
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virol. , 26 February 2024
Sec. Viral Disease Investigation
Volume 4 - 2024 | https://doi.org/10.3389/fviro.2024.1358963
This article is part of the Research Topic Enterovirus Surveillance in Europe and Beyond View all 6 articles
 Elisabeth Toverud Landaas1,2*
Elisabeth Toverud Landaas1,2* Ingvild Klundby2
Ingvild Klundby2 Per Kristian Knudsen3
Per Kristian Knudsen3 Anne-Marte Bakken Kran4
Anne-Marte Bakken Kran4 Susanne Dudman1,2
Susanne Dudman1,2 Andreas Lind2
Andreas Lind2 Mona Holberg-Petersen2
Mona Holberg-Petersen2Background: Enterovirus D68 (EV-D68) primarily causes respiratory infection, occasionally manifesting with neurological symptoms. Outbreak reports have been published from various countries including Norway, but a longitudinal study on EV-D68 prevalence in Northern Europe is lacking.
Methods: Respiratory samples from children ≤14 years received at Oslo University Hospital in the years 2012-2022 were examined for EV-D68. Samples from 2012-2015 were retrospectively screened using a semi-specific RT-PCR, with positive samples confirmed by an EV-D68 specific RT-PCR. Samples from 2016-2022 underwent routine diagnostics with the EV-D68 specific RT-PCR.
Results: Among the 22,911 samples tested, EV-D68 was detected in 338 samples (324 patients). Most EV-D68 cases occurred in August to December. The highest detection rate was recorded in 2014, 2016 and 2022 (6.0%, 7.8% and 6.6% of samples from August-December). Lower frequencies were observed in 2018 and 2019 (1.0% and 2.4%), and in the years before the 2014 outbreak (2012: 1.3%, 2013: 0.8%). Few cases were identified in 2020-2021. Children aged 0-1 years accounted for 40%, and 0-4 years for 78%, of the EV-D68 positive patients. Most of the patients with EV-D68 (83%) were hospitalised.
Discussion: Also in Norway, EV-D68 has caused outbreaks with significant disease burden, especially among the youngest children. The detection rate varies, with a trend towards biennial outbreaks, except for low numbers in 2018 and during the COVID-19 restrictions (2020-2021). Due to its potential for severe respiratory illness and significant neurological complications, conducting EV-D68 testing is essential both for diagnosing clinically suspected cases, and for monitoring the disease burden.
Enterovirus D-68 (EV-D68) has emerged as a significant contributor to acute respiratory illness, affecting both young children and individuals with pre-existing health conditions. The spectrum of symptoms ranges from those of a common cold to severe asthma-like presentations necessitating intensive care and mechanical ventilation in critical cases. Additionally, EV-D68 has since 2014 been associated with the neurologic complication acute flaccid myelitis (AFM) (1–3).
From its discovery in 1962 until 2010, EV-D68 was primarily associated with sporadic cases of respiratory illness. However, in the autumn of 2014, large outbreaks of EV-D68 respiratory disease were reported in various countries (4). Coinciding with these outbreaks, there was a marked increase in cases of children developing AFM (4). Similarly, an outbreak of EV-D68 was recognised at our hospital in Norway during the autumn 2014, also linked with cases of severe AFM (5, 6).
The 2014 outbreaks raised awareness of the importance of EV-D68, leading to increased testing and reporting in subsequent years. Outbreaks in 2016 and 2018 were observed in North America and European countries, and a biennial circulation pattern has been suggested (7–10). Strict SARS-CoV-2 infection control measures likely mitigated potential outbreaks in 2020, but following their relaxation in 2021, higher incidences were reported worldwide (7, 11).
Aside from data from 2014, our understanding of the health impact of EV-D68 in Norway is limited. No surveillance is in place, and only a few laboratories specifically test for EV-D68. To investigate the long-term incidence rates and circulation patterns of EV-D68 infection in Norway, including detailed information on seasonal distribution and burden of infection in different age groups, we have examined EV-D68 presence in paediatric respiratory samples from the last eleven years, received at the microbiology laboratory in Norway’s largest hospital. Such information is of importance both for surveillance strategy and preventive measures planning, and to predict burden of disease in a clinical setting to support strategic priorities in health service.
The Department of Paediatric and Adolescent Medicine at Oslo University Hospital (OUH) serves as the primary healthcare facility for the majority of children in Oslo. The Department of Microbiology at OUH is responsible for analysing respiratory samples from OUH as well as from primary care providers and general practitioners in the Oslo region. All respiratory specimens from children aged 0-14 years received at the Department of Microbiology at OUH for routine respiratory virus analysis from 2012 to 2022 were included in this study. Most included samples, 20,699 (90.3%), were specimens from the upper respiratory tract (nasopharynx, nose and throat), while 1672 (7.3%) were lower respiratory tract samples, and the remaining 540 (2.4%) were unspecified specimens.
Between 2012 and 2015, 3,884 samples collected from June to December underwent retrospective screening for EV-D68. Samples from September to November 2014 (n=569) were processed in the same year (5), while most samples were assessed in 2015 and 2016. Out of the 3,315 samples available for evaluation, those obtained from June 2012 to October 2013 (n=1,441) were primary specimens, while the remainder (n=1,874) were RNA eluates. All samples and RNA eluates had been stored at -70°C.
Starting from 1 June 2016 all respiratory samples from children aged ≤14 years from hospitals (n=17,683) and ≤10 years from primary care (n=1,344) were prospectively analysed for EV-D68 along with other respiratory viruses as part of routine diagnostics.
Patients whose samples were requested from emergency departments or other hospital wards were defined as hospitalised.
All samples were analysed with laboratory developed RT-PCR methods. The samples from September to November 2014 were analysed as described by Bragstad et al. (5). The remaining samples from 2012 to 2015 were analysed with a laboratory developed generic enterovirus RT-PCR targeting the conserved 5’ non-translated region (NTR) of the enterovirus genome, using the forward primer 5′- CCCTGAATGCGGCTAATC -3′ and the reverse primer 5′- GAAACACGGACACCCAAAGTA -3′. The probe used, 5′-FAM- CGCCACRGACTTGCGCRTTACG –BHQ-3′, was designed to detect enteroviruses in group C and D. All positive results from 2012 to 2015 were later reanalysed with a specific EV-D68 RT-PCR targeting the VP1 gene, based on the method by Wylie et al. (12). From 2016, all samples were analysed with the RT-PCR based on Wylie et al. as part of routine diagnostics. All protocols and sequences are available upon request.
Data analyses were performed using Microsoft Excel 2016 (Microsoft Corporation).
A total of 22,911 respiratory samples collected from 2012 to 2022 were examined for EV-D68, and the virus was detected in 338 of them. The EV-D68 positive samples were from 324 patients (including 14 patients with two positive samples), and 270 (83.0%) of these were hospitalised (Table 1).
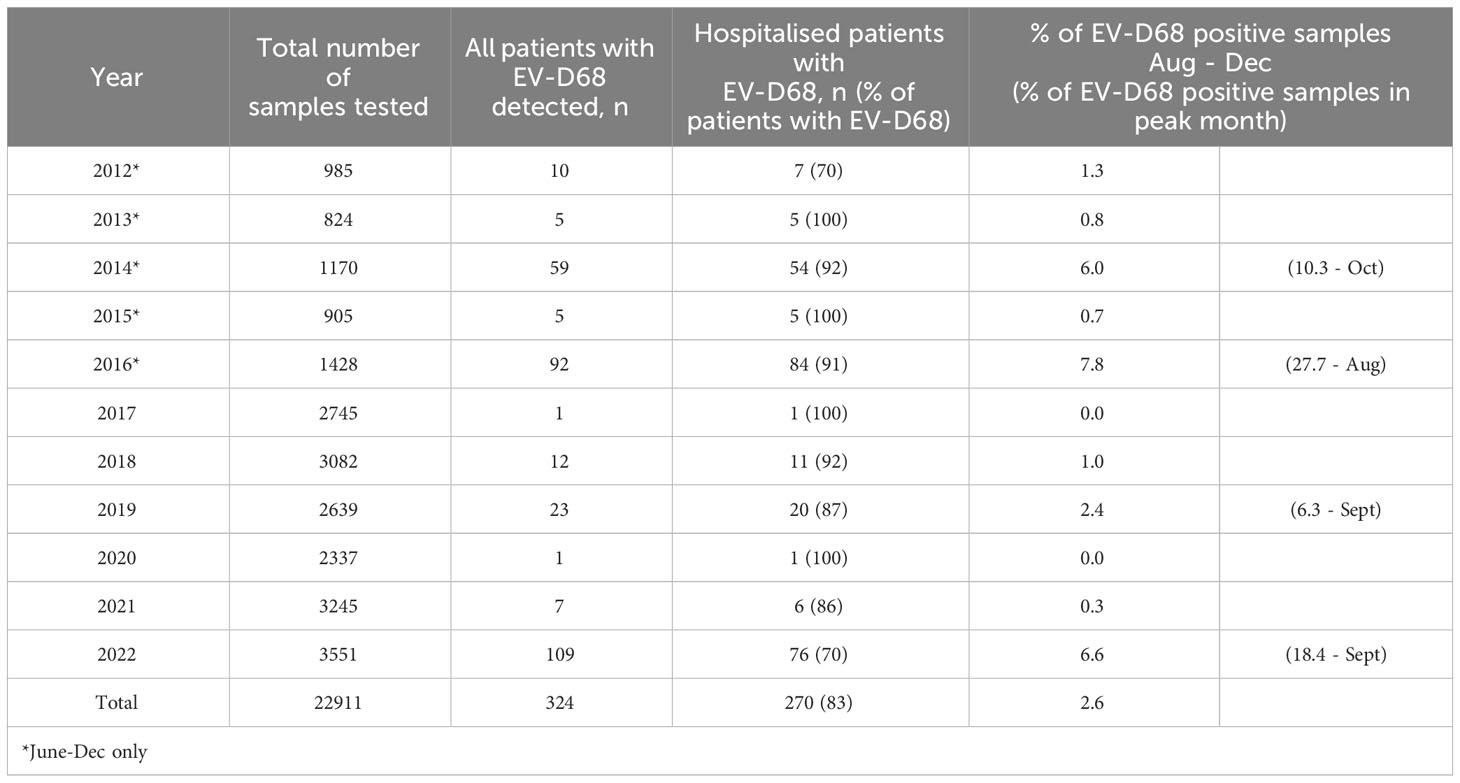
Table 1 Samples examined for EV-D68 per year, with the total number of EV-D68 cases and the number of hospitalised cases, and the percentage of positive samples during the peak months.
Out of the 338 EV-D68 positive samples, 313 (92.6%) were upper respiratory tract specimens, while 17 (5.0%) were from the lower respiratory tract. The total positivity rates in upper versus lower respiratory tract specimens were 1.5% and 1.0%, respectively. EV-D68 was most frequently detected in the months August to October, and only sporadically in the months January to July (ranging from 0 to 4 cases per month for all years combined) (Figure 1).
The number of EV-D68 cases was highest in the years 2014, 2016, and 2022, with 59, 92 and 109 patients testing positive, respectively, constituting 6.0%, 7.8%, and 6.6% of the samples collected between August and December (Table 1). In 2014, the highest number of patients with EV-D68 was recorded in October (n=18, constituting 10.3% of the samples), in 2016, the peak occurred in August (40 patients, 27.7% of the samples), while in 2022, it was observed in September (49 patients, 18.4% of the samples). Notably, no samples were positive for EV-D68 in the summer and autumn of 2017 and 2020. In the years 2012, 2013, 2015, 2018, 2019 and 2021, the number of patients with EV-D68 infection was low (n=5-23), constituting only 0.6-1.1% of the samples collected between August and December (Table 1).
As seen in Figure 2 and Table 2, the majority of patients with EV-D68 belonged to the youngest age groups. Ages 0 and 1 years accounted for 40.1%, while ages 0-4 years constituted 78.4% of the confirmed cases of EV-D68. However, 59.9% of the total number of samples were from the 0–1 year age group, and 81.2% from children aged 0–4 years (data not shown). As seen in Table 3, the prevalence of EV-D68 positive samples was in some years higher among the patients in certain higher age groups, such as 23.3% of the samples from 5-year-olds throughout 2016 (n=6, Table 2).
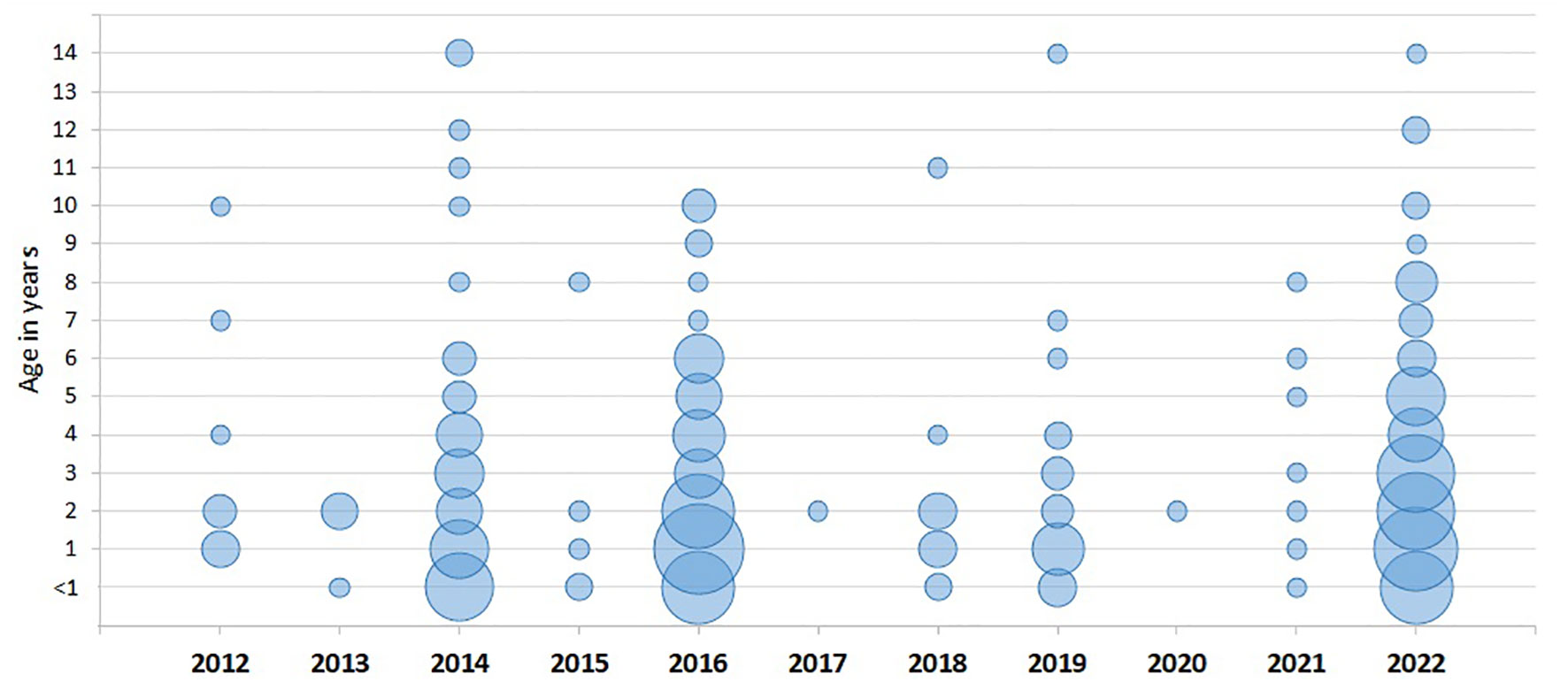
Figure 2 Cases of EV-D68 by age in years, per year. The size of the bubbles reflects the relative number of patients per age and year.
In this first long-term retrospective study on EV-D68 circulation in Norway, we analysed nearly 23,000 respiratory samples from paediatric patients spanning the period 2012 to 2022. Our findings reveal significant variation in the annual incidence of EV-D68. Among the 324 patients with detected EV-D68, the majority were hospitalised, indicating a substantial health burden on children during outbreak years.
Reports from other countries have indicated biennially recurring EV-D68 outbreaks (7–10), and our data suggest a similar circulation pattern. The first globally recognised outbreak of EV-D68 in 2014 drew considerable attention to the virus and affected children also in Oslo (4, 5). Our results show that a greater number of paediatric patients than previously reported were infected with EV-D68 during the 2014 outbreak, with most cases occurring in the hospital setting. Notably, our data from the 2016 season reveal an even higher incidence of EV-D68 compared to the 2014 outbreak, again primarily among hospitalised patients. This high incidence in 2016 aligns with reports of outbreaks from Sweden, France, Spain, the Netherlands and the United States (7, 8, 10, 13, 14). However, our 2018 findings deviate from reports from other countries, as we detected EV-D68 in samples from only a small number of patients. This contrasts with the expected biennial circulation pattern suggested by the 2014 and 2016 outbreaks. There are no published reports documenting the 2018 and 2019 seasons in other Nordic countries, leaving us uncertain whether this deviation in the pattern was unique to our population or also occurred in neighbouring countries. In the summer and autumn of 2020, we found no positive samples for EV-D68, even though the total number of samples tested did not differ from the prepandemic period and the testing strategy was unchanged. Our laboratory managed to maintain test capacity for respiratory pathogens as before the pandemic. Notably, during 2020-2021, all paediatric samples where “respiratory viruses” were requested were tested for other respiratory viruses, including EV-D68, in addition to SARS-CoV-2, providing the same diagnostic service as before. The absence of EV-D68 cases aligns with the marked decline observed in other viral infections during this period (15), and we therefore believe that it is primarily a result of the strict SARS-CoV-2 containment measures, rather than underdiagnosis. In 2021, as measures eased, there was a surge in EV-D68 cases in Europe and the USA (7, 11). However, our data showed only 7 cases that year, all in November and December, possibly due to prolonged infection control measures in Norway.
The highest number of EV-D68 cases was observed in 2022. In that year, we also received the highest number of samples for testing, possibly due to a surge in respiratory infections combined with a lower threshold for testing after the SARS-CoV-2 pandemic. Nevertheless, as many as 76 of the patients were tested in the hospital, suggesting significant symptoms, though clinical data and information on co-infections are not available. Notably, a substantial EV-D68 outbreak was reported in Finland during the autumn of 2022, leading to the hospitalisation of 56 children due to respiratory illness, with one child diagnosed with EV-D68-related encephalitis (16). High numbers of EV-D68 in 2022 have also been reported from the United States (17, 18). Whether the increase in cases observed in 2022 can be attributed to reduced population immunity after years of lower virus circulation, natural variation, or a trend toward increased virus occurrence remains to be determined.
Looking back to 2012 and 2013, we identified 11 and 5 cases, respectively. This indicates that EV-D68 was already present in Norway before the 2014 outbreak. Studies of seropositivity from Finland, England and the Netherlands indicate that EV-D68 was highly prevalent in these countries already in the previous decades (19–21). While we do not have serological data nor respiratory samples for testing prior to 2012, it is unknown how the virus circulated in Norway in earlier years. Nevertheless, our low numbers from 2012 and 2013 indicate that EV-D68 was at least not causing any high burden of disease in these years.
Our data highlight that EV-D68 is predominantly identified in the youngest age group. Nevertheless, the virus also shows relevance in the older children, with a high percentage of EV-D68 positive samples in older age groups some years.
Our study is limited by the absence of detailed clinical information. Nonetheless, insights from previous investigations suggest that EV-D68 is primarily identified in children with severe respiratory illness (4, 5, 7, 16, 17). Among our EV-D68 patients from 2014, two cases had severe EV-D68 associated AFM (6). This is also probably true for one of our cases from 2018, as one patient with detected EV-D68 and AFM was reported from OUH to the National Commission for the Certification of Poliomyelitis Eradication at the Norwegian Institute of Public Health (NIPH) (personal communication from Silje Lae Solberg, NIPH). In 2016, there were three cases of EV-D68 associated AFM in Norway (22), but these occurred in regions not covered by our hospital. Norway upholds a well-established national surveillance program for acute flaccid paralysis in children under 15 years of age, including suspected cases of AFM (23). As part of this system, respiratory specimens are collected to identify cases associated with EV-D68. No further cases of suspected EV-D68 associated AFM has been reported to the surveillance program since 2016 (personal communication).
It is unknown whether the AFM cases in 2014, 2016 and 2018 were caused by neurovirulence of the circulating strain, lack of immunity or simply a rare presentation seen with a larger outbreak of the virus. It has been estimated that <1% of EV-D68 cases develop AFM (2), and according to the detection rates in our study, more cases of AFM should not necessarily have occurred in the years since. However, with trends for increasing numbers of patients suffering from EV-D68 infection, it is likely that there will be new cases in the years to come. Awareness of EV-D68 as a potential cause for this clinical polio-like illness is thus crucial.
To our knowledge this is the first longitudinal study of EV-D68 circulation from any of the Nordic countries. The reports of outbreaks from Sweden in 2016 (14) and Finland in 2022 (16) are in line with our observations. Whether the circulation of EV-D68 in other seasons and other Nordic countries and regions also followed the same pattern as in our material remains to be examined.
As our dataset originates solely from the Oslo area, it might not offer a comprehensive representation of the entire Norwegian population. Nevertheless, our samples were obtained from the Department of Paediatric and Adolescent Medicine at Norway’s largest hospital OUH, which serves as the primary children’s healthcare facility for the greater Oslo region. Additionally, our laboratory analyses respiratory samples not only from OUH, but also from primary care providers and general practitioners in the Oslo region. Collectively, our data thereby represent a substantial share of the Norwegian paediatric population.
In summary, our study on EV-D68 epidemiology in Norway showed significant variations in the annual incidence with peaks in 2014, 2016, and 2022. Though disrupted by low incidence in 2018 and during the COVID-19 pandemic, this indicates a biennial cycle, a trend also observed in other countries. Whether the increase in EV-D68 cases in 2022 represent a genuine rise in virus occurrence in years of outbreaks remains to be seen. Nevertheless, it is apparent that EV-D68 has become a frequently encountered virus in Norway. The knowledge from our study on EV-D68 in children is important not only for planning of health care services, but also for public health policy and outbreak responses. Given its potential to cause severe respiratory illness and significant neurological complications, maintaining vigilance and ensuring EV-D68 screening and testing for clinically suspected cases is crucial.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the studies involving humans because all samples were collected as part of routine diagnostics, and the study contains anonymous, aggregated laboratory data only. The requirement of ethical approval for the studies was waived by the data protection officer at Oslo University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because all samples were collected as part of routine diagnostics, and the study includes anonymous, aggregated laboratory data only. This was approved by the local data protection officer.
ETL: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. IK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – review & editing. PKK: Conceptualization, Resources, Writing – review & editing. A-MBK: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. SD: Conceptualization, Writing – review & editing. AL: Conceptualization, Methodology, Resources, Writing – review & editing. MH-P: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank Bente Krog for her engaged work with the establishment and follow-up of the EV-D68 RT-PCR. We acknowledge Silje Lae Solberg for providing information on AFM cases from the national surveillance program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dyda A, Stelzer-Braid S, Adam D, Chughtai AA, MacIntyre CR. The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68) - what is the evidence for causation? Euro Surveill. (2018) 23(3):17–00310. doi: 10.2807/1560-7917.ES.2018.23.3.17-00310.
2. Hixon AM, Frost J, Rudy MJ, Messacar K, Clarke P, Tyler KL. Understanding enterovirus D68-induced neurologic disease: A basic science review. Viruses. (2019) 11(9):821. doi: 10.3390/v11090821.
3. Messacar K, Asturias EJ, Hixon AM, Van Leer-Buter C, Niesters HGM, Tyler KL, et al. Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality. Lancet Infect Dis. (2018) 18:e239–e47. doi: 10.1016/S1473-3099(18)30094-X.
4. Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: a systematic review. Lancet Infect Dis. (2016) 16:e64–75. doi: 10.1016/S1473-3099(15)00543-5.
5. Bragstad K, Jakobsen K, Rojahn AE, Skram MK, Vainio K, Holberg-Petersen M, et al. High frequency of enterovirus D68 in children hospitalised with respiratory illness in Norway, autumn 2014. Influenza Other Respir Viruses. (2015) 9:59–63. doi: 10.1111/irv.12300.
6. Pfeiffer HC, Bragstad K, Skram MK, Dahl H, Knudsen PK, Chawla MS, et al. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill. (2015) 20:21062. doi: 10.2807/1560-7917.ES2015.20.10.21062.
7. Andres C, Vila J, Creus-Costa A, Pinana M, Gonzalez-Sanchez A, Esperalba J, et al. Enterovirus D68 in hospitalized children, barcelona, Spain, 2014-2021. Emerg Infect Dis. (2022) 28:1327–31. doi: 10.3201/eid2807.220264.
8. Kramer R, Sabatier M, Wirth T, Pichon M, Lina B, Schuffenecker I, et al. Molecular diversity and biennial circulation of enterovirus D68: a systematic screening study in Lyon, France, 2010 to 2016. Euro Surveill. (2018) 23(37):1700711. doi: 10.2807/1560-7917.ES.2018.23.37.1700711.
9. Messacar K, Pretty K, Reno S, Dominguez SR. Continued biennial circulation of enterovirus D68 in Colorado. J Clin Virol. (2019) 113:24–6. doi: 10.1016/j.jcv.2019.01.008.
10. Uprety P, Curtis D, Elkan M, Fink J, Rajagopalan R, Zhao C, et al. Association of enterovirus D68 with acute flaccid myelitis, philadelphia, pennsylvania, USA, 2009-2018. Emerg Infect Dis. (2019) 25:1676–82. doi: 10.3201/eid2509.190468.
11. Benschop KS, Albert J, Anton A, Andres C, Aranzamendi M, Armannsdottir B, et al. Re-emergence of enterovirus D68 in Europe after easing the COVID-19 lockdown, September 2021. Euro Surveill. (2021) 26(45):2100998. doi: 10.2807/1560-7917.ES.2021.26.45.2100998.
12. Wylie TN, Wylie KM, Buller RS, Cannella M, Storch GA. Development and evaluation of an enterovirus D68 real-time reverse transcriptase PCR assay. J Clin Microbiol. (2015) 53:2641–7. doi: 10.1128/JCM.00923-15.
13. Cassidy H, Lizarazo-Forero E, Schuele L, Van Leer-Buter C, Niesters HGM. Off-season circulation and characterization of enterovirus D68 with respiratory and neurological presentation using whole-genome sequencing. Front Microbiol. (2022) 13:1088770. doi: 10.3389/fmicb.2022.1088770.
14. Dyrdak R, Grabbe M, Hammas B, Ekwall J, Hansson KE, Luthander J, et al. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September 2016. Euro Surveill. (2016) 21(46):30403. doi: 10.2807/1560-7917.ES.2016.21.46.30403.
15. Knudsen PK, Lind A, Klundby I, Dudman S. The incidence of infectious diseases and viruses other than SARS-CoV-2 amongst hospitalised children in Oslo, Norway during the Covid-19 pandemic 2020-2021. J Clin Virol Plus. (2022) 2:100060. doi: 10.1016/j.jcvp.2021.100060.
16. Peltola V, Osterback R, Waris M, Ivaska L, Tahtinen PA, Laine M, et al. Enterovirus D68 outbreak in children, Finland, august-september 2022. Emerg Infect Dis. (2023) 29:1258–61. doi: 10.3201/eid2906.221795.
17. Fall A, Han L, Abdullah O, Norton JM, Eldesouki RE, Forman M, et al. An increase in enterovirus D68 circulation and viral evolution during a period of increased influenza like illness, The Johns Hopkins Health System, USA, 2022. J Clin Virol. (2023) 160:105379. doi: 10.1016/j.jcv.2023.105379.
18. Ma KC, Winn A, Moline HL, Scobie HM, Midgley CM, Kirking HL, et al. Increase in acute respiratory illnesses among children and adolescents associated with rhinoviruses and enteroviruses, including enterovirus D68 - United States, july-september 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1265–70. doi: 10.15585/mmwr.mm7140e1.
19. Karelehto E, Koen G, Benschop K, van der Klis F, Pajkrt D, Wolthers K. Enterovirus D68 serosurvey: evidence for endemic circulation in the Netherlands, 2006 to 2016. Euro Surveill. (2019) 24(35):1800671. doi: 10.2807/1560-7917.ES.2019.24.35.1800671.
20. Pons-Salort M, Lambert B, Kamau E, Pebody R, Harvala H, Simmonds P, et al. Changes in transmission of Enterovirus D68 (EV-D68) in England inferred from seroprevalence data. Elife. (2023) 12:e76609. doi: 10.7554/eLife.76609.
21. Smura T, Ylipaasto P, Klemola P, Kaijalainen S, Kyllonen L, Sordi V, et al. Cellular tropism of human enterovirus D species serotypes EV-94, EV-70, and EV-68 in vitro: implications for pathogenesis. J Med Virol. (2010) 82:1940–9. doi: 10.1002/jmv.21894.
22. Knoester M, Helfferich J, Poelman R, Van Leer-Buter C, Brouwer OF, Niesters HGM, et al. Twenty-nine cases of enterovirus-D68-associated acute flaccid myelitis in europe 2016: A case series and epidemiologic overview. Pediatr Infect Dis J. (2019) 38:16–21. doi: 10.1097/INF.0000000000002188.
23. The National Institute of Public Health. National plan of action to sustain a poliomyelitis-free status. National polio outbreak preparedness and response plan (2018). Available online at: https://www.fhi.no/en/publ/2018/national-plan-of-action-to-sustain-a-poliomyelitis-free-status/.
Keywords: enterovirus D68, EV-D68, respiratory infections, paediatric infections, epidemiology
Citation: Landaas ET, Klundby I, Knudsen PK, Kran A-MB, Dudman S, Lind A and Holberg-Petersen M (2024) Emergence of enterovirus D68 in a Norwegian paediatric population 2012-2022. Front. Virol. 4:1358963. doi: 10.3389/fviro.2024.1358963
Received: 20 December 2023; Accepted: 08 February 2024;
Published: 26 February 2024.
Edited by:
Kimberley Benschop, National Institute for Public Health and the Environment, NetherlandsReviewed by:
Omkar Indari, St. Jude Children’s Research Hospital, United StatesCopyright © 2024 Landaas, Klundby, Knudsen, Kran, Dudman, Lind and Holberg-Petersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabeth Toverud Landaas, ZWxpc2xhbmRAbWVkaXNpbi51aW8ubm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.