
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Virol., 13 November 2023
Sec. Virus and Host Immunity
Volume 3 - 2023 | https://doi.org/10.3389/fviro.2023.1227314
Ebola virus disease (EVD) remains a significant public health threat, with sporadic outbreaks occurring in Sub-Saharan Africa. Survivors of EVD may experience various post-infection symptoms, collectively known as post-Ebola virus syndrome (PES), which include chronic arthralgia, uveitis, headache, and psychosocial stressors. In this review, we discuss the persistence of Ebola virus in survivors and its possible role in the reemergence of current outbreaks. We highlight that waning immunity of survivors enhances viral persistence and may lead to viral reactivation and recurrence of disease in previously affected tissues. The delicate equilibrium between diminished immune cell surveillance and limited viral replication may lead to enduring chronic inflammation. Our systematic review, based on an extensive survivor cohort, underscores the importance of continued research and preparedness efforts to combat future outbreaks through adequate surveillance and timely public health interventions. This review serves as a comprehensive guide to understanding the complexities of EVD survivorship, the challenges of PES, and the strategies to mitigate its impact.
Ebola virus (EBOV) is a highly virulent pathogen in the Filoviridae family of negative-sense, single-stranded RNA viruses capable of inducing a severe haemorrhagic fever syndrome in humans and non-human primates (1, 2). The virus causes Ebola virus disease (EVD) and garnered worldwide attention due to the significant number of cases associated with the 2013-2016 outbreak, which marked one of the largest outbreaks of EVD in history (3, 4). Initially identified in 1976 during the first reported EVD epidemic in the Ebola River Valley in Yambuku, Zaire (now the Democratic Republic of the Congo), the virus has since caused sporadic human disease outbreaks in several African countries (5). Since its discovery, there have been at least 37 documented outbreaks of EVD in Africa, with the most recent outbreak being declared over in Uganda on January 11, 2023 (6).
Transmission of EVD to humans predominantly occurs through direct contact with infected humans or animals, such as fruit bats, apes, monkeys, and forest antelopes. The fruit bat is widely regarded as the likely natural host of EBOV, and the close interaction between local communities and these bats renders the local population susceptible to EVD (7–9). While EVD is characterized by fever, severe fatigue, headache, and hemorrhagic conditions leading to multiple organ dysfunction and death, it is noteworthy that some individuals survive despite the disease’s high mortality rate.
However, recent studies indicate that individuals who have overcome Ebola infection can experience relapses and initiate new outbreaks even several years after their initial infection (10, 11). This surprising revelation suggests that the virus can persist in specific bodily sites for extended periods, including the brain’s ventricular system, the eye’s aqueous humor, and the testes’ Sertoli cells in both human and non-human primate survivors of EBOV infection (12–16). Prolonged persistence can result in the virus resurfacing, causing severe and frequently fatal disease (12, 17, 18). Notably, the virus has been observed to enter a dormant state in a small subset of survivors who had previously tested negative, and in rare instances, it can reactivate to initiate new infections and transmissions years later (8, 13). This infrequent yet significant occurrence represents a novel paradigm, emphasizing the significance of understanding this rare transmission route that could contribute to the resurgence of Ebola outbreaks. While acknowledging its rarity, this discovery imparts vital insights into the potential mechanisms driving the resurgence of outbreaks.
Gaining a deeper understanding of the factors behind recent human outbreaks may provide enhanced strategies for their control. This review delves into the role of survivors in the resurgence of EBOV in Sub-Saharan Africa, as well as the factors that amplify the probability of the virus leading to disease recurrence within populations. Furthermore, the review thoroughly examines strategies for managing the ongoing health of survivors and effectively curbing the potential for the reemergence of new outbreaks in the future.
The first documented case of EBOV infection occurred in the Democratic Republic of the Congo (DRC) (19) during an outbreak of severe haemorrhagic fever. Concurrently, a similar outbreak occurred in Sudan (20). Subsequent investigations highlighted differences between the strains isolated from these two countries: Sudan ebolavirus (SUDV) in Sudan and Zaire ebolavirus (ZEBOV) in the DRC (21). Following the initial identification of the virus in 1976, sporadic outbreaks were documented in 1977 and 1979, preceding the occurrence of EVD outbreaks in various other countries.
Reston ebolavirus (RSTV) was discovered in 1989 and 1990, causing disease exclusively in non-human primates and guinea pigs (22, 23). During the same period, the Taï forest ebolavirus (TAFV) was isolated (24). The Bundibugyo ebolavirus (BDBV) first emerged in Uganda in 2007 (25) and again in 2012 (26), capable of inciting epidemics. In 2018, the Bombali virus (BOMV) was identified in Sierra Leone, although its potential to cause human disease remains uncertain (9), in contrast to the ZEBOV, which is the most virulent among all the strains (27).
All the six known EBOV species (SUDV, ZEBOV, RSTV, TAFV, BDBV and BOMV) share common features and gene order (28, 29). Their viral genomes, approximately 19 kb long, encode seven main proteins: nucleoprotein (NP), viral protein (VP) 24, VP30, VP35, VP40, the glycoprotein (GP), and the RNA-dependent RNA polymerase (L) (Figure 1) (30, 32). NP, a multifunctional protein, encapsulates the viral genome into the nucleocapsid (33, 34). VP40, one of the most abundantly expressed proteins, drives viral assembly and budding (35). L functions as an RNA-dependent RNA polymerase (RdRp) and, in association with VP30, forms the RdRp complex responsible for viral genome transcription and replication (36–38). VP24 and VP35 play roles in suppressing interferon signaling, aiding in evading the host’s immune response (39). GP, the sole protein found on the virion’s surface, is essential for binding to target cells, mediating membrane fusion, and facilitating the release of the viral genome. GP is responsible for pathogenic differences among ebolaviruses (30, 40).
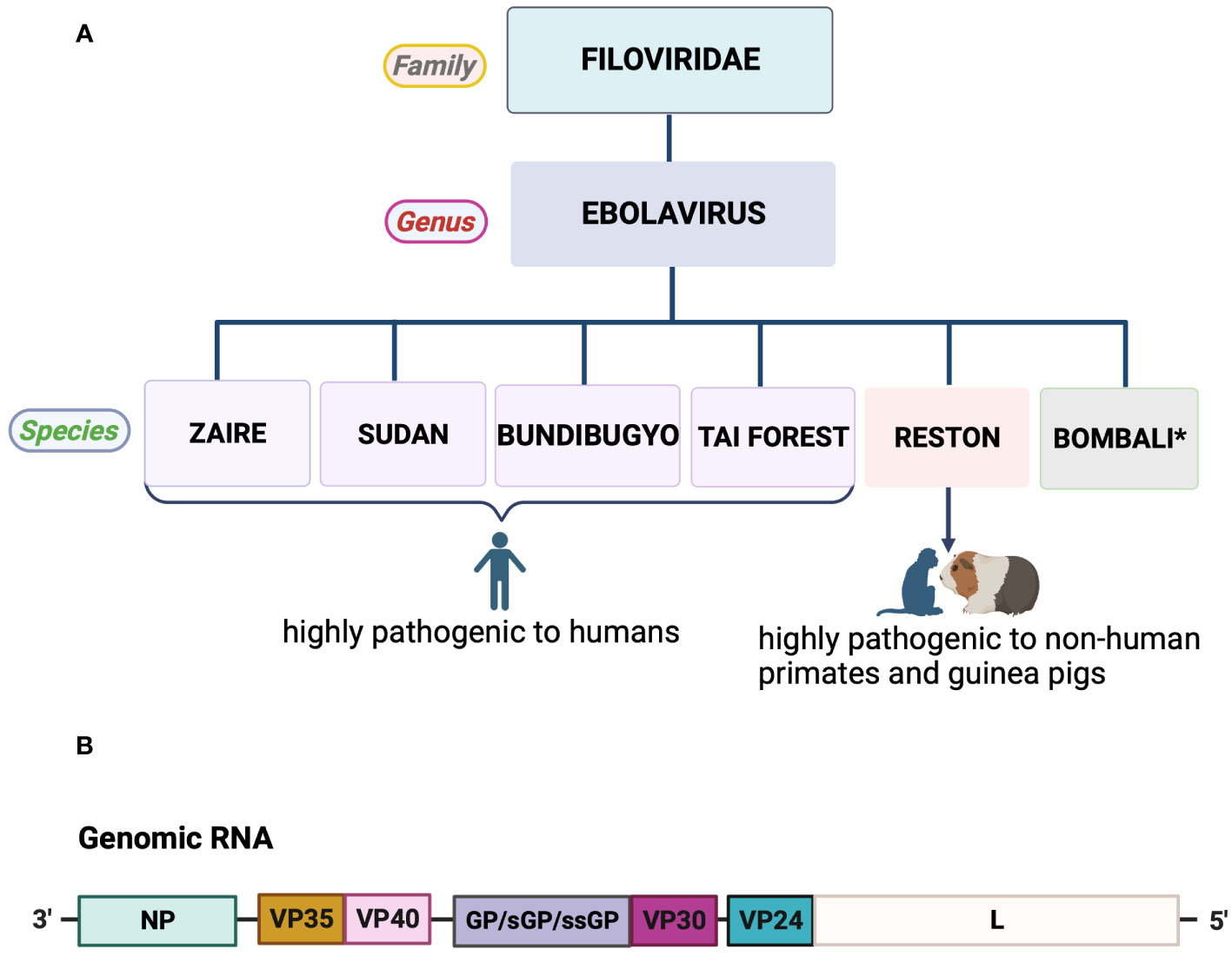
Figure 1 Taxonomy and genome organization of Ebola virus. (A) Most species of the genus Ebolavirus are lethal to humans and non-human primates, with the exception of Bombali virus. *No human infections recorded. (B) Schematic representation of Ebola virus genome organization. All genes encode one protein each, except for GP, which produces three pre-proteins due to transcriptional editing (28, 30, 31). Created with BioRender.com.
EBOV genomes exhibit a remarkable level of similarity, characterized by identical genome organization and often identical sequences within the same strain (Figure 1) (27, 29). Comparative genomics analysis has shown approximately 97% similarity between the genomes of the 1976 DRC outbreak and the 2014 West Africa outbreak, indicating significant genetic sequence homogeneity within the EBOV species (29). Despite these similarities, there is considerable divergence in their sequences, particularly in the intergenic regions and specific areas of genes encoding the GP, NP, and L (25, 29). These genomic variations provide insights into the evolution and dynamics of EBOV, which are crucial for understanding the virus’s virulence and immune responses. Identifying specific regions within the genomes, such as epitope-binding sites, can aid in developing potential vaccine targets and therapeutic strategies.
In summary, the comparative genomics of EBOV species highlights the conservation of genetic organization and diversity within the EBOV genus. This knowledge contributes to our understanding of the virus’s origins, transmission, and potential strategies for combating the resurgence of EVD.
Since the year 2000, the prevalence of EVD outbreaks has firmly established EBOV as a prominent health concern within the African continent, particularly in central and western regions (41). As of 2023, a few cases of Ebola have been reported outside of Africa in countries including the United States, Italy, England, Russia, the Philippines, and Spain (42). These instances are attributed to human mobility, particularly air travel, which can facilitate the cross-border transmission of infectious diseases.
Since its initial appearance in 1976, the continuous emergence of various EBOV species across Sub-Saharan Africa has led to outbreaks of EVD spanning 13 African countries. Notably, significant resurgences have been witnessed in regions such as Sudan, Guinea, Uganda, and the DRC (Figure 2) (4, 41).
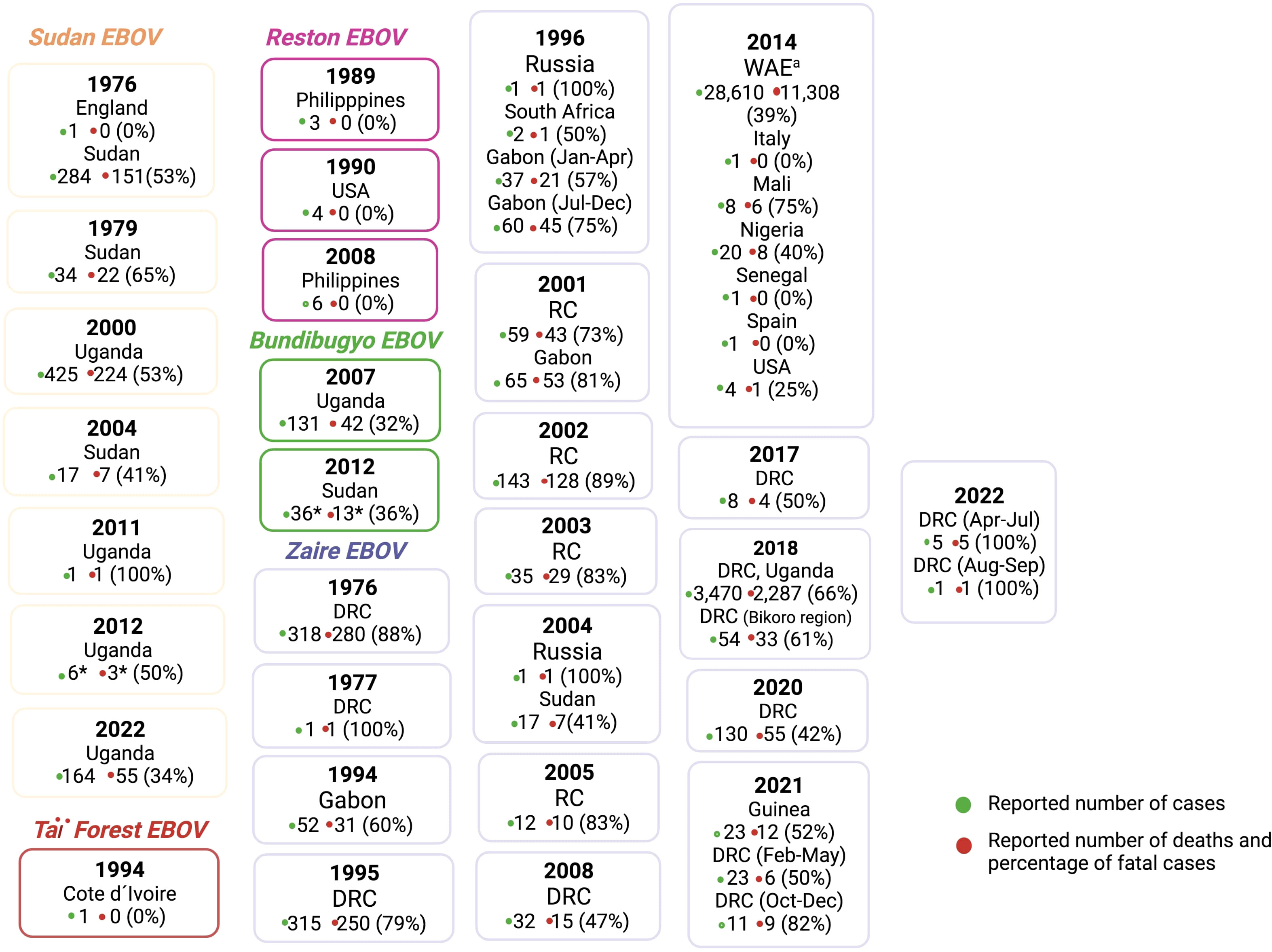
Figure 2 Ebola virus species outbreaks from 1976-2022. RC: Republic of the Congo; *Laboratory confirmed cases only; aWest African Epidemic in Guinea, Liberia and Sierra Leone. The figure was constructed based on information at the Centres for Disease Control and Prevention (CDC) (42). Created with BioRender.com.
Research focusing on survivors who triumphed over the virus across different epidemics throughout the continent has provided intriguing insights into the realm of EBOV RNA persistence, shedding light on its intricate dynamics. EBOV is believed to persist specifically in immune-privileged sites, including the eye, brain, and testes. This persistence is evident in various bodily fluids after recovery, with a comprehensive record, particularly in semen, breast milk, and aqueous humor (14, 15, 43–47). The detectability of EBOV RNA in skin swabs up to 23 days after viral clearance, despite concurrent negative cell culture results, indicates its enduring presence (48). A singular case even revealed the isolation of EBOV from a urine sample, a full 9 days following the cessation of viraemia (48). Strikingly, the virus’s tenacious persistence was confirmed in the aqueous humor of an eye from a survivor afflicted by severe acute EVD, complicated by sight-threatening uveitis and multisystem organ failure (14). During the 2014 Ebola outbreak in Sierra Leone, a nurse experienced meningitis 9 months after recovering from EVD, with subsequent detection of EBOV in her blood and cerebrospinal fluid (49). A similar study conducted on a pregnant woman in Guinea who survived EVD revealed the persistence of a high viral load in both the amniotic fluid and placenta (50). The cycle threshold (Ct) values for the viral load in the amniotic fluid were 22.2, while the placental swab recorded a value of 18.4. These findings strongly suggest that the virus can persist in these reproductive tissues even after the mother’s recovery from the infection (50, 51). This diversity of viral persistence across bodily fluids underscores the virus’s adaptability to distinct physiological niches, yet the selective preference for specific compartments remains an enigmatic puzzle.
In a pilot study involving 93 EVD survivors in Sierra Leone, EBOV RNA was identified in the semen of half of the participants at least once during the follow-up period. Remarkably, viral shedding persisted in 26% of the survivors, remaining evident even after 7-9 months (43).
In a related study, Thorson et al. reported the persistence of Ebola viral RNA in semen of male participants who survived EBOV infection in Sierra Leone (52). Post recovery examinations utilizing quantitative reverse transcription polymerase chain reaction (qRT-PCR) further revealed virus persistence in more than 20% of the survivors (>35 years) at 1 year.
Surprisingly, EBOV RNA persisted in the semen of 11 male survivors for more than two years, raising concerns about prolonged virus carriage and potential sexual transmission by asymptomatic survivors (53). While the exact mechanism behind EBOV RNA persistence in bodily fluids remains elusive, the need for further investigations into viability prevalence is evident.
Infectious virus has been detected in the semen of EVD survivors using Vero E6 cell culture models and qRT-PCR, bearing significant implications for personal and public health (15, 44). It’s worth noting that although the persistence of EBOV in a subset of survivors raises concerns about potential disease reactivation and viral transmission through bodily fluids, the proportion of survivors in whom this occurs is limited. Moreover, modern GeneXpert Ebola assays have effectively revealed prevalence and viability patterns in various fluids and whole blood samples (54, 55), dispelling cases where RNA detection might not necessarily indicate the presence of viable virus (56). These instances provide a fascinating glimpse into the nuanced landscape of EBOV persistence, highlighting its ability to inhabit distinct physiological niches. Understanding the potential events in ongoing outbreaks is crucial for designing effective prevention and control strategies to combat the ongoing threat of EBOV outbreaks.
While the persistence of the virus holds the potential to rekindle human-to-human infections, ultimately leading to the resurgence of Ebola outbreaks (Figure 3), the occurrence of such events remains rare (10, 58–60). In addition, the exact mechanisms and risk factors for such transmission are still being studied, and more research is needed to fully understand this process.
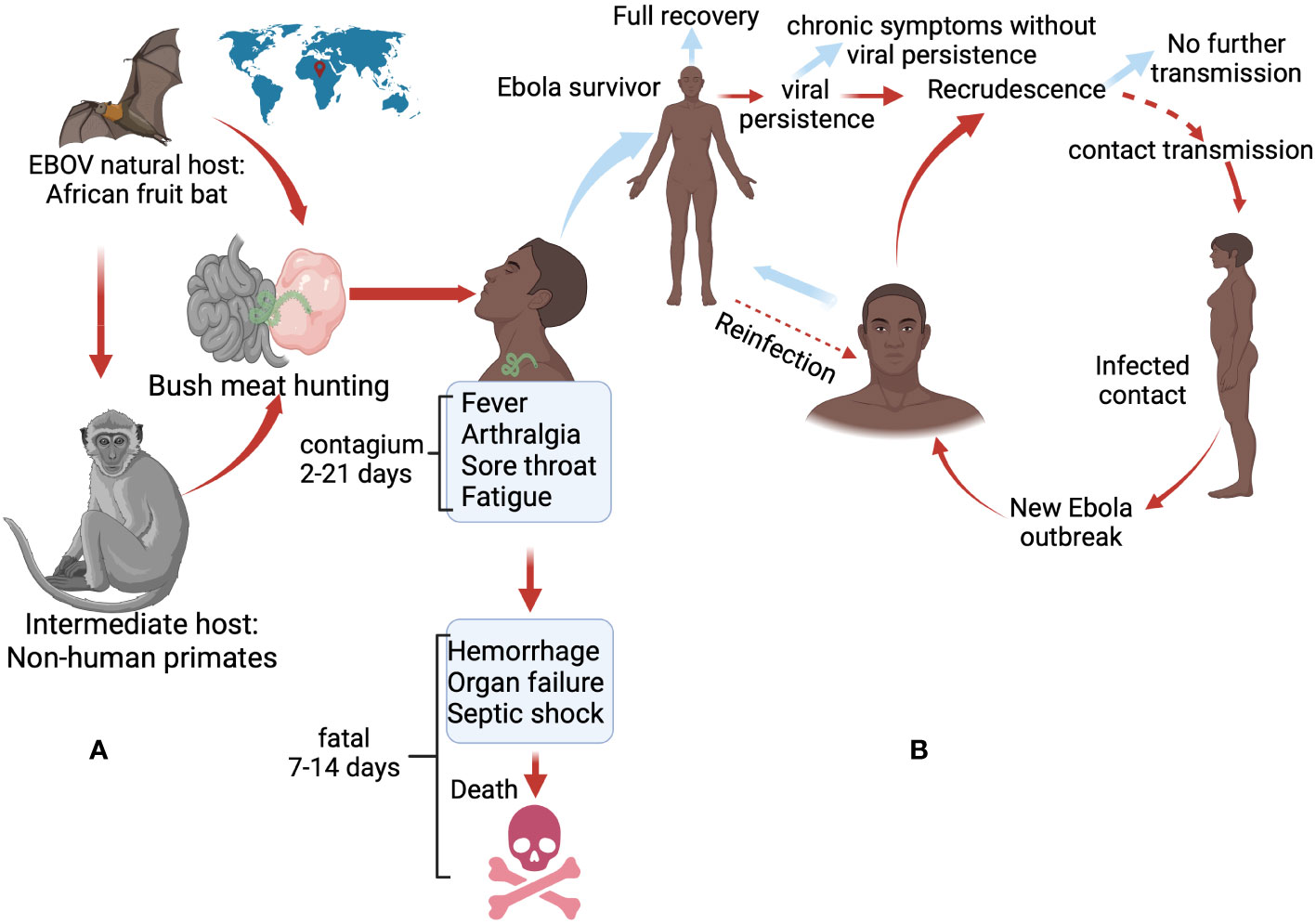
Figure 3 Factors that could potentially trigger current Ebola outbreaks. (A) Primary spillover event from animal reservoirs (bats and non-human primates) to humans, which occurs through bush meat hunting or direct contact with the fluids of infected animals. (B) Outbreak possibility linked to a small subset of survivors who may experience viral persistence in a population. The possibility of transmission is further amplified by viral reactivation and sexual contact. Dotted arrows indicate hypothetical factors that may occur (10, 18, 57). Created with BioRender.com.
For this reason, striking a delicate balance between acknowledging this documented phenomenon and avoiding unwarranted stigma towards survivors, given the exceedingly limited chance of transmission, is essential. The recognition of this rarity reinforce the importance of comprehensive research to fully comprehend its significance.
Noteworthy instances of possible sexual transmission of EBOV infection from male convalescent patients to female sexual partners have been reported (61–63), demonstrating Ebola as a potential sexually transmitted infection (STI). For example, it is reported that a survivor who continued to shed the virus in his semen for over 500 days after contracting EVD, transmitted the virus to his partner through sexual contact, subsequently triggering a reemergence in Guinea in 2016 (64). To further illustrate the role of survivors of Ebola in new outbreaks, genome sequencing analysis and epidemiological evidence provided by Keita et al. showed that the reactivation of a dormant infection in an asymptomatic person for almost 5 years triggered the 2021 Ebola outbreak in Guinea, West Africa (10). The duration of viral persistence following the conclusion of the 2013-2016 West African Ebola outbreak was astonishing, challenging the historical instances of clinically persistent EBOV infections reawakening.
A correlated study provided additional backing to the notion of infectious virus reawakening by tracing the origin of the 2021 EVD outbreak in the DRC to the reactivation of dormant EBOV infections stemming from the 2018-2020 outbreak (65). Further research by Dokubu et al. supports potential for persistent viral infection and transmission (59). Their investigation into an EVD cluster in Liberia revealed phylogenetic relatedness between Ebola virus genomes from the 2015 cluster and epidemiologically linked cases from the 2014–15 West African outbreak. This linkage strongly suggests the survival and transmission of the virus by an individual from a previous outbreak, highlighting the role of viral persistence in fueling recurrences.
Overall, the enduring presence of the virus presents the potential to rejuvenate human-to-human infections, consequently culminating in the resurgence of Ebola outbreaks (Figure 3). In light of these transmission dynamics, it is imperative to conduct further research to gain more insight into this atypical transmission events in the current post-Ebola scenario.
Recovery from EBOV infection triggers the development of both cell-mediated and humoral immune responses. Nonetheless, the strength of these responses may diminish over time (66, 67). In a study involving 115 Ebola survivors in Sierra Leone, IgG levels—the most prevalent antibody in blood serum and extracellular fluids—were closely monitored, revealing intriguing patterns (68). Over a 500-day post-infection period, a substantial drop in IgG levels was observed. However, a fascinating pattern emerged – a subgroup of survivors experienced a resurgence of IgG around 200–300 days after the initial infection. Unfortunately, this resurgence was short-lived, followed by another decline in antibody levels. These findings illuminate the complexities of post-recovery immune responses and hold significant implications.
Ruibal and colleagues also identified activated CD8+ and CD4+ T cells in blood samples from EVD survivors, distinguishing them from fatal cases where higher levels of T cell inhibitory molecules PD-1 and CTLA-4 were expressed on CD4+ and CD8+ T cells. The latter group was more susceptible to disease (69). This suggest that a robust virus-specific T cell response is needed to ensure recovery from the acute phase. However, in scenarios where the virus persists in immune-privileged sites like the brain, eyes, and testes even after recovery, T cells might undergo exhaustion, or their proliferation could be delayed and limited. This phenomenon could be attributed to a high viral load and uncontrolled viral replication (70).
In a longitudinal study of EVD survivors, conducted by Sobarzo et al., significant CD8+ T cell responses were not identified (71). The authors also noted a decline in neutralizing antibodies, providing evidence that survivors might initially exhibit activated adaptive immunity that gradually wanes over a period of at least a decade.
Moreover, weak CD8+ EBOV-specific T cell responses have been detected in survivors for up to 40 days after clearance of viraemia (72). In fact, certain immunodeficiency conditions, such as the loss of primed CD4+ T cells, have previously been linked to EBOV persistence (73). Evidence indicates that some survivors experience substantial lymphocyte loss, combined with disrupted interactions between dendritic cells and T cells (74, 75). The expression of apoptotic genes such as Fas, Fas Ligand (FasL) and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) in EVD patients has been proposed to induce lymphocyte apoptosis, potentially contributing to lymphocyte depletion in survivors over time (76, 77).
Besides the ability of EBOV to evade immune surveillance and ensure persistence (78), it is important to consider the influence of concurrent infections, such as HIV, tuberculosis, and malaria, which are prevalent in Sub-Saharan Africa (79). These co-infections may contribute to EBOV persistence dynamics, affecting immune responses even after recovery from EVD (80). For example, some Ebola patients who are co-infected with HIV may have compromised immune systems, affecting their ability to control EBOV persistence (81). Moreover, malaria and tuberculosis can induce immune-modulating effects that potentially favor EBOV’s ability to persist in a weakened host immune system (82–85). As a result, these concurrent infections could contribute to the gradual waning of immune responses in EVD survivors over time, subsequently enhancing viral persistence dynamics and the potential for reactivation (84).
The concept of reactivation of the initial pathogen through concurrent infections is supported by various studies. When multiple intracellular pathogens infect the same host cell, they can disable cellular immune defenses and evade immune responses, leading to the reactivation of a latent first pathogen and accelerated T-cell exhaustion or suppression regarding a second pathogen (86–89). While there is no confirmed evidence indicating genuine latency of EBOV, the existence of concurrent infections may potentially foster an environment that hinders the immune system’s capacity to manage the persistence of pathogens, including EBOV (87, 90–92). Therefore, understanding the interactions between different pathogens and their impact on the immune system is crucial when studying viral persistence and the potential for reactivation.
Considering the uncertainty surrounding the specific antibody threshold required for achieving optimal protection and nurturing immunological memory, the decline in antibodies among certain Ebola survivors over time warrants attention (93).
Undoubtedly, immunity plays a pivotal role in containing viral infections and preventing recrudescence. The immune system’s response to initial infection is typically robust, with antibodies and immune cells efficiently targeting the virus. Nevertheless, over time, this immune response might wane, potentially enabling shifts in the balance between the host and the virus (68, 94, 95). This weakening of the immune response creates an environment in which the virus, potentially persisting in bodily reservoirs, can reactivate and replicate (68, 73, 96). Consequently, this reactivation could result in the reappearance of symptoms observed in some survivors. The immune system’s once-robust capacity to suppress viral replication might become compromised, potentially facilitating the re-emergence of the virus within a population.
The intricate interplay between immunity, viral persistence, and reemergence underscores the multifaceted nature of Ebola virus outbreaks. In light of these complexities, vigilant monitoring of survivor health becomes imperative. Considering interventions such as administering supplementary vaccinations to invigorate the immune system holds promise in curbing the risk of viral relapse among survivors (97). By proactively supporting survivors’ immune responses, the potential for reseeding outbreaks can be effectively mitigated.
The severity of the disease and its fatality in the context of EBOV infection can largely be attributed to the robust inflammatory response activated by the virus within the host. Generally, this interaction between EBOV and host cells amplifies the release of oxygen-free radicals and various cytokines and chemokines, including TNFα, IL-1, IL-6, IL-8, IL-15, IL-16, IFN-α, IFN-β, IFN-γ, IP-10, CCLs-2, -3, -4, CXCL-10, MCSF ultimately leading to severe illness and death (71, 98–101).
The persistence of cytokine activity may play a critical role in promoting long-term EBOV persistence by dampening T cell activation, creating a conducive environment for the establishment of a pool of infected cells within immune-privileged sites (102). This implies that, beyond the influence of cytokine activity, the specific cells within these immune-privileged sites could also contribute to the extended presence of the virus. In view of this, the hypothesis that certain cell types might act as reservoirs for the virus is currently being investigated, with particular attention on myeloid cells. Resident tissue macrophages and dendritic cells, both originating from the adult bone marrow, are vital components (103). The bone marrow’s susceptibility to infection during the acute phase is evident from the presence of viral inclusion bodies within its cells and substantial antigen deposition in its stroma. Given the continuous production of cells by the bone marrow throughout life, it becomes plausible that myeloid-dendritic progenitor (MDP) cells could also fall victim to the virus. MDP cells, in their regular lifecycle, enter the bloodstream and populate tissues, eventually differentiating into resident tissue macrophages and dendritic cells. If these cells succumb to infection, they could potentially serve as repositories for viral persistence within tissues, persisting for the cell’s natural lifespan, possibly lasting several weeks to months. This paradigm of myeloid cell involvement resonates with similar mechanisms observed in other infections such as HIV, Dengue, and Marburg virus persistence (104–106). Directing attention towards the myeloid cells holds the potential to address the prolonged EBOV reservoir that has been identified in certain survivors.
In addition, the biochemical strategy employed by EBOV to ensure viral persistence in infected cells is not yet fully understood. Nonetheless, it is hypothesized that specific inflammatory markers present in survivors’ blood samples could indicate ongoing viral persistence and inflammation (96). While some survivors of EBOV infection may display a milder inflammation profile and less pronounced cytokine and chemokine responses compared to fatal cases, the persistence of structural damage to the epithelial barrier and immune dysfunction could characterize a state akin to chronic EVD (96). This raises the critical need to investigate whether these survivors are progressing toward a chronic disease state. In this context, the study conducted by Shantha et al. holds significance as they examined EBOV survivors with uveitis who underwent cataract surgeries to explore viral persistence and chronic inflammation (107). Their research sheds light on how sustained low-level viral replication might contribute to recurring inflammation even after the virus has been cleared. The presence of recurrences in immune-privileged sites prompts inquiries into the dynamics of viral replication and the immune response in these specific contexts. The study’s observations offer compelling evidence that EBOV RNA is frequently detected in extracellular compartments during instances of disease recurrence, and the ability to culture the virus from tissue samples in these sites suggests ongoing replication. This observation aligns seamlessly with the concept that sustained low-level viral replication, coupled with an imbalanced immune response, could foster chronic inflammation and ultimately contribute to recurrence (107, 108).
Collectively, the inflammatory response may contribute to the residual levels of viral persistence in cellular reservoirs and potentially lead to disease recurrences among survivors (101). Further research is essential to delve into the intricate mechanisms behind EBOV persistence, as well as the impact of immune markers and inflammation. These investigations hold the potential to provide valuable insights into prolonged inflammation among EVD survivors.
The emergence of post-Ebola virus syndrome (PES) among survivors further highlights the complex aftermath of Ebola virus infection. This syndrome has been observed in a small subset of individuals who have successfully recovered from the virus (109, 110). Despite overcoming the acute phase of the disease, some survivors may experience long-term health effects that encompass a range of symptoms and conditions. The underlying factors contributing to PES are multifaceted, with persistent virus reactivation in immune-privileged sites playing a pivotal role in the reappearance of infection during the convalescent phase (111–113).
PES manifests with a range of clinical symptoms, encompassing conditions like arthralgia, uveitis, arthritis, and psychological stress (109). The health implications faced by EVD survivors have undergone comprehensive evaluation, shedding light on various aspects of the syndrome.
Pediatric survivors in Sierra Leone showed higher prevalence of ophthalmic complications like uveitis and vernal keratoconjunctivitis (110). Similarly, Qureshi and colleagues observed anorexia (100%), chest pain (31%), joint pain (87%), back pain (46%), and myalgias (27%) among 105 EVD survivors who were discharged from treatment after approximately 3.5 months (114). Musculoskeletal issues, particularly arthralgia and myalgia, have been frequently reported among survivors, marking them as common features of PES (115–117).
Additionally, PES is associated with cognitive and psychological symptoms like headaches, insomnia, memory loss, hearing loss, depression, and anxiety (112, 114, 115, 118). These psychosocial factors significantly affect survivors, leading to stigma and hindrances in societal integration (119, 120).
Despite the prevalence of PES, research has indicated that certain chronic PES symptoms may improve with time. Evidence suggests that headache, sleeping disorder, anxiety, depression and fatigue decreased significantly from 0-3 months to 10-12 months among post-EVD participants in Liberia (121). A decline in chronic symptoms of PES with the exception of uveitis has also been reported by the PREVAIL III study, suggestive of the fact that survivors after surviving acute manifestations may still show symptoms of chronic clinical manifestations, and these manifestations may decrease with time (122). This implies that the clinical symptoms of PES may exhibit improvement as time progresses. In contrast, Tozay and colleagues later reported no reduction in the prevalence of post-EVD symptoms among survivors four years after infection (123). The influence of various methodologies, including the choice of symptom-detection questions, the duration of follow-up, and the use of medications by survivors (such as pain medication), might all contribute to the diverse outcomes observed. Although individual survivors may experience specific PES symptoms, the combination of both physical and psychological manifestations could potentially contribute to a more severe clinical presentation (124, 125).
Regarding skin disorders in Ebola survivors, reports have identified common skin symptoms, including alopecia, which affects more than 30% of Ebola survivors (126–128). However, these cutaneous manifestations could potentially stem from hematological compromises like prolonged anemia and leukopenia.
To comprehensively explore the manifestations of PES, we retrieved online data collected from Ebola survivors reporting symptom manifestations, primarily from endemic areas within the Sub-Saharan region, in order to identify commonly reported complications.
We conducted electronic searches to identify publications reporting on PES among EVD survivors from January 1, 2015, to April 7, 2023. The research methodology followed a systematic approach adhering to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standard (129). No restrictions were applied regarding study period or sample size. The complete search protocol is detailed in supplementary methods (Supplementary 1).
Our systematic review excluded experimental research, reviews, editorials, letters to the editor, and case reports. Inclusion criteria were based on studies providing data on the prevalence of signs and symptoms among EVD survivors during the convalescent phase. Two authors (WA, TN-B) independently reviewed all relevant titles and abstracts, selecting articles for full-text review when inclusion criteria were met. Any discrepancies were resolved through consensus with a third author (PO). Articles that met the inclusion criteria were short-listed for the final systematic review.
The literature search yielded 762 articles, of which 49 underwent full review, and 38 were included in the final analysis (Figure 4 and Supplementary 2).
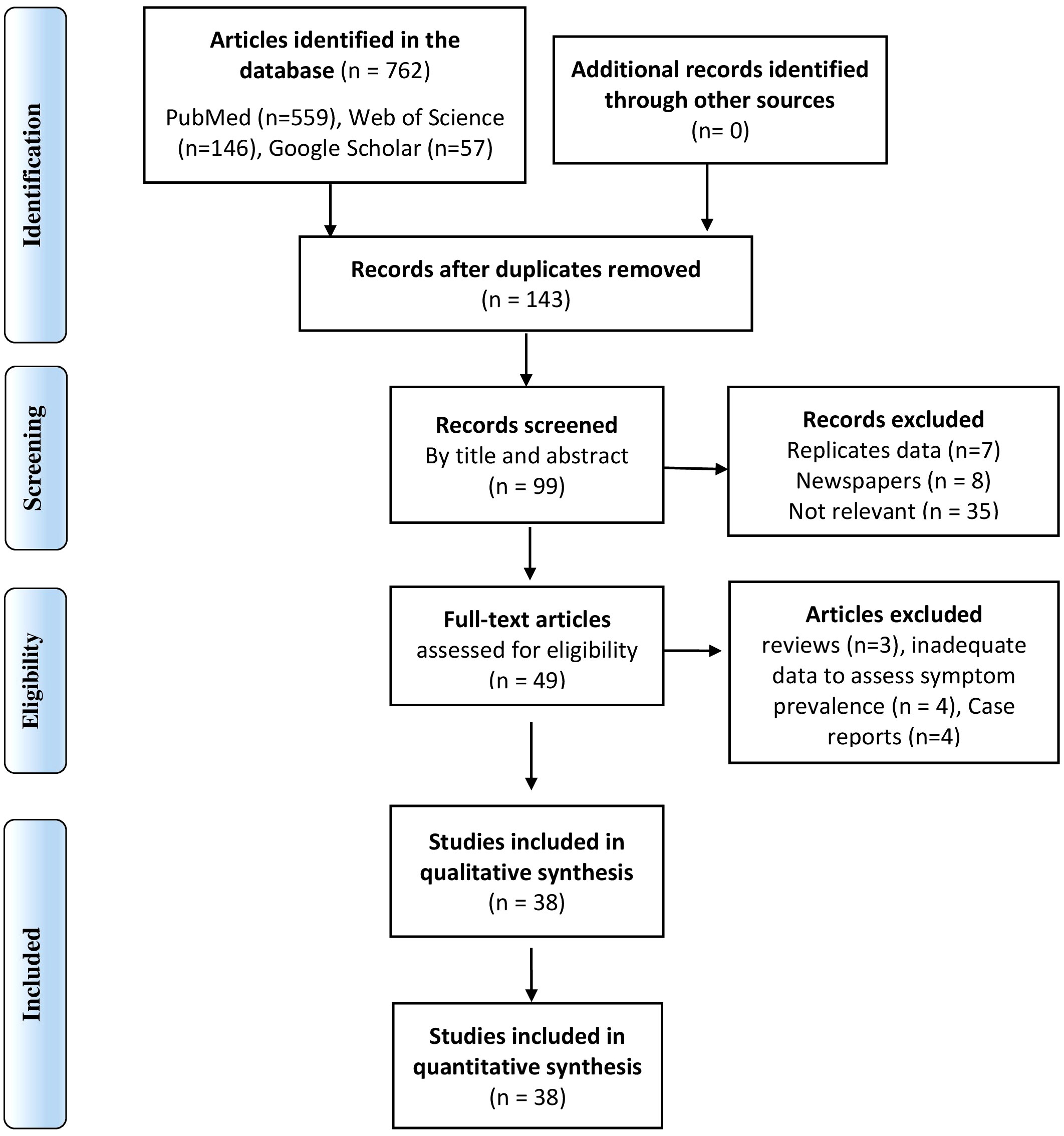
Figure 4 PRISMA flow diagram of the selection of studies included in the systematic review and meta-analysis of PES.
In our analysis, we employed both relative and absolute frequencies to calculate the prevalence percentage of signs and symptoms among survivors of EVD within a given population. The results of our analysis are presented in Table 1, and a visual summary of the findings can be found in Figure 5. Our results offer an overview of the frequency at which various symptoms are commonly manifested by individuals who have survived Ebola, thus contributing to the limited body of literature detailing the symptoms experienced by EVD survivors.
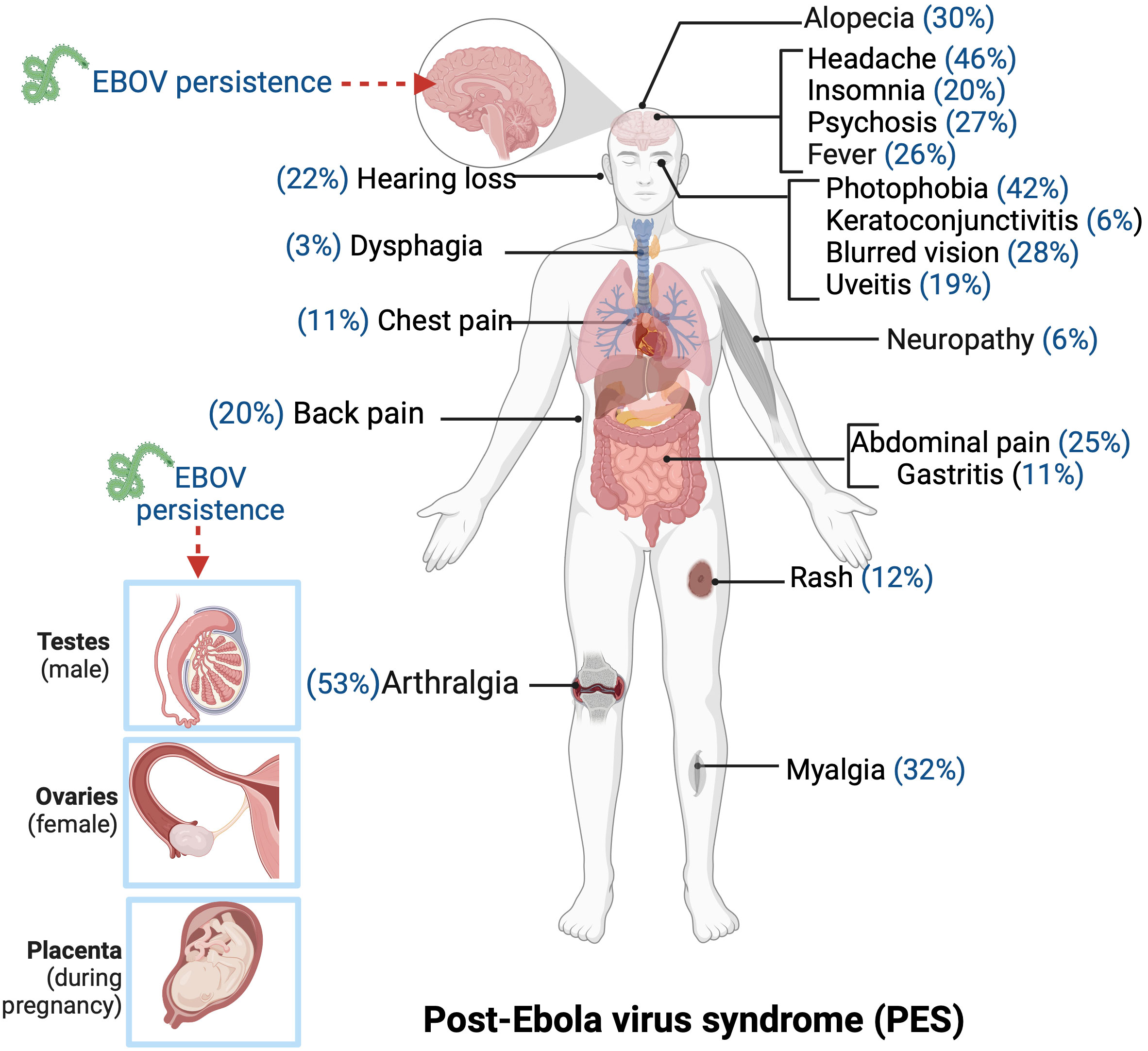
Figure 5 The common chronic clinical manifestations observed among some survivors of Ebola virus disease. The reactivation of persistent Ebola virus in body sites such as testes, ovaries, and placenta can contribute to the development of chronic symptoms in EVD survivors. Created with BioRender.com.
The evidence derived from our systematic review indicates that complications among the thousands of EVD survivors are primarily characterized by a range of disorders that are parallel to those experienced during the acute phase of the disease. Consistent with findings from other studies, arthralgia, headache, and photophobia emerge as the most frequently reported long-term symptoms among survivors, with a prevalence exceeding 40% (4, 112, 114, 118, 121, 122, 127, 128, 130–135).
General symptoms that are frequently identified in survivors during the acute phase, including fever, fatigue, anorexia, and chest pain, have been extensively documented (118, 121, 122, 130, 131). Our research further discloses that these symptoms endure in more than 20% of survivors even during the convalescent phase.
The prevalence of these commonly reported manifestations offers valuable insights into the enduring influence of the EBOV on survivors, serving as a stark reminder of the imperative to sustain vigilant monitoring and support for them as they navigate substantial long-term health challenges. Moreover, this intricacy gains even more significance as it engenders challenges in distinguishing between new cases of Ebola in endemic regions and potential reinfections among survivors (57).
In light of this complexity, addressing the predicaments associated with distinguishing between novel cases and observed symptoms among EVD survivors in Ebola-endemic areas demands further investigation.
It is also evident from our studies that certain symptoms may be underrepresented or overlooked in a population: dysphagia, chest pain, gastritis, and rash. These symptoms might indeed be influenced by other infections, underlying health conditions, or various external factors. Regarding the range of potential causes for these symptoms and their nonspecific nature, it becomes essential to exercise caution when attributing them solely to the lingering symptoms of PES.
Unraveling whether the lingering symptoms of PES can serve as distinctive markers holds the potential to substantially enhance the identification and response to outbreaks (4). Therefore, careful clinical assessment and thorough differential diagnosis are imperative to accurately identify the source of these symptoms and determine appropriate treatment strategies.
Given the hypothesis that the pathogenesis of lingering clinical sequelae stems from systemic damage caused by the initial infection, along with sustained immune responses, autoimmune-like inflammation, and potential viral persistence in immune privilege sites, it is vital to focus on treatment strategies that target the inflammatory cascade and the immune system. This approach could provide a promising foundation for exploring novel therapies (150). However, it’s important to note that, at present, there is no approved drug specifically designed for treating PES in Ebola survivors. Yet researchers speculate that genetic factors may play a role in contributing to prolonged Ebola sequelae, as genes can significantly impact how the body responds to infections (151, 152). In particular, genetic variations in the immune system have been associated with Ebola virus pathogenesis days post-infection (151).
To address the complex issue of genetics in this context, the recently completed PREVAIL VI study (NCT03098862) in Liberia will undergo analysis to determine the safety and efficacy of interventions, including the evaluation of viral persistence in Ebola survivors (153). Additionally, a separate study suggests that cannabidiol (CBD) might offer mitigation for some of the post-Ebola sequelae arising from inflammation or autoimmune responses (150). CBD encompasses a range of pharmacological actions that hold promise in potentially offering therapeutic solutions for mental and somatic well-being of individuals grappling with the aftermath of post-Ebola sequelae (150, 154).
Furthermore, in an endeavor to reduce the number of survivors who may experience viral persistence, health workers could consider the administration of combined therapeutics such as ZMAb, IFN-α, IFN-β and other antiviral drugs during the acute stage of the disease to prevent persistence at immune privilege sites post-infection (155–157). This strategy holds promise in preventing viral persistence, as has been observed in non-human primates that survived EVD (12).
In spite of the small sample size and insufficient data from the aforementioned studies, the long-term complications faced by certain EVD survivors stemming from previous Ebola outbreaks cannot be ignored. While these complications have often been associated with potential viral persistence, there have been few instances highlighting their contribution to new epidemics, raising complex concerns that compound the existing stigma faced by survivors.
The manifestations of PES, primarily reported by certain survivors, may lack the robustness required to definitively establish a direct causal link between these symptoms and the Ebola infection. This is due to the possibility that such symptoms could potentially arise as a consequence of other endemic diseases prevalent in the Sub-Saharan region, such as malaria and Dengue. The similarity in clinical symptoms among various diseases complicates the attribution of symptoms solely to Ebola infection. On account of this, a comprehensive differential diagnosis approach is essential, considering multiple contributing factors to these symptoms among Ebola survivors.
Prioritizing the well-being of survivors is crucial to curtailing cases of EVD relapse, especially within Western and Central Africa, where a significant number of survivors are recorded (120). Achieving this demands a multifaceted approach including long-term follow-up care, pharmaceutical interventions to manage chronic conditions and comorbidities, addressing social stigma, providing mental health support, education, employment opportunities, and implementing rehabilitation programs to minimize the impact on survivors’ health and quality of life.
While viral persistence and reactivation in Ebola survivors may not be the primary driver of current outbreaks due to their rarity, the potential for these occurrences to spark flare-ups and re-ignition highlights the need for further investigation. In this regard, the World Health Organization’s recommendations on semen testing programs and advocating safe sex practices using condoms for at least 12 months among male survivors—potential virus shedders—require strong emphasis (16, 98). Although, instances of viral persistence beyond 12 months have been reported (46, 53, 64), and resource limitations might delay testing in certain endemic regions. Strategically identifying candidates for continued testing, using determinants like age, severity of acute EVD, elevated serum IgG3 levels, and the HLA-C*03:04 allele, is essential in extending testing and counseling beyond established follow-up periods (52, 158).
An essential area for research is the impact of pre-existing immunity from related viruses on post-EVD outcomes (159, 160). While such immunity could potentially bolster viral control, it may also have negative consequences, such as antibody-dependent enhancement (ADE) and original antigenic sin (OAS), potentially exacerbating inflammation and tissue damage—aggravating chronic PES symptoms (161–163). Investigating the impact of pre-existing immunity on the immune response and clinical outcomes in EVD survivors is crucial. This understanding is essential for comprehending the immunological mechanisms underlying PES and evaluating innovative modalities, including immunomodulation and antiviral strategies (164). These approaches aim to alleviate the PES burden and enhance survivor well-being.
In light of the evolving COVID-19 pandemic, re-emerging cases of EVD and other high-threat diseases, a unified approach to managing outbreaks and tackling associated stigma is essential (119, 120, 165). The collaborative synergy between researchers, healthcare practitioners, and policymakers is indispensable in infectious disease management. Investing in building local surge capacities, including rapid response teams and epidemic intelligence, is critical to address the re-emergence of Ebolaviruses (166). Besides, international partnerships play a pivotal role in sharing information, resources, diagnostics, and therapeutics for ongoing EVD survivorship and treatment research in the Sub-Saharan region (167). These endeavors collectively combat EVD and emerging infectious diseases, minimizing their impact and enhancing global public health preparedness against future outbreaks.
Looking forward, the establishment of a comprehensive database containing hematological, immunological, and virological data proves invaluable in identifying common patterns associated with viral persistence and the clinical presentation of PES (168–173). Additionally, the creation of a biobank with DNA samples from patients experiencing viral persistence and PES is essential for genomic studies and the identification of biomarkers linked to these conditions (18, 64, 174). These proposed initiatives would undoubtedly advance our understanding of EVD survivor health and potentially lead to more effective interventions and treatment strategies in the future. As the global community remains vigilant in addressing infectious diseases, these collaborative efforts are crucial in mitigating their impact and enhancing global public health resilience.
EAF and TN-B contributed to the conception of this study. EAF wrote the first draft of the manuscript. PO, WA and TN-B organized databases for the systematic studies and metaanalysis. WA performed the statistical analysis. All authors contributed to the article and approved the submitted version.
The authors declare that financial support was received for the publication of this article. Open access funding for this paper was provided by the Universität Konstanz.
We would like to express our deepest gratitude to Dr. Karla Martinez-Cruz at the Universität Konstanz, for her guidance, comments and suggestions in reviewing this paper. We are also thankful to Svenja Hehn for her comments and contributions to the graphical enhancements of the figures.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2023.1227314/full#supplementary-material
1. Messaoudi I, Amarasinghe GK, Basler CF. Filovirus pathogenesis and immune evasion: insights from Ebola virus and Marburg virus. Nat Rev Microbiol (2015) 13:663–76. doi: 10.1038/nrmicro3524
2. Zampieri CA, Sullivan NJ, Nabel GJ. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat Immunol (2007) 8:1159–64. doi: 10.1038/ni1519
3. Burk R, Bollinger L, Johnson JC, Wada J, Radoshitzky SR, Palacios G, et al. Neglected filoviruses. FEMS Microbiol Rev (2016) 40:494–519. doi: 10.1093/femsre/fuw010
4. Rojas M, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Ansari AA, et al. Ebola virus disease: An emerging and re-emerging viral threat. J Autoimmun (2020) 106:102375. doi: 10.1016/j.jaut.2019.102375
5. Muñoz-Fontela C, McElroy AK. Ebola virus disease in humans: pathophysiology and immunity. In: Mühlberger E, Hensley LL, Towner JS, editors. Marburg- and Ebolaviruses. Cham: Springer International Publishing (2017). p. 141–69.
6. European Centre for Disease Prevention and Control. Ebola outbreak in Uganda, as of 11 January 2023. Available at: https://www.ecdc.europa.eu/en/news-events/ebola-outbreak-Uganda (Accessed March 10, 2023).
7. Rewar S, Mirdha D. Transmission of ebola virus disease: an overview. Ann Glob Health (2015) 80:444. doi: 10.1016/j.aogh.2015.02.005
8. Akem ES, Pemunta NV. The bat meat chain and perceptions of the risk of contracting Ebola in the Mount Cameroon region. BMC Public Health (2020) 20:593. doi: 10.1186/s12889-020-08460-8
9. Goldstein T, Anthony SJ, Gbakima A, Bird BH, Bangura J, Tremeau-Bravard A, et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat Microbiol (2018) 3:1084–9. doi: 10.1038/s41564-018-0227-2
10. Keita AK, Koundouno FR, Faye M, Düx A, Hinzmann J, Diallo H, et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature (2021) 597:539–43. doi: 10.1038/s41586-021-03901-9
11. Wohl DA, Fischer WA, Mei W, Zou F, Tozay S, Reeves E, et al. Post-ebola symptoms 7 years after infection: the natural history of long ebola. Clin Infect Dis (2023) 76:e835–40. doi: 10.1093/cid/ciac732
12. Liu J, Trefry JC, Babka AM, Schellhase CW, Coffin KM, Williams JA, et al. Ebola virus persistence and disease recrudescence in the brains of antibody-treated nonhuman primate survivors. Sci Transl Med (2022) 14:eabi5229. doi: 10.1126/scitranslmed.abi5229
13. Zeng X, Blancett CD, Koistinen KA, Schellhase CW, Bearss JJ, Radoshitzky SR, et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat Microbiol (2017) 2:17113. doi: 10.1038/nmicrobiol.2017.113
14. Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of ebola virus in ocular fluid during convalescence. N Engl J Med (2015) 372:2423–7. doi: 10.1056/NEJMoa1500306
15. Uyeki TM, Erickson BR, Brown S, McElroy AK, Cannon D, Gibbons A, et al. Ebola virus persistence in semen of male survivors. Clin Infect Dis (2016) 62:1552–5. doi: 10.1093/cid/ciw202
16. Sissoko D, Duraffour S, Kerber R, Kolie JS, Beavogui AH, Camara A-M, et al. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health (2017) 5:e80–8. doi: 10.1016/S2214-109X(16)30243-1
17. Martines RB, Ng DL, Greer PW, Rollin PE, Zaki SR. Tissue and cellular tropism, pathology and pathogenesis of Ebola and Marburg viruses: Ebola and Marburg viruses. J Pathol (2015) 235:153–74. doi: 10.1002/path.4456
18. Di Paola N, Sanchez-Lockhart M, Zeng X, Kuhn JH, Palacios G. Viral genomics in Ebola virus research. Nat Rev Microbiol (2020) 18:365–78. doi: 10.1038/s41579-020-0354-7
19. Johnson KM, Lange JV, Webb PA, Murphy FA. Isolation and Partial Characterisation of a new virus causing Acute Haemorrhagic Fever in Zaire. Lancet (1977) 309:569–71. doi: 10.1016/S0140-6736(77)92000-1
20. Bres P. The epidemic of Ebola haemorrhagic fever in Sudan and Zaire, 1976: introductory note. Bull World Health Organ (1978) 56:245.
21. McCormick JB, Bauer SP, Elliott LH, Webb PA, Johnson KM. Biologic differences between strains of ebola virus from zaire and Sudan. J Infect Dis (1983) 147:264–7. doi: 10.1093/infdis/147.2.264
22. Groseth A, Feldmann H, Strong JE. The ecology of Ebola virus. Trends Microbiol (2007) 15:408–16. doi: 10.1016/j.tim.2007.08.001
23. Barrette RW, Metwally SA, Rowland JM, Xu L, Zaki SR, Nichol ST, et al. Discovery of swine as a host for the reston ebolavirus. Science (2009) 325:204–6. doi: 10.1126/science.1172705
24. Le Guenno B, Formenty P, Wyers M, Gounon P, Walker F, Boesch C. Isolation and partial characterisation of a new strain of Ebola virus. Lancet (1995) 345:1271–4. doi: 10.1016/S0140-6736(95)90925-7
25. Towner JS, Sealy TK, Khristova ML, Albariño CG, Conlan S, Reeder SA, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PloS Pathog (2008) 4:e1000212. doi: 10.1371/journal.ppat.1000212
26. Albariño CG, Shoemaker T, Khristova ML, Wamala JF, Muyembe JJ, Balinandi S, et al. Genomic analysis of filoviruses associated with four viral hemorrhagic fever outbreaks in Uganda and the Democratic Republic of the Congo in 2012. Virology (2013) 442:97–100. doi: 10.1016/j.virol.2013.04.014
27. Oluwagbemi O, Awe O. A comparative computational genomics of Ebola Virus Disease strains: In-silico Insight for Ebola control. Inform Med Unlocked (2018) 12:106–19. doi: 10.1016/j.imu.2018.07.004
28. International Committee on Taxonomy of Viruses. Available at: https://ictv.global/report/chapter/filoviridae/filoviridae/orthoebolavirus (Accessed October 17, 2023).
29. Jun S-R, Leuze MR, Nookaew I, Uberbacher EC, Land M, Zhang Q, et al. Ebolavirus comparative genomics. FEMS Microbiol Rev (2015) 39:764–78. doi: 10.1093/femsre/fuv031
30. Jain S, Martynova E, Rizvanov A, Khaiboullina S, Baranwal M. Structural and functional aspects of ebola virus proteins. Pathogens (2021) 10:1330. doi: 10.3390/pathogens10101330
31. Madelain V, Nguyen THT, Olivo A, De Lamballerie X, Guedj J, Taburet A-M, et al. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet (2016) 55:907–23. doi: 10.1007/s40262-015-0364-1
32. Yamaoka S, Ebihara H. Pathogenicity and virulence of ebolaviruses with species- and variant-specificity. Virulence (2021) 12:885–901. doi: 10.1080/21505594.2021.1898169
33. Bharat TAM, Noda T, Riches JD, Kraehling V, Kolesnikova L, Becker S, et al. Structural dissection of Ebola virus and its assembly determinants using cryo-electron tomography. Proc Natl Acad Sci (2012) 109:4275–80. doi: 10.1073/pnas.1120453109
34. Watanabe S, Noda T, Kawaoka Y. Functional mapping of the nucleoprotein of ebola virus. J Virol (2006) 80:3743–51. doi: 10.1128/JVI.80.8.3743-3751.2006
35. Bornholdt ZA, Noda T, Abelson DM, Halfmann P, Wood MR, Kawaoka Y, et al. Structural rearrangement of ebola virus VP40 begets multiple functions in the virus life cycle. Cell (2013) 154:763–74. doi: 10.1016/j.cell.2013.07.015
36. Schmidt ML, Hoenen T. Characterization of the catalytic center of the Ebola virus L polymerase. PloS Negl Trop Dis (2017) 11:e0005996. doi: 10.1371/journal.pntd.0005996
37. Martinez MJ, Volchkova VA, Raoul H, Alazard-Dany N, Reynard O, Volchkov VE. Role of VP30 phosphorylation in the ebola virus replication cycle. J Infect Dis (2011) 204:S934–40. doi: 10.1093/infdis/jir320
38. Biedenkopf N, Lier C, Becker S. Dynamic phosphorylation of VP30 is essential for ebola virus life cycle. J Virol (2016) 90:4914–25. doi: 10.1128/JVI.03257-15
39. Ilinykh PA, Lubaki NM, Widen SG, Renn LA, Theisen TC, Rabin RL, et al. Different temporal effects of ebola virus VP35 and VP24 proteins on global gene expression in human dendritic cells. J Virol (2015) 89:7567–83. doi: 10.1128/JVI.00924-15
40. Jangra RK, Mittler E, Chandran K. Filovirus entry into susceptible cells. In: Biology and Pathogenesis of Rhabdo- and Filoviruses. WORLD SCIENTIFIC (2015). p. 487–514. doi: 10.1142/9789814635349_0019
41. Rugarabamu S, Mboera L, Rweyemamu M, Mwanyika G, Lutwama J, Paweska J, et al. Forty-two years of responding to Ebola virus outbreaks in Sub-Saharan Africa: a review. BMJ Glob Health (2020) 5:e001955. doi: 10.1136/bmjgh-2019-001955
42. Centres for Disease Control and Prevention. History of Ebola Disease Outbreaks. Available at: https://www.cdc.gov/vhf/ebola/history/chronology.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvhf%2Febola%2Foutbreaks%2Fhistory%2Fchronology.html (Accessed March 30, 2023).
43. Deen GF, Broutet N, Xu W, Knust B, Sesay FR, McDonald SLR, et al. Ebola RNA persistence in semen of ebola virus disease survivors — Final report. N Engl J Med (2017) 377:1428–37. doi: 10.1056/NEJMoa1511410
44. Rodriguez LL, De Roo A, Guimard Y, Trappier SG, Sanchez A, Bressler D, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis (1999) 179 Suppl 1:S170–176. doi: 10.1086/514291
45. Kofman A, Linderman S, Su K, Purpura LJ, Ervin E, Brown S, et al. Characteristics of ebola virus disease survivor blood and semen in Liberia: serology and reverse transcription polymerase chain reaction (RT-PCR). Clin Infect Dis (2021) 73:e3641–6. doi: 10.1093/cid/ciaa1331
46. Keita AK, Vidal N, Toure A, Diallo MSK, Magassouba N, Baize S, et al. A 40 months follow-up of Ebola virus disease survivors in Guinea (Postebogui) reveals longterm detection of Ebola viral RNA in semen and breast milk. Open Forum Infect Dis (2019) 6:ofz482. doi: 10.1093/ofid/ofz482
47. Sissoko D, Keïta M, Diallo B, Aliabadi N, Fitter DL, Dahl BA, et al. Ebola virus persistence in breast milk after no reported illness: A likely source of virus transmission from mother to child. Clin Infect Dis (2016) 64:ciw793. doi: 10.1093/cid/ciw793
48. Kreuels B, Wichmann D, Emmerich P, Schmidt-Chanasit J, De Heer G, Kluge S, et al. A case of severe ebola virus infection complicated by gram-negative septicemia. N Engl J Med (2014) 371:2394–401. doi: 10.1056/NEJMoa1411677
49. Jacobs M, Rodger A, Bell DJ, Bhagani S, Cropley I, Filipe A, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet (2016) 388:498–503. doi: 10.1016/S0140-6736(16)30386-5
50. Caluwaerts S, Fautsch T, Lagrou D, Moreau M, Modet Camara A, Günther S, et al. Dilemmas in managing pregnant women with ebola: 2 case reports: table 1. Clin Infect Dis (2016) 62:903–5. doi: 10.1093/cid/civ1024
51. Baggi FM, Taybi A, Kurth A, Van Herp M, Di Caro A, Wölfel R, et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Eurosurveillance (2014) 19:20983. doi: 10.2807/1560-7917.ES2014.19.49.20983
52. Thorson AE, Deen GF, Bernstein KT, Liu WJ, Yamba F, Habib N, et al. Persistence of Ebola virus in semen among Ebola virus disease survivors in Sierra Leone: A cohort study of frequency, duration, and risk factors. PloS Med (2021) 18:e1003273. doi: 10.1371/journal.pmed.1003273
53. Fischer WA, Brown J, Wohl DA, Loftis AJ, Tozay S, Reeves E, et al. Ebola virus ribonucleic acid detection in semen more than two years after resolution of acute ebola virus infection. Open Forum Infect Dis (2017) 4:ofx155. doi: 10.1093/ofid/ofx155
54. Bettini A, Lapa D, Garbuglia AR. Diagnostics of ebola virus. Front Public Health (2023) 11:1123024. doi: 10.3389/fpubh.2023.1123024
55. Loftis AJ, Quellie S, Chason K, Sumo E, Toukolon M, Otieno Y, et al. Validation of the cepheid geneXpert for detecting ebola virus in semen. J Infect Dis (2016) 215:jiw562. doi: 10.1093/infdis/jiw562
56. Jansen Van Vuren P, Grobbelaar A, Storm N, Conteh O, Konneh K, Kamara A, et al. Comparative evaluation of the diagnostic performance of the prototype cepheid geneXpert ebola assay. J Clin Microbiol (2016) 54:359–67. doi: 10.1128/JCM.02724-15
57. MacIntyre CR, Chughtai AA. Recurrence and reinfection—a new paradigm for the management of Ebola virus disease. Int J Infect Dis (2016) 43:58–61. doi: 10.1016/j.ijid.2015.12.011
58. Lee H, Nishiura H. Recrudescence of Ebola virus disease outbreak in West Africa, 2014–2016. Int J Infect Dis (2017) 64:90–2. doi: 10.1016/j.ijid.2017.09.013
59. Dokubo EK, Wendland A, Mate SE, Ladner JT, Hamblion EL, Raftery P, et al. Persistence of Ebola virus after the end of widespread transmission in Liberia: an outbreak report. Lancet Infect Dis (2018) 18:1015–24. doi: 10.1016/S1473-3099(18)30417-1
60. Schindell BG, Webb AL, Kindrachuk J. Persistence and sexual transmission of filoviruses. Viruses (2018) 10:683. doi: 10.3390/v10120683
61. Mate SE, Kugelman JR, Nyenswah TG, Ladner JT, Wiley MR, Cordier-Lassalle T, et al. Molecular evidence of sexual transmission of ebola virus. N Engl J Med (2015) 373:2448–54. doi: 10.1056/NEJMoa1509773
62. Fischer WA, Wohl DA. Confronting ebola as a sexually transmitted infection. Clin Infect Dis (2016) 62:1272–6. doi: 10.1093/cid/ciw123
63. Den Boon S, Marston BJ, Nyenswah TG, Jambai A, Barry M, Keita S, et al. Ebola virus infection associated with transmission from survivors. Emerg Infect Dis (2019) 25:249–55. doi: 10.3201/eid2502.181011
64. Diallo B, Sissoko D, Loman NJ, Bah HA, Bah H, Worrell MC, et al. Resurgence of ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis (2016) 63:1353–6. doi: 10.1093/cid/ciw601
65. Kinganda-Lusamaki E, Black A, Mukadi DB, Hadfield J, Mbala-Kingebeni P, Pratt CB, et al. Integration of genomic sequencing into the response to the Ebola virus outbreak in Nord Kivu, Democratic Republic of the Congo. Nat Med (2021) 27:710–6. doi: 10.1038/s41591-021-01302-z
66. Diallo MSK, Toure A, Sow MS, Kpamou C, Keita AK, Taverne B, et al. Understanding long-term evolution and predictors of sequelae of ebola virus disease survivors in Guinea: A 48-month prospective, longitudinal cohort study (PostEboGui). Clin Infect Dis (2021) 73:2166–74. doi: 10.1093/cid/ciab168
67. Mellors J, Tipton T, Fehling SK, Akoi Bore J, Koundouno FR, Hall Y, et al. Complement-mediated neutralisation identified in ebola virus disease survivor plasma: implications for protection and pathogenesis. Front Immunol (2022) 13:857481. doi: 10.3389/fimmu.2022.857481
68. the Ebola-CP Consortium, Adaken C, Scott JT, Sharma R, Gopal R, Dicks S, et al. Ebola virus antibody decay–stimulation in a high proportion of survivors. Nature (2021) 590:468–72. doi: 10.1038/s41586-020-03146-y
69. Ruibal P, Oestereich L, Lüdtke A, Becker-Ziaja B, Wozniak DM, Kerber R, et al. Unique human immune signature of Ebola virus disease in Guinea. Nature (2016) 533:100–4. doi: 10.1038/nature17949
70. McElroy AK, Akondy RS, Davis CW, Ellebedy AH, Mehta AK, Kraft CS, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci (2015) 112:4719–24. doi: 10.1073/pnas.1502619112
71. Sobarzo A, Eskira Y, Herbert A, Kuehne A, Stonier S, Ochayon D, et al. Immune memory to Sudan virus: comparison between two separate disease outbreaks. Viruses (2015) 7:37–51. doi: 10.3390/v7010037
72. Dahlke C, Lunemann S, Kasonta R, Kreuels B, Schmiedel S, Ly ML, et al. Comprehensive characterization of cellular immune responses following Ebola virus infection. J Infect Dis (2016) 215:jiw508. doi: 10.1093/infdis/jiw508
73. Gupta M, Mahanty S, Greer P, Towner JS, Shieh W-J, Zaki SR, et al. Persistent infection with ebola virus under conditions of partial immunity. J Virol (2004) 78:958–67. doi: 10.1128/JVI.78.2.958-967.2004
74. Hensley L, Jones S, Feldmann H, Jahrling P, Geisbert T. Ebola and marburg viruses: pathogenesis and development of countermeasures. Curr Mol Med (2005) 5:761–72. doi: 10.2174/156652405774962344
75. Falasca L, Agrati C, Petrosillo N, Di Caro A, Capobianchi MR, Ippolito G, et al. Molecular mechanisms of Ebola virus pathogenesis: focus on cell death. Cell Death Differ (2015) 22:1250–9. doi: 10.1038/cdd.2015.67
76. Agrati C, Castilletti C, Casetti R, Sacchi A, Falasca L, Turchi F, et al. Longitudinal characterization of dysfunctional T cell-activation during human acute Ebola infection. Cell Death Dis (2016) 7:e2164–4. doi: 10.1038/cddis.2016.55
77. Baize S, Leroy EM, Georges-Courbot M-C, Capron M, Lansoud-Soukate J, Debré P, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med (1999) 5:423–6. doi: 10.1038/7422
78. McElroy AK, Mühlberger E, Muñoz-Fontela C. Immune barriers of Ebola virus infection. Curr Opin Virol (2018) 28:152–60. doi: 10.1016/j.coviro.2018.01.010
79. Mendelson M. The geography of infectious diseases in Africa: From endemic populations to travelers. Int J Infect Dis (2014) 21:12. doi: 10.1016/j.ijid.2014.03.430
80. Waxman M, Aluisio AR, Rege S, Levine AC. Characteristics and survival of patients with Ebola virus infection, malaria, or both in Sierra Leone: a retrospective cohort study. Lancet Infect Dis (2017) 17:654–60. doi: 10.1016/S1473-3099(17)30112-3
81. Purpura LJ, Rogers E, Baller A, White S, Soka M, Choi MJ, et al. Ebola virus RNA in semen from an HIV-positive survivor of ebola. Emerg Infect Dis (2017) 23:714–5. doi: 10.3201/eid2304.161743
82. Abbate JL, Becquart P, Leroy E, Ezenwa VO, Roche B. Exposure to ebola virus and risk for infection with malaria parasites, Rural Gabon. Emerg Infect Dis (2020) 26:229–37. doi: 10.3201/eid2602.181120
83. Chukwuanukwu RC, Onyenekwe CC, Martinez-Pomares L, Flynn R, Singh S, Amilo GI, et al. Modulation of the immune response to Mycobacterium tuberculosis during malaria/M. tuberculosis co-infection. Clin Exp Immunol (2017) 187:259–68. doi: 10.1111/cei.12861
84. Edwards HM, Counihan H, Bonnington C, Achan J, Hamade P, Tibenderana JK. The impact of malaria coinfection on Ebola virus disease outcomes: A systematic review and meta-analysis. PloS One (2021) 16:e0251101. doi: 10.1371/journal.pone.0251101
85. Hartley M-A, Young A, Tran A-M, Okoni-Williams HH, Suma M, Mancuso B, et al. Predicting ebola severity: A clinical prioritization score for ebola virus disease. PloS Negl Trop Dis (2017) 11:e0005265. doi: 10.1371/journal.pntd.0005265
86. Kurachi M. CD8+ T cell exhaustion. Semin Immunopathol (2019) 41:327–37. doi: 10.1007/s00281-019-00744-5
87. Roe K. Concurrent infections of cells by two pathogens can enable a reactivation of the first pathogen and the second pathogen’s accelerated T-cell exhaustion. Heliyon (2022) 8:e11371. doi: 10.1016/j.heliyon.2022.e11371
88. McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol (2019) 37:457–95. doi: 10.1146/annurev-immunol-041015-055318
89. Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell (2009) 138:30–50. doi: 10.1016/j.cell.2009.06.036
90. Llibre A, Dedicoat M, Burel JG, Demangel C, O’Shea MK, Mauro C. Host immune-metabolic adaptations upon mycobacterial infections and associated co-morbidities. Front Immunol (2021) 12:747387. doi: 10.3389/fimmu.2021.747387
91. Richardson ET, Shukla S, Sweet DR, Wearsch PA, Tsichlis PN, Boom WH, et al. Toll-like receptor 2-dependent extracellular signal-regulated kinase signaling in mycobacterium tuberculosis-infected macrophages drives anti-inflammatory responses and inhibits th1 polarization of responding T cells. Infect Immun (2015) 83:2242–54. doi: 10.1128/IAI.00135-15
92. Garry RF. Ebola virus can lie low and reactivate after years in human survivors. Nature (2021) 597:478–80. doi: 10.1038/d41586-021-02378-w
93. Wauquier N, Becquart P, Gasquet C, Leroy EM. Immunoglobulin G in Ebola outbreak survivors, Gabon. Emerg Infect Dis (2009) 15:1136–7. doi: 10.3201/eid1507.090402
94. Yang X, Li Z, Wang B, Pan Y, Jiang C, Zhang X, et al. Prognosis and antibody profiles in survivors of critical illness from COVID-19: a prospective multicentre cohort study. Br J Anaesth (2022) 128:491–500. doi: 10.1016/j.bja.2021.11.024
95. Woolsey C, Geisbert TW. Antibodies periodically wax and wane in survivors of Ebola. Nature (2021) 590:397–8. doi: 10.1038/d41586-020-03044-3
96. Wiedemann A, Foucat E, Hocini H, Lefebvre C, Hejblum BP, Durand M, et al. Long-lasting severe immune dysfunction in Ebola virus disease survivors. Nat Commun (2020) 11:3730. doi: 10.1038/s41467-020-17489-7
97. Ye W, Ye C, Li J, Lei Y, Zhang F. Lessons from Pasteur may help prevent the deadly relapse of Ebola in patients: Using contingency vaccination to avoid Ebola relapse in immune-privileged organs. Front Immunol (2023) 14:1060481. doi: 10.3389/fimmu.2023.1060481
98. Forrester JV. Ebola virus and persistent chronic infection: when does replication cease? Ann Transl Med (2018) 6:S39–9. doi: 10.21037/atm.2018.09.60
100. Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PloS Negl Trop Dis (2010) 4:e837. doi: 10.1371/journal.pntd.0000837
101. Kuhn JH, Adachi T, Adhikari NKJ, Arribas JR, Bah IE, Bausch DG, et al. New filovirus disease classification and nomenclature. Nat Rev Microbiol (2019) 17:261–3. doi: 10.1038/s41579-019-0187-4
102. Escudero-Pérez B, Volchkova VA, Dolnik O, Lawrence P, Volchkov VE. Shed GP of ebola virus triggers immune activation and increased vascular permeability. PloS Pathog (2014) 10:e1004509. doi: 10.1371/journal.ppat.1004509
103. De Kleer I, Willems F, Lambrecht B, Goriely S. Ontogeny of myeloid cells. Front Immunol (2014) 5. doi: 10.3389/fimmu.2014.00423
104. Supramaniam A, Lui H, Bellette BM, Rudd PA, Herrero LJ. How myeloid cells contribute to the pathogenesis of prominent emerging zoonotic diseases. J Gen Virol (2018) 99:953–69. doi: 10.1099/jgv.0.001024
105. Vandergeeten C, Fromentin R, Chomont N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev (2012) 23:143–9. doi: 10.1016/j.cytogfr.2012.05.001
106. Veenhuis RT, Abreu CM, Costa PAG, Ferreira EA, Ratliff J, Pohlenz L, et al. Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat Microbiol (2023) 8:833–44. doi: 10.1038/s41564-023-01349-3
107. Shantha JG, Mattia JG, Goba A, Barnes KG, Ebrahim FK, Kraft CS, et al. Ebola virus persistence in ocular tissues and fluids (EVICT) study: reverse transcription-polymerase chain reaction and cataract surgery outcomes of ebola survivors in Sierra Leone. EBioMedicine (2018) 30:217–24. doi: 10.1016/j.ebiom.2018.03.020
108. Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol (2011) 11:221–30. doi: 10.1038/nri2940
109. Kelly JD, Van Ryn C, Badio M, Fayiah T, Johnson K, Gayedyu-Dennis D, et al. Clinical sequelae among individuals with pauci-symptomatic or asymptomatic Ebola virus infection and unrecognised Ebola virus disease in Liberia: a longitudinal cohort study. Lancet Infect Dis (2022) 22:1163–71. doi: 10.1016/S1473-3099(22)00127-X
110. Shantha JG, Canady D, Hartley C, Cassedy A, Miller C, Angeles-Han ST, et al. Ophthalmic sequelae and psychosocial impact in pediatric ebola survivors. EClinicalMedicine (2022) 49:101483. doi: 10.1016/j.eclinm.2022.101483
111. Bond NG, Grant DS, Himmelfarb ST, Engel EJ, Al-Hasan F, Gbakie M, et al. Post-ebola syndrome presents with multiple overlapping symptom clusters: evidence from an ongoing cohort study in eastern Sierra Leone. Clin Infect Dis (2021) 73:1046–54. doi: 10.1093/cid/ciab267
112. Clark DV, Kibuuka H, Millard M, Wakabi S, Lukwago L, Taylor A, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis (2015) 15:905–12. doi: 10.1016/S1473-3099(15)70152-0
113. Eghrari OA, Burkholder B, Ross R, Tawse K, Prakalapakorn GS, Reilly C, et al. Factors associated with uveitis among Ebola survivors in the PREVAIL III study. Invest Ophthalmol Vis Sci (2017) 58:2178.
114. Qureshi AI, Chughtai M, Loua TO, Pe Kolie J, Camara HFS, Ishfaq MF, et al. Study of ebola virus disease survivors in Guinea: table 1. Clin Infect Dis (2015) 61:1035–42. doi: 10.1093/cid/civ453
115. Scott JT, Sesay FR, Massaquoi TA, Idriss BR, Sahr F, Semple MG. Post-ebola syndrome, Sierra Leone. Emerg Infect Dis (2016) 22:641–6. doi: 10.3201/eid2204.151302
116. Pers Y-M, Sow MS, Taverne B, March L, Izard S, Étard JF, et al. Characteristics of the musculoskeletal symptoms observed among survivors of Ebola virus disease in the Postebogui cohort in Guinea. Rheumatology (2017) 56:2068–72. doi: 10.1093/rheumatology/kex074
117. Amuzu C, James PB, Bah AJ, Bayoh AVS, Singer SR. Post-Ebola sequelae among Ebola child survivors in Sierra Leone. BMC Pediatr (2021) 21:482. doi: 10.1186/s12887-021-02957-w
118. de St. Maurice A, Ervin E, Orone R, Choi M, Dokubo EK, Rollin PE, et al. Care of ebola survivors and factors associated with clinical sequelae—Monrovia, Liberia. Open Forum Infect Dis (2018) 5:ofy239. doi: 10.1093/ofid/ofy239
119. James PB, Wardle J, Gyasi RM, Steel A, Adams J, Kabba JA, et al. Health-related quality of life among Ebola survivors in Sierra Leone: the role of socio-demographic, health-related and psycho-social factors. Health Qual Life Outcomes (2022) 20:10. doi: 10.1186/s12955-022-01916-y
120. Crea TM, Collier KM, Klein EK, Sevalie S, Molleh B, Kabba Y, et al. Social distancing, community stigma, and implications for psychological distress in the aftermath of Ebola virus disease. PloS One (2022) 17:e0276790. doi: 10.1371/journal.pone.0276790
121. Wilson HW, Amo-Addae M, Kenu E, Ilesanmi OS, Ameme DK, Sackey SO. Post-ebola syndrome among ebola virus disease survivors in montserrado county, Liberia 2016. BioMed Res Int (2018) 2018:1–8. doi: 10.1155/2018/1909410
122. The PREVAIL III Study Group. A longitudinal study of ebola sequelae in Liberia. N Engl J Med (2019) 380:924–34. doi: 10.1056/NEJMoa1805435
123. Tozay S, Fischer WA, Wohl DA, Kilpatrick K, Zou F, Reeves E, et al. Long-term complications of ebola virus disease: prevalence and predictors of major symptoms and the role of inflammation. Clin Infect Dis (2020) 71:1749–55. doi: 10.1093/cid/ciz1062
124. Secor A, Macauley R, Stan L, Kagone M, Sidikiba S, Sow S, et al. Mental health among Ebola survivors in Liberia, Sierra Leone and Guinea: results from a cross-sectional study. BMJ Open (2020) 10:e035217. doi: 10.1136/bmjopen-2019-035217
125. Cénat JM, Rousseau C, Bukaka J, Dalexis RD, Guerrier M. Severe anxiety and PTSD symptoms among ebola virus disease survivors and healthcare workers in the context of the COVID-19 pandemic in eastern DR congo. Front Psychiatry (2022) 13:767656. doi: 10.3389/fpsyt.2022.767656
126. Tiffany A, Vetter P, Mattia J, Dayer J-A, Bartsch M, Kasztura M, et al. Ebola virus disease complications as experienced by survivors in Sierra Leone. Clin Infect Dis (2016) 62:1360–6. doi: 10.1093/cid/ciw158
127. Wing K, Oza S, Houlihan C, Glynn JR, Irvine S, Warrell CE, et al. Surviving Ebola: A historical cohort study of Ebola mortality and survival in Sierra Leone 2014-2015. PloS One (2018) 13:e0209655. doi: 10.1371/journal.pone.0209655
128. Shantha JG, Crozier I, Hayek BR, Bruce BB, Gargu C, Brown J, et al. Ophthalmic manifestations and causes of vision impairment in ebola virus disease survivors in Monrovia, Liberia. Ophthalmology (2017) 124:170–7. doi: 10.1016/j.ophtha.2016.10.011
129. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
130. Etard J-F, Sow MS, Leroy S, Touré A, Taverne B, Keita AK, et al. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis (2017) 17:545–52. doi: 10.1016/S1473-3099(16)30516-3
131. Mohammed H, Vandy AO, Stretch R, Otieno D, Prajapati M, Calderon M, et al. Sequelae and other conditions in ebola virus disease survivors, Sierra Leone, 2015. Emerg Infect Dis (2017) 23:66–73. doi: 10.3201/eid2301.160631
132. Epstein L, Wong KK, Kallen AJ, Uyeki TM. Post-ebola signs and symptoms in U.S. Survivors. N Engl J Med (2015) 373:2484–6. doi: 10.1056/NEJMc1506576
133. Mattia JG, Vandy MJ, Chang JC, Platt DE, Dierberg K, Bausch DG, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis (2016) 16:331–8. doi: 10.1016/S1473-3099(15)00489-2
134. Mwanza KA, Mukadi D, Mukadi P, Hoff AN, Doshi HR, Wasiswa J, et al. Clinical charateristics of ebola survivors 40 years post infection in the democratic republic of Congo. Am J Trop Med Hyg (2017) 95:1–651. doi: 10.4269/ajtmh.abstract2016
135. Guetiya Wadoum RE, Samin A, Mafopa NG, Giovanetti M, Russo G, Turay P, et al. Mobile health clinic for the medical management of clinical sequelae experienced by survivors of the 2013–2016 Ebola virus disease outbreak in Sierra Leone, West Africa. Eur J Clin Microbiol Infect Dis (2017) 36:2193–200. doi: 10.1007/s10096-017-3045-1
136. Howlett PJ, Walder AR, Lisk DR, Fitzgerald F, Sevalie S, Lado M, et al. Case series of severe neurologic sequelae of ebola virus disease during epidemic, Sierra Leone. Emerg Infect Dis (2018) 24:1412–21. doi: 10.3201/eid2408.171367
137. Jagadesh S, Sevalie S, Fatoma R, Sesay F, Sahr F, Faragher B, et al. Disability among ebola survivors and their close contacts in Sierra Leone: A retrospective case-controlled cohort study. Clin Infect Dis (2018) 66:131–3. doi: 10.1093/cid/cix705
138. Kelly JD, Hoff NA, Spencer D, Musene K, Bramble MS, McIlwain D, et al. Neurological, cognitive, and psychological findings among survivors of ebola virus disease from the 1995 ebola outbreak in Kikwit, Democratic Republic of Congo: A cross-sectional study. Clin Infect Dis (2019) 68:1388–93. doi: 10.1093/cid/ciy677
139. Cénat JM, Noorishad P-G, Dalexis RD, Rousseau C, Derivois D, Kokou-Kpolou CK, et al. Prevalence and risk factors of depression symptoms among rural and urban populations affected by Ebola virus disease in the Democratic Republic of the Congo: a representative cross-sectional study. BMJ Open (2022) 12:e053375. doi: 10.1136/bmjopen-2021-053375
140. Hugo M, Declerck H, Fitzpatrick G, Severy N, Gbabai OB-M, Decroo T, et al. Post-traumatic stress reactions in ebola virus disease survivors in Sierra Leone. Emerg Med Open Access (2015) 05:1000285. doi: 10.4172/2165-7548.1000285
141. the PostEboGui Study Group, Keita MM, Taverne B, Sy Savané S, March L, Doukoure M, et al. Depressive symptoms among survivors of Ebola virus disease in Conakry (Guinea): preliminary results of the PostEboGui cohort. BMC Psychiatry (2017) 17:127. doi: 10.1186/s12888-017-1280-8
142. Keita MM, Doukouré M, Chantereau I, Sako FB, Traoré FA, Soumaoro K, et al. Les Survivants De L’épidémie Récente de la Maladie À Virus Ebola Au Service De Psychiatrie De L’hôpital National Donka En Guinée-Conakry: Étude Psychopathologique Et Psychothérapeutique. LÉvolution Psychiatr (2017) 82:127–42. doi: 10.1016/j.evopsy.2016.07.004
143. Hereth-Hebert E, Bah MO, Etard JF, Sow MS, Resnikoff S, Fardeau C, et al. Ocular complications in survivors of the ebola outbreak in Guinea. Am J Ophthalmol (2017) 175:114–21. doi: 10.1016/j.ajo.2016.12.005
144. Steptoe PJ, Scott JT, Baxter JM, Parkes CK, Dwivedi R, Czanner G, et al. Novel retinal lesion in ebola survivors, Sierra Leone, 2016. Emerg Infect Dis (2017) 23:1102–9. doi: 10.3201/eid2307.161608
145. Vandy MJ, Chang J, Mattia GJ, Molleh B, Charles BD, Dierberg K, et al. Burden, timing, and outcomes of uveitis from a retrospective cohort of Ebola survivors in Sierra Leone. Invest Ophthalmol Vis Sci (2018) 59(9):4172.
146. Vandy M, Mattia J, Chang J, Platt D, Mansaray Y, Kamara A, et al. Patterns of ocular manifestations in ebola virus disease survivors in the port loko, Sierra Leone. Invest Ophthalmol Vis Sci (2016) 57(12):4145.
147. Yeh S, Shantha J, Mattia J, Garry R, Vandy MJ. Ebola virus persistence in ocular tissues and fluids (EVICT) study: baseline characteristics and primary findings from ocular fluid of ebola survivors in Sierra Leone. Invest Ophthalmol Vis Sci (2017) 58(8):3609.
148. Shantha GJ, Weil N, Miller C, Bastien G, Yeh S. Ocular complications in pediatric ebola survivors. J Womens Health (2018) 27:1415–33. doi: 10.1089/jwh.2018.29020.abstracts
149. Hereth Hebert E, Sow S, Ethard FJ, Toure A, Msellati P, Taverne B, et al. A case of 11 uveitis in patients who recovered from Ebola in Guinea. Trop Med Int Health (2015) 20:171–441. doi: 10.1016/j.ajo.2016.12.005
150. Reznik SE, Gardner EL, Ashby CR. Cannabidiol: a potential treatment for post Ebola syndrome? Int J Infect Dis (2016) 52:74–6. doi: 10.1016/j.ijid.2016.09.020
151. Rasmussen AL, Okumura A, Ferris MT, Green R, Feldmann F, Kelly SM, et al. Host genetic diversity enables Ebola hemorrhagic fever pathogenesis and resistance. Science (2014) 346:987–91. doi: 10.1126/science.1259595
152. Leroy E, Baize S, Volchkov V, Fisher-Hoch S, Georges-Courbot M-C, Lansoud-Soukate J, et al. Human asymptomatic Ebola infection and strong inflammatory response. Lancet (2000) 355:2210–5. doi: 10.1016/S0140-6736(00)02405-3
153. National Library of Medicine. PREVAIL VI: Identification of Host Genetic Factors Underlying Ebola Virus Disease Risk, Mortality, Long-term Sequelae, Viral RNA Persistence, Humoral Immunity, and Ebola Vaccine Response. Available at: https://beta.clinicaltrials.gov/study/NCT03098862 (Accessed May 17, 2023).
154. Chrobak W, Pacut DW, Blomgren F, Rodin A, Swenson J, Ermilova I. Component of cannabis, cannabidiol, as a possible drug against the cytotoxicity of Aβ(31–35) and Aβ(25–35) peptides: an investigation by molecular dynamics and well-tempered metadynamics simulations. ACS Chem Neurosci (2021) 12:660–74. doi: 10.1021/acschemneuro.0c00692
155. Konde MK, Baker DP, Traore FA, Sow MS, Camara A, Barry AA, et al. Interferon β-1a for the treatment of Ebola virus disease: A historically controlled, single-arm proof-of-concept trial. PloS One (2017) 12:e0169255. doi: 10.1371/journal.pone.0169255
156. Qiu X, Wong G, Fernando L, Audet J, Bello A, Strong J, et al. mAbs and ad-vectored IFN-α Therapy rescue ebola-infected nonhuman primates when administered after the detection of viremia and symptoms. Sci Transl Med (2013) 5207ra143. doi: 10.1126/scitranslmed.3006605
157. Smith LM, Hensley LE, Geisbert TW, Johnson J, Stossel A, Honko A, et al. Interferon-β Therapy prolongs survival in rhesus macaque models of ebola and marburg hemorrhagic fever. J Infect Dis (2013) 208:310–8. doi: 10.1093/infdis/jis921
158. Dyal J, Kofman A, Kollie JZ, Fankhauser J, Orone R, Soka MJ, et al. Risk factors for ebola virus persistence in semen of survivors in Liberia. Clin Infect Dis (2023) 76:e849–56. doi: 10.1093/cid/ciac424
159. Longet S, Mellors J, Carroll MW, Tipton T. Ebolavirus: comparison of survivor immunology and animal models in the search for a correlate of protection. Front Immunol (2021) 11:599568. doi: 10.3389/fimmu.2020.599568
160. Ferguson N, Anderson R, Gupta S. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc Natl Acad Sci (1999) 96:790–4. doi: 10.1073/pnas.96.2.790
161. Ziganshina MM, Shilova NV, Khalturina EO, Dolgushina NV, V. Borisevich S, Yarotskaya EL, et al. Antibody-dependent enhancement with a focus on SARS-coV-2 and anti-glycan antibodies. Viruses (2023) 15:1584. doi: 10.3390/v15071584
162. Wang G, Xiang Z, Wang W, Chen Z. Seasonal coronaviruses and SARS-CoV-2: effects of preexisting immunity during the COVID-19 pandemic. J Zhejiang Univ-Sci B (2022) 23:451–60. doi: 10.1631/jzus.B2200049
163. Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms andin vivo implications. Rev Med Virol (2003) 13:387–98. doi: 10.1002/rmv.405
164. Choi JH, Schafer SC, Zhang L, Juelich T, Freiberg AN, Croyle MA. Modeling pre-existing immunity to adenovirus in rodents: immunological requirements for successful development of a recombinant adenovirus serotype 5-based ebola vaccine. Mol Pharm (2013) 10:3342–55. doi: 10.1021/mp4001316
165. Osei E, Amu H, Appiah PK, Amponsah SB, Danso E, Oppong S, et al. Stigma and discrimination tendencies towards COVID-19 survivors: Evidence from a nationwide population-based survey in Ghana. PloS Glob Public Health (2022) 2:e0000307. doi: 10.1371/journal.pgph.0000307
166. Stehling-Ariza T, Lefevre A, Calles D, Djawe K, Garfield R, Gerber M, et al. Establishment of CDC global rapid response team to ensure global health security. Emerg Infect Dis (2017) 23. doi: 10.3201/eid2313.170711
167. Baral P. Health systems and services during COVID-19: lessons and evidence from previous crises: A rapid scoping review to inform the United Nations research roadmap for the COVID-19 recovery. Int J Health Serv (2021) 51:474–93. doi: 10.1177/0020731421997088
168. Halfmann PJ, Eisfeld AJ, Watanabe T, Maemura T, Yamashita M, Fukuyama S, et al. Serological analysis of Ebola virus survivors and close contacts in Sierra Leone: A cross-sectional study. PloS Negl Trop Dis (2019) 13:e0007654. doi: 10.1371/journal.pntd.0007654
169. Lanini S, Portella G, Vairo F, Kobinger GP, Pesenti A, Langer M, et al. Blood kinetics of Ebola virus in survivors and nonsurvivors. J Clin Invest (2015) 125:4692–8. doi: 10.1172/JCI83111
170. Rimoin AW, Lu K, Bramble MS, Steffen I, Doshi RH, Hoff NA, et al. Ebola Virus Neutralizing Antibodies Detectable in Survivors of theYambuku, Zaire Outbreak 40 Years after Infection. J Infect Dis (2018) 217:223–31. doi: 10.1093/infdis/jix584
171. Koch T, Rottstegge M, Ruibal P, Gomez-Medina S, Nelson EV, Escudero-Pérez B, et al. Ebola virus disease survivors show more efficient antibody immunity than vaccinees despite similar levels of circulating immunoglobulins. Viruses (2020) 12:915. doi: 10.3390/v12090915
172. Rowe AK, Bertolli J, Khan AS, Mukunu R, Muyembe-Tamfum JJ, Bressler D, et al. Clinical, virologic, and immunologic follow-up of convalescent ebola hemorrhagic fever patients and their household contacts, Kikwit, Democratic Republic of the Congo. J Infect Dis (1999) 179:S28–35. doi: 10.1086/514318
173. Green E, Hunt L, Ross JCG, Nissen NM, Curran T, Badhan A, et al. Viraemia and Ebola virus secretion in survivors of Ebola virus disease in Sierra Leone: a cross-sectional cohort study. Lancet Infect Dis (2016) 16:1052–6. doi: 10.1016/S1473-3099(16)30060-3
Keywords: post-Ebola syndrome, reemergence, survivors, public health, outbreak
Citation: Asare Fenteng E, Ossei PPS, Ayibor WG and Narh-Bedu T (2023) Beyond survival: unraveling the dynamics of Ebola virus resurgence in Sub-Saharan Africa and the remarkable journey of survivors. Front. Virol. 3:1227314. doi: 10.3389/fviro.2023.1227314
Received: 23 May 2023; Accepted: 26 October 2023;
Published: 13 November 2023.
Edited by:
Antonio Caruz, University of Jaén, SpainCopyright © 2023 Asare Fenteng, Ossei, Ayibor and Narh-Bedu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Asare Fenteng, ZXJpYy5hc2FyZS1mZW50ZW5nQHVuaS1rb25zdGFuei5kZQ==; ZmVuYXNhcmVAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.