- 1Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, TN, United States
- 2Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN, United States
- 3Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, TN, United States
- 4Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, United States
Background: Patterns of respiratory syncytial virus (RSV) co-detection with other viruses may have been disrupted during the coronavirus disease 2019 (COVID-19) pandemic, but the clinical impact of viral co-detections with RSV is not well-established. We aimed to explore the frequency and clinical outcomes associated with RSV single detection and co-detection before and during the pandemic.
Methods: We conducted a single-center retrospective cohort study of all children and adults with respiratory samples tested using a respiratory pathogen panel (RPP; 01/01/2018–11/30/2022), a provider-ordered polymerase chain reaction–based assay that detects respiratory pathogens. We stratified our cohort into age groups: 0–4, 5–17, 18–64, and ≥65 years old. Among RSV-positive samples, we compared the proportion of samples with single RSV detection before and during the pandemic and the patterns of specific viral co-detections. We compared the odds of hospitalization, oxygen use, intensive care unit admission, and intubation between individuals with RSV single detection and those with co-detection.
Results: Among 57,940 samples collected during the study period, 3,986 (6.9%) were RSV-positive. RSV was co-detected with at least one other virus in 1,231/3,158 (39.0%), 104/348 (29.9%), 49/312 (15.7%), and 21/168 (12.5%) of samples from individuals 0–4, 5–17, 18–64, and ≥65 years old, respectively. The relative frequencies of RSV single detection and co-detection were comparable before and during the pandemic except in children 0–4 years old, in whom single RSV detections were more prevalent before (63.7%) than during (59.5%) the pandemic (p=0.021). In children 0–4 years old, RSV co-detection was associated with lower odds of hospitalization compared to single RSV detection, and RSV co-detection with parainfluenza viruses or human rhinovirus/enterovirus was associated with significantly lower odds of hospitalization, while RSV/SARS-CoV-2 co-detection was associated with higher odds of ICU admission. In adults ≥65 years old, RSV co-detection was associated with lower odds of oxygen use.
Conclusion: The proportion of RSV co-detection did not appreciably vary before and during the pandemic, except in young children, though the combinations of co-detected viruses did vary. Our findings suggest that the clinical impact of RSV co-detection with other viruses may be age-associated and virus-specific.
1 Introduction
Respiratory syncytial virus (RSV) is a common cause of acute respiratory illnesses (ARI) across all age groups. RSV is the most common cause of lower respiratory tract illnesses in young children, with a particularly high disease burden in those <2 years old (1). In vulnerable adult populations, such as the elderly, immunocompromised, and those with underlying medical conditions, RSV infections are associated with severe illness and exacerbations of pre-existing medical conditions such as chronic obstructive pulmonary disease and congestive heart failure (2–5).
Before the coronavirus disease 2019 (COVID-19) pandemic, many respiratory viruses circulated with relatively predictable seasonal patterns, and the circulation periods for many respiratory viruses, including influenza and human rhinovirus, often overlapped with RSV (6). Furthermore, community outbreaks of RSV typically occurred between October and April in the Northern Hemisphere, with peak circulation during the winter months (7). However, during the pandemic, the seasonality of RSV and many other respiratory viruses was disrupted due to nonpharmaceutical interventions implemented to control the circulation of SARS-CoV-2, such as social distancing, mask mandates, improved hand-hygiene compliance, and school and business closures (6). Unlike prior seasons, as community measures were relaxed, RSV circulation occurred during the spring and summer (8). Therefore, off-season circulation of RSV may have led to combinations of co-detected viruses that were previously uncommon.
According to a systematic review and meta-analysis, RSV is co-detected with another respiratory virus in up to 40% of young children with an RSV-associated respiratory illness (9). Data on RSV co-detection in older age groups are limited, but when reported, the frequency is less than in children (1). The clinical implications of RSV co-detection with other respiratory viruses are not well established, and the impact of viral co-detection with RSV may vary according to the type of co-detected virus. One study reported that RSV/HMPV co-detection was associated with a high risk of ICU admission in children (10), and another showed that infants with RSV/HRV co-detection experienced lengthier hospital stay and more frequent oxygen use than infants with a single virus detection (11). Other studies have not reported greater severity with RSV co-detection or have yielded conflicting results (12–14). Furthermore, data describing RSV co-detection during the pandemic are scarce, and many studies have described the circulation of RSV over a single season, included a small sample size, or restricted their population to either children or adults.
To address this research gap, we aimed to explore the frequency of RSV co-detection with other respiratory viruses across different age groups over four consecutive years before and during the pandemic at a tertiary medical center. We also aimed to determine the clinical outcomes of illnesses associated with single RSV detection compared to those with RSV co-detection with other viruses.
2 Methods
This retrospective study included all children and adults with respiratory samples tested in the outpatient, emergency, and inpatient settings using a provider-ordered multiplex respiratory pathogen panel (RPP) between January 1, 2018, and November 30, 2022, at Vanderbilt University Medical Center (VUMC) in Nashville, Tennessee. We included all encounters in which an RPP was ordered by a VUMC provider, regardless of the indication. We defined an encounter as the interval between the dates of presentation to and discharge from the setting during which an RPP was ordered. Settings were classified as outpatient (e.g., primary and specialty clinics), emergency department, or inpatient. If an RPP was obtained in an emergency room setting and the patient was hospitalized during the same encounter, the encounter was classified as a hospital setting encounter. Most respiratory specimens were collected by either a nasopharyngeal swab or bronchoalveolar lavage and tested using an RPP. The RPP targets several common respiratory viruses, including RSV; human rhinovirus/enterovirus (HRV/EV); adenovirus (AdV); human coronaviruses HKU1, NL63, 229E, and OC43 (HCoV); influenza A and B (Flu); parainfluenza viruses (PIV)-1–4; and human metapneumovirus (HMPV). The GenMark eSensor XT-8 Respiratory Viral Panel was used from January to April 2018, BioFire Respiratory Panel 2 (RP2) from April 2018 to October 2021, and BioFire RP2.1 (which incorporates SARS-CoV-2 as an additional target) from October 2021 onward. These assays are performed on nasopharyngeal swab specimens collected in viral transport media. They represent sample-to-result style assays in which nucleic acid extraction, end-point polymerase chain reaction amplification, and fluorometric detection occur microfluidically within a self-contained cartridge. They carry in vitro diagnostic status in the United States, including Food and Drug Administration clearance for the RP2 and emergency use authorization for the RP2.1. We supplemented the RPP data with SARS-CoV-2 test results by any method (antigen-based, molecular, or serologic) if the test was performed within 72 hours before or after RPP testing. Encounters that occurred >14 days after a previous encounter in the same individual were considered new, distinct encounters, while those that occurred ≤14 days were excluded. The Vanderbilt University Institutional Review Board reviewed and approved the study protocol.
Using Epic Clarity, we systematically extracted data from electronic health records across the Vanderbilt Health system to capture demographic and clinical data. Data were encounter-based and included an individual-level identifier, date of testing, age at testing, sex assigned at birth, viral testing results, hospital admission status, and among hospitalized patients, supplemental oxygen use, intensive care unit (ICU) admission status, and intubation status. For this analysis, we considered April 1, 2020, the start date of the pandemic period, given that this was the first day Tennessee’s stay-at-home order was in effect.
We summarized the demographic characteristics and clinical outcomes of individuals in the cohort using absolute and relative frequencies. To illustrate the seasonality of RSV infections, we plotted the monthly proportion of samples positive for RSV during the study period and defined the beginning and end of each RSV season by a minimum monthly threshold of 20 RSV detections. We stratified the cohort into four age groups: 0–4, 5–17, 18–64, and ≥65 years old, and used Pearson’s χ2 test to assess sex differences in RSV detection and compare the proportion of RSV single detection before and during the pandemic among RSV-positive samples within each age group. To account for individuals with more than one encounter during the study period, we used generalized estimating equations with a logistic link and a working independence correlation structure to estimate odds ratios (ORs) and 95% confidence intervals (CIs) comparing the odds of hospitalization between individuals with RSV single detection and those with co-detection, with no covariate adjustment (15). Among those hospitalized, we used the same method to estimate ORs and 95% CIs comparing the odds of oxygen use, ICU admission, and intubation between individuals with RSV single detection and those with co-detection. We then replicated these analyses in children 0–4 years old, including age at testing as a covariate. Finally, among children 0–4 years old, we compared the odds of the same outcomes between those with single RSV detection and those with RSV co-detected with a specific virus or combination of viruses. We selected comparator groups for these analyses if all four expected cell counts in the 2×2 contingency table of detection status and outcome were ≥5.
3 Results
Between January 1, 2018, and November 30, 2022, 57,940 samples were tested using an RPP; 20,272 (35.0%) were tested in the 27-month pre-pandemic period, and 37,668 (65.0%) were tested during the 32-month pandemic period of observation. SARS-CoV-2 test results were available within 72 hours of an RPP in 35,710 (94.8%) encounters during the pandemic. These samples were tested from the outpatient, emergency department, and inpatient settings in 10,723 (18.5%), 11,311 (19.5%), and 35,906 (62.0%) cases, respectively. The most frequently tested age group was children 0–4 years old (n=21,463; 37.0%; Table 1). Of all samples, 3,986 (6.9%) were positive for RSV, and the majority were collected from those 0–4 years old (n=3,158; 79.2%; Table 1). In children and adolescents, RSV-positive males outnumbered RSV-positive females, while females with a positive RSV sample outnumbered males in the adult age groups (Table 1). There was no sufficient evidence for sex differences in RSV detection within any age group (Table 1). Of all RSV-positive samples, 922 (23.1%), 1,078 (27.0%), and 1,986 (49.8%) were collected from encounters in the outpatient, emergency, and inpatient settings, respectively.
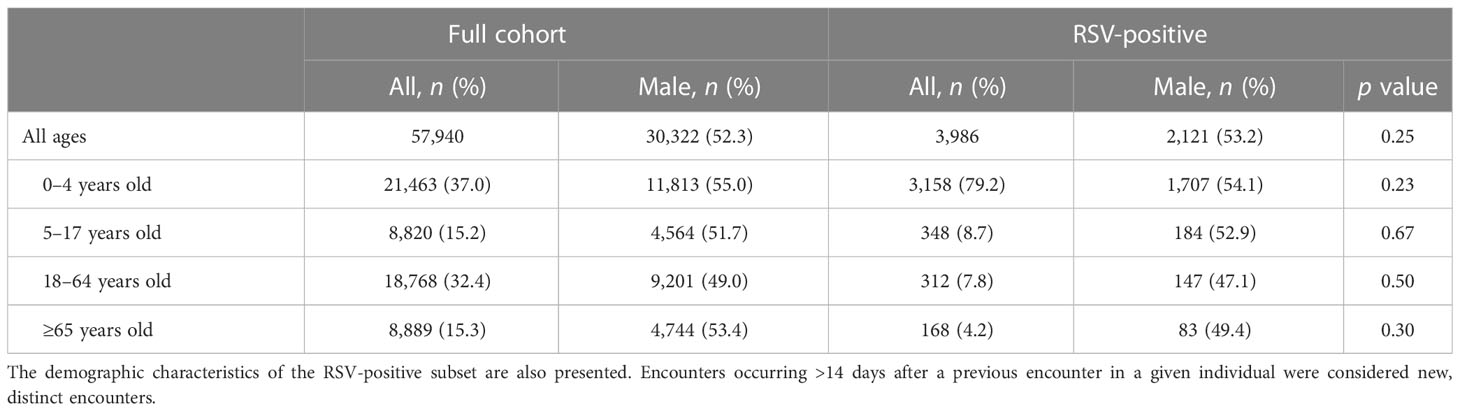
Table 1 Demographic characteristics of all individuals who had a sample tested using a provider-ordered respiratory pathogen panel at Vanderbilt University Medical Center between January 1, 2018, and November 30, 2022.
Before the pandemic (January 1, 2018 - March 31, 2020), RSV primarily circulated between October and March and peaked in December (Figure 1). Relative to previous seasons, RSV circulation was substantially delayed during the 2020-2021 season. During the early pandemic period, RSV was last detected in April 2020 but resumed circulation in late March 2021 and continued through January 2022. RSV then circulated off-season again starting May 2022 and continued to circulate as of the end of the period.

Figure 1 Monthly proportions of respiratory pathogen panels positive for respiratory syncytial virus in Nashville, Tennessee, before and during the COVID-19 pandemic (January 2018 to November 2022). The dashed line represents the first day Tennessee’s stay-at-home order was in effect.
Throughout the 4-year study period, RSV was co-detected with at least one other virus in 39.0% (n=1,231/3,158), 29.9% (n=104/348), 15.7% (n=49/312), and 12.5% (n=21/168) of samples collected from encounters among individuals 0–4, 5–17, 18–64, and ≥65 years old, respectively. Figure 2 displays the proportions, within each age group, of samples positive for RSV only, before and during the pandemic. The proportions of single RSV detections before and during the pandemic remained similar for all but the 0–4 age group, which had a significantly higher proportion of single RSV detections before the pandemic (63.7% vs. 59.5%, Figure 2). Figure 3 shows the proportions, within each age group, of samples positive for RSV and at least one other respiratory virus, before and during the pandemic. RSV/HRV/EV was the most common combination before and during the pandemic, and this combination increased in frequency during the pandemic across all age groups. During the pandemic, the relative frequency of RSV co-detection with influenza or human coronaviruses decreased more than any other combination. The proportions of RSV co-detections, stratified by age group and the setting of care, are shown in Table S1.
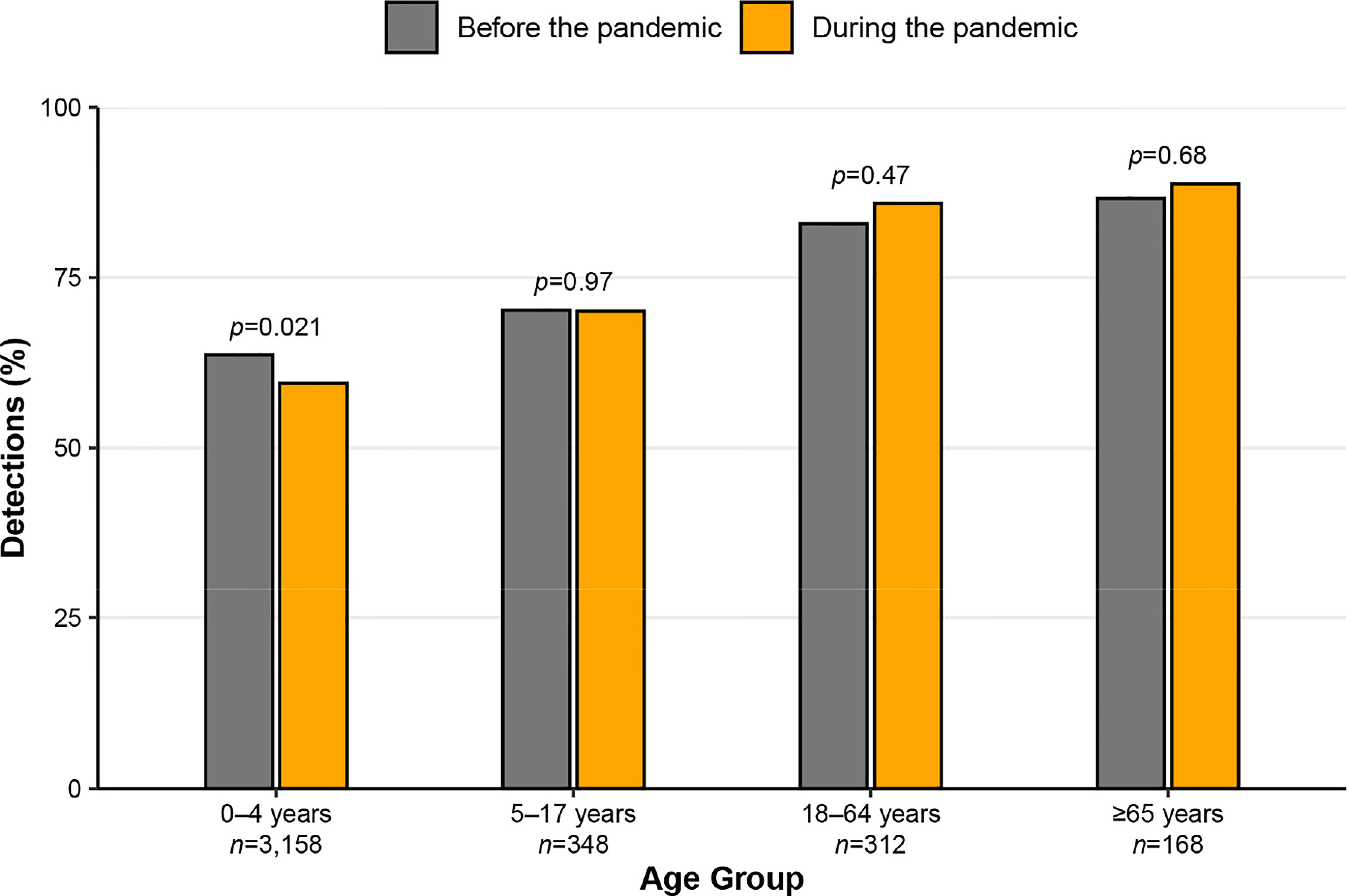
Figure 2 Proportions of samples testing positive for only respiratory syncytial virus (RSV; among all RSV-positive samples) before and during the COVID-19 pandemic, stratified by age group. Samples were tested from January 2018 to November 2022 in Nashville, Tennessee.

Figure 3 Proportions of samples testing positive for respiratory syncytial virus (RSV) and other respiratory viruses (among all RSV-positive samples) before and during the COVID-19 pandemic, stratified by age group. Samples were tested from January 2018 to November 2022 in Nashville, Tennessee. RSV, respiratory syncytial virus; HRV/EV, human rhinovirus/enterovirus; HCoV, human coronaviruses; AdV, adenovirus; PIV, parainfluenza viruses 1–4; Flu, influenza A and B; HMPV, human metapneumovirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Of all RSV-positive individuals, 1,986/3,986 (49.8%) were hospitalized. Among those who were hospitalized, 1,412 (71.1%) received supplemental oxygen, 718 (36.2%) were admitted to the ICU, and 152 (7.7%) were intubated. We compared hospitalization, ICU admission, oxygen use, and mechanical ventilation among individuals with RSV single detection to those with co-detection within each age group. In children 0–4 years old, RSV co-detection with another virus or viruses, was associated with lower odds of hospitalization compared with single RSV detection (Table 2). In adults ≥65 years old, RSV co-detection was associated with lower odds of oxygen use among those hospitalized. Otherwise, we did not find evidence of statistically significant associations between RSV co-detection and the clinical outcomes we defined (Table 2). Results from subgroup analyses in children 0–4 years old, in which age is a covariate, were comparable to the results of the main outcomes analyses—we again found that RSV co-detection was associated with lower odds of hospitalization in children 0–4 years old (aOR, 0.73; 95% CI, 0.63–0.84; p<0.001; Table S2). When specific co-detecting virus combinations were analyzed in this age group, we found that RSV/PIV and RSV/HRV/EV co-detections were associated with significantly lower odds of hospitalization compared to single RSV detection (aOR, 0.55; 95% CI, 0.34–0.89; p=0.014 and aOR, 0.74; 95% CI, 0.62–0.89; p=0.001, respectively). Among children 0–4 years old who were hospitalized, RSV/SARS-CoV-2 co-detection was associated with higher odds of ICU admission (aOR, 3.47; 95% CI, 1.06–11.34; p=0.040; Table 3).

Table 2 Logistic regression models comparing the age group–specific odds of hospitalization, and among those hospitalized, oxygen use, ICU admission, and intubation between individuals with RSV single detection (reference group) and those with co-detection.
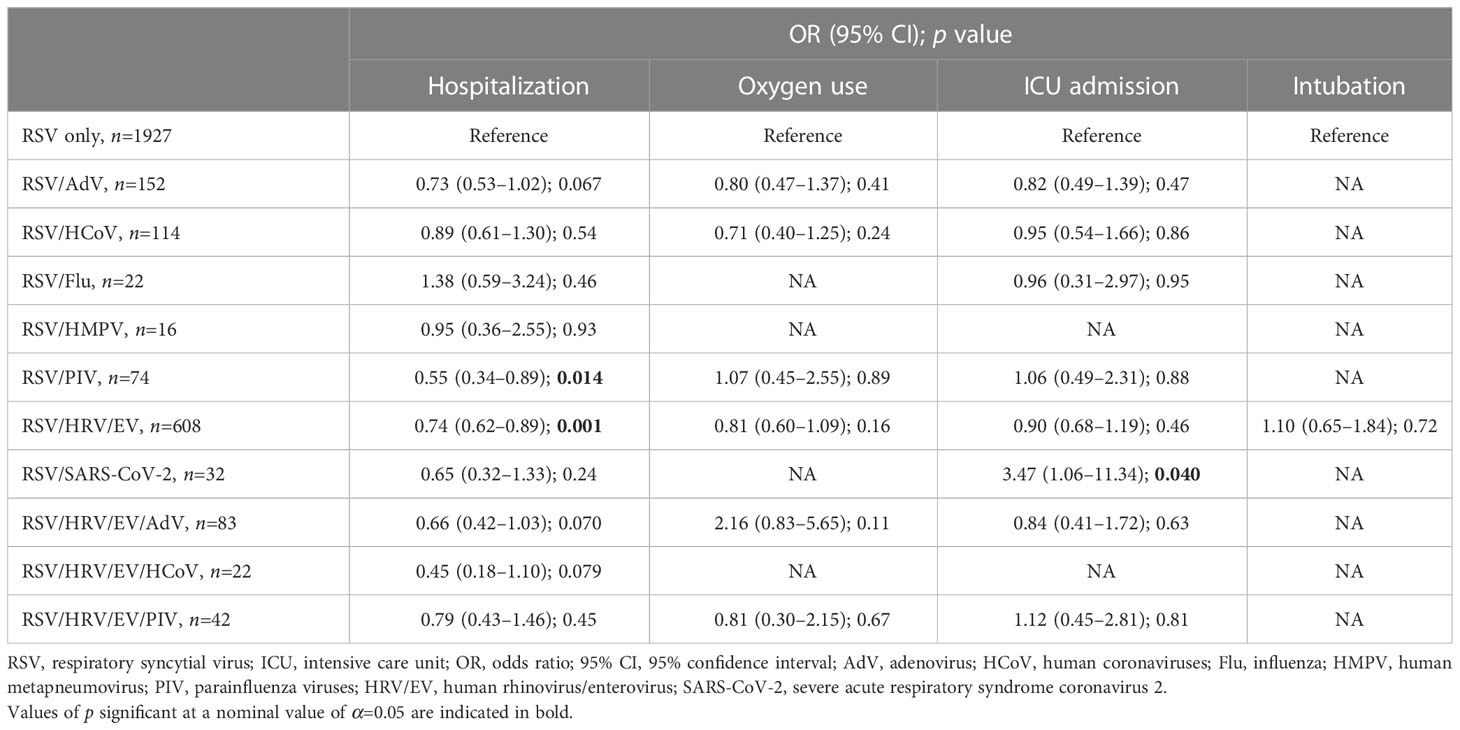
Table 3 Logistic regression models comparing the odds of hospitalization, and among those hospitalized, oxygen use, ICU admission, and intubation between children 0–4 years old with RSV single detection (reference group) and those with co-detection with specific viral combinations.
4 Discussion
In our study, we found that RSV was detected in 6.9% of respiratory samples collected from children and adults across the outpatient, emergency department, and inpatient settings in a single center over four respiratory seasons before and during the pandemic. We observed disrupted seasonal patterns of circulation of RSV during the pandemic relative to prior years. The relative frequencies of RSV co-detections across age groups did not differ during the pandemic compared with the pre-pandemic period, except for children 0–4 years old, among whom single RSV detections were slightly less common in the pandemic period. However, the relative frequencies of certain specific viral combinations differed before compared to during the pandemic. We also compared clinical outcomes of individuals with RSV single detection to individuals with RSV co-detection and found evidence to suggest age- and virus-specific associations.
We observed a substantial delay in RSV circulation during the 2020-2021 season compared to the prior years of observation, a resurgence out-of-season (relative to pre-pandemic circulation), followed by circulation for an extended period. The introduction of nonpharmaceutical interventions, such as stay-at-home orders, masking, and social distancing, during the pandemic has been associated with alterations in the circulation patterns of several common respiratory viruses, including RSV (16). Studies in various regions, including the United States, reproduced these findings (17–20). The disruption in the circulation of common respiratory viruses during the pandemic may also explain why the differences observed in the distribution of viruses co-detected with RSV before compared with during the pandemic. For example, we found that the combination of RSV/Flu was detected less frequently during the pandemic. This finding is likely at least partially attributable to near-absent influenza circulation in the first winter during the pandemic in the study region, resulting in minimal opportunities for RSV/Flu co-detection (6). On the other hand, HRV/EV circulation persisted relatively unchanged during the pandemic, and the concurrent lesser burden of other respiratory viruses may have contributed to the increase in the relative frequency of RSV/HRV/EV co-detection during the pandemic (6).
Since our study included comprehensive respiratory viral testing in both children and adults, we were able to study RSV co-detection within age strata that collectively spanned all ages. We observed a higher proportion of co-detection in children and adolescents compared with adults ≥18 years old, a finding that has also been reported in previous studies (21, 22). Children contract viral infections, both asymptomatic and symptomatic, more so than adults, which may be partially attributable to both increased exposure among children in day care and school settings, as well as to pre-existing immunity in older individuals with a naturally lengthier history of viral exposure.
Interestingly, despite the pandemic-related disruption in the circulation of RSV and other respiratory viruses, we found that children 0–4 years old had a higher percentage of RSV co-detection during the pandemic compared with the pre-pandemic period, and HRV/EV was the most common co-detection. In adolescents and adults, we observed no differences in the proportion of RSV co-detection before and during the pandemic, likely because the increase in HRV/EV co-detection was offset by a lower relative burden of human coronaviruses and influenza to a larger extent than in young children. Young children, especially those born during the pandemic, are likely to be more immunologically naïve to respiratory viruses than adolescents and older adults and, therefore, at a higher risk of contracting multiple viruses once exposed. Further studies are needed to assess whether the increase in RSV co-detection among young children will persist post-pandemic.
We found that RSV co-detection in children 0–4 years old was associated with lower odds of hospitalization. Previously reported results are conflicting; one study showed no association between RSV co-detection and hospitalization, another showed a negative association, and yet another showed a positive association (8). Nascimento et al. also showed that RSV co-detection in infants was not a risk factor for hospitalization (23). In addition to variation in study designs, case definitions, and populations included in these prior studies, we s2581peculate that heterogeneity in the reported findings may be partially a consequence of the heterogeneity of RSV co-detection, a term used to describe a broad range of viral combinations. In support, our subgroup analyses showed some virus-specific differences in clinical features of illnesses, specifically that RSV/PIV and RSV/HRV/EV co-detections, but not other viral combinations, were associated with lower odds of hospitalization. Similarly, Noyola et al. also showed that, compared with RSV alone, RSV/PIV co-detection was associated with lower hospitalization in people of all ages with influenza-like illness. However, while we show that RSV/HRV/EV co-detections were associated with lower hospitalization, they found no association (24). The association of RSV co-detection with lower odds of hospitalization may result from differential virulence of the pathogen “driving” the illness or a consequence of virus–virus interaction. For example, Dee et al. demonstrated that prior infection with HRV inhibits SARS-CoV-2 replication within the respiratory epithelium (25). Whether similar interactions between HRV and RSV exist is unclear, and further investigation is needed. We did, however, observe that RSV co-detection with SARS-CoV-2 was associated with higher odds of ICU admission in children 0–4 years old. This finding is supported by another study, in which among hospitalized children <2 years old, RSV/SARS-CoV-2 co-detection was associated with an increased likelihood of admission to the ICU and subsequent mechanical ventilation compared to single SARS-CoV-2 detection (26). Our study highlights the importance of assessing individual viral combinations, which may explain the conflicting results of the association between RSV co-detection and clinical outcomes in prior studies that assessed RSV co-detection in aggregate.
We also studied the clinical significance of virus-specific RSV co-detection in adults, which has been indirectly studied and remains poorly understood (27). We observed that RSV co-detection was associated with lower odds of oxygen use in adults ≥65 years old. A previous study of adults that compared single and multiple viral detections in aggregate reported insufficient evidence of an association between the number of detections and supplemental oxygen use. The discrepancy between their findings and ours is likely due to differences in study design, study population, and the specific viral combinations under investigation (25). Further studies of specific viral combinations are required in the adult population to elucidate the clinical significance of viral co-detection.
Our study had several limitations. First, we included all RSV-positive individuals regardless of the indication for testing. In addition, RPP tests were ordered more frequently during the pandemic for a broader range of indications. Therefore, we might be including individuals with asymptomatic or subclinical RSV infection. Nevertheless, we anticipate the contribution of asymptomatic RSV detections to be relatively small, given the low frequency of RSV detection in individuals without respiratory symptoms (1, 28, 29). Second, although stratifying our analysis by age group enabled us to assess age-specific associations, we did not perform subgroup analyses in any but the 0–4 age group because of sample size limitations. Third, we were not able to adjust for potentially important patient-specific confounders, including underlying medical conditions such as prematurity or chronic lung disease, since we were unable to retrieve these data systematically. Future studies should study the clinical significance of RSV co-detection in the context of these important factors. Fourth, we analyzed data collected from a single healthcare system, which may limit the generalizability of our findings. Fifth, the sequence of viral infections may impact the virologic and clinical characteristics of illnesses associated with viral co-detections; however, we were unable to assess the sequence of viral infections in co-detections in this cross-sectional assessment. Sixth, we identified cases solely based on RPP testing; if an individual presented again within 14 days for escalation of care without another RPP test, our case-finding strategy would not have identified them. However, we anticipate roughly equal distributions of these instances across exposure groups.
In conclusion, though the typical seasonality of RSV appeared to be disrupted in our center during the pandemic, the proportion of RSV co-detection did not appreciably vary before and during the pandemic, except in young children. In certain age groups, RSV co-detection was associated with varying clinical outcomes. The clinical impact of RSV co-detection with other viruses may be virus-specific. Further studies are needed to elucidate the impact of RSV co-detection with other viruses, with an emphasis on the influence of the pandemic on the nature of viral interactions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Vanderbilt University Institutional Review Board. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization: HH, JA, NH, LH. Formal Analysis: JA, AS, TS. Investigation: HH, JA, JS, JW, NH. Methodology: HH, JA, AS, NH, LH. Supervision: NH, LH. Visualization: JA. Writing – original draft: HH, JA, YQ, NH, LH. Writing – review & editing: HH, JA, YQ, AK, TS, JS, JC, JW, AS, NH, LH. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2023.1156012/full#supplementary-material
Supplementary Table 1 | Counts and proportions of samples testing positive for respiratory syncytial virus (RSV) and other respiratory viruses (among all RSV-positive samples) before and during the COVID-19 pandemic, stratified by age group and setting of care. Samples were collected from January 2018 to November 2022 in Nashville, Tennessee. RSV, respiratory syncytial virus; HRV/EV, human rhinovirus/enterovirus; HCoV, human coronaviruses; AdV, adenovirus; PIV, parainfluenza viruses 1–4; Flu, influenza A and B; HMPV, human metapneumovirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Supplementary Table 2 | Logistic regression models comparing the odds of hospitalization, and among those hospitalized, oxygen use, ICU admission, and intubation between children 0–4 years old with RSV single detection (reference group) and those with co-detection, including age at testing as a covariate. Values of p significant at a nominal value of α=0.05 are indicated in bold. RSV, respiratory syncytial virus; ICU, intensive care unit; OR, odds ratio; 95% CI, 95% confidence interval.
References
1. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med (2015) 372(9):835–45. doi: 10.1056/NEJMoa1405870
2. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med (2005) 352(17):1749–59. doi: 10.1056/NEJMoa043951
3. Pastula ST, Hackett J, Coalson J, Jiang X, Villafana T, Ambrose C, et al. Hospitalizations for respiratory syncytial virus among adults in the united states, 1997-2012. Open Forum Infect Dis (2017) 4(1):ofw270. doi: 10.1093/ofid/ofw270
4. Englund JA, Sullivan CJ, Jordan MC, Dehner LP, Vercellotti GM, Balfour HH Jr. Respiratory syncytial virus infection in immunocompromised adults. Ann Intern Med (1988) 109(3):203–8. doi: 10.7326/0003-4819-109-3-203
5. McManus TE, Marley AM, Baxter N, Christie SN, O’Neill HJ, Elborn JS, et al. Respiratory viral infection in exacerbations of COPD. Respir Med (2008) 102(11):1575–80. doi: 10.1016/j.rmed.2008.06.006
6. Haddadin Z, Spieker AJ, Rahman H, Rankin DA, Talj R, Yanis A, et al. Respiratory pathogens during the COVID-19 pandemic: Alterations in detection and seasonality in Nashville, Tennessee. PloS One (2022) 17(8):e0270469. doi: 10.1371/journal.pone.0270469
7. Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PloS One (2013) 8(2):e54445. doi: 10.1371/journal.pone.0054445
8. Mosscrop LG, Williams TC, Tregoning JS. Respiratory syncytial virus after the SARS-CoV-2 pandemic - what next? Nat Rev Immunol (2022) 22(10):589–90. doi: 10.1038/s41577-022-00764-7
9. Li Y, Pillai P, Miyake F, Nair H. The role of viral co-infections in the severity of acute respiratory infections among children infected with respiratory syncytial virus (RSV): A systematic review and meta-analysis. J Glob Health (2020) 10(1):10426. doi: 10.7189/jogh.10.010426
10. Semple MG, Cowell A, Dove W, Greensill J, McNamara PS, Halfhide C, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis (2005) 191(3):382–6. doi: 10.1086/426457
11. da Silva ER, Pitrez MC, Arruda E, Mattiello R, Sarria EE, de Paula FE, et al. Severe lower respiratory tract infection in infants and toddlers from a non-affluent population: viral etiology and co-detection as risk factors. BMC Infect Dis (2013) 13:41. doi: 10.1186/1471-2334-13-41
12. Calvo C, García-García ML, Pozo F, Paula G, Molinero M, Calderón A, et al. Respiratory syncytial virus coinfections with rhinovirus and human bocavirus in hospitalized children. Med (Baltimore) (2015) 94(42):e1788. doi: 10.1097/MD.0000000000001788
13. Mazur NI, Bont L, Cohen AL, Cohen C, von Gottberg A, Groome MJ, et al. Severity of respiratory syncytial virus lower respiratory tract infection with viral coinfection in HIV-uninfected children. Clin Infect Dis (2017) 64(4):443–50. doi: 10.1093/cid/ciw756
14. De Paulis M, Gilio AE, Ferraro AA, Ferronato AE, do Sacramento PR, Botosso VF, et al. Severity of viral coinfection in hospitalized infants with respiratory syncytial virus infection. J Pediatr (Rio J) (2011) 87(4):307–13. doi: 10.2223/JPED.2100
15. Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of longitudinal data (Oxford statistical science series). 2nd ed. (Oxford, United Kingdom: Oxford University Press) (2013).
16. Haddadin Z, Schuster JE, Spieker AJ, Rahman H, Blozinski A, Stewart L, et al. Acute respiratory illnesses in children in the SARS-CoV-2 pandemic: Prospective multicenter study. Pediatrics (2021) 148(2):e2021051462. doi: 10.1542/peds.2021-051462
17. Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic - united states, 2020-2021. MMWR Morb Mortal Wkly Rep (2021) 70(29):1013–9. doi: 10.15585/mmwr.mm7029a1
18. Groves HE, Piché-Renaud PP, Peci A, Farrar DS, Buckrell S, Bancej C, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: A population-based study. Lancet Reg Health Am (2021) 1:100015. doi: 10.1016/j.lana.2021.100015
19. Gomez GB, Mahé C, Chaves SS. Uncertain effects of the pandemic on respiratory viruses. Science (2021) 372(6546):1043–4. doi: 10.1126/science.abh3986
20. Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol (2022) 17:1–16. doi: 10.1038/s41579-022-00807-9
21. Mandelia Y, Procop GW, Richter SS, Worley S, Liu W, Esper F. Dynamics and predisposition of respiratory viral co-infections in children and adults. Clin Microbiol Infect (2021) 27(4):631.e1–6. doi: 10.1016/j.cmi.2020.05.042
22. Ching NS, Kotsanas D, Easton ML, Francis MJ, Korman TM, Buttery JP. Respiratory virus detection and co-infection in children and adults in a large Australian hospital in 2009-2015. J Paediatr Child Health (2018) 54(12):1321–8. doi: 10.1111/jpc.14076
23. Nascimento MS, Souza AV, Ferreira AV, Rodrigues JC, Abramovici S, Silva Filho LV. High rate of viral identification and coinfections in infants with acute bronchiolitis. Clinics (Sao Paulo) (2010) 65(11):1133–7. doi: 10.1590/s1807-59322010001100014
24. Noyola DE, Hunsberger S, Valdés Salgado R, Powers JH 3rd, Galindo-Fraga A, Ortiz-Hernández AA, et al. Comparison of rates of hospitalization between single and dual virus detection in a Mexican cohort of children and adults with influenza-like illness. Open Forum Infect Dis (2019) 6(11):ofz424. doi: 10.1093/ofid/ofz424
25. Dee K, Goldfarb DM, Haney J, Amat JAR, Herder V, Stewart M, et al. Human rhinovirus infection blocks severe acute respiratory syndrome coronavirus 2 replication within the respiratory epithelium: Implications for COVID-19 epidemiology. J Infect Dis (2021) 224(1):31–8. doi: 10.1093/infdis/jiab147
26. Agathis NT, Patel K, Milucky J, Taylor CA, Whitaker M, Pham H, et al. Codetections of other respiratory viruses among children hospitalized with COVID-19. Pediatrics (2023) 151(2):e2022059037. doi: 10.1542/peds.2022-059037
27. Choi SH, Chung JW, Kim HR. Clinical relevance of multiple respiratory virus detection in adult patients with acute respiratory illness. J Clin Microbiol (2015) 53(4):1172–7. doi: 10.1128/JCM.03298-14
28. Howard LM, Johnson M, Williams JV, Zhu Y, Gil AI, Edwards KM, et al. Respiratory viral detections during symptomatic and asymptomatic periods in young Andean children. Pediatr Infect Dis J (2015) 34(10):1074–80. doi: 10.1097/INF.0000000000000812
Keywords: respiratory syncytial virus infections, coinfection, seasonal variation, COVID-19 pandemic, clinical course
Citation: Hayek H, Amarin JZ, Qwaider YZ, Khanfar A, Stopczynski T, Schmitz J, Chappell JD, Wrenn JO, Spieker AJ, Halasa NB and Howard LM (2023) Co-detection of respiratory syncytial virus with other respiratory viruses across all age groups before and during the COVID-19 pandemic. Front. Virol. 3:1156012. doi: 10.3389/fviro.2023.1156012
Received: 01 February 2023; Accepted: 14 March 2023;
Published: 24 March 2023.
Edited by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaReviewed by:
Andreu Comas-Garcia, Autonomous University of San Luis Potosí, MexicoAlessandra Pierangeli, Sapienza University of Rome, Italy
Copyright © 2023 Hayek, Amarin, Qwaider, Khanfar, Stopczynski, Schmitz, Chappell, Wrenn, Spieker, Halasa and Howard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natasha B. Halasa, bmF0YXNoYS5oYWxhc2FAdnVtYy5vcmc=; Leigh M. Howard, bGVpZ2guaG93YXJkQHZ1bWMub3Jn
 Haya Hayek
Haya Hayek Justin Z. Amarin
Justin Z. Amarin Yasmeen Z. Qwaider
Yasmeen Z. Qwaider Asim Khanfar
Asim Khanfar Tess Stopczynski2
Tess Stopczynski2 Jonathan Schmitz
Jonathan Schmitz Jesse O. Wrenn
Jesse O. Wrenn Leigh M. Howard
Leigh M. Howard