
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Water , 03 February 2022
Sec. Water and Human Health
Volume 3 - 2021 | https://doi.org/10.3389/frwa.2021.786487
This article is part of the Research Topic Emerging Water Contaminants in Developing Countries: Detection, Monitoring, and Impact of Xenobiotics View all 5 articles
 Mudalige R. D. L. Kulathunga1,2
Mudalige R. D. L. Kulathunga1,2 M. A. Ayanka Wijayawardena1,3*
M. A. Ayanka Wijayawardena1,3* Ravi Naidu1,3
Ravi Naidu1,3 Sunil J. Wimalawansa4
Sunil J. Wimalawansa4 Mohammad Mahmudur Rahman1,3
Mohammad Mahmudur Rahman1,3Rice is the staple food of most Asians, including Sri Lankans. It is cultivated extensively in the dry zonal regions in Sri Lanka such as the Polonnaruwa district, where the prevalence of chronic kidney disease of unknown etiology (CKDu) is higher. We investigated the concentrations of potentially toxic heavy metal(loid)s in groundwater and locally produced rice and correlated their exposure with the prevalence of CKDu. We studied human health exposure risks such as total daily intake (TDI), hazard quotient, hazard index, and carcinogenic risk (CR) from the consumption of groundwater and rice. In well-water, the concentrations of heavy metal(loid)s, cadmium (Cd), arsenic (As), and lead (Pb) were below the World Health Organization (WHO) stipulated allowable limits. Except for Pb, contents of other heavy metal(loid) in all rice samples were lower than maximum permissible limits by the WHO (0.02 mg/kg). Twenty-three per cent (23%) of rice samples analyzed exceeded the permissible limit for TDI of Pb, and analysis of hazard index for Cd, As, and Pb revealed 26% of rice samples could result in a health risk through the consumption of rice in this population. Further, the outcome depicted no CR of Cd, As, and Pb by consuming rice in this study area. We recommended further studies and investigations to minimize or eliminate potential risks from chronic Pb exposure to consumers.
During the past couple of decades, exposure to toxic-heavy metal(loid)s through groundwater and food has garnered attention in Sri Lanka and elsewhere. This is in part due to the expanding chronic kidney disease of unknown etiology (CKDu) in dry zone regions, mostly affecting male paddy farmers in the north central province (NCP) in Sri Lanka. Most important risk factors for the common chronic kidney disease (CKD) are long-standing diabetes, hypertension, and glomerulonephritis (Wimalawansa, 2014). The majority of CKD patients in the NCP of Sri Lanka, however, is not associated with the above pre-existing risk factors (Athuraliya et al., 2011). Considering the published data, environmental exposure to multiple risk factors over time, is considered to be the main etiology for CKDu incidents in Sri Lanka (Wimalawansa, 2016; Wimalawansa and Dissanayake, 2019). The literature shows numerous researchers proposing a relationship between the occurrence of CKDu with exposure to Cd, As and Pb (Jayatilake et al., 2013; Jayasumana et al., 2015a). Nevertheless, no study to date has supported this hypothesis (Vlahos et al., 2018; Nanayakkara et al., 2019; Wimalawansa and Dissanayake, 2019).
People are exposed to these elements via drinking water and food, and also through inhalation (air pollution) from vaporized metal particles (Jaishankar et al., 2014; Islam et al., 2021). Chronic dietary exposure to higher doses of heavy metal(loid)s could pose a considerable health risk to humans (Wijayawardena et al., 2017). Most heavy metals are bio-accumulative, not only in plants but also in humans with chronic exposure. Therefore, to avoid adverse effects World Health Organization (WHO) has recommended maximum allowable limits (MAL) (Wimalawansa, 2016). The researches have reported nephrotoxic heavy metal(loid)s such as Cd, As, and Pb in blood, nails, and urine of CKDu patients. These include, Cd in blood, concentrations between 0.25 μg/L (Nanayakkara et al., 2014) and 2.5 μg/L (Jayatilake et al., 2013; Jayasumana et al., 2015a) and concentration of 0.017 mg/kg in patients' nails (Jayatilake et al., 2013). Further, As content in nails was 0.144 mg/kg (Jayatilake et al., 2013) and in urine ranged from 20 to 37 μg/L (Nanayakkara et al., 2014; Jayasumana et al., 2015a) among CKDu patients. Importantly, 3.6 μg/L of Pb in blood (Levine et al., 2016) and 0.69 μg/L of Pb in urine were detected in patients (Jayatilake et al., 2013). Those studies indicated that residents in CKDu endemic areas were exposed to multiple heavy metal(loid)s which potentially can cause serious health problems. However, comprehensive information on the total daily intake and exposure pathways of those elements was limited (Mahendranathan and Thayaruban, 2018) to understand the health impacts of those contaminants.
Rice is the staple diet; on average, a Sri Lankan adult consumes 300 g/day in dry weight basis (Jayawardena et al., 2014). Only 11.6 and 2.1% of adults in Sri Lanka consume the minimum daily recommended servings of vegetables and fruits, respectively (Jayawardena et al., 2013). Thus, rice for Sri Lankans is the primary source of carbohydrate, proteins, phosphorus, iron, and some vitamins, and covers 45% of daily total calories and 40% of total daily protein in adults (Senanayake and Premaratne, 2016). Therefore, consumption of rice could have a significant impact on dietary exposure to toxic elements (Islam et al., 2016).
It is worth noting that the ability to bio-accumulate toxic-heavy metal(loid)s was greater in edible part of the rice crop compared to other cereal crops (Zhao et al., 2012). However, most metal(loid)s remain in the lower part of the rice plant and a lesser proportion are embedded in rice seeds (Satpathy et al., 2014; Kong et al., 2018). Other common food items consumed by the residents in CKDu endemic area are either imported from other countries, i.e., lentil, milk powder, and sugar, or from various other parts of the country (e.g., vegetables and tea from upcountry) as with other parts of the country.
The rice consumed in CKDu endemic areas is mostly local origin. Considering CKDu is endemic to geographically demarcated areas (Chandrajith et al., 2011a; Wimalawansa, 2014) of Sri Lanka, heavy metal(loid) exposure through locally originated food items is feasible. We hypothesized that in addition to groundwater, locally produced food, especially rice, could have an impact on CKDu. Therefore, we opted to investigate the local groundwater and locally-produced food items, especially rice, to assess potential linkage between dietary exposure to heavy metal(loid)s and the prevalence of CKDu.
Previously reported, comparative studies of South Asian rice have shown that Sri Lankan rice contains nephrotoxic metals such as Pb and Cd (Meharg et al., 2013; Norton et al., 2014; Mwale et al., 2018). However, human health risk impact, particularly the exposure over a longer period through rice consumption has not been explored in CKDu endemic areas in Sri Lanka. Over the past decade, several studies have reported chemical composition of groundwater used in CKDu affected regions in Sri Lanka, in comparison with non-endemic areas (Chandrajith et al., 2011b; Jayasumana et al., 2015b; Diyabalanage et al., 2016; Wasana et al., 2016; Herath et al., 2018). None of these studies, however, reported higher levels of metal(loid)s toxins in groundwater or varying concentrations across the region.
The primary water source for the inhabitants in the NCP region's inhabitants in Sri Lanka is shallow, dug-wells (Silva et al., 2015). These well water are subjected to leaching contaminants from irrigation water from agricultural lands, natural processes like weathering, mineral dissolution, ion exchanges and redox reactions (Saha et al., 2020). Heavy metal(loid)s in groundwater is mostly present in ionic and (more toxic) inorganic forms (Habib et al., 2020). Hence, bioavailability can be higher than in other environmental media. However, there is no scientific evidence to suggest a change of heavy metal(loid) content of groundwater in the country. Therefore, metal(loid)s exposure through groundwater is unlikely to have changed over the lifetime of people living in CKDu endemic area. The current cross-sectional assessment therefore, can be extrapolated to previous exposure to water-derived exposure of people to heavy metal(loid)s in the NCP. Therefore, in this study, we have explored the overall exposure–health risks from groundwater and rice consumption by the residents in the CKDu affected areas.
This study was carried out from February to April 2018 in Medirigiriya divisional secretariat (DS) of Polonnaruwa district, where 23% of adults affected by CKDu (Weaver et al., 2015). Medirigiriya DS comprises of 54,404 hectares of land area with ~ 40% used for agriculture and settlements. About 51% of the land area is covered by forest, small reservoirs, and the archeologically-protected regions. The approximate annual cultivation for paddy was 14,000 ha, and 102 hectares for vegetables and other cereals.
This region belongs to poorly-drained low-land, dry zonal agro-ecological zone, that is subjected to east-west monsoon as the main cultivation season. The maximum and minimum temperatures in this area were 36.5 and 22°C respectively. People accessed drinking water from many sources, but dug-wells is the predominant source. For instance, protective dug-wells were used by 71.30% (8,923 wells), unprotected dug-wells by 18.66% (2,335 wells), tube wells used by 4.26% (534 tube wells), village community water supply (pipeline) by 0.29% (36 projects), and rainwater harvesting by 5.48% (686 tanks).
According to the hospital records of district hospital in Polonnaruwa, Medirigiriya DS area has a total of 1,914 CKD patients who were obtaining medical treatments for the disease as at the end of November 2017; of these, 396 patients were diagnosed as CKDu patients. For this study, we visited randomly identified 224 CKDu patients' homes for interviews and sample collection.
The total of 71 dug-well water samples from CKDu patients' houses were collected; some of these wells are shared by adjoining houses. Since 2016, people are using treated water by reverse osmosis for drinking purpose as a prevention method for CKDu. This has led to the abandonment of many dug wells, and thus, those abandoned wells were not used for sampling in this study. However, some people properly maintained these wells and continue to use dug-well water for drinking, cooking, and washing. These wells were selected for sampling in this study. The GPS coordinates of each sampling point were recorded using Magellan eXplorist for mapping and future reference purposes.
Polyethylene bottles (500 ml), pre-washed with acid-water were used for collecting water samples and filtered through a 0.45 μm filter paper into 60 ml sampling bottles to remove debris; 3–4 drops of concentrated HNO3 acid were added as preservative into each of these 60 ml sampling bottles. These acidified aliquots were transported to our laboratories in Australia for trace element analysis. During the same visit 53 samples of raw rice grains were collected from homes of CKDu patients. The husk was removed mechanically, and grains were ground to obtain a homogenized powdered sample using a stainless-steel grinder. Powdered grain samples were also transported to Australia for trace metal analysis.
Original water samples were utilized to analyze pH, electrical conductivity, and bicarbonate. Bicarbonate was examined using a titrimetric method with 0.1 M H2SO4, first adding phenolphthalein, followed by adding a mixed indicator of methyl red and bromocresol green. Total dissolved solids (TDS – mg/kg) was estimated using by EC (μS/cm) values were multiplied by 0.64. The equation for this calculation was presented by Lloyd and Heathcote in their previous research (Lloyd and Heathcote, 1985). Rice grain powder was digested using block digestion. First 0.5 g of raw rice grain samples were treated with 5 ml of trace metal-grade HNO3 (70%) in a glass digestion tube and kept overnight. Next digestion was performed as per a published procedure (Rahman et al., 2009). A digested clear aliquot was transferred to 50 ml falcon tubes and diluted to 20 ml with milli-Q water. The solution was well mixed using a vortex mixture and filtered through 0.45 μm syringe filter into 10 ml tubes, and trace metal analysis was performed.
Trace metal concentrations in acidified water samples and digested rice samples were measured using inductively-coupled plasma mass spectrometry (ICPMS 7900, Agilent Technologies, Japan), and inductively coupled plasma emission spectrometer (PerkinElmer, Avio 200). The instrument detection limits for trace metals were Cd−0.03, Co−0.01, Mn−0.02, Pb 0.05, Cu−0.02, As−0.01, Se−0.2, and Zn−0.01 μg/L.
Human exposure risk (HE) on daily basis from groundwater was estimated by using the following equation based on US EPA (2006) quoted by (Wu et al., 2009):
Where,
HEWater = human exposure risk through drinking water pathway (μg/kg/day),
CW = heavy metal(loid)s concentration in water (μg/L),
IRW = daily average ingestion rate of water (L/day),
BWA = average body weight (kg).
Hazard quotient for drinking water was calculated using the following equation based on USA EPA (Wu et al., 2009): the oral toxicity reference dose (RfD) values used for As, Cd and Pb were 0.3, 0.5 and 1.4 (μg/kg/day) respectively (US EPA, 2006) quoted by Wu et al. (2009).
Where,
HQWater = hazard quotient for water (μg/kg/day),
RfD = oral toxicity reference dose (μg/kg/day).
Total daily intake (TDI—mg/kg) of heavy metal(loid)s through consumption of rice was calculated using the following equations based on (US EPA, 2006), and the reference oral dose (RfD) for Pb, Cd, and As were 3.5, 1.0, and 3 (μg/kg/day) respectively (US EPA, 2021) were used in this calculation.
Where,
CR = concentration of heavy metal(loid) in rice grain (μg/kg),
IRR = daily average rice consumption (0.3 kg/person/day) dry weight basis,
BWA = body weight (60 kg).
Hazardous quotient (HQ) oral (μg/kg/day) for rice consumption was calculated by multiplying TDIRise (μg/kg/day) by oral toxicity reference dose for heavy metal(loid)s that an individual can be exposed. Hazard index (HI) is calculated as the summation of HQ oral of selected heavy metal(loid)s (Cd, As, and Pb) which has a nephrotoxic effect.
Carcinogenic risk (CR) associated with the exposure to Cd, As, and Pb was calculated as total daily intake [mg/(kg day)−1] multiplying by cancer slope factor (SF). Respective SF for Cd and As were 15, 1.5, and 0.0085 mg/(kg day)−1 (US EPA, 2010) quoted by Fan et al. (2017) and Ullah et al. (2017) were used in this study.
Visual data analysis statistical software (JMP, 2013) was used for statistical data analysis. Firstly, descriptive statistical analysis was carried out for heavy metal(loid) content found in rice and groundwater to compare the results with safety guidelines. The relationship to the distribution of CKDu patients at the village level was evaluated using multiple regression analysis for heavy metal(loid)s content found in raw rice and groundwater samples.
The pH of water varied between 6.5 and 8.5 which is considered to be the desirable range for drinking purposes (WHO, 2008). Geometric mean pH in groundwater in this study was 8.4 (mean 8.4 ± 0.23). The pH value for drinking well water collected in study area was closer to the upper limit of the desirable range.
In a comparative study of groundwater quality (Jayawardana et al., 2010), average pH value for wet zone of Sri Lanka was reported to be 7.1 (n = 45). Comparatively, the dry zone (present study) showed a higher mean pH value for groundwater, indicating a more alkaline nature of groundwater in CKDu endemic areas. Gunatilake (2016) reported same value as the present study for pH and mean value was 8.22 for the dry zone area. Following analysis, elevated levels of ammonia were detected in dry zone water, which is attributed to contamination of water with fallen leaves or ammonia-based agrochemicals used in this region resulting elevated pH values.
Geometric mean for EC in groundwater was 584 μS/cm with a mean value of 624 ± 220 μS/cm. EC of groundwater <750 μS/cm is considered to be desirable, while 750–1,500 μS/cm is considered to be permissible for drinking purposes (Elumalai et al., 2017). According to this EC classification, 70% of wells in this study belonged to the desirable range of EC, and 30% belongs to the permissible range. Previous studies in CKDu endemic areas have found EC values in well waters around 500 μS/cm (Jayasumana et al., 2015b), and ranging from 330 to 1,000 μS/cm in water reservoirs (Chandrajith et al., 2011a) which are similar to this study. Well water, EC values for CKDu non-endemic areas were found to be 239.35 μS/cm (Gunatilake, 2016), and the non-endemic urbanized area reported to be 347.2 μS/cm (Rajapakshe and Rathnayake, 2018), which is much lower than the CKDu endemic area. Therefore, higher EC in groundwater is a common occurrence in CKDu endemic regions compared to non-endemic areas. EC reflect the dissolved mineral content in water. While increased ionicity of groundwater was proposed as a causative factor for CKDu (Dharma-Wardana et al., 2015), there is no confirmatory evidence to support it.
Dissolved Ca and Mg causes hardness in water. The maximum permissible water hardness level in drinking water is 500 mg/L (WHO, 2008). Geometric mean value for TDS found in this study was 374 mg/L with a mean value of 399 ±140 mg/L. About 21% of wells in this study exceeded the maximum allowable water hardness level of 500 mg/L, whereas groundwater hardness in CKDu non-endemic areas is reported to be 77 mg/L (Gunatilake, 2016). Water hardness is also classified into four categories. Based on calcium carbonate content of water <60, 60–120, 120–180 and >180 mg/L, it is classified as soft water, moderately hard water, hard water and very hard water respectively (WHO, 2011). As per this classification, 91% of drinking well water samples of in the study area belonged to very hard water category, and only six locations had hard water (n = 3) and moderately hard water (n = 3). Therefore, groundwater in this area range from “hard” to “very hard” water.
Water hardness has both positive and negative impact on human health. Water hardness has several human health effects (Sengupta, 2013); digestive track issues, including constipation, negative effects on reproductive health and kidney stones. There is no evidence of direct effect of water hardness alone on CKDu. It is speculated that hard water containing Mg+2, fluoride, and traces of Cd enhancing nephrotoxicity (Dharma-Wardana, 2018). Even though Pb has valency and chemical properties somewhat similar to Cd, and could cause nephrotoxicity (Satarug et al., 2020), it is not a recognize cause for CKD. Nevertheless, we hypothesized hard water containing Mg+2, fluoride, and trace levels of Pb, increases the risk of nephrotoxicity and could increases the risk of suffering from CKDu.
Means of heavy metal(loid)s content (Table 1) in well water indicated that the heavy metal(loid)s, such as Cd, As, and Pb were below the WHO drinking water safety guidelines (WHO, 2008), indicating minimal health risks from these elements.
Recent findings concerning these metal(loid)s content in well water of CKDu endemic areas were also below the permissible levels (Herath et al., 2018). For instance, maximum levels for Cd, Pb, and As in well water were 0.02, 0.003, and 1.94 μg/L respectively according to recent research conducted by Nanayakkara et al. (2019), which is comparable to the results of the present study. Thus, heavy metal(loid)s levels found in groundwater per se unlikely to cause CKDu.
Manganese, Co, Cu, Zn, and Se are essential micronutrients and found in trace levels in groundwater (Sandeep et al., 2012). In the Medirigiriya study area, those heavy metal(loid)s content also were lower than WHO allowable limits (Table 1). These elements, in decreasing order of quantities are as follow: Mn > Zn > Cu > Se > Co. Previous studies also reported comparable results for shallow well water in CKDu endemic areas (Chandrajith et al., 2011b; Jayasumana et al., 2015b; Nanayakkara et al., 2019), and lower metal contents in CKDu non-prevalence areas (Jayasumana et al., 2015b). Hence, trace metal contents in CKDu endemic areas were higher than other parts of the country.
Mahaweli River is the primary water source for irrigation of CKDu endemic areas and is rich in metals. Diyabalanage et al. (2016) reported the amount of Mn, Co, Cu, Zn, and Se in river water were 1.47, 0.52, 54, 21.3, and 2.77 μg/L, respectively.
Figure 1 illustrates the mean As, Cd, Co, Cu, Mn, Pb, and Zn levels found in rice of neighboring countries and compared the metal(loid) concentrations found in rice grains. Mean Mn concentration in our study was higher than in other countries (Figure 1), and the mean Zn concentration was higher in China, followed by this study. Indian rice grain showed more Cu and Pb than other countries and was followed by Sri Lanka. Other heavy metals, Cd and Co concentrations, were similar in all the countries (Figure 1), while Bangladesh rice grain showed a higher mean concentration of As compared to other countries. This comparison also indicated that Pb content in the edible part of rice was higher than Cd and As content in all countries.
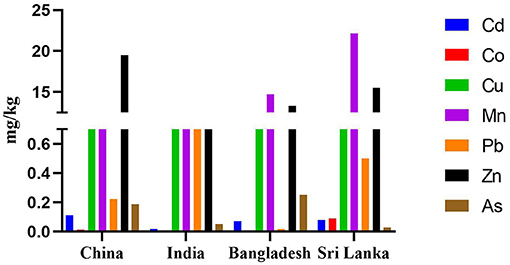
Figure 1. Comparative mean heavy metal(loid)s concentrations (mg/kg) in edible part of rice reported in this study (Sri Lanka), with other studies published in China (Luo et al., 2010; Huang et al., 2013; Fan et al., 2017), India (Kumar et al., 2016), and Bangladesh (Khan et al., 2010; Rahman et al., 2014).
Since, Cd, As and Pb have been reported as exerting nephrotoxic effects, the current study is focused on these metal(loid)s. In this study maximum metal(loid)s content in rice samples (n = 53) for As, Cd, and Pb were 0.098, 0.12, and 1.47 mg/kg, respectively. Mean concentration of As and Cd levels were lower than the maximum allowable limits set by Joint FAO/WHO food standards program codex committee on contaminants in foods (JECFA, 2018) (Table 1), indicating a minimal or no health risk. However, mean Pb content in rice was higher than the maximum allowable limit (Table 1), suggesting potential adverse health effects from rice grown in the study area. Findings of heavy metal(loid)s content in raw rice in CKDu studies are shown in the Table 2. Similar levels of heavy metal(loid)s content were reported in all other studies indicating only the concentration of Pb was greater than WHO's recommended permissible level (Tables 1, 2). Other studies have concluded no significant differences in heavy metal(loid) contents in rice grains between CKDu disease-prone areas and less CKDu disease-prone areas (Diyabalanage et al., 2016; Nanayakkara et al., 2019). Therefore, considering overall data, the slightly increased Pb levels and very low Cd concentrations in rice found in the current study, make As and Cd unlikely to contribute to causing CKDu. However, chronic exposure to Pb can cause negative neurological effect, including lowering of IQ in children (Canfield et al., 2003; Lanphear et al., 2005; Nakashima et al., 2011). Despite the finding that chronic exposure to Pb in this region via daily rice consumption, no excess or abnormal neurological effects are reported in this region.
Regression analysis was used to evaluate the relationship between toxic heavy metal(loid)s (Cd, As and Pb) and prevalence of CKDu. The CKDu patients' information (number of patients in each village), heavy metal(loid)s content in rice and in water (at village level) were used as variables for the correlation analysis. The correlation coefficients in Table 3 showed that there was no significant relationship between the prevalence of CKDu and Cd, As and Pb concentrations either in rice or groundwater. However, the statistical power was insufficient (nine villages) to assess correlation. Therefore, one cannot categorically state the safety of drinking water and rice in this region.
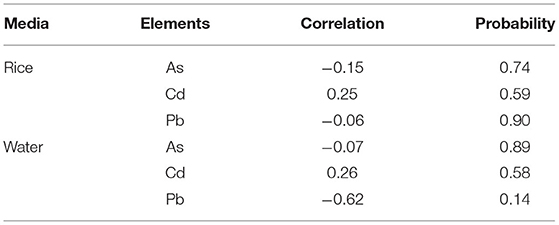
Table 3. Relationship between CKDu prevalence in villages (n = 9) and heavy metal(loid)s content in raw rice and Groundwater.
Chronic exposure to excess heavy metal(loid)s are hazardous and cause CKD (Nanayakkara et al., 2019). For instance, Cd exposure (Wanigasuriya et al., 2011; Jayatilake et al., 2013) and As were suggested as causative factors (Jayasumana et al., 2014), but there are no credible scientific data to support these (Wimalawansa and Dissanayake, 2019). Besides, as per the exposure levels found for Cd, As and Pb in the current risk assessment, is un likely contributing to cause CKDu, derived from groundwater and/or rice consumption. Besides, the toxicity of Cd is further reduced by the presence of excess Zn and Se in the diet (Brzóska and Moniuszko-Jakoniuk, 2001). Absorption of heavy metal(loid)s reduced by chelation in the gastrointestinal tract and the minute quantities absorbed (if any) are counteracted by excess Zn and Se, making them no-toxic.
The health risk was assessed using TDI, the HQ, hazardous index (HI), and CR to examined the potential health risk associated with dietary intake of rice and water. Since rice is a staple food in daily diet, we assessed the health risk from consuming rice. Total daily intake was calculated and compared with tolerable daily intake guidelines, for different trace metals established by the FAO/WHO (2004). If TDI of nephrotoxic metal(loid)s is above the recommendation, one could assume that chronic adverse effects, from the consumption of rice could induce other organ-specific diseases.
Estimated TDI of rice for nephrotoxic heavy metal(loid)s (Table 4) was lower than the permissible daily intake (WHO, 2008). However, 22% of rice samples showed TDI for Pb was higher than the recommended safety level. A study of TDI in marketed rice collected from a non-endemic area, specifically the Kandy district in Sri Lanka, reveals 12% of samples (n = 53) exceeded the permissible level of Pb (Magamage et al., 2017), while other heavy metal(loid)s were within the safe range.
A large percentage of rice bran is removed in the milling process of market rice. Rice bran was not totally removed in the present study as in market rice since the researchers' de-husked rice mechanically. That could be a reason for the differences in heavy metal content with other studies.
Non-carcinogenic health effects of different metals were determined by calculating the HQ. If the HQ of metal exceeds 1, it is suggested to have adverse health outcomes (Fan et al., 2017) chronic exposure from the particular metal(loid). The HQs estimated for CKDu patients' drinking well water are summarized in Table 4, and the rate of water consumption used in the calculation was 2 L/day (Ileperuma et al., 2009). HQ for individual metal(loid) all were much lower than one. Therefore, we concluded that there is no exposure risk to toxic heavy metal(loid)s per se through groundwater in this study area.
Hazard quotient for heavy metal(loid)s through ingestion shows a decrease in the order of Pb > Cd > As (Table 4). The HQ for groundwater and rice together for Cd and As were also below one. In the doses and chronic exposure to Cd and As from groundwater and rice consumption identified in this study was minimal. Although the average value of HQ for Pb content in rice was less than one, 23% of studied rice samples had HQ for Pb higher than one, which indicates a possible non-carcinogenic risk. Therefore, Pb exposure in CKDu endemic area needs to be further investigated for health hazards, other than CKDu with prevention of potential diseases, as because Pb can also be inhaled via air with dust particles and other dietary components, which were not accounted in this study.
Exposure to chronic toxicity through a mixture of several metal(loid)s were also evaluated by using hazard index (HI). It is an estimation of total exposure to several metals over time. The average HI of Cd, As, and Pb metals observed in this study was 0.824, and 26% of rice samples showed a value greater than one. Clinical studies of CKDu patients' biological samples revealed Cd, Pb, and As are at very low concentrations, an unlikely to cause renal damage from single elements (Jayatilake et al., 2013; Jayasumana et al., 2015b; Levine et al., 2016).
However, only a few research studies globally provided evidence for chronic health outcomes risks due to low-level of exposure to combination of heavy metal(loid)s as a risk factor leading to CKD (Navas-Acien et al., 2009; Tsai et al., 2017). Wasana et al. (2016) and Dharma-Wardana (2018) suggested that the Mg2+ in hard water in the CKDu endemic areas might increase the toxicity of heavy metal(loid)s, where such hard water was fed to mice. They speculated a possible synergistic, toxic effects of trace metal(loid)s and water hardness increase the risk for disease in treated mice (Wasana et al., 2016). However, Mg2+ in general protective from causing renal disease. What is more likely is the combined effect of low-level exposure of fluoride with Ca2+ containing hard water and PO4, over an extended period (over 10 years) precipitin CaPO4 apatite/crystals and nanotube complexes, causing chronic renal damage (Wimalawansa and Dissanayake, 2019). In this scenario, heavy metal(loid)s are not involved and, the damage is induced by natural, Geo-Bio interaction.
Cadmium and As are considered possible carcinogenic metals in humans. Carcinogenic risk (CR) indicates the probability of developing cancer over a lifetime (Fan et al., 2017). A CR-value between 10−6 and 10−4 is considered to be of low health risk, while amounts greater than 10−4 likely to cause health risks (Ullah et al., 2017). Therefore, CR were calculated for Cd, As, and Pb. The results showed Cd 2.5 × 10−5, As 4 × 10−6 and Pb 3.5 × 10−7. These data indicate no CR from the consumption of rice produced in the CKDu endemic areas.
Health risk for Pb exposure to an individual person is determined by the Pb concentration in food items, frequency of ingestion, and individual body mass index (Meharg et al., 2013). The rate of consumption of rice and water vs. Pb concentration (Figure 2) makes it possible to identify the health risk for an individual person since consumption rates are dependable. Figure 2 depicts the daily ingestion of Pb in CKDu endemic areas, which indicate a potential health risk through the increased consumption level, although the Pb concentration of rice grains and well water is within safe limits.
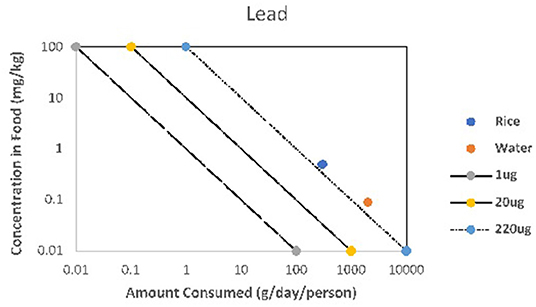
Figure 2. Amount consumed: rice and water with Pb concentration of rice and well water in CKDu endemic areas.
The present study demonstrated drinking water from wells in CKDu endemic areas is safe, with reference to heavy metal(loid)s exposure. The contribution from heavy metal(loid)s to groundwater TDI is very low and unlikely to cause health hazards including CKDu. However, higher ionicity of groundwater may not be appropriate for consumption for people with impaired renal functions. Calculated TDI for toxic metal(loid)s from rice and well water is below the respective recommendations by the WHO daily dietary limits; hence safe for consumption. Therefore, rice and groundwater in CKDu disease-prone areas in Sri Lanka are safe and devoid of harmful level of heavy metal(loid)s, except for Pb in rice.
We would recommend further studies to be conducted on the combination effects of chronic exposure to hard water and Pb in water on CKDu disease in the future. Besides, steps to be taken to reduce the consumption of hard water, together with a system to further reduce the Pb levels in well water or potable water to safeguard human health.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee (HREC) of the University of Newcastle, Australia (H-2017-0250), and Ethics Review Committee of the Faculty of Medicine, Rajarata University of Sri Lanka (ERC/2017/62). The patients/participants provided their written informed consent to participate in this study.
MK: conceptualization, methodology, investigation, writing original draft, and formal analysis. MW: supervision, writing, reviewing, and editing. RN: supervision, assisting with conceptualization, validation, project administration, and resources. SW and MR: reviewing and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MK is grateful to the University of Newcastle, Australia for providing UNIPRS and UNRSC scholarship and higher degree research candidate funding support and GCER (Global Centre for Environmental Remediation), the University of Newcastle for providing laboratory and analytical facilities to complete this study. The authors are also thankful to Mr. Kim Cloyvas, the University of Newcastle for this assistance with the statistical analysis. We are thankful to the staff of RARDC, Aralaganwila, Sri Lanka for support during sample preparation.
Athuraliya, N. T., Abeysekera, T. D., Amerasinghe, P. H., Kumarasiri, R., Bandara, P., Karunaratne, U., et al. (2011). Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 80, 1212–1221. doi: 10.1038/ki.2011.258
Brzóska, M., and Moniuszko-Jakoniuk, J. (2001). Interactions between cadmium and zinc in the organism. Food Chem. Toxicol. 39, 967–980. doi: 10.1016/S0278-6915(01)00048-5
Canfield, R. L., Henderson, C. R., Cory-Slechta, D. A., Cox, C., Jusko, T. A., and Lanphear, B. P. (2003). Intellectual impairment in children with blood Lead concentrations below 10 μg per deciliter. New Engl.J. Med. 348, 1517–1526. doi: 10.1056/NEJMoa022848
Chandrajith, R., Dissanayake, C. B., Ariyarathna, T., Herath, H. M., and Padmasiri, J. P. (2011a). Dose-dependent Na and Ca in fluoride-rich drinking water—another major cause of chronic renal failure in tropical arid regions. Sci. Total Environ. 409, 671–675. doi: 10.1016/j.scitotenv.2010.10.046
Chandrajith, R., Nanayakkara, S., Itai, K., Aturaliya, T., Dissanayake, C., Abeysekera, T., et al. (2011b). Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: geographic distribution and environmental implications. Environ. Geochem. Health 33, 267–278. doi: 10.1007/s10653-010-9339-1
Dharma-Wardana, M.. (2018). Chronic kidney disease of unknown etiology and the effect of multiple-ion interactions. Environ. Geochem. Health 40, 705–719. doi: 10.1007/s10653-017-0017-4
Dharma-Wardana, M., Amarasiri, S. L., Dharmawardene, N., and Panabokke, C. (2015). Chronic kidney disease of unknown aetiology and ground-water ionicity: study based on Sri Lanka. Environ. Geochem. Health 37, 221–231. doi: 10.1007/s10653-014-9641-4
Diyabalanage, S., Abekoon, S., Watanabe, I., Watai, C., Ono, Y., Wijesekara, S., et al. (2016). Has irrigated water from Mahaweli River contributed to the kidney disease of uncertain etiology in the dry zone of Sri Lanka? Environ. Geochem. Health 38, 679–690. doi: 10.1007/s10653-015-9749-1
Elumalai, V., Brindha, K., and Lakshmanan, E. (2017). Human exposure risk assessment due to heavy metals in groundwater by pollution index and multivariate statistical methods: a case study from South Africa. Water 9, 234. doi: 10.3390/w9040234
Fan, Y., Zhu, T., Li, M., He, J., and Huang, R. (2017). Heavy metal contamination in soil and Brown Rice and human health risk assessment near three mining areas in Central China. J. Healthc. Eng. 2017, 4124302. doi: 10.1155/2017/4124302
FAO/WHO (2004). Joint FAO/WHO Expert Standards Program Codex Alimentation Commission. Geneva, Switzerland: World Health Organization.
Gunatilake, S.. (2016). N and P variation in groundwater in wet zone and dry zone in Sri Lanka due to fertilization of paddy crop. Int. J. Sci. Res. Publ. 6, 1–5.
Habib, M. A., Islam, A. R. M. T., Bodrud-Doza, M., Mukta, F. A., Khan, R., Siddique, M. A. B., et al. (2020). Simultaneous appraisals of pathway and probable health risk associated with trace metals contamination in groundwater from Barapukuria coal basin, Bangladesh. Chemosphere 242:125183. doi: 10.1016/j.chemosphere.2019.125183
Herath, H. M. A., Kawakami, T., Nagasawa, S., Serikawa, Y., Motoyama, A., Chaminda, G. G. T., et al. (2018). Arsenic, cadmium, lead, and chromium in well water, rice, and human urine in Sri Lanka in relation to chronic kidney disease of unknown etiology. J. Water Health 16, 212–222. doi: 10.2166/wh.2018.070
Huang, Z., Pan, X.-D., Wu, P.-G., Han, J.-L., and Chen, Q. (2013). Health risk assessment of heavy metals in rice to the population in Zhejiang, China. PLoS ONE 8, e75007. doi: 10.1371/journal.pone.0075007
Ileperuma, O., Dharmagunawardhane, H., and Herath, K. (2009). Dissolution of aluminium from sub-standard utensils under high fluoride stress: a possible risk factor for chronic renal failure in the North-Central Province. J. Natl. Sci. Found. Sri Lanka 37, 219–222. doi: 10.4038/jnsfsr.v37i3.1217
Islam, A. R. M. T., Kabir, M. M., Faruk, S., Al Jahin, J., Bodrud-Doza, M., Didar-ul-Alam, M., et al. (2021). Sustainable groundwater quality in southeast coastal Bangladesh: co-dispersions, sources, and probabilistic health risk assessment. Environ. Dev. Sustain. 23, 18394–18423. doi: 10.1007/s10668-021-01447-4
Islam, S., Rahman, M. M., Islam, M., and Naidu, R. (2016). Arsenic accumulation in rice: consequences of rice genotypes and management practices to reduce human health risk. Environ. Int. 96, 139–155. doi: 10.1016/j.envint.2016.09.006
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., and Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7, 60–72. doi: 10.2478/intox-2014-0009
Jayasumana, C., Gajanayake, R., and Siribaddana, S. (2014). Importance of Arsenic and pesticides in epidemic chronic kidney disease in Sri Lanka. BMC Nephrol. 15, 124. doi: 10.1186/1471-2369-15-124
Jayasumana, C., Gunatilake, S., and Siribaddana, S. (2015a). Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 16, 103. doi: 10.1186/s12882-015-0109-2
Jayasumana, C., Paranagama, P., Agampodi, S., Wijewardane, C., Gunatilake, S., and Siribaddana, S. (2015b). Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ. Health 14, 6. doi: 10.1186/1476-069x-14-6
Jayatilake, N., Mendis, S., Maheepala, P., and Mehta, F. R. (2013). Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 14, 180. doi: 10.1186/1471-2369-14-180
Jayawardana, D., Pitawala, H. M. T. G.A., and Ishiga, H. (2010). Groundwater quality in different climatic zones of Sri Lanka: focus on the occurrence of fluoride. Int. J. Environ. Sci. Dev. 1, 244–250. doi: 10.7763/IJESD.2010.V1.47
Jayawardena, R., Byrne, N. M., Soares, M. J., Katulanda, P., and Hills, A. P. (2013). Food consumption of Sri Lankan adults: an appraisal of serving characteristics. Public Health Nutr. 16, 653–658. doi: 10.1017/S1368980012003011
Jayawardena, R., Thennakoon, S., Byrne, N., Soares, M., Katulanda, P., and Hills, A. (2014). Energy and nutrient intakes among Sri Lankan adults. Int. Arch. Med. 7, 34. doi: 10.1186/1755-7682-7-34
JECFA (2018). Joint FAO/WHO Food Standars Programe Codex Committee on Contaminants in Foods. 12th Session. Utrecht: Codex Alimenturius Commision.
Khan, S. I., Ahmed, A. M., Yunus, M., Rahman, M., Hore, S. K., Vahter, M., et al. (2010). Arsenic and cadmium in food-chain in Bangladesh—an exploratory study. J. Health Popul. Nutr. 28, 578–584. doi: 10.3329/jhpn.v28i6.6606
Kong, X., Liu, T., Yu, Z., Chen, Z., Lei, D., Wang, Z., et al. (2018). Heavy metal bioaccumulation in rice from a high geological background area in Guizhou Province, China. Int. J. Environ. Res. Public Health 15, 2281. doi: 10.3390/ijerph15102281
Kumar, M., Rahman, M. M., Ramanathan, A., and Naidu, R. (2016). Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci. Total Environ. 539, 125–134. doi: 10.1016/j.scitotenv.2015.08.039
Lanphear, B. P., Hornung, R., Khoury, J., Yolton, K., Baghurst, P., Bellinger, D. C., et al. (2005). Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ. Health Perspect. 113, 894. doi: 10.1289/ehp.7688
Levine, K. E., Redmon, J. H., Elledge, M. F., Wanigasuriya, K. P., Smith, K., Munoz, B., et al. (2016). Quest to identify geochemical risk factors associated with chronic kidney disease of unknown etiology (CKDu) in an endemic region of Sri Lanka-a multimedia laboratory analysis of biological, food, and environmental samples. Environ. Monit. Assess. 188, 548. doi: 10.1007/s10661-016-5524-8
Lloyd, J. W., and Heathcote, J. (1985). Natural Inorganic Hydrochemistry in Relation to Ground Water. U.S. Department of Energy.
Luo, D., Zheng, H., Chen, Y., Wang, G., and Fenghua, D. (2010). Transfer characteristics of cobalt from soil to crops in the suburban areas of Fujian Province, southeast China. J. Environ. Manage. 91, 2248–2253. doi: 10.1016/j.jenvman.2010.06.001
Magamage, C., Waidyaratna, W. H. M. C. U., Dhanapala, W. P. A. P., and Panampitiya, D. M. (2017). Determination of heavy metals in rice available in Kandy district, Sri Lanka. Ann. Dept. Agric. 19, 351–368.
Mahendranathan, C., and Thayaruban, T. (2018). Evidences of heavy metal contamination and their consequences in Sri Lanka with special reference to Agriculture: a review. World J. Agric.Soil Sci. 5, 14–28.
Meharg, A. A., Norton, G., Deacon, C., Williams, P., Adomako, E. E., Price, A., et al. (2013). Variation in rice cadmium related to human exposure. Environ. Sci. Technol. 47, 5613–5618. doi: 10.1021/es400521h
Mwale, T., Rahman, M. M., and Mondal, D. (2018). Risk and benefit of different cooking methods on essential elements and arsenic in rice. Int. J. Environ. Res. Public Health 15, 1056. doi: 10.3390/ijerph15061056
Nakashima, T., Matsuno, K., Matsushita, M., and Matsushita, T. (2011). Severe lead contamination among children of samurai families in Edo period Japan. J. Archaeol. Sci. 38, 23–28. doi: 10.1016/j.jas.2010.07.028
Nanayakkara, S., Senevirathna, S., Harada, K. H., Chandrajith, R., Hitomi, T., Abeysekera, T., et al. (2019). Systematic evaluation of exposure to trace elements and minerals in patients with chronic kidney disease of uncertain etiology (CKDu) in Sri Lanka. J. Trace Elem. Med. Biol. 54, 206–213. doi: 10.1016/j.jtemb.2019.04.019
Nanayakkara, S., Stmld, S., Abeysekera, T., Chandrajith, R., Ratnatunga, N., Edl, G., et al. (2014). An integrative study of the genetic, social and environmental determinants of chronic kidney disease characterized by tubulointerstitial damages in the North Central Region of Sri Lanka. J. Occup. Health 56, 28–38. doi: 10.1539/joh.13-0172-oa
Navas-Acien, A., Tellez-Plaza, M., Guallar, E., Muntner, P., Silbergeld, E., Jaar, B., et al. (2009). Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am. J. Epidemiol. 170, 1156–1164. doi: 10.1093/aje/kwp248
Norton, G. J., Williams, P. N., Adomako, E. E., Price, A. H., Zhu, Y., Zhao, F.-J., et al. (2014). Lead in rice: analysis of baseline lead levels in market and field collected rice grains. Sci. Total Environ. 485, 428–434. doi: 10.1016/j.scitotenv.2014.03.090
Rahman, M. A., Rahman, M. M., Reichman, S. M., Lim, R. P., and Naidu, R. (2014). Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: health hazard. Ecotoxicol. Environ. Saf. 100, 53–60. doi: 10.1016/j.ecoenv.2013.11.024
Rahman, M. M., Ng, J. C., and Naidu, R. (2009). Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ. Geochem. Health 31, 189–200. doi: 10.1007/s10653-008-9235-0
Rajapakshe, A., and Rathnayake, U. (2018). Urbanization and initial groundwater quality investigation in Malabe, Sri Lanka. Eng. Appl. Sci. Res. 45, 74–82. doi: 10.14456/easr.2018.22
Saha, N., Bodrud-Doza, M., Islam, A. R. M. T., et al. (2020). Hydrogeochemical evolution of shallow and deeper aquifers in central Bangladesh: arsenic mobilization process and health risk implications from the potable use of groundwater. Environ. Earth Sci. 79, 477. doi: 10.1007/s12665-020-09228-4
Sandeep, G., Sangita, A., Kumar, S. S., Rakhi, G., and Dinesh, K. (2012). Biological effect of heavy metal in drinking water samples of Western Uttar Pradesh region in India. J. Appl. Pharmaceut. Sci. 2, 177–181. doi: 10.7324/JAPS.2012.2727
Satarug, S., Gobe, G. C., Vesey, D. A., and Phelps, K. R. (2020). Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics 8, 86. doi: 10.3390/toxics8040086
Satpathy, D., Reddy, M. V., and Dhal, S. P. (2014). Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the East Coast of India. Biomed Res. Int. 2014, 545473. doi: 10.1155/2014/545473
Senanayake, S., and Premaratne, S. (2016). An analysis of the paddy/rice value chains in Sri Lanka. Asia Pac. J. Rural Dev. 26, 105–126. doi: 10.1177/1018529120160104
Silva, N. R. N., Weerasinghe, P., and Gunathilake, J. (2015). Water quality in agro wells and surface water bodies in Anuradhapura district of Sri Lanka. Ann. Sri Lanka Dept. Agric. 17, 24–27.
Tsai, T.-L., Kuo, C.-C., Pan, W.-H., Chung, Y.-T., Chen, C.-Y., Wu, T.-N., et al. (2017). The decline in kidney function with chromium exposure is exacerbated with co-exposure to lead and cadmium. Kidney Int. 92, 710–720. doi: 10.1016/j.kint.2017.03.013
Ullah, A. A., Maksud, M., Khan, S., Lutfa, L., and Quraishi, S. B. (2017). Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 4, 574–579. doi: 10.1016/j.toxrep.2017.10.002
US EPA (2006). Risk-Based Concentration Table. United States Environmental Protection Agency (US EPA). Available online at: http://www.epa.gov/reg3hwmd/risk/human/rbc/rbc1006.pdf (accessed December 27, 2021).
US EPA (2010). Risk-Based Concentration Table. United States Environmental Protection Agency (US EPA). Available online at: http://www.epa.gov/reg3hwmd/risk/human/index.htm (accessed December 27, 2021).
US EPA (2021). Risk-Based Concentration Table. United States Environmental Protection Agency (US EPA). Available online at: https://semspub.epa.gov/work/HQ/401635.pdf (accessed December 27, 2021).
Vlahos, P., Schensul, S. L., Nanayakkara, N., Chandrajith, R., Haider, L., Anand, S., et al. (2018). Kidney progression project (KiPP): Protocol for a longitudinal cohort study of progression in chronic kidney disease of unknown etiology in Sri Lanka. Glob. Publ. Health 14, 214–226. doi: 10.1080/17441692.2018.1508480
Wanigasuriya, K. P., Peiris-John, R. J., and Wickremasinghe, R. (2011). Chronic kidney disease of unknown aetiology in Sri Lanka: is cadmium a likely cause? BMC Nephrol. 12, 32. doi: 10.1186/1471-2369-12-32
Wasana, H. M. S., Aluthpatabendi, D., Kularatne, W. M. T. D., Wijekoon, P., Weerasooriya, R., and Bandara, J. (2016). Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water. Environ. Geochem. Health 38, 157–168. doi: 10.1007/s10653-015-9699-7
Weaver, V. M., Fadrowski, J. J., and Jaar, B. G. (2015). Global dimensions of chronic kidney disease of unknown etiology (CKDu): a modern era environmental and/or occupational nephropathy? BMC Nephrol. 16, 145. doi: 10.1186/s12882-015-0105-6
WHO (2011). Hardness in Drinking Water, Background Document for Development of WHO Guidelines for Drinking Water Quality. Geneva: World Health Organization.
Wijayawardena, M. A., Megharaj, M., and Naidu, R. (2017). Bioaccumulation and toxicity of lead, influenced by edaphic factors: using earthworms to study the effect of Pb on ecological health. J. Soils Sediments 17, 1064–1072. doi: 10.1007/s11368-016-1605-0
Wimalawansa, S. J.. (2014). Escalating chronic kidney diseases of multi-factorial origin in Sri Lanka: causes, solutions, and recommendations. Environ. Health Prev. Med. 19, 375–394. doi: 10.1007/s12199-014-0395-5
Wimalawansa, S. J.. (2016). The role of ions, heavy metals, fluoride, and agrochemicals: critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environ. Geochem. Health 38, 639–678. doi: 10.1007/s10653-015-9768-y
Wimalawansa, S. J., and Dissanayake, S. B. (2019). Factors affecting the environmentaly induced, chronic kidney disease of unknown aetiology in dry zonal regions in tropical countries-noval findings. Environments 7, 1–29. doi: 10.3390/environments7010002
Wu, B., Zhao, D., Jia, H., Zhang, Y., Zhang, X., and Cheng, S. (2009). Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 82, 405–409. doi: 10.1007/s00128-008-9497-3
Keywords: carcinogenic risk, chronic kidney disease of unknown etiology, hazard index, heavy metals, total daily intake
Citation: Kulathunga MRDL, Wijayawardena MAA, Naidu R, Wimalawansa SJ and Rahman MM (2022) Health Risk Assessment From Heavy Metals Derived From Drinking Water and Rice, and Correlation With CKDu. Front. Water 3:786487. doi: 10.3389/frwa.2021.786487
Received: 30 September 2021; Accepted: 26 November 2021;
Published: 03 February 2022.
Edited by:
Katherine A. James, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Abu Reza Md. Towfiqul Islam, Begum Rokeya University, BangladeshCopyright © 2022 Kulathunga, Wijayawardena, Naidu, Wimalawansa and Rahman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. A. Ayanka Wijayawardena, YXlhbmthLndpamF5YXdhcmRlbmFAbmV3Y2FzdGxlLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.