
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virtual Real. , 08 March 2023
Sec. Virtual Reality in Medicine
Volume 4 - 2023 | https://doi.org/10.3389/frvir.2023.926679
This article is part of the Research Topic The role of perceptual manipulations of XR in neurological rehabilitation View all 6 articles
Rationale: Social factors are considered important for the initiation and maintenance of drug abuse. Virtual reality (VR) research on cue reactivity and exposure frequently incorporates social stimuli as part of complex drug-intake scenarios. Attempts are rarely made to dissect the impact of the different components and their interactive effects. The present study critically extends this line of research by investigating the modulatory effects of social context on the reactivity evoked by proximal smoking cues.
Methods: Thirty-two smokers and 33 never-smokers were presented in VR with proximal cues and neutral stimuli, embedded in a social context or a neutral context. A virtual hand model was used to translate real hand movements into VR. Each trial started with the presentation of the different stimulus–context combinations. Discrete stimuli were presented on the table in front of the participants, and contextual stimuli were presented at the end of the table. Afterward, participants were instructed to grasp the target stimulus (a cigarette vs. a pencil) in front of them. After successful contact, the stimulus appeared in the virtual hand. Modulation of cue reactivity by social context was assessed by self-report, physiological measures, and overt approach behavior.
Results: The results revealed modulatory effects of social context on the responses to proximal smoking cues in smokers. In contrast to never-smokers, smoking cues evoked craving in smokers, which was attenuated in a social context. Furthermore, social context increased the latency to approach and contact the cigarette in the group of smokers but did not affect behavioral approach responses in never-smokers. Other data provided indications for interactive, but also main effects of cues and contexts. Interestingly, cue-evoked craving was increased after contact with the virtual cigarette.
Conclusion: The present study critically extends previous research by providing evidence for the modulation of cue reactivity by social context. The results are particularly important given the well-established role of drug-associated environmental contexts in the stimulus control of addictive behaviors. Our results emphasize the need to address social context effects on cue reactivity in basic research and treatment and further suggest that changes in the perceived availability of smoking might enhance or inhibit cue-evoked reactivity.
Regular cigarette smoking is commonly considered a major risk for the development of severe health problems and premature death (Doll et al., 2004; US Department of Health and Human Services, 2014; Reitsma et al., 2017). Smoking cessation has a positive impact on health (Jha et al., 2013), and a significant number of smokers report an intention to quit (Babb et al., 2017). Unfortunately, nicotine dependence (Schnoll et al., 2013) often undermines efforts to stop smoking, and even with effective behavioral or pharmacological interventions, success rates are not as high as one would hope for (Prochaska and Benowitz, 2019). Accordingly, there is a strong need to refine our understanding of the biopsychosocial mechanisms (Skewes and Gonzalez, 2013) involved in the initiation, maintenance, and relapse of smoking to improve prevention and treatment.
Core criteria of substance use disorders, such as an intense desire to take the drug (i.e., craving) and compulsive drug-seeking and consumption, are often precipitated by exposure to drug-associated stimuli (Siegel et al., 2000; Wise and Koob, 2014; Berridge and Robinson, 2016; Koob and Volkow, 2016). Most human research targets the effects of discrete, naturalistic drug stimuli proximal to consumption, e.g., pictures of drug paraphernalia like a newly lit cigarette (Conklin et al., 2008; Mucha et al., 2008; Stippekohl et al., 2010). Accordingly, there is rich evidence that proximal smoking cues evoke craving in smokers (Betts et al., 2021), which appears to be modulated by the perceived opportunity to use the drug (Mucha et al., 2008; Bailey et al., 2010). Cue-evoked increases in skin conductance and heart rate have been reported as general indices of physiological arousal, related to attentional processing and preparation for action (Bradley, 2009; Betts et al., 2021). Furthermore, there is evidence that exposure to smoking-related stimuli may promote approach (Bailey et al., 2010; Wiers et al., 2013; Boecker and Pauli, 2019) and consummatory responses (Hogarth et al., 2010; Winkler et al., 2011), which may be determined by the nature of the stimulus (Mucha et al., 2008) and the context (Conklin et al., 2019; Vollstädt-Klein et al., 2022).
Drug-taking frequently takes place in specific environmental contexts (Conklin and Tiffany, 2002; LeCocq et al., 2020), e.g., a bar, a café, a place at home, and in the presence of other people (Dimoff and Sayette, 2017; de Wit and Sayette, 2018). In this paragraph, we will highlight results from animal and human research on the role of physical contexts in the control of drug-seeking and cue reactivity, as they may be relevant to our understanding of social context effects in addictive behaviors. First, the animal literature shows that drug-associated contexts evoke reactivity by themselves, for instance, in the form of a conditioned place preference (Le Foll and Goldberg, 2005; Napier et al., 2013; Wu et al., 2014). Second, there is evidence that drug-related environments invigorate the reactivity evoked by discrete drug-associated cues (Sciascia et al., 2015; LeCocq et al., 2020; Valyear et al., 2020). Third, drug-associated contexts may renew the effects of cues extinguished in a new context different from the context where drug intake previously took place (Kearns and Weiss, 2007; Crombag et al., 2008; Chesworth and Corbit, 2017). This modulatory context effect in the renewal paradigm is also interesting from a clinical perspective, as it may partly mirror the high-risk situation when abstinent drug users return to their natural drug-taking environment after cue exposure-based treatment in a therapeutic context (LeCocq et al., 2020). Accordingly, contextual stimuli received heightened attention in human research as they may promote drug-taking by themselves or in interaction with discrete drug-associated cues. For instance, evidence for the renewal effect in smokers was shown by the return of cue-evoked craving that had been extinguished in a new context unrelated to drug-taking, when the participants were brought back to the original context where the cues were previously predictive for smoking (Thewissen et al., 2006). Seminal work in smokers realized with picture stimuli demonstrated that smoking contexts, completely devoid of proximal smoking cues, may act as distal smoking stimuli promoting craving (Conklin, 2006; Conklin et al., 2008) and smoking (Stevenson et al., 2017). Moreover, individualized smoking contexts may even have stronger effects on craving, positive affect and heart rate responses (Conklin et al., 2010), neuronal activity, and cigarette consumption (McClernon et al., 2016). Importantly, recent research has started to investigate the interactive effects of combining (personalized) smoking vs. non-smoking contexts with proximal smoking cues vs. neutral stimuli (Conklin et al., 2019). The combination of proximal smoking cues and smoking contexts resulted in increased craving compared to the other stimulus combinations and a decreased latency to initiate smoking.
Human drug use often occurs in social settings, and social factors are generally considered important for our understanding of the initiation, maintenance, and relapse of drug-taking (Baker et al., 2004; Heilig et al., 2016; Dimoff and Sayette, 2017). There is evidence from both animal and human studies that social stimuli affect drug-seeking and the responses to drug-associated stimuli (Bardo et al., 2013; Venniro et al., 2020). Particularly interesting are observations from animal research suggesting that social stimuli can function as discriminative stimuli, setting the occasion for the availability and non-availability of drug-intake (Strickland and Smith, 2014; Smith et al., 2016). In human research, experimental manipulations of the predictive relationship between social stimuli and the drug are rather rare. However, there is rich evidence that the presence or absence of smoking peers is a major predictor for adolescent smoking (Baker et al., 2004). Studies based on ecological momentary assessments of the situational factors related to smoking revealed considerable evidence that socializing or the presence of other people smoking increases the probability to smoke (Dimoff and Sayette, 2017). Social smoking may be present in varying degrees in both intermittent and daily smokers (Shiffman et al., 2014; Shiffman et al., 2015) and thus may represent an important determinant of smoking. Interestingly, smoking behavior might be even suppressed below a baseline rate defined by solitary smoking in a social context involving the presence of other people not smoking (Shiffman and Rathbun, 2011). Although this research provided considerable evidence for the impact of social context on smoking, experimental studies systematically dissecting the effects of different facets of social context on drug-directed and cue-evoked responding are scarce (Dimoff and Sayette, 2017). Social context may affect craving and drug consumption by multiple mechanisms (e.g., by setting the occasion for the availability of drug-intake, by behavioral modeling, social norms, peer pressure, or support), one of them being the mere exposure to proximal cues related to smoking, e.g., the sight of a cigarette. For instance, the presence of an experimental confederate smoking cigarettes increases smoking compared to a non-smoking one, an effect that does not necessarily need to be dependent on social pressure to smoke or not to smoke (Harakeh and Vollebergh, 2011; Harakeh and Vollebergh, 2012). However, there is also evidence that presenting participants with pictures devoid of proximal smoking cues depicting people with whom they regularly smoke or do not smoke increases and decreases craving compared to a baseline provided by pictures of strangers (Conklin et al., 2013). Accordingly, social stimuli related to smoking may affect craving independent of the presence of smoking cues. Overall, this line of research provided considerable evidence for the importance of social factors in the control of drug-motivated behavior. However, it provides no answer to the question of how social contexts interact with the effects of proximal smoking cues. This is important for both our understanding of the precise mechanisms of stimulus control in addictive behaviors and the development of efficient approaches for treatment.
In this regard, the progress of virtual reality (VR) technology during the last decades boosted interest in therapeutic applications for smoking cessation (Hone-Blanchet et al., 2014; Segawa et al., 2019; Trahan et al., 2019; Langener et al., 2021). A particular benefit of VR as a research and therapeutic method is its ability to simulate highly immersive, three-dimensional drug-intake situations with both high experimental control and ecological validity. A high amount of VR-based research investigates the motivational effects of complex drug-related scenarios, which involve various combinations of drug-associated environments, proximal drug stimuli, and the presence of other people taking drugs or offering drugs to the participant, e.g., in a party scene (Segawa et al., 2019). Rarely have attempts been made to separate the individual contributions and interactive effects of the different components, which may be important for the identification of individual triggers to smoke, the development of exposure hierarchies, and unraveling the mechanisms of cue exposure-based treatments (Pericot-Valverde et al., 2014). Pioneering work conducted by Paris et al. (2011) revealed preliminary evidence that a smoking-associated context (a convenience store including an employee) devoid of proximal smoking cues increases craving in smokers. Importantly, craving to smoke was further enhanced when the same environment was presented afterward in combination with discrete smoking cues (a person smoking outside, cigarettes, and cigarette packs in the shop; see also, e.g., Gamito et al., 2011). Regarding the effects of social contexts, there are preliminary suggestions that in participants with a low degree of alcohol dependence, social pressure might increase craving for alcohol in presence of both alcohol and control stimuli, while alcohol cue effects were only reported in the condition without social pressure (Cho et al., 2008). Interestingly, there are indications that in abstinent alcohol-dependent individuals, social pressure might not further increase alcohol craving in situations directly associated with alcohol intake, although strong cue effects were reported (Lee et al., 2008). In contrast, in social drinkers, the effect of social pressure appeared to be more pronounced than the effects of the drug-related environment, which may suggest that social factors could affect drug-related behavior by different mechanisms in different populations (Lee et al., 2008).
Overall, previous studies provided considerable evidence for the importance of social context in the control of drug-directed responses. However, systematic research on the impact of social context on the reactivity evoked by proximal smoking cues is lacking. Accordingly, the present study aims to close this gap by investigating the interactive effects of social context and discrete smoking cues and the dependency of the effects on the perceived proximity to smoke intake. A second objective of the study was to test which smoking-related scenario—one with or without social context–might be better suited for exposure treatment. In the experiment, smokers and never-smokers were immersed in virtual reality and presented with discrete smoking vs. neutral stimuli in a social or neutral context. Modulation of cue reactivity was assessed by multiple measures, including self-report (craving, pleasure, and arousal), physiological indices of arousal (heart rate and skin conductance), motivational valence (affect-modulation of the startle response), and overt behavior (latency of behavioral approach). Given the role of incentive proximity in the functional organization of behavior systems (Timberlake, 1994; Mucha et al., 2008; Perusini and Fanselow, 2015) and previous reports that smoking a virtual cigarette increases craving (Garcia-Rodriguez et al., 2013), craving to smoke was registered twice before and after completion of the approach response, when the target stimulus (a cigarette vs. a pencil) appeared in the virtual hand. We hypothesized that social context would specifically enhance the reactivity to smoking cues.
Sixty-nine participants were recruited for the study. General inclusion criteria comprised an age between 18 and 40 years, the absence of any self-reported major physical or mental problems, and right-handedness (as the virtual hand used in this study has only been available as a right-handed version). Smokers were included if they smoked an average of at least 10 cigarettes per day for at least one year. Never-smokers were included if they reported having smoked less than a pack of cigarettes in their lifetime. Smokers and never-smokers were matched according to age and sex. Participants were mainly recruited from the student population of the university and received either course credit or a monetary compensation of 12 euros for their participation. Four recruited participants had to be excluded from the analyses due to technical problems or a high number of artifacts in the psychophysiological recordings. Accordingly, the final sample comprised 32 smokers (sex: 16 males, 16 females; age: M = 24.19 years, SD = 3.16) and 33 never-smokers (sex: 15 males, 16 females; age: M = 24.09 years, SD = 3.45). As intended, both groups did not differ with regard to age, t(63) = 0.12, p = 0.907, and sex ratio, χ2(1) = 0.14, p = 0.714. Smokers reported regular smoking for 8.32 (SD = 3.13) years with a current average of 14.06 (SD = 4.36) cigarettes per day and scored 3.19 (SD = 1.82) on the Fagerström Test for Nicotine Dependence (FTND). Last cigarette consumption was 2.16 (SD = 4.19) hours before the start of the experiment. Never-smokers reported having smoked an average of 5.06 (SD = 5.47) cigarettes in their lifetime.
The Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) was used to assess the degree of nicotine dependence in smokers. Smokers’ self-reported motives to smoke were assessed by the Smoking Motives Questionnaire (SMQ; Russell et al., 1974). Dispositional tendencies related to the activation of two hypothesized motivational systems (Gray, 1990), a behavioral inhibition system (BIS) and a behavioral activation system (BAS), were assessed by the German version (Strobel et al., 2001) of the BIS/BAS scales (BIS/BAS; Carver and White, 1994). The iGroup Presence Questionnaire (IPQ; Schubert, 2003) was used to measure the degree of presence experienced in the virtual environments, i.e., the sense of “actually being there.” Self-reported symptoms of simulator sickness, such as fatigue, nausea, and dizziness, which could sometimes occur from immersion in virtual environments, were assessed by the Simulator Sickness Questionnaire (SSQ; Kennedy et al., 1993). Finally, the German version (Sosic et al., 2008) of the Social Phobia Inventory (SPIN; Connor et al., 2000) was used as a measure of the individual degree of social phobia.
Changes in baseline craving during the study were assessed by the German version (QSU-G; Müller et al., 2001) of the Questionnaire on Smoking Urges (QSU; Tiffany and Drobes, 1991). Self-reported pleasure, arousal, and craving for cigarettes, alcohol, food, and water were monitored by Self-Assessment Manikins (SAM; Lang, 1980) and nine-point scales (Mucha et al., 1999), respectively.
The basic virtual environment consisted of an immersive 3D model of the real laboratory in which the experiment took place. The model was created in vrml97 (Web3DConsortium) by our working group (see Peperkorn et al., 2016) and comprised a chair with armrests and a table, where the participants were seated in both the real and virtual world (see Figure 1).
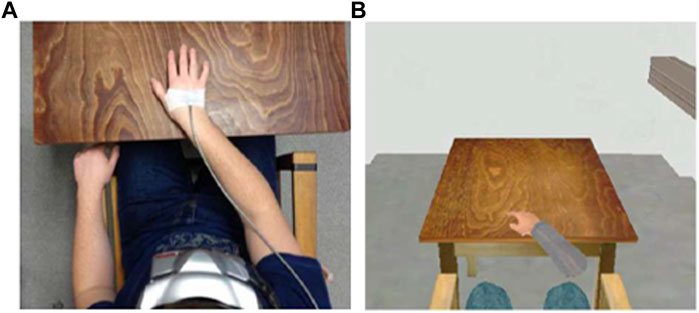
FIGURE 1. Laboratory setup and corresponding VR scenario. (A) Laboratory setup, including head-mounted display (HMD) and hand tracking. (B) VR scenarios including VR hands. Pictures are adapted from our previous study (Peperkorn et al., 2016).
Sex-specific virtual legs and a virtual model of a right hand and arm were exported from Poser 6 (Curious Labs, Inc., Santa Cruz, United States of America). The virtual legs were depicted in a bent position resting at the virtual chair and were visible from the seating position of the participants.
The hand-arm model was available in three versions: before the initiation and after the completion of the approach response to the target stimulus (cigarette vs. pencil). After successful contact, the cigarette vs. the pencil appeared in the virtual hand (see Figure 2). Hand movements of the participants in the real world were tracked using a sensor placed on top of the right hand. Physical movements of the sensor were rendered in terms of rotation and location data of the complete hand-arm model (see Peperkorn et al., 2016). This approach allowed us to measure the latency to approach and contact virtual target stimuli with varying motivational content (cigarette vs. pencil) and provided the participants an opportunity for interaction, which affected the perceived proximity of the incentive stimuli.
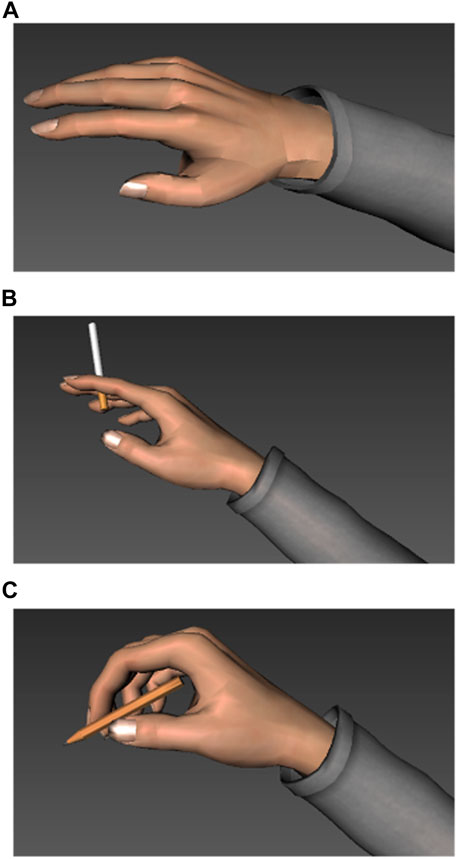
FIGURE 2. Virtual hand used in the experiment in three versions. (A) Before the initiation of the approach response without object. (B) After completion of the approach response, holding a cigarette. (C) After completion of the approach response, holding a pencil.
The 3D stimulus material was created with Autodesk Maya 2009 (Autodesk, Inc., San Rafael, CA, United States of America) or downloaded from internet archives and partially implemented with modifications.
Smoking stimuli comprised an unlit cigarette in an ashtray, a pack of cigarettes, and a lighter. Smokers were allowed to choose the cigarette box used in the experiment from three frequently consumed brands (Marlboro, Lucky Strike, or Gauloises) according to their personal preference. Never-smokers were matched according to the brand preferences of the smokers. Smoking stimuli were presented as sets created by varying the color of the ashtray (black or metal), the position of the cigarette box (right or left to the ashtray), the color (green or lavender), and position (right or left to the ashtray) of the lighter.
Neutral stimuli comprised a pencil on a notepad, a pencil box, and a sharpener. Stimulus sets were created by varying the texture of the notebook (lined or checkered with a red or green upper border), the position of the pencil case (right or left to the notebook), the color (green or blue), and the position (left or right to the notebook) of the sharpener. The stimuli were presented on the virtual table in front of the participants, within their reach. A white cross on the table marked the starting position of the virtual (and real) hand of the participants.
The social context was manipulated by presenting agents of the same sex as the participants with a seat at the table vis-à-vis the participants. The virtual agents looked in the direction of the participants. Four different agents with neutral facial expressions were used, which varied in physiognomic characteristics, hair color, hairstyles, and clothing. Indoor plants were used as a neutral context placed at the table vis-à-vis the participant. Four different foliage plants, varying in size and shape were used. Larger plants were located on the floor, smaller plants were placed in a plant pot on top of a stele.
Four experimental conditions were realized by combining the two (smoking vs. neutral) stimulus types and the two (social vs. neutral) contexts. Thus, the participants were presented with proximal smoking stimuli in a social context, proximal smoking stimuli in a neutral context, neutral stimuli in a social context, and neutral stimuli in a neutral context, respectively (see Figure 3 for examples). The sets were realized as a unique combination of stimuli, varying in appearance, color, position, and the corresponding contexts. Each experimental condition consisted of eight sets of stimulus-context combinations.
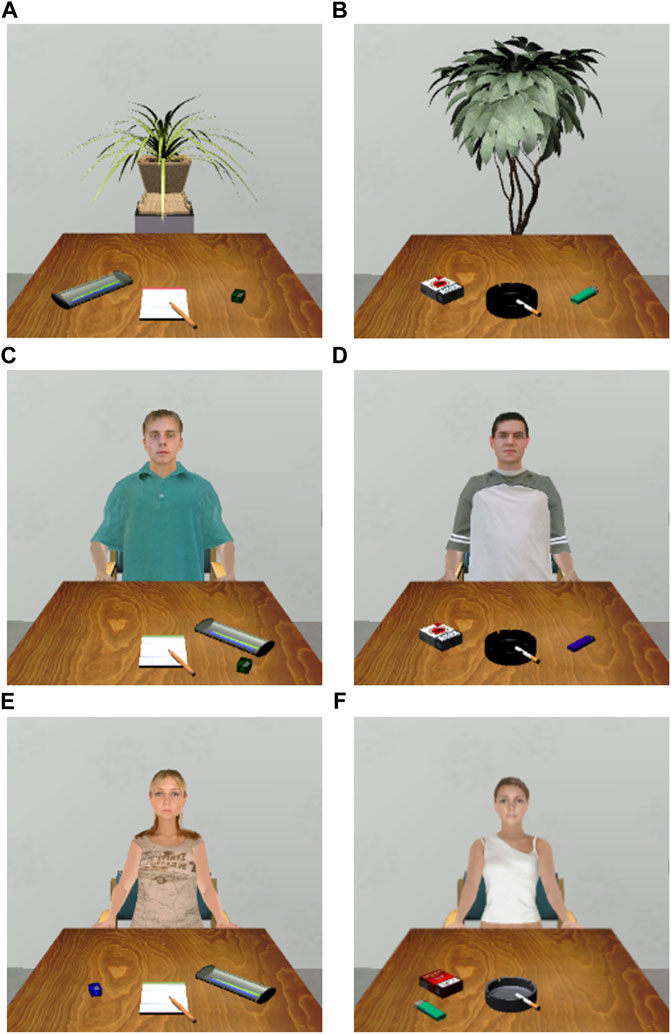
FIGURE 3. Examples of the four stimulus conditions used in the study (male and female versions). (A) Neutral stimuli in a neutral context. (B) Smoking stimuli in a neutral context. (C) Neutral stimuli in a social context (male version). (D) Smoking stimuli in a smoking context (male version). (E) Neutral stimuli in a social context (female version). (F) Smoking stimuli in a social context (female version).
Each trial started with an inter-trial interval with an average duration of 21 s (16.5–25.5 s). During the inter-trial interval, only the virtual room, including the virtual arm and legs, and the blank table, without any stimuli, were presented. Then, the smoking or neutral stimuli appeared on the table together with the social or neutral contextual stimuli at the end of the table. A startle probe was presented during 75 percent of the trials of each experimental condition, at 2.5, 4.0, or 5.5 s after stimulus onset. After an average of 7.5 s (7–8 s) of stimulus presentation, participants were automatically prompted via headphones to rate their momentary state of pleasure, arousal, and craving to smoke (T1). Afterward, participants were asked to grasp the cigarette and the pencil lying in front of them on the table, respectively. Successful contact with the target was signaled by a tone, and the corresponding object (cigarette vs. pencil) appeared in the hand of the participant. Then, the craving to smoke was rated again (T2). Each trial ended after 10 s with the instruction to resume the starting position, i.e., to put their (virtual) hand on the white cross on the table, to sit up straight, and to look straight ahead. The trials were presented in four pseudo-randomized sequences (matched for smokers and never-smokers) with the following restrictions: no more than two times, the same experimental condition; no more than four times, a trial with a startle probe; and no more than two times, the same startle time in a row. During the experiment, each participant completed a total of 32 trials (eight trials for each of the four conditions), divided into two blocks of 16 trials.
The three-dimensional virtual environments were shown via a head-mounted display (HMD; Z800 3DVisor, eMagin, Bellevue, Washington, US). Headphones (HD 215; Sennheiser; Wedemark–Wennebostel) were used for presenting instructions and startle stimuli. Tracking sensors were placed on top of the headphones and on the right hand of the participants. An electromagnetic position tracking system (3space Fastrak; Polhemus, Colchester, VT, United States) was used to register head and hand positions and to transfer them into the virtual environments (see Peperkorn et al., 2016). Rendering was realized by Cortona VRML Client (ParallelGraphics, Dublin, Ireland). Experimental control and data recording was established using the in-house written VR-software CyberSession (Version 5.3.38, VTplus GmbH, Würzburg, Germany; see www.cybersession.info for detailed information).
A Varioport-B system (Becker Meditec, Karlsruhe, Germany) was used for psychophysiological recordings. Acoustic startle responses were evoked by 50 ms, 96 dB, white noise with an almost instantaneous rise time, presented binaurally via headphones. Startle responses were measured by recording electromyographic activity (EMG) via two Ag/AgCl miniature electrodes (Ø = 5 mm) placed above the M. orbicularis oculi of the left eye (Blumenthal et al., 2005). The signal was sampled at a frequency of 1,024 Hz. An electrocardiogram (ECG) was recorded from two pre-filled, disposable electrodes attached to the sternum and the left lower costal arch. A ground electrode was placed below the chest. ECG data were recorded at a sampling rate of 512 Hz. Electrode impedance was kept below 10 kΩ. Electrodermal activity (EDA) was recorded with two Ag/AgCl electrodes (Ø 8 mm) placed at the middle phalanges of the fore and middle finger of the left hand. The electrodes were filled with a 0.05 M sodium chloride electrolyte paste. The Varioport system constantly delivered 0.5 V across the two electrodes. Skin conductance was sampled at a rate of 128 Hz. Alveolar carbon monoxide (CO) samples were taken by a Bedfont Micro Smokerlyzer.
Participants arrived in the lab, which was arranged to resemble its virtual counterpart as much as possible. A notebook and an ashtray stood on the table. After providing informed consent, the first alveolar carbon monoxide (CO) sample was taken, and the participants filled out a short sociodemographic questionnaire, the FTND (smokers only), the SAM plus additional rating scales, and the QSU-G. The participants were familiarized with the oral rating procedure used in VR and trained on the standard position of their hand on the table. Next, the electrodes for physiological recordings, the tracking sensors, the HMD, and headphones were attached to the participants. The participants were seated comfortably in the chair at the table and were immersed in the virtual environment, where they received further instructions on the procedure. Two startle probes were presented before the participants completed one practice trial, including one startle probe, of each of the four experimental conditions. Then, the experiment started. During the break after the first block, the HMD was removed, a CO sample was taken, and the participants filled in the following questionnaires: the SAM plus additional rating scales, the QSU-G, and the BIS/BAS scales. At the end of the second block, technical equipment was removed, a last CO sample was obtained, and the participants filled out the final questionnaires: the SAM plus additional rating scales, the QSU-G, the SSQ, the IPQ, the SPIN, and the SMQ (smokers only). Overall, the study lasted about 2 h.
Psychophysiological recordings were processed with the software Vision Analyzer Version 1.05 (BrainProducts, Munich, Germany). The raw EMG was filtered with a high cutoff filter of 500 Hz (24 dB/oct) and a low cutoff filter of 28 Hz (24 dB/oct). The data were rectified and smoothed (50 ms moving average window). The peak amplitude of the startle response was identified within a time window between 50 ms and 180 ms after startle probe onset. Artifacts and null responses were detected manually. Null responses were set to zero, and artifact-contaminated trials were excluded from further analyses. The magnitude of the startle response was calculated as the difference between the peak and the mean of 50 ms baseline activity before probe onset. Individual startle magnitudes were standardized (T-values) to normalize the distribution.
The raw ECG was filtered with a high cutoff filter of 70 Hz (24 dB/oct) and a low cutoff filter of 0.48 Hz (24 dB/Oct). R-waves were detected by an algorithm implemented in Vision Analyzer software. Next, interbeat intervals were computed and interpolated into a continuous heart rate (bpm). The data were baseline corrected by subtracting the average activity during a 1-s period before stimulus onset. Heart rate responses for each trial were calculated as the peak within a time window between 3 and 7 s after stimulus onset.
Skin conductance data were filtered with a low-pass filter with a cutoff of 1 Hz. Skin conductance responses (SCRs) were calculated as the largest increase between 1 and 7 s after stimulus onset, relative to 1 s mean activity before stimulus onset. Responses smaller than 0.01 µS were scored as zero (null responses). Trials with activity larger than 0.01 µS within a time window of 0.1 s before and after stimulus onset were scored as artifacts and rejected from further analyses. The data were logarithmized (ln (SCR+1)) to normalize the distribution (Venables and Christie, 1980).
Latencies to contact the target stimulus on the table (cigarette vs. pencil) were calculated by subtracting the time at the end of the instruction to grasp from the time the virtual object was contacted.
Scores for statistical analyses were derived by averaging all trials for each experimental condition. We tested our hypotheses (SPSS Statistics 25.0, IBM Corp. United States) using mixed analyses of variance (ANOVAs) with group (smokers vs. never-smokers) as between factor and stimulus (smoking vs. neutral) and context (social vs. neutral) as within factors. As stimulus-evoked craving to smoke was assessed two times, before and after completion of the approach response, when the virtual cigarette vs. the pencil appeared in the virtual hand, the within factor of time was additionally included in the analysis of self-reported craving. Follow-up analyses were conducted by ANOVAs and paired t-tests (uncorrected). The alpha level was set to 0.05 (two-tailed) for all analyses. Effect sizes are stated as partial eta2 (ηp2).
Smokers and never-smokers did not differ with regard to their scores on the BIS/BAS scales; BIS: t(63) = 0.92, p < 0.361; BAS: t(63) = 1.06, p < 0.295; the SSQ, t(63) = 0.16, p < 0.871; and the SPIN, t(63) = 1.49; p < 0.141 (see Table 1). Both groups marginally differed in their IPQ scores, t(63) = 1.75, p = 0.085. Never-smokers reported a slightly higher feeling of being present in VR than smokers. It might be relevant that all participants who scored above the German cutoff score for social phobia (25) were never-smokers (see Discussion). A descriptive analysis of the SMQ showed that smokers scored highest for sedative smoking and lowest for automatic and psychosocial smoking (see Supplementary Table S1).
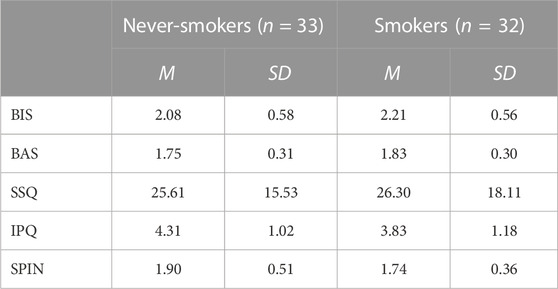
TABLE 1. Means (SD) of the scores of never-smokers and smokers on the Behavioral Inhibition System/Behavioral Approach System scales (BIS/BAS scales), the Simulator Sickness Questionnaire (SSQ), the iGroup Presence Questionnaire (IPQ), and the Social Phobia Inventory (SPIN).
Changes in motivational state during the study are depicted in Table 2. The analysis of the alveolar carbon monoxide levels revealed significant effects of group, F(1,63) = 88.06, p < 0.001, ηp2 = 0.58, time, F(2,126) = 47.68, p < 0.001, ηp2 = 0.43, and group x time, F(2,126) = 42.19, p < 0.001, ηp2 = 0.40. Compared to never-smokers, CO levels were higher in smokers and showed a reliable decrease during the experiment, F(2,62) = 45.82, p < 0.001, ηp2 = 0.60, which was evident as a decline from the first to the second time point, t(31) = 6.87, p < 0.001, and from the second to the third time point, t(31) = 4.77, p < 0.001. The CO levels of never-smokers did not change during the study, F(2,64) = 8.63, p = 0.410, ηp2s = 0.03.
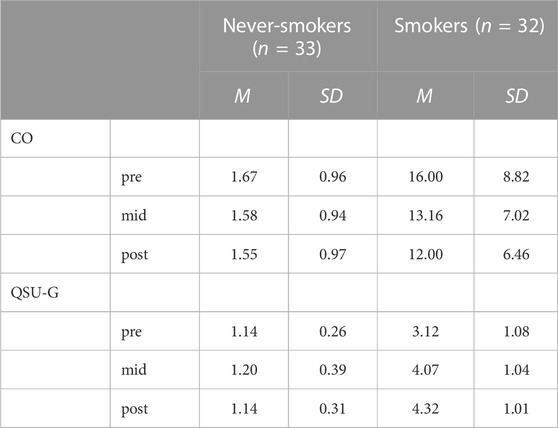
TABLE 2. Mean (SD) of alveolar carbon monoxide levels CO (ppm) and scores on the Questionnaire of Smoking Urges (QSU-G) in never-smokers and smokers before the start of the experiment (pre), during the break after the first block of the experiment (mid) and at the end of the experiment (post).
The analysis of urges to smoke as assessed by the QSU-G revealed significant effects of group, F(1,63) = 245.50, p < 0.001, ηp2 = 0.80, time, F(2,126) = 39.48, p < 0.001, ηp2 = 0.39, and group x time, F(2,126) = 36.64, p < 0.001, ηp2 = 0.37. Compared to never-smokers, urges to smoke were higher in smokers and showed a reliable increase during the experiment, F(2,62) = 39.70, p < 0.001, ηp2 = 0.56, which was evident as an increase from the first to the second time point, t(31) = 6.87, p < 0.001, and from the second to the third time point, t(31) = 3.32, p = 0.002. QSU-G levels of never-smokers did not change during the study; F(2,64) = 1.35, p = 0.265, ηp2 = 0.04. The analyses of the self-reported desire to smoke, pleasure, arousal, desire to eat, desire to drink water, and desire to drink alcohol are reported in the (see Supplementary Table S2).
In the present study, craving to smoke was rated twice: before (T1) and after (T2) completion of the approach response, when the cigarette or pencil appeared in the virtual hand of the participant (see Figure 4). We first conducted a group x stimulus x context ANOVA for craving at T1 (before approaching the target stimulus) to allow for direct comparisons with the pleasure and arousal ratings. Then, we further analyzed the effect of time on craving by conducting a group x stimulus x context x time ANOVA and restricted follow-up analyses to significant effects involving the factor of time.
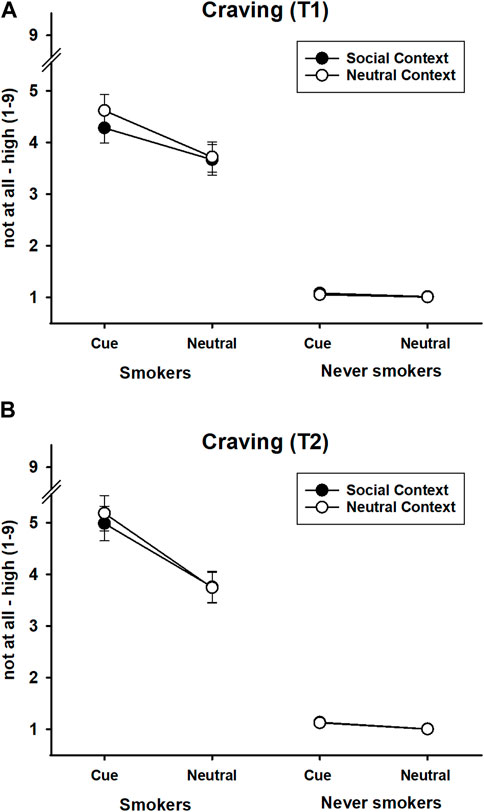
FIGURE 4. Craving in response to smoking and neutral stimuli presented in a social or neutral context in smokers and never-smokers. (A) Craving at T1, before initiation of the approach response to the target stimulus (cigarette vs. pencil). (B) After completion of the approach response, holding the target stimulus (cigarette vs. pencil) in the virtual hand. Error bars represent standard errors of the mean.
The ANOVA for craving at T1 returned significant main effects of group, F(1,63) = 114.63, p < 0.001, ηp2 = 0.65, stimulus, F(1,63) = 37.58, p < 0.001, ηp2 = 0.37, context, F(1,63) = 5.48, p = 0.022, ηp2 = 0.08; significant two-way interactions of group x stimulus, F(1,63) = 27.80, p < 0.001, ηp2 = 0.31, group x context, F(1,63) = 8.11, p = 0.006, ηp2 = 0.11; and stimulus x context, F(1,63) = 6.05, p = 0.017, ηp2 = 0.09. Importantly, these results were qualified by a reliable three-way interaction of group x stimulus x context, F(1,63) = 8.33, p = 0.005, ηp2 = 0.117.
A 2 × 2 follow-up ANOVA in the group of smokers revealed significant effects of stimulus, F(1,31) = 34.17, p < 0.001, ηp2 = 0.52, context, F(1,31) = 6.74, p = 0.014, ηp2 = 0.18, and stimulus x context, F(1,31) = 7.21, p = 0.012, ηp2 = 0.19. Smoking stimuli evoked greater craving than neutral stimuli. Importantly, social context reduced the craving response to smoking stimuli, t(31) = 3.29, p = 0.003, but not to neutral stimuli, t(31) = 0.640, p = 0.527. In other words, smoking cue reactivity was stronger in the neutral than in the social context (see Figure 4A). The corresponding follow-up analysis in the group of never-smokers returned no significant effects: all Fs(1,32) < 2.43, all ps > 0.129, all ηp2s < 0.07.
The ANOVA of the effect of time on craving revealed significant effects of group, F(1,63) = 124.81, p < 0.001, ηp2 = 0.67, stimulus, F(1,63) = 42.57, p < 0.001, ηp2 = 0.40, and time, F(1,63) = 23.94, p < 0.001, ηp2 = 0.28, and a marginally significant effect of context, F(1,63) = 3.51, p = 0.065, ηp2 = 0.05. All two-way interactions were significant: group x time, F(1,63) = 17.22, p < 0.001, ηp2 = 0.22; group x stimulus, F(1,63) = 29.57, p < 0.001, ηp2 = 0.32; group x context, F(1,63) = 5.10, p = 0.027, ηp2 = 0.08; stimulus x time, F(1,63) = 25.65, p < 0.001, ηp2 = 0.29; context x time, F(1,63) = 5.82, p = 0.019, ηp2 = 0.09, and stimulus × context, F(1,63) = 5.38, p = 0.024, ηp2 = 0.08. Also, the three-way interactions between group x stimulus x time, F(1,63) = 15.97, p < 0.001, ηp2 = 0.20; group x context x time, F(1,63) = 9.12, p = 0.004, ηp2 = 0.13; and group x stimulus x context, F(1,63) = 6.86, p = 0.011, ηp2 = 0.10, were significant. The four-way interaction between group x stimulus x context x time missed significance, F(1,63) = 2.76, p = 0.102, ηp2 = 0.04.
To follow-up on the significant three-way interactions involving the factor time, separate analyses in the groups of smokers and never-smokers were conducted. A stimulus x time ANOVA in the group of smokers revealed significant effects of stimulus, F(1,31) = 37.31, p < 0.001, ηp2 = 0.55, time, F(1,31) = 20.67, p < 0.001, ηp2 = 0.40, and stimulus x time, F(1,31) = 21.61, p < 0.001, ηp2 = 0.41. Smoking stimuli evoked stronger craving than neutral stimuli at both T1, t(31) = 5.85, p < 0.001, and T2, t(31) = 5.99, p < 0.001. However, craving to smoke increased from T1 to T2 only in case of smoking stimuli, t(31) = 4.74, p < 0.001, but not in case of neutral stimuli, t(31) = 1.55, p = 0.131. In never-smokers, the corresponding ANOVA returned a significant effect of stimulus, F(1,32) = 4.38, p = 0.044, ηp2 = 0.12, and marginally significant effects of time, F(1,32) = 3.43, p = 0.073, ηp2 = 0.10, and stimulus x time, F(1,32) = 3.71, p = 0.063, ηp2 = 0.10. Overall, smoking stimuli evoked slightly more craving than neutral stimuli, which was only evident as a minimal deviation from the endpoint of the scale (no craving). Interestingly, the stimulus effect in never-smokers appeared to be mainly driven by the virtual contact with the cigarette. Smoking stimuli appeared to elicit slightly stronger craving than neutral stimuli at T2, t(32) = 2.26, p = 0.031, but not at T1, t(32) = 1.56, p = 0.129. Similarly, craving to smoke marginally increased from T1 to T2 in case of smoking stimuli, t(32) = 1.92, p = 0.062, but not in case of neutral stimuli, t(32) = 1.00, p = 0.325. Given the selection criteria of the present study, it might be possible that this effect reflects previous, but rare, experiences with smoking. Regarding the interaction of group x context x time, the follow-up ANOVA for context x time in the group of smokers returned significant effects of context, F(1,31) = 4.22, p = 0.049, ηp2 = 0.12; time F(1,31) = 20.67, p < 0.001, ηp2 = 0.40; and context x time, F(1,31) = 7.95, p = 0.008, ηp2 = 0.20. The social context evoked lower craving than the neutral context only at T1, t(31) = 2.60, p = 0.014, but had no reliable effect at T2, t(31) = 1.33, p = 0.192. In never-smokers, the corresponding ANOVA revealed only a trend for an effect of time, F(1,32) = 3.43, p = 0.073, ηp2 = 0.10, evident as higher craving at T2. The other effects did not reach significance, all Fs(1,32) < 2.10, all ps > 0.160, all ηp2s < 0.06.
The ANOVA of the pleasure ratings revealed significant main effects of stimulus, F(1,63) = 11.23, p = 0.001, ηp2 = 0.15, and context, F(1,63) = 19.76, p < 0.001, ηp2 = 0.24. In addition, the ANOVA returned a significant interaction of group x stimulus, F(1,63) = 13.55, p < 0.001, ηp2 = 0.18, and a trend for a group × context interaction, F(1,63) = 3.56, p = 0.064, ηp2 = 0.05. Other effects did not reach significance, all Fs(1,63) < 2.47, all ps > 0.121, all ηp2s < 0.04. Never-smokers rated the smoking stimuli as less pleasant than the neutral stimuli, t(32) = 4.41, p < 0.001. In contrast, smokers did not discriminate between smoking and neutral stimuli, t(31) = 0.28, p = 0.783. Furthermore, both groups rated the social context as less pleasant than the neutral context, although this effect appeared to be slightly less pronounced in smokers, t(31) = 2.78, p = 0.009, than in never-smokers, t(32) = 3.61, p = 0.001 (see Figure 5A).

FIGURE 5. Pleasure (A) and arousal (B) in response to smoking and neutral stimuli presented in a social or neutral context in smokers and never-smokers. Error bars represent standard errors of the mean.
Regarding the arousal data, the ANOVA returned significant effects of group, F(1,63) = 7.25, p = 0.009, ηp2 = 0.10; stimulus, F(1,63) = 10.21, p = 0.002, ηp2 = 0.14; and context; F(1,63) = 13.28, p = 0.001, ηp2 = 0.17. Other effects were not significant, all Fs(1,63) < 1.83, all ps > 0.181, all ηp2s < 0.03. Both groups rated smoking stimuli as more arousing than neutral stimuli and the social context as more arousing than the neutral context. Generally, smokers reported higher arousal than never-smokers (see Figure 5B).
The ANOVA of the startle data returned only a significant interaction of group x context, F(1,63) = 4.46, p = 0.039, ηp2 = 0.07. All other effects failed to reach significance (all other Fs(1,63) < 1.20, all ps > 0.278, all ηp2s < 0.02). The interaction was mainly driven by a marginally significant effect of context in the group of never-smokers, t(32) = 1.94, p = 0.061, which was evident as a potentiation of the startle response in the social context (see Figure 6A). The corresponding comparison was not significant in the group of smokers, t(31) = 0.99, p = 0.330.
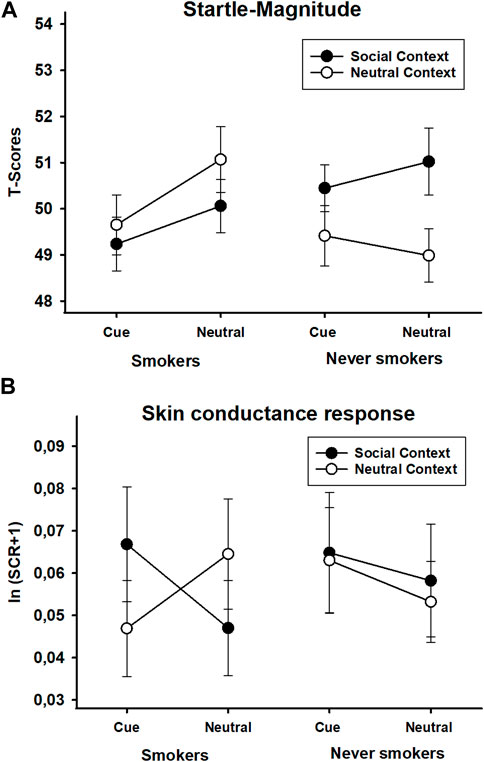
FIGURE 6. Startle magnitude (T-scores) (A) and skin conductance responses [ln (SCR+1)] (B) during smoking and neutral stimuli presented in a social or neutral context in smokers and never-smokers. Error bars represent standard errors of the mean.
The ANOVA of the skin conductance data revealed a significant interaction of group x context, F(1,63) = 4.84, p = 0.031, ηp2 = 0.07, which was qualified by a reliable interaction of group x stimulus x context, F(1,63) = 6.82, p = 0.011, ηp2 = 0.01. The other effects did not reach significance, all Fs(1,63) < 0.70, all ps > 0.405, all ηp2s < 0.02. The 2 × 2 ANOVA in the group of smokers resulted only in a significant interaction of stimulus x context, F(1,31) = 12.03, p = 0.002, ηp2 = 0.28 (all other Fs(1,32) < 0.87, all other ps > 0.359, all other ηp2s < 0.03). Follow-up tests showed that compared to neutral stimuli, skin conductance responses to smoking stimuli were slightly lower in the neutral context, t(31) = 2.58, p = 0.015, but higher in the social context, t(31) = 1.99, p = 0.056. Separate comparisons of stimulus effects for each context revealed trends for an increased response to smoking stimuli, t(31) = 1.84, p = 0.075, and a decreased response to neutral stimuli in the social context, t(31) = 1.73, p = 0.093, respectively. Thus, social context affected the responses to smoking and neutral stimuli in opposite ways (see Figure 6B). The corresponding analysis in the group of never-smokers yielded no reliable effects, all Fs(1,32) < 0.87, all ps > 0.359, all ηp2s < 0.03.
The analysis of the heart rate data revealed a main effect of group, F(1,63) = 2.96, p = 0.090, ηp2 = 0.05, and a group × stimulus interaction, F(1,63) = 3.75, p = 0.057, ηp2 = 0.06, of marginal significance. All other effects did not reach significance, all Fs(1,31) < 1.27, all ps > 0.264, all ηp2s < 0.02. The main effect of the group was evident as a generally increased heart rate response in smokers compared to never-smokers. The group × stimulus interaction was mainly driven by a trend for higher heart rate responses to smoking stimuli compared to neutral stimuli in the group of smokers, t(31) = 2.00, p = 0.055, which was not seen in the group of never-smokers, t(32) = 0.85, p = 0.404 (see Figure 7A).
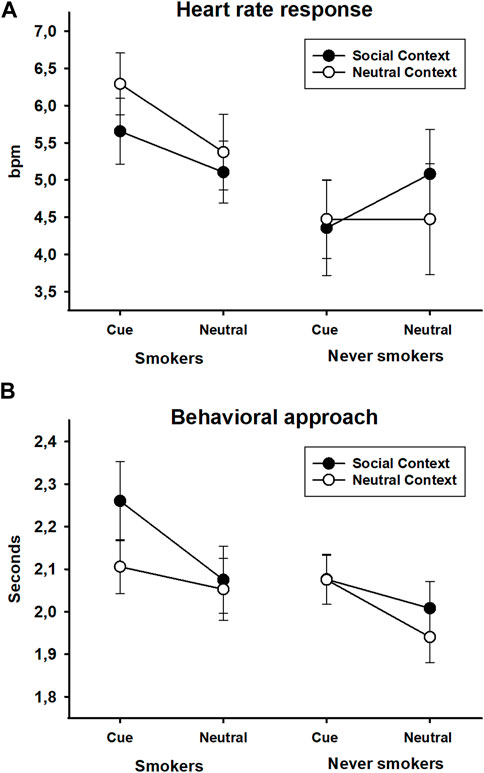
FIGURE 7. Heart rate responses (bpm) (A) and behavioral approach responses (time to target contact in seconds) (B) to smoking and neutral stimuli presented in a social or neutral context in smokers and never-smokers. Error bars represent standard errors of the mean.
The ANOVA of latency to approach the target stimulus revealed significant effects of stimulus, F(1,63) = 8.94, p = 0.004, ηp2 = 0.12, and context, F(1,63) = 7.18, p = 0.009, ηp2 = 0.10, which were qualified by a three-way interaction of group x stimulus x context, F(1,63) = 7.58, p = 0.008, ηp2 = 0.11. All other effects were not reliable, all Fs(1,31) < 1.39, all ps > 0.243, all ηp2s < 0.02. A 2 × 2 ANOVA in the group of smokers yielded a marginally significant main effect of stimulus, F(1,31) = 3.40, p = 0.075, ηp2 = 0.10, a significant effect of context, F(1,31) = 4.64, p = 0.039, ηp2 = 0.13, and a significant context × stimulus interaction, F(1,31) = 5.02, p = 0.032, ηp2 = 0.14. In the social context, smokers showed a delayed response to approaching smoking stimuli compared to neutral stimuli, t(31) = 2.61, p = 0.014, which was not evident in the neutral context, t(31) = 0.56, p = 0.579. The corresponding analysis in never-smokers only returned a significant effect of stimulus, F(1,32) = 7.59, p = 0.010, ηp2 = 0.19. The other effects were not reliable, all Fs(1,31) < 2.61, all ps > 0.116, all ηp2s < 0.08. Independent of the context, never-smokers were slower to approach smoking stimuli compared to neutral stimuli (see Figure 7B).
The present study investigated the impact of social context on smoking cue reactivity in virtual reality. Smokers and never-smokers were exposed to proximal smoking cues and neutral stimuli in a social and neutral context, respectively. To our knowledge, this study is the first to have systematically probed the modulatory effects of social context on the reactivity evoked by proximal smoking stimuli as assessed by self-reported, physiological, and behavioral measures. In line with our hypothesis, social context specifically affected cue-elicited craving to smoke and cue-directed behavioral approach in smokers. However, contrary to our assumption, the presence of a virtual agent decreased the craving response to proximal smoking cues and increased the latency to contact the cigarette on the table with the virtual hand. Moreover, cue-evoked craving was increased when holding the cigarette in the virtual hand.
Regarding cue-evoked craving, the present study met several benchmarks in the literature and extended previous research. First, our results showed that proximal smoking cues trigger robust craving in smokers, which is consistent with previous studies using virtual reality (Pericot-Valverde et al., 2016) or more traditional stimulus presentation methods (Betts et al., 2021). Second, we found an increase in cue-evoked craving when the participants held the virtual cigarette in their virtual hand. These results may partly mirror previous observations that smoking a virtual cigarette increases craving in smokers (Garcia-Rodriguez et al., 2013) and may point to the dependency of cue effects on the perceived proximity to smoke intake (Mucha et al., 2008; Stippekohl et al., 2010). Third, and most importantly, we provided evidence for the modulation of cue reactivity by social context, which was evident in decreased cue-evoked craving and behavioral approach in the group of smokers. This effect was found in the absence of explicit social pressure or support applied by the virtual agent (Cho et al., 2008; Lee et al., 2008; Pfaller et al., 2021) and only in the presence of discrete smoking cues. These findings critically extend previous research with pictorial social stimuli associated with smoking and not smoking, which directly increased or decreased craving in the absence of discrete smoking cues (Conklin et al., 2013). Our results may be relevant for both basic and clinical research as they suggest that cue-evoked craving and approach may be affected by apparently “inert” (contextual) stimuli, which do not evoke intense craving by themselves (Mucha et al., 2008). The present findings further suggest that measures of overt behavioral approach and avoidance, which are less frequently implemented in virtual reality-based cue reactivity paradigms, may show promise for future research and treatment (Girard et al., 2009; Kim and Lee, 2019; Machulska et al., 2021). Previous research with speeded reaction time tasks demonstrated that pictures of discrete smoking cues may evoke a stronger approach bias in smokers than in never-smokers (Wiers et al., 2013; Machulska et al., 2015), which can be modified by training (Wittekind et al., 2019). Moreover, results from the cue availability paradigm indicate that the latency to approach a naturalistic proximal smoking cue (a real cigarette) critically depends on the probability of actual consumption. In particular, smokers were shown to be faster to approach a cigarette when the probability for smoke intake was high, whereas no differences in the control condition (Carter and Tiffany, 2001) or even opposite effects (Bailey et al., 2010) were found when smoking was unavailable. The results of the present study add to and extend these findings by showing that social context has a significant impact on cue-evoked overt behavioral approach, which may be related to a perceived reduction of the probability to consume. Finally, the skin conductance data also revealed differential effects of social context in the group of smokers only. Previous research established skin conductance as a sensitive measure of autonomic arousal and orientation in preparation for action (Bradley, 2009; Betts et al., 2021). In the present study, skin conductance responses to the proximal smoking cues were increased in the presence and decreased in the absence of a virtual agent, respectively, which may indicate higher resource mobilization in case of the former. Increases in skin conductance have also been related to the anticipation of response outcomes during risky decision-making (Dawson et al., 2011), which might point to the engagement of processes involved in the assessment of a social situation.
Regarding the putative mechanisms involved, it may be fruitful to discuss our findings in light of previous research with pictorial stimuli in smokers. Recent work by Conklin et al. (2019) demonstrated potentiation of drug-directed responding when proximal smoking stimuli were presented in combination with (personalized) smoking environments, as indicated by an increase in craving, a decrease in the latency to initiate smoking, and invigoration of smoke intake. In discussing their results, the authors address the predictive relationship of discrete and contextual stimuli to smoking and drug availability. Thus, combining two smoking-associated stimuli may have had additive effects on craving and smoking by increasing the signaled availability of the drug. Although summation of drug-directed responding is not always seen when two drug-associated stimuli are combined (Mucha et al., 2008), there are cases in the animal literature, which indicate additive effects (Panlilio et al., 1996). Furthermore, human research suggests that cue reactivity increases with the perceived availability of the drug (Wertz and Sayette, 2001; Bailey et al., 2010). Thus, a decrease in signaled smoke availability may partly account for the reduction of craving during incongruent stimulus–context combinations, with the lowest craving being reported when neutral stimuli were presented in an environment (almost) not related to smoking (Conklin et al., 2019). Interestingly, there are reports in the animal literature, which suggest that stimuli explicitly associated with the absence of drug reinforcement may not simply function as “neutral” stimuli but may acquire inhibitory properties and suppress drug-seeking (Kearns et al., 2005). The results from the present study extend this line of research on the combined effects of smoking-associated environments and stimuli by showing that social context moderates the reactivity to proximal smoking cues. Although the mechanisms involved in the attenuation of cue-evoked craving and approach in the present study are hypothetical, the presence of an unfamiliar virtual agent may have resulted in a reduction of the perceived opportunity to engage in smoking. Previous research suggests that personalized pictures of individuals a smoker regularly associates with smoking can function as cues for smoking and evoke robust cravings in comparison to pictures of strangers with an unknown relationship to smoking (Conklin et al., 2013). Interestingly, in this study, smokers reported the lowest craving in response to pictures of people they regularly associate with not smoking. Extending this line of research, the present study suggests that social stimuli not only affect craving per se but also modulate the reactivity evoked by proximal smoking cues, perhaps by reducing the perceived availability of smoking. Partly related are results provided by ecological momentary assessments, which showed that cigarette use falls below a baseline rate of smoking alone in the presence of others not smoking (Shiffman and Rathbun, 2011). The authors suggest that the effect may be mediated by the local creation of a non-smoking norm that results in compliant behavior. Thus, the presence of a non-smoking virtual agent in our study may have activated self-regulatory processes, which affected not only overt behavior but also the self-reported motivation for drug intake. Accordingly, the smokers in our study may have relied on several strategies (McRae and Gross, 2020) to downregulate cue reactivity, for instance, by actively controlling attentional resources (Yang et al., 2021), intentionally inhibiting craving (Brody et al., 2007), or (re-)interpreting the situation (Wu et al., 2015). However, animal studies also provide rich evidence for the impact of social stimuli on drug-seeking and intake, which may not necessarily be mediated by higher-order cognitive processes (Strickland and Smith, 2014).
Regarding clinical implications, the present paradigm may be informative for the enhancement of cue exposure-based therapies in virtual reality (Segawa et al., 2019). Cue exposure treatments commonly follow the rationale of extinguishing cue reactivity by repeatedly exposing patients to drug-associated cues without the delivery of the drug. There is considerable evidence that extinction learning does not simply erase the association between the drug and associated stimuli, but involves a new form of learning, which is highly context dependent (Conklin and Tiffany, 2002). Thus, individuals with substance use disorders might frequently relapse after having conducted cue exposure therapy in a new (social) environment, e.g., in the presence of a therapist in a therapist’s office unrelated to smoking, when reentering a (social) context previously associated with the drug (Crombag et al., 2008). The results of the present experiment suggest that it may be important to carefully consider the effects of social context in cue exposure situations. Following the rationale of the study, we expected to find increased cue reactivity when proximate smoking cues were embedded in a social context, which could be a promising approach to creating strong cravings in smokers undergoing cue exposure treatment. In contrast to our hypotheses, the social context specifically decreased cue-evoked craving and behavioral approach. These findings suggest that it might be fruitful to extend the therapeutic focus to stimuli that do not directly trigger (strong) craving by themselves, although they can nevertheless modulate the effects of proximate drug cues. Furthermore, it might be important to carefully consider the effects of social context when it comes to the development of graded exposure hierarchies. In this regard, it might be useful to use people individually associated with smoking in virtual exposure paradigms to boost the reactivity to proximal smoking cues (Conklin et al., 2013). Alternatively, it might be important to further investigate the putative protective functions of individuals explicitly associated with not smoking or functioning as attachment figures for smokers (Le et al., 2020). Furthermore, the present study showed an increase in cue-evoked craving when participants were holding the cigarette in their virtual hand. Thus, manipulating the perceived proximity to smoking might have a significant effect on cue reactivity in smokers (Mucha et al., 2008), which emphasizes the need to incorporate opportunities to interact with proximal smoking stimuli in a virtual environment. Extinguishing the association between drug-associated stimuli and behavioral approach and consumption or replacing the maladaptive behavior with a newly acquired alternative response may have a beneficial impact on treatment. For instance, seminal previous work reported that crushing virtual cigarettes had a positive effect on nicotine dependence, abstinence, and dropout rates (Girard et al., 2009). Recently, implementations of approach-avoidance trainings in virtual reality received heightened interest and showed some promising results (Kim and Lee, 2019; Mellentin et al., 2020; Machulska et al., 2021).
Interestingly, in the present study, the effects of social context on cue-evoked craving to smoke were partly decoupled from self-reported pleasure and arousal. Thus, social context decreased subjective pleasure and increased self-reported arousal in both groups. However, the context manipulation did not interact with the responses to the proximal smoking stimuli, and the impact on self-reported pleasure was less pronounced in the group of smokers. This may indicate either that these measures were less sensitive to the experimental manipulation or that the mechanisms involved in the modulation of cue-evoked craving may target different processes. For instance, although there are indications that proximal smoking cues may enhance self-reported pleasure in smokers (Stippekohl et al., 2010), in particular when smoking appears to be available (Bailey et al., 2010), this effect is not always seen (Mucha et al., 1999), and theoretical reasons may account for this (Robinson and Berridge, 1993). In interpreting the results of the physiological measures in the present study, it might be important to note that the stimulus parameters were basically derived from passive picture viewing paradigms (Lang, 1995) and translated to more complex, three-dimensional environments, including higher degrees of freedom (Müller et al., 1998; Löw et al., 2008). Nevertheless, the present study revealed a general effect of social context in the form of a trend for a potentiation of the startle response in never-smokers, indicative of negative affect (Lang, 1995), which was also seen in the rating data. In this regard, it might be informative that several never-smokers in our study reached an extreme score on the Social Phobia Inventory, which may account for these effects (Boecker and Pauli, 2019). Furthermore, the smoking group showed a trend for an increase in heart rate during exposure to proximal smoking cues, which may indicate heightened physiological arousal. Similar results were reported previously in smokers while “smoking” a virtual cigarette in a virtual pub (Garcia-Rodriguez et al., 2013), although the drug-associated environment had no influence on heart rate by itself. Another study reported an increase in heart rate in deprived smokers during specific exposure situations in complex drug-associated scenarios (Thompson-Lake et al., 2015), while Garcia-Rodriguez et al. (2012) found a partial decrease during exposure to smoking-associated environments in non-deprived smokers. Overall, these results emphasize the need to further investigate the impact of group characteristics (e.g., degree of nicotine dependence and deprivation) and the effects of specific stimulus configurations on drug-directed responding.
Areas for improvement and further research comprise the sample size of the present study, which could be increased to enhance statistical power to reliably detect more subtle effects. Also, the implementation of the social and environmental contexts could receive further attention. The virtual room used in our study was a simulation of the real laboratory where the experiment took place. In Germany, smoking is normally not allowed in public buildings, which may have diminished cue reactivity (Mucha et al., 2008; Lu et al., 2022). It is also possible that this environment affected the modulation of cue effects by social context, for instance, in a direction opposite to environments where social smoking frequently takes place. Regarding the basic mechanisms mediating the effect of social context on cue reactivity, it might be informative to probe the dynamic time course of visual attention, e.g., via eye-tracking. Drug-associated stimuli are commonly hypothesized to capture the attention of users (Field et al., 2014), which might compete with the processing of social stimuli (Cho et al., 2008; Wechsler et al., 2021), in particular when drug use appears to be imminent. Furthermore, in our study, the participants were not familiar with the virtual agent, which may represent a new social context only partially resembling the situations smokers are used to smoking or not smoking in. Thus, one could provide explicit information about the smoking status of the agent, attitudes toward smoking, or the availability of smoking to disambiguate the situation. Moreover, recent developments in VR technology may allow to present smokers with personalized models of individuals (Waltemate et al., 2018) they routinely engage in or refrain from smoking with (Conklin et al., 2013; Le et al., 2020), which could be used to probe their impact on cue reactivity and to improve strategies for treatment.
In sum, the present study crucially extends laboratory-based human research on the effects of social context on the motivation to smoke and smoking-related behavior by revealing a modulatory effect of social context on the reactivity evoked by proximal smoking cues. Previous work suggests that pictures of people individually associated with smoking or not smoking can enhance or decrease craving independently from the presence of proximal cues for smoking. The current study broadens the scope of this line of research by revealing a decrease in cue-evoked craving and behavioral approach tendencies only in the presence of a virtual agent. These findings may contribute to a better understanding of the interplay between social contexts and proximate drug cues in the stimulus control of addictive behaviors. In unraveling the underlying mechanisms, it might be fruitful for future research to address social context-dependent changes in the perceived availability of drug intake, which may provide a link to inhibitory processes.
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The study was carried out in accordance with the ethical standards of the fifth revision of the Declaration of Helsinki. The experimental procedures are comparable to previous designs approved by the ethics committee of the German Psychological Association (DGPs). The patients/participants provided their written informed consent to participate in this study.
MW and AM contributed to the concept, design, and implementation of the study. They supervised the collection and pre-processing of the data. MW analyzed the data and drafted the manuscript. All authors contributed to the article by providing critical revisions and feedback. All authors approved the submitted version and agreed to be accountable for the content of the work.
This study was partially supported by the German Research Foundation (DFG), the research group “Emotion and Behavior” (FOR 605, PA 566/9-1, PA 566/9-2), and the Sino-German Center for Research Promotion (M-0039). This publication was supported by the Open Access Publication Fund of the University of Würzburg.
The authors are very grateful to Julia Kreß for her distinguished contribution to this study. This article is partly based on her diploma thesis submitted to the University of Würzburg, which was supervised by AM, PP, and MW. The authors also thank Henrik M. Peperkorn and Mathias Müller (VTplus GmbH) for excellent technical support in implementing the virtual paradigm.
AM and PP are shareholders of a commercial company (VTplus GmbH), which develops medical devices for virtual reality exposure and virtual environment research systems for empirical studies in the fields of psychology, psychiatry, and psychotherapy.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frvir.2023.926679/full#supplementary-material
Babb, S., Malarcher, A., Schauer, G., Asman, K., and Jamal, A. (2017). Quitting smoking among adults - United States, 2000-2015. Mmwr-Morbidity Mortal. Wkly. Rep. 65 (52), 1457–1464. doi:10.15585/mmwr.mm6552a1
Bailey, S. R., Goedeker, K. C., and Tiffany, S. T. (2010). The impact of cigarette deprivation and cigarette availability on cue-reactivity in smokers. Addiction 105 (2), 364–372. doi:10.1111/j.1360-0443.2009.02760.x
Baker, T. B., Brandon, T. H., and Chassin, L. (2004). Motivational influences on cigarette smoking. Annu. Rev. Psychol. 55, 463–491. doi:10.1146/annurev.psych.55.090902.142054
Bardo, M. T., Neisewander, J. L., and Kelly, T. H. (2013). Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev. 65 (1), 255–290. doi:10.1124/pr.111.005124
Berridge, K. C., and Robinson, T. E. (2016). Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 71 (8), 670–679. doi:10.1037/amp0000059
Betts, J. M., Dowd, A. N., Forney, M., Hetelekides, E., and Tiffany, S. T. (2021). A meta-analysis of cue reactivity in tobacco cigarette smokers. Nicotine Tob. Res. 23 (2), 249–258. doi:10.1093/ntr/ntaa147
Blumenthal, T. D., Cuthbert, B. N., Filion, D. L., Hackley, S., Lipp, O. V., and van Boxtel, A. (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42 (1), 1–15. doi:10.1111/j.1469-8986.2005.00271.x
Boecker, L., and Pauli, P. (2019). Affective startle modulation and psychopathology: Implications for appetitive and defensive brain systems. Neurosci. Biobehav Rev. 103, 230–266. doi:10.1016/j.neubiorev.2019.05.019
Bradley, M. M. (2009). Natural selective attention: Orienting and emotion. Psychophysiology 46 (1), 1–11. doi:10.1111/j.1469-8986.2008.00702.x
Brody, A. L., Mandelkern, M. A., Olmstead, R. E., Jou, J., Tiongson, E., Allen, V., et al. (2007). Neural substrates of resisting craving during cigarette cue exposure. Biol. Psychiatry 62 (6), 642–651. doi:10.1016/j.biopsych.2006.10.026
Carter, B. L., and Tiffany, S. T. (2001). The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Exp. Clin. Psychopharmacol. 9 (2), 183–190. doi:10.1037//1064-1297.9.2.183
Carver, C. S., and White, T. L. (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Personality Soc. Psychol. 67 (2), 319–333. doi:10.1037/0022-3514.67.2.319
Chesworth, R., and Corbit, L. H. (2017). Recent developments in the behavioural and pharmacological enhancement of extinction of drug seeking. Addict. Biol. 22 (1), 3–43. doi:10.1111/adb.12337
Cho, S., Ku, J., Park, J., Han, K., Lee, H., Choi, Y. K., et al. (2008). Development and verification of an alcohol craving-induction tool using virtual reality: Craving characteristics in social pressure situation. Cyberpsychol Behav. 11 (3), 302–309. doi:10.1089/cpb.2007.0149
Conklin, C. A. (2006). Environments as cues to smoke: Implications for human extinction-based research and treatment. Exp. Clin. Psychopharmacol. 14 (1), 12–19. doi:10.1037/1064-1297.14.1.12
Conklin, C. A., McClernon, F. J., Vella, E. J., Joyce, C. J., Salkeld, R. P., Parzynski, C. S., et al. (2019). Combined smoking cues enhance reactivity and predict immediate subsequent smoking. Nicotine Tob. Res. 21 (2), 241–248. doi:10.1093/ntr/nty009
Conklin, C. A., Perkins, K. A., Robin, N., McClernon, F. J., and Salkeld, R. P. (2010). Bringing the real world into the laboratory: Personal smoking and nonsmoking environments. Drug Alcohol Depend. 111 (1-2), 58–63. doi:10.1016/j.drugalcdep.2010.03.017
Conklin, C. A., Robin, N., Perkins, K. A., Salkeld, R. P., and McClernon, F. J. (2008). Proximal versus distal cues to smoke: The effects of environments on smokers' cue-reactivity. Exp. Clin. Psychopharmacol. 16 (3), 207–214. doi:10.1037/1064-1297.16.3.207
Conklin, C. A., Salkeld, R. P., Perkins, K. A., and Robin, N. (2013). Do people serve as cues to smoke? Nicotine Tob. Res. 15 (12), 2081–2087. doi:10.1093/ntr/ntt104
Conklin, C. A., and Tiffany, S. T. (2002). Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97 (2), 155–167. doi:10.1046/j.1360-0443.2002.00014.x
Connor, K. M., Davidson, J. R., Churchill, L. E., Sherwood, A., Foa, E., and Weisler, R. H. (2000). Psychometric properties of the social phobia inventory (SPIN). New self-rating scale. Br. J. Psychiatry 176 (4), 379–386. doi:10.1192/bjp.176.4.379
Crombag, H. S., Bossert, J. M., Koya, E., and Shaham, Y. (2008). Context-induced relapse to drug seeking: A review. Philos. Trans. R. Soc. Lond B Biol. Sci. 363 (1507), 3233–3243. doi:10.1098/rstb.2008.0090
Dawson, M. E., Schell, A. M., and Courtney, C. G. (2011). The skin conductance response, anticipation, and decision-making. J. Neurosci. Psychol. Econ. 4 (2), 111–116. doi:10.1037/a0022619
de Wit, H., and Sayette, M. (2018). Considering the context: Social factors in responses to drugs in humans. Psychopharmacol. Berl. 235 (4), 935–945. doi:10.1007/s00213-018-4854-3
Dimoff, J. D., and Sayette, M. A. (2017). The case for investigating social context in laboratory studies of smoking. Addiction 112 (3), 388–395. doi:10.1111/add.13503
Doll, R., Peto, R., Boreham, J., and Sutherland, I. (2004). Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 328 (7455), 1519. doi:10.1136/bmj.38142.554479.AE
Field, M., Marhe, R., and Franken, I. H. A. (2014). The clinical relevance of attentional bias in substance use disorders. Cns Spectrums 19 (3), 225–230. doi:10.1017/S1092852913000321
Gamito, P., Oliveira, J., Baptista, A., Pereira, E., Morais, D., Saraiva, T., et al. (2011). Virtual reality exposure on nicotine craving. Stud. Health Technol. Inf. 167, 63–68.
Garcia-Rodriguez, O., Pericot-Valverde, I., Gutierrez-Maldonado, J., Ferrer-Garcia, M., and Secades-Villa, R. (2012). Validation of smoking-related virtual environments for cue exposure therapy. Addict. Behav. 37 (6), 703–708. doi:10.1016/j.addbeh.2012.02.013
Garcia-Rodriguez, O., Weidberg, S., Gutierrez-Maldonado, J., and Secades-Villa, R. (2013). Smoking a virtual cigarette increases craving among smokers. Addict. Behav. 38 (10), 2551–2554. doi:10.1016/j.addbeh.2013.05.007
Girard, B., Turcotte, V., Bouchard, S., and Girard, B. (2009). Crushing virtual cigarettes reduces tobacco addiction and treatment discontinuation. Cyberpsychol Behav. 12 (5), 477–483. doi:10.1089/cpb.2009.0118
Gray, J. A. (1990). Brain systems that mediate both emotion and cognition. Cognition Emot. 4 (3), 269–288. doi:10.1080/02699939008410799
Harakeh, Z., and Vollebergh, W. A. M. (2011). Actions speak louder than words: An experiment on the impact of peers discouraging young adult smoking. Eur. Addict. Res. 17 (6), 316–320. doi:10.1159/000330318
Harakeh, Z., and Vollebergh, W. A. (2012). The impact of active and passive peer influence on young adult smoking: An experimental study. Drug Alcohol Depend. 121 (3), 220–223. doi:10.1016/j.drugalcdep.2011.08.029
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., and Fagerstrom, K. O. (1991). The fagerstrom test for nicotine dependence: A revision of the fagerstrom tolerance questionnaire. Br. J. Addict. 86 (9), 1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x
Heilig, M., Epstein, D. H., Nader, M. A., and Shaham, Y. (2016). Time to connect: Bringing social context into addiction neuroscience. Nat. Rev. Neurosci. 17 (9), 592–599. doi:10.1038/nrn.2016.67
Hogarth, L., Dickinson, A., and Duka, T. (2010). The associative basis of cue-elicited drug taking in humans. Psychopharmacol. Berl. 208 (3), 337–351. doi:10.1007/s00213-009-1735-9
Hone-Blanchet, A., Wensing, T., and Fecteau, S. (2014). The use of virtual reality in craving assessment and cue-exposure therapy in substance use disorders. Front. Hum. Neurosci. 8, 844. doi:10.3389/fnhum.2014.00844
Jha, P., Ramasundarahettige, C., Landsman, V., Rostron, B., Thun, M., Anderson, R. N., et al. (2013). 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 368 (4), 341–350. doi:10.1056/NEJMsa1211128
Kearns, D. N., and Weiss, S. J. (2007). Contextual renewal of cocaine seeking in rats and its attenuation by the conditioned effects of an alternative reinforcer. Drug Alcohol Depend. 90 (2-3), 193–202. doi:10.1016/j.drugalcdep.2007.03.006
Kearns, D. N., Weiss, S. J., Schindler, C. W., and Panlilio, L. V. (2005). Conditioned inhibition of cocaine seeking in rats. J. Exp. Psychol. Anim. Behav. Process 31 (2), 247–253. doi:10.1037/0097-7403.31.2.247
Kennedy, R. S., Lane, N. E., Berbaum, K. S., and Lilienthal, M. G. (1993). Simulator sickness questionnaire: An enhanced method for quantifying simulator sickness. Int. J. Aviat. Psychol. 3 (3), 203–220. doi:10.1207/s15327108ijap0303_3
Kim, D. Y., and Lee, J. H. (2019). The effects of training to reduce automatic action tendencies toward alcohol using the virtual alcohol approach-avoidance task in heavy social drinkers. Cyberpsychology, Behav. Soc. Netw. 22 (12), 794–798. doi:10.1089/cyber.2019.0121
Koob, G. F., and Volkow, N. D. (2016). Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 3 (8), 760–773. doi:10.1016/S2215-0366(16)00104-8
Lang, P. J. (1980). “Behavioral treatment and bio-behavioral assessment: Computer applications,” in Technology in mental health care delivery systems. Editors J. B. Sidowski, J. H. Johnson, and T. A. Williams (Norwood: Ablex), 119–137.
Lang, P. J. (1995). The emotion probe. Studies of motivation and attention. Am. Psychol. 50 (5), 372–385. doi:10.1037//0003-066x.50.5.372
Langener, S., Van Der Nagel, J., van Manen, J., Markus, W., Dijkstra, B., De Fuentes-Merillas, L., et al. (2021). Clinical relevance of immersive virtual reality in the assessment and treatment of addictive disorders: A systematic review and future perspective. J. Clin. Med. 10 (16), 3658. doi:10.3390/jcm10163658
Le Foll, B., and Goldberg, S. R. (2005). Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacol. Berl. 178 (4), 481–492. doi:10.1007/s00213-004-2021-5
Le, T. L., Conklin, C. A., George, T. P., Levitan, R. D., Mann, R. E., and Maunder, R. G. (2020). Exposure to attachment figure cue reduces cigarette craving. Exp. Clin. Psychopharmacol. 28 (1), 81–86. doi:10.1037/pha0000284
LeCocq, M. R., Randall, P. A., Besheer, J., and Chaudhri, N. (2020). Considering drug-associated contexts in substance use disorders and treatment development. Neurotherapeutics 17 (1), 43–54. doi:10.1007/s13311-019-00824-2
Lee, J. S., Namkoong, K., Ku, J., Cho, S., Park, J. Y., Choi, Y. K., et al. (2008). Social pressure-induced craving in patients with alcohol dependence: Application of virtual reality to coping skill training. Psychiatry Investig. 5 (4), 239–243. doi:10.4306/pi.2008.5.4.239
Löw, A., Lang, P. J., Smith, J. C., and Bradley, M. M. (2008). Both predator and prey: Emotional arousal in threat and reward. Psychol. Sci. 19 (9), 865–873. doi:10.1111/j.1467-9280.2008.02170.x
Lu, W., Wu, Q., Liu, Y., Wang, Y., Wei, Z., Li, Y., et al. (2022). No smoking signs with strong smoking symbols induce weak cravings: An fMRI and EEG study. Neuroimage 252, 119019. doi:10.1016/j.neuroimage.2022.119019
Machulska, A., Eiler, T. J., Kleinke, K., Grunewald, A., Bruck, R., Jahn, K., et al. (2021). Approach bias retraining through virtual reality in smokers willing to quit smoking: A randomized-controlled study. Behav. Res. Ther. 141, 103858. doi:10.1016/j.brat.2021.103858
Machulska, A., Zlomuzica, A., Adolph, D., Rinck, M., and Margraf, J. (2015). A cigarette a day keeps the goodies away”: Smokers show automatic approach tendencies for smoking—but not for food-related stimuli. PloS one 10 (2), e0116464. doi:10.1371/journal.pone.0116464
McClernon, F. J., Conklin, C. A., Kozink, R. V., Adcock, R. A., Sweitzer, M. M., Addicott, M. A., et al. (2016). Hippocampal and insular response to smoking-related environments: Neuroimaging evidence for drug-context effects in nicotine dependence. Neuropsychopharmacology 41 (3), 877–885. doi:10.1038/npp.2015.214
Mellentin, A. I., Nielsen, A. S., Ascone, L., Wirtz, J., Samochowiec, J., Kucharska-Mazur, J., et al. (2020). A randomized controlled trial of a virtual reality based, approach-avoidance training updates program for alcohol use disorder: A study protocol. Bmc Psychiatry 20 (1), 1–12. doi:10.1186/s12888-020-02739-1
Mucha, R. F., Geier, A., and Pauli, P. (1999). Modulation of craving by cues having differential overlap with pharmacological effect: Evidence for cue approach in smokers and social drinkers. Psychopharmacol. Berl. 147 (3), 306–313. doi:10.1007/s002130051172
Mucha, R. F., Pauli, P., Weber, M., and Winkler, M. (2008). Smoking stimuli from the terminal phase of cigarette consumption may not be cues for smoking in healthy smokers. Psychopharmacol. Berl. 201 (1), 81–95. doi:10.1007/s00213-008-1249-x
Müller, V., Mucha, R. F., Ackermann, K., and Pauli, P. (2001). Die Erfassung des Cravings bei Rauchern mit einer deutschen Version des "Questionnaire on Smoking Urges" (QSU-G)/The assessment of craving in smokers with a German version of the "Questionnaire on Smoking Urges" (QSU-G). Z. fuer Klin. Psychol. Psychother. Forsch. Prax. 30 (3), 164–171. doi:10.1026/0084-5345.30.3.164
Müller, V., Mucha, R. F., and Pauli, P. (1998). Dependence on smoking and the acoustic startle response in healthy smokers. Pharmacol. Biochem. Behav. 59 (4), 1031–1038. doi:10.1016/s0091-3057(97)00508-x
Napier, T. C., Herrold, A. A., and de Wit, H. (2013). Using conditioned place preference to identify relapse prevention medications. Neurosci. Biobehav Rev. 37 (9), 2081–2086. doi:10.1016/j.neubiorev.2013.05.002
Panlilio, L. V., Weiss, S. J., and Schindler, C. W. (1996). Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacol. Berl. 125 (3), 202–208. doi:10.1007/BF02247329
Paris, M. M., Carter, B. L., Traylor, A. C., Bordnick, P. S., Day, S. X., Armsworth, M. W., et al. (2011). Cue reactivity in virtual reality: The role of context. Addict. Behav. 36 (7), 696–699. doi:10.1016/j.addbeh.2011.01.029
Peperkorn, H. M., Diemer, J. E., Alpers, G. W., and Mühlberger, A. (2016). Representation of patients' hand modulates fear reactions of patients with spider phobia in virtual reality. Front. Psychol. 7, 268. doi:10.3389/fpsyg.2016.00268
Pericot-Valverde, I., Germeroth, L. J., and Tiffany, S. T. (2016). The use of virtual reality in the production of cue-specific craving for cigarettes: A meta-analysis. Nicotine Tob. Res. 18 (5), 538–546. doi:10.1093/ntr/ntv216
Pericot-Valverde, I., Secades-Villa, R., Gutierrez-Maldonado, J., and Garcia-Rodriguez, O. (2014). Effects of systematic cue exposure through virtual reality on cigarette craving. Nicotine Tob. Res. 16 (11), 1470–1477. doi:10.1093/ntr/ntu104
Perusini, J. N., and Fanselow, M. S. (2015). Neurobehavioral perspectives on the distinction between fear and anxiety. Learn Mem. 22 (9), 417–425. doi:10.1101/lm.039180.115
Pfaller, M., Kroczek, L. O. H., Lange, B., Fülöp, R., Müller, M., and Mühlberger, A. (2021). Social presence as a moderator of the effect of agent behavior on emotional experience in social interactions in virtual reality. Front. Virtual Real. 2, 741138. doi:10.3389/frvir.2021.741138
Prochaska, J. J., and Benowitz, N. L. (2019). Current advances in research in treatment and recovery: Nicotine addiction. Sci. Adv. 5 (10), eaay9763. doi:10.1126/sciadv.aay9763
Reitsma, M. B., Fullman, N., Ng, M., Salama, J. S., Abajobir, A., Abate, K. H., et al. (2017). Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: A systematic analysis from the global burden of disease study 2015. Lancet 389 (10082), 1885–1906. doi:10.1016/S0140-6736(17)30819-X
Robinson, T. E., and Berridge, K. C. (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 18 (3), 247–291. doi:10.1016/0165-0173(93)90013-p
Russell, M., Peto, J., and Patel, U. (1974). The classification of smoking by factorial structure of motives. J. R. Stat. Soc. Ser. A General. 137 (3), 313–333. doi:10.2307/2344953
Schnoll, R. A., Goren, A., Annunziata, K., and Suaya, J. A. (2013). The prevalence, predictors and associated health outcomes of high nicotine dependence using three measures among US smokers. Addiction 108 (11), 1989–2000. doi:10.1111/add.12285
Schubert, T. W. (2003). The sense of presence in virtual environments: A three-component scale measuring spatial presence, involvement, and realness. Z. für Medien. 15 (2), 69–71. doi:10.1026//1617-6383.15.2.69
Sciascia, J. M., Reese, R. M., Janak, P. H., and Chaudhri, N. (2015). Alcohol-seeking triggered by discrete pavlovian cues is invigorated by alcohol contexts and mediated by glutamate signaling in the basolateral amygdala. Neuropsychopharmacology 40 (12), 2801–2812. doi:10.1038/npp.2015.130
Segawa, T., Baudry, T., Bourla, A., Blanc, J. V., Peretti, C. S., Mouchabac, S., et al. (2019). Virtual reality (VR) in assessment and treatment of addictive disorders: A systematic review. Front. Neurosci. 13, 1409. doi:10.3389/fnins.2019.01409
Shiffman, S., Dunbar, M. S., Li, X., Scholl, S. M., Tindle, H. A., Anderson, S. J., et al. (2014). Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One 9 (3), e89911. doi:10.1371/journal.pone.0089911
Shiffman, S., Li, X., Dunbar, M. S., Ferguson, S. G., Tindle, H. A., and Scholl, S. M. (2015). Social smoking among intermittent smokers. Drug Alcohol Dependence 154, 184–191. doi:10.1016/j.drugalcdep.2015.06.027
Shiffman, S., and Rathbun, S. L. (2011). Point process analyses of variations in smoking rate by setting, mood, gender, and dependence. Psychol. Addict. Behav. 25 (3), 501–510. doi:10.1037/a0022178
Siegel, S., Baptista, M. A., Kim, J. A., McDonald, R. V., and Weise-Kelly, L. (2000). Pavlovian psychopharmacology: The associative basis of tolerance. Exp. Clin. Psychopharmacol. 8 (3), 276–293. doi:10.1037//1064-1297.8.3.276
Skewes, M. C., and Gonzalez, V. M. (2013). The biopsychosocial model of addiction. Princ. Addict. 1, 61–70.
Smith, M. A., Zhang, H., and Robinson, A. M. (2016). The effects of excitatory and inhibitory social cues on cocaine-seeking behavior. Front. Behav. Neurosci. 10, 217. doi:10.3389/fnbeh.2016.00217
Sosic, Z., Gieler, U., and Stangier, U. (2008). Screening for social phobia in medical in- and outpatients with the German version of the Social Phobia Inventory (SPIN). J. Anxiety Disord. 22 (5), 849–859. doi:10.1016/j.janxdis.2007.08.011
Stevenson, J. G., Oliver, J. A., Hallyburton, M. B., Sweitzer, M. M., Conklin, C. A., and McClernon, F. J. (2017). Smoking environment cues reduce ability to resist smoking as measured by a delay to smoking task. Addict. Behav. 67, 49–52. doi:10.1016/j.addbeh.2016.12.007
Stippekohl, B., Winkler, M., Mucha, R. F., Pauli, P., Walter, B., Vaitl, D., et al. (2010). Neural responses to BEGIN- and END-stimuli of the smoking ritual in nonsmokers, nondeprived smokers, and deprived smokers. Neuropsychopharmacology 35 (5), 1209–1225. doi:10.1038/npp.2009.227
Strickland, J. C., and Smith, M. A. (2014). The effects of social contact on drug use: Behavioral mechanisms controlling drug intake. Exp. Clin. Psychopharmacol. 22 (1), 23–34. doi:10.1037/a0034669
Strobel, A., Beauducel, A., Debener, S., and Brocke, B. (2001). Eine deutschsprachige Version des BIS/BAS-Fragebogens von Carver und White. Z. für Differ. Diagn. Psychol. 22 (3), 216–227. doi:10.1024//0170-1789.22.3.216
Thewissen, R., Snijders, S. J., Havermans, R. C., van den Hout, M., and Jansen, A. (2006). Renewal of cue-elicited urge to smoke: Implications for cue exposure treatment. Behav. Res. Ther. 44 (10), 1441–1449. doi:10.1016/j.brat.2005.10.010
Thompson-Lake, D. G., Cooper, K. N., Mahoney, J. J., Bordnick, P. S., Salas, R., Kosten, T. R., et al. (2015). Withdrawal symptoms and nicotine dependence severity predict virtual reality craving in cigarette-deprived smokers. Nicotine Tob. Res. 17 (7), 796–802. doi:10.1093/ntr/ntu245
Tiffany, S. T., and Drobes, D. J. (1991). The development and initial validation of a questionnaire on smoking urges. Br. J. Addict. 86 (11), 1467–1476. doi:10.1111/j.1360-0443.1991.tb01732.x
Timberlake, W. (1994). Behavior systems, associationism, and Pavlovian conditioning. Psychon. Bull. Rev. 1 (4), 405–420. doi:10.3758/BF03210945
Trahan, M. H., Maynard, B. R., Smith, K. S., Farina, A. S. J., and Khoo, Y. M. (2019). Virtual reality exposure therapy on alcohol and nicotine: A systematic review. Res. Soc. Work Pract. 29 (8), 876–891. doi:10.1177/1049731518823073
US Department of Health Human Services (2014). The health consequences of smoking—50 years of progress: A report of the surgeon general. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion.
Valyear, M. D., Glovaci, I., Zaari, A., Lahlou, S., Trujillo-Pisanty, I., Andrew Chapman, C., et al. (2020). Dissociable mesolimbic dopamine circuits control responding triggered by alcohol-predictive discrete cues and contexts. Nat. Commun. 11 (1), 3764. doi:10.1038/s41467-020-17543-4
Venables, P. H., and Christie, M. J. (1980). “Electrodermal activity,” in Techniques in psychophysiology. Editors I. Martin, and P. H. Venables (New York: Wiley), 4–67.
Venniro, M., Banks, M. L., Heilig, M., Epstein, D. H., and Shaham, Y. (2020). Improving translation of animal models of addiction and relapse by reverse translation. Nat. Rev. Neurosci. 21 (11), 625–643. doi:10.1038/s41583-020-0378-z
Vollstädt-Klein, S., Nees, F., Wieland, A., Karl, D., Diener, C., Smolka, M. N., et al. (2022). Contexts enhance ratings of craving and psychophysiological responses of cue-reactivity in tobacco use disorder. medRxiv. doi:10.1101/2022.07.12.22277347
Waltemate, T., Gall, D., Roth, D., Botsch, M., and Latoschik, M. E. (2018). The impact of avatar personalization and immersion on virtual body ownership, presence, and emotional response. IEEE Trans. Vis. Comput. Graph 24 (4), 1643–1652. doi:10.1109/TVCG.2018.2794629
Wechsler, T. F., Pfaller, M., van Eickels, R. E., Schulz, L. H., and Muhlberger, A. (2021). Look at the audience? A randomized controlled study of shifting attention from self-focus to nonsocial vs. Social external stimuli during virtual reality exposure to public speaking in social anxiety. Front. Psychiatry 12, 751272. doi:10.3389/fpsyt.2021.751272
Wertz, J. M., and Sayette, M. A. (2001). A review of the effects of perceived drug use opportunity on self-reported urge. Exp. Clin. Psychopharmacol. 9 (1), 3–13. doi:10.1037/1064-1297.9.1.3
Wiers, C. E., Kuhn, S., Javadi, A. H., Korucuoglu, O., Wiers, R. W., Walter, H., et al. (2013). Automatic approach bias towards smoking cues is present in smokers but not in ex-smokers. Psychopharmacol. Berl. 229 (1), 187–197. doi:10.1007/s00213-013-3098-5
Winkler, M. H., Weyers, P., Mucha, R. F., Stippekohl, B., Stark, R., and Pauli, P. (2011). Conditioned cues for smoking elicit preparatory responses in healthy smokers. Psychopharmacol. Berl. 213 (4), 781–789. doi:10.1007/s00213-010-2033-2
Wise, R. A., and Koob, G. F. (2014). The development and maintenance of drug addiction. Neuropsychopharmacology 39 (2), 254–262. doi:10.1038/npp.2013.261
Wittekind, C. E., Reibert, E., Takano, K., Ehring, T., Pogarell, O., and Ruther, T. (2019). Approach-avoidance modification as an add-on in smoking cessation: A randomized-controlled study. Behav. Res. Ther. 114, 35–43. doi:10.1016/j.brat.2018.12.004
Wu, L., Winkler, M. H., Wieser, M. J., Andreatta, M., Li, Y., and Pauli, P. (2015). Emotion regulation in heavy smokers: Experiential, expressive and physiological consequences of cognitive reappraisal. Front. Psychol. 6, 1555. doi:10.3389/fpsyg.2015.01555
Wu, Y., Li, Y., Yang, X., and Sui, N. (2014). Differential effect of beta-adrenergic receptor antagonism in basolateral amygdala on reconsolidation of aversive and appetitive memories associated with morphine in rats. Addict. Biol. 19 (1), 5–15. doi:10.1111/j.1369-1600.2012.00443.x
Keywords: cue reactivity, social context, cue exposure therapy, cue availability, smoking, substance use disorders, addiction, virtual reality
Citation: Winkler MH, Li Y, Pauli P and Mühlberger A (2023) Modulation of smoking cue reactivity by social context—Implications for exposure therapy in virtual reality. Front. Virtual Real. 4:926679. doi: 10.3389/frvir.2023.926679
Received: 22 April 2022; Accepted: 08 February 2023;
Published: 08 March 2023.
Edited by:
Katie A. Ragsdale, Emory University, United StatesReviewed by:
Laura E. Watkins, Emory University, United StatesCopyright © 2023 Winkler, Li, Pauli and Mühlberger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Markus H. Winkler, bWFya3VzLndpbmtsZXJAdW5pLXd1ZXJ6YnVyZy5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.