
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Virol. , 03 March 2023
Sec. Antivirals and Vaccines
Volume 3 - 2023 | https://doi.org/10.3389/fviro.2023.1065991
This article is part of the Research Topic The perpetual challenge of Antiviral drug-resistance View all 4 articles
Introduction: Vaccination is one of the most crucial strategies in the control of pandemics such as COVID-19. Although a couple of research has been conducted to assess the willingness of the population to accept the COVID-19 vaccine, the findings are inconsistent and inconclusive. This study aimed to assess the pooled willingness to uptake the COVID-19 vaccine and its determinants in Ethiopia.
Methods: Published and unpublished articles were accessed from various electronic databases and digital libraries. A random-effects model was used to estimate the pooled effect size with a 95% confidence interval. Inverse variance (I2) was used to visualize the presence of heterogeneity. Publication bias was assessed using funnel plots and Egger’s statistical test.
Results: A total of 2345 studies were identified from several databases and 16 studies fulfilled the eligibility criteria and were included in the final meta-analysis. The pooled magnitude of willingness to accept the COVID-19 vaccine in Ethiopia was 55.19% (95% CI: 42.91, 67.48). The current meta-analysis indicated that age greater than 25 years (OR=1.49, 95% CI: 1.12, 1.98) and having a good attitude towards the COVID-19 vaccine (3.57, 95% CI: 1.46, 8.72) were significantly associated with the COVID-19 vaccine uptake.
Conclusions and recommendations: In general, the magnitude of the COVID-19 vaccine acceptance rate among the public is unacceptably low in Ethiopia. Therefore, there is a need to build public trust through the provision of reliable and consistent information about vaccines using different media outlets.
Coronavirus disease (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome 2 (SARS-CoV-2) viruses. It mainly affects an immunosuppressed group of people, who have chronic medical conditions and older ages. It can also affect all age groups and sex leading to mild illness to death in severity (1). Since the emergence of SARS-COV-2 in late 2019 in Wuhan city, Hubei province of China, billions of human life has been affected and lost (2). It is an emerging virus that is highly pathogenic and has the recent worldwide pandemic officially named coronavirus disease (COVID-19). On 16th January 2023, the world has more than 662 million confirmed cases of COVID-19 and more than 6.7 million deaths due to the disease (3).
COVID-19 vaccines are effective at preventing people from getting sick with COVID-19. It prevents severe illness, hospitalizations, and death. Getting vaccinated is the best way to reduce the transmission of SARS-COV-2, the virus that can cause COVID-19. Therefore, it is recommended for any eligible group to stay up-to-date on their COVID-19 vaccine, including people with reduced immunity (4).
World health organization (WHO) is working tirelessly with partners to develop, manufacture and deploy safe and effective vaccines. Currently, COVID-19 vaccines have reached billions of people worldwide, the evidence is overwhelming that no matter which type is administered, the vaccines offer life-saving protection against a disease that has killed millions (5). The current focus of WHO remains on reducing the severity of the diseases and death as well as capacitating health care systems to combat negative consequences of COVID-19 (6). Around the globe, there are more than 137 types of COVID-19 vaccine candidates undergoing clinical trials and 194 candidates under pre-clinical development (5, 7, 8).
WHO and other organizations were suggesting the best global vaccination approach so that all the nations of the globe would be covered in an equitable manner. Among the approaches, vaccinating the most vulnerable people first is an important one. This is followed by distributing safe and effective vaccines that are backed by sciences through exercising global cooperation and generosity, cooperating with ministries of health, civil society partners, community and religious leaders to encourage voluntary vaccinations are the most commonly suggested ones (8, 9).
To date (17 Jun 2022) globally, 9.37 billion doses of the COVID-19 vaccine were administered among which 8.6% and 52% of people were fully and partly vaccinated respectively. This can represent 60.2% of the world population. Only 9.4% of the people in lower-income countries have received at least one dose. Since the delivery of the vaccine on 7th March 2021 in Ethiopia, where about 20 million people are residing, 11 million doses of the COVID-19 vaccine were administered to about 1.6 million people (1.35% fully and 6.59% partly) (10).
Few studies conducted in Ethiopia recently utilized limited number of studies, and missed some variables like attitude of the public towards the vaccine uptake (11–14). A number of associated factors were identified so far regarding the willingness of people to take COVID-19 vaccines; perceived susceptibility, safety (fear of side effects), willingness to pay for the vaccine, history of contracting COVID-19, knowledge regarding COVID-19, sex, occupation, religion, age and marital status (9, 15, 16).
Showing a comprehensive willingness to receive the vaccine and the determinants is paramount needed for Ethiopian people who will in turn helps to develop and implement effective means of promoting COVID-19 vaccine uptake and to curb the recent shocking increase in COVID-19 transmission. Different pocket studies done so far might not represent the whole picture of the level of willingness and the associated factors in Ethiopia as most of them were limited to small sample size, limited populations, and limited study area. Despite several studies conducted in Ethiopia, their findings are inconsistent and inconclusive. Revealing information related to the vaccine uptake is crucial to increasing the acceptability of vaccines and reducing factors contributing to the hesitancy of COVID-19 vaccines among health professionals (17, 18). Hence, this study aimed to assess the pooled willingness to accept the COVID-19 vaccine and its determinants in Ethiopia.
This systematic review and meta-analysis assessed the willingness to receive the COVID-19 vaccine and its determinant factors in Ethiopia. Meta-analysis was reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (19). We checked for the presence of systematic reviews and meta-analyses on this topic to prevent duplication. Both published and unpublished studies were searched thoroughly using electronic databases such as Medline, Embase, Hinari, Pub Med, Cochrane library, Web of Science, and Google Scholar using different key terms. To find unpublished papers, some research centers, including the Digital Library of universities in Ethiopia were searched. All articles published and unpublished until January 15, 2022 were included in the review.
Pre-defined search terms were used to enable a comprehensive search strategy that included all relevant studies. All fields within records and Medical Subject Headings (MeSH terms) were used to expand the advanced Pub Med search. The search strategy was prepared and modified for the various databases using important Boolean operators with initial keywords “COVID-19 vaccine” OR “COVID-19 vaccine uptake” OR “willingness to receive COVID-19 vaccine” OR “willingness to accept COVID-19 vaccine” AND “associated factors” OR “determinants” AND “Ethiopia”. Literature was downloaded to Endnote (version X7.2), to maintain and manage citation and facilitate the review process.
This systematic review and meta-analysis included studies conducted on the willingness to accept the COVID-19 vaccine and its determinants in Ethiopia. This review considered all observational study designs that were written in the English. Participants were all residents of Ethiopia. This review included only studies conducted in Ethiopia. There were no limits on the studies based on sample size. We excluded articles, with low-quality assessment scales owing to methodological problems.
This systematic review and meta-analysis had two main outcomes. The primary outcome of review was the willingness to accept COVID-19 vaccine which was estimated as the total number of individuals with willing to take COVID-19 vaccine divided by the total number of COVID-19 vaccine takers multiplied by 100%. It is defined as those respondents who had responded “Yes” for a question “Do you accept COVID-19 vaccination if it is available?” and those who responded “No” were considered as vaccine hesitant” (20, 21).
The other outcome was the determinant factor of willingness to accept the COVID-19 vaccine which was determined in the form of an odds ratio and calculated based on the binary outcome from the included primary studies. The major factors identified after reviewing all primary articles were age (≤ 25 years or >25 years), marital status (married or not married), residence (rural/urban), knowledge towardsCOVID-19 vaccine (good knowledge/poor knowledge), attitude towards COVID-19 vaccine (good attitude/poor attitude), and history of chronic medical disorder (yes/no).
Citation management software (Endnote version X7.2) was used to combine the database search results and manually remove duplicate articles. The Newcastle-Ottawa Scale (NOS) adapted for cross-sectional studies was used for the quality assessment. Data were extracted by TT and GF. Then, checked by BW using standardized data extraction checklists on a Microsoft excel spreadsheet. For the first outcome (willingness to accept the vaccine), the data extraction checklist included the title, author name, year of publication, date of data collection, region, study design, sample size, participants, method of data collection, and number of subjects with the outcome variable. For the second outcome (determinant factors), data were extracted in a format of two by two tables, and the log odds ratio for each factor was calculated based on the findings of the original studies. Discrepancies between the two independent reviewers were resolved by including the third reviewer (BW) after discussion of the consensus.
A systematic review was conducted to compare, contrast as well as to describe the results of the primary studies. The meta-analysis findings were analyzed using STATA version 14. The prevalence, standard error of prevalence, logarithm of odds ratio and standard error of the odds ratio (OR) for each included study were generated using the “generate” command in STATA. Heterogeneity was evaluated using the Q test and the inverse variance index (I2). A random-effects model was used to estimate the pooled magnitude of the willingness to accept the COVID-19 vaccine. In addition, we conducted meta-regression and sub-group analyses to identify the source of heterogeneity. For subgroup analysis, the heterogeneity within groups was tested, using the same statistical methods. A subgroup analysis was conducted by the participants of the study and the data collection method.
A funnel plot of asymmetry was used to assess publication bias. Furthermore, Egger’s statistical test was used to check the statistical significance of publication bias, and the I-squared statistic (I2 = 100% × (Q-df)/Q). For the Q test, a P-value of 0.10 or less was considered statistically significant, indicating marked heterogeneity among studiescand was considered statistically significant.
A total of 2345 published and unpublished articles related to titles were accessed in our initial literature search. Of the total identified, 1467 duplicate records were removed, leaving 878 articles. After initial screening, 845 articles were excluded based on their titles and abstracts. The abstracts and full texts of the remaining 33 studies were assessed and screened for eligibility criteria. Finally, 16 articles fulfilled all eligibility criteria and were included in the final analysis (Figure 1).
From a total of 16 studies, 15 of the included studies were published in peer-reviewed journals and one was a preprint study (18). All the 16 articles included in this study were published in 2021. A total of 9560 participants were included in this systematic review and meta-analysis. The smallest sample size was 301 from a study conducted in Gondar town, Amhara region (22) and the largest sample size was 2178 from a study conducted by an online survey (23). All studies considered in the final review had a cross-sectional study design. Of the 16 studies included in the final analysis, six were conducted in SNNP (20, 24–28), four in the Amhara region (21, 22, 29, 30), three conducted online surveys at the national level (9, 16, 23), two in Addis Ababa (18, 31) and one in the Oromia region (32). Regarding the participants of the study, HCWs (16, 25, 30–32), general population (9, 18, 20, 23, 26, 28, 29), teachers and students (21, 22), and pregnant and lactating women (24, 27) were participated in the study. Four studies were collected via online surveys (9, 16, 23, 32), seven studies by interview (face to face) administered questionnaires (18, 22, 24, 26–29), and five studies were collected by self-administered questionnaires (20, 21, 25, 30, 31) (Table 1).
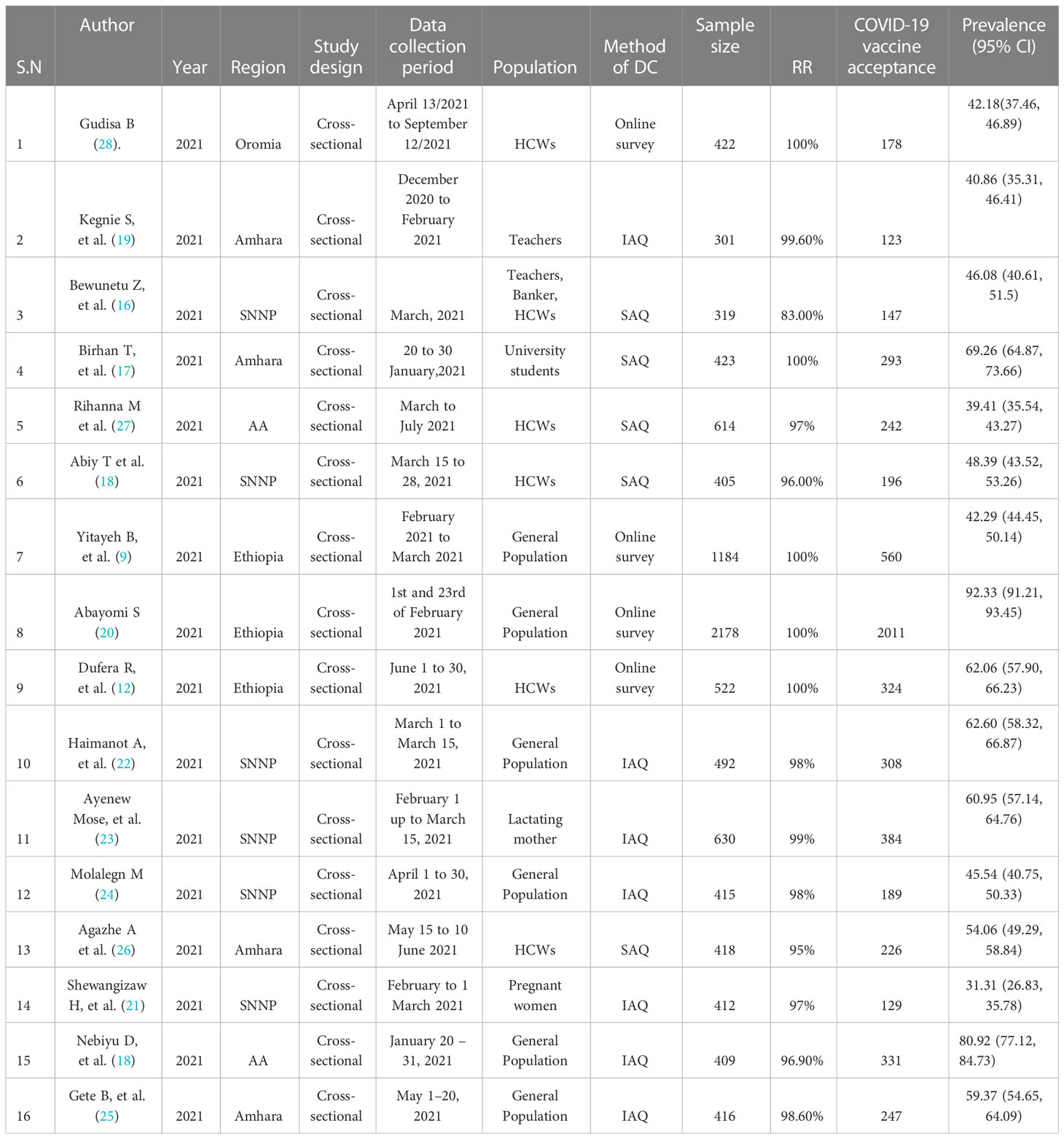
Table 1 Summary of Included Studies regarding willingness to accept COVID-19 vaccine in Ethiopia, 2022Willingness to accept COVID-19 vaccine.
Substantial heterogeneity was found using χ2 and Cochrane Q test statistics (I2 = 99.5, p < 0.001), and a random-effects model was used to estimate the pooled magnitude of willingness to accept COVID-19 vaccine. The overall pooled magnitude of willingness to accept the COVID-19 vaccine in Ethiopia was 55.19% (95% CI: 42.91, 67.48). The highest (92.33% (95%CI: 91.21, 93.45)) was observed in study conducted at national level by online survey (23), and the lowest 31.31% (95%CI: 26.83, 35.78) was reported in SNNP region (24) (Figure 2). To check for underlying heterogeneity, meta-regression models were performed using sample size and year of publication, but there was statistical insignificant for underlying heterogeneity (p = 0.546) and (p = 0.765), respectively.
To assess heterogeneity among the included studies, subgroup analysis was performed based on the population that participated in the study and the method of data collection utilized in the primary studies. Accordingly, the highest magnitude of willingness to receive COVID-19 vaccine was reported among adult general population 62.06% (95% CI: 43.41, 80.72) whereas the lowest was observed in pregnant and lactating women 46.16% (95% CI: 17.11, 75.20) (Figure 3). According to the method of data collection utilized in the primary studies, the magnitude of willingness to accept COVID-19 was high among studies collected through online surveys 61.06% (95%CI: 32.31, 89.70) and the lowest was from studies collected by self-administered questionnaires 51.45% (95%CI: 40.79, 61.11) (Figure 4).
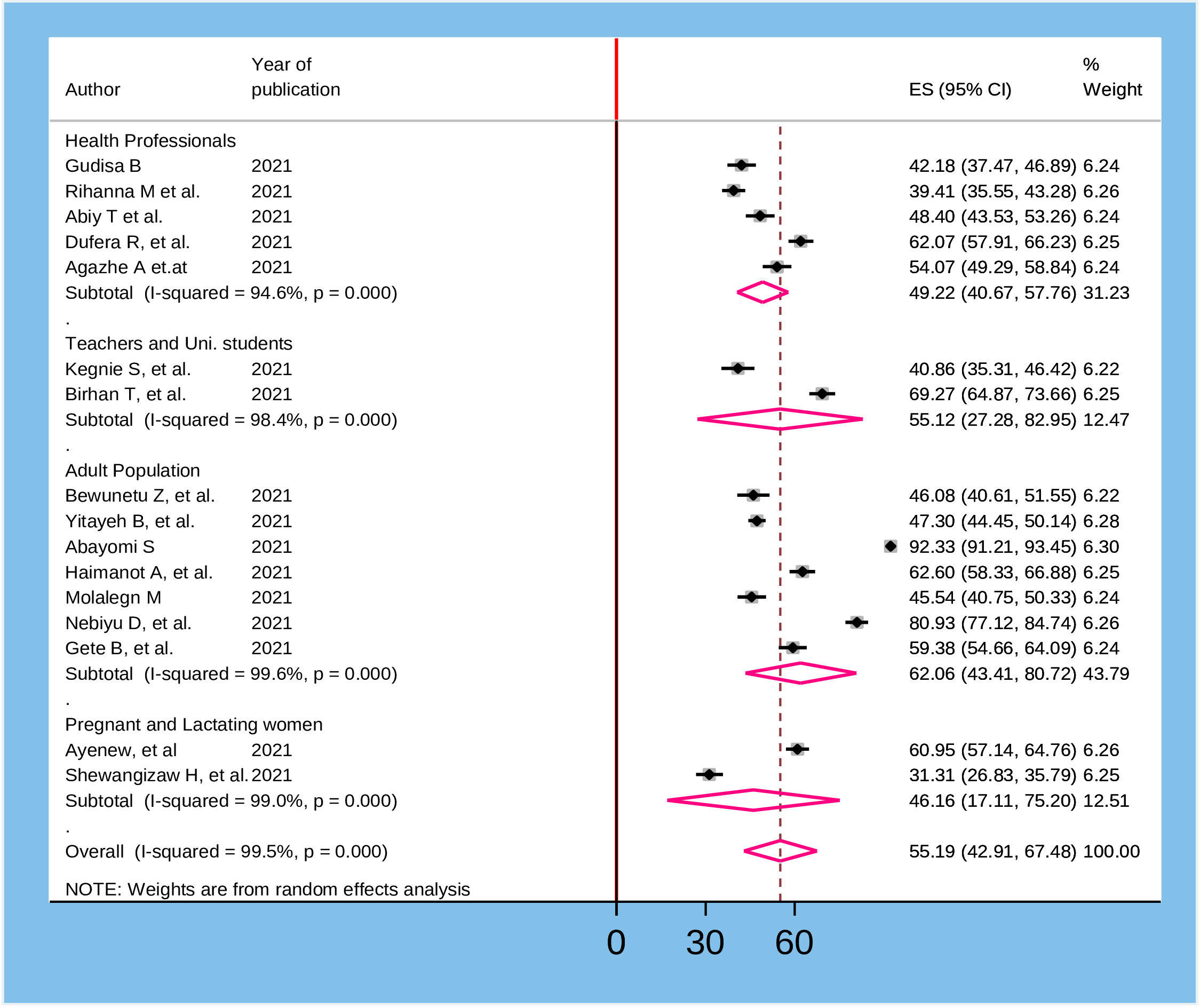
Figure 3 Sub group analysis based on the participants of the study for willingness to accept COVID-19 vaccine in Ethiopia, 2022.
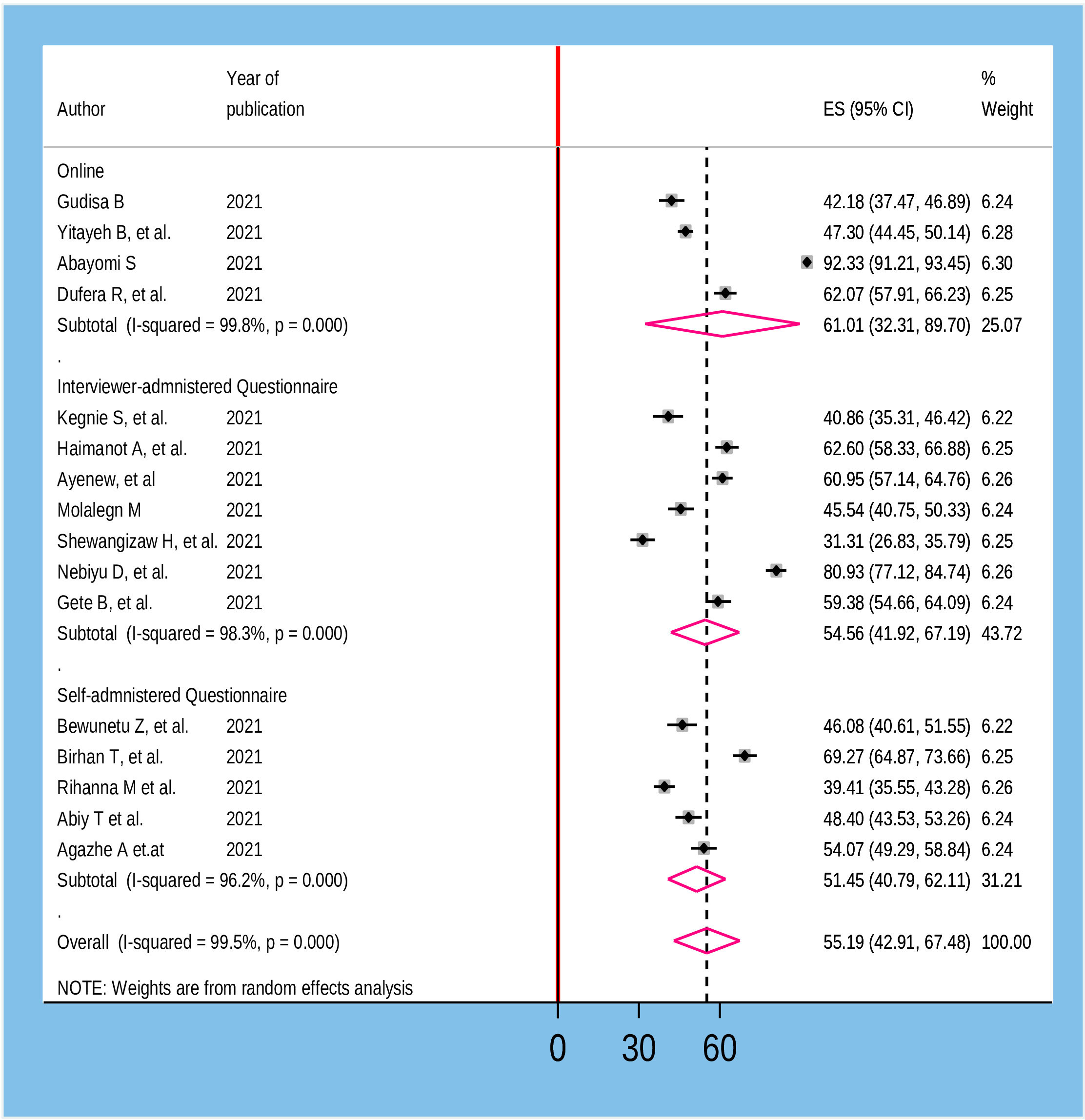
Figure 4 Sub group analysis based on the method of data collection for willingness to accept COVID-19 vaccine in Ethiopia, 2022.
To assess publication bias, a traditional funnel plot was used. The plot showed asymmetry of the plot which was suggestive of publication bias (Figure 5). Egger’s test’s weighted regression test and Begg’s correlation test were performed to determine the significance of publication bias and there was no significant publication bias from both tests (p= 0.0.075 and 0.106), respectively. To see the effect of a single study on overall studies and outliers, a sensitivity analysis was performed and which showed that there was no single study influence on the overall included studies (Figure 6).
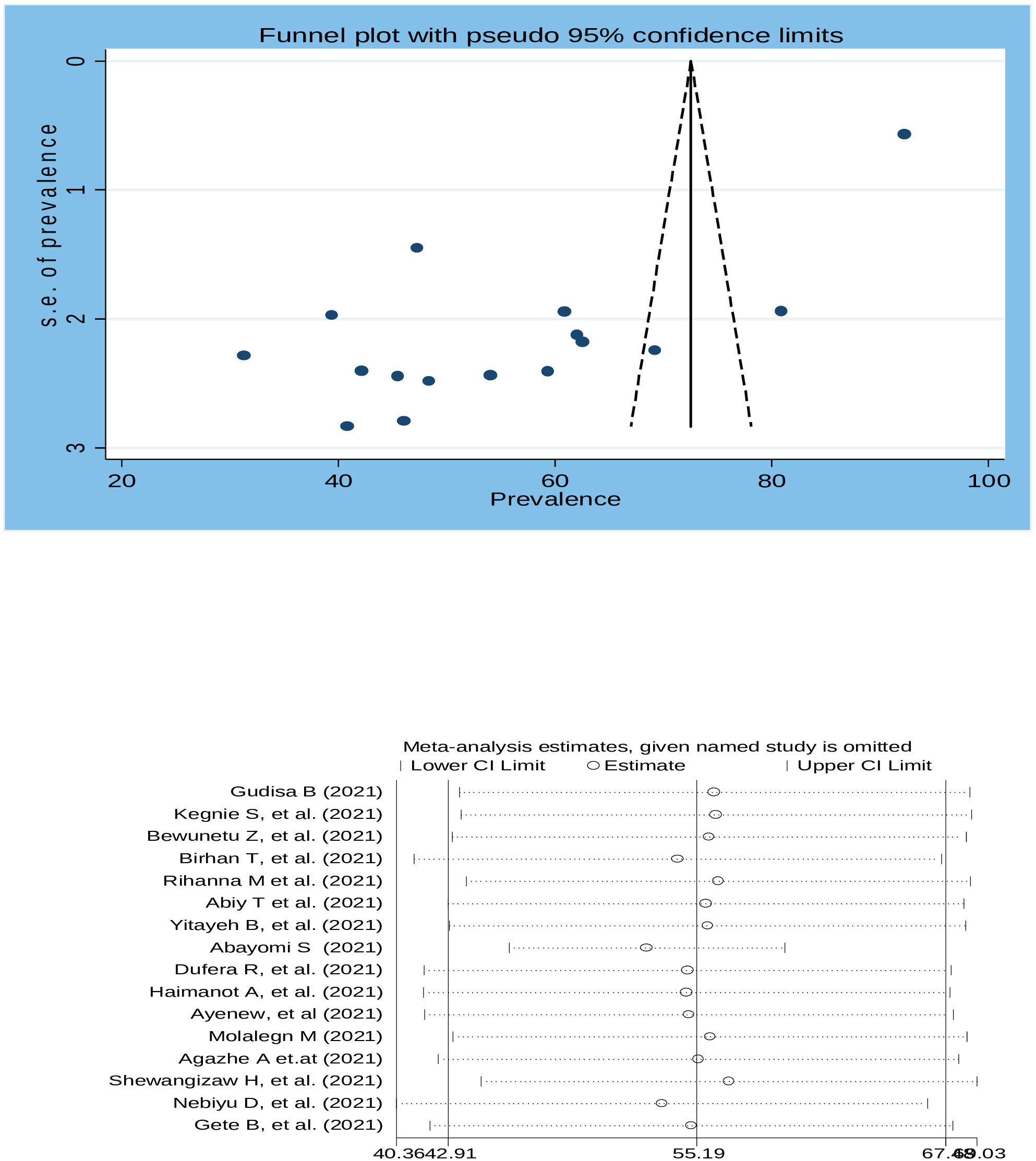
Figure 5 Funnel plot with 95% confidence limits of willingness to accept COVID-19 vaccine in Ethiopia, 2022.
To identify the determinants of willingness to accept the COVID-19 vaccine, several variables from the primary studies were reviewed. Thus, we identified seven main variables that strongly predicted the willingness to accept the COVID-19 vaccine. These variables were sex of participants, age of participants, marital status, residence, knowledge about COVID-19 vaccine, attitude about COVID-19 vaccine, and having a chronic medical disease (Table 2). In the pooled analysis, only two factors (age of participants and attitude about COVID-19 vaccine) were significantly associated with willingness to accept the COVID-19 vaccine (Figure 7), while the remaining five variables were not significantly associated with the outcome variable (Figures 8–12).
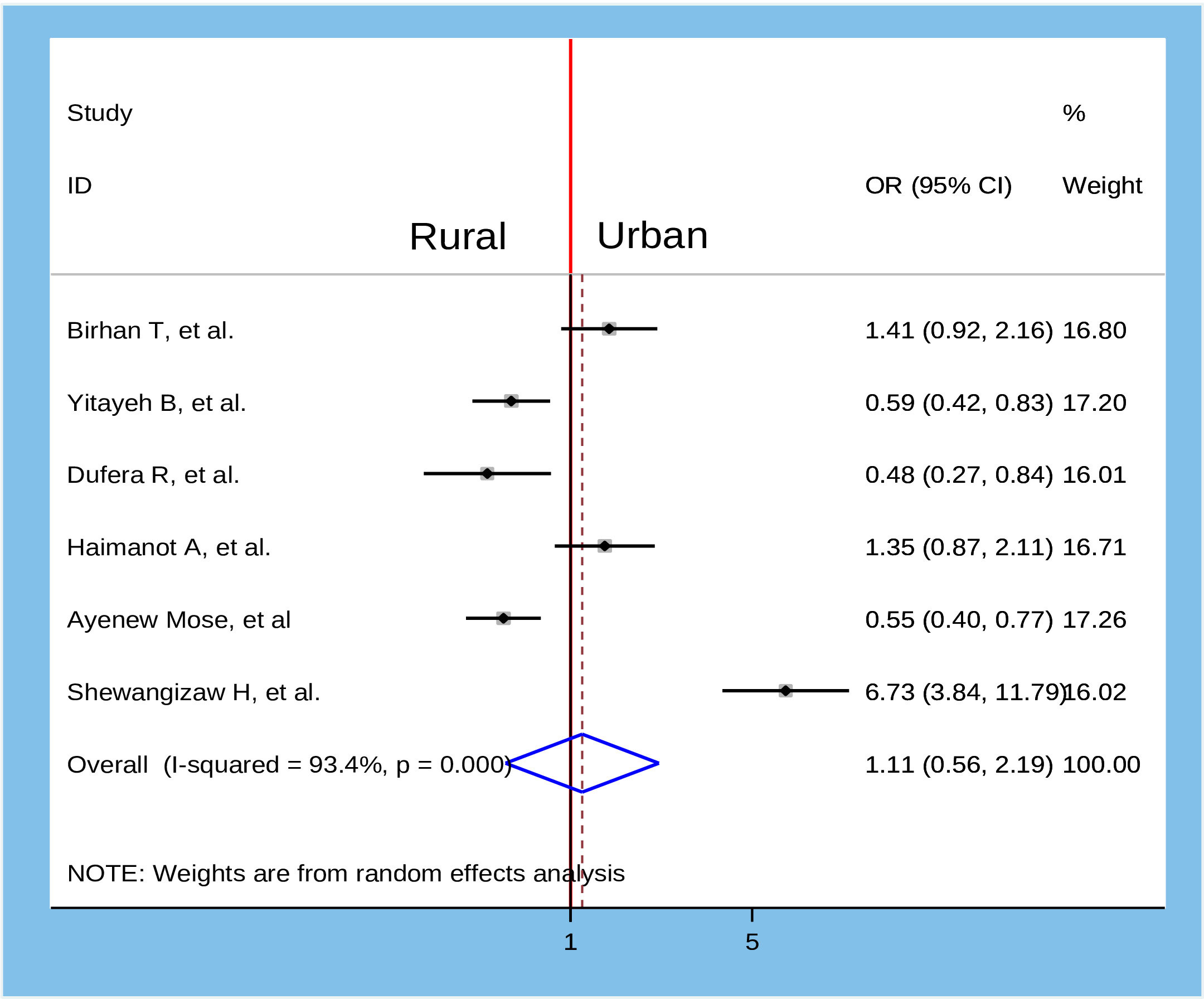
Figure 9 Association between marital status and willingness to accept COVID-19 vaccine in Ethiopia, 2022.
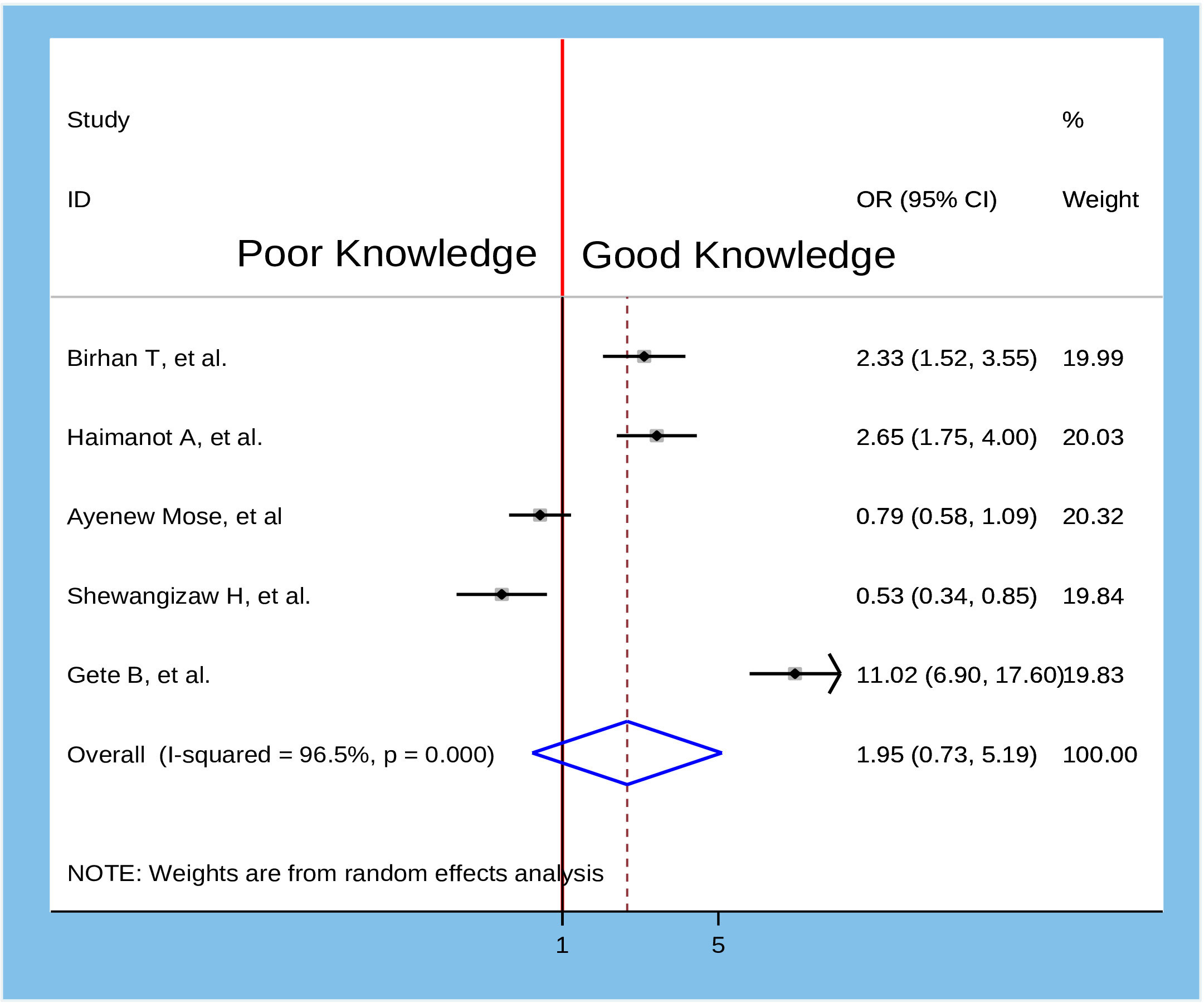
Figure 10 Association between residence and willingness to accept COVID-19 vaccine in Ethiopia, 2022.
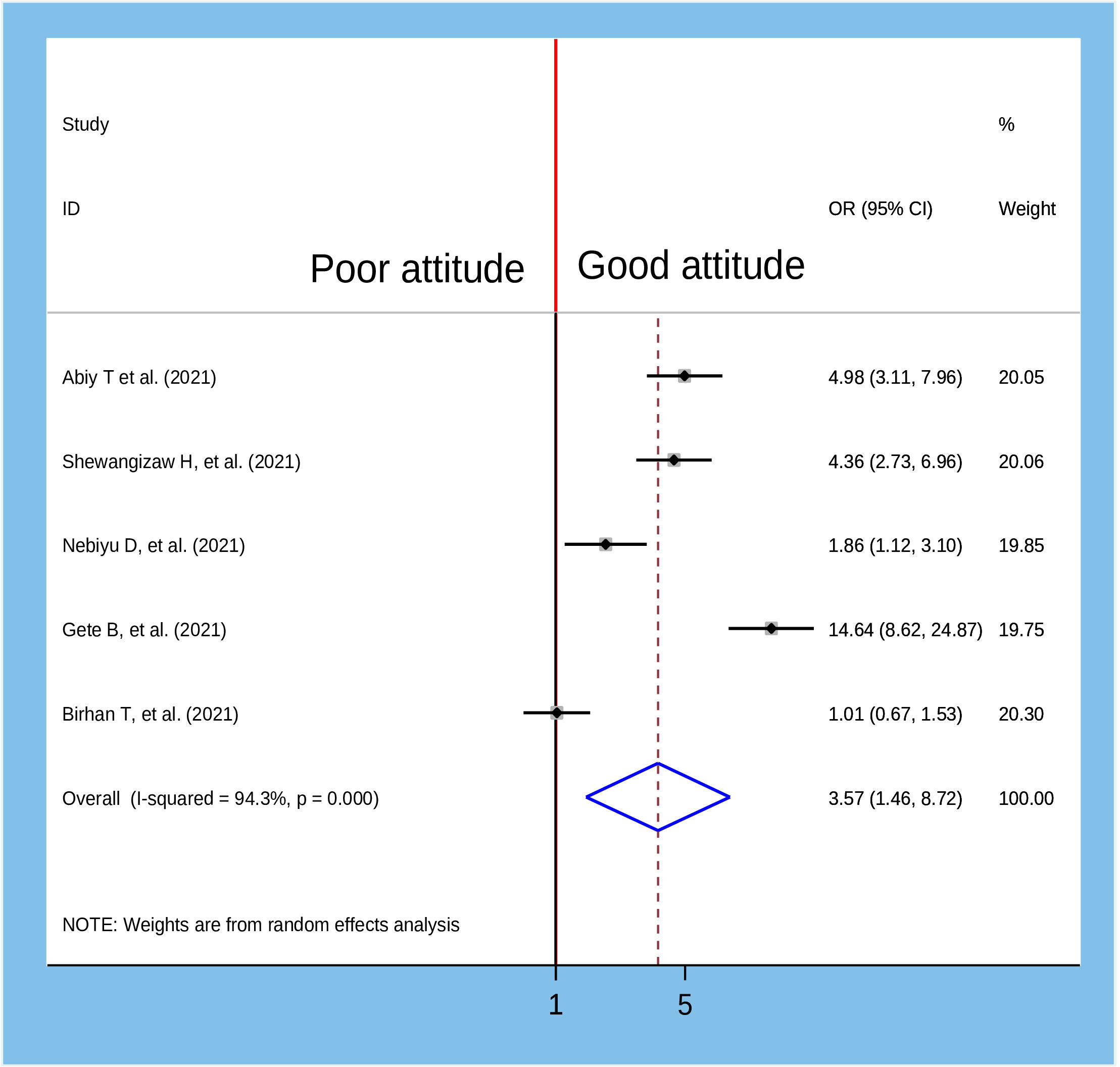
Figure 11 Association between knowledge and willingness to accept COVID-19 vaccine in Ethiopia, 2022.
Figure 12 Association between attitude towards COVID-19 vaccine and willingness to accept COVID-19 vaccine in Ethiopia, 2022.
To identify the association between the age of participants and their willingness to accept the COVID-19 vaccine, six studies were included in the meta-analysis (9, 21, 21, 26, 27, 30). We found moderate heterogeneity across studies (I2 = 64.0%, P = 0.016), which is an indicator of the use of a random-effects model to estimate the pooled association between age of participants and their willingness to accept the COVID-19 vaccine reported by the six studies with inverse variance. The pooled result of the analysis showed that there was a statistically significant association between the age of the participants and their willingness to accept the COVID-19 vaccine. Accordingly, the likelihood of receiving the COVID-19 vaccine was 1.49 times higher among the population aged >25years than in the younger age group AOR=1.49, 95%CI: 1.12, 1.98) (Figure 9).
To examine the relationship between the attitude of participants towards the COVID-19 vaccine and their willingness to accept the COVID-19 vaccine, five studies were reviewed (18, 21, 24, 25, 29). A random-effects model was used to estimate the pooled association between attitude and willingness to accept the COVID-19 vaccine (I2 = 94.3, p-value <0.001). The pooled results of the analysis indicated that there was a statistically significant association between the attitude of participants and willingness to accept the COVID-19 vaccine. Population who had good attitude towards COVID-19 vaccine was 3.57 times more likely to accept COVID-19 vaccine as compared to their counterparts AOR=3.57, 95%CI: 1.46, 8.72).
Despite the recent good news about the development of a vaccine for coronavirus disease, the world continues to face enormous crises due to COVID-19 vaccine hesitancy. It is straightforward that vaccination is the most cost-effective strategy for preventing and controlling highly contagious diseases. Hence, without enhancing the effective uptake of the COVID-19 vaccine, adherence to coronavirus preventive measures alone cannot end the spread of the virus and its crisis. Although there are plenty of primary studies on the acceptance level of a vaccine against the coronavirus in different parts of Ethiopia, they reported inconsistent and inconclusive findings. Therefore, the current review aimed to determine the pooled magnitude of the COVID-19 vaccine acceptance rate and its determinants among the public in Ethiopia. Certainly, understanding the pooled estimate of the COVID-19 vaccine acceptance level and its determinants among the public plays a vital role in controlling the spread of the virus.
The present meta-analysis indicated that the pooled magnitude of COVID-19 vaccine acceptance among the public was 55.19% (95% CI: 42.19, 67.48). This is congruent with the results of studies conducted in Africa (48.93%) (33), Bangladesh (65%) (34), Egypt (43%) (35), Saudi Arabia (64.7%) (36), USA(66%) (37), China(60.4-63%) (38, 39), Iran (64.2%) (40), and Malaysia(48.2%) (41). Perhaps this is due to the time during which those studies conducted was early in the introduction of the vaccine. However, it is by far lower than the studies done in Indonesia (86.8%) (42), Tonga (93%) (35), Italy (86%) (43), Turkey (84.6%) (44), Mexico (88%) (45), Brazil (83%) (45), Australia (80%) (46), and United Kingdom (86%) (47). Furthermore, it is lower than the study done in Mozambique (71.4%) (48), Pakistan (70.25-72%) (34, 49), Nepal (74%) (34), South Africa (76%) (50), France (77.6%) (51), and Vietnam (76.10%) (52). The observed discrepancy might be due to the variation between the populations in terms of access to information and educational level. In addition, it might also be due to most of the previous studies being done through an online survey in which the respondents of the study were necessarily educated population. Nevertheless, the present study finding is higher compared to the study done in Nigeria (40.5%) (53), Jordan (36.8-37.4%) (54, 55), Palestine (40%) (56), and Haiti (43%) (45). This may be due to variation in the study period and the population of interest.
In the current meta-analysis, subgroup analysis by the population of interest indicated that there were variations in the willingness to accept vaccine against COVID-19 among different included Populations in the primary studies. Accordingly, the highest magnitude of willingness to receive the COVID-19 vaccine was reported among the adult general population 62.06% (95% CI: 43.41, 80.72) while the lowest was observed among pregnant and lactating women 46.16% (95% CI: 17.11, 75.20). This could be due to the feared side-effects of the vaccine among pregnant and lactating women on their fetus or infant than in the adult general population. Moreover, the finding of subgroup analysis by the method of data collection in the primary studies revealed that the magnitude of willingness to accept COVID-19 vaccine was high among studies done through online survey 61.06% (95%CI: 32.31, 89.70) and low among studies conducted by self-administered questionnaire 51.45% (95%CI: 40.79, 61.11). This is due to the fact that respondents of the online survey have a relatively better understanding of the importance of the vaccine, as they might have access to different social media outlets.
In this systematic review and meta-analysis, only two factors (age of participants and attitude about COVID-19 vaccine) were significantly associated with willingness to accept COVID-19 vaccine. This result is also similar with different systematic review and meta-analysis conducted to assess vaccination willingness for COVID-19 in which age were most frequently observed to be significantly associated with vaccine acceptance or refusal (53, 57, 58). This is due to the fact that age has impact on the willingness of people towards this vaccine. Particularly, increasing age of individuals might have a sense of responsibility and accountability for themselves and their families’ relative to the youngest age groups.
Attitude of participants towards COVID-19 vaccine was also another factor which predicts the likelihood of willingness to accept COVID-19 vaccine. This is in line with studies conducted in different settings such as a systematic review and meta-analysis in Africa (58), and a global systematic review (59). This may be due to the fact that population those who had good attitude of COVID-19 vaccine may take care of themselves to minimize the risk, also they know the advantage of taking the vaccine and hence, they might accept to receive a vaccine.
The strength of this study was that various databases were used to search literature, and both published and unpublished studies were included in the study. This study also had some limitations, of which all of the included studies in the final analysis were cross-sectional study design which may decrease causal conclusion between selected variables and outcome variable. Furthermore, most of the studies selected for final analysis were conducted only in some regions of the country, which is not the true representative of the remaining regions. Hence, the consumers of this findings need to take in to considerations regarding its generalizability.
In general, the magnitude of the COVID-19 vaccine acceptance rate among the public was unacceptably low in Ethiopia. Having a positive attitude towards COVID-19 vaccine uptake and being in the age category of above 25 years were independent determinants of the willingness of the public to accept the vaccine. Therefore, there is a need to build public trust through the provision of reliable and consistent information about the vaccine uptake using different media outlets. Moreover, there should be continuous media programs insisting on vaccination against coronavirus to enhance the public to have a positive attitude and perception towards the vaccine utilization.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
TT and BW involved in proposal development. TT andGF participated in statistical analysis. TT, GF, BW, and BRF involved inthe design, selection of articles, and data extraction. TT, BRF, GF, BW,and ML involved in developing the initial drafts of the manuscript. Allauthors contributed to the article and approved the submitted version.
We would like to thank all authors of the studies included in this systematic review and meta-analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2023.1065991/full#supplementary-material
AA, Addis Ababa; AOR, Adjusted Odds Ratio; CI, Confidence Interval; HCW, Health Care Worker; IAQ, Interview Administered Questionnaire; J and J, Johnson and Johnson; SAQ, Self-Administered Questionnaire; SNNP, Southern Nation, Nationalities and People.
2. Shu Z, Chang K, Zhou Y, Peng C, Li X, Cai W, et al. Add-on chinese medicine for coronavirus disease 2019 (accord): A retrospective cohort study of hospital registries. Am J Chin Med (2021) 49(03):543–75. doi: 10.1142/S0192415X21500257
4. Basch CH, Hillyer GC, Meleo-Erwin ZC, Jaime C, Mohlman J, Basch CE, et al. Preventive behaviors conveyed on YouTube to mitigate transmission of COVID-19: Cross-sectional study. JMIR Public Health Surveillance (2020) 6(2):e18807. doi: 10.2196/18807
5. Westrop SJ, Whitaker HJ, Powell AA, Power L, Whillock C, Campbell H, et al. Real-world data on immune responses following heterologous prime-boost COVID-19 vaccination schedule with pfizer and AstraZeneca vaccines in England. J Infect (2022). doi: 10.1101/2021.12.14.21267562
6. Roy P, Chattopadhyay S, Das H. A review on-SARS-CoV-2 omicron variant. doi: 10.47191/ijcsrr/V5-i2-26
7. Barry M, BaHammam AS. COVID-19 vaccine in the kingdom of Saudi Arabia: A true operation warp speed. J Nat Sci Med (2021) 4(2):92. doi: 10.4103/jnsm.jnsm_8_21
8. Russell FM, Greenwood B. Who should be prioritised for COVID-19 vaccination? Hum Vaccines Immunotherapeut (2021) 17(5):1317–21. doi: 10.1080/21645515.2020.1827882
9. Belsti Y, Gela YY, Akalu Y, Dagnew B, Getnet M, Abdu Seid M, et al. Willingness of Ethiopian population to receive COVID-19 vaccine. J Multidiscip Healthc (2021) 14:1233. doi: 10.2147/JMDH.S312637
10. Blakney AK, Bekker L-G. DNA Vaccines join the fight against COVID-19. Lancet (2022) 399(10332):1281–2. doi: 10.1016/S0140-6736(22)00524-4
11. Sahile AT, Gizaw GD, Mgutshini T, Gebremariam ZM, Bekele GE. COVID-19 vaccine acceptance level in Ethiopia: A systematic review and meta-analysis. Can J Infect Dis Med Microbiol (2022) 2313367:1–7. doi: 10.1155/2022/2313367
12. Belay GM, Alemu TG, Techane MA, Wubneh CA, Assimamaw NT, Tamir TT, et al. COVID-19 vaccine acceptance rate and its predictors in Ethiopia: A systematic review and meta-analysis, Human vaccines & immunotherapeutics. (2022) 18:6. doi: 10.1080/21645515.2022.2114699
13. Mose A, Wasie A, Shitu S, Haile K, Timerga A, Melis T, et al. Determinants of COVID-19 vaccine acceptance in Ethiopia: A systematic review and meta-analysis. PloS One (2022) 17(6):e0269273. doi: 10.1371/journal.pone.0269273
14. Birye DM, Banchigizie AM. COVID-19 vaccine acceptance and its associated factors in Ethiopia: A systematic review and meta-analysis. Clin Epidemiol Global Health (2022) 14(1):101001. doi: 10.1016/j.cegh.2022.101001
15. Banik R, Islam MS, Pranta MUR, Rahman QM, Rahman M, Pardhan S, et al. Understanding the determinants of COVID-19 vaccination intention and willingness to pay: Findings from a population-based survey in Bangladesh. BMC Infect Dis (2021) 21(1):1–15. doi: 10.1186/s12879-021-06406-y
16. Terefa DR, Shama AT, Feyisa BR, Ewunetu Desisa A, Geta ET, Chego Cheme M, et al. COVID-19 vaccine uptake and associated factors among health professionals in Ethiopia. Infect Drug Resistance (2021) 14:5531. doi: 10.2147/IDR.S344647
17. Papagiannis D, Rachiotis G, Malli F, Papathanasiou IV, Kotsiou O, Fradelos EC, et al. Acceptability of COVID-19 vaccination among Greek health professionals. Vaccines (Basel) (2021) 9(3). doi: 10.3390/vaccines9030200
18. Dereje N, Tesfaye A, Tamene B, Alemeshet D, Abe H, Tesfa N, et al. COVID-19 vaccine hesitancy in Addis Ababa, Ethiopia: A mixed-methods study. medRxiv (2021). doi: 10.1101/2021.02.25.21252443
19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
20. Zewude B, Habtegiorgis T. Willingness to take COVID-19 vaccine among people most at risk of exposure in southern Ethiopia. Pragmat Obs Res (2021) 12:37–47. doi: 10.2147/POR.S313991
21. Taye BT, Amogne FK, Demisse TL, Zerihun MS, Kitaw TM, Tiguh AE, et al. Coronavirus disease 2019 vaccine acceptance and perceived barriers among university students in northeast Ethiopia: A cross-sectional study. Clin Epidemiol Global Health (2021) 12:100848. doi: 10.1016/j.cegh.2021.100848
22. Shitu K, Wolde M, Handebo S, Kassie A. Acceptance and willingness to pay for COVID-19 vaccine among school teachers in gondar city, Northwest Ethiopia. Trop Med Health (2021) 49(1):1–12. doi: 10.1186/s41182-021-00337-9
23. Oyekale AS. Willingness to take COVID-19 vaccines in Ethiopia: An instrumental variable probit approach. Int J Environ Res Public Health (2021) 18(17):8892. doi: 10.3390/ijerph18178892
24. Hailemariam S, Mekonnen B, Shifera N, Endalkachew B, Asnake M, Assefa A, et al. Predictors of pregnant women’s intention to vaccinate against coronavirus disease 2019: A facility-based cross-sectional study in southwest Ethiopia. SAGE Open Med (2021) 9. 20503121211038454. doi: 10.1177/20503121211038454
25. Angelo AT, Alemayehu DS, Dachew AM. Health care workers intention to accept COVID-19 vaccine and associated factors in southwestern Ethiopia, 2021. PloS One (2021) 16(9):e0257109. doi: 10.1371/journal.pone.0257109
26. Abebe H, Shitu S, Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinates of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect Drug Resistance (2021) 14:2015. doi: 10.2147/IDR.S312116
27. Mose A. Willingness to receive COVID-19 vaccine and its determinant factors among lactating mothers in Ethiopia: A cross-sectional study. Infect Drug Resistance (2021) 14:4249. doi: 10.2147/IDR.S336486
28. Mesele M. COVID-19 vaccination acceptance and its associated factors in sodo town, wolaita zone, southern Ethiopia: Cross-sectional study. Infect Drug Resistance (2021) 14:2361. doi: 10.2147/IDR.S320771
29. Berihun G, Walle Z, Berhanu L, Teshome D. Acceptance of COVID-19 vaccine and determinant factors among patients with chronic disease visiting dessie comprehensive specialized hospital, northeastern Ethiopia. Patient Preference Adherence (2021) 15:1795. doi: 10.2147/PPA.S324564
30. Aemro A, Amare NS, Shetie B, Chekol B, Wassie M. Determinants of COVID-19 vaccine hesitancy among health care workers in amhara region referral hospitals, Northwest Ethiopia: A cross-sectional study. Epidemiol Infect (2021) 149. doi: 10.1017/S0950268821002259
31. Mohammed R, Nguse TM, Habte BM, Fentie AM, Gebretekle GB. COVID-19 vaccine hesitancy among Ethiopian healthcare workers. PloS One (2021) 16(12):e0261125. doi: 10.1371/journal.pone.0261125
32. Bareda. G. Enthusiasm to acceptance of SARS_COV_2 vaccine among health care workers in oromia regional state, ethiopia. an online based cross-sectional study, 2021. J Subst Abuse Alcoholism (2021) 8(3). doi: 10.26420/austinjpulmrespirmed.2021.1077
33. Wake AD. The acceptance rate toward COVID-19 vaccine in Africa: A systematic review and meta-analysis. Global Pediatr Health (2021) 8. 2333794X211048738. doi: 10.1177/2333794X211048738
34. Hawlader MDH, Rahman ML, Nazir A, Ara T, Haque MMA, Saha S, et al. COVID-19 vaccine acceptance in south Asia: A multi-country study. Int J Infect Dis (2022) 114:1–10. doi: 10.1016/j.ijid.2021.09.056
35. Mannan DKA, Farhana KM. Knowledge, attitude and acceptance of a COVID-19 vaccine: A global cross-sectional study. Int Res J Business Soc Sci (2020) 6(4). doi: 10.2139/ssrn.3763373
36. Al-Mohaithef M, Padhi BK. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: A web-based national survey. J Multidiscip Healthcare (2020) 13:1657. doi: 10.2147/JMDH.S276771
37. Ehde DM, et al. Willingness to obtain COVID-19 vaccination in adults with multiple sclerosis in the united states. Multiple Sclerosis Related Disord (2021) 49:102788. doi: 10.1016/j.msard.2021.102788
38. Gan L, Chen Y, Hu P, Wu D, Zhu Y, Tan J, et al. Willingness to receive SARS-CoV-2 vaccination and associated factors among Chinese adults: A cross sectional survey. Int J Environ Res Public Health (2021) 18(4):1993. doi: 10.3390/ijerph18041993
39. Kwok KO, Li KK, Wei WI, Tang A, Wong SYS, Lee SS, et al. Influenza vaccine uptake, COVID-19 vaccination intention and vaccine hesitancy among nurses: A survey. Int J Nurs Stud (2021) 114:103854. doi: 10.1016/j.ijnurstu.2020.103854
40. Askarian M, Fu LY, HosseinTaghrir M, Borazjani R, Shayan Z, Fard ET, et al. Factors affecting COVID-19 vaccination intent among iranians: COVID-19 vaccination acceptance. (2020).
41. Wong LP, Alias H, Wong PF, Lee HY, AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum Vaccines Immunotherapeut (2020) 16(9):2204–14. doi: 10.1080/21645515.2020.1790279
42. Yanto TA, Octavius GS, Heriyanto RS, Ienawi C, Nisa H, Pasai HE. Psychological factors affecting COVID-19 vaccine acceptance in Indonesia. Egyptian J Neurol Psychiatry Neurosurg (2021) 57(1):1–8. doi: 10.1186/s41983-021-00436-8
43. Barello S, Nania T, Dellafiore F, Graffigna G, Caruso R. ‘Vaccine hesitancy’ among university students in Italy during the COVID-19 pandemic. Eur J Epidemiol (2020) 35(8):781–3. doi: 10.1007/s10654-020-00670-z
44. Kaplan AK, Sahin MK, Parildar H, Adadan Guvenc I. The willingness to accept the COVID-19 vaccine and affecting factors among healthcare professionals: A cross sectional study in Turkey. Int J Clin Pract (2021):e14226. doi: 10.1111/ijcp.14226
45. Urrunaga-Pastor D, Bendezu-Quispe G, Herrera-Añazco P, Uyen-Cateriano A, Toro-Huamanchumo CJ, Rodriguez-Morales AJ, et al. Cross-sectional analysis of COVID-19 vaccine intention, perceptions and hesitancy across Latin America and the Caribbean. Travel Med Infect Dis (2021) 41:102059. doi: 10.1016/j.tmaid.2021.102059
46. Seale H, Heywood AE, Leask J, Sheel M, Durrheim DN, Bolsewicz K, et al. Examining Australian public perceptions and behaviors towards a future COVID-19 vaccine. BMC Infect Dis (2021) 21(1):1–9. doi: 10.1186/s12879-021-05833-1
47. Williams L, Gallant AJ, Rasmussen S, Brown Nicholls LA, Cogan N, Deakin K, et al. Towards intervention development to increase the uptake of COVID-19 vaccination among those at high risk: Outlining evidence-based and theoretically informed future intervention content. Br J Health Psychol (2020) 25(4):1039–54. doi: 10.1111/bjhp.12468
48. Dula J, Mulhanga A, Nhanombe A, Cumbi L, Júnior A, Gwatsvaira J, et al. COVID-19 vaccine acceptability and its determinants in Mozambique: An online survey. Vaccines (2021) 9(8):828. doi: 10.3390/vaccines9080828
49. Malik A, Malik J, Ishaq U. Acceptance of COVID-19 vaccine in Pakistan among health care workers. medRxiv (2021). doi: 10.1101/2021.02.23.21252271
50. Cooper S, van Rooyen H, Wiysonge CS. COVID-19 vaccine hesitancy in south Africa: How can we maximize uptake of COVID-19 vaccines? Expert Rev Vaccines (2021) 20(8):921–33. doi: 10.1080/14760584.2021.1949291
51. Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A, et al. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine (2020) 38(45):7002–6. doi: 10.1016/j.vaccine.2020.09.041
52. Huynh G, Tran TT, Nguyen HT, Pham LA. COVID-19 vaccination intention among healthcare workers in Vietnam. Asian Pacific J Trop Med (2021) 14(4):159. doi: 10.4103/1995-7645.312513
53. Eniade OD, Olarinmoye A, Otovwe A, Akintunde FE, Okedare OO, Aniyeloye AO, et al. Willingness to accept COVID-19 vaccine and its determinants among Nigeria citizens: A web-based cross-sectional study. J Adv Med Med Res (2021), 13–22. doi: 10.9734/jammr/2021/v33i830881
54. Al-Qerem WA, Jarab AS. COVID-19 vaccination acceptance and its associated factors among a middle Eastern population. Front Public Health (2021) 9:34. doi: 10.3389/fpubh.2021.632914
55. El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ. Acceptance and attitudes toward COVID-19 vaccines: A cross-sectional study from Jordan. PloS One (2021) 16(4):e0250555. doi: 10.1371/journal.pone.0250555
56. Rabi R, Maraqa B, Nazzal Z, Zink T. Factors affecting nurses' intention to accept the COVID-19 vaccine: A cross-sectional study. Public Health Nurs (2021). doi: 10.1111/phn.12907
57. Nehal KR, Steendam LM, Campos Ponce M, van der Hoeven M, Smit GSA. Worldwide vaccination willingness for COVID-19: A systematic review and meta-analysis. Vaccines (2021) 9(10):1071. doi: 10.3390/vaccines9101071
58. Wake AD. The willingness to receive COVID-19 vaccine and its associated factors: “vaccination refusal could prolong the war of this pandemic”–a systematic review. Risk Manage Healthcare Policy (2021) 14:2609. doi: 10.2147/RMHP.S311074
Keywords: COVID-19 vaccine, COVID-19 vaccine uptake, Ethiopia, systematic review, meta-analysis
Citation: Tolossa T, Fetensa G, Feyisa BR, Wakuma B and Lema M (2023) Willingness to accept COVID-19 vaccine and its determinants in Ethiopia: A systematic review and meta-analysis. Front. Virol. 3:1065991. doi: 10.3389/fviro.2023.1065991
Received: 10 October 2022; Accepted: 15 February 2023;
Published: 03 March 2023.
Edited by:
Graciela Andrei, KU Leuven, BelgiumReviewed by:
Ajay Kumar Yadav, Indian Veterinary Research Institute (IVRI), IndiaCopyright © 2023 Tolossa, Fetensa, Feyisa, Wakuma and Lema. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadesse Tolossa, eWFkYW5vdG9sYXNhQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.