
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virol., 14 July 2022
Sec. Virus and Host Immunity
Volume 2 - 2022 | https://doi.org/10.3389/fviro.2022.906271
This article is part of the Research TopicViral-host interactions in pregnancy and early lifeView all 5 articles
 Kerina Duri1*
Kerina Duri1* Hope Mataramvura1
Hope Mataramvura1 Panashe Chandiwana1
Panashe Chandiwana1 Arthur John Mazhandu1
Arthur John Mazhandu1 Simeon Banhwa2
Simeon Banhwa2 Privilege Tendai Munjoma1
Privilege Tendai Munjoma1 Lovemore Ronald Mazengera1
Lovemore Ronald Mazengera1 Felicity Zvanyadza Gumbo3
Felicity Zvanyadza Gumbo3 for The UZ Birth Cohort Study Team
for The UZ Birth Cohort Study Team
Introduction: Mother-to-child-transmission (MTCT) of human immunodeficiency virus (HIV) can occur in pregnancy/in utero (IU), during childbirth/intrapartum (IP), or postpartum (PP) through breastfeeding from an infected mother to her infant. Burden of PP-MTCT and associated risk factors remain poorly described, especially in adolescent girls and young women (AGYW) aged 15–24 years. Furthermore, despite concerns on high postnatal seroconversions, there is paucity of data on the burden of subsequent MTCT rates.
Methods: Pregnant women ≥20 weeks of gestation were enrolled into the University of Zimbabwe Birth Cohort from four primary health centers in Harare, Zimbabwe. Mother–infant dyads were followed up from delivery, week(s) 1, 6, 10, 14, 24, 36, 48, 72, and 96 after birth. Women who were uninfected at baseline were re-tested for HIV on subsequent visits. Plasma HIV RNA was quantified using reverse transcriptase polymerase chain reaction. Exposed babies were tested for HIV using qualitative/quantitative proviral DNA PCR on dried blood spots. Maternal–infant factors were tested in univariable/multivariable regression analyses for HIV-MTCT predictors.
Results: A total of 600 HIV-uninfected and 608 HIV-infected pregnant women on Tenofovir/Lamivudine/Efavirenz regimen were enrolled from 2016 to 2019. Postnatal HIV incidence was 0.42 cases/100 women-years [95% confidence interval (CI): 0.12–1.1]. Postnatal seroconverters were less likely to have children/pregnancies sharing same father and unaware of their spouses/intimate partner’s HIV status: p = 0.008 and p = 0.02, respectively, compared with non-seroconverters.
Overall HIV-MTCT rate was (15/549): 2.7% (CI: 1.3–4.1%); (7/93) 7.5% observed in AGYW against 1.7%; in women aged >24, p = 0.008. PP-MTCT was the predominant 9/15 (60%) route, followed by IP-MTCT 4/15 (26.6%), whereas IU and postnatal MTCT rates each contributed 6.7% of all infant infections. Postnatal MTCT incidence was 12.8 (CI: 0.3–71.4) infant HIV infections/100 child-years of breastfeeding; a rate 14 times higher than PP-MTCT rate in babies born to women HIV-infected pre/post-conception whose babies were HIV DNA PCR–negative at six weeks.
Antenatal HIV RNA >1,000 copies/ml was independently associated with MTCT; odds ratio [CI: 9.3 (2.6–43.1)]. Infected infants’ pre–HIV treatment HIV RNA levels correlated positively with maternal viral load; Spearman’s rank correlation. r = 0.6; p = 0.03.
Discussion: Mothers were 9.3 times more likely to transmit if HIV RNA was >1,000 copies/ml, disproportionately occurring in vulnerable AGYW. Breastfeeding-associated PP-MTCT remains high; therefore, it is imperative that HIV-infected women commence antiretroviral therapy early in pregnancy to suppress HIV RNA until weaning to decrease the risk of MTCT and possibly reduce the severity of disease in infected infants. HIV-uninfected lactating mothers should be continuously counseled on the risks of postnatal seroconversion.
www.clinicaltrials.gov, trial registration number: NCT04087239.
Before the advent of antiretroviral therapy (ART), 35%–49% of babies born to human immunodeficiency virus (HIV)–infected women became infected, with 8%, 15%, and up to 26% being infected in pregnancy/in utero (IU), during childbirth/intrapartum (IP), and postpartum (PP) through breastfeeding, respectively (1, 2). HIV-infected pregnant women have been shown to have higher rates of adverse birth outcomes compared with their HIV-uninfected counterparts (3, 4). Hence, undiagnosed maternal HIV mainly due to poor social-economic circumstances remains a challenge in both resource rich and limited settings, more so in adolescent girls and young women (AGYW) aged 15–24 years) (5–7).
A positive maternal HIV status has been the strongest predictor of infant mortality (8). By the age of 1 and 2 years, 35% and 53% of the HIV-infected babies would have died, respectively (9). Even in the absence of mother-to-child transmission (MTCT), just mere exposure to maternal HIV has been associated with adverse fetal/infant outcomes. Despite their negative HIV status, mortality of HIV-exposed and uninfected (HEU) infants remained high, as by the age of 1 and 2 years, 4.9% and 7.7% of HEU babies would have died, respectively (9).
ART strategies for the treatment of HIV-infected pregnant women for their own health and for prevention of MTCT (PMTCT), alongside other complementary interventions, have reduced HIV transmission rates to less than 1% in resource rich countries and greatly improved maternal–infant survival (10). One mechanism of reducing MTCT risk involves lowering maternal viral load (VL) using nucleoside reverse transcriptase inhibitors (NRTIs) with high transplacental passage that inhibits viral replication in the fetus (11). NRTIs also provide post-exposure prophylaxis to infants during the breastfeeding period (12). Despite the current universal access to effective ART prophylaxis, 1,500 child HIV cases still occur each day globally as a result of MTCT from the 1.5 million HIV-infected women who get pregnant annually worldwide, with 9 out of 10 pediatric HIV cases occurring in Sub-Saharan Africa (13).
Trophoblast and villi have been shown to have cluster of differentiation-4 (CD4) receptors that make them potential candidates for HIV infection in pregnancy (14). Maternal-fetal microtransfusion during labor and delivery, including prolonged rupture of membranes may also explain the mechanisms of IP transmission of HIV (15, 16). Despite its nutritional and immunological benefits, breast milk can serve as a vector for PP-MTCT, a public health concern in breastfeeding populations (17, 18).
Good maternal health remains critical, since even without any intervention, MTCT rates were about 30%, implying that despite continued HIV exposure(s), maternal protective effects exist. Maternal antibodies of the IgG class are passively transferred to the fetus and these together with plasma broadly neutralizing antibodies confer protection towards neonatal HIV-1 infection (19–21). HIV-1 envelope-specific breast milk IgA antibodies reduce the risk of postnatal HIV-MTCT (22). In addition, the presence of protective bioactive innate immune factors in breast milk, such as lactoferrin and elafin including soluble Toll-like receptor 2, directly inhibit HIV infection, integration, and inflammation (23, 24). Breast milk HIV RNA levels have been shown to correlate strongly with systemic plasma VL (25).
In resource rich countries, breastfeeding is not recommended in mothers living with HIV/AIDS (MLWHA) to mitigate PP-MTCT. Interestingly, a 2019 Germany study of MLWHA has shown that breast feeding related MTCT cases are increasing (26). Thus, despite being discouraged to breastfeed, some MLWHA in resource rich settings continue this practice instinctively, in the face of MTCT risks. On the other hand for resource limited settings, World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of life followed by gradual introduction of supplementary feeds to offset the higher risks to infants’ life posed by diarrheal diseases and pneumonias (27). In such settings, exclusive replacement feeding is neither feasible nor safe due to limited access to clean water and other concerns around food insecurity (28). Such observations in both rich and limited resource settings of MLWHA continuing this breastfeeding practice underscores the urgent need to have a deeper understanding of the correlates of PP-MTCT of HIV with the aim to prevent infant HIV acquisition through breast milk.
In Zimbabwe, without any HIV treatment, a third of the infected infants rapidly progressed to acquired immunodeficiency syndrome (AIDS), with only 50% surviving beyond the age of two (29). During the era of short course ART that was administered from 36 weeks gestation until 1 week PP, the overall HIV-MTCT rates decreased from 31% before the advent of ART to 3.6% in 2014 (30–32). Pediatric HIV cases drastically declined from 51,000 in the year 2000 to 8,100 in 2020 (33, 34).
Despite the adoption of the lifelong HIV treatment strategy in 2013, coupled with the establishments of 1,560 health facilities offering comprehensive PMTCT services nationwide, new pediatric HIV infections remain unacceptably high, with a prevalence rate of 1.6% in children aged 0–14 years (32). Breastfeeding-associated PP-MTCT rates beyond 6 months of life and/or until weaning, including the associated risk factors, remain poorly described. Furthermore, despite concerns on high postnatal seroconversions (35), there is paucity of data on the burden of subsequent MTCT rates, especially in AGYW. Understanding the risk factors of PP-MTCT in MLWHA already infected in pregnancy vis-à-vis those seroconverting after delivery is key to identifying effective protective mechanisms with the aim to develop evidence-based innovative strategies to reduce PP-MTCT rates in breastfeeding populations.
The main objective was to determine HIV-MTCT burden during this current lifelong HIV treatment strategy and to compare PP-MTCT rates in MLWHA aware of their HIV status in pregnancy versus those seroconverting after delivery, including assessing HIV-MTCT rates and risk factors in AGYW (15–24 years) versus older women >24 years of age over the 24-month follow-up period.
Depending on the risk period for HIV-MTCT, we specifically aimed the following:
1. To determine the overall HIV-MTCT rate, including IU-, IP-, and early PP-MTCT rates in women who were HIV-infected in pregnancy at enrollment and their exposed infants at risk of acquiring HIV in pregnancy, during labor/delivery and through breastfeeding up to 6 months of age (predominantly exclusive breastfeeding period), and describe the associated risk factors.
2. To determine the late PP-MTCT rate in women HIV-infected in pregnancy, at enrollment with exposed infants testing HIV DNA PCR–negative at 6 weeks of age but continued breastfeeding beyond 6 months of age (mixed feeding effect).
3. To determine maternal HIV incidence in uninfected women during pregnancy but seroconverting after delivery over the 24 months follow-up period and estimate the subsequent PP-MTCT rate, timing, and describe the associated risk factors.
4. To compare the cumulative breastfeeding PP-MTCT rates between women HIV-infected in pregnancy (as in point 2 above whose infants were DNA PCR–negative 6 weeks) relative to the subsequent PP-MTCT rate of postnatal seroconverters’ as in point 3 above.
The University of Zimbabwe College of Health Sciences Birth Cohort study (UZBCS) is a prospective observational cohort of HIV-infected and HIV-uninfected pregnant women in a resource limited setting of high-density suburbs west of Harare, Zimbabwe. The UZBCS aims to investigate the role of maternal comorbidities, including co-infections with persistent viruses such as HIV, cytomegalovirus (CMV), hepatitis B virus (HBV), and other infectious diseases like syphilis and non-communicable diseases including maternal nutritional status, hypertensive disorders on pregnancy outcomes, infant mortality, and health in general. By design, at baseline approximately 50% of the expecting women, the UZBCS was HIV-infected as previously described (36).
Potential participants for the cohort were identified during routine antenatal care visits at any one of the four selected out of a total of twelve City of Harare Polyclinics, namely, Kuwadzana, Dzivaresekwa (Rujeko), Glenview, and Budiriro. Study sites were selected on the basis of the higher volumes of maternal and child health services, frequency of HIV sero-positivity, and lack of competing research activities targeting the same population.
Pregnant women had to be ≥15 years of age, at least 20 weeks of gestation at enrollment and planning to deliver at any of the four selected study sites. All pregnant women with a documented positive HIV status were encouraged to enroll in the study. For every HIV-infected pregnant woman recruited, the 10th HIV-uninfected woman presenting to the same primary health clinic for the initial antenatal registration was recruited, accounting for the 12% HIV prevalence within this population. The pregnancy was dated by the standard way of using menstrual history together with ultrasound scans whenever available as documented in the antenatal booklet that is issued to each pregnant woman upon registration.
Exclusion criteria included the presence of severe maternal mental health disorders, rendering the women incapacitated to provide informed consent or comply with study procedures as was assessed by the study clinicians.
Briefly, at enrollment, all women answered a structured questionnaire, aiming at a comprehensive clinical, socio-demographic, and household characterization of the research participants, including monthly amounts of money set aside for food and general household food security. Other questions addressed maternal life style such as alcohol use and spouse/intimate partner factors.
Date for ART commencement, compliance, and HIV status disclosure were recorded in HIV-infected women. According to the Zimbabwean national guidelines, all HIV-infected women should be on lifelong ART, but in practice, pregnant women often do not receive a timely HIV diagnosis (6). By then in 2016, the current standard of care for all HIV-infected pregnant women for PMTCT of HIV consisted of (non-)NRTIs, TENOLAM-E (Tenofovir, Lamivudine and Efavirenz). Mothers were encouraged to breastfeed for as long as they wished, with exclusive breastfeeding encouraged during the first 6 months of life. Exclusive breastfeeding classification allowed for medicines, vitamins, vaccines, and/or oral re-hydration solutions to be given to infants.
At enrollment, the study midwife performed a full physical examination, blood pressure checks, assessment of edema, and anthropometry. The examination also included measurement of symphysis fundal height for calculation of gestational age in comparison with the last menstrual period that is subject to considerable margin of error, particularly late in pregnancy.
Assessment of nutritional status was done using standard anthropometric index of mid-upper arm circumference (MUAC) (37, 38). Blood pressure, both systolic and diastolic, was measured after allowing the mother to rest for at least 5 min.
WHO clinical staging was done in all HIV-infected women.
Mothers were reminded of their upcoming follow-up study visits by phone call or through text message. Follow-up was scheduled at delivery, 1, 6, 10, 14, 24, 36, 48, 72, and 96 week(s) PP. If a mother failed to turn up for their scheduled follow-up appointments, then attempts to contact her or her next of kin by phone and/or home visit was done strictly adhering to the documented mother’s preferences. Data and samples were collected, provided that the visit was rescheduled within the following 7 days. If two consecutive study visits were missed without any prior communication, then a discontinuation option was discussed with the mother.
The WHO definition of stillbirth as birth of fetus at ≥28 weeks of gestation with birth weight of ≥1,000 g or body length of ≥35 cm was used (39). Information on place, date of birth, and mode of delivery were extracted from “Road to Health Child’ cards” issued at birth for monitoring growth and vaccinations records until 5 years of age. Longitudinal infant feeding practices and information on health and development were collected. Extensive questionnaires more or less similar to the one administered at enrollment was re-administered at 6, 12, 18, and 24 months.
Infants’ physical examinations were done at every visit. Sick infants were seen by the study pediatrician. Study procedures included bio-sampling of blood, dried blood spot (DBS), and breast milk at every study visit.
Ten milliliters of maternal venous blood was collected from enrollment. All samples were processed accordingly and stored in a bio-bank at −80°C within a maximum of 6 h after acquisition.
Full blood counts were determined from whole ethylenediaminetetraacetic acid (EDTA) blood samples using a Mindray© Haematology three-part differential, 16-parameter BC3600 Analyser (Shenzhen, China). For diagnosis of anemia in pregnancy, the WHO definition was used (hemoglobin <11.0 g/dl) (40).
All mothers were tested for CMV and syphilis in pregnancy using Euroimmun ELISA (Germany) and SD Bioline rapid kits respectively and HBV using Advanced Quality, HighTop and CTK Biotech rapid test kits.
Maternal HIV diagnosis and/or confirmation was done using qualitative rapid immunochromatographic assays, SD Bioline HIV-1/2 3.0 (Standard Diagnostics Inc., Kyonggi-do, South Korea), and Abbott’s Determine® HIV-1/2. Western Blot was the tie breaker for any indeterminate test results.
HIV-uninfected mothers were continuously counseled and retested for HIV infection every 3 months until 24 months after delivery. Seroconverting women were referred to the national PMTCT program for immediate commencement of ART and immunological and virological assessments and monitoring.
Absolute CD4+ T-lymphocyte counts were enumerated in EDTA blood samples within a maximum of 6 h following sample acquisition for all HIV-infected mothers using a Partec Cyflow counter (Cyflow, Partec, Munster, Germany).
For analysis of HIV RNA, whole blood was centrifuged for 5 min at 3,000 rpm, and the plasma was aliquoted in appropriately labelled cryo-vials and stored at −80°C until testing. Viral nucleic acids were extracted from 1 ml of maternal baseline plasma. HIV-1 RNA was quantified using an automated TaqMan Roche Amplicor 1.5 Monitor Test (Cobas AmpliPrep/Cobas TaqMan, Roche Diagnostics, Branchburg NJ), according to the manufacturer’s instructions. The detection limit was 20 copies/ml.
Venous EDTA blood or heel prick blood spots were collected and preserved on 903 protein saver card (number 105311018) at delivery, week(s) 1, 6, 10, 14, 24, 36, 48, and 96 of age and stored at −80°C for evaluations of HIV-MTCT. The volume of blood collected was within recommended amounts depending on the weight of the infant (i.e., ≤1%–5% of total blood volume over 24 h and ≤10% over 8 weeks) (41). Blood was not collected from severely sick infants, including those with suspected severe anemia according to clinical assessment.
As per the national guidelines, all HIV-exposed infants were commenced on Nevirapine and Cotrimoxazole prophylaxis until they stopped breastfeeding or tested HIV DNA PCR–positive, whichever occurred first.
Infants’ HIV-1 infection was detected using a qualitative/quantitative 1.5 Roche Amplicor HIV-1 proviral DNA PCR kit (Roche Diagnostics Incorporation, Branchburg, New Jersey) performed on DBS within the first 10 days of life, at weeks 6, 10, 14, 24, 36, 48, 72, and 96 or until cessation of breast feeding, whichever came first. The infant was considered infected if the DNA PCR tested positive in at least two different specimens. Infants testing DNA PCR–positive within the first 10 days of life were considered infected in pregnancy, before onset of labor, thus IU-MTCT. Those testing DNA PCR–negative within the first 10 days of life but testing positive at 6 weeks of age were considered infected during labor and/or delivery, thus IP-MTCT. IU-MTCT cases were excluded from the IP-MTCT analysis. IU and IP (perinatal) MTCT cases were excluded in the PP-MTCT analysis.
Upon detection of HIV infection, infants were referred appropriately for immediate commencement of ART, by then, regimen comprised the protease inhibitors Lopinavir/ritonavir and NRTIs, Zidovudine, and Lamivudine.
Data were entered and managed using Research Electronic Data Capture (REDCap v 8.0, © 2020); https://www.redcap.uzchs.ac.zw/redcap/. Quality assurance on the accuracy of data entry included independent double entries and verification in case of discrepancies.
Continuous variables were presented as medians and interquartile ranges (IQR). Standard clinical thresholds of 350 cells/µl for absolute maternal CD4+ T-lymphocyte counts and >11 g/dl for hemoglobin levels were used. Different thresholds of HIV RNA load: ≤50 (undetectable VL), 50–1,000 (low-level viremia), >1,000 (unsuppressed VL) (42), including 10,000 copies/ml, were assessed in univariate analysis, and threshold of only >1,000 copies/ml was included in the regression analysis.
Categorical variables were presented as frequencies and percentages.
To investigate the role of ART, we categorized use as >126 days or sub-optimum (≤126 days) by the time of delivery as previously described (6). Differences in the groups of women; non-transmitters versus transmitters after stratification by timing of MTCT: IU, IP, and PP, were tested by Kruskal–Wallis, χ2, or Fishers exact tests, where appropriate.
For postnatal seroconverters, the mid-point between the latest negative and the earliest positive test dates was used as the estimated date of the event of infection, whereas the single random method was used in cases of mothers missing at least two consecutive scheduled study visits, as previously described (43).
For infant outcomes, all infants (including both twins) were included, and for each infant, the respective maternal parameters were used. For maternal outcomes (e.g., prediction of maternal HIV RNA load), the mothers with twin deliveries were counted once.
The probability of HIV free survival was estimated using the Kaplan–Meier curves including assessing the role of VL, >126 days of ART use by the time of delivery, VL >1,000 copies/ml, and exclusive breastfeeding practice with differences between groups assessed using the Log-Rank test and p-values <0.05 deemed statistically significant. In all Kaplan–Meier analyses, infants who never had a positive HIV test were censored at the time of their last negative result, 60 days after breastfeeding cessation or their mother’s date of death, or at 24 months of age, whichever occurred first.
Spearman’s rank test was used to assess the correlation between antenatal VL and pre-treatment infant VL, at the time of HIV diagnosis, with p-value <0.05 deemed significant.
Multivariate logistic regression analysis was carried out to assess risk factors for MTCT. Variables associated with transmission risk were selected, provided that there was low to no correlation between them using Spearman’s rank correlation, Cramér’s V, and Point-Biserial correlations. Testing for interactions between variables that remained significant was done for PP-MTCT and the overall MTCT. Logistic regression analyses outputs were expressed as odds ratios (ORs) and the respective 95% confidence intervals (CIs). The p-values were two-sided, and associations were considered statistically significant at p <0.05. Automated parameter elimination for optimization of the Akaike information criterion using the “glmulti” R package was done. All statistical analyses were performed in R software version 4.1.1.
A total of 600 HIV-uninfected and 608 HIV-infected pregnant women on mainly TENOLAM-E regimen were enrolled from March 2016 to June 2019.
HIV-infected women were older and had lower level of education and were more likely to be unmarried compared with the HIV-uninfected (Table 1; Supplementary Table 1). In addition, they had three and two times higher rates of stillbirths and neonatal deaths, respectively, compared with their HIV-uninfected counterparts. This was a predominantly breastfeeding cohort with only 1.8% of MLWHA reporting to never had breastfed their HIV-exposed babies (not shown). HIV-uninfected women were more likely to breastfeed their babies beyond 18 months of age (Supplementary Table 1).
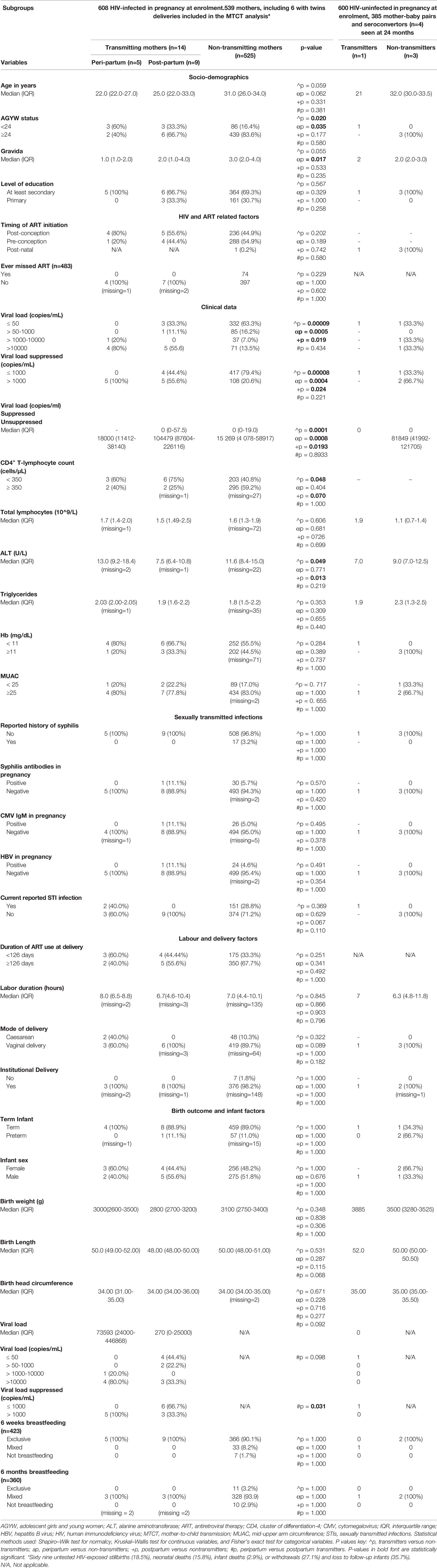
Table 1 Demographic and clinical characteristics, HIV- and ART-related factors, and delivery outcomes including infant factors of HIV-infected transmitting (n = 14) and non-transmitting mothers (n = 525) already infected in pregnancy at enrollment plus four mothers sero-converting after delivery from whom one transmitted at 6 months after seroconversion.
Almost a quarter [300/1,208 (24.8%)] of the pregnant women in our study met the AGYW criterion, and of these 104/300 (34.7%) were already HIV-infected at enrollment, thus constituting up 17.1% (104/608) of all the HIV-infected pregnant women (Supplementary Table 1).
From the 608 HIV-infected women at baseline, 545 together with their exposed babies were assessed for HIV-MTCT, whereas from the 600 HIV-uninfected group, four postnatal seroconverters were recorded. All in all, 15/549 MTCT events were observed, resulting in an overall HIV-MTCT rate of 2.7% (CI:1.3–4.1), with PP-MTCT (60%) being the predominant route, followed by IP-MTCT (26.6%), whereas IU and subsequent postnatal MTCT rates each contributed 6.7% of all infant infections. Thus, the subsequent postnatal MTCT rate was one-fouth (25%). The single postnatal MTCT case was not included in further analyses since her disease course was more of a primary HIV infection relative to generally more chronic HIV in women who were infected before or during pregnancy. However, for comparison sake, the respective solitary results of the transmitting seroconverter are shown alongside with the median (IQR) for the three non-transmitting seroconverters (Table 1). However, no further statistical tests were done due to the small numbers observed.
IU, IP, and PP transmission rates were 0.2%, 0.8%, and 1.8%, respectively (Figure 1), and for the transmission timing, see Figure 2. Sixty-nine (11%) HIV-exposed infants were not tested for HIV due to deaths: stillbirths (18.5%), neonatal deaths (15.8%), infant deaths (2.9%), or withdrawals (27.1%), and loss to follow-up (35.7%). It is plausible that some of the deaths could have been due to the unascertained HIV infection.
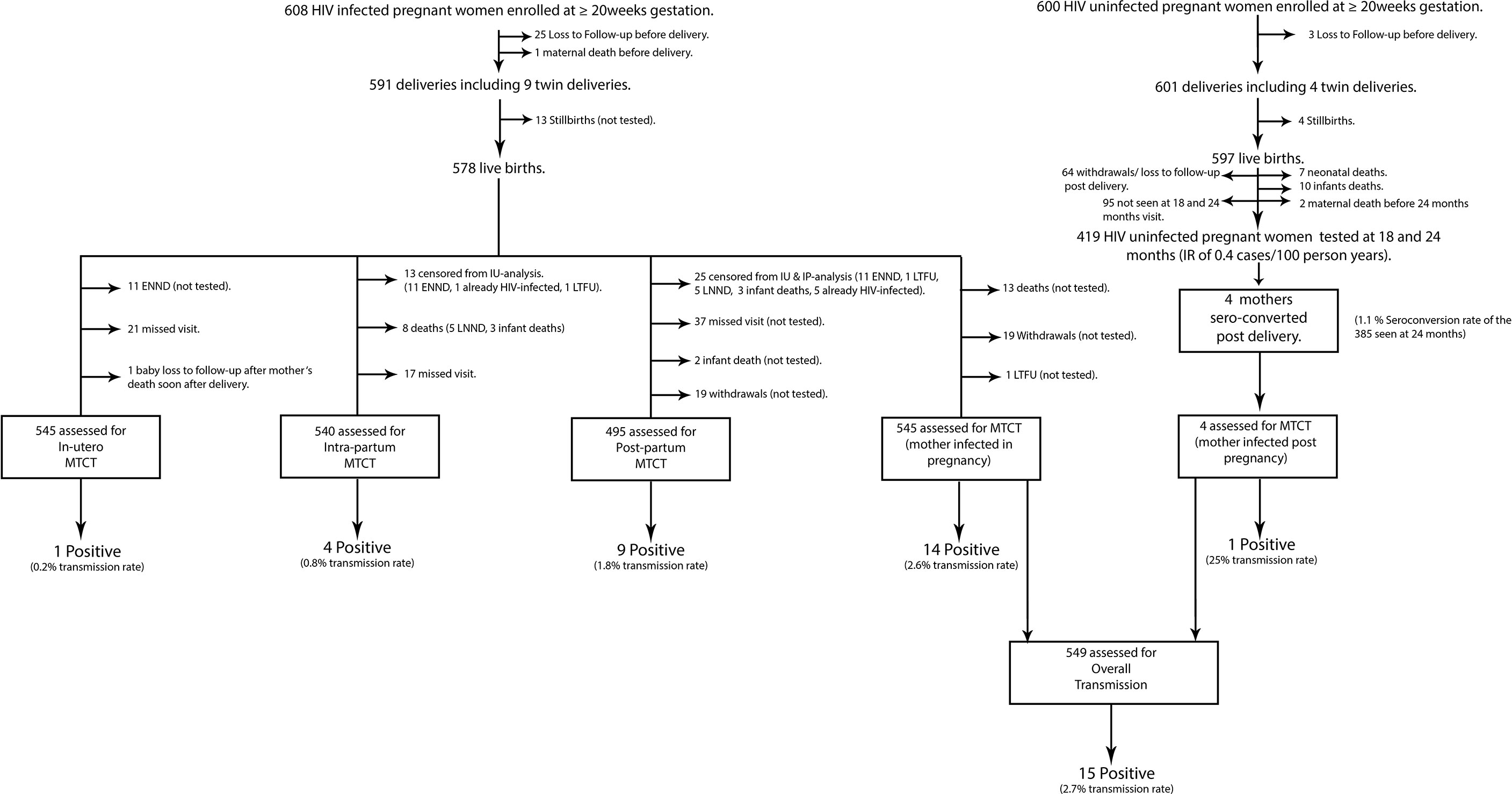
Figure 1 Flow chart showing analysis steps to determine rates of mother-to-child transmission of HIV in women infected in pregnancy and those seroconverting postnatally. HIV, human immunodeficiency virus; ENND, early neonatal deaths (death of a newborn occurring within 7 days after birth); LNND, late neonatal deaths (death of a live born infant within 28 days of life); infant death, death of an infant occurring after 28 days of life; IU-analysis, in-utero analysis; IP-analysis, intra-partum analysis; MTCT, mother-to-child-transmission; LTFU, lost to follow-up.
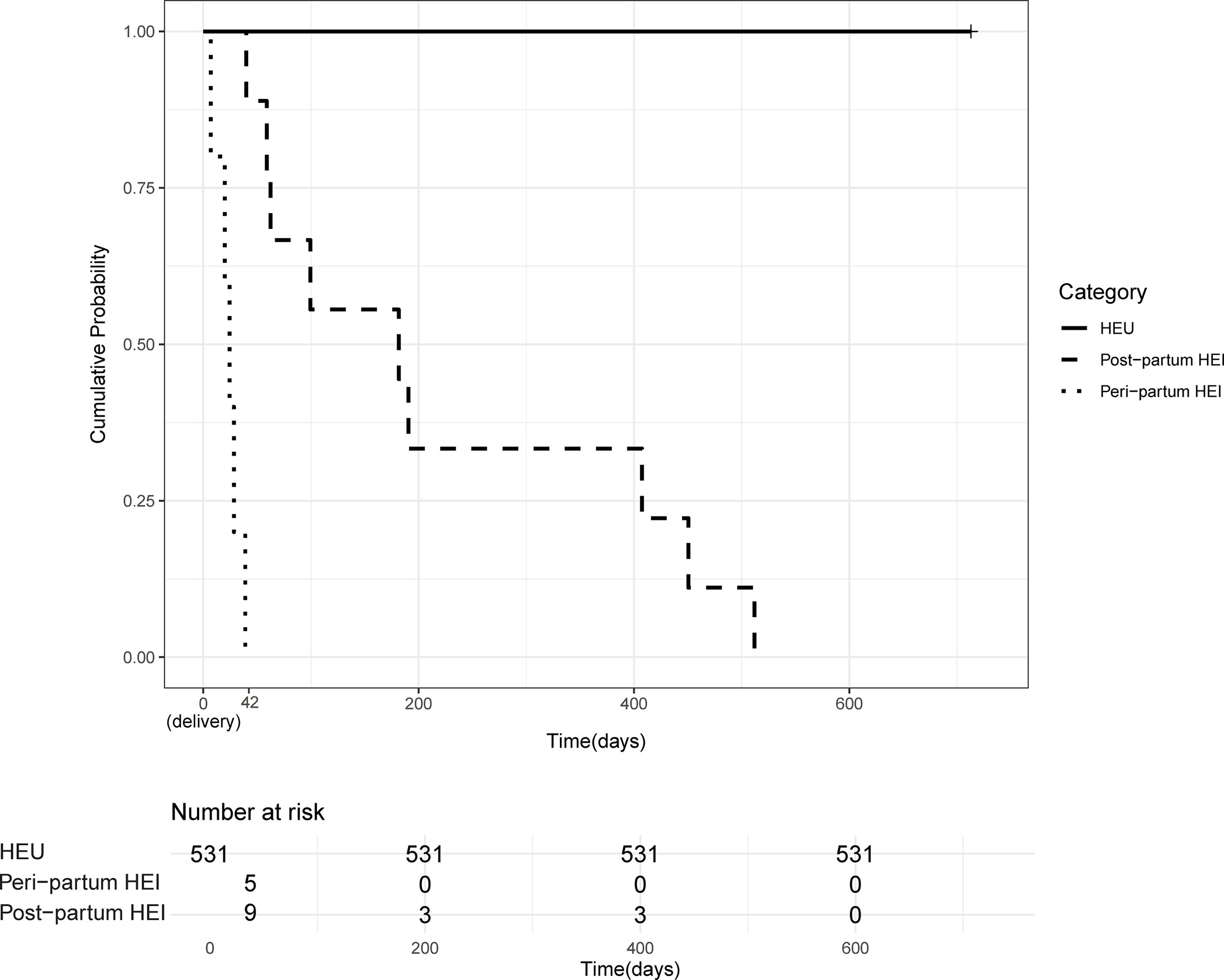
Figure 2 Timing of HIV mother-to-child-transmission. Kaplan–Meier probability of HIV transmission among HIV-exposed infants over the 24-month follow-up period. Cases of HIV-MTCT were grouped according to timing of transmission; peripartum (from birth to 6 weeks of age), postpartum (after 6 weeks of age), and the exposed but uninfected (HEU) groups. Time line in the x axis is in days and time zero is at birth.
In a univariate analysis comparing transmitters who were HIV-infected in pregnancy (n = 14) and non-transmitters also infected in pregnancy (n = 525); 56% transmitters had unsuppressed VL (>1,000 copies/ml) relative to 21% in non-transmitters; p < 0.001. As expected, transmitters were more likely to be immunocompromised with CD4+ T-lymphocyte counts <350 cells/µl (p = 0.048, Table 1).
Interestingly, infected infants’ pre–HIV treatment RNA load correlated positively with antenatal VL, Spearman’s rank correlation: 0.6; p = 0.03 (not shown). There were no differences in infant sex distribution, median birth weight, head circumference, and body length between transmitters and non-transmitters (Table 1).
Alanine transaminase (ALT) levels were significantly lower in transmitting mothers, median IQR 8.0 (6.2–11.0) U/L (against adult normal ranges of 10–45 U/L) versus 11.6 (8.4–15.0) U/L in HIV-infected non-transmitters (p = 0.049, Table 1), and both levels were lower than median IQR of 12.8 (7–14) U/L observed the HIV-uninfected controls (p ≤ 0.001; Supplementary Table 1). Furthermore, a strong negative correlation was observed between ALT levels and plasma antenatal VL (r = −0.087; p = 0.035).
Transmitters were younger, hence generally lower gravida and more likely be anemic with shorter median duration of breastfeeding, all with borderline significance, probably due to the small numbers observed. Otherwise, there were no significant differences between transmitters and non-transmitters with respect to the level of education, timing of ART initiation, or whether fetal exposure to ART was at least 126 days by the time of delivery or not, presence of IgM CMV and syphilis antibodies, MUAC, levels of hemoglobin, the total lymphocyte count, and triglyceride levels late in pregnancy including mode of delivery and duration of labor (Table 1).
There were no differences in the probability of peripartum-MTCT by duration of ART exposure (Supplementary Figure 1), an observation underscoring the importance of adherence not necessarily duration of ART use.
Of the 543 women included in the AGYW-MTCT sub-analysis, the overall MTCT rate was 7/93 (7.5%), versus 1.7% in older women >24 years old (p = 0.008, Table 2). In a univariate analysis, comparing AGYW (n = 93) versus women >24 years old (n = 450), AGYW were more likely to have initiated ART late post-conception and, as expected, had suboptimal duration (<126 days) of ART exposure by the time of delivery and, hence, less likely to be HIV RNA supressed; all variables p < 0.0001 (Table 2). In addition, AGYW were more likely to be undernourished (MUAC <25 cm), p = 0.0002 with approximately $10 less money set aside every month for food compared with their older counterparts (p ≤ 0.0001, Table 2). However, there were no differences in hemoglobin levels, alcohol use in pregnancy, presence of sexually transmitted infections, spouse factors, immune competence, and reported ART adherence, including birth outcomes between the two groups (Table 2). Because of the small MTCT cases, observed predictors of transmission for AGYW were not determined.
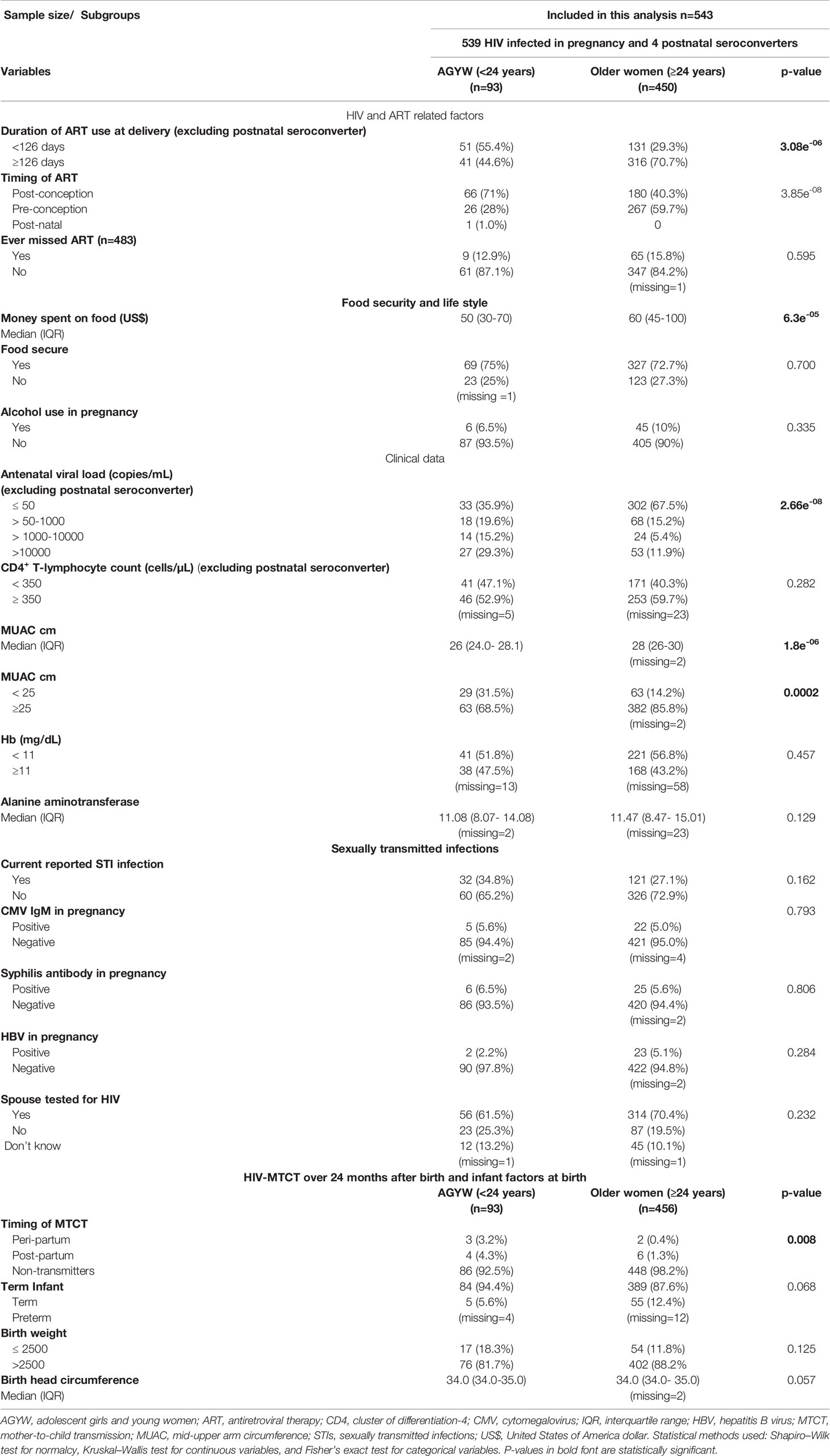
Table 2 Demographic and clinical characteristics, HIV- and ART-related factors, clinical data, presence of sexually transmitted infections, and birth outcome/infant factors of HIV-infected adolescent girls and young women (15–24 years old) versus HIV-infected older women >24 years.
Interestingly, the only postnatal MTCT case observed also fell in the AGYW subgroup (Table 1).
In a multivariable logistic regression analysis for predictors of MTCT comparing transmitters (N = 14) and non-transmitters (N = 525), all women HIV-infected pre/post conception, we first computed complete regression models for risk factors of transmission without variable elimination and further optimized shorter models upon automated variable elimination. The most important predictor for transmission before variable elimination was VL >1,000 copies/ml OR (CI): 11.4 (2.7–90) (p = 0.007) which remained significant even after variable elimination: 9.3; (2.6–43) (p = 0.001). Another relevant predictor was ALT levels, where a unit increase in ALT was associated with a 1.1 times reduced risk of transmission but did not reach statistical significance in both models (Table 3).

Table 3 Predictors of overall and postpartum mother-to-child transmission of HIV in infected pregnant women at least 20 weeks of gestational age without variable elimination (423 observations) and with variable elimination (514 observations).
In a univariate analyses comparing peripartum (IU and IP) transmitters (N = 5) and non-transmitters (525), all peripartum transmitters (100%) had unsuppressed antenatal VL > 1,000 copies/ml versus 21% among non-transmitters (p = 0.0004). Thus, a threshold below 1,000 copies/ml was observed below, which lack of peripartum transmission was ensured. Unsuppressed VL significantly contributed to peripartum MTCT (Figure 3).
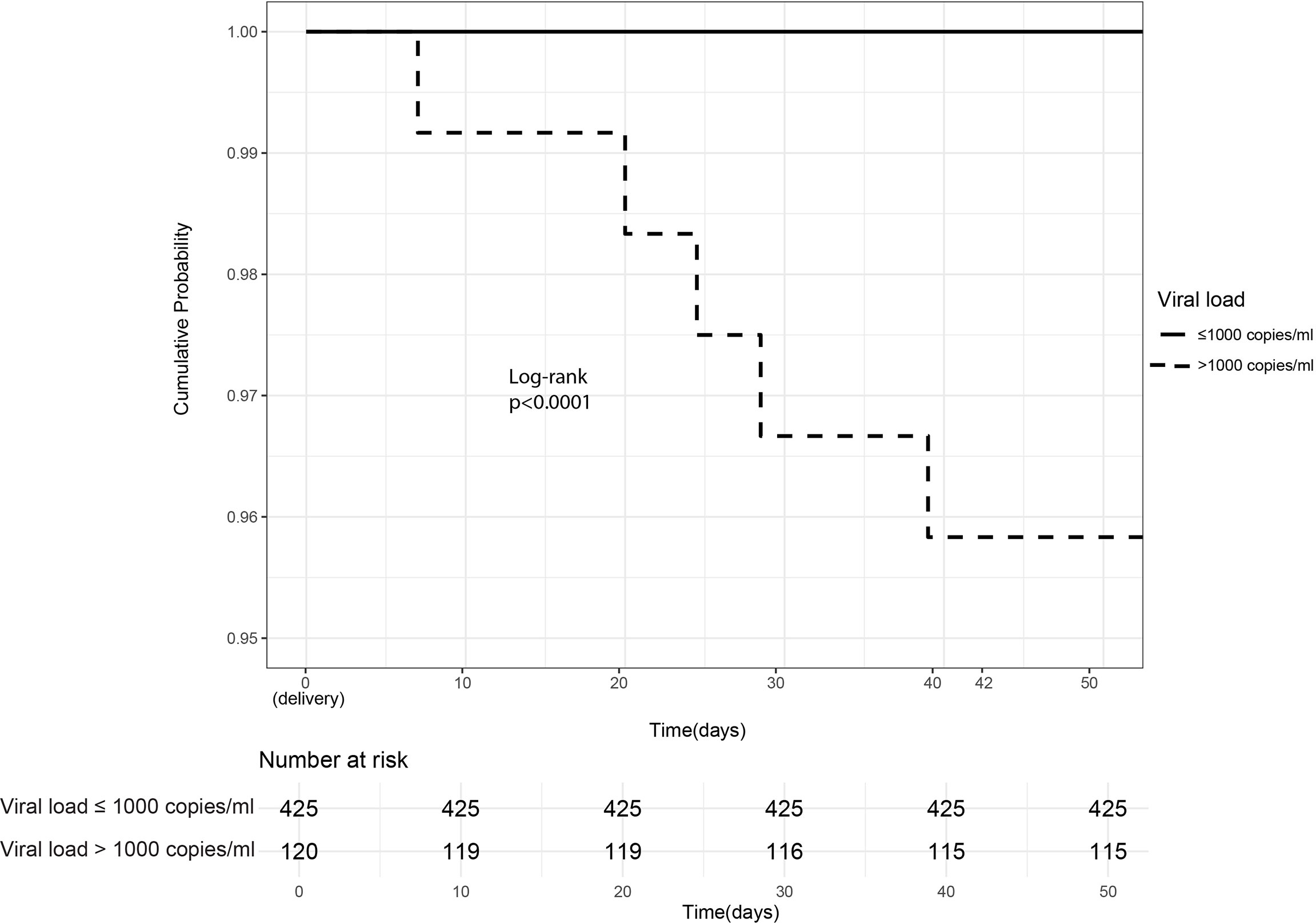
Figure 3 Intrauterine/Intrapartum HIV mother-to-child-transmission. Cumulative probability of peripartum (intrauterine and intrapartum) HIV transmission in infants born of women already HIV-infected during pregnancy stratified by maternal viral load ≤1,000 copies/ml (suppressed) versus >1,000 copies/ml (unsuppressed).
Peripartum transmitters were more likely to be younger with lower gravida, more likely be anemic, and to have cesarean deliveries, all with borderline significance (Table 1). In addition, they were more likely to present with abnormal vaginal discharge or vulval itchiness in pregnancy but also with borderline significance (p = 0.07). Maternal factors in pregnancy and at delivery, as well as infant factors, were comparable between the two groups (Table 1).
In mothers who were HIV-infected in pregnancy whose infants were DNA PCR–negative at 6 weeks, a 1.8% PP transmission rate was observed, thus an incidence rate of 9 PP-MTCT cases/982.8 total child years, resulting in 0.92: 95% CI (0.42–1.7) infant HIV cases per 100 child-years of breastfeeding.
Of the nine PP-MTCT events, six (67%) occurred between 6 weeks and 6 months of life, the period mothers were encouraged to exclusively breastfeed. Thus, the protective effects of exclusive breastfeeding within the first 6 months of life were not observed in our study (Figure 4). Interestingly, there were no PP-MTCT events from about 6 months to ~410 days (13.5 months) of age. The last PP-MTCT events (33%) were observed between 410 days (13 months) and 510 days (17 months), a predominantly mixed feeding period, and no other MTCT events occurred beyond 17 months of age. As expected, none of the seven HEU infants who were not breastfed was infected.
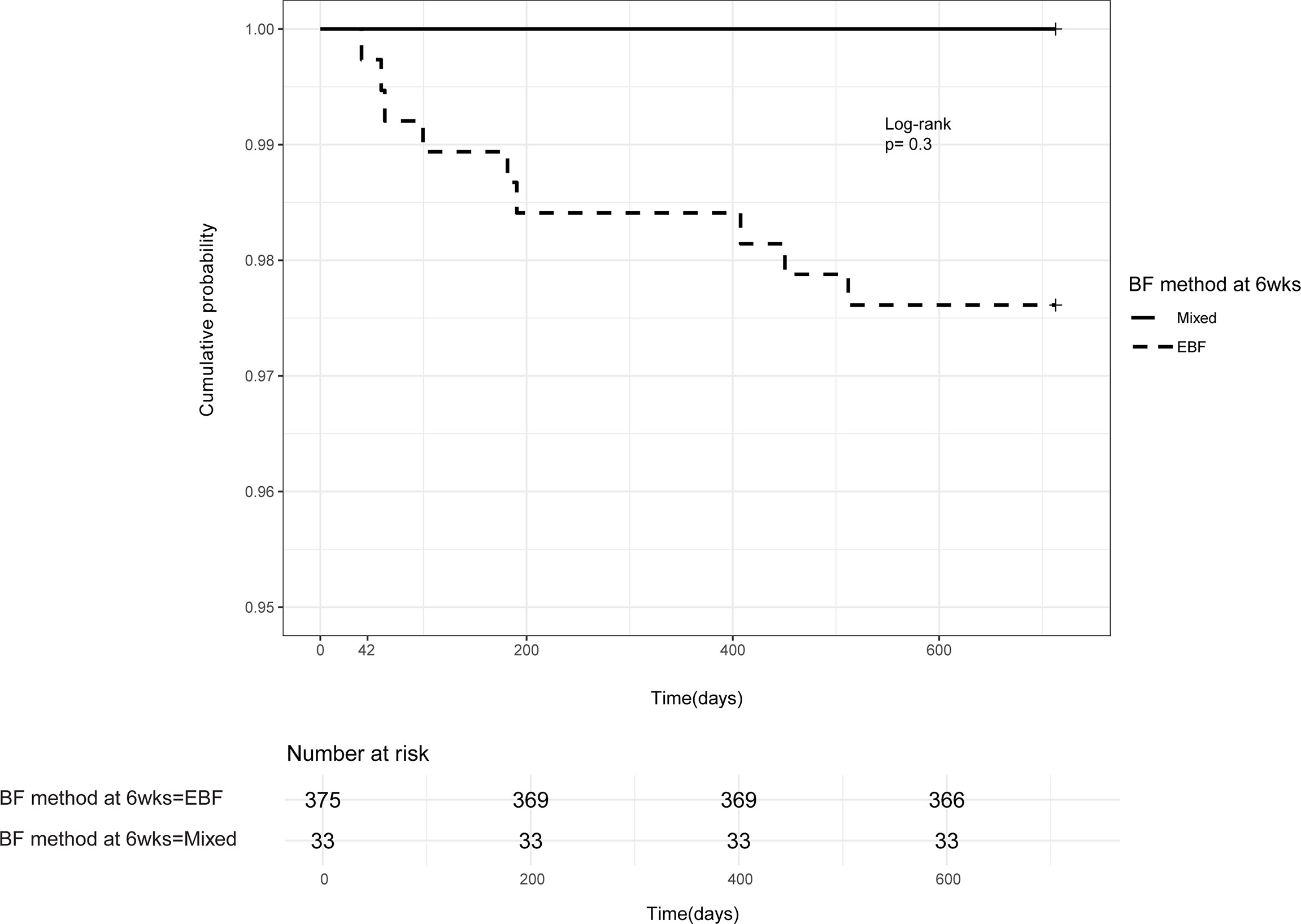
Figure 4 Postpartum HIV mother-to-child-transmission. Kaplan–Meier probability of postpartum HIV transmission in infants born of women already HIV-infected during pregnancy and one woman infected postnatally—based on the method of infant feeding at the 6 weeks follow-up visit. EBF, exclusive breastfeeding; mixed, breastfeeding and complimentary feeds.
In a univariate analyses comparing the nine women who were HIV-infected in pregnancy who transmitted HIV to their infants (who had a negative PCR at 6 weeks) with non-transmitting women (N = 525), the PP-MTCT transmitters were more likely to have HIV RNA levels >1,000 copies/ml and more likely to have lower levels of ALT; median IQR 7.5 (6.4–10.9) U/L (p = 0.013), against adult normal ranges; 10–45 U/L. PP transmitters were less likely to have vaginal discharge during pregnancy, and, as expected, they were not as immunocompetent, both maternal factors with borderline significance (Table 1).
In a multivariable regression analysis without variable elimination of nine PP transmitters versus 525 non-transmitters, VL > 1,000 copies had an OR (CI): 5.4 (0.90–46.3) (p = 0.08), and after variable elimination 7.4 (1.5–54.0) (p = 0.022). Thus the expecting women were 7.4 times more likely to transmit PP if they were not virally suppressed; >1,000 copies/ml late in pregnancy, Table 3.
Other relevant factors observed after variable elimination were CD4 count ≥350 and ALT levels, both without significance. Women with CD4+ T-lymphocytes counts ≥350 were 2.2 times less likely to transmit relative to their immunocompromised peers. Furthermore, a unit increase of ALT was associated with 1.2 reduced odds of PP-MTCT (Table 3).
There were no differences in maternal socio-demographic characteristics, clinical data such CD4+ T-lymphocyte counts, VL, MUAC, presence or absence of sexually transmitted infections, and ART- and HIV-related factors in pregnancy, including delivery and breastfeeding patterns in the univariate analysis between five peripartum transmitters and nine PP transmitters. However, PP-infected infants were more likely to have lower VL, ≤ 1,000 copies/ml at the time of HIV diagnosis (p = 0.031); furthermore, they tended to have shorter birth length (p = 0.098). No further multivariate regression analyses were computed for the IU, IP, and PP due to the relatively lower frequencies observed.
From the 600 HIV-uninfected pregnant women, 385 were seen at 24 months resulting in seroconversion rate of 1.1% (Figure 1).
A total of 419 women were seen up to 24 months after delivery of whom four mothers seroconverted, thus a postnatal HIV incidence of 0.4 cases/100 women-years (95% CI: 0.12–1.1) (Figure 1).
Compared with non-seroconverters, postnatal seroconverters were less likely to be aware of their spouse/intimate partner’s HIV status in pregnancy and more likely to have children/pregnancies not sharing same fathers (p = 0.022 and p = 0.008, respectively; Supplementary Table 2).
Unlike the three non-transmitting seroconverters, the single postnatal seroconverter who transmitted to her infant was younger; 21 years versus median IQR of 32 (30–33.5), hemoglobin, and ALT levels were lower and had an abnormal vaginal discharge in pregnancy (Table 1). The seroconversion interval was 19 weeks after delivery; the midpoint between the last negative HIV test results was at 14 weeks and the first seropositive HIV test observed at 6 months PP. She initiated ART after 6 months of HIV diagnosis. The male term, normal birth weight baby, received mixed feeds at 8 weeks of age and was HIV-infected at 12 months of age. The total child years of follow-up was 7.5, resulting in a postnatal incidence rate of 12.8 CI: (0.33–71.43) of infant HIV infections per 100 child-years of breastfeeding; a rate 14 times higher compared with PP-MTCT rate of babies who were DNA PCR–negative at six weeks of age.
Interestingly, other than drastically reducing the overall MTCT rate to 2.7%, ART had a positive impact on mortality, as evidenced by no differences in maternal and infant mortality rates at 24 months in HIV-infected women and their infants compared with their HIV uninfected and their respective unexposed babies (p = 1.000 and p = 0.163 respectively; Supplementary Table 1).
In our cohort of 608 HIV-infected and 600 HIV-uninfected pregnant women, the overall MTCT rate was 2.7%, a figure much lower compared with 3.7% observed during the short dose ART era (29). Encouragingly, this 2.7% rate is well below the joint United Nations Programme on HIV/AIDS global targets for MTCT elimination of <5% final MTCT (44).
When stratified by timing of maternal HIV diagnosis, the MTCT rate for the women who were HIV-infected in pregnancy was 2.6%, again well below the global target for MTCT elimination. Our estimate is much lower than the 4.3% observed in a South African cohort of HIV positive pregnant women (45), but higher than the cumulative MTCT rate of 1.6% observed within the first 6 months of life in a recent Harare cohort of 451 HIV-infected pregnant women (46). Should we evaluate our MTCT rate limiting the analysis to the first 6 months of life, our cumulative MTCT rate becomes 2.1%, quite comparable to the rate of 1.6% observed in this recent study. In the same study, IU-MTCT was the major route of transmission, whereas in our study, PP-MTCT through breastfeeding was the major route: 1.8% versus 0.44%. Differences could be the shorter follow-up periods reported: 6 months versus our 24 months.
The postnatal MTCT rate was 25%, a figure almost five times higher than the global MTCT elimination target of <5%. Our postnatal MTCT rate of 25% was comparable to the 21%–28% observed in a South African, Malawian, and Cameroonian breastfeeding cohort (47–49), disproportionately occurring in women with a last positive HIV test result recorded during the breastfeeding period (49).
The median postnatal case rate of 1,280 infant HIV infections per 100,000 live births observed on our study is much higher than targeted global case rate of ≤50 new pediatric HIV infections/100,000 live births. Our case rate is comparable to the 1,290 postnatal MTCT case rates observed in a South African study also done during this era of Option B+. In the same study, the postnatal MTCT was 1.7% which is also comparable to ours at 1.1% (45, 50). These observations underscore the critical need to stop new postnatal HIV infections. The health delivery system/policymakers should make concerted effort to continuously counsel and educate HIV-uninfected women on the risks of MTCT.
Antenatal HIV RNA > 1,000 copies/ml was independently associated with MTCT risk: OR (CI): 9.3 (2.6–43.1), thus complementing previous findings (49, 51–53). A Brazilian study showed an almost similar degree of risk but the only difference was most of babies were infected during the IU and not PP-MTCT as in our case study (54).
In our study, interestingly, none of the mothers with low-level viremia VL: 51–1,000 copies/ml late in pregnancy transmitted perinatally. Low-level viremia has been shown to have positive clinical significance in terms of reduced MTCT rates (55). It is therefore imperative that MLWHA commence ART in early pregnancy to suppress HIV RNA to undetectable levels as rapidly as possible to reduce the risk of MTCT (56). Viral suppression rates might now improve with the new TENOLAM-D regimen (Dolutegravir), currently being offered since 2018, which has been shown to suppress VL more rapidly with at least a 2log10 decrease by week 2 of therapy (57).
Infected infants’ pre–HIV treatment RNA load positive correlation with antenatal VL observed in our study resonated well with previous reports hypothesizing that maternal ART use during pregnancy may mask the severity of disease in infected infants (58).
Since VL evaluation was done once late in pregnancy, it is plausible that adherence issues could have negatively affected viral suppression after delivery as the PP-MTCT rate was the highest in our study. Composite adherence measures such as combining self-report with tablet recount or pharmacy refill records would have been more accurate. A review of large studies in South Africa, Kenya, Zambia, and even in the USA observed that about 76% of pregnant women adhered to ART in pregnancy but proportions fall to only 53% post-delivery (59). Furthermore, ART initiated in pregnancy suppresses breast milk cell free HIV-1 RNA but has no effect on cell associated DNA in breast milk (60). Thus, ensuring HIV-infected women of child bearing age have a VL of 1,000 copies/ml before conception and continue to maintain viral suppression during pregnancy and breastfeeding is crucial if elimination of MTCT of HIV is to be achieved
In our study, we did not observe the protective effect of exclusive breast feeding probably because of too few MTCT events observed. It also possible that mothers are misreported practicing exclusive breastfeeding while introducing early mixed feeds. A previous huge study done during the pre-ART era showed that 25% of MLWHA never practiced exclusive breastfeeding (61). The practice of exclusive breastfeeding is not common as it is a cultural norm to introduce complementary feeds quite early in Zimbabwe (62). It remains critical to identify means of making breastfeeding safer for HIV-infected women who have no choice other than to continue breastfeeding. Concerted efforts are required to retain HIV-infected women in PMTCT programs from conception, delivery until weaning.
As expected, transmitters were more likely to be immunocompromised with CD4 count less that 350 cells/µl relative to non-transmitters as previously reported (63).
Elevated ALT (defined as >40 IU/L) has been observed in HIV-infected persons, even without viral hepatitis infection, and remains a major concern for the HIV-infected population (64). Such trend seem to be inconsistent our study findings, where ALT levels were highest in the HIV-uninfected (Supplementary Table 1).
Interestingly, ALT levels differed significantly between transmitters and non-transmitters. Previous studies demonstrated that serum ALT levels decrease following initiating antiretroviral treatment in HIV-infected patients (65). In our study, we observed a strong negative correlation between ALT and plasma antenatal VL, implying that the deranged serum ALT levels in addition to its utility as a biomarker for hepatic damage could be a relatively simple and cheaper diagnostic and/or prognostic biomarker for viral suppression. However, in our regression models, ALT is protective against transmission but did not reach statistical significance in both models, hence not much can be hypothesized. Normal reference values of biochemical assays for pregnant African women and in new-borns are needed to guide HIV care and treatment programs to help interpret clinical data and assist in patient monitoring in a Sub-Saharan African context.
Poorer economic circumstances including nutritional outcomes in AGYW could be due to the fact that AGYW are less likely to be in established positions in family structures, with less economic resources within their control, thus making them more vulnerable, and may in turn lead to a lower adherence to ART. This underscores the need for education and social support systems including economic empowerment for the AGYW, and policymakers should allocate resources, depending on the level of risk and shun one-size-fits-all HIV prevention strategies.
Our study has several strengths and limitations. The main strength of this study relatively large sample size with appropriate controls and HIV-positive non-transmitters alongside HIV-negative pregnant women recruited simultaneously from the same community. Thus, all research participants reside in highly similar environmental conditions in high-density areas of Harare, resulting in an unusually homogenous study population. The assessment of both pre/post conception and postnatal MTCT rates, together with the evaluation of pre-treatment infants’ HIV RNA load are unique aspects of our study. The novelty include describing MTCT rate and risk factors in the vulnerable AGYW population constituting about 25% of the HIV-infected population with unmet needs in the fight against HIV/AIDS. In addition, a threshold below 1,000 copies/ml was observed below which lack of peripartum transmission was ensured.
However, maternal HIV load, the most significant factor determining HIV-MTCT, was assessed once in pregnancy. It would have been more edifying if plasma VL was also evaluated 6 weeks after delivery to effectively assess the trend in HIV RNA load and possibly better explain the later PP-MTCT events. Sixty-nine exposed infants were not tested for HIV due to deaths or withdrawals or loss to follow-up; hence, we may have underestimated the MTCT rates.
A mother was 9.3 times more likely to transmit if her HIV RNA was >1,000 copies/ml and levels positively correlated with infant’s pre-treatment HIV RNA load. Breastfeeding-associated PP-MTCT remains high. It is therefore imperative that HIV-infected women commence and adhere to ART early in pregnancy until weaning to suppress HIV RNA to reduce the risk of MTCT and reduce the severity of disease in infected infants. HIV-uninfected lactating mothers should be continuously counseled on the risks of postnatal seroconversion. Our observations underscore the critical need to stop new maternal HIV infections and roll out effective PMTCT programs for MLWHA to mitigate MTCT. In particular, it is critical to address the socio-economic disadvantages observed in AGYW by crafting, implementing, monitoring, and evaluating young people–friendly policies for HIV prevention as they transition into adulthood.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Joint Research Ethics Committee (JREC) of the University of Zimbabwe (reference number: JREC/18/15) and the Medical Research Council of Zimbabwe (MRCZ/A/1968). The participants provided their written informed consent to participate in this study.
The study was conceived by KD and FZG designed by KD, LRM and FZG. PTM, PC, HM, AJM, SB were responsible for data collection, entry and validation overseen by KD and FZG. PTM, HM, PC and AJM performed the statistical analysis. KD, PTM, HM, PC and AJM were involved in the interpretation of findings. The draft manuscript was written by KD. All authors contributed to the article and approved the submitted version.
The study was supported by the Wellcome Trust under the University of Zimbabwe College of Health Sciences Southern Africa Consortium for Research Excellence (SACORE) grant number 087537/F/08/A. The funding body was not involved in the study design, data collection, and analysis nor in the interpretation of data and manuscript writing. Supplemental sponsorships was from the Norwegian Programme for Capacity Development in Higher Education and Research for Development under the University of Zimbabwe NORHED grant (NORHED QZA-0484MWI-13/0032), Botnar Foundation and the Department of Visceral Surgery and Medicine, Bern University, Switzerland. None of the funding bodies were or will be involved in the study design, data collection, data analysis, interpretation of findings, and/or manuscript writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the research participants of the UZ-CHS birth cohort study and the research support team for their commitment; Professor E. Gomo (Medical laboratory Sciences Unit); Dr. A. Ziruma and Dr. T. Marere (Obstetrics and Gynaecology Unit); Dr. G.Q. Kandawasvika, Dr. S. Chimhuya, and Dr. P. Kuona (Paediatrics and Child care Unit); and Ms. E. Mazengera, Mr. N. Taremeredzwa, Sr. M.M. Chipiti, Sr. N. Sibiya, and Sr. M. Ngoweni (Immunology Unit). We are very grateful to Professor Benjamin Misselwitz and Dr. Bruno Sebastian Jordi for the capacity building in data analysis using R in our Immunology Unit.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2022.906271/full#supplementary-material
AGYW, adolescence girls and young women; ART, antiretroviral therapy; CD4, cluster of differentiation 4; CI, confidence interval; HIV, human immunodeficiency virus; IP, intrapartum; IQR, interquartile range; IU, intrauterine; MTCT, mother-to-child transmission; MLWHA, mothers living with HIV/AIDs; MUAC, mid-upper arm circumference; PP, postpartum; RNA, ribonucleic acid; RT-PCR, real-time polymerase chain reaction; UZBCS, University of Zimbabwe Birth Cohort Study; VL, viral load.
1. Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the Timing of HIV Transmission From Mother to Infant. JAMA (2001) 285(6):709–12. doi: 10.1001/jama.285.6.709
2. Leroy V, Newell ML, Dabis F, Peckham C, Van de Perre P, Bulterys M, et al. International Multicentre Pooled Analysis of Late Postnatal Mother-to-Child Transmission of HIV-1 Infection. Ghent International Working Group on Mother-To-Child Transmission of HIV. Lancet (1998) 352(9128):597–600. doi: 10.1016/S0140-6736(98)01419-6
3. Hofer CB, Keiser O, Zwahlen M, Lustosa CS, Frota AC, de Oliveira RH, et al. In Utero Exposure to Antiretroviral Drugs: Effect on Birth Weight and Growth Among HIV-Exposed Uninfected Children in Brazil. Pediatr Infect Dis J (2016) 35(1):71–7. doi: 10.1097/INF.0000000000000926
4. Sofeu CL, Warszawski J, Ateba NF, Penda IC, Tetang NS, Guemkam G, et al. Low Birth Weight in Perinatally HIV-Exposed Uninfected Infants: Observations in Urban Settings in Cameroon. PLoS One (2014) 9(4):e93554. doi: 10.1371/journal.pone.0093554
5. Peters HP, et al. Vertical Transmissions in the UK- Insights and Remaining Challenges. In: 5th Joint Conference of the British HIV Association (BHIVA) With the British Association for Sexual Health and HIV (BASHH). 19–21 April 2021. Poster abstract P027. Available at: https://www.bhiva.org/file/6091178219915/AbstractBook2021.pdf
6. Duri K, Munjoma PT, Mazhandu AJ, Marere T, Gomo E, Banhwa S, et al. Predictors and Timing to Viral Suppression in HIV-Infected Pregnant Women in the University of Zimbabwe Birth Cohort Study During the Era of Lifelong Antiretroviral Therapy (Option B+ Treatment Strategy). Front Virol (2022) 2:838234. doi: 10.3389/fviro.2022.838234.2022
7. UNAIDS. Adolescent Girls and Young Women. (2014). Available online at: https://www.unaids.org/en/resources/documents/2014/Adolescentgirlsandyoungwomen.
8. Kurewa EN, Gumbo FZ, Munjoma MW, Mapingure MP, Chirenje MZ, Rusakaniko S, et al. Effect of Maternal HIV Status on Infant Mortality: Evidence From a 9-Month Follow-Up of Mothers and Their Infants in Zimbabwe. J Perinatol (2010) 30(2):88–92. doi: 10.1038/jp.2009.121
9. Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of Infected and Uninfected Infants Born to HIV-Infected Mothers in Africa: A Pooled Analysis. Lancet (2004) 364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7
10. Pitzen IC, Otten LA, Dresbach T, Boesecke C, Wasmuth JC, Mueller A, et al. [Treatment of HIV in Pregnancy - Progress Over One Decade]. Z Geburtshilfe Neonatol (2019) 223(1):26–32. doi: 10.1055/a-0741-7700
11. Chappuy H, Treluyer JM, Jullien V, Dimet J, Rey E, Fouche M, et al. Maternal-Fetal Transfer and Amniotic Fluid Accumulation of Nucleoside Analogue Reverse Transcriptase Inhibitors in Human Immunodeficiency Virus-Infected Pregnant Women. Antimicrob Agents Chemother (2004) 48(11):4332–6. doi: 10.1128/AAC.48.11.4332-4336.2004
12. Hurst SA, Appelgren KE, Kourtis AP. Prevention of Mother-to-Child Transmission of HIV Type 1: The Role of Neonatal and Infant Prophylaxis. Expert Rev Anti Infect Ther (2015) 13(2):169–81. doi: 10.1586/14787210.2015.999667
13. UNAIDS. AIDS Epidemic Update: Geneva, Joint United Nations Programme on HIV/AIDS. (1998). Available online at: http://data.unaids.org/publications/irc-pub06/epiupdate98_en.pdf
14. Al-Husaini AM. Role of Placenta in the Vertical Transmission of Human Immunodeficiency Virus. J Perinatol (2009) 29(5):331–6. doi: 10.1038/jp.2008.187
15. De Vries BS, Cossart YE, Murray H, Peek MJ. Transplacental Haemorrhage may Explain the Intrapartum Transmission of HIV. A Pilot Study Uses Flow Cytometry to Quantify Maternal Red Blood Cells in Infants Born Vaginally or by Caesarean Section. Aust N Z J Obstet Gynaecol (2008) 48(6):575–9. doi: 10.1111/j.1479-828X.2008.00913
16. Peters H, Byrne L, De RA, Francis K, Harding K, Taylor GP, et al. Duration of Ruptured Membranes and Mother-to-Child HIV Transmission: A Prospective Population-Based Surveillance Study. BJOG (2016) 123(6):975–81. doi: 10.1111/1471-0528.13442
17. Willumsen JF, Filteau SM, Coutsoudis A, Newell ML, Rollins NC, Coovadia HM, et al. Breastmilk RNA Viral Load in HIV-Infected South African Women: Effects of Subclinical Mastitis and Infant Feeding. AIDS (2003) 17(3):407–14. doi: 10.1097/00002030-200302140-00015
18. Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, et al. Human Immunodeficiency Virus Load in Breast Milk, Mastitis, and Mother-to-Child Transmission of Human Immunodeficiency Virus Type 1. J Infect Dis (1999) 180(1):93–8. doi: 10.1086/314854
19. Martinez DR, Tu JJ, Kumar A, Mangold JF, Mangan RJ, Goswami R, et al. Maternal Broadly Neutralizing Antibodies Can Select for Neutralization-Resistant, Infant-Transmitted/Founder HIV Variants. mBio (2020) 11(2):e00176–20. doi: 10.1128/mBio.00176-20
20. Mishra N, Sharma S, Dobhal A, Kumar S, Chawla H, Singh R, et al. Broadly Neutralizing Plasma Antibodies Effective Against Autologous Circulating Viruses in Infants With Multivariant HIV-1 Infection. Nat Commun (2020) 11(1):4409. doi: 10.1038/s41467-020-18225-x
21. Williams WB, Wiehe K, Saunders KO, Haynes BF. Strategies for Induction of HIV-1 Envelope-Reactive Broadly Neutralizing Antibodies. J Int AIDS Soc (2021) 24 Suppl 7:e25831. doi: 10.1002/jia2.25831
22. Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, et al. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses With Reduced Risk of Postnatal Mother-To-Child Transmission of HIV-1. J Virol (2015) 89(19):9952–61. doi: 10.1128/JVI.01560-15
23. Henrick BM, Yao XD, Nasser L, Roozrogousheh A, Rosenthal KL. Breastfeeding Behaviors and the Innate Immune System of Human Milk: Working Together to Protect Infants Against Inflammation, HIV-1, and Other Infections. Front Immunol (2017) 8:1631. doi: 10.3389/fimmu.2017.01631
24. Henrick BM, Yao XD, Drannik AG, Abimiku A, Rosenthal KL. Soluble Toll-Like Receptor 2 is Significantly Elevated in HIV-1 Infected Breast Milk and Inhibits HIV-1 Induced Cellular Activation, Inflammation and Infection. AIDS (2014) 28(14):2023–32. doi: 10.1097/QAD.0000000000000381
25. Wahl A, Baker C, Spagnuolo RA, Stamper LW, Fouda GG, Permar SR, et al. Breast Milk of HIV-Positive Mothers Has Potent and Species-Specific In Vivo HIV-Inhibitory Activity. J Virol (2015) 89(21):10868–78. doi: 10.1128/JVI.01702-15
26. Haberl L, Audebert F, Feiterna-Sperling C, Gillor D, Jakubowski P, Jonsson-Oldenbuttel C, et al. Not Recommended, But Done: Breastfeeding With HIV in Germany. AIDS Patient Care STDS (2021) 35(2):33–8. doi: 10.1089/apc.2020.0223
27. World Health Organisation. Guidelines Om HIV and Infant Feeding. Principles and Recommendations for Infant Feeding in the Context of HIV and a Summary of Evidence. In: Geneva: World Health Organisation, vol. 2010. (2010). Available online at: http://apps.who.int/iris/bitstream/handle/10665/44345/9789241599535_eng.pdf;jsessionid=70A1E5407E684F082D48FBA0B0A859AA?sequence=1
28. Creek TL, Kim A, Lu L, Bowen A, Masunge J, Arvelo W, et al. Hospitalization and Mortality Among Primarily Nonbreastfed Children During a Large Outbreak of Diarrhea and Malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr (2010) 53(1):14–9. doi: 10.1097/QAI.0b013e3181bdf676
29. Shepherd BL, Ferrand R, Munyati S, Folkard S, Boyd K, Bandason T, et al. HLA Correlates of Long-Term Survival in Vertically Infected HIV-1-Positive Adolescents in Harare, Zimbabwe. AIDS Res Hum Retroviruses (2015) 31(5):504–7. doi: 10.1089/aid.2014.0338
30. World Data Atlas Zimbabwe Health (2019). Available online at: https://knoema.com/atlas/Zimbabwe/topics/Health.
31. World Data Atlas Zimbabwe. Zimbabwe Mortality Rates. (2018) Available online at: https://knoema.com/atlas/Zimbabwe/topics/Health/Health-Status/Infant-mortality-rate
32. UNAIDS. UNAIDS DATA 2018. (2018). Available online at: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf
33. UNAIDS. UNAIDS 2021 Estimates (2000-2020). (2021). Available online at: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf.
34. Zimbabwe Ministry of Health. Zimbabwe Ministry of Health and Child Care, 2012. In: Prevention of Mother to Child Transmission of HIV 2012 Annual Report (2012). Available online at: https://medbox.org/document/prevention-of-mother-to-child-transmission-of-hiv-2012-annual-report#GO
35. Munjoma MW, Mhlanga FG, Mapingure MP, Kurewa EN, Mashavave GV, Chirenje MZ, et al. The Incidence of HIV Among Women Recruited During Late Pregnancy and Followed Up for Six Years After Childbirth in Zimbabwe. BMC Public Health (2010) 10:668. doi: 10.1186/1471-2458-10-668
36. Duri K, Gumbo FZ, Munjoma PT, Chandiwana P, Mhandire K, Ziruma A, et al. The University of Zimbabwe College of Health Sciences (UZ-CHS) BIRTH COHORT Study: Rationale, Design and Methods. BMC Infect Dis (2020) 20(1):725. doi: 10.1186/s12879-020-05432-6
37. Roy NC. Use of Mid-Upper Arm Circumference for Evaluation of Nutritional Status of Children and for Identification of High-Risk Groups for Malnutrition in Rural Bangladesh. J Health Popul Nutr (2000) 18(3):171–80.
38. Fakier A, Petro G, Fawcus S. Mid-Upper Arm Circumference: A Surrogate for Body Mass Index in Pregnant Women. S Afr Med J (2017) 107(7):606–10. doi: 10.7196/SAMJ.2017.v107i7.12255
39. Tosif S, Jatobatu A, Maepioh A, Subhi R, Francis KL, Duke T. Cause-Specific Neonatal Morbidity and Mortality in the Solomon Islands: An Assessment of Data From Four Hospitals Over a Three-Year Period. J Paediatr Child Health (2020) 56(4):607–14. doi: 10.1111/jpc.14699
40. WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment Of Severity. In: Vitamin and Mineral Nutrition Information System, vol. 2011. . Geneva: World Health Organization (2011).
41. Howie SR. Blood Sample Volumes in Child Health Research: Review of Safe Limits. In: Bull World Health Organ (2011) 89:46–53. doi: 10.2471/BLT.10.080010
42. World Health Organisation. Updated Recommentation on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring (2021). Available online at: https://www.who.int/publications/i/item/9789240022232
43. Vandormael A, Dobra A, Barnighausen T, de OT, Tanser F. Incidence Rate Estimation, Periodic Testing and the Limitations of the Mid-Point Imputation Approach. Int J Epidemiol (2018) 47(1):236–45. doi: 10.1093/ije/dyx134
44. World Health Organization. Global Guidance on Criteria and Processes for Validation: Elimination of Mother-to-Child Transmission (EMTCT) of HIV and Syphilis. World Health Organization (2014). Available at: https://apps.who.int/iris/handle/10665/112858
45. Goga AE, Lombard C, Jackson D, Ramokolo V, Ngandu NK, Sherman G, et al. Impact of Breastfeeding, Maternal Antiretroviral Treatment and Health Service Factors on 18-Month Vertical Transmission of HIV and HIV-Free Survival: Results From a Nationally Representative HIV-Exposed Infant Cohort, South Africa. J Epidemiol Community Health (2020) 74(12):1069–77. doi: 10.1136/jech-2019-213453
46. Zijenah LS, Bandason T, Bara W, Chipiti MM, Katzenstein DA. Mother-To-Child Transmission of HIV-1 and Infant Mortality in the First Six Months of Life, in the Era of Option B Plus Combination Antiretroviral Therapy. Int J Infect Dis (2021) 109:92–8. doi: 10.1016/j.ijid.2021.06.036
47. le Roux SM, Abrams EJ, Nguyen KK, Myer L. HIV Incidence During Breastfeeding and Mother-to-Child Transmission in Cape Town, South Africa. AIDS (2019) 33(8):1399–401. doi: 10.1097/QAD.0000000000002224
48. Nguefack HL, Gwet H, Desmonde S, Oukem-Boyer OO, Nkenfou C, Tejiokem M, et al. Estimating Mother-to-Child HIV Transmission Rates in Cameroon in 2011: A Computer Simulation Approach. BMC Infect Dis (2016) 16:11. doi: 10.1186/s12879-016-1336-2
49. Chagomerana MB, Edwards JK, Zalla LC, Carbone NB, Banda GT, Mofolo IA, et al. Timing of HIV Testing Among Pregnant and Breastfeeding Women and Risk of Mother-to-Child HIV Transmission in Malawi: A Sampling-Based Cohort Study. J Int AIDS Soc (2021) 24(3):e25687. doi: 10.1002/jia2.25687
50. Goga AE, Van de Perre P, Ngandu N, Nagot N, Abrams EJ, Moodley D, et al. Eliminating HIV Transmission Through Breast Milk From Women Taking Antiretroviral Drugs. BMJ (2021) 374:n1697. doi: 10.1136/bmj.n1697
51. Technau KG, Kalk E, Coovadia A, Black V, Pickerill S, Mellins CA, et al. Timing of Maternal HIV Testing and Uptake of Prevention of Mother-to-Child Transmission Interventions Among Women and Their Infected Infants in Johannesburg, South Africa. J Acquir Immune Defic Syndr (2014) 65(5):e170–8. doi: 10.1097/QAI.0000000000000068
52. Myer L. Initiating Antiretroviral Therapy in Pregnancy: The Importance of Timing. J Acquir Immune Defic Syndr (2011) 58(2):125–6. doi: 10.1097/QAI.0b013e31822ad573
53. Duri K, Gumbo FZ, Kristiansen KI, Kurewa NE, Mapingure MP, Rusakaniko S, et al. Antenatal HIV-1 RNA Load and Timing of Mother to Child Transmission; a Nested Case-Control Study in a Resource Poor Setting. Virol J (2010) 7:176. doi: 10.1186/1743-422X-7-176
54. Menegotto M, Magdaleno AM, da Silva CLO, Friedrich L, da Silva CH. Mother-To-Child HIV Transmission Among Pregnant Women in a City With the Highest Rates of HIV in Brazil. Am J Perinatol (2021) 10.1055/s-0040-1722605. doi: 10.1055/s-0040-1722605
55. Duri K, Chimhuya S, Gomo E, Munjoma PT, Chandiwana P, Yindom LM, et al. Role of Antenatal Plasma Cytomegalovirus DNA Levels on Pregnancy Outcome and HIV-1 Vertical Transmission Among Mothers in the University of Zimbabwe Birth Cohort Study (UZBCS). Virol J (2021) 18(1):30. doi: 10.1186/s12985-021-01494-3
56. Patel M, Tedaldi E, Armon C, Nesheim S, Lampe M, Palella F Jr, et al. HIV RNA Suppression During and After Pregnancy Among Women in the HIV Outpatient Study, 1996 to 2015. J Int Assoc Provid AIDS Care (2018) 17:2325957417752259. doi: 10.1177/2325957417752259
57. Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, et al. Safety and Pharmacokinetics of Dolutegravir in Pregnant Mothers With HIV Infection and Their Neonates: A Randomised Trial (DolPHIN-1 Study). PLoS Med (2019) 16(9):e1002895. doi: 10.1371/journal.pmed.1002895
58. Patel F, Shiau S, Strehlau R, Shen Y, Burke M, Paximadis M, et al. Low Pretreatment Viral Loads in Infants With HIV in an Era of High-Maternal Antiretroviral Therapy Coverage. Pediatr Infect Dis J (2021) 40(1):55–9. doi: 10.1097/INF.0000000000002897
59. UNAIDS. Miles to Go: Global AIDS Update, (2018). Available online at: https://www.unaids.org/en/20180718_GR2018#:~:text=Miles%20to%20go%20%2D%20Global%20AIDS%20update%202018%20%7C%20UNAIDS&text=GENEVA%2C%2018%20July%202018%E2%80%94UNAIDS,is%20at%20a%20precarious%20point
60. Shapiro RL, Ndung'u T, Lockman S, Smeaton LM, Thior I, Wester C, et al. Highly Active Antiretroviral Therapy Started During Pregnancy or Postpartum Suppresses HIV-1 RNA, But Not DNA, in Breast Milk. J Infect Dis (2005) 192(5):713–9. doi: 10.1086/432489
61. Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early Exclusive Breastfeeding Reduces the Risk of Postnatal HIV-1 Transmission and Increases HIV-Free Survival. AIDS (2005) 19(7):699–708. doi: 10.1097/01.aids.0000166093.16446.c9
62. Piwoz EG, Iliff PJ, Tavengwa N, Gavin L, Marinda E, Lunney K, et al. An Education and Counseling Program for Preventing Breast-Feeding-Associated HIV Transmission in Zimbabwe: Design and Impact on Maternal Knowledge and Behavior. J Nutr (2005) 135(4):950–5. doi: 10.1093/jn/135.4.950
63. Nydal SM, Munyaw Y, Bruun JN, Brantsaeter AB. Achievements and Challenges in the Prevention of Mother-To-Child Transmission of HIV-A Retrospective Cohort Study From a Rural Hospital in Northern Tanzania. Int J Environ Res Public Health (2021) 18(5):2751. doi: 10.3390/ijerph18052751
64. Alghamdi S, Alrbiaan A, Alaraj A, Alhuraiji A, Alghamdi M, Alrajhi A. Elevated Alanine Aminotransferase Levels in HIV-Infected Persons Without Hepatitis B or C Virus Coinfection. Ann Saudi Med (2016) 36(4):288–91. doi: 10.5144/0256-4947.2016.288
Keywords: in utero HIV transmission, intrapartum HIV transmission, postpartum HIV transmission rates and risk factors, breastfeeding population during option B+ era, postnatal HIV seroconversion and HIV transmission, infants’ pretreatment viral load, adolescent girls and young women
Citation: Duri K, Mataramvura H, Chandiwana P, Mazhandu AJ, Banhwa S, Munjoma PT, Mazengera LR and Gumbo FZ (2022) Mother-to-Child Transmission of HIV Within 24 Months After Delivery in Women Initiating Lifelong Antiretroviral Therapy Pre/Post-Conception or Postnatally; Effects of Adolescent Girl and Young Woman Status and Plasma Viremia Late in Pregnancy. Front.Virol. 2:906271. doi: 10.3389/fviro.2022.906271
Received: 28 March 2022; Accepted: 20 May 2022;
Published: 14 July 2022.
Edited by:
Caroline T. Tiemessen, National Institute of Communicable Diseases (NICD), South AfricaReviewed by:
Ria Goswami, Cornell University, United StatesCopyright © 2022 Duri, Mataramvura, Chandiwana, Mazhandu, Banhwa, Munjoma, Mazengera and Gumbo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerina Duri, a2R1cmlAbWVkc2NoLnV6LmFjLnp3; a2VyaW5hLmR1cmlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.