
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Virol., 20 February 2025
Sec. Antivirals and Vaccines
Volume 5 - 2025 | https://doi.org/10.3389/fviro.2025.1537821
 Benedetta Trentacapilli1*
Benedetta Trentacapilli1* Angelo Roberto Raccagni1
Angelo Roberto Raccagni1 Sara Diotallevi2
Sara Diotallevi2 Silvia Nozza1,2
Silvia Nozza1,2 Riccardo Lolatto2
Riccardo Lolatto2 Anna Carole D’Amelio3
Anna Carole D’Amelio3 Gaia Catalano1
Gaia Catalano1 Giacomo Ponta1
Giacomo Ponta1 Vincenzo Spagnuolo1,2
Vincenzo Spagnuolo1,2 Massimo Cernuschi2
Massimo Cernuschi2 Nicola Gianotti2
Nicola Gianotti2 Antonella Castagna1,2
Antonella Castagna1,2 Diana Canetti2
Diana Canetti2People living with HIV (PLWH) are at high risk of herpes zoster (HZ). The recombinant anti-HZ vaccine (RZV), approved by European Medicines Agency (EMA) in 2018, has been shown to be effective and safe in PLWH. This study aims to describe the implementation of RZV in our center. Prospective cohort study on PLWH in care at the Infectious Diseases Unit of San Raffaele Hospital, Milan, Italy, receiving RZV between January 2022 and October 2023. To establish three priority criteria for identifying three groups among PLWH and to implement a proactive approach by offering immediate on-site vaccination during routine HIV medical visits. The three priority criteria identified were PLWH older than 65 years, PLWH had at least one previous episode of HZ, and PLWH had a CD4+ T lymphocytes count <200 cells/microL. Among the 599 PLWH vaccinated, 287 (48%) belonged to the Priority Group. The prioritization strategy facilitated the immunization program. The different implementation strategies showed different degrees of success. On-site vaccination during routine HIV medical examinations and prioritization of specific groups are effective strategies to increase vaccine uptake. We believe that collaboration between motivated clinicians and individuals paves the way for opportunities for prevention. These methods are crucial for ensuring effective prevention as new vaccines are introduced.
Vaccines have historically played a crucial role in preventing and controlling the spread of several infectious diseases (1). Improving universal access to preventive treatments and healthcare is one of the Sustainable Development Goals (SDGs) related to HIV, as formulated by UNAIDS (the United Nations Program on HIV/AIDS), with the aim of eliminating AIDS as a cause of death by 2030 (2). To implement health programs, especially those aimed at enhancing equity, is important to consider prioritizing the protection of the most fragile individuals (3). It is shown that immunization is a cost-effective health intervention, with the economic benefits of prevention far outweighing the costs of vaccination programs (4). Vaccination policies include the establishment of priority groups to maximize the cost-effectiveness by protecting those most at risk. Elderly and immunocompromised individuals are two key groups at high risk of developing infections due to immune senescence, age-related specific organ physiological changes, immunosuppression, and co-morbidities (5). Regarding access to vaccines, one of the key populations requiring particular focus are people living with HIV (PLWH) (6). The European AIDS Clinical Society (EACS) provides clinical guidelines on HIV/AIDS management in Europe, with focus also on vaccinations. Immunization is preferably indicated after achieving suppressed viremia and immune reconstitution (CD4+ T lymphocytes count >200 cells/microL or >15% and undetectable HIV-RNA). Recommended vaccinations for PLWH include influenza, severe acute respiratory syndrome coronavirus-2. (SARS-CoV-2), human papillomavirus, pneumococcus, meningococcus, viral hepatitis, pertussis, varicella and herpes zoster (HZ) (7).
HZ is an infectious disease caused by the reactivation of varicella-zoster virus (VZV), a common human alpha-herpesvirus. After contracting chickenpox, the virus remains dormant in the nerve ganglia and reactivate causing HZ. The main disease features are the appearance of an itchy and painful skin rash, involving one or adjacent dermatomes, that usually evolves into fluid-filled blisters, that can then rupture and form scabs (8, 9). HZ symptoms can last for several weeks, but in some cases, pain persist following rash resolution, a phenomenon known as post-herpetic neuralgia (PHN) which manifests as paroxysmal, lightning-like and knife-like pain (10). Some studies have shown that PLWH have a 5-10 times higher risk of developing herpes zoster compared to the general population, and a significantly higher risk of complications, such as PHN (11). PHN has been identified as the leading cause of long-term disability, significantly contributing to the increase in DALY along with the higher incidence (12). ART has substantially reduced the risk of HZ among PLWH, as ART lowers viral load and enhances immune responses, thereby reducing the risk of developing herpes zoster. However, this key population remains at higher risk of VZV reactivation, and its complications compared to the general population. Additionally, among PLWH, aging is one of the most frequent factors contributing to viral reactivation (13).
Historically, the live zoster vaccine (ZVL) was recommended in the general population (14, 15). However, this vaccine is contraindicated in immunocompromised individuals, including those receiving moderate to high dose immunosuppressive medications, PLWH with CD4+ lymphocytes <200cells/microL, or those receiving chemotherapy (16). Since 2017, different studies evaluated the use of novel recombinant zoster vaccine (RZV), a subunit vaccine based on viral glycoprotein E, among immunocompromised populations, including PLWH, proving to be safe and effective, with comparable immunogenicity data (17). Currently, the Centers for Disease Control and Prevention (CDC) recommends RZV to all people over 50 years, including those who received previous ZVL, and to all individuals over 18 years of age at increased risk of HZ due to immunodeficiency or immunosuppression, including PLWH (18).
Strategies adopted for vaccine distribution involve optimizing vaccination and prioritizing at-risk groups to ensure that the most vulnerable subjects have overriding access to vaccines, to limit disease burden (19).
In the context of this study, the aim is to describe a single-center experience on the implementation of RZV among PLWV, to illustrate the different strategies adopted to ensure active immunization for the rapid implementation of vaccination.
This is a prospective cohort-study on active RZV vaccine implementation; data from our center are presented. PLWH in care at Centro San Luigi (CLS), the Infectious Diseases Unit of San Raffaele Hospital (Milan, Italy) who belong to the HIV-CSL cohort were included. Moreover, proactive vaccination is provided within the outpatient clinic, in line with EACS recommendations, and RZV is part of the routinely offered vaccinations. Participants provided written informed consent; Centro San Luigi HIV cohort was approved by the Ethics Committee of the IRCCS San Raffaele Scientific Institute on December 4, 2017 (protocol number 34). Its follow-up accrued from January 2022, the date of first RZV vaccination, to October 2023, the freezing date. Individuals’ characteristics, including demographic factors, were considered at the date of first RZV dose (baseline) and within 6 months before vaccination. People with ad interim vaccination (e.g. received one RZV dose with scheduled second dose at date of freezing) were also considered vaccinated for the purposes of this study. Priority to RZV was defined according to Italian Guidelines for PLWH and EACS recommendations for HZ vaccination delivery and based on risk of HZ reactivation and related complications. PLWH with at least one of the following criteria were considered at increased risk of HZ and therefore part of the “Priority group”: a) CD4+ T lymphocytes <200 cells/microL; b) ≥1 previous HZ episode or related complications; c) age >65 years. Different vaccination delivery strategies have been used over follow-up with the intent to effectively deliver the higher number of RZV doses, bearing in mind the need to vaccinate firstly people of the Priority groups.
At the beginning of RZV vaccination campaign in January 2022, PLWH in the Priority group were asked if they were interested in vaccination during outpatient visits and signed informed consent. Subsequently, vaccination was scheduled, and they had to return on a second date [Strategy 1. Routine Visit Immunization Booking System (January 2022-December 2022)]. To improve vaccination coverage, since January 2023, a proactive outreach strategy was implemented. Individuals were contacted, based on their risk profile, and a designated healthcare workers (HCW) centrally coordinated the vaccination calendar; appointments for delivery of RZV were scheduled on specific days once a week [Strategy 2. Proactive Medical Outreach Vaccination System (January 2023-March 2023)]. Due to the excellent adherence and the high demand, the vaccination approach was changed from April 2023. The RZV service was integrated into routine HIV care visits: the first dose was injected to eligible PLWH during their routine clinical care by the referring HCW and the second dose was scheduled after 2 to 6 months [Strategy 3. Integrated In-Visit Vaccination System (April 2023- October 2023)].
Anonymized data were collected by means of the electronic health records of PLWH in care at the Infectious Diseases Unit of San Raffaele Hospital, which includes demographic data, risk factors, clinical history, antiretrovirals, co-medications, previous vaccinations, laboratory, serologic and virologic data. Data are registered and updated at each medical visit., including episodes of HZ defined as the development of a unilateral vesicular rash, typically confined to a single dermatome, and related complications e.g. PHN. The median (quartile 1, quartile 3) and the frequency (%) were used, as appropriate, to describe the individuals’ characteristics.
Overall, 5167 PLWH were included and 599 received ≥1 dose of RZV in the study period. Vaccinated PLWH were 84.3% (n=505) male, with a median (IQR) age of 59.8 [55.6-65.1]. The main risk factors for HIV infection were being a man who has sex with men (MSM) in 48.7% (n=298) and being an injection drug user (IDU) in 11.2% (n=67). Vaccinated people were living with HIV from 23.8 median years [16.5-30.5], with 21.2 median years of ART [12.8-26.3] and all were currently receiving ART. Referring to comorbidities, a previous AIDS event -like the onset of specific opportunistic infections, certain malignancies, or a CD4+ T cell count below 200 cells/µL, even in the absence of clinical symptoms- was recorded in 154 (25.7%) PLWH, chronic obstructive pulmonary disease (COPD) in 8 (1.34%), diabetes among 55 (9.18%), major adverse cardiovascular events (MACEs) in 43 (7.18%) and malignancies among 107 (17.9%). Co-infection with HBV was found in 38 people (6.34%) and previous HCV infection in 126 (21%). Cardiovascular medications were reported by 403 (67.3%), diabetes medications by 50 (8.35%); median BMI was 24.7 [22.7-27.2]. Regarding the immunovirological status of vaccinated PLWH at baseline, 575 (96.0%) had HIV RNA <50 copies/mL, median CD4+ T lymphocytes count was 714 cells/microL [546-962] and median CD4+/CD8+ T lymphocytes ratio 0.93 [0.63-1.27]. Among people with undetectable HIV RNA, median years of virologic suppression were 11.6 [6.96-16.0] and median CD4+ T lymphocytes nadir was 248 cells/microL [133-368]. Previous episodes of HZ were reported in 173 (28.88%) PLWH, including 151 (25.2%) with a single episode, 20 (3.34%) with two episodes, and 2 (0.33%) with three episodes. During the 12 months following vaccination, only one individual (0.19%) reported an episode of HZ. From first RZV dose to second dose median days were 77 [70-126].
Out of the 547 PLWH who had only age as a risk factor, 112 were vaccinated, out of the 519 who had at least one previous episode of HZ as a priority criterion, 131 people were vaccinated. Meanwhile, of the 63 PLWH with CD4+ T lymphocytes <200 cells/microL, only two individuals were vaccinated. However, when we intersect the priority criteria, we see that out of the 5 individuals meeting all 3 criteria, 4 were successfully vaccinated. These data suggest that, despite the relatively low number of vaccinated individuals among the groups with more severe criteria (such as CD4+ T <200), those who meet all three criteria have a good likelihood of being vaccinated. Distribution of PLWH according to risk profile and status of RZV is presented in Figure 1.
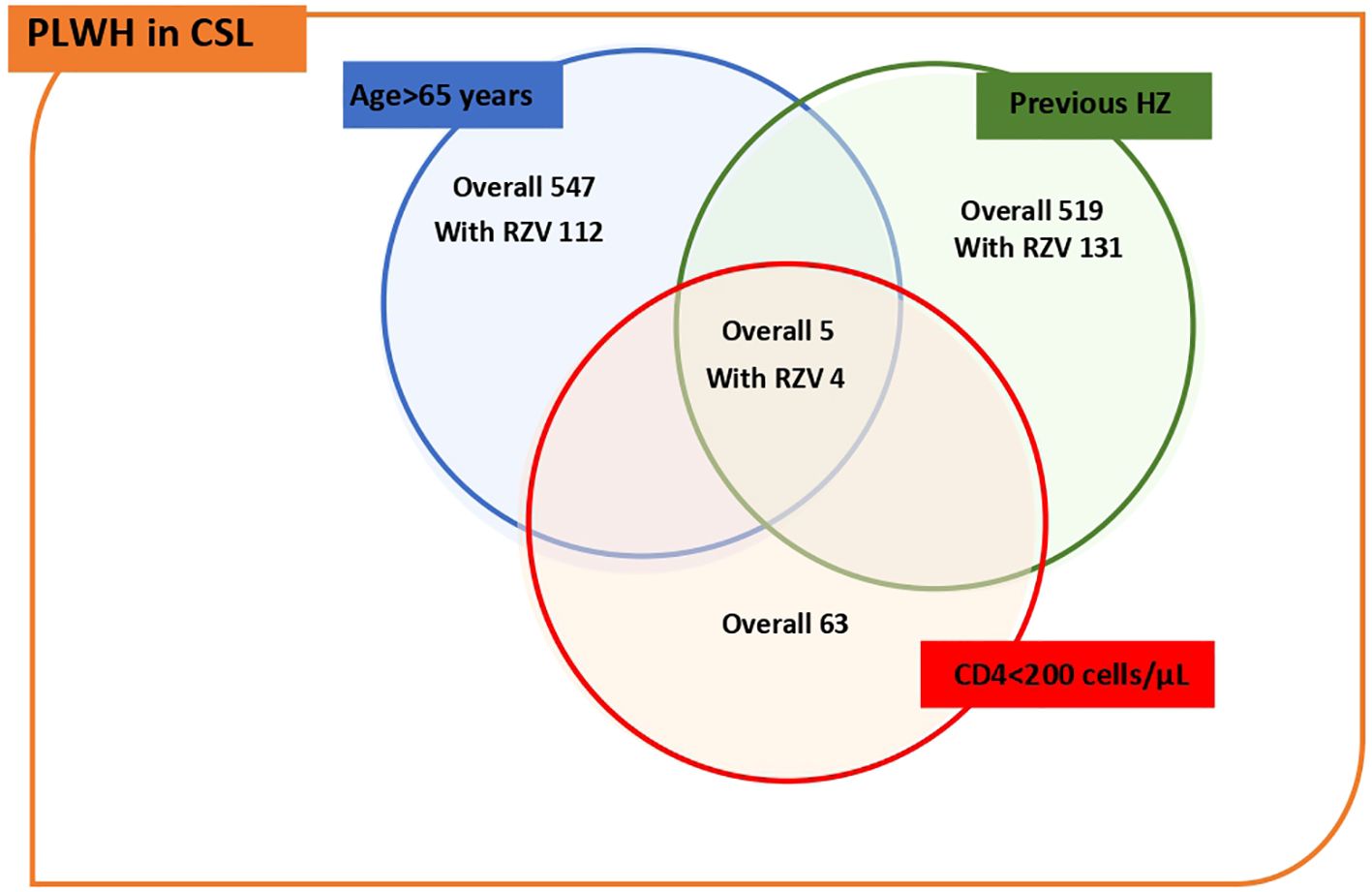
Figure 1. Distribution of PLWH according to risk profile for HZ and status of RZV. The figure shows a Venn diagram, which divides numbers of PLWH and the doses of RZV received by each risk factor, and intersects them to show how many high-risk PLWH have been vaccinated.
The distribution of first RZV dose over the study period is presented in Figure 2; 96/599 (16%) people were vaccinated during the Routine Visit Immunization Booking System (Strategy 1) between January and December 2022 (12 months). During the Proactive Medical Outreach Vaccination System (Strategy 2), 249/599 (43.2%) PLWH received RZV between January and March 2023 [50 in January; 99 in February; 100 in March]. During the Integrated In-Visit Vaccination System (Strategy 3), from April to October 2023, 254 (42.4%) PLWH were vaccinated.
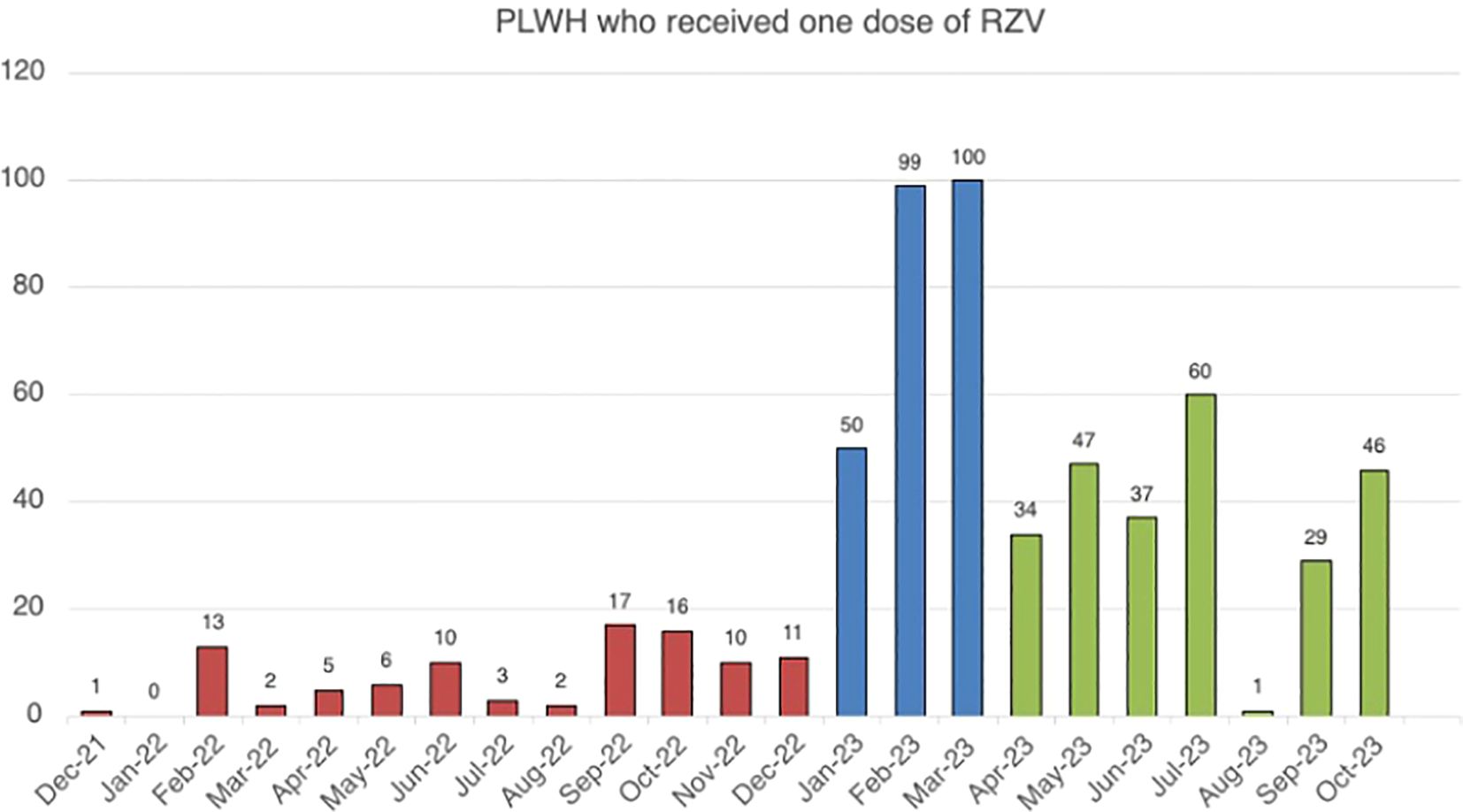
Figure 2. PLWH administered at last one RZV dose over according to different vaccination strategies. The figure shows the number of PLWH vaccinated for each month included in the study period, and different colors were used to indicate the various vaccination strategies.
In this study, we presented the implementation of RZV vaccination among PLWH and proposed prioritization of immunization among HZ high risk group people, by means of a dedicated team, contributing to boost the vaccination coverage. Other examples of implementation strategies regarding RZV immunization in other cohorts such as The Ohio State University which had investigated the Functions of an Electronic Medical Record, in combination with a pharmacist as part of the care team, to increase vaccination coverage (20), and the experience of the Tufts Medical Center (TMC), Medford, United States where users who needed RZV were identified and contacted by a certified designated pharmacy technician (CPhT) and then immunized in dedicated clinics (19).
Most likely, one of the major success factors was attachment towards the infectious disease center of reference, as they were vaccinated at the same center and by the same. Additionally, easy and immediate access to healthcare providers has facilitated the opportunity to address specific questions, clarify concerns, and minimize misunderstandings, thereby helping to build trust and reduce hesitancy (21). The analysis of the study population’s characteristics and the median age of this cohort underscores the importance of tailored vaccination strategies for an aging population and the need for targeted healthcare interventions for long-term HIV management, which further emphasizes the multifaceted health challenges faced by this population, necessitating a holistic approach. Although the majority of PLWH had undetectable viremia and sufficient CD4+ T lymphocyte counts, the observed common history of previous HZ underscores the heightened vulnerability of the study population (22), reinforcing the importance of this vaccination in this setting.
The study effectively leveraged electronic records to identify and categorize high-priority PLWH, possibly providing a blueprint for future vaccination initiatives. The three different implementation strategies demonstrated varying degrees of success. The Routine Visit Immunization Booking System (Strategy 1) resulted in a steady but modest vaccination rate over 12 months. In contrast, the Proactive Medical Outreach Vaccination System (Strategy 2) and the Integrated In-Visit Vaccination System (Strategy 3) increased vaccination rates, with 43.2 percent and 42.4 percent of doses administered, respectively. Although the results achieved so far are encouraging, there were limitations to the implementation of vaccination. Firstly, the timing of co-administration with other vaccines, indicated by the EACS guidelines, resulted in RZV administration delay. Another limitation of the study pertains to the people’ willingness to receive the vaccine. Despite the high risk, some PLWH either refused the vaccine or were unable to receive it due to concurrent personal issues. As a result, for instance, among the third group (PLWH with CD4+ <200 cells/microL), only 3% were vaccinated, and this was a random occurrence. Moreover, RZV implementation was delayed to January 2023 due to COVID-19 pandemic and the related involvement of HCW in Sars-CoV-2 vaccination campaigns. Also, because of the limited availability of RZV from local health authorities, a maximum number of total monthly doses and this determined a further delay in the implementation project. Limitations of the study also include manual data entry on electronic health records, which slowed down each visit and imposed a maximum number of administrations per day.
Overall, we believe that effective collaboration between motivated clinicians and PLWH, thanks to clear information and a deep understanding of the benefits, fosters a better acceptance of vaccines, including RZV, paving the way for numerous opportunities for prevention. The strategy of implementing the RZV vaccine among the PLWH included in the study, through proactive offering, has proven to be effective. In a scenario of growing knowledge and availability of vaccines, as also observed in the context of the Mpox outbreak, we believe that integrating vaccine administration during routine HIV visits is the best approach to deliver vaccinations. Our intention is to continue proactively administering the RZV vaccine to increase vaccine coverage during routine visits and value the lesson of this implementation study for the future. In addition familiarity with healthcare workers and the vaccination center may present a potential strategy to mitigate vaccine hesitancy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the IRCCS San Raffaele Scientific Institute on December 4, 2017 (protocol number 34). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
BT: Writing – original draft, Writing – review & editing. AR: Writing – review & editing. SD: Data curation, Methodology, Resources, Writing – review & editing. SN: Project administration, Supervision, Writing – review & editing. RL: Data curation, Formal Analysis, Software, Writing – review & editing. AD: Investigation, Methodology, Writing – review & editing. GC: Visualization, Writing – review & editing. GP: Visualization, Writing – review & editing. VS: Resources, Writing – review & editing. MC: Conceptualization, Writing – review & editing. NG: Resources, Validation, Writing – review & editing. AC: Conceptualization, Funding acquisition, Visualization, Writing – review & editing. DC: Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Data presented in this paper has been produced within the project “Implementation of anti-herpes zoster immunization coverage in HIV-infected individuals over fifty years old in care at the Infectious Diseases Unit, San Raffaele Scientific Institute, Milan, Italy” which has been unconditionally funded by GlaxoSmithKline SpA (GSK S.p.A.) within the “Call for Prevention” tender notice published in 2021, aimed to support adult vaccination.
The nursing staff for help in vaccine management, the database for data analysis, and the residents and physicians of the CSL HIV clinic for their help shown in the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. (2014) 369. doi: 10.1098/rstb.2013.0433
2. UNAIDS. Fast-Track Strategy to End AIDS . Available online at: www.unaids.org (Accessed November 15, 2024).
3. Gavazzi G, Krause KH. Spécificités des infections chez le sujet agé [Infections in the elderly. Praxis (Bern 1994). (2004) 93. doi: 10.1024/0369-8394.93.33.1297
4. Koplan JP. Benefits, risks and costs of immunization programmes. Ciba Found Symp. (1985) 110:55–68. doi: 10.1002/9780470720912.ch5
5. Addario A, Célarier T, Bongue B, Barth N, Gavazzi G, Botelho-Nevers E. Impact of influenza, herpes zoster, and pneumococcal vaccinations on the incidence of cardiovascular events in subjects aged over 65 years: a systematic review. Geroscience. (2023) 3:1–29. doi: 10.1007/s11357-023-00807-4
6. El Chaer F, El Sahly HM. Vaccination in the adult patient infected with HIV: A review of vaccine efficacy and immunogenicity. Am J Med. (2019) 132:437–46. doi: 10.1016/j.amjmed.2018.12.011
7. EACS. Guidelines; v12.0. Brussels, Belgium: European AIDS Clinical Society. (2023). doi: 10.1111/hiv.13542
9. Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, et al. Varicella zoster virus infection. Nat Rev Dis Primers. (2015) 1:15016. doi: 10.1038/nrdp.2015.16
10. Wollina U, Machetanz J. Herpes zoster und postzosterische Neuralgie. Hautarzt. (2016) 67:653–65. doi: 10.1007/s00105-016-3834-y
11. GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1260–344. doi: 10.1016/S0140-6736(17)32130-X
12. McKay SL, Guo A, Pergam SA, Dooling K, Zoster H. Risk in immunocompromised adults in the United States: A systematic review. Clin Infect Dis. (2020) 71:e125–34. doi: 10.1093/cid/ciz1090
13. De Vito A, Colpani A, Trunfio M, Fiore V, Moi G, Fois M, et al. Living with HIV and getting vaccinated: A narrative review. Vaccines. (2023) 11:896. doi: 10.3390/vaccines11050896
14. Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. (2005) 352:2271–84. doi: 10.1056/NEJMoa051016
15. Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, et al. Centers for Disease Control and Prevention . Zostavax (Zoster vaccine live) recommendations (Accessed June 15, 2017).
16. Geretti AM, Brook G, Cameron C, Chadwick D, French N, Heyderman R, et al. BHIVA guidelines on the use of vaccines in HIV-positive adults. (2015). doi: 10.1111/hiv.12424
17. Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. Am J Transplant. (2018) 18. doi: 10.15585/mmwr.mm6703a5
18. Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. (2018) 67:103–8. doi: 10.15585/mmwr.mm6703a5
19. Flaspohler NK, Grossman K, Martin A, Tang S, Abourjaily PN. Implementation of a herpes zoster immunization program in an outpatient hospital-based pharmacy. J Am Pharm Assoc. (2003) 63:S88–S92.e1. doi: 10.1016/j.japh.2022.11.010
20. Otsuka SH, Tayal NH, Porter K, Embi PJ, Beatty SJ. Improving herpes zoster vaccination rates through use of a clinical pharmacist and a personal health record. Am J Med. (2013) 126:832.e1–6. doi: 10.1016/j.amjmed.2013.02.018
21. Romate J, Rajkumar E, Gopi A, Abraham J, Rages J, Lakshmi R, et al. What contributes to COVID-19 vaccine hesitancy? A systematic review of the psychological factors associated with COVID-19 vaccine hesitancy. Vaccines (Basel). (2022) 10:1777. doi: 10.3390/vaccines10111777
Keywords: herpes zoster, proactive vaccination, HIV, implementation, prioritization
Citation: Trentacapilli B, Raccagni AR, Diotallevi S, Nozza S, Lolatto R, D’Amelio AC, Catalano G, Ponta G, Spagnuolo V, Cernuschi M, Gianotti N, Castagna A and Canetti D (2025) Implementation of recombinant anti-herpes zoster vaccination in people living with HIV: a single-center experience. Front. Virol. 5:1537821. doi: 10.3389/fviro.2025.1537821
Received: 01 December 2024; Accepted: 03 February 2025;
Published: 20 February 2025.
Edited by:
Arif Nur Muhammad Ansori, Universitas Airlangga, IndonesiaReviewed by:
A. Raj Kumar Patro, Kalinga Institute of Medical Sciences (KIMS), IndiaCopyright © 2025 Trentacapilli, Raccagni, Diotallevi, Nozza, Lolatto, D’Amelio, Catalano, Ponta, Spagnuolo, Cernuschi, Gianotti, Castagna and Canetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benedetta Trentacapilli, dHJlbnRhY2FwaWxsaS5iZW5lZGVAaHNyLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.