
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virol. , 11 March 2022
Sec. Fundamental Virology
Volume 2 - 2022 | https://doi.org/10.3389/fviro.2022.861628
This article is part of the Research Topic HIV/SIV basic research update View all 10 articles
 Christopher B. Whitehurst1,2†‡
Christopher B. Whitehurst1,2†‡ Monica Rizk3,4,5†
Monica Rizk3,4,5† Adonay Teklezghi3,4,5
Adonay Teklezghi3,4,5 Rae Ann Spagnuolo3,4,5
Rae Ann Spagnuolo3,4,5 Joseph S. Pagano1,2,6
Joseph S. Pagano1,2,6 Angela Wahl3,4,5*
Angela Wahl3,4,5*In most individuals, EBV maintains a life-long asymptomatic latent infection. However, EBV can induce the formation of B cell lymphomas in immune suppressed individuals including people living with HIV (PLWH). Most individuals who acquire HIV are already infected with EBV as EBV infection is primarily acquired during childhood and adolescence. Although antiretroviral therapy (ART) has substantially reduced the incidence of AIDS-associated malignancies, EBV positive PLWH are at an increased risk of developing lymphomas compared to the general population. The direct effect of HIV co-infection on EBV replication and EBV-induced tumorigenesis has not been experimentally examined. Using a humanized mouse model of EBV infection, we demonstrate that HIV co-infection enhances systemic EBV replication and immune activation. Importantly, EBV-induced tumorigenesis was augmented in EBV/HIV co-infected mice. Collectively, these results demonstrate a direct effect of HIV co-infection on EBV pathogenesis and disease progression and will facilitate future studies to address why the incidence of certain types of EBV-associated malignancies are stable or increasing in ART treated PLWH.
Epstein-Barr Virus (EBV) is a ubiquitous virus infecting over 90% of adults in developed and developing countries (1). EBV is an oncogenic herpesvirus that primarily infects B cells (2–4). Primary infection is characterized by the rapid proliferation of infected B cells until an effective immune response is elicited and/or EBV-infected B cells differentiate into latently-infected memory-like cells establishing a life-long infection of the B cell compartment (2–4).
In most individuals, EBV maintains a latent infection with intermittent periods of subclinical virus reactivation and shedding (5). However, EBV can induce the formation of B cell lymphomas in immune suppressed individuals including people living with HIV (PLWH) (4, 6, 7). Most individuals who acquire HIV are already infected with EBV as EBV infection is primarily acquired during childhood and adolescence (1). Without any interventions, EBV positive PLWH have over a 60-fold higher risk of developing lymphomas compared to the general population (8). While antiretroviral therapy (ART) use has significantly decreased the incidence of AIDS-associated malignancies in PLWH, the incidence of certain types of EBV-associated cancers has remained elevated in PLWH (9–11). HIV mediated immune dysfunction may contribute to lymphomagenesis (12). A better understanding of how HIV co-infection affects EBV replication and tumorigenesis would facilitate the development and testing of novel interventions to ameliorate EBV-associated pathologies in PLWH.
Here, a humanized mouse model of EBV infection was used to evaluate the effect of HIV co-infection on EBV replication and tumorigenesis in vivo. Results demonstrate that HIV co-infection enhanced systemic EBV replication in vivo resulting in higher levels of virus in the peripheral blood and tissues. HIV co-infection also resulted in an enhancement of EBV-mediated CD8+ T cell activation. Importantly, data directly demonstrated that HIV co-infection augmented EBV-associated tumorigenesis in vivo. These results will facilitate future studies to address how HIV-associated enhancement of EBV replication and EBV mediated immune activation and tumorigenesis is impacted by suppressive ART and why the incidence of certain types of EBV-associated malignancies are stable or increasing in ART treated PLWH.
Humanized mice reconstituted with human B cells and T cells were first exposed to EBV (Supplementary Table 1). To evaluate the effect of HIV co-infection on EBV replication and tumorigenesis in vivo, a subset of mice was subsequently exposed to HIV. EBV infection was assessed in mice by measuring EBV-DNA levels in peripheral blood (cells and plasma) longitudinally and in tissues at necropsy (16 weeks post exposure or earlier if mice experienced 20% weight loss and/or appeared lethargic). HIV infection was monitored over time by measuring peripheral blood plasma HIV-RNA levels. HIV-RNA was detected in the plasma of all mice exposed to HIV and virus replication sustained over time (Supplementary Figure 1A). By six weeks post-exposure, plasma HIV-RNA levels were higher in EBV/HIV co-infected mice (P = 0.0317) compared to mice infected with HIV-only (Supplementary Figure 1A).
EBV-DNA was detected in the peripheral blood of all mice exposed to EBV only and 6/7 mice (86%) exposed to EBV and HIV (Figure 1A). No significant difference in EBV acquisition, as measured by the presence of detectable EBV-DNA in peripheral blood, was observed between groups of mice (P = 0.5007). While cell-free (plasma) and cell-associated EBV-DNA was detected in the peripheral blood of all EBV-positive mice, peak levels of cell-free and cell-associated EBV-DNA were 15-fold and 5-fold higher (P = 0.0082 and P = 0.0350, respectively) in EBV/HIV co-infected mice (Figures 1B–D). These results demonstrate that HIV co-infection enhanced EBV viremia.
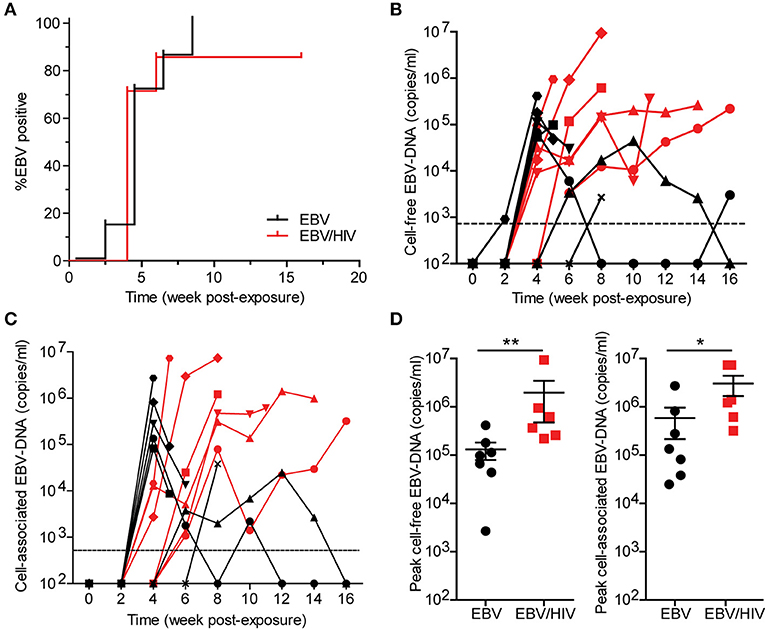
Figure 1. HIV co-infection enhances EBV viremia. Humanized mice were inoculated intraperitoneally with EBV B95.8 (n = 14). Following EBV exposure, a group of mice (n = 7) were subsequently inoculated intravenously with HIV-1 LAI. EBV-DNA levels were monitored longitudinally in peripheral blood plasma (cell-free EBV-DNA) and cells (cell-associated EBV-DNA) of mice with real-time PCR. (A) A Kaplan Meier plot depicts the proportion of mice inoculated with EBV (black) or EBV/HIV (red) that became systemically infected with EBV as determined by the presence of EBV-DNA in peripheral blood. The (B) cell-free and (C) cell-associated EBV-DNA levels for individual mice systemically infected with EBV (n = 7 mice, black symbols) or EBV/HIV (n = 6 mice, red symbols) are shown. A dashed line indicates the assay limit of detection. (D) Peak cell-free and cell-associated EBV-DNA levels in the peripheral blood of EBV (n = 7 mice, black circles) and EBV/HIV (n = 6 mice, red squares) infected mice. The mean and standard error mean EBV-DNA levels are shown. Peak EBV-DNA levels between EBV and EBV/HIV infected animals were compared with a two-tailed Mann-Whitney test (*p < 0.05 and **p < 0.01).
EBV infection induces a CD8+ T cell response in peripheral blood that is characterized by the expansion and activation of CD8+ T cells and acquisition of a memory phenotype (13). At necropsy, regardless of HIV co-infection status, CD8+ T cell levels were significantly higher in the peripheral blood of EBV positive mice (baseline: 4.5 ± 0.7% s.e.m.; necropsy: 29.6 ± 6.5% s.e.m.; P = 0.0009) compared to baseline pre-exposure levels (Supplementary Table 2). In addition, the frequencies of memory (baseline: 43.5 ± 6.9% s.e.m.; necropsy: 94.3 ± 2.2% s.e.m.; P < 0.0001) and activated (baseline: 12.9 ± 2.9% s.e.m.; necropsy: 47.1 ± 7.2% s.e.m.; P = 0.0002) CD8+ T cells in peripheral blood were also significantly higher at necropsy compared to baseline pre-exposure levels (Supplementary Table 2). At necropsy, no significant differences in the levels of CD8+ T cells in peripheral blood and tissues were observed between mice infected with EBV only or co-infected with EBV and HIV (Figure 2A; Supplementary Table 2). Notably, the vast majority of CD8+ T cells in EBV positive mice expressed a memory phenotype in peripheral blood and tissues at necropsy (Figure 2B; Supplementary Table 2). However, the levels of activated CD8+ T cells were significantly higher in the peripheral blood (P = 0.0087), spleen (P = 0.0221), liver (P = 0.0140), and lung (P = 0.0047) of EBV positive mice co-infected with HIV (Figure 2C; Supplementary Table 2). In the peripheral blood of mice infected with HIV only, the levels of activated CD8+ T cells only transiently increased peaking at 2 weeks post-exposure indicating that EBV and HIV contribute to the higher levels of CD8+ T cell activation observed in EBV/HIV co-infected mice (Supplementary Figure 1B). Collectively, these results suggest that HIV co-infection enhanced EBV-mediated immune activation.
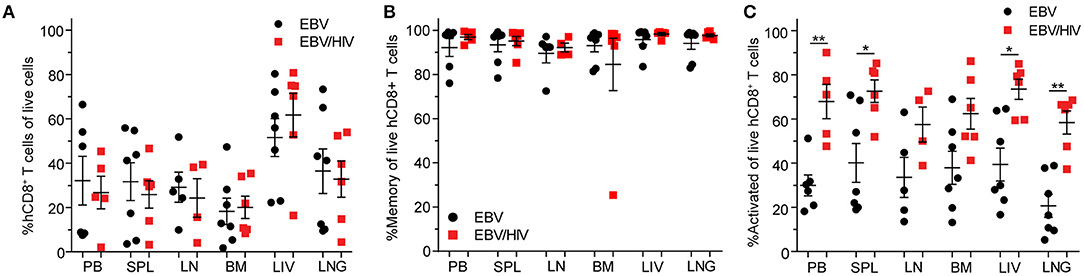
Figure 2. Systemic EBV-induced CD8+ T cell activation is amplified by HIV co-infection. (A) CD8+ T cell, (B) memory (CD45RAneg) CD8+ T cell and (C) CD8+ T cell activation (HLA-DR+CD38+) levels as determined by flow cytometric analysis in the peripheral blood (PB) and spleen (SPL), lymph node (LN), bone marrow (BM), liver (LIV) and lung (LNG) of EBV (SPL, BM, LIV, LNG: n = 7 mice, PB: n = 6 mice, LN: n = 5 mice, black circles) and EBV/HIV (SPL, BM, LIV, LNG: n = 6 mice, PB: n = 5 mice, LN: n = 4 mice, red squares) infected mice at necropsy. The mean and standard error mean are shown. CD8+ T cell, memory CD8+ T cell, and CD8+ T cell activation levels in the PB and tissues of EBV and EBV/HIV infected animals were compared with a two-tailed Mann-Whitney test (*p < 0.05 and **p < 0.01).
To evaluate the effect of HIV on systemic EBV replication, EBV-DNA levels were measured in the peripheral blood, spleen, lymph nodes, bone marrow, liver, lung, and brain of EBV and EBV/HIV co-infected mice at necropsy. Significantly higher levels of cell-free and cell-associated EBV-DNA were observed in peripheral blood of EBV/HIV co-infected mice (P = 0.0022). EBV-DNA was detected in 39/41 tissues samples analyzed from mice infected with EBV only and in 42/42 tissue samples analyzed from EBV/HIV co-infected mice (Supplementary Table 3). EBV-DNA levels were significantly higher in the spleen (P = 0.0047), lymph nodes (P = 0.0140), bone marrow (P = 0.0350), liver (P = 0.0082), and lung (P = 0.0221) of EBV/HIV co-infected mice (Figure 3; Supplementary Table 3). Notably, EBV-DNA levels were also 60-fold higher in the brain of EBV/HIV co-infected mice (Figure 3, Supplementary Table 3).
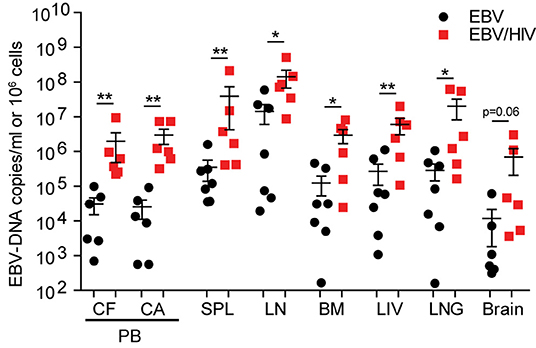
Figure 3. HIV co-infection augments systemic EBV replication. EBV-DNA levels in the peripheral blood (PB, CF, cell-free; CA, cell-associated) and tissues (SPL, spleen; LN, lymph nodes; BM, bone marrow; LIV, liver; LNG, lung and brain) of EBV (n = 7 mice, black circles) and EBV/HIV (n = 6 mice, red squares) infected mice at necropsy. The mean and standard error mean EBV-DNA levels are shown. EBV-DNA levels between EBV and EBV/HIV infected animals were compared with a two-tailed Mann-Whitney test (*p < 0.05 and **p < 0.01).
The presence of tumors in EBV and EBV/HIV infected mice was also analyzed at necropsy. HIV co-infection enhanced EBV-induced tumorigenesis in vivo. Macroscopic tumors were readily observed in 7/7 EBV/HIV co-infected mice (Figure 4A; Supplementary Table 3). In contrast, only 4/7 mice infected with EBV only had macroscopic tumors (Figure 4A; Supplementary Table 3). Tumors were located in a greater number of distinct anatomical sites per mouse in EBV/HIV co-infected mice compared to mice infected with EBV only (P = 0.0023) (Figure 4B). Tumors were identified in one or two different tissues in mice infected with EBV only. In contrast, tumors were identified in up to five different tissues per mouse in mice co-infected with EBV and HIV (Figure 4B). In mice infected with EBV only tumors were only observed on the spleen and/or liver (Figures 4C,D; Supplementary Table 3). However, in EBV/HIV co-infected mice tumors could be observed on the spleen, liver, kidney, gastrointestinal tract, and/or salivary glands (Figures 4C–E; Supplementary Table 3). An analysis of viral gene expression in tumor samples collected from mice demonstrated that all tumors analyzed from EBV infected mice regardless of HIV co-infection status expressed LMP1 and EBNA2 (Figure 4F). This is characteristic of type III latency gene expression. Although HIV co-infection enhanced systemic EBV replication and EBV-induced tumorigenesis, no significant difference in survival (P = 0.8372) was observed between mice infected with EBV only or co-infected with EBV and HIV (Supplementary Figure 2).
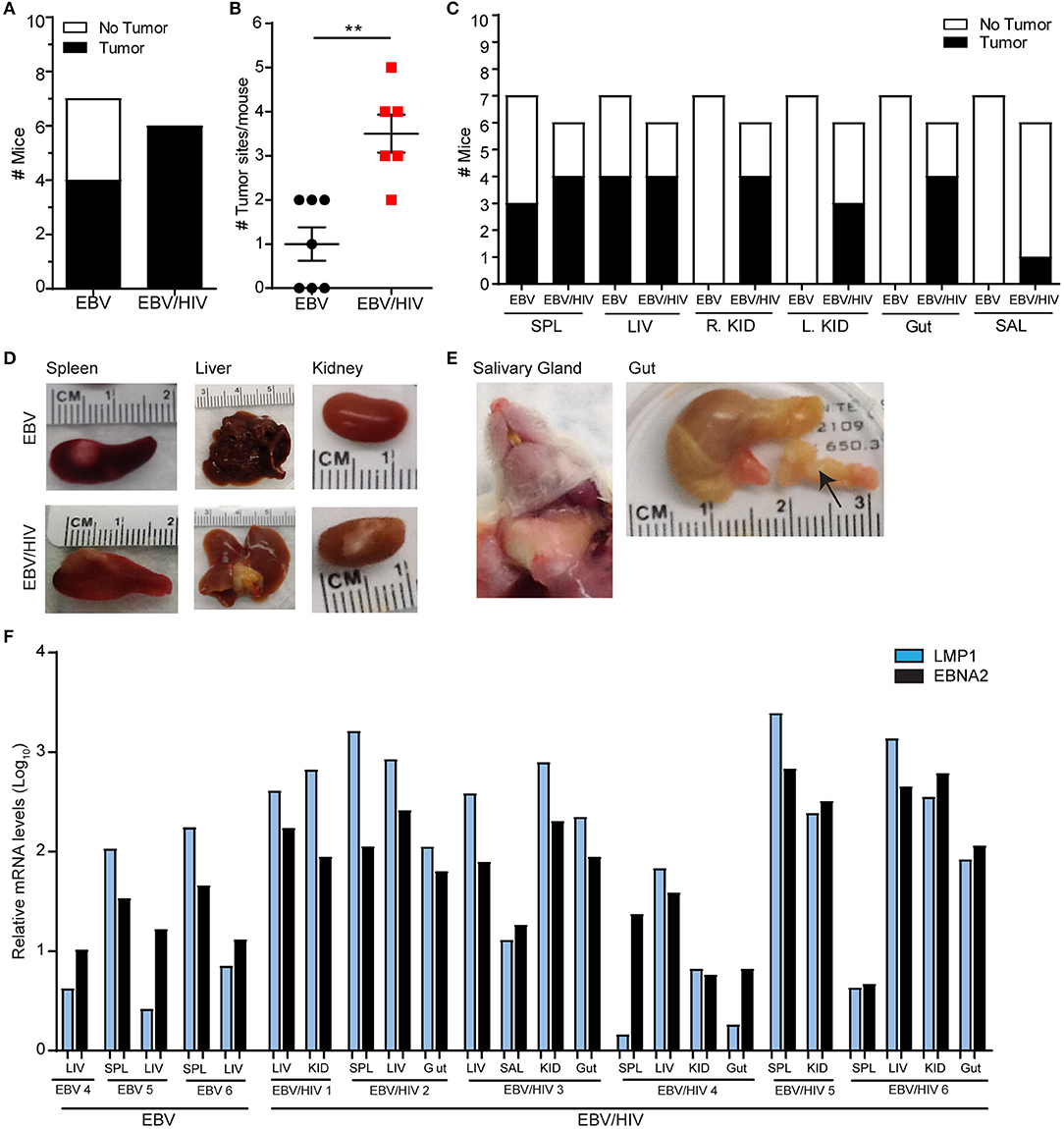
Figure 4. HIV co-infection augments EBV-induced tumorigenesis in vivo. The presence of macroscopic tumors in the organs of EBV (n = 7 mice) and EBV/HIV (n = 6 mice) infected animals were noted at necropsy. (A) Tumor incidence in EBV and EBV/HIV infected mice (white: no tumors detected, black: tumors detected). (B) The number of different tissues with macroscopic tumors for each EBV (black circles) and EBV/HIV (red squares) infected mouse. The mean and standard error mean are shown. The number of tumor sites detected in EBV and EBV/HIV infected mice was compared with a two-tailed Mann-Whitney test (**p < 0.01). (C) Tumor incidence in the spleen (SPL), liver (LIV), right kidney (R.KID), left kidney (L.KID), gut and salivary glands (SAL) of EBV and EBV/HIV infected mice (white: no tumors detected, black: tumors detected). (D) Representative images of the spleen, liver, and kidney harvested from EBV and EBV/HIV infected mice. (E) Images of tumors detected in the salivary glands and gut (noted with an arrow) of EBV/HIV infected mice. (F) LMP1 and EBNA2 mRNA levels in tumors isolated from the spleen (SPL), liver (LIV), kidney (KID), gut and salivary glands (SAL) of EBV and EBV/HIV infected mice. Relative LMP1 (blue bars) and EBNA2 (black bars) mRNA levels were quantified using β-actin as an internal reference.
Using a humanized mouse model of EBV infection (14–17), we directly demonstrated that HIV co-infection enhances systemic EBV replication, immune activation, and EBV-induced tumorigenesis in vivo. These data are consistent with studies reporting increased detection of EBV-DNA in the peripheral blood and saliva and the increased incidence of certain types of EBV-associated cancers in PLWH without any interventions (6–8, 18–21). In mice infected with EBV only, macroscopic tumors were only observed on the spleen and liver. In EBV/HIV co-infected animals, tumors were observed on the liver, spleen, kidneys, gastrointestinal tract, and salivary glands. These results suggest that HIV co-infection facilitates the spread of EBV-associated lymphomas and/or the simultaneous development of tumors at multiple different sites. Similar results were recently observed by another laboratory in humanized mice during a study focused on evaluating the effect of EBV infection on the cellular tropism of HIV (22). Several types of EBV positive lymphomas on extra nodal sites have been observed in PLWH including Hodgkin's lymphoma and diffuse large B cell lymphomas (6, 8). While we did not define the type of malignancies present, expression of EBV latency genes LMP1 and EBNA2 were detected in all tumors analyzed from humanized mice albeit at different levels. Co-expression of LMP1 and EBNA2 are indicative of type III latency (e.g. immunoblastic diffuse large B cell lymphomas) (4, 6).
Suppressive antiretroviral therapy (ART) reduces the incidence of AIDS-associated malignancies in PLWH (9, 10). The effect of ART on EBV reactivation and replication is less clear. For example, one study reported that incidence of EBV reactivation (as measured by the detection of EBV-DNA in saliva) was higher in PLWH compared to HIV negative controls regardless of ART use. However, the incidence of EBV reactivation was reduced in PLWH (ART +/–) with high CD4+ T cell counts (23). Despite ART, the incidence of Hodgkin's lymphoma in PLWH has not decreased (9, 11). Most Hodgkin's lymphomas in PLWH are associated with EBV (7). While ART efficiently suppresses systemic HIV replication in PLWH, systemic immune activation and dysfunction persist presumably in part due to residual HIV replication and gut dysbiosis (24). Chronic immune activation and dysfunction in ART-suppressed PLWH may impair the formation of a robust immune response allowing for the formation of EBV-associated malignancies. Humanized mice could be used in the future to directly evaluate the effect of ART on systemic EBV replication and EBV-associated tumor formation and correlations between markers of chronic immune activation (e.g. LPS, sCD14, CRP, neopterin, TNFα, IL-6, IL-10, IFNγ, etc.) and EBV-associated disease.
Most PLWH acquire EBV infection prior HIV infection (1). Therefore, our study focused on the effect of HIV co-infection on EBV pathogenesis. However, EBV may influence the course of HIV infection. Here, we observed higher levels of HIV-RNA in the plasma of EBV/HIV co-infected mice compared to mice infected with HIV only by six weeks post-exposure. Even in healthy individuals, EBV periodically reactivates in the body which could stimulate local immune activation, potentially enhancing HIV replication (5). Studies in PLWH have attempted to evaluate associations between herpesvirus shedding (as determined by detectable virus in peripheral blood, throat washes, urine, stool, and/or semen) and HIV viral loads, however, given the high prevalence of other herpesviruses in PLWH, it is difficult to directly determine the effect of EBV on HIV replication and pathogenesis (25, 26). Humanized mice could serve as a model to directly evaluate the effect of EBV infection on HIV replication, pathogenesis and latency in vivo.
Limitations to our study include no uninfected control animals to serve as a comparison for the changes in the CD8+ T cell compartment observed in EBV-infected mice. However, the effect of EBV infection on the CD8+ T cell compartment including expansion, activation, and acquisition of a memory phenotype, has been well-documented in humans and in multiple humanized mouse models when compared to baseline levels and/or uninfected controls (13–15, 27–30). Furthermore, it has been shown that while the levels of human hematopoietic cells (hCD45+) in the peripheral blood of mice is stable between 16 and 32 weeks post engraftment, the percentage of T cells that are CD8+ decreases over time which is the opposite of what was observed in EBV-infected humanized mice (31). In addition, the short lifespan of humanized mice compared to humans requires experimental timelines to be condensed. Despite the shortened timeline between EBV and HIV exposures, our experimental data supports the clinical observation that the incidence of EBV-associated malignancies is higher in PLWH compared to the general population (6–8). In addition, while we anticipate that infection with a CCR5 or CXCR4 tropic strain of HIV would enhance EBV-associated tumorigenesis, a CXCR4 strain of HIV was chosen for this study to accelerate the development of HIV-associated pathogenesis.
In summary, we have implemented a model that permits the analysis of the effect of HIV infection on EBV. Our results demonstrate that HIV co-infection enhances EBV replication and the formation of EBV-associated malignancies. This work will facilitate future studies using humanized mouse models to address why the incidence of certain types of EBV-associated malignancies are stable or increasing in ART treated PLWH in contrast to the decline observed for many types of AIDS-associated malignancies.
Humanized mice were constructed by transplanting 12–15-week-old female and male irradiated (200 rads) NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (NSG; The Jackson Laboratory) with 2–3.5 x 105 human CD34+ hematopoietic stem cells intravenously. Reconstitution of animals with human hematopoietic cells was monitored longitudinally with flow cytometry as previously described (14). Animals were maintained by the Division of Comparative Medicine at the University of North Carolina-Chapel Hill.
Infectious EBV was reactivated by transfection of BZLF1 and gp110 into 293 cells harboring a B95.8 EBV bacmid (32). Cell supernatant fluid was passed through a 0.4 uM filter and concentrated with an Amicon ultra 100-kDa-molecular-mass-cutoff filter (Millipore). EBV stocks were titered on Raji cells as previously described (33). Stocks of HIV-1LAI were generated by transient transfection of 293T cells and titered on TZM-BL indicator cells as previously described (34–40). Mice (16–24 weeks post-transplant) were exposed to 1–1.2 x 105 green Raji units (GRU) EBV via intraperitoneal injection. On the same day and following EBV exposure, a group of mice was also exposed intravenously to 3 x 104 TCIU HIV-1LAI via tail vein injection.
HIV-RNA levels were measured longitudinally in the peripheral blood plasma of mice using real-time PCR as previously described (34–40).
EBV-DNA levels were measured longitudinally in peripheral blood and in tissues at necropsy using a real-time PCR assay as previously described (14). Flow cytometry was used to evaluate levels of CD8+ T cells, memory (CD45RAneg) CD8+ T cells and CD8+ T cell activation (HLA-DR+CD38+) in peripheral blood and tissues as previously described (14). Live cell gates were determined by forward and side scatter. Plasma was collected from peripheral blood following centrifugation (375 x g, 5 min). Cells were isolated from the peripheral blood, spleens, lymph nodes, bone marrow, livers, lungs and brains collected from mice for analysis by real-time PCR and flow cytometry as previously described (34–40). Liver, lung, and brain mononuclear cells were purified with a Percoll gradient. Mice were euthanized at 16 weeks post-exposure or earlier if they experienced 20% weight loss and/or appeared lethargic and a necropsy performed to assess the presence of tumors. Tumors were harvested, snap-frozen and stored at −80C for subsequent nucleic acid extraction and gene expression analysis. Tissue and tumor images were taken with an iSight color camera and the brightness adjusted in Adobe Photoshop CS6.
RNA was isolated from 100 mg of tissue from tumor samples using the TRIzol reagent (Invitrogen) according to manufacturer's instructions. Total RNA quantity and quality was determined using a Nanodrop 1000 (ThermoScientific). Total RNA (1 ug) was converted to cDNA using the iScript cDNA kit (BioRad) and random primers according to manufacturer's guidelines. qPCR was performed using iTaq Universal SYBR Green Supermix (BioRad) and the QuantStudio6 Real-Time PCR system (Applied Biosystems) under standard conditions. Samples were monitored for LMP1, EBNA2 and B-actin with the following primers: LMP1, 5′-AATTTGCACGGACAGGCATT-3′; (forward) and 5′-AAGGCCAAAAGCTGCCAGAT-3′ (reverse); EBNA2, 5′-GCTTAGCCAGTAACCCAGCACT-3′ (forward) and 5′-TGCTTAGAAGGTTGTTGGCATG-3′ (reverse), B-Actin, 5′-GTCTGCCTTGGTAGTGGATAATG-3′ (forward) and 5′-TCGAGGACGCCCTATCATGG-3′ (reverse). Relative levels of LMP1 and EBNA2 were quantified using β-actin as an internal reference.
Statistical analyses were performed in Prism, version 6 (Graph Pad). A Log-rank Mantel-Cox test was used to compare the rates of EBV-DNA detection in the peripheral blood and survival between EBV and EBV/HIV exposed mice. A two-tailed Mann-Whitney U test was used to compare the peak peripheral blood cell-associated and cell-free HIV-DNA levels, tissue EBV-DNA levels, levels of CD8+ T cells, memory CD8+ T cells, and CD8+ T cell activation in peripheral blood and tissues, and the number of tumor sites per mouse between EBV and EBV/HIV exposed mice. A two-tailed Mann-Whitney U test was also used to compare CD8+ T cells, memory CD8+ T cells, and CD8+ T cell activation levels in the peripheral blood of EBV infected mice (regardless of HIV co-infection status) at baseline and at necropsy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
CW produced and titered stocks of EBV, performed the analysis of EBV latency gene expression, contributed to the experimental design, data interpretation, and manuscript writing. MR longitudinally monitored mouse health and survival, processed samples from EBV-exposed mice, performed flow cytometric analyses, performed the real-time PCR analysis of EBV-DNA levels in peripheral blood, tissue samples, and analyzed data. AT longitudinally monitored mouse health, survival and processed samples from EBV-exposed mice. RS performed and supervised the real-time PCR analysis of EBV-DNA. JP contributed to the experimental design and data interpretation. AW conceived, designed experiments, coordinated the study, contributed to data interpretation, data presentation, and manuscript writing.
This work was supported in part by funding from the National Institutes for Heath grants P01-CA019014-37, R01-AI123010, R01-AI140799, R01-DK131585, and R01-MH108179. This research was also supported by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI050410.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank current and past members of the UNC International Center for the Advancement of Translational Science for technical assistance. We also thank husbandry and veterinary staff in the UNC Division of Comparative Medicine.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2022.861628/full#supplementary-material
1. Hjalgrim H, Friborg J, Melbye M. The epidemiology of EBV and its association with malignant disease. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press (2007). p. 929–959.
2. Rickinson AB, Callan MF, Annels NE. T-cell memory: lessons from Epstein-Barr virus infection in man. Philos Trans R Soc Lond B Biol Sci. (2000) 355:391–400. doi: 10.1098/rstb.2000.0579
3. Thorley-Lawson DA. EBV persistence–introducing the virus. Curr Top Microbiol Immunol. (2015) 390(Pt 1):151–209. doi: 10.1007/978-3-319-22822-8_8
4. Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. (2004) 350:1328–37. doi: 10.1056/NEJMra032015
5. Maurmann S, Fricke L, Wagner HJ, Schlenke P, Hennig H, Steinhoff J, et al. Molecular parameters for precise diagnosis of asymptomatic Epstein-Barr virus reactivation in healthy carriers. J Clin Microbiol. (2003) 41:5419–28. doi: 10.1128/JCM.41.12.5419-5428.2003
6. Bibas M, Antinori A. EBV and HIV-related lymphoma. Mediterr J Hematol Infect Dis. (2009) 1:e2009032. doi: 10.4084/MJHID.2009.032
7. Carbone A, Volpi CC, Gualeni AV, Gloghini A. Epstein-Barr virus associated lymphomas in people with HIV. Curr Opin HIV AIDS. (2017) 12:39–46. doi: 10.1097/COH.0000000000000333
8. Grogg KL, Miller RF, Dogan A. HIV infection and lymphoma. J Clin Pathol. (2007) 60:1365–72. doi: 10.1136/jcp.2007.051953
9. Simard EP, Pfeiffer RM, Engels EA. Cumulative incidence of cancer among individuals with acquired immunodeficiency syndrome in the United States. Cancer. (2011) 117:1089–96. doi: 10.1002/cncr.25547
10. Rubinstein PG, Aboulafia DM, Zloza A. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. AIDS. (2014) 28:453–65. doi: 10.1097/QAD.0000000000000071
11. Powles T, Robinson D, Stebbing J, Shamash J, Nelson M, Gazzard B, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. (2009) 27:884–90. doi: 10.1200/JCO.2008.19.6626
12. Epeldegui M, Vendrame E, Martinez-Maza O. HIV-associated immune dysfunction and viral infection: role in the pathogenesis of AIDS-related lymphoma. Immunol Res. (2010) 48:72–83. doi: 10.1007/s12026-010-8168-8
13. Roos MT, van Lier RA, Hamann D, Knol GJ, Verhoofstad I, van Baarle D, et al. Changes in the composition of circulating CD8+ T cell subsets during acute epstein-barr and human immunodeficiency virus infections in humans. J Infect Dis. (2000) 182:451–8. doi: 10.1086/315737
14. Wahl A, Linnstaedt SD, Esoda C, Krisko JF, Martinez-Torres F, Delecluse HJ, et al. A cluster of virus-encoded microRNAs accelerates acute systemic Epstein-Barr virus infection but does not significantly enhance virus-induced oncogenesis in vivo. J Virol. (2013) 87:5437–46. doi: 10.1128/JVI.00281-13
15. Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. (2009) 206:1423–34. doi: 10.1084/jem.20081720
16. Yajima M, Imadome K, Nakagawa A, Watanabe S, Terashima K, Nakamura H, et al. A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis. (2008) 198:673–82. doi: 10.1086/590502
17. Whitehurst CB Li G, Montgomery SA, Montgomery ND, Su L, Pagano JS. Knockout of Epstein-Barr virus BPLF1 retards B-cell transformation and lymphoma formation in humanized mice. MBio. (2015) 6:e01574–15. doi: 10.1128/mBio.01574-15
18. Lucht E, Biberfeld P, Linde A. Epstein-Barr virus (EBV) DNA in saliva and EBV serology of HIV-1-infected persons with and without hairy leukoplakia. J Infect. (1995) 31:189–94. doi: 10.1016/S0163-4453(95)80025-5
19. Ling PD, Vilchez RA, Keitel WA, Poston DG, Peng RS, White ZS, et al. Epstein-Barr virus DNA loads in adult human immunodeficiency virus type 1-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. (2003) 37:1244–9. doi: 10.1086/378808
20. Pan R, Liu X, Zhou S, Ning Z, Zheng H, Gao M, et al. Differential prevalence and correlates of whole blood Epstein-Barr virus DNA between HIV-positive and HIV-negative men who have sex with men in Shanghai, China. Epidemiol Infect. (2017) 145:2330–40. doi: 10.1017/S0950268817001054
21. Byrne CM, Johnston C, Orem J, Okuku F, Huang ML, Rahman H, et al. Examining the dynamics of Epstein-Barr virus shedding in the tonsils and the impact of HIV-1 coinfection on daily saliva viral loads. PLoS Comput Biol. (2021) 17:e1009072. doi: 10.1371/journal.pcbi.1009072
22. McHugh D, Myburgh R, Caduff N, Spohn M, Kok YL, Keller CW, et al. EBV renders B cells susceptible to HIV-1 in humanized mice. Life Sci Alliance. (2020) 3:e202000640. doi: 10.26508/lsa.202000640
23. Yan Y, Ren Y, Chen R, Hu J, Ji Y, Yang J, et al. Evaluation of Epstein-Barr virus salivary shedding in HIV/AIDS patients and HAART use: a retrospective cohort study. Virol Sin. (2018) 33:227–33. doi: 10.1007/s12250-018-0028-z
24. Williams LD, Amatya N, Bansal A, Sabbaj S, Heath SL, Sereti I, et al. Immune activation is associated with CD8 T cell interleukin-21 production in HIV-1-infected individuals. J Virol. (2014) 88:10259–63. doi: 10.1128/JVI.00764-14
25. Agudelo-Hernandez A, Chen Y, Bullotta A, Buchanan WG, Klamar-Blain CR, Borowski L, et al. Subclinical herpesvirus shedding among HIV-1-infected men on antiretroviral therapy. AIDS. (2017) 31:2085–94. doi: 10.1097/QAD.0000000000001602
26. Gianella S, Moser C, Vitomirov A, McKhann A, Layman L, Scott B, et al. Presence of asymptomatic cytomegalovirus and Epstein–Barr virus DNA in blood of persons with HIV starting antiretroviral therapy is associated with non-AIDS clinical events. AIDS. (2020) 34:849–57. doi: 10.1097/QAD.0000000000002484
27. Zdimerova H, Murer A, Engelmann C, Raykova A, Deng Y, Gujer C, et al. Attenuated immune control of Epstein-Barr virus in humanized mice is associated with the multiple sclerosis risk factor HLA-DR15. Eur J Immunol. (2021) 51:64–75. doi: 10.1002/eji.202048655
28. Heuts F, Rottenberg ME, Salamon D, Rasul E, Adori M, Klein G, et al. T cells modulate Epstein-Barr virus latency phenotypes during infection of humanized mice. J Virol. (2014) 88:3235–45. doi: 10.1128/JVI.02885-13
29. Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. (2004) 304:104–7. doi: 10.1126/science.1093933
30. Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. (2006) 12:1316–22. doi: 10.1038/nm1431
31. Audige A, Rochat MA Li D, Ivic S, Fahrny A, Muller CKS, et al. Long-term leukocyte reconstitution in NSG mice transplanted with human cord blood hematopoietic stem and progenitor cells. BMC Immunol. (2017) 18:28. doi: 10.1186/s12865-017-0209-9
32. Delecluse HJ, Hilsendegen T, Pich D, Zeidler R, Hammerschmidt W. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc Natl Acad Sci U S A. (1998) 95:8245–50. doi: 10.1073/pnas.95.14.8245
33. Kumar R, Whitehurst CB, Pagano JS. The Rad6/18 ubiquitin complex interacts with the Epstein-Barr virus deubiquitinating enzyme, BPLF1, and contributes to virus infectivity. J Virol. (2014) 88:6411–22. doi: 10.1128/JVI.00536-14
34. Nixon CC, Mavigner M, Sampey GC, Brooks AD, Spagnuolo RA, Irlbeck DM, et al. Systemic HIV and SIV latency reversal via non-canonical NF-kappaB signalling in vivo. Nature. (2020) 578:160–5. doi: 10.1038/s41586-020-1951-3
35. Honeycutt JB, Liao B, Nixon CC, Cleary RA, Thayer WO, Birath SL, et al. T cells establish and maintain CNS viral infection in HIV-infected humanized mice. J Clin Invest. (2018) 128:2862–76. doi: 10.1172/JCI98968
36. Wahl A, Ho PT, Denton PW, Garrett KL, Hudgens MG, Swartz G, et al. Predicting HIV pre-exposure prophylaxis efficacy for women using a preclinical pharmacokinetic-pharmacodynamic in vivo model. Sci Rep. (2017) 7:41098. doi: 10.1038/srep41098
37. Kovarova M, Shanmugasundaram U, Baker CE, Spagnuolo RA De C, Nixon CC, et al. HIV pre-exposure prophylaxis for women and infants prevents vaginal and oral HIV transmission in a preclinical model of HIV infection. J Antimicrob Chemother. (2016) 71:3185–94. doi: 10.1093/jac/dkw283
38. Olesen R, Swanson MD, Kovarova M, Nochi T, Chateau M, Honeycutt JB, et al. ART influences HIV persistence in the female reproductive tract and cervicovaginal secretions. J Clin Invest. (2016) 126:892–904. doi: 10.1172/JCI64212
39. Wahl A, Baker C, Spagnuolo RA, Stamper LW, Fouda GG, Permar SR, et al. Breast Milk of HIV-positive mothers has potent and species-specific in vivo HIV-inhibitory activity. J Virol. (2015) 89:10868–78. doi: 10.1128/JVI.01702-15
Keywords: Epstein-Barr Virus (EBV), human immunodeficiency virus (HIV), co-infection, replication, tumorigenesis, B cell lymphoma, humanized mice
Citation: Whitehurst CB, Rizk M, Teklezghi A, Spagnuolo RA, Pagano JS and Wahl A (2022) HIV Co-infection Augments EBV-Induced Tumorigenesis in vivo. Front. Virol. 2:861628. doi: 10.3389/fviro.2022.861628
Received: 24 January 2022; Accepted: 11 February 2022;
Published: 11 March 2022.
Edited by:
Akio Adachi, Kansai Medical University, JapanReviewed by:
Ramesh Akkina, Colorado State University, United StatesCopyright © 2022 Whitehurst, Rizk, Teklezghi, Spagnuolo, Pagano and Wahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Wahl, YW5nZWxhX3dhaGxAbWVkLnVuYy5lZHU=
†Present address: Christopher B. Whitehurst, Department of Pathology, Microbiology, and Immunology, New York Medical College, Valhalla, NY, United States
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.