- 1Department of Microbiology, All India Institute of Medical Sciences (AIIMS), Raipur, Chhattisgarh, India
- 2Department of General Medicine, All India Institute of Medical Sciences (AIIMS), Raipur, Chhattisgarh, India
We have reported here the fatal outcome of Epstein-Barr virus (EBV) infection in a 58-year-old male who had probably developed reactivation/chronic active EBV (CAEBV) which gave rise to various neurological deficits, pancytopenia, and a lower CD4 count in the patient. The decreased immune response helped Cryptococcus neoformans (C. neoformans) to manifest a disseminated infection. Although he was exclusively provided with antifungal treatment and the patient appeared to be successfully treated for cryptococcal infection, no coverage of EBV appeared detrimental as the patient died the very next day. This report highlights the need for clinical suspicion of EBV in unexplained cases of neurological manifestation, the hematological disorder of pancytopenia, a lower CD4 count, and multiorgan involvement such as pleural effusion, coarse liver echotexture, and splenomegaly.
1 Introduction
The prevalence of Epstein-Barr virus (EBV) infection is observed to be more than 90% in the adult population worldwide (1). It is mostly asymptomatic in childhood, while nearly half of the infected population tend to develop symptoms by the time they reach adulthood, which is mainly self-limiting. Furthermore, EBV is established as a latent infection awaiting reactivation/chronic active EBV (CAEBV) upon decreased immunity. Reactivation of EBV/CAEBV leads to various clinical manifestations, including meningitis, encephalitis, optic neuritis, autoimmune diseases, and various malignancies (2). These manifestations also hamper CD4 T cell count to weaken cellular immunity. Weakened cellular immunity provides a conducive environment for other pathogens such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and Cryptococcus spp., making both diagnosis and treatment management complex and difficult. CAEBV is rare and characterized by a high viral load, increased anti-EBV antibody response, pancytopenia, and T/natural killer (NK) cell lymphoproliferative diseases (1).
Cryptococcus neoformans (C. neoformans) causes various infections such as disseminated cryptococcosis and meningitis, which are mainly observed in HIV-positive patients with increased mortality. In HIV-negative patients, the clinical significance of the co-occurrence of EBV with cryptococcal infection has been scarcely reported (3, 4). Herein, we provide a case report of a 58-year-old deceased patient with a coinfection of EBV and C. neoformans after receiving the due consent from his wife. The requirement of ethical approval was waived by the Institutional Ethics Committee, AIIMS Raipur, as per their policy of not requiring ethical permission in case reports and only consent from the patients is sufficient with due information to the ethical committee. The studies were conducted in accordance with the local legislation and institutional requirements.
2 Case history
A 58-year-old male presented to the All India Institute of Medical Sciences (AIIMS) Raipur Trauma and Emergency in April 2024 with complaints of bilateral lower limb weakness for 1 year with recent onset of breathlessness, mMRC 2 (Modified Medical Research Council) for 10 months, which progressed to mMRC 4 in the previous 6 weeks, and intermittent low-grade fever associated with chills that were relieved with symptomatic treatment. The bilateral lower limb weakness (right > left) started distally and progressed proximally, followed by bilateral upper limb weakness for 3 months. On clinical history evaluation, the patient was found to be non-diabetic and non-hypertensive but hypothyroid for 2 years and an alcoholic for 20 years. A history of bilateral pneumonia was noted in the last week of January 2024, which improved upon treatment in a private hospital with levofloxacin, amoxy-clav, prednisolone, and hydrocortisone nebulization, and the patient was discharged. However, similar symptoms recurred once again in mid-March and the patient was treated similarly. However, with the progression of his worsening condition, he was referred to our hospital. We followed the CARE case report guidelines for the diagnostic investigations, treatment, and outcome (Supplementary Table 1).
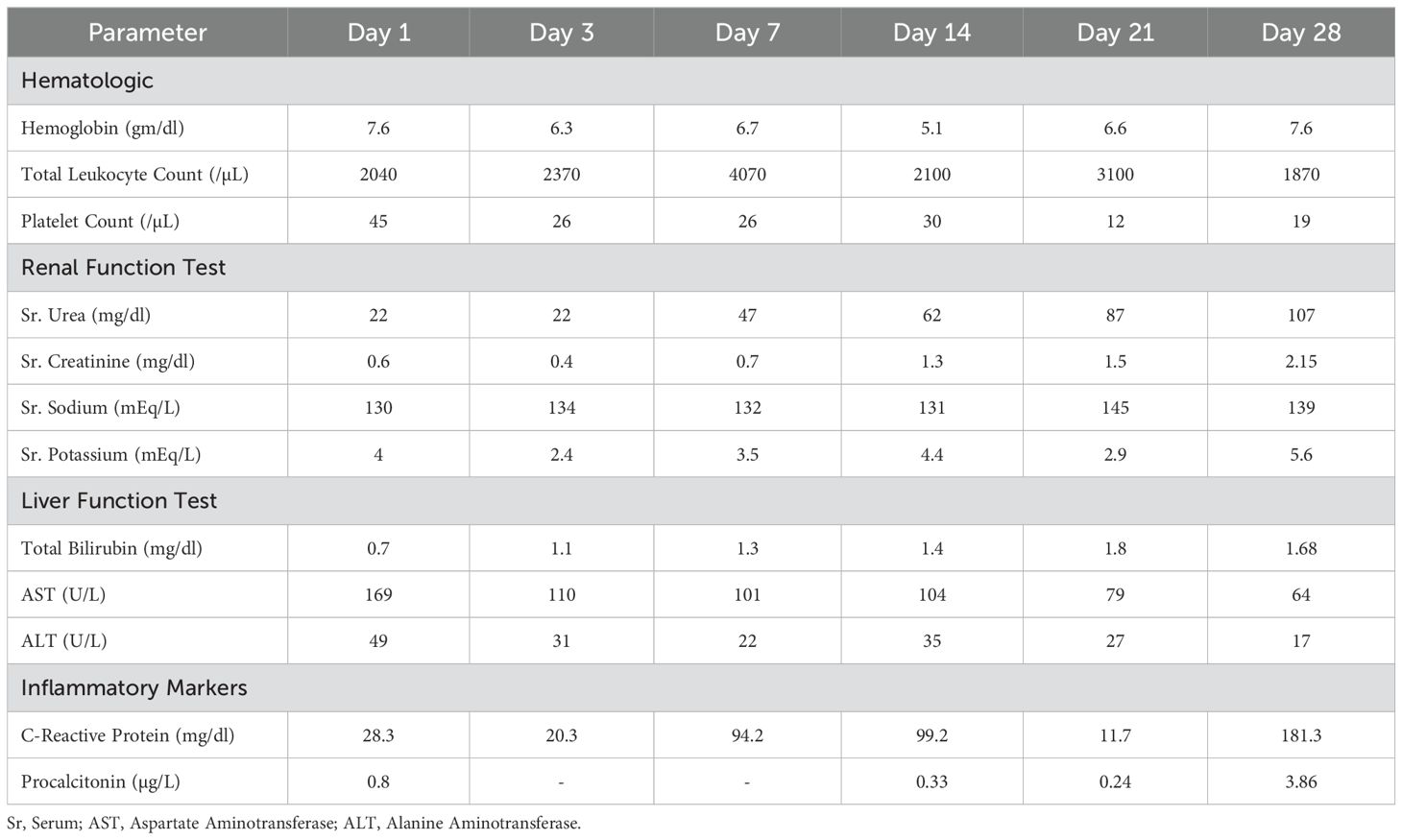
The day-wise hematological, renal, liver, and inflammatory investigations were recorded (Table 1A). On examination, his Pulse Rate (PR) was 61/min, Blood Pressure (BP) 104/74, RR 17/min, and saturation of peripheral oxygen (SpO2) of 99%. Bilateral pitting edema, pallor, and clubbing were present with bilateral inspiratory coarse crepitations. The patient was subsequently shifted to the Medical High Dependency Unit (HDU) and was treated for hospital-acquired pneumonia with vancomycin 1gm q8h iv and meropenem 1 gm 8h iv. The summarized timeline of the patient’s clinical condition, treatment, and investigation is provided in Table 1B.
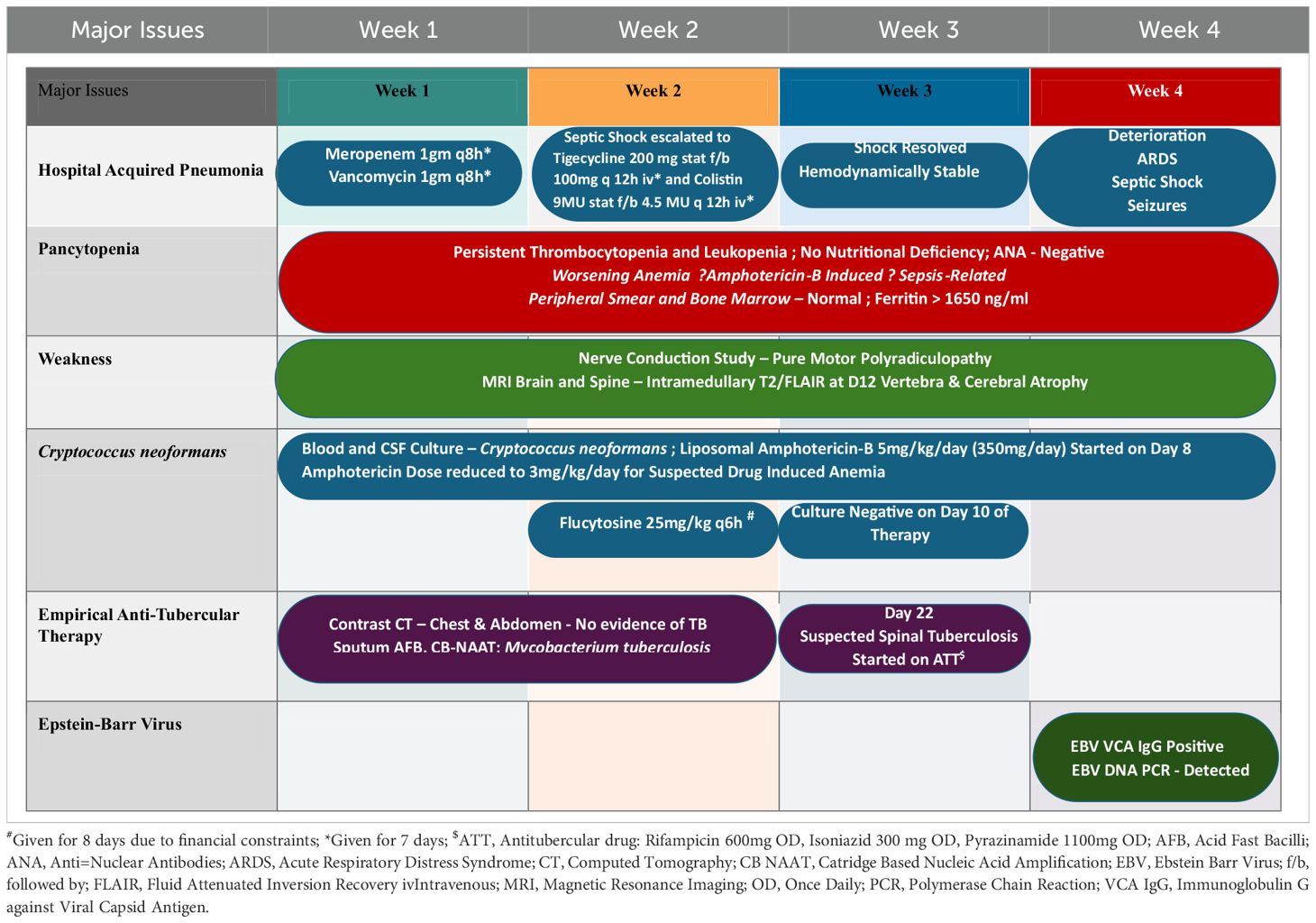
Hence, in view of the pancytopenia, bone marrow aspiration and bone marrow biopsy were conducted, the results of which were found to be insignificant. The patient was started on romiplostim 250mcg once weekly subcutaneously with repeated random donor platelet transfusions. CD4+ T helper cell count was 149. A nerve conduction study revealed pure motor polyradiculoneuropathy with the neurogenic pattern on an electromyogram (EMG). In contrast-enhanced computed tomography (CECT), lung consolidation with bilateral pleural effusion and splenomegaly were noted. Ultrasonography (USG) of the abdomen revealed a coarse echotexture of the liver with a prominent portal vein and splenomegaly.
The patient was non-reactive to HIV (HIV1/2 Immunochromatography Lateral flow Assay kit, (Pathkits, India, Lot No. PKRK/014/0424-07), Hepatitis B (HBsAg Rapid Immunochromatography lateral flow assay, Med Source Ozone Bio Pvt. Ltd., Lot No. HBSC210324L), Hepatitis C (HCV Rapid Immunochromatography lateral flow Assay kit, BIOGENIX INC.PVT.LTD, Lucknow India, Lot No. HCV 0324), and Dengue (ErbaQik Dengue DUO NS1 Ag with IgG/IgM Immunochromatography assay, Erba Manheim, India, Lot No. DRDDO2302B). Multiple blood cultures were collected and incubated in a BacT/Alert automated blood culture system which flagged positive after 48 hours of incubation. Gram staining from the positive blood culture bottle revealed the presence of gram-positive budding yeast cells. The positive blood culture bottles were then subcultured onto blood agar and Sabourauds dextrose agar (SDA) which grew mucoid white creamy pasty round colonies after 48 hours of aerobic incubation at 37°C and was found rapid urease positive. It was also identified as C.neoformans in the Vitek2 automated system (bioMerieux Inc, USA with the use of Vitek yeast (YST) card Lot No. 2432243503). The cerebrospinal fluid (CSF) cytology reported slightly elevated leukocytosis (150 cells/ml) with a protein level of 127mg/dl and glucose level of 69mg/dl. A rapid Cryptococcal antigen test of the patient’s CSF (Crypto PS Rapid Immunochromatography lateral flow Assay, BIOSYNEX SA, France with Lot No. KCC240226) was found to be positive. CSF India ink microscopy identified capsulated round budding yeast cells while the culture grew C. neoformans (Figure 1). The isolate was sensitive to fluconazole, flucytosine, and amphotericin B with minimum inhibitory concentrations (MICs) of 1μg/ml, 2μg/ml, and 0.5μg/ml respectively tested by the Vitek2 system using Vitek2 antibiotic sensitivity testing (AST)-YS08 card, Lot No. 2882831503. He was started on flucytosine 25mg/kg q6h and liposomal amphotericin B 5mg/kg/day (350 mg) which was reduced to 3 mg/kg/day due to suspected drug-induced thrombocytopenia. Despite laboratory evidence of the clearing of the cryptococcal infection in the blood culture, the patient’s condition worsened. He was urgently started on tigecycline and colistin. Magnetic resonance imaging (MRI) of the brain with the spine showed a short segment with ill-defined intramedullary T2/Fluid-Attenuated Inversion Recovery (T2/FLAIR) of 14.3 mm at the Thoracic Vertebral level 12 (T12) region and brain atrophy. With a clinical suspicion of spine tuberculosis, the patient was started on antitubercular (ATT) drugs. However, M. tuberculosis was not detected in a cartridge-based nucleic acid amplification test (CBNAAT) on Genexpert (Cepheid, USA) in the clinical sample of endotracheal aspirate, pleural fluid, and CSF with the absence of acid-fast bacilli in Ziehl-Neelsen (ZN) microscopy. The patient developed altered sensorium for 2 days with decreased speech and responsiveness and was unable to identify the attendants. This was followed by one episode of a generalized tonic-clonic seizure (GTCS) with a postictal loss of consciousness. The patient was intubated with GCS E3VTM5 with fentanyl sedation. Non-contrast computed tomography (NCCT) of the patient’s head revealed frontoparietal variable densities and anisocoria. An ophthalmology consultation revealed a bilateral dot and blot hemorrhage. Meanwhile, an anti-EBV Immunoglobulin G test against viral capsid antigen (VCA) (tested outside) was found reactive and DNA was detected by real-time PCR in the blood by targeting the BNRF1 gene (Figure 2). Within a day, the patient had refractory septic shock, sudden onset bradycardia, and expired.
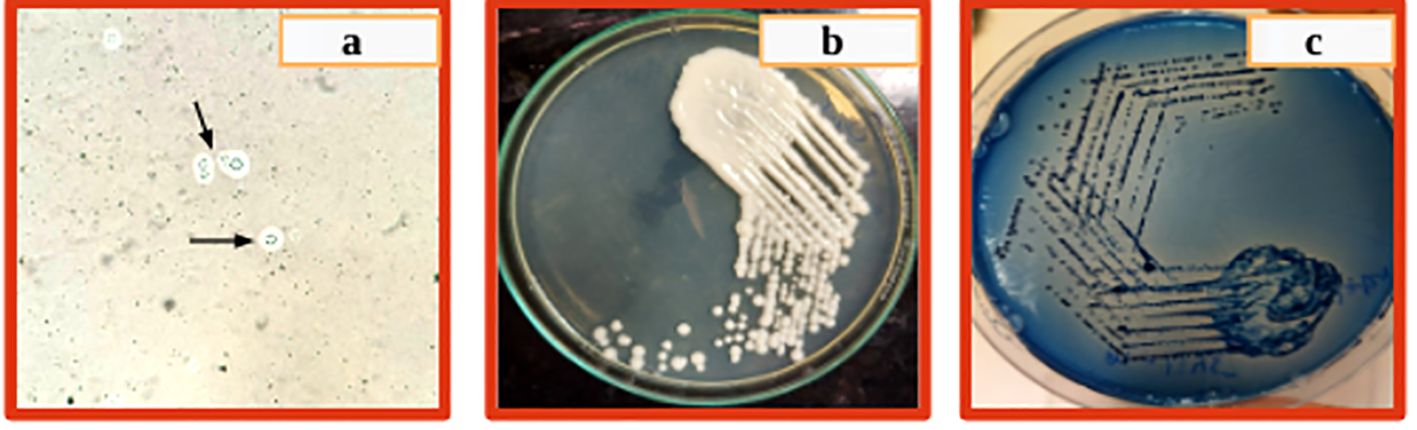
Figure 1. (A) Circular yeast cells with capsule and narrow-based budding, (B) White creamy pasty mucoid colony on SDA after 48 hrs incubation, (C) Blue colonies on Cryptococcal differential media after 48 hrs incubation.
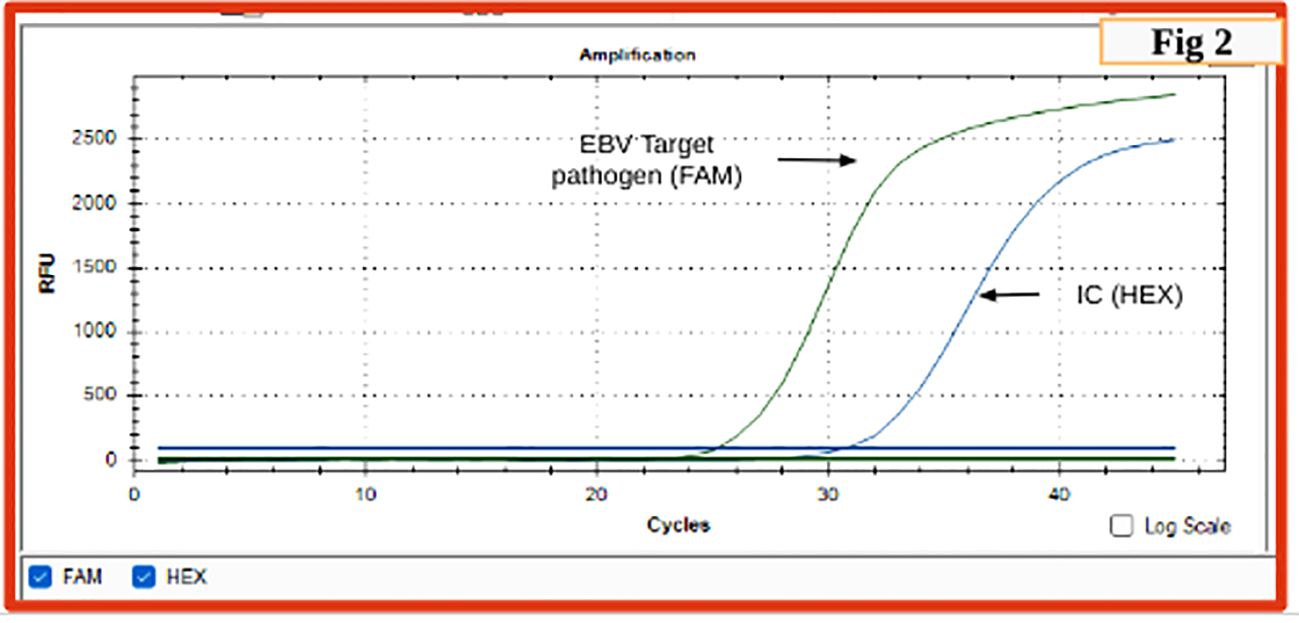
Figure 2. Real-time PCR graph indicating sigmoid graph at 25 cycles indicating positivity for EBV in the test sample (FAM) (cutoff is 35 cycles); sigmoid curve for internal control (HEX).
3 Discussion
The given case history, clinical manifestation, and investigation findings reasonably suggest that this patient was probably primarily infected by EBV in the past which eventually progressed to CAEBV/reactivation, leading to rare CNS complications as also reported previously (5). Rare CNS complications have been reported in 0.5% to 7.5% of cases and occur either as the sole or first clinical manifestation of primary EBV infection (5, 6). EBV has been well-documented to cause meningitis, encephalitis, myelitis, and vasculitis. It is also known to cause various complications such as Guillain Barre Syndrome (GBS), acute demyelinating encephalomyelitis (ADEM), transverse myelitis, and polyradiculomyelitis (1, 6, 7). The nerve conduction test also established pure motor polyradiculoneuropathy. Although most individuals recover from primary EBV infection and enter into a virus-host balance state, leading to the healthy carrier stage, a small proportion has been reported to undergo further progression to CAEBV, hemophagocytic lymphohistiocytosis (EBV-HLH), diffuse B cell lymphoma, Hodgkin lymphoma, and other EBV-related natural killer/T cell lymphoproliferative-related diseases (1). In our patient, CAEBV appears a more likely possibility as it causes pancytopenia, decreased CD4+ T cell count, and HLH, and is evident clinically with coarse echotexture of the liver, splenomegaly, and prolonged fever (8). EBV has also been reported to stimulate primary CD4+ T cells, resulting in many effector cells either dying or losing functional capacity and leaving a small proportion of effector cells to mediate protection during EBV persistent infection which has been found to have fallen as low as 0.33% (8). CAEBV is further substantiated with a reactive anti-EBV IgG test against VCA. CAEBV most likely resulted in encephalitis in this patient which could be substantiated by his predominant clinical features of memory impairment and focal neurological deficits of limb weakness and numbness, vestibule ataxia disorder, altered mental status, lymphocytic pleocytosis, and slightly elevated protein in CSF. There was no EBV investigation in CSF and the bacterial and fungal cultures and sensitivity tests provided a diagnosis of Cryptococcus and its treatment. This unfortunately leaves the exact cause of death unidentified as planning of the EBV diagnosis in CSF was left unattempted due to his death the very next day after confirming EBV in blood. This we consider a limitation in this case report. However, the various discussed investigations may be considered as reasonably establishing our hypothesis of a CAEBV-derived weakened immune system facilitating C. neoformans infection in this patient. Various earlier reports also report that EBV in conjunction with C. neoformans severely affects the CNS and other organs (3, 4). EBV has been reported to increase mortality in HIV-negative Cryptococcal meningitis cases (3). Our case report appears to be the first-ever case of coinfection of EBV and C. neoformans in an HIV-negative adult from Chhattisgarh and even from India.
We have presented this case to showcase the importance of clinical suspicion of EBV in cases of prolonged neurological deficit, pancytopenia, multiorgan involvement, bilateral pleural effusion, and splenomegaly. EBV encephalitis probably occurred due to direct viral invasion into neurons and endothelial cells of the brain blood vessels by increasing the pro-inflammatory molecules and enhancing the surface adhesion molecule expression, thereby creating a probable inflammatory blood-brain barrier (BBB) breach to augment Cryptococcus entry into CSF (3, 9). The immune injury to CNS could be caused by molecular mimicry between EBV and autoantigens of the CNS and antigen-antibody complex deposition (10). EBV encephalitis has also been reported earlier in HIV-negative adult patients with both mono and mixed infections (11). It is suggested that the antiviral ganciclovir targeting EBV along with corticosteroids and immunoglobulin could prove therapeutic benefits to these patients as also reported previously (12).
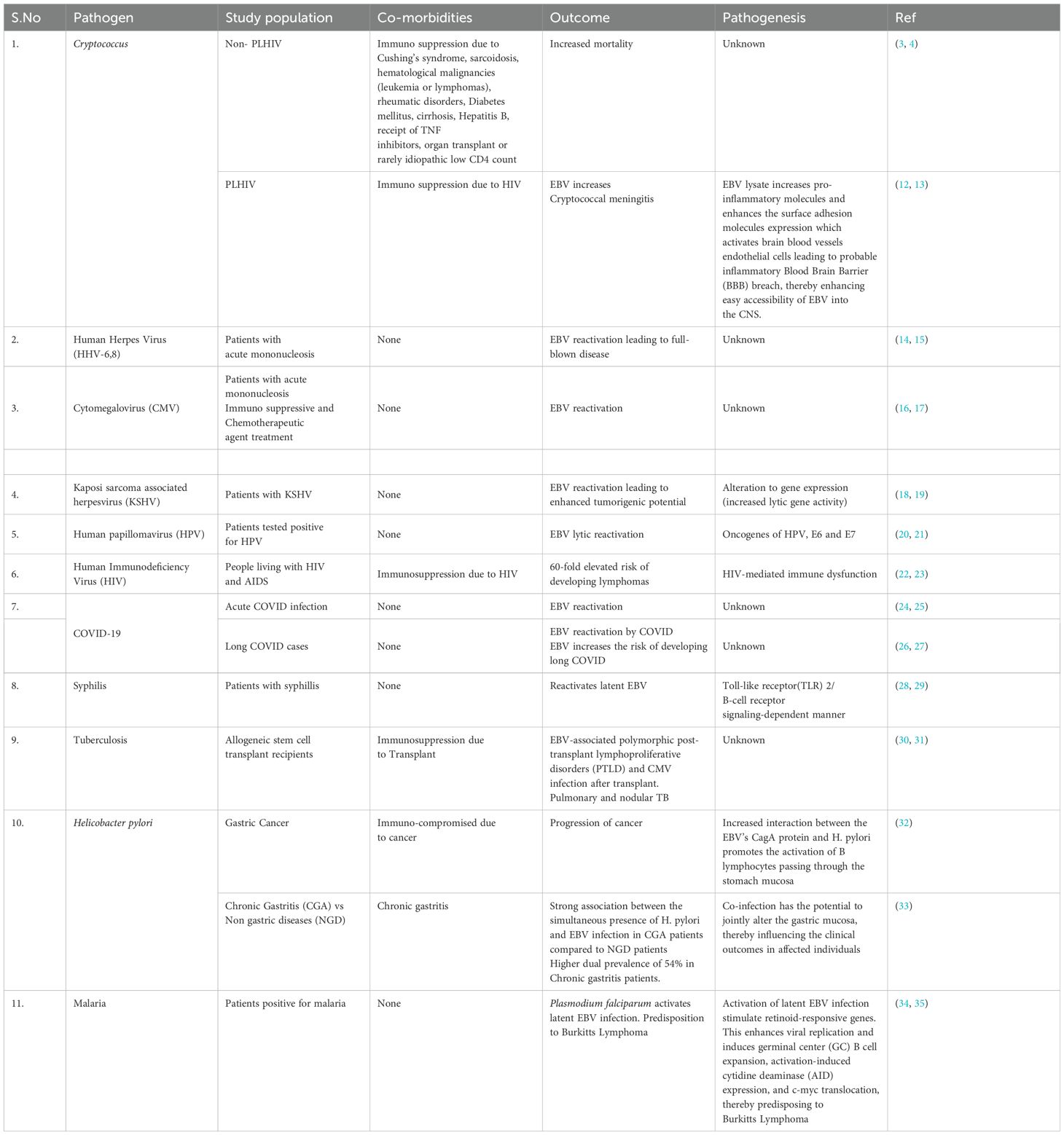
We have reviewed reported coinfections of EBV and found that EBV has been reported in conjunction with other viral, bacterial, fungal, and parasitic etiological agents such as Human Immunodeficiency Virus (HIV), cytomegalovirus (CMV), human herpes virus (HHV-6), severe acute respiratory syndrome (SARS-CoV-2), human papillomavirus (HPV), Cryptococcus neoformans, Mycobacterium tuberculosis, Treponema pallidum, and Plasmodium falciparum to cause dual and triple infection (Table 2). EBV has been reported to undergo reactivation by enhancing lytic gene expression in presence of these different viral, bacterial, fungal, and parasitic agents (Table 1). The findings of EBV and Cryptococcus sp as the predominant pathogen in CSF and its more frequent occurrence than CMV in HIV patients have been reported previously (13).
This case report is concluded with a reminder of the utmost need for high clinical suspicion of EBV in unexplained neurological deficit disorders, pancytopenia, and multiorgan involvement along with screening for all possible bacterial and fungal etiologies.
Data availability statement
The datasets presented in this article are not readily available because no genetic sequential data was obtained. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
SN: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. GM: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. GS: Formal analysis, Investigation, Methodology, Writing – original draft. JS: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. AK: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2024.1485608/full#supplementary-material
References
1. Wang Y, Yang J, Wen Y. Lessons from Epstein-Barr virus DNA detection in cerebrospinal fluid as a diagnostic tool for EBV induced central nervous system dysfunction. BioMed Pharmacotherapy. (2022) 145:112392. doi: 10.1016/j.biopha.2021.112392
2. Kert JB. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol. (2019) 72:651–8. doi: 10.1136/jclinpath-2019-205822
3. Lu Yi, Li S, Su Z, Luo C, Gu M, Yuan D, et al. Presence of Epstein–Barr virus in cerebrospinal fluid is associated with increased mortality in HIV-negative cryptococcal meningitis. Med Mycology. (2024) 62. doi: 10.1093/mmy/myae052
4. Zhang N, Chen K, Zhu J, Li H, Shao J, Jiang H. Sequential infection of Epstein-Barr virus and cryptococcal encephalitis after umbilical cord blood transplantation in a child with X-linked adrenoleukodystrophy. Pediatr Transplant. (2020) 25. doi: 10.1111/petr.13956
5. Tsuruyama Y, Mori N, Yoshida S, Hayashi T. Epstein-Barr virus-related encephalitis in a young women: a case report. J Infect Chemother: Off J Jpn Soc.Chemother. (2020) 26:741–4. doi: 10.1016/j.jiac.2020.02.005
6. Portegies P, Corssmit N. Epstein-Barr virus and the nervous system. Curr.Opion. Neurol. (2000) 13:301–4. doi: 10.1097/00019052-200006000-00012
7. Fujimoto H, Asaoka K, Imaizumi T, Ayabe M, Shoji H, Kaji M. Epstein-Barr virus infection of the central nervous system. Intern Med. (2003) 42:33–40. doi: 10.2169/internalmedicine.42.33
8. Liu M, Wang R, Xie Z. T cell mediated immunity during Epstein-Barr virus infections in children. Infect Genet Evolution. (2023) 112:105443. doi: 10.1016/j.meegid.2023.105443
9. Kanno H, Watabe D, Shimazu N. Adhesion of Epstein-Barr virus-positive natural killer cell lines to cultured endothelial cells stimulated with inflammatory cytokines. Clin Exp Immunol. (2008) 151:519. doi: 10.1111/j.1365-2249.2007.03584.x
10. Shen Y, Tu J, Liu H, Dai T, Wu W. Epstein- Barr virus infection involving bilateral middle cerebellar peduncles in an old women: a case report. Neurol Sci. (2016) 37:479–81. doi: 10.1007/s10072-015-2431-7
11. Dyachenko P, Smianova O, Kurkanskaya V, Oleshko A, Dyachenko A. Epstein Barr virus associated encephalitis in a case series of more than 40 patients. Wiad Lek. (2018) 71:1224–30. Available online at: https://api.semanticscholar.org/CorpusID:52884028.
12. Peuchmaur M, Voisin J, Vaillant M, Truffot A, Lup J, Morand P, et al. Epstein Barr virus encephalitis: A review of case reports from the last 25 years. Microorganism. (2023) 11:2825. doi: 10.3390/microorganisms11122825
13. Dai F, Zhang L, Lu X, Lou J, Yu Y, Chen M. Epstein-Barr virus and cryptococcus are more predominant pathogens in cerebrospinal fluid of people living with HIV with low CD4 + cell counts. Research Square. (2022). doi: 10.21203/rs.3.rs-1696252/v1.
14. Traore L, Nikiema O, Ouattara AK, Compaore TR, Soubeiga ST, Diarra B, et al. EBV and hhv-6 circulating subtypes in people living with hiv in Burkina Faso, impact on cd4 T cell count and hiv viral load. Mediterr J Hematol Infect Dis. (2017) 9:e2017049. doi: 10.4084/mjhid.2017.049
15. Bertram G, Dreiner N, Krueger GR, Ramon A, Ablashi DV, Salahuddin SZ, et al. Frequent double infection with Epstein-Barr virus and human herpesvirus-6 in patients with acute infectious mononucleosis. In Vivo. (1991) 5:271–9.
16. Hatayama Y, Hashimoto Y, Motokura T. Frequent co-reactivation of Epstein-Barr virus in patients with cytomegalovirus viremia under immunosuppressive therapy and/or chemotherapy. J Int Med Res. (2020) 48:300060520972880. doi: 10.1177/0300060520972880
17. Olson D, Huntington MK. Co-infection with cytomegalovirus and Epstein-Barr virus in mononucleosis: case report and review of literature. S D Med. (2009) 62:351–3.
18. Böni M, Rieble L, Münz C. Co-infection of the epstein-barr virus and the kaposi sarcoma-associated herpesvirus. Viruses. (2022) 14:2709. doi: 10.3390/v14122709
19. McHugh D, Caduff N, Barros MHM, Ramer PC, Raykova A, Murer A, et al. Persistent KSHV infection increases EBV-associated tumor formation in vivo via enhanced EBV lytic gene expression. Cell Host Microbe. (2017) 22:61–73. doi: 10.1016/j.chom.2017.06.009
20. Blanco R, Carrillo-Beltrán D, Corvalán AH, Aguayo F. High-risk human papillomavirus and epstein-barr virus coinfection: A potential role in head and neck carcinogenesis. Biol (Basel). (2021) 10:1232. doi: 10.3390/biology10121232
21. Makielski KR, Lee D, Lorenz LD, Nawandar DM, Chiu YF, Kenney SC, et al. Human papillomavirus promotes Epstein-Barr virus maintenance and lytic reactivation in immortalized oral keratinocytes. Virology. (2016) 495:52–62. doi: 10.1016/j.virol.2016.05.005
22. Wan Z, Chen Y, Hui J, Guo Y, Peng X, Wang M, et al. Epstein-Barr virus variation in people living with human immunodeficiency virus in southeastern China. Virol J. (2023) 20:107. doi: 10.1186/s12985-023-02078-z
23. Epeldegui M, Vendrame E, Martinez-Maza O. HIV-associated immune dysfunction and viral infection: role in the pathogenesis of AIDS-related lymphoma. Immunol Res. (2010) 48:72–83. doi: 10.1007/s12026-010-8168-8
24. Naendrup JH, Garcia Borrega J, Eichenauer DA, Shimabukuro-Vornhagen A, Kochanek M, Boll B. Reactivation of EBV and CMV in severe COVID-19-epiphenomena or trigger of hyperinflammation in need of treatment? A large case series of critically ill patients. J Intensive Care Med. (2022) 37:1152–8. doi: 10.1177/08850666211053990
25. Saade A, Moratelli G, Azoulay E, Darmon M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect Dis Now. (2021) 51:676–9. doi: 10.1016/j.idnow.2021.07.005
26. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. (2022) 185:881–895.e20. doi: 10.1016/j.cell.2022.01.014
27. Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to epstein-barr virus reactivation. Pathogens. (2021) 10:763. doi: 10.3390/pathogens10060763
28. Zakafonetis C. Occurrence of infectious mononucleosis and Syphillis in the same individual. Ann Intern Med. (1953) 38:1053–7. doi: 10.7326/0003-4819-38-5-1053
29. Hirsiger JR, Fuchs PS, Hausermann P, Muller-Durovic B, Daikeler T, Recher M, et al. Syphilis reactivates latent epstein-barr virus reservoir via toll-like receptor 2 and B-cell receptor activation. Open Forum Infect Dis. (2019) 6(9). doi: 10.1093/ofid/ofz317
30. Ostendorf BN, Jehn CF, Vuong LG, Nogai H, Hemmati PG, Gebauer B, et al. Synchronous tuberculosis, Epstein-Barr virus-associated lymphoproliferative disorder and cytomegalovirus infection in an allogeneic transplant recipient: a case report. Springerplus. (2014) 3:278. doi: 10.1186/2193-1801-3-278
31. Gledhill RF, Greyling M. Epstein-Barr virus infection in a patient with active pulmonary tuberculosis. A case report. S Afr Med J. (1986) 70:761–2.
32. Singh S, Jha HC. Status of Epstein-barr virus coinfection with helicobacter pylori in gastric cancer. J Oncol. (2017) 2017:3456264. doi: 10.1155/2017/3456264
33. Fasciana T, Capra G, Calà C, Zambuto S, Mascarella C, Colomba C, et al. Helicobacter pylori and epstein–barr co-infection in gastric. Helicobacter. (2017) 1:73–82.
34. Samayoa-Reyes G, Weigel C, Koech E, Waomba K, Jackson C, Onditi I, et al. Effect of malaria infection on epstein-barr virus persistence in Kenyan children. J Infect Dis. (2024) 229:73–82. doi: 10.1093/infdis/jiad264
Keywords: EBV (Epstein-Barr virus), chronic active EBV, reactivation, immunosuppression, disseminated cryptococcosis
Citation: Mishra G, Sarnaik G, Samanta J, Keche A and Negi SS (2025) The probable progression of Epstein-Barr virus (EBV) to chronic active EBV/reactivation weakens the immune response and stimulates Cryptococcus neoformans infection, which invariably proves fatal: a case report and review of the literature. Front. Virol. 4:1485608. doi: 10.3389/fviro.2024.1485608
Received: 24 August 2024; Accepted: 30 December 2024;
Published: 28 January 2025.
Edited by:
Patricia A. Barril, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Erica Diani, University of Verona, ItalyPalmer Masumbe Netongo, Navajo Technical University, United States
Copyright © 2025 Mishra, Sarnaik, Samanta, Keche and Negi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanjay Singh Negi, bmVnaWRyQGFpaW1zcmFpcHVyLmVkdS5pbg==
 Gargee Mishra
Gargee Mishra Gaurav Sarnaik
Gaurav Sarnaik Joydeep Samanta
Joydeep Samanta Archana Keche1
Archana Keche1 Sanjay Singh Negi
Sanjay Singh Negi