.
Viral infections that are commonly reported in humans in Malaysia include dengue, hepatitis and enterovirus. Here, we reviewed published articles on water-related viral infections in Malaysia.
Dengue fever is caused by RNA virus from the Flaviviridae family and is transmitted through mosquito vectors commonly Aedes aegypti and Aedes albopictus (Kyle and Harris, 2008). The WHO has classified dengue fever as a water-related disease as Aedes mosquitoes breed in areas where there is stagnant water such as flowerpots, water containers, discarded tires and mud pots (Ferede et al., 2018; World Health Organization, 2021a). The Global Burden of Disease Study 2013 reported that dengue fever is highly prevalent in the Southeast Asian region (Stanaway et al., 2016). In Malaysia, dengue remains endemic since its outbreak in the 1980's with its highest concentration of cases in the state of Selangor. A 61.4% increase in dengue cases were reported in 2019 compared to 2018 in Malaysia, with Selangor state accounting for the highest number of reported cases (n = 72,543) [Crisis Preparedness and Response Centre (CPRC), 2020]. The symptoms of dengue fever are flu-like, however a severe infection may lead to dengue haemorrhagic fever or dengue shock syndrome (Hasan et al., 2016). Practices such as improving household environmental sanitation and eliminating Aedes breeding sites have shown to be effective methods of controlling and preventing dengue (Chandren et al., 2015).
Since 2010, there have been few published cross-sectional studies evaluating the general public's knowledge, attitude, and practices (KAP) on dengue prevention (Naing et al., 2011; Mohamad et al., 2014; Wong et al., 2014, 2015; Chandren et al., 2015; Wan Rosli et al., 2018; Yeo and Shafie, 2018). Three articles reported the association of health beliefs and knowledge toward dengue prevention practices among Orang Asli (indigenous) communities (Chandren et al., 2015) and the nationwide population (Wong et al., 2014, 2015). Another two studies, Naing et al. (2011) and Mohamad et al. (2014) reported the level of knowledge and practice of dengue control and factors affecting these practices in a semi-urban town in Mantin, Seremban (Naing et al., 2011) and in a dengue outbreak-prone area in Selangor (Mohamad et al., 2014). In 2018, there are two cross-sectional studies that investigated the willingness among people to pay for vaccinations (Yeo and Shafie, 2018) and the impact of educational intervention on the levels of knowledge, attitude, and practice toward dengue prevention among university students (Wan Rosli et al., 2018).
The aforementioned cross-sectional studies used self-administered questionnaires (Wan Rosli et al., 2018), a computer assisted telephone survey (Wong et al., 2014, 2015) or interviewer administered survey (Naing et al., 2011; Mohamad et al., 2014; Chandren et al., 2015; Yeo and Shafie, 2018). The questionnaire in all seven studies included a section on self-reported preventive practices that are undertaken to combat the spread of dengue. In the context of WASH practices involving safe use of water for combating dengue, there were several recurrent concerns found among the aforementioned reports. Firstly, dengue prevention practices were found to be inadequate in some study populations. For example, just over half of the indigenous participants (n = 280, 55.4%) showed good prevention practices but only 52.1% of the study population would examine for Aedes larvae in their water containers (Chandren et al., 2015). Even in the semi-urban population of Mantin, Seremban, it was reported that only 44.5% of the study population had covered their stored water properly (Naing et al., 2011). Thus, these studies demonstrate the KAP of general public on dengue prevention and the need for improving awareness around WASH practices in Malaysia.
In the same theme of evaluating the health related perceptions and behavior in the context of dengue, two studies, that adopted the Health Belief Model (HBM), which is recognized for its ability to predict health behavior by looking at four main elements: perceived susceptibility of the disease, perceived seriousness of the disease, perceived benefits of an action and perceived barriers carrying out said action, were reported (Hayden, 2019). These two studies found that participants with lower knowledge and lower perceived susceptibility were less likely to perform dengue prevention practices. In contrast, participants with lower perceived barriers to perform dengue prevention practices were more likely to perform dengue prevention practices (Wong et al., 2014; Chandren et al., 2015). Another recurrent concern that was identified from these studies are the lack of use of Abate or chemicals in stored water to prevent mosquito breeding, which is key toward dengue prevention (Mohamad et al., 2014; Chandren et al., 2015; Wong et al., 2015; Wan Rosli et al., 2018). This is likely due to the misconception that Abate is harmful to humans or Aedes can breed in dirty/contaminated water only (Naing et al., 2011; Chandren et al., 2015; Yeo and Shafie, 2018), suggesting a lack of awareness on the life cycle of Aedes mosquitoes and their preferred breeding environments. It is important to note that participants with good knowledge scores of dengue tend to have better prevention practices score (Chandren et al., 2015; Wong et al., 2015), although this is not the case among the university students (Wan Rosli et al., 2018). In general, high dengue prevention practice scores were associated with factors such as a high density of mosquitoes in neighborhood, lower income (Wong et al., 2015), younger age with higher level of education (Naing et al., 2011), and attendance to health campaigns (Mohamad et al., 2014).
Besides studying human behavior toward dengue and dengue prevention, we also found reports that described other factors such as environment, land use, climate and weather (Dieng et al., 2012; Dickin et al., 2013; Dom et al., 2013; Roslan et al., 2013; Aziz et al., 2014; Cheong et al., 2014; Mallhi et al., 2015; Lau et al., 2017). Dengue incidence was found to be significantly associated with land areas that had water bodies (Cheong et al., 2014). Aziz et al. (2014) and Roslan et al. (2013) described the spatial density of Aedes distribution in urban areas situated in Kuala Lumpur and its positive association with monthly rainfall (Roslan et al., 2013; Aziz et al., 2014).
Indeed, a study that investigated the impact of environmental factors on dengue incidence found that high temperature and rainfall led to increased incidence of dengue (Dom et al., 2013). Mallhi et al. (2015) studied the clinic-laboratory spectrum of dengue viral infection and the associated risk factors such as heavy rainfall and exposure to stagnant water resources (Mallhi et al., 2015). Similarly, Lau et al. (2017) reported that a rise in dengue cases positively correlate to heavy downpour (Lau et al., 2017). Interestingly, they also found that Aedes aegypti could breed in water tanks on the rooftop of high-rise buildings. A similar finding was previously reported by Dieng et al. (2012) where Aedes were found to breed in water tanks and even in water bowls used for pets (Dieng et al., 2012). Although many water sources harbor and breed Aedes aegypti, higher susceptibility and vulnerability to dengue was reported in areas of higher use of pour-flush toilets (Dickin et al., 2013). Despite these useful observations, several limitations to these studies including selection bias around convenience sampling of residents accessible by land transport, recall bias from questionnaires, bias in selecting socially desired behaviors when answering survey questions and lack of correlation of timing of survey distribution with Dengue outbreaks may impact the findings.
In summary, these recent studies focus on defining the various environmental factors and water sources that contribute to the spreading of the disease and the perceptions and practices that influence the implementation of the prevention measures. Education intervention through health campaigns by government representatives implemented throughout Malaysia with emphasis on the common rural population practice could be implemented (Wong et al., 2014; Wan Rosli et al., 2018) highlighting the need to increase awareness of dengue and dengue prevention through social media or other visual aids (posters/billboards).
Hepatitis is a form of liver inflammation (Centers for Disease Control Prevention, 2020) that affects liver functions acutely or chronically. Overconsumption of alcohols, toxins, certain medications and medical conditions may cause hepatitis. The most common cause of hepatitis is due to hepatitis viruses A, B, C, D, and E exposure. Viral hepatitis may lead to complications such as: fibrosis, cirrhosis and liver cancer (Ministry of Health Malaysia, 2019, 2020). Patients with viral hepatitis may experience fever, fatigue, loss of appetite, nausea and vomiting, abdominal pain, jaundice and dark urine. Certain patients with chronic Hepatitis B or Hepatitis C may not show any symptoms at all (Ministry of Health Malaysia, 2020).
In Malaysia, the most common types of viral hepatitis are: Hepatitis A, Hepatitis B and Hepatitis C (Ministry of Health Malaysia, 2020). The Ministry of Health Malaysia highlighted that it is compulsory to notify viral hepatitis infections under the First Schedule of Control and Prevention of Communicable Disease Act 1988 (Ministry of Health Malaysia, 2019). Hepatitis A virus (HAV) infection is usually acute and transmitted through food and water, but it is much easier to control through good personal hygiene and sanitation practices. On the other hand, Hepatitis B (HBV) and Hepatitis C viral (HCV) infection are more serious in Malaysia, where both viruses are spread through sex, exposure to contaminated blood and mother-to-child transmission. Most patients with HBV or HCV infection are diagnosed until chronic stages, as they are often asymptomatic in early infection (Ministry of Health Malaysia, 2020). Currently, three doses of vaccination for Hepatitis B have been mandatory to new-born babies and booster given in the 1st and 6th months after birth since 1989, but not for Hepatitis C. Hepatitis A is suggested for immunization but is currently not listed in the National Immunization Program (Yeong, 2015). For Hepatitis E (HEV), a vaccine has been developed and is licensed in China, but is not yet available elsewhere (World Health Organization, 2021b).
There were a few reported outbreaks of Hepatitis A infection in Malaysia since 2010. In 2011, three villages in Hulu Terengganu, Terengganu had an outbreak of HAV infection where their water source was contaminated affecting Orang Asli settlement and two other villages (Zolkepli, 2011). In 2012, there were 78 confirmed cases of HAV infection in Manjung district, Perak identifying unhygienic toddy processing places, procedures and contaminated well water as potential sources of the outbreak (Yusoff et al., 2015).
In 2011, Ahmad et al. enrolled 119 patients with viral origin chronic liver disease (CLD) between July and September 2009 in Kelantan to determine the seroprevalence of anti-HAV antibodies (Ahmad et al., 2011). 80.7% (96) patients were with HBV infection and 19.3% (23) patients were with HCV infection while the overall prevalence of anti-HAV was 88.2% (105/119). The seroprevalence rate of HAV was higher in the age group of more than 30 years, and all 17 patients with liver cirrhosis were anti-HAV positive and were in the older age group of mean age 52.4 years. The authors suggested that patients with CLD who are younger than 30 years old will benefit from Hepatitis A vaccination.
From April 2011 to February 2013, Wong et al. (2020) collected 207 blood samples from healthy aboriginal communities and analyzed for anti-HEV IgG and IgM in 2018 and conducted semi-structured interviews through a cross-sectional study. Usage of river water for daily washing and recreational swimming occasionally or when clean water sources was not available, seems to be one of the major reasons causing HEV infection in the aboriginal villages at Negeri Sembilan and Selangor. Participants also claimed that outbreaks of HEV were not uncommon, this could imply that WASH practices and surveillance in the aboriginal communities need to be improved to prevent transmission of HEV (Wong et al., 2020). A cross-sectional study was conducted on 120 university students in Klang Valley to evaluate KAP, which is important to reduce the risk of viral hepatitis infection. The mean total scores of KAP regarding viral hepatitis were significantly higher in medical students as compared to non-medical science-based participants (Mohd Nazri et al., 2019).
Hepatitis A and Enterovirus (EV) are both Picornaviruses in the family of picornaviridae. EV are classified into 13 species, EV-A to D are the most commonly known species; where poliovirus is EV-C species. Non-polio enteroviruses (NPEVs) include coxsackieviruses, echoviruses, numbered enteroviruses and rhinoviruses. The infection usually causes mild illness including fever, runny nose, sneezing, cough, skin rash, mouth blisters, body and muscle aches. EVs are also associated with outbreaks of more serious diseases, such as hand, foot, and mouth disease (HFMD). Enteroviruses spread either through the fecal–oral route or via respiratory transmission. Enterovirus A71 (EV-A71) is one of the major causes of HFMD together with coxsackievirus A16 (CVA-16) and coxsackievirus A6 (CVA-6) (Xing et al., 2014). EV-A71 can cause a wide range of disorders with varying presentation and severity, most often in infants, young children and immunocompromised individuals (Baggen et al., 2018).
NikNadia et al. (2016) conducted a study to compare the seroepidemiology of EV-A71 among rural Orang Asli and urban Kuala Lumpur populations, and determined the risk factors associated with EV-A71 seropositivity in rural Orang Asli (NikNadia et al., 2016). They collected 460 serum samples from Diagnostic Virology Laboratory, University of Malaya Medical Center, in Kuala Lumpur and 298 samples previously collected from aboriginal communities in Selangor, Pahang, Perak, Malacca, and Negeri Sembilan between 2010 and 2012. The seropositivity rates of Orang Asli children ≤ 12 years were significantly higher than children living in urban area. Using untreated water (e.g., river, well and rainwater) and age ≤ 12 years were important risk factors for EV-A71 seropositivity among Orang Asli confirmed by univariate and multivariate analysis and this could be due to poor hygiene and sanitation practices.
In this section, we reviewed the articles of major parasitic human infections in Malaysia, including free-living amoeba, amoebiasis, giardiasis, cryptosporidiosis, helminths, Blastocystis sp. and sarcocystosis.
Free-living amoeba (FLA) that cause human disease include Acanthamoeba spp, Naegleria fowleri, Balamuthia mandrillaris, and Sappinia diploidea (Visvesvara et al., 2007). Acanthamoeba spp. are opportunistic pathogens known to cause granulomatous amoebic encephalitis (GAE) and more commonly severe keratitis among contact lens wearers. N. fowleri, on the other hand, causes primary amoebic meningoencephalitis (PAM), which is an acute and potentially lethal disease of the central nervous system. These FLAs are present ubiquitously in the environment including soil and water bodies, and hence poses a serious health risk to humans.
While human diseases caused by Acanthamoeba spp. and Naegleria spp. are rarely reported in Malaysia, several reports showing the presence of these FLAs in water bodies have been published. A report in 2010 by Init and colleagues described the detection of Acanthamoeba spp. and Naegleria spp. in 14 swimming pools around urban cities of Petaling Jaya and Kuala Lumpur (Init et al., 2010). They reported that Acanthamoeba spp. was more widespread than Naegleria spp. and that Acanthamoeba spp. is resistant to dry-hot areas and chlorinated water, but no confirmation of FLA species was performed. However, a study in 2018 by Basher et al. found that Acanthamoeba spp. was indeed widespread as it was detected in all water samples from 15 recreational rivers around urban Selangor and Kuala Lumpur (Basher et al., 2018). In fact, the detected Acanthamoeba spp. strains have been previously associated with GAE and severe keratitis. Similarly, water-borne parasites including Acanthamoeba spp. and Naegleria spp. were detected in various processing sites of two drinking water treatment plants in Sarawak (Richard et al., 2016). The detection of Naegleria spp. in swimming pools, recreational lakes and rivers, and water tanks in mosques around Selangor and Kuala Lumpur was also reported (Ithoi et al., 2011a) but none of them included any pathogenic strains. Collectively, reports on FLA stress the importance of proper monitoring of drinking water treatment plants and recreational sites such as rivers, streams, and swimming pools.
Unlike studies on the aforementioned FLAs that were mostly conducted in waters from urban areas, studies on Entamoeba infections focused on indigenous ethnic groups in rural areas (Anuar et al., 2012a; Ngui et al., 2012; Lau et al., 2013). The genus Entamoeba includes many species but only E. histolytica is a confirmed human pathogen that causes intestinal disease. E. histolytica is commonly observed in tropical regions and is transmitted via contaminated water or food. In Malaysia, Entamoeba infection is sporadically observed in rural areas particularly among indigenous ethic groups where access to clean water and hygiene practices are still subpar (Tengku and Norhayati, 2011). Although common in Malaysia, most studies on the prevalence of Entamoeba infection relied on data from microscopic analysis of stool samples. As such, the true prevalence of E. histolytica remains unknown especially since E. histolytica, E. dispar and E. moshkovskii cannot be distinguished under the microscope.
Our search that was limited to recent years resulted in three reports that aim to study the true prevalence of Entamoeba species using the molecular method, PCR (Anuar et al., 2012a; Ngui et al., 2012; Lau et al., 2013). Anuar et al. (2012a) studied the risk factors of Entamoeba infection, i.e., behavioral risks (personal hygiene and food consumption), environmental sanitation and characteristics of living conditions. The overall prevalence of Entamoeba infection among the indigenous ethnic group determined by microscopy ranges between 17.6 and 19.5%. Lau et al. (2013) reported that the most common infection was either a single E. histolytica infection or mixed E. histolytica and E. dispar (Lau et al., 2013). Similarly, Ngui et al. (2012) reported that E. histolytica was the most common Entamoeba infection among indigenous people (Ngui et al., 2012), but Anuar et al. on the other hand found that E. dispar was most common followed by E. histolytica (Anuar et al., 2012a). A recurring observation that was reported in all three studies was the lack of hygienic practices.
Although none of the three reports have studied water samples specifically, current WASH practices suggest that Entamoeba infection among the native Orang Asli may have been acquired through contaminated water sources. Therefore, educational intervention is very much needed to improve their knowledge, attitude, and practice toward Entamoeba infection prevention and the safe use of water. Besides that, the true incidence of pathogenic Entamoeba species among the population as well as its pathogenicity must be investigated. Since Entamoeba can be found in aquatic environment and water bodies, it also remains to be elucidated whether other factors such as environment, climate and weather have an effect on Entamoeba survival in the environment.
Giardiasis and cryptosporidiosis are protozoan parasitic intestinal infections, in which the transmission route could be water-borne, through ingestion of drinking water or recreational water or contaminated food. Giardia, together with Cryptosporidium are mentioned in the WHO's “Neglected Diseases Initiative” in 2004, which exhibit an increasing global burden (Savioli et al., 2006). The prevalence of Giardia in developed countries is nearly 2% for adults and 8% for children, while the estimation in developing countries is almost 33% (Dunn N, 2020). In the European Union (EU), there were 5.4 confirmed cases per 100,000 population in the year 2014 (European Centre for Disease Prevention Control, 2016). On the other hand, the prevalence in Malaysia varies and has been increased, where it was 1.4–11% in 1992 (Shekhar et al., 1996).
Patients with parasitic intestinal infections share similar signs and symptoms. Ministry of Health Malaysia listed patients with cryptosporidiosis may suffer diarrhea, stomach cramps/pain, nausea, vomiting, fever, headache and loss of appetite (Ministry of Health Malaysia, 2017b). Meanwhile, for acute cases of Giardia infection, CDC included symptoms like diarrhea, abdominal pain, bloating, nausea and vomiting (Centers for Disease Control Prevention, 2017). Less common symptoms like fever, itchy skin, hives, swelling of the eyes and joints has been reported as well (Centers for Disease Control Prevention, 2021). Patients with chronic Giardia infections might suffer from fatigue and weight loss (Centers for Disease Control Prevention, 2017; Wang et al., 2019). However, Dixon (2021) highlighted that there is an ongoing debate that challenges the link between diarrhea and Giardia infection, due to the increased reporting of asymptomatic infection (Dixon, 2021).
There are several articles that report infections from intestinal parasite species, prevalence of polyparasitism and mixed infections with one or more parasite species in Malaysia. From 2010 to 2012, Lee et al. carried out a study in five indigenous villages located in Selangor, Pahang and Perak using One Health (Mackenzie and Jeggo, 2019) approach to investigate occurrence of Giardia in humans, animals and river water (Lee et al., 2014b, 2017). The overall prevalence of Giardia infection (G. duodenalis) among humans was 6.7% (18 out of 269) and some participants were co-infected with other protozoa and helminths. Fecal samples from free roaming and companion animals, such as dogs, chickens, ducks, birds, rodents, otters and cows were also tested but only dogs and cats harbored Giardia infection at 4.7% (Lee et al., 2017). When the river water at same locations were tested, 51.3% of the samples had Giardia cysts and 23.1% samples had Cryptosporidium (oo)cysts (Lee et al., 2014b).
The co-infection in children have been reported by another study in Pahang where 374 children aged 7–12 were enrolled to investigate the burden of giardiasis and its effect on the growth of the children in rural, indigenous community (Al-Mekhlafi et al., 2013). Among these children, 22.2% had Giardia infection and 60% of the children with giardiasis had co-infection with other parasites. The children in these communities were found to be malnourished including 28.3% severely underweight, 23.8% severely stunting and 21.0% severely wasting. Lower weight was significantly associated with Giardia infection compared to their counterparts and treatment with albendazole improved the children's weight and height at 6 months assessment.
Another study of 498 children in Pahang also highlighted the polyparasitism and found 98.4% (490 of 498) infected with at least one intestinal parasite species (between January and April 2012), with higher prevalence of Trichuris trichiura at 95.6% (Al-Delaimy et al., 2014). Giardiasis was prevalent in 28.3% of the study sample and Cryptosporidium infection had 5.2% prevalence. Polyparasitism was however common and present in 71.4% study participants. Children <10-year-old, those living in houses without a toilet, those using unsafe sources (river) for drinking water, not washing hands before eating and presence of infected family members had higher prevalence of polyparasitism.
When the prevalence and risk factors of giardiasis in three indigenous communities in Perak, Pahang and Negeri Sembilan (Anuar et al., 2012b) were investigated, the prevalence of giardiasis was 20% (100 of 500) in those populations. Drinking untreated water, bathing and washing in the river were identified as significant risk factors in two of the tribes, but this was not confirmed in a multivariate model. Presence of other family members infected with giardiasis was the variable confirmed by logistic regression as the most significant predictor of giardiasis among all the tested tribal communities.
Choy et al. (2014) undertook a larger study on aboriginal communities from 28 villages distributed in Pahang, Selangor, Negeri Sembilan, Kelantan, Kedah, Malacca and Sabah where 1,330 participants were screened for prevalence of Giardia infection (Choy et al., 2014). The majority of participants were aged <12 years (69.9%) and had acquired a significantly higher prevalence of Giardia infection than older children. The overall prevalence of Giardia in this study was 11.6% (154/1,330) yet some participants had co-infections of other intestinal parasitic infections where about two thirds of (104/154) were mixed infections with one or more parasite species (Trichuris, Ascaris, Entamoeba) while one third were Giardia single infections (50/154). In contrast to the study by Anuar et al. (2012b) but similar to the study by Al-Delaimy et al. (2014), in both univariate and multivariate analyses, no toilet in the house, not boiling water before consumption, bathing in river and not washing hands before eating were significantly associated with increased levels of Giardia infection which manifested with symptoms of diarrhea, abdominal pain and vomiting.
A 6-month long survey of the river water in Sungai Lopo, Hulu langat was carried out by Lim and Aahmad (2004) to study the occurrence of Giardia cysts and Cryptosporidium oocysts in the Temuan Orang Asli river system (Lim and Aahmad, 2004). The findings showed that while Giardia cysts and fecal coliform was detected in all the water samples collected, Cryptosporidium oocysts were detected only in one water sample which was collected downstream. Overall, the results implied that the river is contaminated with fecal-oral transmitted parasites, indicating a possible route for Giardia and Cryptosporidium transmission through open defecation to the river at this location. Similarly, a study by Bilung et al. (2017) revealed that higher concentrations of Cryptosporidium and cyclospora in environmental water used for abstraction of drinking water treatment plant (Sungai Sarawak Kanan and Sungai Sarawak Kiri) and recreational activities (Ranchan recreational park and UNIMAS lake) in Sarawak (Bilung et al., 2017). However, the study had a small sample size at a specific point in time and hence it is difficult to draw any general conclusions from this study.
When the water quality of the two main rivers in Kuantan, Pahang, namely Sungai Kuantan and Sungai Balok were investigated by studying the levels of Cryptosporidium oocysts, the physicochemical and heavy metal parameters, the Sungai Kuantan and Sungai Balok were reported as major risks for Cryptosporidium infection (Zainutdin et al., 2017). This may be due improper waste management and poor hygiene practices among the population near the two rivers but there was no direct evidence provided in the study (Zainutdin et al., 2017). Further, a cross-sectional study of 25 eligible water treatment plants (WTPs) across 11 administrative divisions in Sarawak, Malaysia (Ting Lo et al., 2018) also suffered lack of direct evidence and insufficient participation as only eight of the 25 WTPs in Sarawak and one WTP in Peninsular Malaysia participated. Thus, the data collectively indicate the impact of unhygienic WASH practices and river/drinking water contamination as major risk factors of Giardiasis and cryptosporidiosis intestinal infection in various parts of Malaysia. Regular monitoring of the rivers and water treatment plants along with awareness programs that educate the local communities on proper sanitation and proper waste disposal are necessary to combat these parasitic infections.
Soil-transmitted helminth (STH) infections are caused by mainly 3 types of STH species: Ascaris lumbricoides, Trichuris trichuria, and hookworms (Ancylostoma duodenale and Necator americanus). As these worms feed on host tissues, the host develops protein-energy malnutrition, iron-deficiency anemia, and vitamin A deficiency (American International Medical University, 2017), causing symptoms to arise including abdominal pain, nausea, diarrhea, bloody stools, worm in stools, and weight loss. As one of the NTD, STH has infected approximately 2 billion people globally by 2012 (World Health Organization, 2012), resulting in about 135,000 deaths annually (Pasaribu et al., 2019), and has been exclusively prevalent in the lower income developing countries. In Malaysia, STH prevalence varies across different geographical locations/populations, mainly affecting the aboriginal Orang Asli tribes and the rural Malay, mostly located in Perak, Kedah, Kelantan, and Pahang (Northern Peninsular Malaysia). Factors that contribute to this are poor sanitation systems, lack of de-worming programmes, preventive health education and in some cases inadequate personal hygiene practices (Nasr et al., 2013a).
Nasr et al. (2013a,b) carried out a two-part cross-sectional study among 484 Orang Asli children aged ≤ 15 years belonging to 215 households from 13 villages in Lipis district, Pahang, Malaysia. The study showed a high prevalence of STH in these children and key factors were using unsafe drinking water supply, absence of a toilet in the house, large family size (≥ 7 members), as well as not washing hands before eating, and after defecation (Nasr et al., 2013a). The second part of the study shows that the KAP on STH infections among the participants were inadequate (Nasr et al., 2013b). Overall, the entire study exposed the need for school-based deworming programmes, proper sanitation, treated drinking water supply, proper health education on personal hygiene and transmission & prevention of STH; as a more holistic STH prevention and elimination strategy. In the same way, Lee et al. (2014a) reported a cross sectional study of 269 people from two different subtribes of indigenous communities: the Temuan and Temiar subtribes (Lee et al., 2014a). They discovered that overall STH infections were higher in the Temuan subtribe, with Trichuris trichiura (46.2%) as the most prevalent parasite, followed by Ascaris spp. (25.7%) and hookworm (4.1%). Comparatively, Ascaris spp. (39.8%) was more prevalent among the Temiar subtribe, preceded by T. trichiura (35.7%) and finally hookworm (8.3%). Co-infections of helminthiasis and intestinal protozoa were three times higher among the tribes Temiar compared to Temuan. This study takes into account the variations in infections and also cultural practices in each subtribe of the indigenous population, rather than considering the indigenous communities as a homogenous group, and this enables more customisable control measures (Lee et al., 2014a).
Another cross-sectional survey from May 2016 to April 2017, in eight villages comprising all six Negrito indigenous sub-tribes located in the northern states of Peninsular Malaysia (Muslim et al., 2019). The survey aimed to compare STH infections in 416 participants who were grouped into two categories of communities based on location; Inland Jungle Villages (IJV); and Resettlement Plan Scheme (RPS). Their findings proved that prevalence of STH was significantly higher in IJV than in RPS. The prevalence of moderate to severe hookworm infections and other intestinal parasitic infections (e.g., Entamoeba sp., Blastocystis sp. and flukes) were also higher in IJV than in RPS. However, the percentage of individuals with severe T. trichiura infections and severe Ascaris lumbricoides infection were significantly higher in the RPS compared to IJV. Therefore, all these suggested that the development plan by RPS does not result in a great impact in terms of STH reduction among the indigenous communities. A biannual mass albendazole intervention, community empowerment to both communities, and also recruitment of more Orang Asli individuals in the health-care taskforce were suggested as a long-term strategy (Muslim et al., 2019).
In these studies, Ascaris spp., T. trichiura, and hookworm infections are the most prevalent parasites causing STH infections in Malaysia. Although Lee et al. (2014b) isolates different subtribes of the indigenous population, all the studies were conducted in few villages with limited number of sampling sites (Lee et al., 2014a). Furthermore, demarginalisations and resettlements per se will not bring about impactful outcomes in STH elimination. More holistic approaches in the health-care taskforce, raising awareness and education about STH transmission and prevention, water quality monitoring and drinking water treatment, and proper sanitation are required to eliminate helminth infections.
Blastocystis sp. is also an enteric parasite, and is found to be more prevalent than other intestinal protozoan parasites (Wawrzyniak et al., 2013). Although the prevalence varies widely from country to country, higher prevalence in developing countries appears in univariate and multivariate models to be due to poor hygiene, exposure to animals, and consumption of contaminated food or water (Tan, 2008; Wawrzyniak et al., 2013). Different subtypes of Blastocystis are found worldwide with nine subtypes detected in humans to date (Tan, 2008; Alfellani et al., 2013). Ministry of Health Malaysia (2020) stated that infection with Blastocystis can lead to symptoms like watery or loose stools, diarrhea, abdominal pain, anal itching, weight loss and constipation, although asymptomatic infections were reported. Abdullah et al. (2017a) reported that Blastocystis infection can cause other gastrointestinal symptoms like nausea, vomiting, anorexia pruritus as well as tenesmus (Abdullah et al., 2017b).
A study carried out among 300 primary school children (aged 6–12 years) living in rural communities of Pahang reported a 25.7% (77/300) overall prevalence of Blastocystis (Abdulsalam et al., 2012). Other infections were also reported among these children including G. duodenalis (15.3%), co-infection of Blastocystis and giardiasis (2.3%), helminth T. trichiura (47.0%) and Entamoeba histolytica/dispar (4.3%). Univariate analysis and logistic regression analysis confirmed that absence of piped water supply was significantly associated with the occurrence of Blastocystis infection and those children who do not have a tap water supply were three times more likely to get Blastocystis infection.
Similarly, when the prevalence of Blastocystis infection among aboriginal communities in Pahang was investigated during dry and wet season (Abdullah et al., 2017b), the overall prevalence of Blastocystis infection was 40.4% and was not statistically significant different between wet and dry seasons. Among the subtypes, ST1 significantly had greater prevalence during the wet season while ST2, ST3 and ST4 had no significant difference between two seasons. More than half of the participants used untreated tap water supply (63.6%) and river water (50.5%) for their daily use. 43.3% of the people do not have proper latrine systems and practice defecation in the river and bushes. Univariate analysis revealed that use of untreated tap water supply, use of untreated tap water for washing, use of stored river water for domestic use and the absence of latrine system during the wet season, while use of stored river water in containers for domestic use during the dry season was significantly associated with Blastocystis infection. In logistic regression analysis, presence of other family members infected and use of river water stored in containers for domestic activities were significant risk factors during dry and wet seasons.
Two further studies tested the occurrence of Blastocystis and fecal coliforms in river water samples from indigenous community settlements (Abdullah et al., 2017a) in both wet and dry seasons (Abdullah et al., 2016) at Sungai Krau and Sungai Lompat and reported significant association between the infection and concentration of fecal coliforms (Abdullah et al., 2017a). Blastocystis sp. subtype ST3 were detected at all sampling points during dry and wet season (Abdullah et al., 2016). High concentration of fecal coliform was also detected in both the rivers during both seasons, although only occurrence of Blastocystis sp. subtype ST2 was significantly associated with fecal coliform (Abdullah et al., 2016). Similar results were demonstrated by a study done earlier in 2004 to 2005 where they detected Blastocystis sp. in river water of recreational areas (Ithoi et al., 2011b). It was detected at the rate of 33.3% (Sungai Congkak) and 22.1% (Sungai Batu) where the highest concentration was at the downstream of both rivers. The significant correlation between fecal coliforms and Blastocystis was also evident in both rivers. Both the studies raise the potential health risk to the indigenous community if they use river water as drinking water or other purposes and the need of river water monitoring. The study did not evaluate presence of symptoms or human health impact with the presence of fecal coliforms or Blastocystis sp.
In selected villages at Negeri Sembilan, Perak and Pahang, Peninsular Malaysia indigenous communities, the overall prevalence of Blastocystis infection was reported as 20.4% (102 of 500) where most of the infected individuals were <15 years old (Anuar et al., 2013). Of all the samples with Blastocystis, 82.4% had co-infection with one or more other parasites such as T. trichiura (53.9%), E. histolytica/dispar/moshkovskii (26.5%), E. hartmanni (22.6%), E. coli (20.6%), G. intestinalis (17.7%) and others. Like other studies, univariate analysis and logistic regression analysis showed that drinking untreated water and presence of other infected family members significantly associated with presence and higher numbers of Blastocystis infection among the study population. Further, a focused study on school children aged 7–12 years that tested the prevalence of intestinal parasitic infection (IPI) (Nithyamathi et al., 2016) found that 13.3% of the school children had IPI, with 10.6% (n = 186) of children had Blastocystis sp. detected in both rural (13.7%) and urban (7.2%) area. Other parasites such as Giardia sp. and T. trichiura were found only in rural areas. There were higher number of ST1 (22.6%) infecting these school children detected in both urban and rural areas compared to other subtypes.
In contrast to the studies in children, where children had higher prevalence, study by Mohammad et al. (2017) showed those aged 15 years or above having significantly higher prevalence of Blastocystis infection. Among the 253 participants, 40.7% of 253 participants had Blastocystis infection (Mohammad et al., 2017). Drinking untreated water was significantly associated with occurrence of Blastocystis infection in univariate analysis but not multivariate analysis. Presence of other family members with Blastocystis infection was a significant risk factor shown by both univariate and multivariate analysis, which is consistently reported in all the studies. Together, these reports highlight the various risk factors, coinfections and the significance of water quality monitoring of rivers, drinking water and need for WASH practice awareness in rural communities.
Sarcocystis spp. being the causing agent of sarcocystosis, is a parasite that could infect intestine or muscles which could be transmitted through food or water. This is due to the fact that swine and cow might serve as an intermediate host of Sarcocystis, thus infecting human through undercooked meat (Fayer et al., 2015). Depending on the site of infection, different clinical presentations were observed, ranging from diarrhea, vomiting for intestinal sarcocystosis, to muscle weakness and myalgia for muscular sarcocystosis (Latif and Muslim, 2016). The prevalence of Sarcocystis infection was relatively unknown, but there is <100 humans infected with muscular sarcocystosis reported in the literature and intestinal sarcocystosis was more prevalent in Europe (Fayer, 2004). It was found in a seroepidemiological survey that almost 20% of 243 participants had antibodies to Sarcocystis (Thomas and Dissanaike, 1978).
Sarcocystosis were only reported by travelers who returned from vacation in Tioman Island from 2011 to 2013 (Maizura et al., 2012; Tappe et al., 2013; Esposito et al., 2014). Some of the patients had prolonged symptoms of >6 months (Slesak et al., 2014). Another wave of muscular sarcocystosis outbreak was suspected in May 2014 involving six patients after returning from Tioman Island, although no muscle biopsies were taken (Tappe et al., 2014). An outbreak of muscular sarcocystosis occurred after a retreat at Pangkor Island in 2012 involving 89 students and teachers (Italiano et al., 2014). A subsequent study on two symptomatic patients also detected sarcocysts in the skeletal muscle biopsy and the species was identified as S. nesbitti (Lau et al., 2014). Thereafter, Shahari et al. collected sediment samples (indirect screening of water samples) from rivers, water tanks, wells and seawater in 2015 at Tioman Island, the hotspot of sarcocystosis outbreak (Shahari et al., 2016). Of the 157 samples, 19 samples tested positive of sarcocysts.
The same searches were performed in PubMed on fungal infections related to water in Malaysia. However, to the best of our knowledge, no articles were published between 2010 and 2021.
According to the data from Ministry of Health Malaysia in 2018, dengue has the highest incidence rate per 100,000 population, leptospirosis recorded 15.39 incidence rate with a mortality rate of 0.11 per 100,000 population and typhoid and paratyphoid fever recorded 0.53 incidence rate with a mortality rate of 0.02 per 100,000 population (Ministry of Health Malaysia, 2018). While there are reports of occasional outbreaks and patients admitted to hospital in Malaysia, the burden of melioidosis in Malaysia, as other places in the world, is unknown and most probably under-reported (Limmathurotsakul et al., 2016). Leptospirosis also had a significantly higher incidence rate and death rate among the infectious diseases and can be co-infected with other diseases, hence more precautions are needed especially in Southeast Asia countries, including Malaysia, where the climate is humid and flooding is relatively common (Pappas et al., 2008; Mohd Radi et al., 2018). Studies proved that seroprevalence toward leptospirosis was high, for example, among municipal service and wet market workers, indicating work exposure is one of the important exposure routes. There are not many reports about S. typhi infection or determination of S. typhi concentration in the water environment. Other than that, H. pylori infection is associated with clean water and sanitation as contaminated water could be a source (Bahrami et al., 2013; Aziz et al., 2015) and with the improved facilities in Malaysia, the prevalence is relatively low as concluded by Rahim et al. (2010). Most of the studies carried out in the indigenous communities' settlements concluded that some of the settlements do not have toilet facilities or piped-water supply, instead they might use untreated water from nearby river and/or defecate indiscriminately. Consequently, the indigenous communities were more prone to amoeba (Tengku and Norhayati, 2011; Anuar et al., 2012a, 2013; Ngui et al., 2012; Lau et al., 2013), giardiasis and/or cryptosporidiosis (Lim et al., 2011; Anuar et al., 2012b, 2013; Al-Mekhlafi et al., 2013; Al-Delaimy et al., 2014; Choy et al., 2014; Lee et al., 2017), helminth and hookworm (Anuar et al., 2013; Nasr et al., 2013a; Lee et al., 2014a; Muslim et al., 2019), Blastocystis sp. infection (Anuar et al., 2013; Abdullah et al., 2017b) and even co-infections. Safely managed water, sanitation and hygiene (WASH) is essential but remain a major challenge in low-and-middle income countries (World Health Organization Regional Office for the Western Pacific, 2018).
From the findings of this study, it is apparent that there is no standardized methodology for examining the relationship between microorganisms and health in Malaysia. This is a common finding in many countries where water sampling for health-related issues is only undertaken after an event has come to light, by which point it is difficult to directly attribute symptoms and health outcomes to a specific organism, time point or location and identify causality (Hill, 1965). When sites are selected for water sampling, it is important to also think about the sources of infection that cause disease and the route of the infection. For example, food, latrines and other sources can transmit similar microorganisms to water bodies. In the reviewed papers, there has been little or no attempt to identify other sources of infection that may have confounded the findings of those studies. For most microorganisms, there is little or no baseline data and in Malaysia, only E. coli and dengue are regularly measured at specific locations decided in the government sector and these are often the same locations chosen for research projects; whereas this increases the frequency of sampling, it does not improve the general understanding of a basin. Sharing and making access to data easier, alongside more strategically designed sampling campaigns, would help pinpoint infection sources and make source tracking studies more feasible. Multivariate predictive models could use existing data to predict optimum sites for future sampling and inform regularity of sampling in relation to environmental, physical and hydro-climatic related variables. A multi or transdisciplinary systems approach to disease modeling incorporating environmental and health data would improve existing models and enable health practitioners and environmental bodies to work together in tackling water quality issues affecting health of vulnerable populations. Such a model could involve citizen scientists in data collection, which would increase public awareness and input into maintaining water security locally (Siew et al., 2016; Nardi et al., 2020).
When infectious diseases as described above are symptomatic in communities, reporting of cases largely falls on general practitioners in family medicine clinics, but only when a symptomatic individual presents themselves for treatment. To report a case formally, an objective diagnosis, for example, from a stool sample is required. Without this, cases will not get reported to the center for disease control. It is therefore likely that many cases are unreported, and the scale of water related infection in communities residing by lakes, ponds and rivers, for example, is considerably higher than official data shows. In most studies, controls are not evaluated for presence of microorganisms, studies are affected by recall bias as interviewing is typically days to weeks after an outbreak and there is no long term follow up. In many cases, the number, site and frequency of samplings undertaken for water quality studies is not justified rather, simply determined by funding and existing practice. Since symptoms of diarrhoeal disease from any source are similar, it is likely that such symptoms are normalized, and specific infections are not ever identified. Those living with these conditions may consider them common and minor, as such, do not seek medical assistance, hence cases will not be identified and recorded. There is little information in the articles reviewed in this paper on recording pre-existing immunocompromised in infected individuals. With the exception of diabetes mellitus, few other conditions are asked about and severity of diabetes mellitus is not recorded or measured. As such, it is difficult to know the impact diabetes mellitus had on infection outcomes in those patients. Informing citizens of symptoms to watch out for might alert vulnerable populations including poorly controlled diabetics to seek medical help and gain formal diagnoses rather than staying home with manageable symptoms typically lasting only a few days. Without more accurate data to demonstrate a causal relationship between presence of microorganisms and disease (Hill, 1965) it is difficult to evidence base a policy change or health initiative to improve existing situations.
It is important to note that in a natural disaster such as a flooding episode, other issues may be considered more than acute emergency priorities, such as trauma, mobilizing communities and, preventing hypothermia. As such, water sampling may be a later event that only commences when symptomatic disease becomes more widespread. By the time, conditions are sufficiently settled to sample water and focus on health outcomes, sites of infection will have altered and point sources of infection will have changed or moved (Watkins et al., 2012; Johanning et al., 2014). Such a situation can occur with sewage overflow following pluvial flooding, but remedying the situation is difficult in the short term as it requires structural changes at some expense to existing drainage systems, which may not be a priority for emergency task forces and future planning where maintaining transport links to schools and businesses and supply chains are priorities (Patterson and Jeffrey, 2011).
During the COVID-19 pandemic, regular collecting of baseline data has not been possible in many countries, with lockdowns, lack of access to laboratory facilities and suchlike. It has been shown however, that drinking water pollutants may affect response to COVID-19 in susceptible individuals (Quinete and Hauser-Davis, 2021). Environmental hazards such as monsoon flooding however continues compounding difficulties for inhabitants of river basins and those living near water bodies.
A strategic approach to better understand and manage the relationship between water quality and health is needed. This is particularly important as the latest IPCC report (IPCC, 2021) provide clear evidence that climate change is intensifying the water cycle, with changing rainfall patterns and intensities affecting flood and drought risk. Taking into account whole system interdisciplinary understanding of basins, this strategic approach should consider and include:
• Identification of point and diffuse sources of infection and transport/transmission routes, this requires an integrated understanding of the basin hydrology;
• Standardized data collection protocols and sharing mechanisms amongst the relevant authorities and decision makers;
• Procedures for data analysis and interpretation that could inform predictive models, improve understanding of health-related challenges and in turn iteratively design data collection strategies;
• Empowering citizens and communities to collect their own data, understand and identify problems, promote behavior change and provide more frequent data points;
• Protocols that prioritize sampling during extreme flood events, which have shown to cause more widespread disease; although not identified in this review, there is growing evidence of links between experiencing flooding and mental health (e.g., Fernandez et al., 2015; French et al., 2019);
• Exploration for data proxies or alternative mechanisms to collect samples e.g., using drones during periods of inaccessibility.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
JH, AL, and DK conducted data collection and analysis, prepared figures, and wrote the manuscript. CL wrote the manuscript. AR conducted data collection and analysis. CW, MG, and JE provided resources, suggestions, conceptualized the ideas and methodology, managed the overall project administration and coordinated the data analysis, wrote original draft, reviewed, and edited the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by Newcastle University Medicine (NUMed) Malaysia Biomedical Science Research Fellowship Programme, Water Security and Sustainable Development Hub funded by the UK Research and Innovation's Global Challenges Research Fund (GCRF) [Grant No. ES/S008179/1].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to Water Security and Sustainable Development Hub and Newcastle University Medicine (NUMed) Malaysia.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frwa.2022.779860/full#supplementary-material
Abdullah, N. S., Lee, I. L., Anuar, T. S., Salleh, F. M., Abdul Manap, S. N., Mohd Mohtar, N. S., et al. (2016). Occurrence of Blastocystis sp. in water catchments at Malay villages and Aboriginal settlement during wet and dry seasons in Peninsular Malaysia. PeerJ 4:e2541. doi: 10.7717/peerj.2541
Abdullah, N. S., Lee, I. L., Anuar, T. S., Salleh, F. M., Manap, S. N. A., Husnie, N. S., et al. (2017a). Blastocystis spp. contaminated water sources in aboriginal settlements. Trop. Biomed. 34, 110–117.
Abdullah, N. S., Moktar, N., Anuar, T. S., Lee, I. L., Salleh, F. M., Manap, S. N. A. A., et al. (2017b). Molecular epidemiology of blastocystosis in Malaysia: does seasonal variation play an important role in determining the distribution and risk factors of Blastocystis subtype infections in the Aboriginal community? Parasit. Vect. 10, 360–360. doi: 10.1186/s13071-017-2294-2
Abdulsalam, A. M., Ithoi, I., Al-Mekhlafi, H. M., Ahmed, A., Surin, J., and Mak, J. W. (2012). Drinking water is a significant predictor of Blastocystis infection among rural Malaysian primary schoolchildren. Parasitology 139, 1014–1020. doi: 10.1017/S0031182012000340
Abu Hassan, M. R., Aziz, N., Ismail, N., Shafie, Z., Mayala, B., Donohue, R. E., et al. (2019). Socio-epidemiological and land cover risk factors for melioidosis in Kedah, Northern Malaysia. PLoS Negl. Trop. Dis. 13:e0007243. doi: 10.1371/journal.pntd.0007243
Ahmad, F., Hamzah, N. A. C., Mustaffa, N., and Gan, S. H. (2011). Anti-hepatitis A seroprevalence among chronic viral hepatitis patients in Kelantan, Malaysia. World J. Gastroenterol. 17, 4130–4134. doi: 10.3748/wjg.v17.i36.4130
Aiello, A. E., and Larson, E. L. (2002). What is the evidence for a causal link between hygiene and infections? Lancet Infect. Dis. 2, 103–110. doi: 10.1016/S1473-3099(02)00184-6
Al-Badaii, F., and Shuhaimi-Othman, M. (2015). Water pollution and its impact on the prevalence of antibiotic-resistant E. coli and total coliform bacteria: a study of the semenyih river, Peninsular Malaysia. Water Q. Exposure Health 7, 319–330. doi: 10.1007/s12403-014-0151-5
Al-Delaimy, A. K., Al-Mekhlafi, H. M., Nasr, N. A., Sady, H., Atroosh, W. M., Nashiry, M., et al. (2014). Epidemiology of intestinal polyparasitism among orang asli school children in rural Malaysia. PLoS Negl. Trop. Dis. 8:e3074. doi: 10.1371/journal.pntd.0003074
Alfellani, M. A., Stensvold, C. R., Vidal-Lapiedra, A., Onuoha, E. S., Fagbenro-Beyioku, A. F., and Clark, C. G. (2013). Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 126, 11–18. doi: 10.1016/j.actatropica.2012.12.011
Al-Mekhlafi, H. M., Al-Maktari, M. T., Jani, R., Ahmed, A., Anuar, T. S., Moktar, N., et al. (2013). Burden of giardia duodenalis infection and its adverse effects on growth of schoolchildren in rural Malaysia. PLoS Negl. Trop. Dis. 7:e2516. doi: 10.1371/journal.pntd.0002516
American International Medical University (2017). Helminthiasis (Soil-transmitted helminth infections): Symptoms, Diagnosis and Management [Online]. USA: American International Medical University. Available online at: https://www.aimu.us/2017/11/10/helminthiasis-soil-transmitted-helminth-infections-symptoms-diagnosis-and-management/ (accessed September 15, 2021).
Anita, S., Amir, K. M., Fadzilah, K., Ahamad, J., Noorhaida, U., Marina, K., et al. (2012). Risk factors for typhoid outbreak in Sungai Congkak Recreational Park, Selangor 2009. Med. J. Malaysia 67, 12–16.
Antillón, M., Warren, J. L., Crawford, F. W., Weinberger, D. M., Kürüm, E., Pak, G. D., et al. (2017). The burden of typhoid fever in low- and middle-income countries: A meta-regression approach. PLoS Negl. Trop. Dis. 11:e0005376. doi: 10.1371/journal.pntd.0005376
Anuar, T. S., Al-Mekhlafi, H. M., Abdul Ghani, M. K., Abu Bakar, E., Azreen, S. N., Salleh, F. M., et al. (2012a). Molecular epidemiology of amoebiasis in Malaysia: highlighting the different risk factors of Entamoeba histolytica and Entamoeba dispar infections among Orang Asli communities. Int. J. Parasitol. 42, 1165–1175. doi: 10.1016/j.ijpara.2012.10.003
Anuar, T. S., Al-Mekhlafi, H. M., Ghani, M. K. A., Osman, E., Yasin, A. M., Nordin, A., et al. (2012b). Giardiasis among different tribes of Orang Asli in Malaysia: Highlighting the presence of other family members infected with Giardia intestinalis as a main risk factor. Int. J. Parasitol. 42, 871–880. doi: 10.1016/j.ijpara.2012.07.003
Anuar, T. S., Ghani, M. K., Azreen, S. N., Salleh, F. M., and Moktar, N. (2013). Blastocystis infection in Malaysia: evidence of waterborne and human-to-human transmissions among the Proto-Malay, Negrito and Senoi tribes of Orang Asli. Parasit. Vect. 6:40. doi: 10.1186/1756-3305-6-40
Atil, A., Jeffree, M. S., Syed Abdul Rahim, S. S., Hassan, M. R., Awang Lukman, K., and Ahmed, K. (2020). Occupational determinants of leptospirosis among Urban service workers. Int. J. Environ. Res. Public Health 17:427. doi: 10.3390/ijerph17020427
Azali, M. A., Yean Yean, C., Harun, A., Aminuddin Baki, N. N., and Ismail, N. (2016). Molecular characterization of Leptospira spp. in environmental samples from north-eastern Malaysia revealed a pathogenic strain, Leptospira alstonii. J. Trop. Med. 2016:2060241. doi: 10.1155/2016/2060241
Aziz, R. K., Khalifa, M. M., and Sharaf, R. R. (2015). Contaminated water as a source of Helicobacter pylori infection: A review. J. Adv. Res. 6, 539–547. doi: 10.1016/j.jare.2013.07.007
Aziz, S., Aidil, R. M., Nisfariza, M. N., Ngui, R., Lim, Y. A., Yusoff, W. S., et al. (2014). Spatial density of Aedes distribution in urban areas: a case study of breteau index in Kuala Lumpur, Malaysia. J. Vector Borne Dis. 51, 91–96.
Baggen, J., Thibaut, H. J., Strating, J. R. P. M., and van Kuppeveld, F. J. M. (2018). The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 16, 368–381. doi: 10.1038/s41579-018-0005-4
Bahrami, A. R., Rahimi, E., and Ghasemian Safaei, H. (2013). Detection of Helicobacter pylori in city water, dental units' water, and bottled mineral water in Isfahan, Iran. Sci. World J. 2013:280510. doi: 10.1155/2013/280510
Barati, A., Ghaderpour, A., Chew, L. L., Bong, C. W., Thong, K. L., Chong, V. C., et al. (2016). Isolation and characterization of aquatic-borne klebsiella pneumoniae from tropical estuaries in Malaysia. Int. J. Environ. Res. Public Health 13:426. doi: 10.3390/ijerph13040426
Basher, M. H. A., Ithoi, I., Mahmud, R., Abdulsalam, A. M., Foead, A. I., Dawaki, S., et al. (2018). Occurrence of acanthamoeba genotypes in central west Malaysian environments. Acta Trop. 178, 219–228. doi: 10.1016/j.actatropica.2017.11.015
Batra, P., Mathur, P., and Misra, M. C. (2016). Aeromonas spp.: An emerging nosocomial pathogen. J. Lab. Physicians 8, 1–4. doi: 10.4103/0974-2727.176234
Benacer, D., Mohd Zain, S. N., Sim, S. Z., Mohd Khalid, M. K., Galloway, R. L., Souris, M., et al. (2016a). Determination of Leptospira borgpetersenii serovar Javanica and Leptospira interrogans serovar Bataviae as the persistent Leptospira serovars circulating in the urban rat populations in Peninsular Malaysia. Parasit. Vect. 9:117. doi: 10.1186/s13071-016-1400-1
Benacer, D., Thong, K. L., Verasahib, K. B., Galloway, R. L., Hartskeerl, R. A., Lewis, J. W., et al. (2016b). Human leptospirosis in Malaysia: reviewing the challenges after 8 decades (1925-2012). Asia Pac. J. Public Health 28, 290–302. doi: 10.1177/1010539516640350
Benacer, D., Woh, P. Y., Mohd Zain, S. N., Amran, F., and Thong, K. L. (2013). Pathogenic and saprophytic Leptospira species in water and soils from selected urban sites in peninsular Malaysia. Microbes Environ. 28, 135–140. doi: 10.1264/jsme2.ME12154
Bilung, L. M., Tahar, A. S., Yunos, N. E., Apun, K., Lim, Y. A.-L., Nillian, E., et al. (2017). Detection of cryptosporidium and cyclospora oocysts from environmental water for drinking and recreational activities in Sarawak, Malaysia. Biomed Res. Int. 2017:4636420. doi: 10.1155/2017/4636420
Birnie, E., Virk, H. S., Savelkoel, J., Spijker, R., Bertherat, E., Dance, D. A. B., et al. (2019). Global burden of melioidosis in 2015: a systematic review and data synthesis. Lancet. Infect. Dis. 19, 892–902. doi: 10.1016/S1473-3099(19)30157-4
Blancas, L. C., and El-Hifnawi, M. B. (2014). Facilitating Trade Through Competitive, Low-Carbon Transport: The Case for Vietnam's Inland and Coastal Waterways. Washington, DC: World Bank. doi: 10.1596/978-1-4648-0105-1
Blasdell, K. R., Morand, S., Perera, D., and Firth, C. (2019). Association of rodent-borne Leptospira spp. with urban environments in Malaysian Borneo. PLOS Negl. Trop. Dis. 13:e0007141. doi: 10.1371/journal.pntd.0007141
Centers for Disease Control Prevention (2017). Giardiasis [Online]. Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/dpdx/giardiasis/index.html (accessed August 19, 2021).
Centers for Disease Control Prevention (2020). What is Viral Hepatitis? [Online]. Available online at: https://www.cdc.gov/hepatitis/abc/index.htm (accessed August 19, 2021).
Centers for Disease Control Prevention (2021). Parasites - Giardiasis [Online]. Centers for Disease Control and Prevention. Available online at: https://www.cdc.gov/parasites/giardia/illness.html (accessed August 19, 2021).
Chandren, J. R., Wong, L. P., and AbuBakar, S. (2015). Practices of dengue fever prevention and the associated factors among the Orang Asli in Peninsular Malaysia. PLoS Negl. Trop. Dis. 9, e0003954. doi: 10.1371/journal.pntd.0003954
Chang, C. Y., Lau, N. L. J., Currie, B. J., and Podin, Y. (2020). Disseminated melioidosis in early pregnancy - an unproven cause of foetal loss. BMC Infect. Dis. 20:201. doi: 10.1186/s12879-020-4937-8
Cheong, Y. L., Leitão, P. J., and Lakes, T. (2014). Assessment of land use factors associated with dengue cases in Malaysia using Boosted Regression Trees. Spat. Spatiotemporal. Epidemiol. 10, 75–84. doi: 10.1016/j.sste.2014.05.002
Choy, S. H., Al-Mekhlafi, H. M., Mahdy, M. A., Nasr, N. N., Sulaiman, M., Lim, Y. A., et al. (2014). Prevalence and associated risk factors of Giardia infection among indigenous communities in rural Malaysia. Sci. Rep. 4:6909. doi: 10.1038/srep06909
Crisis Preparedness Response Centre (CPRC) (2020). iDengue for Community [Online]. Available online at: https://idengue.mysa.gov.my/ (accessed September 15, 2021).
Crump, J. A., and Mintz, E. D. (2010). Global trends in typhoid and paratyphoid Fever. Clin. Infect. Dis. 50, 241–246. doi: 10.1086/649541
Dada, A. C., Ahmad, A., Usup, G., and Heng, L. Y. (2013). Speciation and antimicrobial resistance of Enterococci isolated from recreational beaches in Malaysia. Environ. Monit. Assess. 185, 1583–1599. doi: 10.1007/s10661-012-2653-6
Daniel, D. S., Lee, S. M., Gan, H. M., Dykes, G. A., and Rahman, S. (2017). Genetic diversity of Enterococcus faecalis isolated from environmental, animal and clinical sources in Malaysia. J. Infect. Public Health 10, 617–623. doi: 10.1016/j.jiph.2017.02.006
Daud, A. B., Mohd Fuzi, N. M. H., Wan Mohammad, W. M. Z., Amran, F., Ismail, N., Arshad, M. M., et al. (2018). Leptospirosis and workplace environmental risk factors among cattle farmers in Northeastern Malaysia. Int. J. Occup. Environ. Med. 9, 88–96. doi: 10.15171/ijoem.2018.1164
Dickin, S. K., Schuster-Wallace, C. J., and Elliott, S. J. (2013). Developing a vulnerability mapping methodology: applying the water-associated disease index to dengue in Malaysia. PLoS ONE 8, e63584. doi: 10.1371/journal.pone.0063584
Dieng, H., Saifur, R. G. M., Ahmad, A. H., Salmah, M. R. C., Aziz, A. T., Satho, T., et al. (2012). Unusual developing sites of dengue vectors and potential epidemiological implications. Asian Pac. J. Trop. Biomed. 2, 228–232. doi: 10.1016/S2221-1691(12)60047-1
Dixon, B. R.. (2021). Giardia duodenalis in humans and animals – Transmission and disease. Res. Vet. Sci. 135, 283–289. doi: 10.1016/j.rvsc.2020.09.034
Dom, N. C., Ahmad, A. H., Latif, Z. A., Ismail, R., and Pradhan, B. (2013). Coupling of remote sensing data and environmental-related parameters for dengue transmission risk assessment in Subang Jaya, Malaysia. Geocarto Int. 28, 258–272. doi: 10.1080/10106049.2012.696726
Drancourt, M.. (2010). “Acute diarrhea,” in Infectious Diseases (Third Edition), eds J. Cohen, S. M. Opal, and W. G. Powderly (London: Mosby) 381–388. doi: 10.1016/B978-0-323-04579-7.00035-6
Esposito, D. H., Stich, A., Epelboin, L., Malvy, D., Han, P. V., Bottieau, E., et al. (2014). Acute muscular sarcocystosis: an international investigation among ill travelers returning from Tioman Island, Malaysia, 2011–2012. Clin. Infect. Dis. 59, 1401–1410. doi: 10.1093/cid/ciu622
European Centre for Disease Prevention and Control (2016). Annual Epidemiological Report 2016 – Giardiasis. (Stockholm).
Fayer, R.. (2004). Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 17, 894–902. doi: 10.1128/CMR.17.4.894-902.2004
Fayer, R., Esposito, D. H., and Dubey, J. P. (2015). Human infections with sarcocystis species. Clin. Microbiol. Rev. 28, 295–311. doi: 10.1128/CMR.00113-14
Feng, W., Teh, W., Hitam, W.-H., and Ali, H. (2018). Endogenous endophthalmitis secondary to melioidosis in paediatric patients: Case series and review article. J. Acute Dis. 7, 234–240. doi: 10.4103/2221-6189.248027
Ferede, G., Tiruneh, M., Abate, E., Kassa, W. J., Wondimeneh, Y., Damtie, D., et al. (2018). Distribution and larval breeding habitats of Aedes mosquito species in residential areas of northwest Ethiopia. Epidemiol. Health 40, e2018015–e2018015. doi: 10.4178/epih.e2018015
Fernandez, A., Black, J., Jones, M., Wilson, L., Salvador-Carulla, L., Astell-Burt, T., et al. (2015). Flooding and mental health: a systematic mapping review. PLoS ONE 10, e0119929. doi: 10.1371/journal.pone.0119929
Florence, L. C., Hakim, S. L., Kamaluddin, M. A., and Thong, K. L. (2011). Determination of toxinotypes of environmental Clostridium perfringens by Polymerase Chain Reaction. Trop. Biomed. 28, 171–174.
Fong, S. M., Wong, K. J., Fukushima, M., and Yeo, T. W. (2015). Thalassemia major is a major risk factor for pediatric melioidosis in Kota Kinabalu, Sabah, Malaysia. Clin. Infect. Dis. 60, 1802–1807. doi: 10.1093/cid/civ189
French C. E. Waite T. D. Armstrong B. Rubin G. J. English National Study of F. Health Study G. . (2019). Impact of repeat flooding on mental health and health-related quality of life: a cross-sectional analysis of the English National Study of Flooding and Health. BMJ Open 9:e031562. doi: 10.1136/bmjopen-2019-031562
Ghaderpour, A., Mohd Nasori, K. N., Chew, L. L., Chong, V. C., Thong, K. L., and Chai, L. C. (2014). Detection of multiple potentially pathogenic bacteria in Matang mangrove estuaries, Malaysia. Mar. Pollut. Bull. 83, 324–330. doi: 10.1016/j.marpolbul.2014.04.029
Golob, M., Pate, M., Kušar, D., Dermota, U., Avberšek, J., Papi,ć, B., et al. (2019). Antimicrobial resistance and virulence genes in Enterococcus faecium and Enterococcus faecalis from humans and retail red meat. Biomed Res. Int. 2019:2815279. doi: 10.1155/2019/2815279
Gomez, M., Perdiguero, J., and Sanz, A. (2019). Socioeconomic factors affecting water access in rural areas of low and middle income countries. Water 11:20202. doi: 10.3390/w11020202
Hasan, S., Jamdar, S. F., Alalowi, M., and Al Ageel Al Beaiji, S. M. (2016). Dengue virus: A global human threat: Review of literature. J. Int. Soc. Prevent. Commun. Dentistry 6, 1–6. doi: 10.4103/2231-0762.175416
Hassan, M. R. A., Pani, S. P., Peng, N. P., Voralu, K., Vijayalakshmi, N., Mehanderkar, R., et al. (2010). Incidence, risk factors and clinical epidemiology of melioidosis: a complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect. Dis. 10:302. doi: 10.1186/1471-2334-10-302
Hayden, J.. (2019). Introduction to Health Behaviour Theory. Burlington, MA: Jones & Bartlett Learning.
Hill, A. B.. (1965). The environment and disease: association or causation? Proc. R. Soc. Med. 58, 295–300. doi: 10.1177/003591576505800503
Ho, J. Y., Jong, M.-C., Acharya, K., Liew, S. S. X., Smith, D. R., Noor, Z. Z., et al. (2021). Multidrug-resistant bacteria and microbial communities in a river estuary with fragmented suburban waste management. J. Hazard. Mater. 405:124687. doi: 10.1016/j.jhazmat.2020.124687
Huang, Y., Tian, Z., Ke, Q., Liu, J., Irannezhad, M., Fan, D., et al. (2020). Nature-based solutions for urban pluvial flood risk management. WIREs Water 7:e1421. doi: 10.1002/wat2.1421
Init, I., Lau, Y. L., Arin Fadzlun, A., Foead, A. I., Neilson, R. S., and Nissapatorn, V. (2010). Detection of free living amoebae, Acanthamoeba and Naegleria, in swimming pools, Malaysia. Trop. Biomed. 27, 566–577.
IPCC (2021). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Italiano, C. M., Wong, K. T., AbuBakar, S., Lau, Y. L., Ramli, N., Syed Omar, S. F., et al. (2014). Sarcocystis nesbitti causes acute, relapsing febrile myositis with a high attack rate: description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS Negl. Trop. Dis. 8:e2876. doi: 10.1371/journal.pntd.0002876
Ithoi, I., Ahmad, A. F., Nissapatorn, V., Lau, Y. L., Mahmud, R., and Mak, J. W. (2011a). Detection of Naegleria species in environmental samples from Peninsular Malaysia. PLoS ONE 6, e24327. doi: 10.1371/journal.pone.0024327
Ithoi, I., Jali, A., Mak, J. W., Wan Sulaiman, W. Y., and Mahmud, R. (2011b). Occurrence of blastocystis in water of two rivers from recreational areas in Malaysia. J. Parasitol. Res. 2011:123916. doi: 10.1155/2011/123916
Johanning, E., Auger, P., Morey, P. R., Yang, C. S., and Olmsted, E. (2014). Review of health hazards and prevention measures for response and recovery workers and volunteers after natural disasters, flooding, and water damage: mold and dampness. Environ. Health Prev. Med. 19, 93–99. doi: 10.1007/s12199-013-0368-0
Kau, A. L., Martin, S. M., Lyon, W., Hayes, E., Caparon, M. G., and Hultgren, S. J. (2005). Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73, 2461–2468. doi: 10.1128/IAI.73.4.2461-2468.2005
Khor, W. C., Puah, S. M., Tan, J. A., Puthucheary, S. D., and Chua, K. H. (2015). Phenotypic and genetic diversity of aeromonas species isolated from fresh water lakes in Malaysia. PLoS ONE 10, e0145933. doi: 10.1371/journal.pone.0145933
Kishimoto, M., Brown, J. D., Chung, H. H., and Howman, S. (2004). Leptospirosis misdiagnosed as pulmonary-renal syndrome. Am. J. Med. Sci. 328, 116–120. doi: 10.1097/00000441-200408000-00008
Kyle, J. L., and Harris, E. (2008). Global spread and persistence of dengue. Annu. Rev. Microbiol. 62, 71–92. doi: 10.1146/annurev.micro.62.081307.163005
Laishram, N., and Singh, P. A. (2016). Clinical profile of enteric fever in children. J. Evolut. Med. Dental Sci. 5:114. doi: 10.14260/jemds/2016/28
Latif, B., and Muslim, A. (2016). Human and animal sarcocystosis in Malaysia: A review. Asian Pac. J. Trop. Biomed. 6, 982–988. doi: 10.1016/j.apjtb.2016.09.003
Lau, S. M., Chua, T. H., Sulaiman, W. Y., Joanne, S., Lim, Y. A., Sekaran, S. D., et al. (2017). A new paradigm for Aedes spp. surveillance using gravid ovipositing sticky trap and NS1 antigen test kit. Parasit Vect 10:151. doi: 10.1186/s13071-017-2091-y
Lau, Y. L., Anthony, C., Fakhrurrazi, S. A., Ibrahim, J., Ithoi, I., and Mahmud, R. (2013). Real-time PCR assay in differentiating Entamoeba histolytica, Entamoeba dispar, and Entamoeba moshkovskii infections in Orang Asli settlements in Malaysia. Parasit. Vect. 6, 250–250. doi: 10.1186/1756-3305-6-250
Lau, Y. L., Chang, P. Y., Tan, C. T., Fong, M. Y., Mahmud, R., and Wong, K. T. (2014). Sarcocystis nesbitti infection in human skeletal muscle: possible transmission from snakes. Am. J. Trop. Med. Hyg. 90, 361–364. doi: 10.4269/ajtmh.12-0678
Lee, S. C., Ngui, R., Tan, T. K., Muhammad Aidil, R., and Lim, Y. A. L. (2014a). Neglected tropical diseases among two indigenous subtribes in peninsular malaysia: highlighting differences and co-infection of helminthiasis and sarcocystosis. PLoS ONE 9, e107980. doi: 10.1371/journal.pone.0107980
Lee, S. C., Ngui, R., Tan, T. K., Roslan, M. A., Ithoi, I., and Lim, Y. A. (2014b). Aquatic biomonitoring of Giardia cysts and Cryptosporidium oocysts in peninsular Malaysia. Environ. Sci. Pollut. Res. Int. 21, 445–453. doi: 10.1007/s11356-013-1925-1
Lee, S. C., Ngui, R., Tan, T. K., Roslan, M. A., Ithoi, I., Mahdy, M. A. K., et al. (2017). Understanding Giardia infections among rural communities using the one health approach. Acta Trop. 176, 349–354. doi: 10.1016/j.actatropica.2017.08.030
Lee, Y. Y., Ismail, A. W., Mustaffa, N., Musa, K. I., Majid, N. A., Choo, K. E., et al. (2012). Sociocultural and dietary practices among Malay subjects in the north-eastern region of Peninsular Malaysia: a region of low prevalence of Helicobacter pylori infection. Helicobacter 17, 54–61. doi: 10.1111/j.1523-5378.2011.00917.x
Leung, V., Luong, M.-L., and Libman, M. (2011). Leptospirosis: pulmonary hemorrhage in a returned traveller. CMAJ 183, E423–E427. doi: 10.1503/cmaj.092203
Lim, Y. A., and Aahmad, R. A. (2004). Occurrence of giardia cysts and Cryptosporidium oocysts in the Temuan Orang Asli (aborigine) River System. Southeast Asian J. Trop. Med. Public Health 35, 801–810.
Lim, Y. A. L., Iqbal, A., Surin, J., Sim, B. L. H., Jex, A. R., Nolan, M. J., et al. (2011). First genetic classification of Cryptosporidium and Giardia from HIV/AIDS patients in Malaysia. Infect. Genet. Evol. 11, 968–974. doi: 10.1016/j.meegid.2011.03.007
Limmathurotsakul, D., Golding, N., Dance, D. A. B., Messina, J. P., Pigott, D. M., Moyes, C. L., et al. (2016). Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 1:15008. doi: 10.1038/nmicrobiol.2015.8
Mackenzie, J. S., and Jeggo, M. (2019). The one health approach-why is it so important? Trop. Med. Infect. Dis. 4:88. doi: 10.3390/tropicalmed4020088
Maizura, A. M., Verasahib, K., Chong, C. K., Shah, A. M., Azri, A., and Sulaiman, L. (2012). Surveillance for sarcocystosis in Tioman Island, Malaysia. Malaysian J. Public Health Med. 12, 39–44.
Mallhi, T. H., Khan, A. H., Adnan, A. S., Sarriff, A., Khan, Y. H., and Jummaat, F. (2015). Clinico-laboratory spectrum of dengue viral infection and risk factors associated with dengue hemorrhagic fever: a retrospective study. BMC Infect. Dis. 15:399. doi: 10.1186/s12879-015-1141-3
Mariappan, V., Thavagnanam, S., Vellasamy, K. M., Teh, C. J. S., Atiya, N., Ponnampalavanar, S., et al. (2018). Relapse of chronic melioidosis in a paediatric cystic fibrosis patient: first case report from Malaysia. BMC Infect. Dis. 18:455. doi: 10.1186/s12879-018-3371-7
McEniry, D. W., Gillespie, S. H., and Felmingham, D. (1988). Susceptibility of Pseudomonas pseudomallei to new beta-lactam and aminoglycoside antibiotics. J. Antimicrob. Chemother. 21, 171–175. doi: 10.1093/jac/21.2.171
Ministry of Health Malaysia, (2016). Helicobacter Pylori In Peptic Ulcers. Malaysia: Ministry of Health Malaysia.
Ministry of Health Malaysia, (2017a). Guidelines for Typhoid Epidemic/Case Management. Malaysia: Ministry of Health Malaysia.
Ministry of Health Malaysia, (2017b). Precautions For People Exposed To Wildlife. Malaysia: Ministry of Health Malaysia.
Ministry of Health Malaysia, (2018). Incidence Rate and Mortality Rate of Communicable Diseases 2018 (per 100,000 Population) [Online]. Available online at: https://www.moh.gov.my/index.php/pages/view/324 (accessed September 15, 2021).
Ministry of Health Malaysia, (2019). National Strategic Plan for Hepatitis B and C 2019 – 2023. Malaysia: Ministry of Health Malaysia.
Ministry of Health Malaysia, (2020). Towards Elimination of Viral Hepatitis in Malaysia Through Mutisectoral Collaboration, 2019. Malaysia: Ministry of Health Malaysia.
Mohamad Ikbal, N., Bhassu, S., Simarani, K., Uni, S., Chan, C., and Omar, H. (2019). Prevalence and genetic divergence of Leptospira interrogans and Leptospira borgpetersenii in house rats (Rattus rattus) from Peninsular Malaysia. Asian Pac. J. Trop. Med. 12, 463–471. doi: 10.4103/1995-7645.269906
Mohamad, M., Selamat, M. I., and Ismail, Z. (2014). Factors associated with larval control practices in a dengue outbreak prone area. J. Environ. Public Health 2014:459173. doi: 10.1155/2014/459173
Mohammad, N. A., Al-Mekhlafi, H. M., Moktar, N., and Anuar, T. S. (2017). Prevalence and risk factors of Blastocystis infection among underprivileged communities in rural Malaysia. Asian Pac. J. Trop. Med. 10, 491–497. doi: 10.1016/j.apjtm.2017.05.001
Mohan, A., Podin, Y., Tai, N., Chieng, C.-H., Rigas, V., Machunter, B., et al. (2017). Pediatric melioidosis in Sarawak, Malaysia: Epidemiological, clinical and microbiological characteristics. PLoS Negl. Trop. Dis. 11:e0005650. doi: 10.1371/journal.pntd.0005650
Mohapatra, P. R., Behera, B., Mohanty, S., Bhuniya, S., and Mishra, B. (2019). Melioidosis. Lancet Infect. Dis. 19, 1056–1057. doi: 10.1016/S1473-3099(19)30480-3
Mohd Ali, M. R., Mohamad Safiee, A. W., Yusof, N. Y., Fauzi, M. H., Yean Yean, C., and Ismail, N. (2018). Isolation of Leptospira kmetyi from residential areas of patients with leptospirosis in Kelantan, Malaysia. J. Infect. Public Health 11, 578–580. doi: 10.1016/j.jiph.2017.12.008
Mohd Nazri, N., Rahman, N., Mohd Shafri, M., and Rahman, N. (2019). Knowledge, attitude and practice of Malaysian public university students on viral hepatitis. Adv. Hum. Biol. 9, 46–53. doi: 10.4103/AIHB.AIHB_21_18
Mohd Radi, M. F., Hashim, J. H., Jaafar, M. H., Hod, R., Ahmad, N., Mohammed Nawi, A., et al. (2018). Leptospirosis outbreak after the 2014 major flooding event in kelantan, Malaysia: a spatial-temporal analysis. Am. J. Trop. Med. Hyg. 98, 1281–1295. doi: 10.4269/ajtmh.16-0922
Moher D. Liberati A. Tetzlaff J. Altman D. G. The P. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. doi: 10.1371/journal.pmed.1000097
Muhammad, E. N., Abdul Mutalip, M. H., Hasim, M. H., Paiwai, F., Pan, S., Mahmud, M. A. F., et al. (2020). The burden of typhoid fever in Klang Valley, Malaysia, 2011–2015. BMC Infect. Dis. 20:843. doi: 10.1186/s12879-020-05500-x
Musa, H. I., Hassan, L., Shamsuddin, Z. H., Panchadcharam, C., Zakaria, Z., Abdul Aziz, S., et al. (2015). Case-control investigation on the risk factors of melioidosis in small ruminant farms in Peninsular Malaysia. J. Appl. Microbiol. 119, 331–341. doi: 10.1111/jam.12830
Musa, H. I., Hassan, L., Shamsuddin, Z. H., Panchadcharam, C., Zakaria, Z., and Abdul Aziz, S. (2016). Physicochemical properties influencing presence of Burkholderia pseudomallei in soil from small ruminant farms in Peninsular Malaysia. PLoS ONE 11, e0162348. doi: 10.1371/journal.pone.0162348
Musa, H. I., Hassan, L., Shamsuddin, Z. H., Panchadcharam, C., Zakaria, Z., and Aziz, S. A. (2018). Physicochemical properties associated with the presence of Burkholderia pseudomallei in small ruminant farm water supplies in Peninsular Malaysia. Environ. Monit. Assess. 190:241. doi: 10.1007/s10661-018-6613-7
Muslim, A., Mohd Sofian, S., Shaari, S. A., Hoh, B.-P., and Lim, Y. A.-L. (2019). Prevalence, intensity and associated risk factors of soil transmitted helminth infections: A comparison between Negritos (indigenous) in inland jungle and those in resettlement at town peripheries. PLoS Negl. Trop. Dis. 13:e0007331. doi: 10.1371/journal.pntd.0007331
Naing, C., Ren, W. Y., Man, C. Y., Fern, K. P., Qiqi, C., Ning, C. N., et al. (2011). Awareness of dengue and practice of dengue control among the semi-urban community: a cross sectional survey. J. Community Health 36, 1044–1049. doi: 10.1007/s10900-011-9407-1
Nardi, F., Cudennec, C., Abrate, T., Allouch, C., Annis, A., Assumpção, T., et al. (2020). Citizens AND HYdrology (CANDHY): conceptualizing a transdisciplinary framework for citizen science addressing hydrological challenges. Hydrol. Sci. J. 2020, 1–18. doi: 10.1080/02626667.2020.1849707
Nasr, N. A., Al-Mekhlafi, H. M., Ahmed, A., Roslan, M. A., and Bulgiba, A. (2013a). Towards an effective control programme of soil-transmitted helminth infections among Orang Asli in rural Malaysia. Part 1: prevalence and associated key factors. Parasit. Vect. 6:27. doi: 10.1186/1756-3305-6-27
Nasr, N. A., Al-Mekhlafi, H. M., Ahmed, A., Roslan, M. A., and Bulgiba, A. (2013b). Towards an effective control programme of soil-transmitted helminth infections among Orang Asli in rural Malaysia. Part 2: Knowledge, attitude, and practices. Parasit. Vect. 6:28. doi: 10.1186/1756-3305-6-28
N'Cho, H., Masunda, K. P. E., Mukeredzi, I., Manangazira, P., and Govore, E. (2019). Notes from the field: typhoid fever outbreak - Harare, Zimbabwe, October 2017-February 2018. MMWR Morb. Mortal. Wkly. Rep. 68, 44–45. doi: 10.15585/mmwr.mm6802a5
Neela, V. K., Azhari, N. N., Joseph, N., Mimie, N. P., Ramli, S. N. A., Mustapha, N. F., et al. (2019). An outbreak of leptospirosis among reserve military recruits, Hulu Perdik, Malaysia. Eur. J. Clin. Microbiol. Infect. Dis. 38, 523–528. doi: 10.1007/s10096-018-03450-6
Ngui, R., Angal, L., Fakhrurrazi, S. A., Lian, Y. L. A., Ling, L. Y., Ibrahim, J., et al. (2012). Differentiating Entamoeba histolytica, Entamoeba dispar and Entamoeba moshkovskii using nested polymerase chain reaction (PCR) in rural communities in Malaysia. Parasit. Vect. 5, 187–187. doi: 10.1186/1756-3305-5-187
NikNadia, N. M. N., Sam, I. C., Khaidir, N., Ngui, R., Lim, Y. A. L., Goh, X. T., et al. (2016). Risk factors for enterovirus a71 seropositivity in rural indigenous populations in West Malaysia. PLoS ONE 11, e0148767. doi: 10.1371/journal.pone.0148767
Nithyamathi, K., Chandramathi, S., and Kumar, S. (2016). Predominance of Blastocystis sp. Infection among school children in Peninsular Malaysia. PLOS ONE 11, e0136709. doi: 10.1371/journal.pone.0136709
Pangare, G., Das, B., Arriens, W. L., and Makin, I. (2014). Waterwealth? Investing in Basin Management in Asia and the Pacific. International Union for Conservation of Nature and Asian Development Bank.
Pappas, G., Papadimitriou, P., Siozopoulou, V., Christou, L., and Akritidis, N. (2008). The globalization of leptospirosis: worldwide incidence trends. Int. J. Infect. Dis. 12, 351–357. doi: 10.1016/j.ijid.2007.09.011
Pasaribu, A. P., Alam, A., Sembiring, K., Pasaribu, S., and Setiabudi, D. (2019). Prevalence and risk factors of soil-transmitted helminthiasis among school children living in an agricultural area of North Sumatera, Indonesia. BMC Public Health 19:1066. doi: 10.1186/s12889-019-7397-6
Patterson, C. L. A., and Jeffrey, Q. (2011). Emergency response planning to reduce the impact of contaminated drinking water during natural disasters. Front. Earth Sci. 5, 341–349. doi: 10.1007/s11707-011-0196-8
Philip, N., Bahtiar Affendy, N., Ramli, S. N. A., Arif, M., Raja, P., Nagandran, E., et al. (2020). Leptospira interrogans and Leptospira kirschneri are the dominant Leptospira species causing human leptospirosis in Central Malaysia. PLoS Negl. Trop. Dis. 14:e0008197. doi: 10.1371/journal.pntd.0008197
Podin, Y., Sarovich, D. S., Price, E. P., Kaestli, M., Mayo, M., Hii, K., et al. (2014). Burkholderia pseudomallei Isolates from Sarawak, Malaysian Borneo, are predominantly susceptible to aminoglycosides and macrolides. Antimicrob. Agents Chemotherapy 58, 162–166. doi: 10.1128/AAC.01842-13
Prüss-Ustün, A., Bartram, J., Clasen, T., Colford, J. M., Cumming, O., Curtis, V., et al. (2014). Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop. Med. Int. Health 19, 894–905. doi: 10.1111/tmi.12329
Pui, C. F., Bilung, L. M., Apun, K., and Su'ut, L. (2017). Diversity of Leptospira spp. in rats and environment from urban areas of Sarawak, Malaysia. J. Trop. Med. 2017, 3760674–3760674. doi: 10.1155/2017/3760674
Quinete, N., and Hauser-Davis, R. A. (2021). Drinking water pollutants may affect the immune system: concerns regarding COVID-19 health effects. Environ. Sci. Pollut. Res. 28, 1235–1246. doi: 10.1007/s11356-020-11487-4
Rahim, A. A., Lee, Y. Y., Majid, N. A., Choo, K. E., Raj, S. M., Derakhshan, M. H., et al. (2010). Helicobacter pylori infection among Aborigines (the Orang Asli) in the northeastern region of Peninsular Malaysia. Am. J. Trop. Med. Hyg. 83, 1119–1122. doi: 10.4269/ajtmh.2010.10-0226
Rahman, M. H. A. A., Hairon, S. M., Hamat, R. A., Jamaluddin, T. Z. M. T., Shafei, M. N., Idris, N., et al. (2018). Seroprevalence and distribution of leptospirosis serovars among wet market workers in northeastern, Malaysia: a cross sectional study. BMC Infect. Dis. 18:569. doi: 10.1186/s12879-018-3470-5
Rasul, F., Sughra, K., Mushtaq, A., Zeeshan, N., Mehmood, S., and Rashid, U. (2017). Surveillance report on typhoid fever epidemiology and risk factor assessment in district Gujrat, Punjab, Pakistan. Biomed. Res. Tokyo 28, 6921–6926.
Richard, R. L., Ithoi, I., Abd Majid, M. A., Wan Sulaiman, W. Y., Tan, T. C., Nissapatorn, V., et al. (2016). Monitoring of waterborne parasites in two drinking water treatment plants: a study in Sarawak, Malaysia. Int. J. Environ. Res. Public Health 13:641. doi: 10.3390/ijerph13070641
Ridzlan, F. R., Bahaman, A. R., Khairani-Bejo, S., and Mutalib, A. R. (2010). Detection of pathogenic Leptospira from selected environment in Kelantan and Terengganu, Malaysia. Trop. Biomed. 27, 632–638.
Roslan, M. A., Shafie, A., Ngui, R., Lim, Y. A., and Sulaiman, W. Y. (2013). Vertical infestation of the dengue vectors Aedes aegypti and Aedes albopictus in apartments in Kuala Lumpur, Malaysia. J. Am. Mosq. Control Assoc. 29, 328–336. doi: 10.2987/13-6363.1
Roy, J., Saikia, L., Medhi, M., and Tassa, D. (2016). Epidemiological investigation of an outbreak of typhoid fever in Jorhat town of Assam, India. Ind. J. Med. Res. 144, 592–596. doi: 10.4103/0971-5916.200902
Samsudin, S., Masri, S. N., Tengku Jamaluddin, T. Z. M., Saudi, S. N. S., Md Ariffin, U. K., Amran, F., et al. (2015). Seroprevalence of leptospiral antibodies among healthy municipal service workers in selangor. Adv. Public Health 2015:208145. doi: 10.1155/2015/208145
Sanderson, C., and Currie, B. J. (2014). Melioidosis: a pediatric disease. Pediatr. Infect. Dis. J. 33, 770–771. doi: 10.1097/INF.0000000000000358
Sapian, M., Khair, M. T., How, S. H., Rajalingam, R., Sahhir, K., Norazah, A., et al. (2012). Outbreak of melioidosis and leptospirosis co-infection following a rescue operation. Med. J. Malaysia 67, 293–297.
Savioli, L., Smith, H., and Thompson, A. (2006). Giardia and cryptosporidium join the 2018 'neglected diseases initiative'. Trends Parasitol. 22, 203–208. doi: 10.1016/j.pt.2006.02.015
Shafei, M. N., Sulong, R., Azwany, Y., Hasan, H., Wan Mohammad, W. M. Z., Daud, A., et al. (2012). Seroprevalence of leptospirosis among town service workers on northeastern State of Malaysia. Int. J. Collaborative Res. Internal Med. Public Health 4, 395–403.
Shahari, S., Tengku-Idris, T. I. N., Fong, M. Y., and Lau, Y. L. (2016). Molecular evidence of Sarcocystis nesbitti in water samples of Tioman Island, Malaysia. Parasit. Vect. 9:598. doi: 10.1186/s13071-016-1883-9
Shekhar, K. C., Prathapa, S., and Gurpreet, K. (1996). Prevalence of giardiasis among Malaysian primary school children. Med. J. Malaysia 51, 475–479.
Siew, T. F., Aenis, T., Spangenberg, J. H., Nauditt, A., Döll, P., Frank, S. K., et al. (2016). Transdisciplinary research in support of land and water management in China and Southeast Asia: evaluation of four research projects. Sustainab. Sci. 11, 813–829. doi: 10.1007/s11625-016-0378-0
Singh, K. G., and Sundar, J. S. (2019). A study on clinical profile of typhoid fever at Government General Hospital, Nizamabad, Telangana, India. Int. J. Contemp. Pediat. 6:4. doi: 10.18203/2349-3291.ijcp20194746
Slesak, G., Schäfer, J., Langeheinecke, A., and Tappe, D. (2014). Prolonged clinical course of muscular sarcocystosis and effectiveness of cotrimoxazole among travelers to Tioman Island, Malaysia, 2011–2014. Clin. Infect. Dis. 60, 329–329. doi: 10.1093/cid/ciu791
Stanaway, J. D., Shepard, D. S., Undurraga, E. A., Halasa, Y. A., Coffeng, L. E., Brady, O. J., et al. (2016). The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 16, 712–723. doi: 10.1016/S1473-3099(16)00026-8
Sulaiman, H., Ponnampalavanar, S., Mun, K. S., and Italiano, C. M. (2013). Cervical abscesses due to co-infection with Burkholderia pseudomallei, Salmonella enterica serovar Stanley and Mycobacterium tuberculosisin a patient with diabetes mellitus. BMC Infect. Dis. 13:527. doi: 10.1186/1471-2334-13-527
Suppiah, J., Chan, S.-Y., Ng, M.-W., Khaw, Y.-S., Ching, S.-M., Mat-Nor, L. A., et al. (2017). Clinical predictors of dengue fever co-infected with leptospirosis among patients admitted for dengue fever - a pilot study. J. Biomed. Sci. 24, 40–40. doi: 10.1186/s12929-017-0344-x
Suut, L., Mazlan, M. N.-A., Arif, M. T., Yusoff, H., Abdul Rahim, N.-A., Safii, R., et al. (2016). Serological prevalence of leptospirosis among rural communities in the Rejang Basin, Sarawak, Malaysia. Asia Pacific J. Public Health 28, 450–457. doi: 10.1177/1010539516648003
Tan, K. S. W.. (2008). New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 21, 639–665. doi: 10.1128/CMR.00022-08
Tang, R. Y., Lim, S. H., Lam, J. E., Nurasykin, S., Eileen, T., and Chan, Y. W. (2019). A 5-year retrospective study of melioidosis cases treated in a district specialist hospital. Med. J. Malaysia 74, 472–476.
Tappe, D., Ernestus, K., Rauthe, S., Schoen, C., Frosch, M., Müller, A., et al. (2013). Initial patient cluster and first positive biopsy findings in an outbreak of acute muscular sarcocystis-like infection in travelers returning from Tioman Island, Peninsular Malaysia, in 2011. J. Clin. Microbiol. 51, 725–726. doi: 10.1128/JCM.03063-12
Tappe, D., Stich, A., Langeheinecke, A., von Sonnenburg, F., Muntau, B., Schäfer, J., et al. (2014). Suspected new wave of muscular sarcocystosis in travellers returning from Tioman Island, Malaysia, May 2014. Eurosurveillance 19:20816. doi: 10.2807/1560-7917.ES2014.19.21.20816
Tengku, S. A., and Norhayati, M. (2011). Public health and clinical importance of amoebiasis in Malaysia: a review. Trop. Biomed. 28, 194–222.
Thomas, V., and Dissanaike, A. S. (1978). Antibodies to sarcocystis in Malaysians. Trans. R. Soc. Trop. Med. Hyg. 72, 303–306. doi: 10.1016/0035-9203(78)90211-0
Ting Lo, N., Abul Bashar Sarker, M., Ai Lian Lim, Y., Harun-Or-Rashid, M., and Sakamoto, J. (2018). Inadequate water treatment quality as assessed by protozoa removal in Sarawak, Malaysia. Nagoya J. Med. Sci. 80, 165–174. doi: 10.18999/nagjms.80.2.165
Troeger, C., Forouzanfar, M., Rao, P. C., Khalil, I., Brown, A., Reiner, R. C. Jr., et al. (2017). Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 17, 909–948. doi: 10.1016/S1473-3099(17)30276-1
Visvesvara, G. S., Moura, H., and Schuster, F. L. (2007). Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 50, 1–26. doi: 10.1111/j.1574-695X.2007.00232.x
Wan Rosli, W. R., Abdul Rahman, S., Parhar, J. K., and Suhaimi, M. I. (2018). Positive impact of educational intervention on knowledge, attitude, and practice towards dengue among university students in Malaysia. J. Public Health 27, 461–471. doi: 10.1007/s10389-018-0971-z
Wang, Y., Gonzalez-Moreno, O., Roellig, D. M., Oliver, L., Huguet, J., Guo, Y., et al. (2019). Epidemiological distribution of genotypes of Giardia duodenalis in humans in Spain. Parasit. Vect. 12:432. doi: 10.1186/s13071-019-3692-4
Watkins, R., Meiers, M. W., and Visser, Y. L. (2012). A Guide to Assessing Needs: Essential Tools for Collecting Information, Making Decisions, and Achieving Development Results (English). Washington, DC. doi: 10.1596/978-0-8213-8868-6
Wawrzyniak, I., Poirier, P., Viscogliosi, E., Dionigia, M., Texier, C., Delbac, F., et al. (2013). Blastocystis, an unrecognized parasite: an overview of pathogenesis and diagnosis. Therap. Adv. Infect. Dis. 1, 167–178. doi: 10.1177/2049936113504754
Wiersinga, W. J., Virk, H. S., Torres, A. G., Currie, B. J., Peacock, S. J., Dance, D. A. B., et al. (2018). Melioidosis. Nat. Rev. Dis. Primers 4:17107. doi: 10.1038/nrdp.2017.107
Wong, L. P., AbuBakar, S., and Chinna, K. (2014). Community knowledge, health beliefs, practices and experiences related to dengue fever and its association with IgG seropositivity. PLoS Negl. Trop. Dis. 8:e2789. doi: 10.1371/journal.pntd.0002789
Wong, L. P., Alias, H., Choy, S. H., Goh, X. T., Lee, S. C., Lim, Y. A. L., et al. (2020). The study of seroprevalence of hepatitis E virus and an investigation into the lifestyle behaviours of the aborigines in Malaysia. Zoonoses Public Health 67, 263–270. doi: 10.1111/zph.12681
Wong, L. P., Shakir, S. M., Atefi, N., and AbuBakar, S. (2015). Factors affecting dengue prevention practices: nationwide survey of the Malaysian public. PLoS ONE 10, e0122890. doi: 10.1371/journal.pone.0122890
World Health Organization (WHO) and United Nations Children's Fund (UNICEF) (2017). Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines [Online]. Geneva: World Health Organization.
World Health Organization Regional Office for the Western Pacific (2018). Drinking-Water, Sanitation and Hygiene in the Western Pacific Region: Opportunities and Challenges on the Threshold of the SDG era. Manila, Philippines.
World Health Organization, (2003). Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. Geneva.
World Health Organization, (2012). Soil-Transmitted Helminthiases: Eliminating as Public Health Problem Soil-Transmitted Helminthiases in Children: Progress Report 2001-2010 and Strategic Plan 2011-2020. Geneva: World Health Organization.
World Health Organization, (2019). Drinking-Water [Online]. World Health Organization. Available online at: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed January 23, 2022).
World Health Organization, (2020). Neglected tropical diseases [Online]. World Health Organization. Available online at: https://www.who.int/neglected_diseases/diseases/en/ (accessed September 15, 2021).
World Health Organization, (2021a). Dengue and Severe Dengue [Online]. Available online at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed September 15, 2021).
World Health Organization, (2021b). Hepatitis E [Online]. World Health Organization. Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e (accessed August 27, 2021).
Wu, C.-H., Liu, C.-F., Huang, S.-H., Lee, C.-H., and Kung, C.-T. (2012). Clinical characteristics of patients with melioidosis treated in an emergency department. J. Acute Med. 2, 13–18. doi: 10.1016/j.jacme.2012.02.001
Xing, W., Liao, Q., Viboud, C., Zhang, J., Sun, J., Wu, J. T., et al. (2014). Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect. Dis. 14, 308–318. doi: 10.1016/S1473-3099(13)70342-6
Yap, K. P., Gan, H. M., Teh, C. S., Chai, L. C., and Thong, K. L. (2014). Comparative genomics of closely related Salmonella enterica serovar Typhi strains reveals genome dynamics and the acquisition of novel pathogenic elements. BMC Genomics 15:1007. doi: 10.1186/1471-2164-15-1007
Yazid, M. B., Fauzi, M. H., Hasan, H., Md Noh, A. Y., and Deris, Z. Z. (2017). An 11-year analysis of emergency presentations of melioidosis in Northeastern Malaysia. J. Immigr. Minor. Health 19, 774–777. doi: 10.1007/s10903-016-0429-8
Yeo, H. Y., and Shafie, A. A. (2018). The acceptance and willingness to pay (WTP) for hypothetical dengue vaccine in Penang, Malaysia: a contingent valuation study. Cost Eff. Resour. Alloc. 16:60. doi: 10.1186/s12962-018-0163-2
Yusoff, F. A., Rahman, R. A., May, L. H., Budart, S. B., and Sulaiman, L. H. (2015). Investigation of hepatitis A outbreak in district of Manjung, Perak, Malaysia, October 2012. Western Pacific Surveill. Res. J. 6, 27–31. doi: 10.5365/wpsar.2015.6.1.012
Zainutdin, F. K., Barudin, M. A., Jainul, M. A., Isa, M., and Yusof, A. (2017). The association of Cryptosporidium from three different points of Balok River and Kuantan River by using physico-chemical and heavy metal assessments. Asian Pacific J. Trop. Dis. 7, 449–454. doi: 10.12980/apjtd.7.2017D7-103
Keywords: water, health, Malaysia, infectious diseases, systems approach
Citation: Ho JY, Lavinya AA, Kay DSW, Lee CIS, Razmi AH, Walsh CL, Goodson ML and Eswaran J (2022) Towards an Integrated Approach to Improve the Understanding of the Relationships Between Water-Borne Infections and Health Outcomes: Using Malaysia as a Detailed Case Study. Front. Water 4:779860. doi: 10.3389/frwa.2022.779860
Received: 20 September 2021; Accepted: 31 January 2022;
Published: 03 March 2022.
Edited by:
Miguel R. Peña, University of Valle, ColombiaReviewed by:
Chaoqun Li, Yellow River Engineering Consulting Co., Ltd., ChinaCopyright © 2022 Ho, Lavinya, Kay, Lee, Razmi, Walsh, Goodson and Eswaran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michaela L. Goodson, TWljaGFlbGEuR29vZHNvbkBuZXdjYXN0bGUuZWR1Lm15; Jeyanthy Eswaran, SmV5YW50aHkuRXN3YXJhbkBuZXdjYXN0bGUuZWR1Lm15
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.