
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virol., 02 June 2023
Sec. Virus and Host Immunity
Volume 3 - 2023 | https://doi.org/10.3389/fviro.2023.1157659
 Satish K. Mehta1*
Satish K. Mehta1* Douglass M. Diak2
Douglass M. Diak2 Bridgette V. Rooney3
Bridgette V. Rooney3 Stephanie S. Krieger4
Stephanie S. Krieger4 Mayra Nelman-Gonzalez4
Mayra Nelman-Gonzalez4 James P. Locke5
James P. Locke5 Maria A. Nagel6
Maria A. Nagel6 Millennia Young5
Millennia Young5 Brian E. Crucian5
Brian E. Crucian5Introduction: Reactivation of herpes viruses, such as Epstein–Barr virus (EBV), herpes simplex virus 1 (HSV1), and varicella zoster virus (VZV), increases in astronauts during spaceflight, compared with their preflight and postflight levels. Reactivations can increase the risk of associated clinical conditions, such as herpes zoster, chronic neuropathic pain, vision loss, stroke, cognitive impairment, and cold sores. Furthermore, continued viral shedding for longer periods after space travel may increase the risk of viral transmission to uninfected crew contacts, including, but not limited to, the immunocompromised and newborn infants. Thus, it is essential to develop spaceflight countermeasures to prevent herpes viral reactivations to ensure the health of crewmembers and their contacts. One such countermeasure is the prophylactic administration of an antiviral drug (valacyclovir) against the alpha herpesviruses (VZV and HSV1). To determine the effectiveness of this countermeasure, we studied the shedding of EBV, VZV, and HSV1 in Antarctic expeditioners, who have similar salivary viral shedding patterns during winter-over to astronauts during long spaceflights.
Methods: The efficacy of this antiviral drug as a countermeasure was determined using three major parameters in the saliva of expeditioners during winter-over with and without administration of this drug: (i) viral load and frequency, (ii) physiological stress biomarkers [i.e., levels of cortisol, dehydroepiandrosterone (DHEA), and amylase), and (iii) immune markers (i.e., inflammatory cytokines)]. Thirty-two volunteers from two Antarctic stations (McMurdo and South Pole) participated in this study. Participants were randomly assigned to either the treatment group (valacyclovir HCl: 1 g/day) or placebo group (oyster calcium: 500mg/day).
Results: Viral shedding of EBV reduced significantly (> 24-fold) in the treatment group compared with the placebo group. HSV1 was also reduced by more than fivefold, but this was not statistically significant. No VZV shedding was observed in any of the participants. In the placebo group 50% of the saliva samples had measurable viral DNA (EBV, HSV1, or both), compared with 19% of the treatment group. There was no significant change in the ratio of cortisol to DHEA or levels of alpha-amylase, indicating that physiological stress was similar between the groups. No difference was detected in levels of salivary cytokines, except IL-10, which was found in significantly lower levels in the treatment group.
Discussion: These data indicate that valacyclovir is a safe and successful intervention to reduce EBV and HSV1 shedding in individuals subjected to extreme environments and stressors.
More than 90% of human adults are infected with one or more of the known eight members of the Herpesviridae family. These viruses establish lifelong latency in various reservoirs throughout the human body (1), but can reactivate due to physiological/psychological stressors and/or reduced immunological control. Once reactivated, these viruses can spread both intrinsically (cell to cell) and extrinsically (person to person). In most cases, this reactivation is asymptomatic to the carrier, as evidenced by prolonged periods of variable Epstein–Barr Virus (EBV) shedding in otherwise healthy individuals (2). However, these viruses can also produce debilitating symptoms, such as the painful vesicular rash associated with reactivation of latent varicella zoster virus (VZV). Continued replication and shedding, whether symptomatic or not, may indirectly lead to increased risk of developing other malignancies, including stroke (caused by VZV) (3), nasopharyngeal carcinoma, and Hodgkin’s lymphoma (caused by EBV) (4), or Alzheimer’s disease (linked to HSV-1) (5, 6). In addition, continued shedding of an infectious virus for a long period of time may increase the risk of transmitting the virus to uninfected and/or immunocompromised contacts, and to newborn infants. Thus, developing countermeasures is crucial in protecting the health and wellbeing of deep-space crews and their professional and personal contacts in space and on the ground (7, 8).
Humans living in extreme environments for prolonged periods of time are optimal models for evaluating countermeasures for herpes viral reactivations due to the increased physiological and psychological pressures experienced under these conditions. Our group has reported the reactivation and shedding of latent herpes viruses in astronauts during both short-duration shuttle spaceflights (10–16 days) (9) and long-duration (up to 180 days) inhabitation of the International Space Station (ISS) (10). Following reactivation, viruses are shed in saliva, blood, and urine in significantly larger quantities than normal (11, 12). Surprisingly, the higher viral loads still represent an asymptomatic phenomenon in astronauts; however, in some cases, live, infectious viruses (VZV and HSV-1) have been recovered in saliva associated with persistent atopic dermatitis (13), herpes zoster (12), and cold sores during a spaceflight. Although simple countermeasures [i.e., physical exercise (14) or nutritional improvements (15–17)] have been applied to flight programs with some success, more experimental measures are normally first tested for efficacy in ground analogs before use in astronauts. Expeditioners endure many of the same environmentally induced physiological and psychological pressures during winter-over in Antarctica as astronauts do in space. Therefore, Antarctic expeditioners provide an excellent ground analog for evaluating the effects of countermeasures mitigating clinical responses to extreme environments before application in astronauts (18–20).
In this study, we recruited participants participating in Antarctic winter-over to evaluate the physiological and viral reactivation responses to prophylactic administration of the antiviral drug Valtrex (valacyclovir). Valtrex is clinically used to treat cold sores around the mouth (caused by herpes simplex) as well as chickenpox and shingles (caused by varicella zoster). Valtrex is also known to reduce detectable EBV load in saliva for EBV-seropositive runners when compared with a placebo treatment (p = 0.04) (21). Long-term valacyclovir treatment (up to 1 year) has also been used previously in immunocompetent individuals and was well tolerated (22) (23). We hypothesized that the prophylactic administration of valacyclovir (1 g daily) to Antarctic expeditioners, who have been reported to have similar viral shedding patterns in their saliva during winter-over as astronauts, would significantly reduce shedding of herpes viruses when compared with placebo controls. Countermeasure efficacy was determined by comparing viral load and frequency (EBV, HSV-1, and VZV), physiological stress biomarkers (i.e., levels of cortisol, DHEA, and alpha-amylase), and immune markers (inflammatory cytokines) between the treatment group (valacyclovir HCl: 1 g/day) and the placebo group (oyster calcium 500mg/day) during winter-over. The study was carried out in 32 participants from two Antarctic stations (McMurdo and South Pole). Participants were randomly assigned to either the treatment group or placebo group. Results from the current study found that valacyclovir taken daily decreased EBV and HSV1 viral loads by > 24-fold and > 5-fold, respectively, in the treatment group compared with the placebo group over the course of the entire winter-over. Thus, the results suggest that the long-term administration of a prophylactic antiviral drug (valacyclovir) may be a good and safe method to reduce viral reactivation and shedding in astronauts during long space journeys.
Forty-six Antarctic expeditioners (30% female; ages ranging from 27 to 63 years, with a mean of 44 years) were enrolled in this study. However, only 32 completed the study in full and only their data are presented hereafter. Non-compliance with study parameters (six participants), as well as any treatment-related adverse events (two participants) or non-treatment-related health events (six participants) that warranted the concern of the subject or clinical staff, served as criteria for withdrawal from the study and the exclusion of the subject’s data. Twenty-two of the participants were from the (coastal) McMurdo Antarctic station and 10 were from the (inland) South Pole Antarctic station. Participants were excluded if they had a history of renal dysfunction, chronic headaches, or allergies to valacyclovir or similar drugs. In addition, individuals > 65 years of age with declining renal function, those with known renal dysfunction, and those on other potentially nephrotoxic medications were not included in the study. This study was approved by the JSC Institutional Review Board (IRB) and by the National Science Foundation’s Office of Polar Programs (NSF/OPP) Antarctic Infrastructure and Logistics (AIL) and Antarctic Sciences (ANT) sections and executed during winter-over from March to October 2020. Subject privacy and data confidentiality were maintained in accordance with (i) NASA Policy Directive (NPD) 7100.8, “Protection of Human Research Subjects”; (ii) NASA Procedural Requirements (NPR) 7100.1, “Protection of Human Research Subjects”; and (iii) to the extent allowed by federal law. Collected data were stored on secure databases and in locked housing within JSC and only non-identifiable data were released to the scientific community. Informed consent was obtained from all participants before the study. Participants were randomly assigned to either the treatment group (valacyclovir: 1g/day) or placebo group (oyster calcium: 500mg/day) at both stations. Participants at the McMurdo station collected samples for 6 months, whereas the participants from the South Pole collected for 8 months during winter-over. Saliva samples were collected from each subject 5 consecutive days each month throughout the study (Figure 1). These participants were seropositive for EBV, HSV-1, and VZV and had not been vaccinated with Shingrix or Zostavax. A total of 1,054 saliva samples were collected from these 32 participants from both stations: 656 from McMurdo and 398 from South Pole. After collection, samples were stored frozen until they were shipped to the Immunology/Virology Laboratory of the Johnson Space Center, NASA. These samples were processed for determining viral load and frequency (i.e., EBV, HSV-1, and VZV), physiological stress biomarkers (i.e., levels of cortisol, DHEA, and salivary amylase) and immune markers (i.e., inflammatory cytokines) using the following specific assays.
Latent herpes virus reactivation and shedding was measured in the saliva samples collected from these expeditioners by extracting DNA from 1 mL of saliva using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany; Cat. No. 51106) according to the manufacturer’s instructions. Extracted viral DNA was then amplified by real-time quantitative PCR using a QuantStudio 3 (Thermo Fisher Scientific, Waltham, MA, USA) with specific primers and probes unique to EBV, HSV-1, and VZV reactivation genes. The sequences of primers and probes for different viruses have been previously published (9, 10). The genes that were probed and amplified for these viruses were as follows: for EBV, the BALF5 gene encoding viral DNA polymerase; for HSV1, the intergenic region between UL42 and UL43; and for VZV, VZV ORF28. All the samples were run in duplicate, and the data (copies/mL) were normalized to the volume of starting saliva. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a positive control.
Tests for salivary cortisol, dehydroepiandrosterone (DHEA), and alpha-amylase were performed on these samples by enzyme-linked immunosorbent assay (ELISA) using Salimetrics kits (cortisol Catalog # 1–3002; DHEA Catalog # 1–1202; α-amylase Catalog # 1–1902) as described in previous studies (24, 25).
Salivary cytokines were measured using a MILLIPLEX® MAP Human High Sensitivity T Cell Panel Premixed 13-plex Multiplex Assay (EMD Millipore, Burlington, MA, USA) according to the manufacturer’s instructions on a Luminex MAGPIX instrument (DiaSorin, Austin, TX, USA). The 13-cytokine panel is presented in Table 1. We have also documented cytokine stability, with minimum loss of signal following up to three freeze–thaw cycles in our laboratory (unpublished data). All saliva cytokines with a measurable baseline concentration were found to be generally stable, except for IL-12.
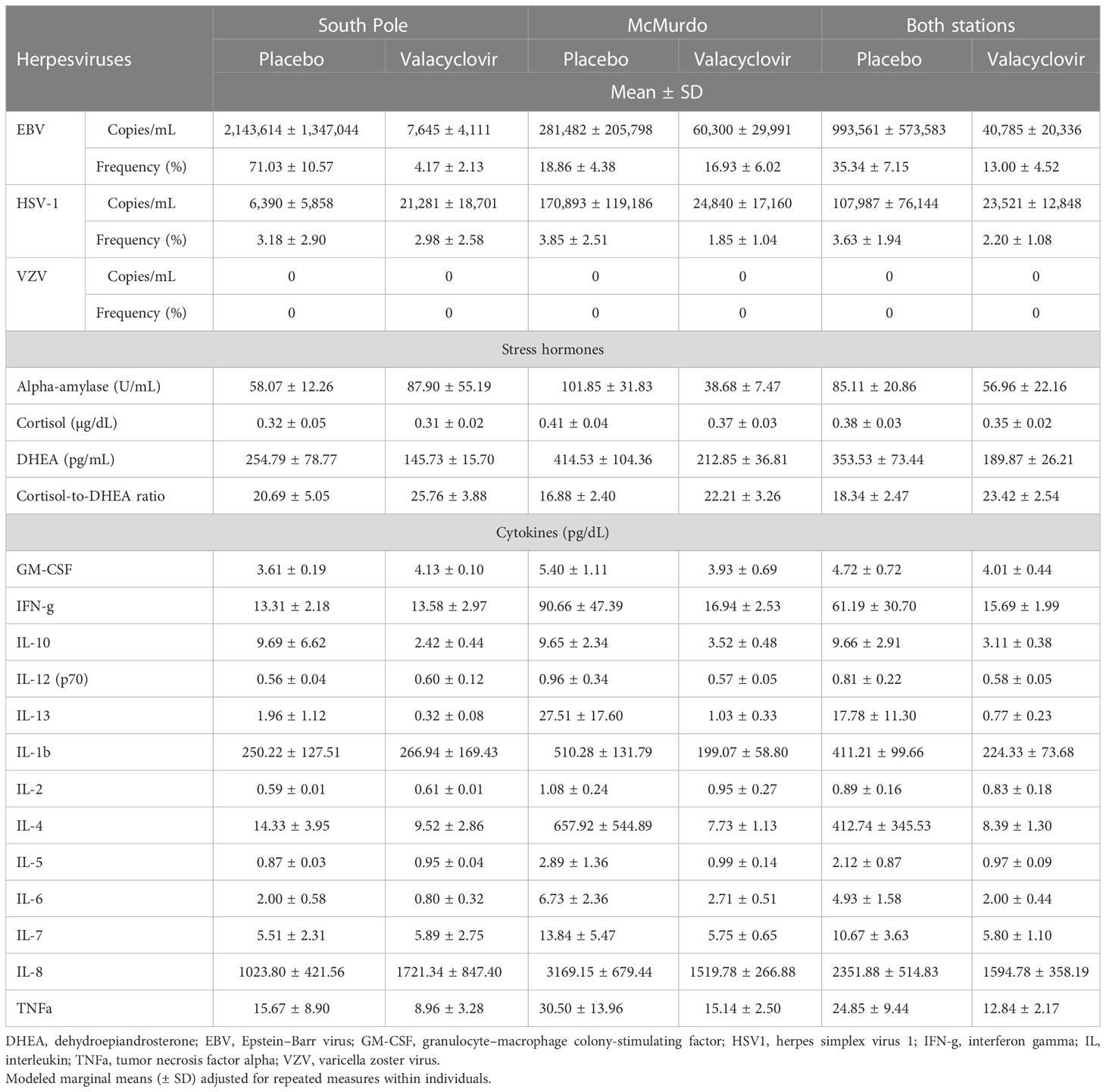
Table 1 Salivary viral shedding, stress hormones, and cytokine profiles in Antarctica expeditioners with and without prophylactic antiviral administration during winter-over.
The data were analyzed with generalized linear mixed-effects models. EBV and HSV-1 were modeled using logistic models with events defined by shedding counts greater than 0. The level of shedding was modeled using Poisson regression among those that did shed. Other immune measures were analyzed via standard normally distributed continuous models. As we wanted to make inferences within and between sites for viral activity, models for EBV and HSV-1 were defined by the interaction of site (McMurdo or South Pole) and treatment (antiviral or placebo) as categorical fixed effects. Subject-specific random generalized estimating equations (GEE) effects were included to address the repeated measures. When making inferences across sites, we incorporated site into the random GEE effect. Similarly, for immune measures, we incorporated the subject nested within sites into the random effects to focus on treatment effects. Robust standard errors addressed non-homogeneous variance across treatment, site, and time. The estimation of means, standard errors, and comparisons between treatments and sites was performed via the marginal means. Models were fit using the GLIMMIX procedure in SAS v9.4.
Repeated measures correlation was used to estimate the association between all measures. All data (from both the placebo and antiviral groups) were used to organize dendrograms based on hierarchical clustering using the distance metric of 1 – abs(ρ). Separate heatmaps of the correlations were created for each group (control and treatment). Repeated measures correlations were estimated with the rmcorr R package, and visualizations built with the heatmap.2 function in the R package gplots.
After orally administering 1 g of valacyclovir or 500 mg of oyster calcium to the Antractica expeditioners daily for 6–8 months of winter-over, only two participants had experienced treatment-related adverse events to the drug or placebo. One subject had an adverse event to the drug, reporting fatigue and nausea, and one subject in the placebo group reported indigestion/gastroesophageal reflux disease (GERD), possibly due to an allergy to shellfish (as the placebo was oyster calcium). None of the major adverse symptoms to valacylovir, which include headache, dizziness, depression, vomiting, stomach pain, joint pain, confusion, agitation, hallucinations (seeing or hearing things that are not real), problems with speech, seizure, or kidney problems (little or no urination, swelling in the feet or ankles, or feeling tired or short of breath) were reported during the winter-over at both the stations.
Twenty-eight of the 32 participants had a reactivation of at least one herpes virus throughout the duration of the study. One subject in the placebo group and three participants in the treatment group did not shed any virus in any sample analyzed. The frequency of EBV reactivation events was significantly less in the treatment group (13%) than in the placebo group (35%) across both the McMurdo and South Pole stations [odds ratio (OR) 3.7, 95% CI 1.3 to 10.3; p = 0.016]. However, when analyzing the frequency of EBV by site, this reduction was heavily influenced by the results from the South Pole station (p < 0.0001) and not by the results from the McMurdo station (p = 0.7999) (Table 1). Viral loads (are presented throughout as mean ± SD copies/mL) of EBV were 24 times lower in the treatment group (40,785 ± 20,336 copies/mL) than in the placebo group (993,561 ± 573,583 copies/mL) (F = 17.52; p = 0.0002). The South Pole station had a significant reduction in EBV viral load of about 280-fold [placebo 2,143,614 ± 1,347,044 copies/ml; treatment 7,645 ± 4,111 copies/mL (t = 6.81, p < 0.0001)], whereas the fivefold reduction at the McMurdo station was not significant [placebo 281,482 ± 205,798 copies/mL; treatment 60,300 ± 29,991 copies/mL (t = 1.74, p = 0.0924)]. The frequency of HSV1 reactivation did not change between the treatment (2%) and placebo (4%) groups across both stations (OR 1.7, 95% CI 0.36 to 7.76; p = 0.50). Although there were five times fewer HSV1 copies/mL detected in the treatment group (23,521 ± 12,848) than in the placebo group (107,987 ± 76,144), this result was not significant (F = 2.92; p = 0.0978). No VZV was detected in any sample.
The level of salivary DHEA was reduced significantly from 353.5 pg/mL in the placebo group to 189.9 pg/mL in the treatment group (mean difference 163.66 pg/mL, SE 77.8 pg/mL; p = 0.044). The levels of cortisol (F = 0.58; p = 0.4519) and alpha-amylase (F = 0.86; p = 0.3624) did not show any significant changes, nor did the cortisol-to-DHEA ratio (F = 2.06; p = 0.1619).
Twelve of the 13 cytokines tested did not show any significant changes due to the antiviral treatment. However, levels of IL-10 reduced with antiviral treatment from 9.7 pg/dL in the placebo group to 3.1 pg/dL in the treatment group (mean difference 6.6 pg/dL; F = 4.99; p = 0.033). In addition, levels of interferon gamma (IFN-γ) and IL-6 were four times and 2.5 times less, respectively, in the treatment group than in the placebo. However, these changes in levels of cytokines did not reach statistical significance.
Figure 2 illustrates the correlation heat maps for all analyses for the placebo and treatment groups. Hierarchical clustering of both groups combined produced the dendrogram organization of variables. Dendrograms (organization trees of variables) were estimated through hierarchical clustering of all data combined using the distance metric of one minus the absolute value of the repeated measures correlation. This distance metric presents strong correlations, whether positive or negative, as closer, whereas correlations around zero are more distant. This approach groups more strongly associated variables closer together. Separate heatmaps are shown for placebo and antiviral groups, highlighting the differences between them. Antiviral use appeared to reduce the correlation between the block of IL parameters and changed the direction of the association between DHEA and IL-10 (negative for the placebo group and positive for the treatment group) (Figure 2).
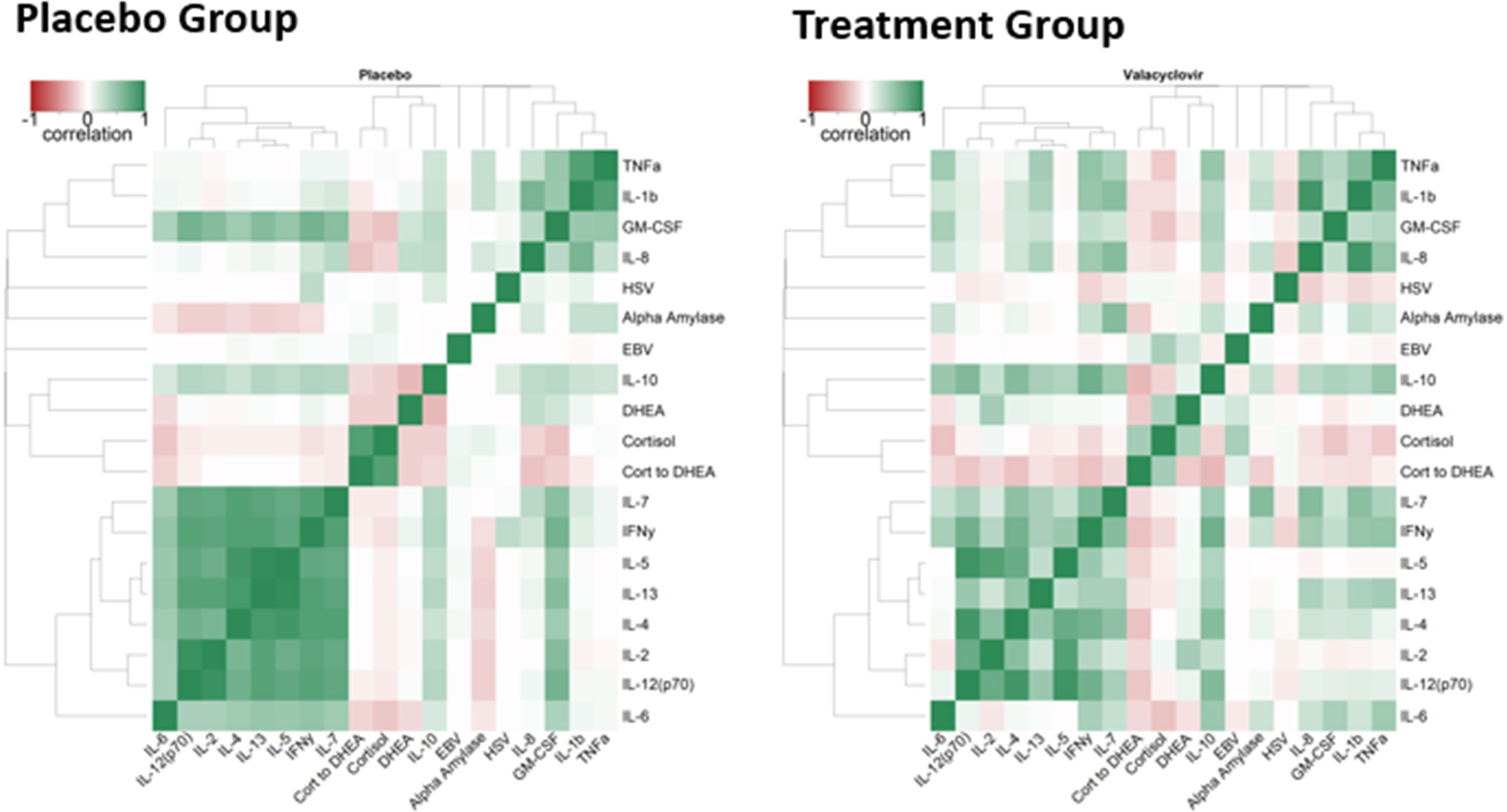
Figure 2 Correlation Heatmaps. Color represents the range of correlation between – 1 (red) to +1 (green) `with 0 being represented by white. The darker the color, the stronger correlation.
Our previous studies show that 52% of Antarctic expeditioners reactivate and shed latent herpes viruses (including VZV) through their saliva during winter-over, which is a similar rate to astronauts (50%–65%) (10, 11). Therefore, Antarctica is the best terrestrial–space analog for studying immune dysregulation and viral reactivation under stressful conditions. Persistent increased viral shedding during and post spaceflight may present some clinical risk to crew contacts, including uninfected or immunocompromised individuals and newborn infants; it is therefore important to develop countermeasures to prevent such reactivations in astronauts during current and future long-term space missions. Although valacyclovir is used to treat herpes virus infections, including herpes labialis (cold sores), herpes zoster (shingles), and herpes simplex (genital herpes) in adults, and chickenpox and cold sores in children, the drug has never been used prophylactically in spaceflight under zero gravity or under extreme terrestrial conditions like Antarctica. As valacyclovir treatment has been successfully used for long periods of 6 months or more as a treatment for HSV-1 infections in otherwise healthy adults, we undertook this study to (i) examine the efficacy of Valtrex as a prophylactic therapy to prevent viral reactivation and (ii) assess any adverse symptoms associated with such a therapy in apparently healthy adults.
In the current study, we found a significant decrease in the viral shedding of EBV in participants in response to prophylactic administration of Valtrex. Combined data for both the South Pole and McMurdo stations illustrated a 24-fold decrease in the average EBV viral load (copies/mL in saliva) for the treatment groups (antiviral valacyclovir administration) when compared with the placebo groups. The South Pole station had a 280-fold decrease in viral load, whereas the McMurdo station had a fivefold decrease in viral load. For HSV-1, the fivefold reduction across both stations was not statistically significant. When examining each location separately for HSV-1 viral load, the South Pole station had a threefold increase in viral load and the McMurdo station had an 8.5-fold decrease. Interestingly, there was no VZV shedding in the saliva of expeditioners from both groups, so the effect of valacyclovir on VZV reactivation and shedding cannot be inferred in this case. In general, VZV does not reactivate as frequently as HSV-1 or EBV, particularly in the younger population. Our previous studies have shown approximately 50% of astronauts shed VZV during space travel (10). Although this may appear inconsistent with our current study, it must be noted that no ground analog perfectly replicates the unique stressors of spaceflight. It may be likely that more VZV shedding occurs in astronauts due to increased radiation exposure or microgravity, neither of which are simulated in the Antarctic winter-over analog. In addition, VZV shedding has decreased significantly in astronauts recently due to the administration of a VZV vaccine to every astronaut before a space flight (unpublished data).
These data are in agreement with an earlier study, in which an 82% reduction of EBV load in saliva was reported for EBV seropositive runners treated with Valtrex when compared with the placebo treatment (p = 0.04) (21). In another non-placebo-controlled study, valacyclovir treatment did not decrease peripheral blood EBV viral loads (26). Valacyclovir is a commonly prescribed antiviral drug used to treat clinical herpes viral symptoms, so the viral results of the study were not surprising. We found that the South Pole station generally had a higher frequency and viral load of herpes viruses than the McMurdo station. The location of the stations may be the contributing factor, as the McMurdo station is located on the coast at the South Pacific Ocean-facing point. The South Pole station, on the other hand, is inland, isolated, and truly winter-overed. This could result in a more psychologically stressful experience for the expeditioners and, thus, more viral shedding.
Valacyclovir is a valyl ester of the antiviral drug acyclovir. Acting as an oral prodrug, valacyclovir is converted in vivo to acyclovir. Acyclovir, a nucleoside analog, is phosphorylated by virally encoded thymidine kinase, and, subsequently, by cellular enzymes, creating acyclovir triphosphate, which competitively inhibits viral DNA polymerase. Valacyclovir works by stopping the virus from multiplying and spreading to nearby healthy cells. It does not cure shingles, cold sores, or genital herpes, but it does help the sores to heal more quickly, and it relieves pain and discomfort. Valacyclovir reduces oral shedding of EBV in patients with infectious mononucleosis by inhibiting viral DNA polymerase (22). The adverse effects of valacyclovir treatment at the dose administered in our study in healthy individuals include headache (13%), abdominal pain (1%), nausea (5%), and rash (8%). Very rare side effects include nephrotoxicity and thrombotic thrombocytopenic purpura, but these tend to occur at higher doses, with intravenous administration, with co-administration of other potentially nephrotoxic drugs, or in renally compromised individuals (www.micromedexsolutions.com). In the current study, administration of this drug to our participants at both the South Pole and McMurdo stations produced only one adverse event (headache, fatigue, and nausea) in one subject, which was determined as having a “probable” link to the study product (valacyclovir), and one adverse event in the placebo, possibly owing to a shellfish allergy. This suggests that the long-term administration of a prophylactic antiviral drug (valacyclovir) may be a good and safe choice to reduce viral reactivation and shedding in astronauts during space travel.
Levels of salivary cortisol and alpha-amylase did not change between the two groups of expeditioners. However, there was a significant decrease in DHEA in the treatment group when compared with the placebo at both stations (Table 1). As valacyclovir directly inhibits viral DNA polymerase within infected cells (as described in detail above), we hypothesized that physiological stress hormones would be unaffected and that, consequently, similar levels of DHEA would be found in the treatment and placebo groups. However, it is unknown if valacyclovir has any effect on the production of adrenal androgens in the cortex. Although we find this to be unlikely, it cannot be discounted at this time. Furthermore, DHEA levels are known to correlate with many aspects of human physiology, such as age, sex, and nutritional status (27). In this study, age and sex were equitable between the two groups. Although additional variables may have had a role, we do not want to make any unnecessary conclusions on data that were not obtained. Importantly, however, many published works (28), including our own (9), regard the cortisol-to-DHEA ratio as a more thorough representation of how human beings respond to stress. In the current study, cortisol levels remained unchanged, but DHEA levels decreased. This led to a slight increase in the cortisol-to-DHEA ratio. However, this was not a significant change, and we conclude that stress levels were similar between the groups.
Herpes viruses normally remain latent in healthy humans whose immune systems are robust and intact, specifically those with normal T-cell function. No blood was collected in the present study; therefore, no T-cell function was assessed. However, we were able to analyze the saliva samples for 13 common inflammatory cytokines. Unfortunately, due to assay constraints, the salivary cytokine analysis was limited to one sample per time point, providing only a snapshot of the salivary cytokine levels in this study. This additionally introduces a limitation to the correlation of these data with viral reactivation. Most of the 13 inflammatory cytokines studied in saliva did not show any statistical differences in levels between the groups. Furthermore, almost all the samples were below the detection limit for IL-13, IL-2, and IL-5, so inferences cannot be made specifically for these cytokines. Regardless, it was interesting to observe that interleukin 10 (IL-10) decreased significantly in the treated group at both the stations. Our data show that the placebo group not only had elevated levels of herpes viral shedding but also had substantially more IL-10, and this effect was counteracted by valacyclovir in the treatment group. Both EBV and HSV-1 are known to induce the release of hIL-10 as a strategy to disarm and evade the immune system (29). Furthermore, EBV encodes an indistinguishable ortholog of human IL-10, known as viral IL-10 (vIL-10), that is preferentially released during lytic replication to evade recognition by cytotoxic memory T cells (30). We hypothesize that the IL-10 reduction in the treatment group was because fewer lytic EBV and HSV-1 cells were replicating, and thus the production of vIL-10 and hIL-10, respectively, was reduced.
Finally, we also found reductions in IL-4 (50-fold), IFN-γ (4-fold) and IL-6 (2.5-fold) production in the treatment group. For IL-4, two participants in the placebo group had extremely high values (one outside the range of detection) at each time point, skewing the averages. When these participants values were removed, there was essentially no difference between the groups. Thus, we suspect these values were outliers. For IFN-γ and IL-6, although these cytokines did not reach statistically significant levels in our study, we believe they provide a physiological insight into the effect of the viral burden on the immune system of the Antarctic expeditioners. IFN-γ is a potent antiviral cytokine produced by natural killer cells, γδ T cells, and CD8+ T cells, all of which are important in controlling both primary and reactivated shedding of EBV (31, 32). IL-6 has both pro- and anti-inflammatory properties depending on the source of its production (immune cells vs. muscle cells). When produced by immune cells, IL-6 is largely a pro-inflammatory cytokine and regulates many aspects of the immune response. In addition, IL-6 correlates strongly with inflammatory diseases resulting from an active immune system (33). High levels of both IFN-γ (34) and IL-6 (35) have been seen in disease states associated with EBV. Similar to the reductions in IL-10, we believe the reductions in these cytokines resulted from less EBV and HSV-1 viral burden on the immune system of participants within the treatment group. In other words, we believe participants in the placebo group had a significantly larger “viral burden” being placed on their immune system, leading to increased cellular activity, cytokine production, and possibly DHEA levels. On the other hand, the immune systems of the participants receiving valacyclovir experienced far less “viral stress” and thus decreased production of immunomodulatory components. However, a comprehensive immune profile by blood sampling was outside the scope of this study and further studies are needed to understand the effect of the long-term, prophylactic administration valacyclovir to prevent viral shedding and burden on the immune system, especially regarding cell-mediated immunity.
Our data indicate that 1 g of valacyclovir taken daily is a safe and successful regimen to attenuate herpes viral reactivation (particularly EBV reactivation) in individuals subjected to extreme environments. Thus, lessons learned from such a study may be applied to upcoming spaceflight missions. It is possible that prophylactic Valtrex could reduce the risk of contaminating the internal environment of the spacecraft with live infectious viruses and decrease the risk of crew developing or transmitting herpes viral-related maladies, including zoster, chronic neuropathic pain, malaise, vision loss, stroke, and cognitive impairment. Furthermore, it would reduce the risk of viral transmission risk from crew to their contacts back on Earth.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the JSC Institutional Review Board (IRB) and by the National Science Foundation’s Office of Polar Programs (NSF/OPP) Antarctic Infrastructure and Logistics (AIL) and Antarctic Sciences (ANT) sections, and executed during winter-over from March to October 2020. The patients/participants provided their written informed consent to participate in this study.
SM: conceptualization; data collection; formal analysis; writing—original draft preparation, review, and editing; visualization; and supervision funding acquisition. DD and BR: methodology, investigation, and review and editing. SK: methodology and investigation. MN-G: visualization review and editing. JL and MN: conceptualization, review and editing, and visualization. MY: statistical analysis of data, and review and editing. BC: conceptualization, review and editing, visualization, and supervision funding acquisition. All authors contributed to the article and approved the submitted version.
The study was funded by Human Research Program of NASA grant # 80JSC017N0001-OMNIBUS. Authors acknowledge the National Science Foundation and UTMB, Galveston, Texas staff for coordinating the study and the subjects who participated in the study at McMurdo and South Pole Stations.
SM was employed by company JES Tech. DD was employed by Aegis Aerospace. BR was employed by GeoControl. SK and MNG were employed by company KBR.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J Oral Microbiol (2013) 5. doi: 10.3402/jom.v5i0.22766
2. Hadinoto V, Shapiro M, Sun CC, Thorley-Lawson DA. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog (2009) 5(7):e1000496. doi: 10.1371/journal.ppat.1000496
3. Nagel MA, Gilden D. The relationship between herpes zoster and stroke. Curr Neurol Neurosci Rep (2015) 15(4):16. doi: 10.1007/s11910-015-0534-4
4. Lee AWM, Lee VHF, Ng WT, Strojan P, Saba NF, Rinaldo A, et al. A systematic review and recommendations on the use of plasma EBV DNA for nasopharyngeal carcinoma. Eur J Cancer (2021) 153:109–22. doi: 10.1016/j.ejca.2021.05.022
5. Protto V, Marcocci ME, Miteva MT, Piacentini R, Li Puma DD, Grassi C, et al. Role of HSV-1 in alzheimer's disease pathogenesis: a challenge for novel preventive/therapeutic strategies. Curr Opin Pharmacol (2022) 63:102200. doi: 10.1016/j.coph.2022.102200
6. Piacentini R, De Chiara G, Li Puma DD, Ripoli C, Marcocci ME, Garaci E, et al. HSV-1 and alzheimer's disease: more than a hypothesis. Front Pharmacol (2014) 5:97. doi: 10.3389/fphar.2014.00097
7. Crucian BE, Makedonas G, Sams CF, Pierson DL, Simpson R, Stowe RP, et al. Countermeasures-based improvements in stress, immune system dysregulation and latent herpesvirus reactivation onboard the international space station - relevance for deep space missions and terrestrial medicine. Neurosci Biobehav Rev (2020) 115:68–76. doi: 10.1016/j.neubiorev.2020.05.007
8. Makedonas G, Mehta S, Choukèr A, Simpson RJ, Marshall G, Orange JS, et al. Specific immunologic countermeasure protocol for deep-space exploration missions. Front Immunol (2019) 10:2407. doi: 10.3389/fimmu.2019.02407
9. Mehta SK, Laudenslager ML, Stowe RP, Crucian BE, Sams CF, Pierson DL. Multiple latent viruses reactivate in astronauts during space shuttle missions. Brain Behav Immun (2014) 41:210–7. doi: 10.1016/j.bbi.2014.05.014
10. Mehta SK, Laudenslager ML, Stowe RP, Crucian BE, Feiveson AH, Sams CF, et al. Latent virus reactivation in astronauts on the international space station. NPJ Microgravity (2017) 3:11. doi: 10.1038/s41526-017-0015-y
11. Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol (2004) 72(1):174–9. doi: 10.1002/jmv.10555
12. Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol (2008) 80(6):1116–22. doi: 10.1002/jmv.21173
13. Mehta SK, Szpara ML, Rooney BV, Diak DM, Shipley MM, Renner DW, et al. Dermatitis during spaceflight associated with HSV-1 reactivation. Viruses (2022) 14(4):789. doi: 10.3390/v14040789
14. Agha NH, Mehta SK, Rooney BV, Laughlin MS, Markofski MM, Pierson DL, et al. Exercise as a countermeasure for latent viral reactivation during long duration space flight. FASEB J (2020) 34(2):2869–81. doi: 10.1096/fj.201902327R
15. Zwart SR, Rice BL, Dlouhy H, Shackelford LC, Heer M, Koslovsky MD, Smith SM, et al. Dietary acid load and bone turnover during long-duration spaceflight and bed rest. Am J Clin Nutr (2018) 107(5):834–44. doi: 10.1093/ajcn/nqy029
16. Douglas GL, DeKerlegand D, Dlouhy H, Dumont-Leblond N, Fields E, Heer M, et al. Impact of diet on human nutrition, immune response, gut microbiome, and cognition in an isolated and confined mission environment. Sci Rep (2022) 12(1):20847. doi: 10.1038/s41598-022-21927-5
17. Smith SM, Zwart SR, Heer M. Human adaptation to spaceflight: the role of food and nutrition. 2nd edition (NP-2021-03-003-JSC). (National aeronautics and space administration Lyndon b. Johnson Space Center) (2021).
18. Mehta SK, Pierson DL, Cooley H, Dubow R, Lugg D. Epstein-Barr Virus reactivation associated with diminished cell-mediated immunity in antarctic expeditioners. J Med Virol (2000) 61(2):235–40. doi: 10.1002/(SICI)1096-9071(200006)61:2<235::AID-JMV10>3.0.CO;2-4
19. Zwart SR, Mehta SK, Ploutz-Snyder R, Bourbeau Y, Locke JP, Pierson DL, et al. Response to vitamin d supplementation during Antarctic winter is related to BMI, and supplementation can mitigate Epstein-Barr virus reactivation. J Nutr (2011) 141(4):692–7. doi: 10.3945/jn.110.134742
20. Strewe C, Moser D, Buchheim JI, Gunga HC, Stahn A, Crucian BE, et al. Sex differences in stress and immune responses during confinement in Antarctica. Biol Sex Differ (2019) 10(1):20. doi: 10.1186/s13293-019-0231-0
21. Cox AJ, Gleeson M, Pyne DB, Saunders PU, Clancy RL, Fricker PA. Valtrex therapy for Epstein-Barr virus reactivation and upper respiratory symptoms in elite runners. Med Sci Sports Exerc (2004) 36(7):1104–10. doi: 10.1249/01.MSS.0000131957.40985.2B
22. Hoshino Y, Katano H, Zou P, Hohman P, Marques A, Tyring SK, et al. Long-term administration of valacyclovir reduces the number of Epstein-Barr virus (EBV)-infected b cells but not the number of EBV DNA copies per b cell in healthy volunteers. J Virol (2009) 83(22):11857–61. doi: 10.1128/JVI.01005-09
23. Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir. J Infect Dis (2002) 186 Suppl 1:S40–6. doi: 10.1086/342966
24. Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol (2010) 30(8):1433–40. doi: 10.1007/s10571-010-9606-9
25. Padgett DA, Loria RM, Sheridan JF. Steroid hormone regulation of anti-viral immunity. Ann NY Acad Sci (2000) 917:935–43. doi: 10.1111/j.1749-6632.2000.tb05459.x
26. Ozçay F, Arslan H, Bilezikçi B, Sevmiş S, Moray G, Haberal M. The role of valacyclovir on Epstein-Barr virus viral loads in pediatric liver transplantation patients. Transplant Proc (2009) 41(7):2878–80. doi: 10.1016/j.transproceed.2009.07.059
27. Papadopoulou-Marketou N, Kassi E, Chrousos GP. Adrenal androgens and aging, in endotext. Feingold KR, et al, editors. South Dartmouth (MA: MDText.com, Inc) (2000). Available at: https://www.ncbi.nlm.nih.gov/sites/books/NBK279006/
28. Ahmed T, Qassem M, Kyriacou PA. Measuring stress: a review of the current cortisol and dehydroepiandrosterone (DHEA) measurement techniques and considerations for the future of mental health monitoring. Stress (2023) 26(1):29–42. doi: 10.1080/10253890.2022.2164187
29. La Rosa F, Agostini S, Bianchi A, Nemni R, Piancone F, Marventano I, et al. Herpes simplex virus-1 (HSV-1) infection induces a potent but ineffective IFN-λ production in immune cells of AD and PD patients. J Transl Med (2019) 17(1):286. doi: 10.1186/s12967-019-2034-9
30. Ressing ME, van Gent M, Gram AM, Hooykaas MJ, Piersma SJ, Wiertz EJ. Immune evasion by Epstein-Barr virus. curr top microbiol. Immunol (2015) 391:355–81. doi: 10.1007/978-3-319-22834-1_12
31. Morrison TE, Mauser A, Wong A, Ting JP, Kenney SC. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity (2001) 15(5):787–99. doi: 10.1016/S1074-7613(01)00226-6
32. Jochum S, Moosmann A, Lang S, Hammerschmidt W, Zeidler R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected b cells from immune recognition and elimination. PloS Pathog (2012) 8(5):e1002704. doi: 10.1371/journal.ppat.1002704
33. Salimetrics. Salivary IL-6 background. Available at: https://salimetrics.com/analyte/salivary-interleukin-6/.
34. Uemura Y, Ohashi A, Yoshimori M, Nishio M, Hirakawa T, Shimizu N, et al. Plasma interferon-γ concentration: a potential biomarker of disease activity of systemic chronic active Epstein-Barr virus infection. Front Virol (2022) 2. doi: 10.3389/fviro.2022.999929
Keywords: antiviral, herpes viruses, Antarctic, countermeasure, valacyclovir HCl
Citation: Mehta SK, Diak DM, Rooney BV, Krieger SS, Nelman-Gonzalez M, Locke JP, Nagel MA, Young M and Crucian BE (2023) Antiviral treatment with valacyclovir reduces virus shedding in saliva of Antarctic expeditioners. Front. Virol. 3:1157659. doi: 10.3389/fviro.2023.1157659
Received: 13 February 2023; Accepted: 02 May 2023;
Published: 02 June 2023.
Edited by:
Andrea Lombardi, University of Milan, ItalyReviewed by:
Paul R. Kinchington, University of Pittsburgh, United StatesCopyright © 2023 Mehta, Diak, Rooney, Krieger, Nelman-Gonzalez, Locke, Nagel, Young and Crucian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satish K. Mehta, c2F0aXNoLmsubWVodGFAbmFzYS5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.