- 1Immunology Unit, University of Zimbabwe Faculty of Medicine and Health Sciences (UZ–FMHS), Harare, Zimbabwe
- 2Obstetrics and Gynaecology Unit, UZ–FMHS, Harare, Zimbabwe
- 3Medical Laboratory Sciences Unit, UZ–FMHS, Harare, Zimbabwe
- 4National Microbiology Reference Laboratory, Sally Mugabe Central Hospital, Harare, Zimbabwe
- 5Clinic for Visceral Surgery and Medicine, Inselspital Bern and Bern University, Bern, Switzerland
Background: Achieving and maintaining viral suppression (VS) in people living with HIV/AIDS on antiretroviral therapy (ART) remains a crucial clinical goal, more so in pregnancy to prevent mother-to-child-transmission (MTCT). There is a need to understand VS kinetics and barriers to achieving it in order to meet the target of eliminating HIV-MTCT by 2030.
Methods: HIV-infected pregnant women ≥20 weeks of gestation with different durations of Tenofovir/Lamivudine/Efavirenz exposures seeking antenatal care services at four primary health centres in high-density residential areas in Harare, Zimbabwe were enrolled in the University of Zimbabwe Birth Cohort Study. Plasma viral load (VL) was quantified by reverse transcriptase–polymerase chain reaction. Demographic, clinical, socio-economic and HIV- and ART-related factors were tested in multivariable logistic regression analyses as potential predictors for VS and undetectable VL.
Results: From March 2016 to June 2019, 608 HIV-infected pregnant women were enrolled. 63 (10.4%) were self-reported-ART-naïve; 324 (53.3%) and 221 (36.3%) initiated ART pre- and post-conception, respectively. Time from ART initiation to VS (VL ≤ 1,000 copies/ml) in 95% of the women was 126 days. Overall lack of VS (VL > 1,000 copies/ml) was observed in 133 (21.9%) women being 76.2, 27.4 and 7.7% in self-reported-ART-naïve, post-conception and pre-conception groups, respectively. Undetectable VL (≤ 50 copies/ml) was observed in 371 (61.2%) and low-level viremia (51–1,000 copies/ml) in 102 (16.8%) women.
In multivariable models for all participants regardless of ART exposure, being on ART was the strongest predictor for both VS and undetectable VL (odds ratio 95% confidence interval, OR (CI): 8.9(4.2–19.5) and 8.1(3.2–24.4), respectively). For women on ART, duration of ART use >126 days was the strongest predictor with OR (CI): 6.7(3.3–14.0) for VS and 8.5(5.6–13.1) for undetectable VL. Other relevant predictors for favourable virological outcomes were older maternal age, HIV-status disclosure, absence of ART side effects and self-reported depression. Having a spouse/intimate partner on ART predicted a 4 times higher likelihood for VS.
Discussion: Lack of VS was frequently observed in this Harare cohort of pregnant women, mainly due to new HIV diagnosis, hence not being on ART and suboptimal duration of ART exposure. Since VS for 95% of women needed about 4 months of ART exposure, eliminating HIV-MTCT will require timely screening and commencing women together with their spouses/intimate partners on ART before pregnancy or early after conception.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier: NCT04087239.
Introduction
Current treatment guidelines for human immunodeficiency virus (HIV) infection in pregnant women recommend lifelong antiretroviral therapy (ART) immediately following HIV diagnosis irrespective of any clinical staging or assessment of cluster of differentiation-4 positive (CD4+) T-lymphocytes (Option B+ treatment strategy) (1). The effectiveness, success or failure of ART can be assessed mainly virologically by measuring viral particle genetic material/ribonucleic acid (RNA) or viral load (VL) in the blood (2). In an effort to curb the pandemic, the joint United Nations Programme on HIV/AIDS (UNAIDS) set a global 90–90–90 goal, targeting 90% of people living with HIV/AIDS (PLWHA) to know their HIV status, 90% of the diagnosed to initiate ART and 90% of those on ART to achieve viral suppression (VS) by year 2020 (3). However, there are different cut-offs of HIV RNA levels defining VS or undetectable VL. The World Health Organisation (WHO) 2021 guidelines define undetectable VL as viral RNA levels below the detection limit of an ultra-sensitive assay, currently pegged at ≤ 50 copies/ml (4). Low-level viremia (LLV), i.e. VL of 51–1,000 copies/ml, can predict future virological failure (4). Lack of VS, according to the same guidelines, is the inability of a treatment plan to achieve and/or maintain VL below 1,000 copies/ml after at least 6 months of continuous ART use (4). However, this threshold is in stark contrast to the European and American HIV guidelines that define lack of VS as VL>200 copies/ml (5, 6), with some contemporary low resource setting studies now defining it as VL>400 copies/ml (7).
The public health benefits of ART use are realised if VS is attained and maintained, and this has been associated with lower mortality, near to normal life expectancy and improved general health of PLWHA (8, 9). Monitoring the effectiveness of ART is important to identify adherence challenges and guides decisions on whether ART regimens should be switched to a second-line regimen. However, access to this essential service remains a challenge in resource-limited settings (10), more so in pregnant women, where VL monitoring is critical to reduce the mother-to-child transmission (MTCT) of HIV (11, 12).
Initiating ART early in pregnancy generally outweighs the potential adverse neonatal outcomes and recommended regimens include dual nucleoside reverse transcriptase inhibitors (NRTIs) combination such as Tenofovir disoproxilfumarate plus either Lamivudine or Emtricitabine or an integrase strand transfer inhibitor like Dolutegravir (13, 14). Mechanisms of reducing MTCT include lowering maternal HIV RNA levels as NRTIs have high transplacental passage that inhibits viral replication in the foetus (15). Suppression of maternal VL to undetectable levels should be achieved as rapidly as possible to reduce the risk of HIV-MTCT (16), yet pregnancy as a condition is associated with poor VS (17, 18).
Achieving VS by time of delivery remains critical for pregnant women in both low- and high-income countries since failure to achieve VS puts the unborn babies at high risk of MTCT of HIV (16). In Zimbabwe, there has been a huge scaling up of prevention of MTCT (PMTCT) initiatives, from a couple of sites during the piloting stages in 1999 to the current 1,560 health facilities offering these services (19). According to Zimbabwean national guidelines, all HIV-infected women should be on lifelong ART (1). Since 2013, the standard of care for all Zimbabwean HIV-infected pregnant women for PMTCT has been TENOLAM-E (Tenofovir, Lamivudine and Efavirenz), a combination of (non)-nucleoside reverse transcriptase inhibitors (NNRTIs). However, since 2018, Dolutegravir has been slowly replacing Efavirenz as a fixed dose combination resulting in Tenofovir/Lamivudine/Dolutegravir (TENOLAM-D) now the preferred first-line therapy. Zidovudine is still occasionally offered as an alternative treatment regimen in some PLWHA on second line or salvage regimens in those who develop renal issues with Tenofovir.
Despite these remarkable preventive measures in place, the HIV burden, although in a downward trajectory, remains unacceptably high, at approximately 33,000 new cases every year with annual incidence and prevalence rates of 0.60 and 16%, respectively, among adult female urbanites of the 24- to 35-year age group (20). In addition, up to 54% of Zimbabwean women of child-bearing age living with HIV/AIDS desire to get pregnant in the future (21). Previous Zimbabwean studies on maternal VS lacked more critical data, including potentially modifiable factors such maternal demographics, socio-economic status and family/household/spouse/intimate partner factors, and HIV- and ART-related factors (10). Thus, there is a paucity of comprehensive local data regarding HIV suppression and frequencies of LLV in pregnant women during this era of lifelong ART.
To achieve the UNAIDS 95–95–95-set target of ending the HIV epidemic by 2030, there is therefore a need to understand VS kinetics and barriers/facilitators with the aim to craft more effective public health policies to improve mother–infant care. This study aimed to determine rates and predictors of undetectable VL and VS in HIV-infected pregnant women accessing antenatal care services at 4 City of Harare primary health facilities in high-density residential areas.
Materials and Methods
We aimed to
1. Determine the proportion of pregnant women at least 20 weeks gestational age with undetectable baseline HIV RNA load ≤ 50 copies/ml, LLV (VL of 51–1,000 copies/ml) and lacking VS; VL > 1,000 copies/ml.
2. Estimate the average time to plasma VS (VL ≤ 1,000 copies/ml) from the time of ART initiation in ≥95% of the pregnant women.
3. Determine facilitators of undetectable VL (≤ 50 copies/ml) and barriers to VS; VL > 1,000 copies/ml including clinical characteristics, laboratory markers, ART/HIV infection-related factors, socio-demographic parameters and intimate partner/spouse factors.
Design of University of Zimbabwe Birth Cohort Study (UZBCS)
The aims and methodology of the University of Zimbabwe Birth Cohort Study (UZBCS) have been previously described (22). Briefly, the UZBCS is a prospective cohort study investigating the role of maternal comorbidities, including co-infections with persistent viruses such as HIV, cytomegalovirus, hepatitis B virus and other infectious diseases like syphilis, non-communicable diseases, including maternal nutritional status on pregnancy outcomes, infant mortality, growth and development, immunity and general health.
The study population was HIV-infected and HIV-uninfected pregnant women seeking antenatal care services at four municipal primary health centres in high-density residential areas in Harare. Following a pre-study site feasibility assessment in September 2015, out of the 12 polyclinics, Kuwadzana, Dzivaresekwa (Rujeko), Glenview and Budiriro in South West of Harare city centre were selected based on the higher volumes of maternal and child health services, frequency of HIV sero-positivity and lack of competing research activities targeting the same population. The catchment areas of the selected four polyclinics have relatively stable communities that do not frequently change their accommodation. By design, ~50% of the expecting women in the UZBCS were HIV-infected as previously described (22).
Inclusion and Exclusion Criteria
Inclusion criteria were pregnant women ≥15 years of age, at least 20 weeks of gestation at enrolment and planning to deliver at any of the 4 study sites.
Exclusion criteria were the presence of severe mental health disorders, rendering the women incapacitated to provide informed consent or comply with study procedures as assessed by the study clinicians.
Study Procedures
The study midwife administered a structured questionnaire to all pregnant women at enrolment aiming at a comprehensive clinical and socio-demographics characterisation. Socio-economic information comprising employment status, family monthly income, money set aside for food and general household food security were assessed. Furthermore, questions addressing maternal lifestyle such as alcohol use, concurrent medications, presence of comorbidities, awareness of spouse/intimate partner's HIV status, HIV status disclosure and stock unavailability of ART at health centres, including missed ART doses were asked.
Maternal Physical Examination
At enrolment, the study midwife performed a full physical examination, blood pressure checks, assessment of oedema and anthropometry of body mass index and mid upper arm circumference to assess nutritional status as previously described (22). WHO clinical staging was done to assess immune status.
Blood Collection and Analysis
Ten millilitres of maternal venous blood was collected in EDTA tubes at enrolment. The whole blood EDTA samples were aliquoted for subsequent measurements of CD4+ T-lymphocyte counts within a maximum of 6 h after acquisition. Plasma was processed and stored at −80°C for later HIV RNA testing.
Maternal HIV diagnosis was done from an aliquot of whole-blood EDTA using qualitative rapid immunochromatographic assays, SD Bioline HIV-1/2 3.0 (Standard Diagnostics Inc., Kyonggi-do, South Korea) and Abbott's Determine® HIV-1/2. Western Blot was the tiebreaker for any indeterminate test results.
Absolute CD4+ T-lymphocyte counts in whole-blood EDTA samples were enumerated within a maximum of 6 hours after sample acquisition using a Partec Cyflow counter (Cyflow, Partec, Munster, Germany).
HIV RNA was extracted from 1 ml maternal baseline frozen plasma and quantified using an automated TaqMan Roche Amplicor 1.5 Monitor Test (Cobas AmpliPrep/Cobas TaqMan, Roche Diagnostics, Branchburg NJ), according to the manufacturer's instructions. The detection limit was 20 HIV RNA copies/ml. However, we used a VL of 50 copies/ml as the end point for measuring the efficacy of ART since correlation and concordance at this threshold are comparable between different VL assays (23).
Data Management and Statistical Analysis
Data were entered and managed using Research Electronic Data Capture (REDCap v 8.0, © 2020); http://www.redcap.uzchs.ac.zw/redcap/. Quality assurance on the accuracy of data entry included independent double entries and verification in case of discrepancies.
For continuous variables, normalcy testing was performed using the Shapiro–Wilk test, which revealed a non-normal distribution of all parameters. Descriptive summary statistics were calculated for predictor and outcome variables of interest using frequencies and percentages for categorical variables. Medians and interquartile ranges, minimum and maximum values were used for continuous variables. A p-value < 0.05 was considered significant.
Parameters from groups of HIV-infected self-reported ART naïve, women who initiated ART before or during pregnancy (i.e. the pre-conception and post-conception ART initiation groups, respectively) were compared using the Kruskal–Wallis test, Mann–Whitney U test or Fisher's exact test where appropriate. Multivariable logistic regression analyses were performed to estimate odds ratios (OR) and 95% confidence intervals (95% CI) for factors associated with undetectable VL (≤ 50 copies/ml) and lack of VS, defined as VL >1,000 copies/ml. We used automated variable elimination to obtain simplified models with fewer parameters but high predictive power. Variable elimination was performed for maximising the Akaike information criterion which considers both the model fit and the number of parameters (i.e. the Akaike information criterion increases with a better model fit but decreases according to the number of predictors) (24). For the analysis, missing values for the relevant variables were excluded. To clearly distinguish effects of being on ART and being ART-naïve, the ART defaulters were excluded from group comparisons and the predictive models. The following potential predictors of VS and undetectable VL, all assessed at the time of enrolment, were tested: whether the pregnancy was planned, self-reported depression, mid upper arm circumference, median maternal age, level of education, employment status, ethnicity, gravida, number of days since HIV diagnosis, whether women were currently living with spouses/intimate partners under the same roof, any experience(s) of domestic violence during pregnancy, disclosure of HIV status to anyone and food security. For those on ART, we included the additional predictors of access to constant supply of ART, presence of any ART-related side effects, ART duration and if an ART dose was ever missed. Estimation of the number of days on ART where 95% of the women were VS was done using the receiver operator characteristic (ROC) curve. Statistical analyses were performed in RStudio, version 1.4.1717.
Consent to Participate
UZBCS complied with the ethical principles of the Declaration of Helsinki (updated 2013) and was conducted in compliance with the international council for harmonisation of Good Clinical and Laboratory Practice guidelines and local regulatory requirements. Literacy is nearly universal in Zimbabwe (25), and all potential participants were able to read and comprehend the informed consent form. All study participants provided written informed consent.
Results
Demographic Characteristics of the Study Population
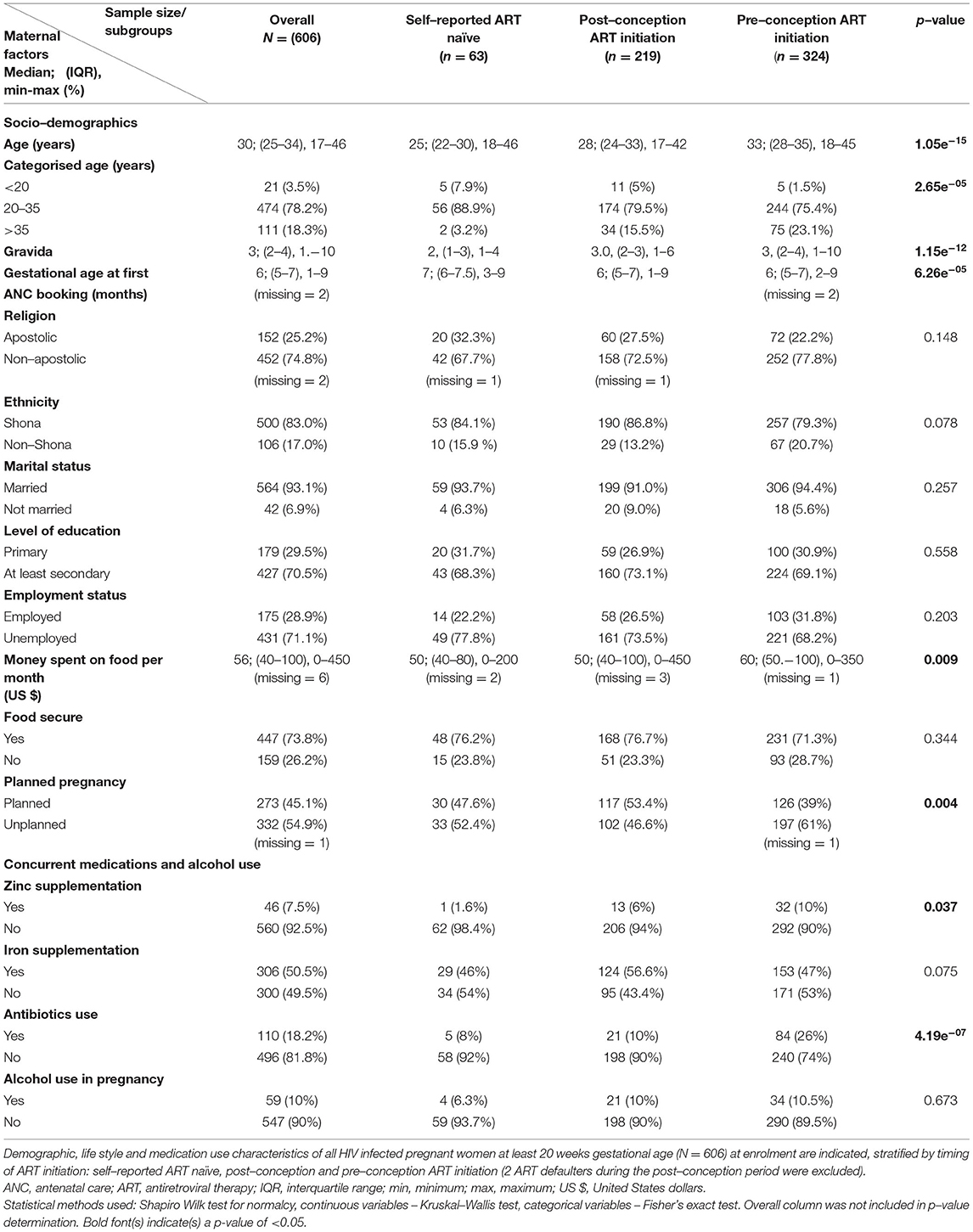
From March 2016 to June 2019, 608 HIV-infected pregnant women were enrolled into UZBCS. A total of 324 (53.3%) and 221 (36.3%) of the participants initiated ART pre- and post-conception, respectively, whilst 63 (10.4%) were self-reported ART-naïve (Table 1). Within the post-conception group, 2 (0.9%) were ART defaulters (i.e. participants had started treatment but stopped due to severe side effects). Commencement of ART within the post-conception group generally coincided with HIV diagnosis, but other pregnant women spent up to 2,925 days without treatment after HIV diagnosis (Table 2). A total of 53 out of 63 (84.1%) of the self-reported ART-naïve pregnant women only knew of their HIV status at enrolment into our study, but overall, 555/608 (91.3%) of the women were aware of their HIV status, exceeding the expectations of the first joint UNAIDS's 90–90–90 goal post. Out of 555 women who knew their HIV status, 543 (97.8%) were on ART, also well above the second joint UNAIDS 90–90–90 goal post by year 2020.
Median maternal age at enrolment was at least 5 years older in women in the pre-conception ART initiation group compared to the other 2 groups. Furthermore, this group of relatively older pregnant women could afford ~$10 more on food every month and also had significantly higher frequencies of unplanned pregnancies (61% compared to the frequencies around 50% observed in the two other groups; Table 1).
Gestational age at first antenatal care booking was much later (at 7 months) for the self-reported ART-naïve women compared to 6 months for the other 2 groups. There were no statistically significant differences in the marital status, level of education, religion, ethnicity, current employment status, reported household food security, mid upper arm circumference and alcohol consumption between self-reported ART-naïve, post- and pre-conception ART initiation groups (Tables 1, 2).
HIV- and ART-Related Factors
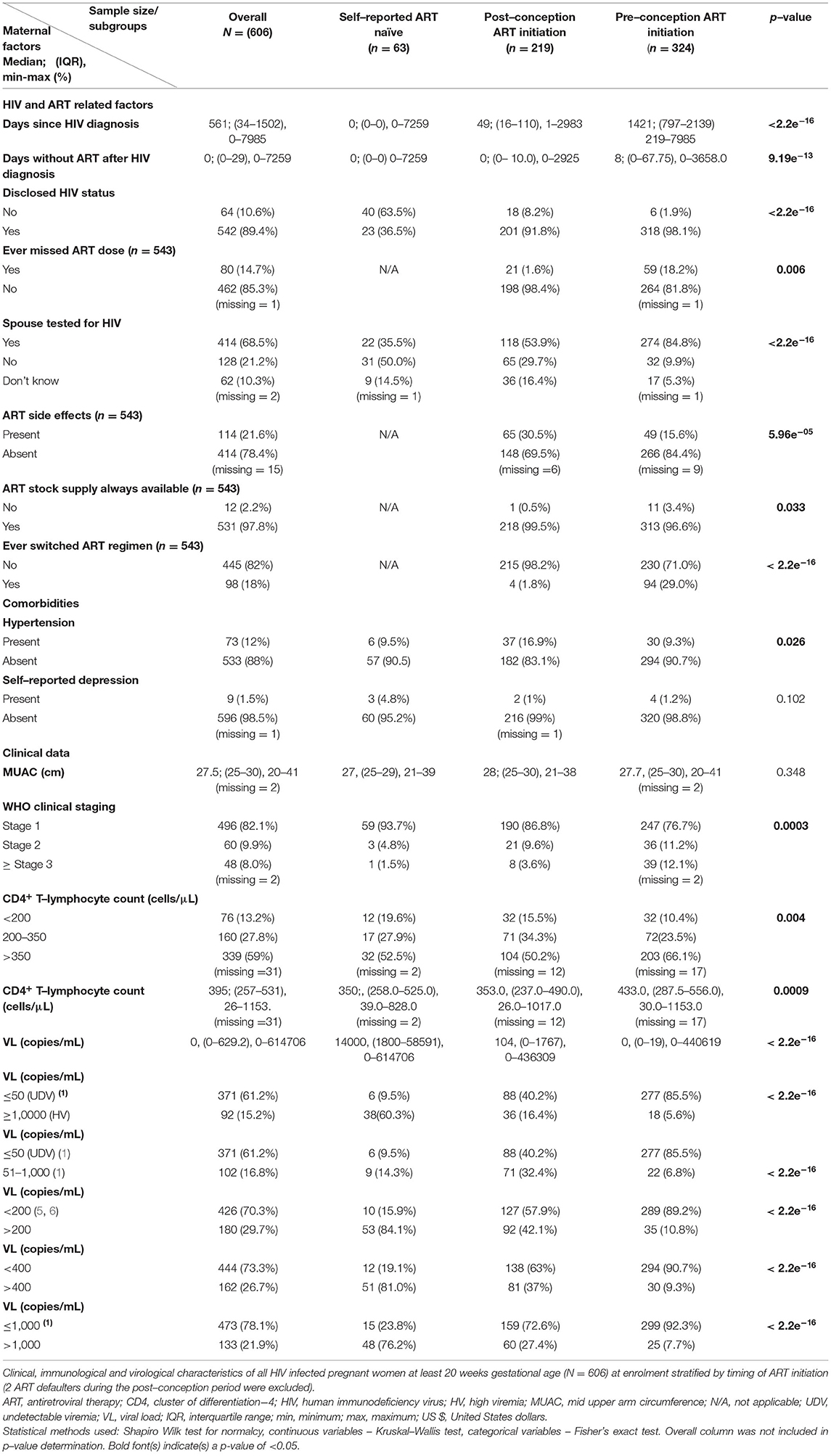
Self-reported adherence to ART differed between groups: only 1.6% in those who initiated ART in pregnancy compared to 18.2%, who were already on ART before conceiving, reported to have ever missed their ART dosages (Table 2). This could be at least partially explained by the longer period of ART use in the pre-conception group. In addition, there were significant differences in the frequencies of presence of ART side effects (15.6% in the pre-conception vs. 30.5% in the post-conception ART initiation group). Constant availability of ART was generally high, with only 0.5 and 3.4% of women in pre- and post-conception ART groups, respectively, reporting ART stock unavailability at the health centres.
Of the 543 women on ART, 536 (98.7%) were on Tenofovir, Lamivudine and Efavirenz (TENOLAM-E) with only 7/543 (1.3%) women, all in the pre-pregnancy ART initiation subgroup, on other different regimens: 2 women on TENOLAM plus Atazanavir, 2 on TENOLAM plus Nevirapine, 1 on Tenofovir, Efavirenz plus Atazanavir, 1 on Zidovudine, Lamivudine plus Nevirapine and 1 on Zidovudine, Lamivudine plus Atazanavir. Overall, 98 (18%) reported ever switching ART regimen, and of these, 83 (84.7%) were VS (VL ≤ 1,000 copies/ml). Since 98.7% of the women were on the same ART regimen and the remaining women were receiving heterogeneous regimens, no subgroup analysis according to the type of ART was done, and the ART regimen was not tested as a predictor for VS.
Overall, 68.5% of the women were aware of their spouses/intimate partners' HIV status including whether or not they were on ART, if infected. Frequency of awareness of spouses/intimate partners' HIV status was highest in women who initiated ART before pregnancy, intermediate in women who initiated during pregnancy and lowest in the self-reported ART-naive (84.8, 53.9 and 35.5%, respectively). Strikingly, 62 (10.3%) women did not know whether their spouses/intimate partners had undergone HIV testing or not, and up to 21.2% reported that their spouses/intimate partners were yet to be tested for HIV (Table 2).
HIV Staging and Presence of Comorbidities
WHO clinical staging assessment showed that an overall 82.1% of HIV-infected pregnant women were asymptomatic. As expected, we observed the highest (12.1%) frequency of advanced disease (WHO stage ≥3) in women in the pre-conception group who probably had been infected for longer periods compared to the self-reported ART-naïve women and the post-conception group (1.5 and 3.6%, respectively; Table 2).
Absolute CD4+ T-lymphocyte assessments showed that most women were immunocompetent (339/575, 59%) with CD4+ T-lymphocyte ≥350 cells/μl. This rate was highest in women who were on ART before pregnancy (66.1%) compared to about 50% in the other two groups, demonstrating the benefit of ART.
Generally, the frequencies of self-reported depression were low at 1.5% but somewhat higher (4.8%) in self-reported ART-naïve individuals (not significant). Moreover, women who initiated ART in pregnancy were more likely to be hypertensive (16.9%) compared to 9.3% of women who were already on ART pre-conception and 9.5% among self-reported ART-naïve women (Table 1).
Estimate of Average Time to VS of at Least 95% of Participants From the Time of ART Initiation
When plotting the number of women with VS (≤ 1,000 copies/ml) and lack of VS (> 1,000 copies/ml) as a function of time after ART initiation, we noted an increase in the proportion of women with VS over time (Figure 1). We estimated the time to VS (≤ 1,000 copies/ml) in 95% of women since ART initiation to be 126 days.
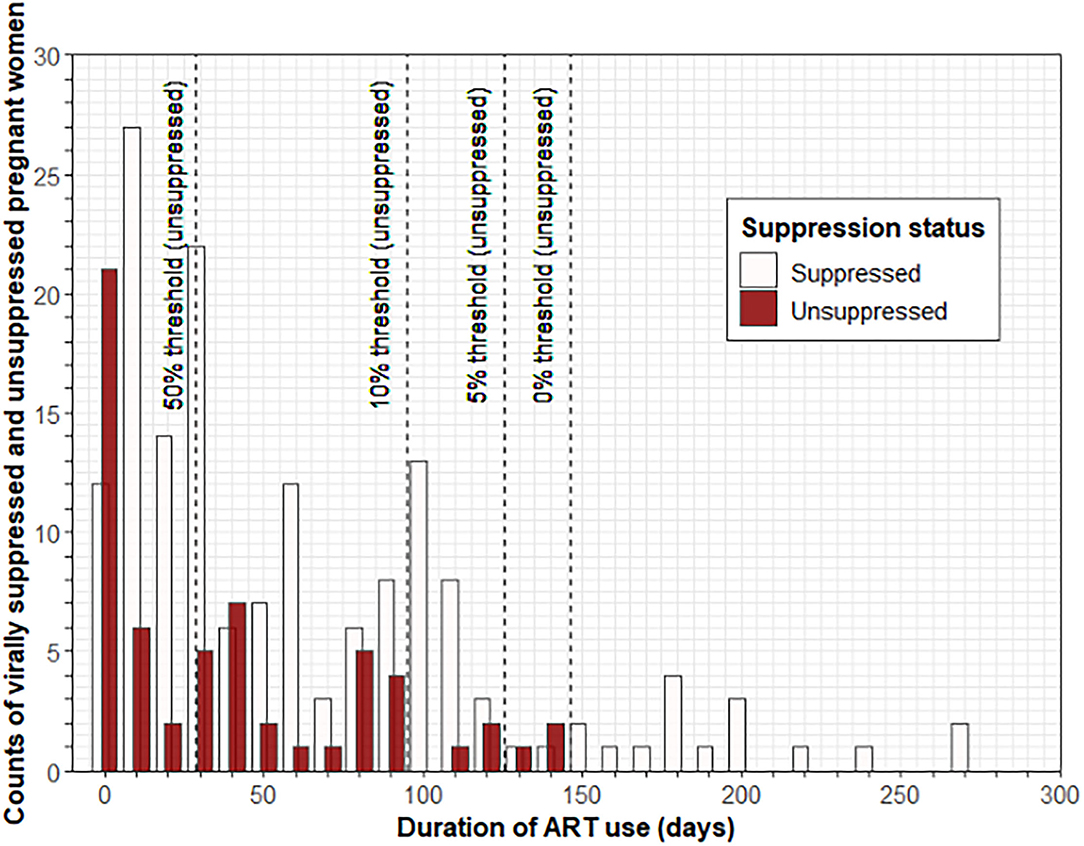
Figure 1. Estimate of average time to VS of at least 95% of participants from the time of ART initiation. Histograms showing the number of pregnant women at least 20 weeks gestational age with VS (≤ 1,000 copies/ml) and lack of VS (>1,000 copies/ml) as a function of time after ART initiation; a decrease in the proportion of women without VS over time is observed. Dashed lines indicate ART duration time thresholds (28.5, 95, 125.5, and 146.5 days, respectively). The estimated time to VS (≤ 1,000 copies/ml) in 95% of the women since ART initiation was 126 days. ART, antiretroviral therapy; VS, viral suppression.
Undetectable VL (≤ 50 Copies/ml)
Overall, 371/606 (61.2%) pregnant women had undetectable VL with frequencies increasing with longer exposure to ART, being 6/63 (9.5%), 88/219 (40.2%) and 277/324 (85.5%) in the self-reported ART-naïve, post- and pre-conception groups, respectively (Table 2).
LLV (51–1,000 Copies/ml)
Overall 102/606 (16.8%) pregnant women had LLV, but the highest frequency was observed in women initiating ART in pregnancy (71/219, 32.4%), intermediate in self-reported ART-naïve women (9/63, 14.3%) and lowest in those who were on ART before conception (22/324, 6.8%; Table 2).
VS (≤ 1,000 Copies/ml) as Per 2021 WHO Guidelines
Overall, 473/606 (78.1%) pregnant women had VS. This rate was considerably lower than the 90% expected by the third joint UNAIDS goal post by year 2020. Women who were on ART before pregnancy surpassed this threshold (299/324) at 92.3%. Frequencies of VS increased with increasing duration of ART exposures, being 15/63 (23.8%) in self-reported ART-naïve individuals (possibly indicating natural immunity), 159/219 (72.6%) in the post-conception group and 299/324 (92.3%) in the pre-conception group, respectively (Table 2).
Among the unsuppressed women, 28/133 (21.1%) were on ART for more than 126 days, with a median duration of ART exposure of 1,619 days since HIV diagnosis, probably demonstrating complacency in self-reported ART adherence associated with long-term duration of ART use. In addition, 25% of these women reported to have missed their ART doses, whilst 53.6% had a history of changing their ART regimens.
Very High Viremia (>10,000 Copies/ml)
Very high viremia was observed in 92/606 (15.2%) of pregnant women. Such high levels of viremia are generally observed either in untreated individuals or in the ART experienced in the presence of HIV drug resistance. In our study, frequencies decreased with increasing duration of ART exposure, being 38/63 (60.3%) in individuals without treatment, 36/219 (16.4%) in the post-conception group and 18/324 (5.6%) in the pre-conception group. In the latter group, ART exposure was more likely >126 days, and persistent very high viremia may reflect drug resistance (Table 2).
Factors Associated With Undetectable VL (≤ 50 Copies/ml)
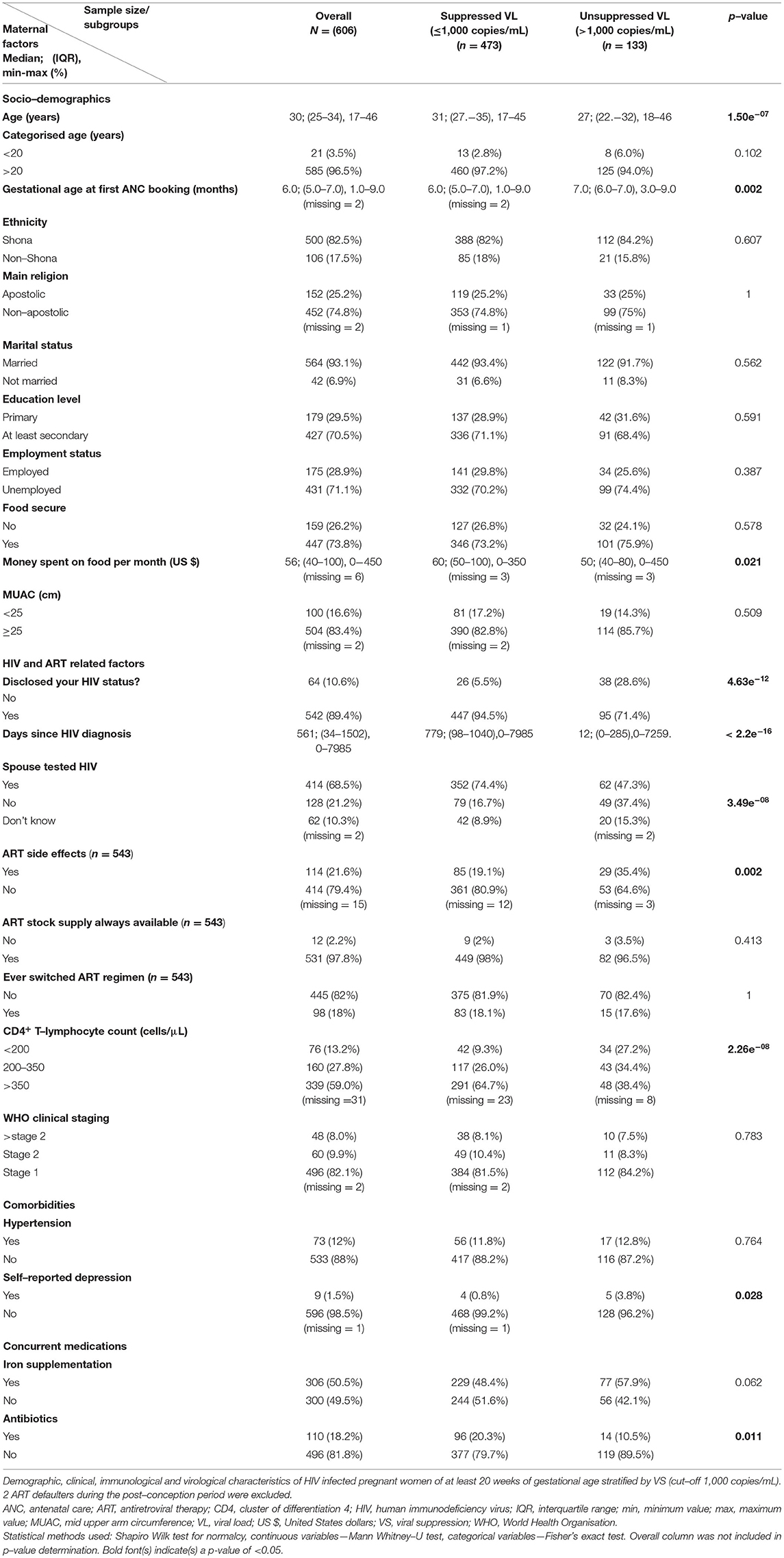
In a bivariate analysis comparing 371 women with undetectable VL (≤ 50 copies/ml) with 235 women with detectable VL, those with detectable VL were approximately 4 years younger, booked for antenatal care 1 month later and were less likely to have their spouses/intimate partners tested for HIV. Furthermore, these women set aside relatively less money aside every month to buy food and less likely to be on concurrent antibiotic treatment (Supplementary Table 1).
Women who had disclosed their HIV status were 2.3 times more likely to have undetectable VL compared to their counterparts with undisclosed HIV status. ART side effects were less frequently reported in women with undetectable VL (17.4% vs. 30.4%), and these women also had lower rates of self-reported depression (1.1% vs. 2.1%). As expected, women with undetectable VL were more immunocompetent with higher counts of CD4+ T-lymphocytes. However, there were no differences between the two groups when immune status was assessed using the less-sensitive WHO clinical staging (Supplementary Table 1).
Factors Associated With LLV (51–1,000 Copies/ml)
In a bivariate analysis comparing 371 women with undetectable VL (≤ 50 copies/ml) with 102 women with LLV; VL 51–1,000 copies/ml, those with LLV were approximately 3 years younger, more likely to be of apostolic religion and unaware of their spouse/intimate partner's HIV status, but less likely to have disclosed their HIV status. In addition, they were more likely to be immunocompromised (CD4+ T lymphocyte count of <350 cells/μl) and had experienced ART-related side effects, both factors with borderline significance (Supplementary Table 2).
Factors Associated With VS (≤ 1,000 Copies/ml)
Differences in clinical and epidemiological parameters were found when we compared 473 women with VS and 133 women without VS. These differences were highly reminiscent of the comparison of detectable (>50 copies/ml) to undetectable VL (≤ 50 copies/ml) as described above. Specifically, we noted a higher maternal age (4-year difference), a lower gestational age at first antenatal care booking (1 month earlier), higher rates of HIV status disclosure (94.5% vs. 71.4%), spouse testing for HIV (74.4% vs. 47.3%), lower rates of ART side effects (19.1% vs. 35.4%) and lower frequencies of self-reported depression (0.8% vs. 3.8%) in suppressed women (Table 3). As expected, unsuppressed women had lower median CD4+ T-lymphocyte counts of 295 cells/μl vs. 428 cells/μl (not shown).
Predictor of Undetectable VL and VS for All Women in Multivariable Regression Analyses
To test for predictors of undetectable VL and VS, we computed multivariable logistic regression models. We first computed models including all 606 HIV-infected pregnant women, regardless of ART exposure (excluding the 2 defaulters). We computed both complete models including all risk factors without variable elimination (Supplementary Table 3) and optimised shorter models upon automated variable elimination (Table 4).
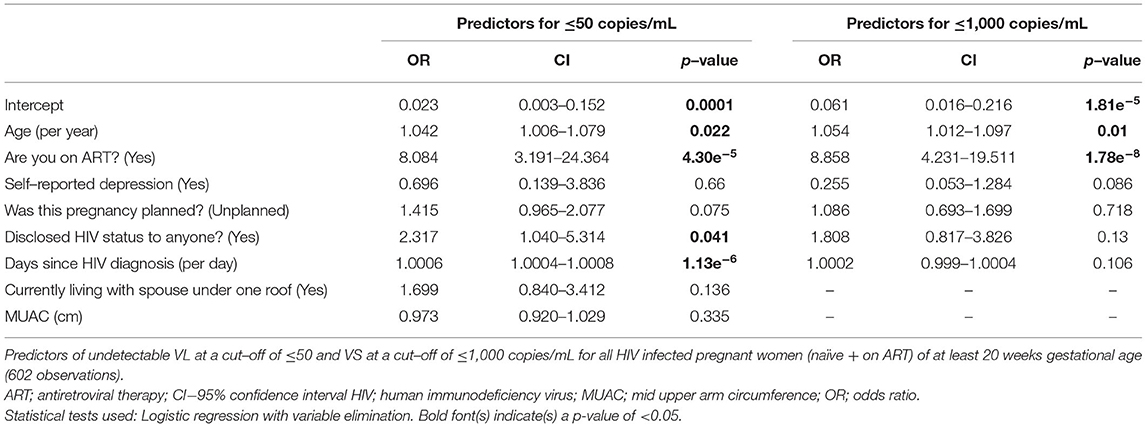
Table 4. Predictors of virological outcomes for all women (naïve + on ART) with variable elimination.
The most important predictor for both undetectable VL and VS was being on ART with an OR (CI): 8.1 (3.2–24.4) for undetectable VL and 8.9 (4.2–19.5) for VS (p < 0.001). Other relevant predictors included older age, longer days after HIV diagnosis and disclosure of HIV status (Table 4; Supplementary Table 3). However, some of these parameters did not reach statistical significance in all models.
Predictors of Undetectable VL and VS for Women on ART in Multivariable Regression Analyses
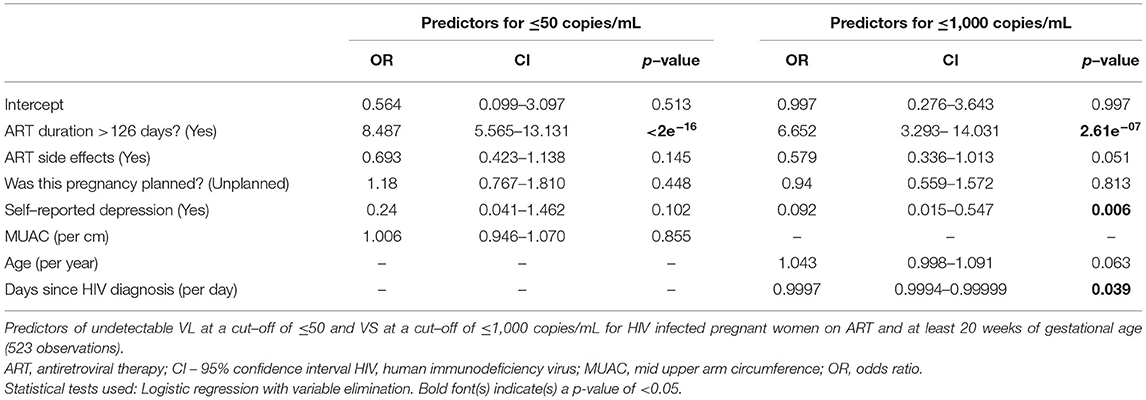
We also computed models including all 543 HIV-infected pregnant women on ART. We computed both complete models including all risk factors without variable elimination (Supplementary Table 4) and optimised shorter models upon automated variable elimination (Table 5). In this analysis, longer duration of ART use (>126 days) was the most important predictor for virus control with an OR (CI) 8.5 (5.6–13.1) for undetectable VL and 6.7 (3.3–14.0) for VS. Other relevant predictors were maternal age at enrolment, days since HIV diagnosis and ART side effects, but not all of these predictors yielded statistical significance in the regression models. Furthermore, self-reported depression (though only significant in the short model) predicted approximately 10.8-fold lower rates of VS.
Spouse-Related Factors as Predictors for Undetectable VL and VS
In another analysis, we included 'spouse on ART' and 'spouse tested for HIV' as additional predictors into the models. Strikingly, after variable elimination, 'spouse on ART' was a consistent and significant strong predictor for VS with OR values ranging from 1.8 to 4.0 in all models independent of the threshold of VL (≤ 50 or ≤ 1,000 copies/ml) in all women whether on ART or not and women on ART.
Discussion
Pregnancy is a period of life with high vulnerability, and any hazards, including HIV infection, will affect both the mother and the unborn child. In that respect, PMTCT is of utmost importance since foetus/infant HIV infection results in poor infant outcomes and higher mortality rates. In Zimbabwe, one in 15 children dies before his or her fifth birthday (69 deaths per 1,000 live births), with 70% of these deaths occurring in infancy (26). Therefore, improving maternal health through effective HIV management is a critical public health task essential for the survival of children.
We report results regarding VS from 608 HIV-infected pregnant women in the UZBCS. The key findings are as follows: (i) In the overall study population, 78.1% of the women had VL ≤ 1,000 copies/ml, but only 61.2% showed optimal controlled HIV RNA levels ≤ 50 copies/ml. (ii) The most important predictors for poor viral control were not being on ART and duration of ART use of <126 days. (iii) Self-reported depression, non-disclosure of HIV status and younger maternal age were additional risk factors, while having a spouse also on ART predicted a better virological outcome.
Virus Suppression
UNAIDS declared the 95–95–95 global HIV management targeted to end the pandemic by 2030, but an intermediate goal of 90–90–90 was set for 2020. In our cohort, (i) 91.3% of pregnant women were aware of their HIV status, (ii) 98.4% of the expecting women with known HIV status were on ART and 84.3 and 61.2% of women on ART had VL below the 1,000 and 50 copies/ml thresholds, respectively. Therefore, at least for this cohort of pregnant women, two out of the three UNAIDS global 90–90–90 targets were met. Considering that only 78.1% and 61.2% of women had VL ≤ 1,000 and ≤ 50 copies/ml, respectively, our study points towards a significant proportion of pregnant women for whom management of HIV should be urgently improved.
Generally, in resource-limited settings, frequencies of undetectable VL (<50 copies/ml) in pregnancy range from 53 to 70% (27–30), well in line with our frequency of 61.2%. Women with detectable VL (>50 copies/ml) were ~4 years younger with lesser money set aside every month to buy food, booked for antenatal care 1 month later and were less likely to be on concurrent antibiotic treatment.
In previous Zimbabwean studies assessing VL in pregnant women, the average rate of undetectable VL (by then <400 copies/ml) was about 28% (31); however, these studies were done during the single-dose Nevirapine era, where single-dose Nevirapine therapy was only administered during labour. A recent Tanzanian study in pregnant women in their third trimester reported a VS rate (<400 copies/ml) of 88% (7). Applying the same threshold to our study population would translate to an overall rate of VL <400 copies/ml of 73.3% and up to 90.7% in women who initiated ART before pregnancy but only 63% in women who initiated ART during pregnancy.
In a review summarising 31 studies examining LLV among adults on ART, the prevalence of LLV ranged from 2.7 to 26%, but the main challenges were differences in the definitions of LLV among studies [67]. Thus, the frequency of 16.8% of LLV observed in our study was within this range being highest among women initiating ART in pregnancy (32.4%) and lowest (6.8%) in those who were on ART before conception. This was expected as the latter group had been on ART for long enough time to facilitate optimal ART exposure to enable complete VS. In any case, LLV is clinically highly relevant since HIV MTCT may occur within this LLV range (31–33), possibly in the presence of co-infections like cytomegalovirus (34). It is therefore essential to evaluate LLV among pregnant women on ART for factors that contribute to failure of reaching undetectable VL.
Rates for lack of VS (VL>1,000 copies/ml) in pregnant women in Sub-Saharan Africa ranged from 8.1 to 15.2% (7, 27, 35–37). Similarly, a previous Zimbabwean study during the Option B+ era demonstrated an 86% VS rate (<1,000 copies/ml) with nearly all HIV-infected pregnant women reporting taking ART (20). Even better VS rates (<1,000 copies/ml) of 94% were observed in a 2017 rural study of 1,112 pregnant women from Mazowe district, 50 km northwest of Harare (10). However, in this study, only 32% of participants underwent VL testing.
In any case, in most studies in low-resource settings, the third goal of UNAIDS of ≥90% VS rate in PLWHA on ART has not been met, just as in the case of our study where the overall (regardless of ART exposure) rate of VS (VL ≤ 1,000 copies/ml) was 78%, improving to 84.3% in pregnant women who knew their HIV status and were already on ART at enrolment. Therefore, more concerted effort is required to improve on the third 90 of the UNAIDS 90–90–90 goal at least in our study population to ensure optimal health outcomes for mothers and babies including PMTCT.
Risk Factors for Lack of VS
When assessing potential risk factors for good HIV control in multivariable regression models, we identified two dominating protective factors: ART treatment (“are you on ART”) in the whole population with an OR of 8.1. Furthermore, in women already on ART, sufficient length of ART treatment (>126 days) was of overriding importance as a predictor for HIV suppression with an OR of 8.5. Therefore, screening all young women for HIV even before pregnancy and starting ART as soon as possible in all infected individuals would help to improve health outcomes of pregnant women as a highly vulnerable population. In that respect, it should be noted that pregnant women registered rather late at antenatal care clinics at 6 months of pregnancy. Furthermore, mothers lacking VS (i.e. with VL >1,000 copies/ml) registered even later at 7 months after conception. Financial hardships with inability to afford US $25 required for antenatal care might have contributed to these late registrations. Therefore, financial support of mothers with very limited resources or abolishing antenatal care user fees might lead to earlier antenatal registration to facilitate an earlier start of HIV management and thus allow more time to achieve optimum VS and might ultimately avert or reduce MTCT and save lives.
The period of 36 weeks of pregnancy to labour is critical since 50% of HIV MTCT occur within this time period (20% occurring before 36 weeks and 30% after delivery) in non-breastfeeding populations (38). However, in breastfeeding populations, intra-uterine transmission is the major route of MTCT (39). Recent observational studies concluded that ART should be initiated at least 16 weeks (112 days) prior to the date of birth to achieve the desirable undetectable HIV VL by the time of delivery (40–42). In light of our finding of VS in >95% of women only after 126 days, an even earlier start of ART might be advantageous. Considering the late antenatal care bookings, VS rates might also improve with the new TENOLAM-D regimen (Dolutegravir), being offered since 2018 which has been shown to suppress VL more rapidly with at least a 2 log10 decrease by week 2 of therapy (43).
ART Side Effects and Self-Reported Adherence to ART
Overall ART side effects were reported by 21.6% of the women, disproportionally occurring in those who initiated ART during pregnancy compared to those initiating before pregnancy. Reported adverse effects included dizziness (56.1%), vomiting and nausea (17.5%) and general body weakness (14%). Furthermore, 2/219 women who initiated ART during pregnancy stopped HIV treatment due to intolerable vomiting and nausea (ART defaulters). ART side effects contributed to lower VS with effects bordering statistical significance. Therefore, frequent consulting with HIV specialists would be desirable, especially for women initiating HIV therapy after conception since rates of adverse effects were higher in this population.
A recent systematic review and meta-analysis found that 46% of the PLWHA receiving NNRTI-based first-line ART regimen re-suppressed at the next VL assessment, indicating that in many cases, elevated baseline VL may have been due to poor adherence (44). Simple and affordable measures recommended by WHO to assess adherence include self-reporting, pill counts and assessment of pharmacy refill records (45). In addition, composite adherence measures such as combining self-report with tablet recount or pharmacy refill records would have been more accurate. In any case, since in our study, 14.7% of women reported ever missing ART dosage, more adherence counselling interventions might be necessary.
Teenage Mothers
In our cohort age <20 years was associated with reduced rates of both VS and undetectable VL. VS rates were relatively lower at 61.9% for the (21/608) teenage pregnant women in our cohort. In line with these observations, in a previous Zimbabwean study of non-pregnant adolescents (10–19 years old) following 12 months of being on ART, only 57% achieved VS at VL ≤ 1,000 copies/ml (46). In the same study, risk factors for lack of VS in non-pregnant adolescents included poor ART adherence, alcohol consumption and non-disclosure of HIV status (46).
Poorer outcomes in younger mothers could be explained since they were less likely to be in an established position in the family structures or had less economic resources within their control, thus making them more vulnerable. Higher economic vulnerability might, in turn, lead to lower adherence to ART. A longitudinal qualitative study on HIV-infected Kenyan adults showed that livelihood interventions such as a microfinance loan or agricultural and financial training improved food security and poverty and consequently led to improved ART adherence, VS and CD4+ T-lymphocyte counts (47, 48). This observation underscores the need for economic empowerment for women since economic insecurity is a major barrier to HIV treatment adherence.
Unplanned Pregnancies
Overall, the rate of unplanned pregnancies observed in our cohort was 54.9%. This seems quite high especially in this urban setting with good contraception coverage. The highest frequencies of unplanned pregnancies (61%) were reported in generally older women who were on ART before pregnancy, underscoring the need for integrating family-planning services into HIV care and treatment settings. These findings are comparable to a previous 1997 Harare study (N = 923) that observed a 41% rate of unplanned and a 9% rate of unwanted pregnancies (regardless of HIV status), with women >35 years being 3 times more likely to present with an unplanned pregnancy compared to the younger ones (49). Rates of contraception failure in women on ART have been shown to be high (29, 50), and unplanned pregnancy might also be an indicator of being on ART before conception.
Self-Reported Depression
Self-reported depression was rare in our cohort (1.5% of all HIV-infected mothers); however, depression was associated with the highest likelihood among all predictors for not achieving VS among women on ART (OR 0.092, corresponding to a 10.8-fold higher likelihood of not being virally suppressed). In line with these observations, mental disorders have previously been described as barriers to efficient HIV therapy in Zimbabwean adolescents (51).
Depression contributes to poor HIV outcomes and every 365 days of depression was shown to increase mortality by 71% (52, 53). Depression has been linked to low adherence to HIV medication (54), most likely explaining suboptimal VS. Nurse-delivered cognitive behavioural therapy has been shown to improve depression and adherence to HIV medication in South Africa (55). However, access to mental health care is limited in Zimbabwe, more so in high-density areas where most eligible women may not be able to afford medication or other psychiatric or psychotherapeutic interventions. Since rates of self-reported depression likely underestimated mental diseases/disorders, the true impact of depression might even be higher in our study population.
Non-disclosure of HIV Status and Stigma
Deliberate non-disclosure of HIV status may be a function of personal interests or priorities to avoid loss of trust and ensure self-preservation while simultaneously (re)constructing self-identity and reaffirm or redefine existing social interactions with the spouse/intimate partner (56). This probably may also have been the case in our study where 1.8% of women had an undisclosed HIV status, up to 7,259 days after being diagnosed of HIV. It is possible that women did not disclose due to fear of potential violence, blame or even divorce. Future studies focusing deeper into women's sexual practises including marriage issues such as extra-marital affairs may inform the dynamics of HIV status disclosure, ultimately improving HIV control in the populations at risk.
In our study, HIV status disclosure increased the likelihood of undetectable VL, confirming findings from previous studies. In a South African study of women with a new HIV diagnosis during pregnancy and immediate initiation of ART, disclosure of HIV status to their spouses/intimate partner was associated with a reduced risk of detectable VL >50 copies/ml at delivery (29). Conversely, non-disclosure of HIV status has also been associated with decreased odds for VS in Ugandan pregnant women initiating lifelong ART (57, 58) and Zimbabwean adolescents with mental disorders (51). This points to HIV-related stigma as possibly one reason for reduced rates of ART adherence.
Having a spouse on ART increased the likelihood of VS and undetectable VL. In our cohort, 21.2% of the women reported that their spouse/intimate partner had not yet undergone HIV testing, whilst 10.3% did not even know whether their spouses/intimate partners had undergone HIV testing or not. This suggests that stigmatisation and lack of efficient/open communication regarding HIV infection was prevalent within couples. The rate of 21.2% of spouses/intimate partners not tested for HIV in our cohort is corroborating the findings of the 2015 Zimbabwe Demographic and Health survey that performed interviews and HIV testing for 3,151 cohabitating couples and found that 15% of the men who tested positive had never been tested before this survey (26). Therefore, in a concerted effort to reduce new HIV infections, there is a great need to sensitise and motivate men to go for regular HIV screening. Male-friendly environments and policies may be key to increase male involvement. Previous studies have observed that when men are not involved, they may be less aware that their actions may put the lives of their spouse/intimate partner and the unborn baby at risk, for example, by stealing the spouse/intimate partner's medication or not allowing them to go for resupplies.
Strengths and Limitations of the Study
Our study has several strengths and some limitations. Strengths include the large size of our cohort with >600 HIV-infected pregnant women. Furthermore, we performed extensive clinical characterisation of participants and provided comprehensive clinical, anthropometric and socio-demographic information. The main limitation is the cross-sectional nature of this analysis with VL testing done only once. Therefore, for most women, HIV diagnosis was already known before inclusion in our cohort, and the time after initiation of ART until VS could not be directly estimated. The ART-naïve status was not verified.
In conclusion, in a cohort of pregnant women in a low-resource setting in Sub-Saharan Africa, 21.9% of the women had HIV VL >1,000 copies/ml and 38.7% had VL >50 copies/ml in late pregnancy. Analysis of risk factors re-confirmed being on ART and a sufficient period of ART use (>126 days) as the most important factors for VS. Maternal depression, ART side effects and non-disclosure of HIV status were additional risk factors of lower importance. Conversely, having a spouse on ART improved VS rates. Thus, HIV screening in all young individuals and initiation of ART immediately after diagnosis are the most important measures to suppress VL in pregnancy and reduce HIV transmission. HIV MTCT rates may be further reduced by addressing challenges of adolescent mothers, late antenatal care presenters, couple HIV testing at antenatal care centres and also economic empowering interventions.
Couples of reproductive age should be encouraged to go for a pre-conceptual visit for HIV counselling and testing for timely diagnosis and early treatment of any new infections before pregnancy. Concerted efforts are needed to optimise pregnancy health outcomes in a positive move towards attaining the ambitious UNAIDS 95–95–95 strategy aiming to end the AIDS epidemic by 2030.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Joint Research Ethics Committee (JREC) for the University of Zimbabwe Faculty of Medicine and Health Sciences and Parirenyatwa Group of Hospitals, reference number: JREC/18/15 and the Medical Research Council of Zimbabwe; reference number: MRCZ/A/1968. The participants provided their written informed consent to participate in this study.
Author Contributions
The study was conceived by KD, EG, and LRM. The study was designed by KD, EG, LRM, and TM. PTM, TM, AJM, and SB were responsible for data collection, entry and validation overseen by KD and EG. PTM and AJM performed the statistical analysis supervised by BM and SBUJ. BM, SBUJ, KD, PTM, and AJM were involved in the interpretation of findings. The manuscript was written by KD and BM. All authors were involved in manuscript revisions and approved the final draft.
Funding
The study was supported by the Wellcome Trust under the University of Zimbabwe College of Health Sciences Southern Africa Consortium for Research Excellence (SACORE) Grant Number 087537/F/08/A. Supplemental sponsorships were from the Norwegian Programme for Capacity Development in Higher Education and Research for Development under the University of Zimbabwe NORHED grant (NORHED QZA-0484MWI-13/0032), Deutsche Forschungsgemeinschaft (DFG) BU 3630/2-1, the Botnar Foundation and the Department of Visceral Surgery and Medicine, Bern University, Switzerland. None of the funding bodies were involved in the study design, data collection, data analysis, interpretation of findings and/or manuscript writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the participants and the staff of the UZBCS for their commitment, Dr. Asaph Ziruma (Obstetrics and Gynaecology Unit, FMHS), the late Dr. Sekesai Zinyowera* (National Microbiology Reference Laboratory, Sally Mugabe Central Hospital, Harare, Zimbabwe), Ms. Precious Chandiwana and Mrs. Progress Muzire (Research Support Centre), Mr. McLeod Madhaka (Research Support Centre, FMHS-UZ), Mr. Panashe Chandiwana, Sr. Mary Maria Chipiti, Sr. Mercy Ngoweni, Sr. Nyaradzo Sibiya, Mr. Ndega Taremeredzwa, and Ms. Edith Mazengera (Immunology Unit-FMHS-UZ). The study has also been supported from the Midwives and Nurses working at Kuwadzana, Rujeko, Glen View and Budiriro City of Harare Poly Clinics. *We are very grateful to Dr. Sekesai Zinyowera for facilitating the existing synergies and collaborations in HIV research between the Immunology Unit at FMHS-UZ and the Ministry of the Health and Child Care. May her soul rest in peace.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fviro.2022.838234/full#supplementary-material
Abbreviations
ART, Antiretroviral therapy; CD4, Cluster of differentiation-4; CI, Confidence interval; HIV, Human immunodeficiency virus; IQR, Interquartile range; LLV, Low-level viremia; PLWHA, People living with HIV/AIDs; MTCT, Mother-to-child transmission; NNRTI, Non-nucleosides reverse transcriptase inhibitors; RNA, Ribonucleic acid; RT-PCR, Real-time polymerase chain reaction; UZBCS, University of Zimbabwe Birth Cohort Study; UNAIDS, The joint United Nations Programme on HIV/AIDS; VL, Viral load; VS, Viral suppression; WHO, World Health Organisation.
References
1. World Health Organisation (WHO). Programmatic Update. “Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants (2012). Available online at: https://apps.who.int/iris/handle/10665/70892
2. Murray JS, Elashoff MR, Iacono-Connors LC, Cvetkovich TA, Struble KA. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS. (1999) 13:797–804. doi: 10.1097/00002030-199905070-00008
3. The Joint United Nations. The Joint United Nations Programme on HIV/AIDS 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. (2014). doi: 10.18356/9a93e1b1-en
4. World Health Organisation (WHO). Updated Recommendation on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring (2021). Available online at: https://www.who.int/publications/i/item/9789240022232
5. Panel on Antiretroviral Guidelines for Adults Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. (2017). Available online at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf.
6. European AIDS. Clinical Society. EACS Guidelines (2017). Available online at: https://www.eacsociety.org/media/guidelines_9.0-english.pdf
7. Lyatuu GW, Mwashemele SZ, Urrio R, Naburi H, Kashmir N, Machumi L, et al. Long-term virological outcomes in women who started option B+ care during pregnancy for prevention of mother-to-child transmission of HIV in Dar es Salaam, Tanzania: a cohort study. Lancet HIV. (2021). doi: 10.1016/S2352-3018(20)30308-8
8. Jensen-Fangel S, Pedersen L, Pedersen C, Larsen CS, Tauris P, Moller A, et al. Low mortality in HIV-infected patients starting highly active antiretroviral therapy: a comparison with the general population. AIDS. (2004) 18:89–97. doi: 10.1097/00002030-200401020-00011
9. Gueler A, Moser A, Calmy A, Gunthard HF, Bernasconi E, Furrer H, et al. Life expectancy in HIV-positive persons in Switzerland: matched comparison with general population. AIDS. (2017) 31:427–36. doi: 10.1097/QAD.0000000000001335
10. Nyakura J, Shewade HD Ade S, Mushavi A, Mukungunugwa SH, Chimwaza A, et al. Viral load testing among women on 'option B+' in Mazowe, Zimbabwe: How well are we doing? PLoS ONE. (2019) 14:e0225476. doi: 10.1371/journal.pone.0225476
11. Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. (2013) 339:966–71. doi: 10.1126/science.1228160
12. He N, Duan S, Ding Y, Rou K, McGoogan JM, Jia M, et al. Antiretroviral therapy reduces HIV transmission in discordant couples in rural Yunnan, China. PLoS ONE. (2013) 8:e77981. doi: 10.1371/journal.pone.0077981
13. Panel on Treatment of Pregnant Women with HIV Infection Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States (2021). Available online at: https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new-guidelines
14. Snijdewind IJM, Smit C, Godfried MH, Bakker R, Nellen JFJB, Jaddoe VWV, et al. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PLoS ONE. (2018) 13:e0191389. doi: 10.1371/journal.pone.0191389
15. Moodley D, Pillay K, Naidoo K, Moodley J, Johnson MA, Moore KH, et al. Pharmacokinetics of Zidovudine and Lamivudine in neonates following coadministration of oral doses every 12 hours. J Clin Pharmacol. (2001) 41:732–41. doi: 10.1177/00912700122010636
16. Patel M, Tedaldi E, Armon C, Nesheim S, Lampe M, Palella F, et al. HIV RNA Suppression during and after Pregnancy among Women in the HIV Outpatient Study, 1996 to 2015. J Int Assoc Provid AIDS Care. (2018) 17:2325957417752259. doi: 10.1177/2325957417752259
17. Westreich D, Cole SR, Nagar S, Maskew M, van der Horst C, Sanne I. Pregnancy and virologic response to antiretroviral therapy in South Africa. PLoS ONE. (2011) 6:e22778. doi: 10.1371/journal.pone.0022778
18. MacCarthy S, Laher F, Nduna M, Farlane L, Kaida A. Responding to her question: a review of the influence of pregnancy on HIV disease progression in the context of expanded access to HAART in sub-Saharan Africa. AIDS Behav. (2009) 13:66–71. doi: 10.1007/s10461-009-9541-2
19. Ministry Of Health and Child Welfare Zimbabwe. The Zimbabwe Programme for Prevention of Mother tp Child Transmission of HIV (PMTCT), Annual Report (2010).
20. Ministry of Health and Child Care (MOHCC) Z. Zimbabwe Population-Based HIV Impact Assessment (ZIMPHIA) 2015-16: First Report. Harare: MOHCC (2017).
21. Moyo W, Mbizvo MT. Desire for a future pregnancy among women in Zimbabwe in relation to their self-perceived risk of HIV infection, child mortality, and spontaneous abortion. AIDS Behav. (2004) 8:9–15. doi: 10.1023/B:AIBE.0000017521.26426.9d
22. Duri K, Gumbo FZ, Munjoma PT, Chandiwana P, Mhandire K, Ziruma A, et al. The University of Zimbabwe College of Health Sciences (UZ-CHS) BIRTH COHORT study: rationale, design and methods. BMC Infect Dis. (2020) 20:725. doi: 10.1186/s12879-020-05432-6
23. Swenson LC, Cobb B, Geretti AM, Harrigan PR, Poljak M, Seguin-Devaux C, et al. Comparative performances of HIV-1 RNA load assays at low viral load levels: results of an international collaboration. J Clin Microbiol. (2014) 52:517–23. doi: 10.1128/JCM.02461-13
24. Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. (1974) 19:716–23. doi: 10.1109/TAC.1974.1100705
25. UNESCO. Literacy is Nearly Universal in Zimbabwe (2019). Available online at: http://uis.unesco.org/en/country/zw
26. Zimbabwe National Statistics Agency ICF International. 2016 Zimbabwe Demographic and Health Survey 2015. Rockville, Maryland (2015). Available online at: https://www.zimstat.co.zw
27. Napyo A, Tumwine JK, Mukunya D, Tumuhamye J, Arach AAO, Ndeezi G, et al. Detectable HIV-RNA Viral Load Among HIV-Infected Pregnant Women on Treatment in Northern Uganda. Int J MCH AIDS. (2020) 9:232–41. doi: 10.21106/ijma.374
28. Yotebieng M, Mpody C, Ravelomanana NL, Tabala M, Malongo F, Kawende B, et al. HIV viral suppression among pregnant and breastfeeding women in routine care in the Kinshasa province: a baseline evaluation of participants in CQI-PMTCT study. J Int AIDS Soc. (2019) 22:e25376. doi: 10.1002/jia2.25376
29. Brittain K, Mellins CA, Remien RH, Phillips TK, Zerbe A, Abrams EJ, et al. Impact of HIV-status disclosure on HIV viral load in pregnant and postpartum women on antiretroviral therapy. J Acquir Immune Defic Syndr. (2019) 81:379–86. doi: 10.1097/QAI.0000000000002036
30. Woldesenbet SA, Kufa T, Barron P, Chirombo BC, Cheyip M, Ayalew K, et al. Viral suppression and factors associated with failure to achieve viral suppression among pregnant women in South Africa. AIDS. (2020) 34:589–97. doi: 10.1097/QAD.0000000000002457
31. Duri K, Gumbo FZ, Kristiansen KI, Kurewa NE, Mapingure MP, Rusakaniko S, et al. Antenatal HIV-1 RNA load and timing of mother to child transmission a nested case-control study in a resource poor setting. Virol J. (2010) 7:176. doi: 10.1186/1743-422X-7-176
32. Technau KG, Kalk E, Coovadia A, Black V, Pickerill S, Mellins CA, et al. Timing of maternal HIV testing and uptake of prevention of mother-to-child transmission interventions among women and their infected infants in Johannesburg, South Africa. J Acquir Immune Defic Syndr. (2014) 65:e170–8. doi: 10.1097/QAI.0000000000000068
33. Myer L. Initiating antiretroviral therapy in pregnancy: the importance of timing. J Acquir Immune Defic Syndr. (2011) 58:125–6. doi: 10.1097/QAI.0b013e31822ad573
34. Duri K, Chimhuya S, Gomo E, Munjoma PT, Chandiwana P, Yindom LM, et al. Role of antenatal plasma cytomegalovirus DNA levels on pregnancy outcome and HIV-1 vertical transmission among mothers in the University of Zimbabwe birth cohort study (UZBCS). Virol J. (2021) 18:30. doi: 10.1186/s12985-021-01494-3
35. Wester CW, Thomas AM, Bussmann H, Moyo S, Makhema JM, Gaolathe T, et al. Non-nucleoside reverse transcriptase inhibitor outcomes among combination antiretroviral therapy-treated adults in Botswana. AIDS. (2010) 24:S27–36. doi: 10.1097/01.aids.0000366080.91192.55
36. Brittain K, Remien RH, Mellins CA, Phillips TK, Zerbe A, Abrams EJ, et al. Determinants of suboptimal adherence and elevated HIV viral load in pregnant women already on antiretroviral therapy when entering antenatal care in Cape Town, South Africa. AIDS Care. (2018) 30:1517–23. doi: 10.1080/09540121.2018.1503637
37. Chetty T, Newell ML, Thorne C, Coutsoudis A. Viraemia before, during and after pregnancy in HIV-infected women on antiretroviral therapy in rural KwaZulu-Natal, South Africa, 2010-2015. Trop Med Int Health. (2018) 23:79–91. doi: 10.1111/tmi.13001
38. Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis. (2006) 6:726–32. doi: 10.1016/S1473-3099(06)70629-6
39. Zijenah LS, Bandason T, Bara W, Chipiti MM, Katzenstein DA. Mother-to-child transmission of HIV-1 and infant mortality in the first six months of life, in the era of Option B Plus combination antiretroviral therapy. Int J Infect Dis. (2021) 109:92–8. doi: 10.1016/j.ijid.2021.06.036
40. Mandelbrot L, Tubiana R, Le CJ, Dollfus C, Faye A, Pannier E, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. (2015) 61:1715–25. doi: 10.1093/cid/civ578
41. Chibwesha CJ, Giganti MJ, Putta N, Chintu N, Mulindwa J, Dorton BJ, et al. Optimal time on HAART for prevention of mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. (2011) 58:224–8. doi: 10.1097/QAI.0b013e318229147e
42. Read PJ, Mandalia S, Khan P, Harrisson U, Naftalin C, Gilleece Y, et al. When should HAART be initiated in pregnancy to achieve an undetectable HIV viral load by delivery? AIDS. (2012) 26:1095–103. doi: 10.1097/QAD.0b013e3283536a6c
43. Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, et al. Safety and pharmacokinetics of Dolutegravir in pregnant mothers with HIV infection and their neonates: A randomised trial (DolPHIN-1 study). PLoS Med. (2019) 16:e1002895. doi: 10.1371/journal.pmed.1002895
44. Ford N, Orrell C, Shubber Z, Apollo T, Vojnov L. HIV viral resuppression following an elevated viral load: a systematic review and meta-analysis. J Int AIDS Soc. (2019) 22:e25415. doi: 10.1002/jia2.25415
45. Mekuria LA, Prins JM, Yalew AW, Sprangers MA, Nieuwkerk PT. Which adherence measure - self-report, clinician recorded or pharmacy refill - is best able to predict detectable viral load in a public ART programme without routine plasma viral load monitoring? Trop Med Int Health. (2016) 21:856–69. doi: 10.1111/tmi.12709
46. Sithole Z, Mbizvo E, Chonzi P, Mungati M, Juru TP, Shambira G, et al. Virological failure among adolescents on ART, Harare City. BMC Infect Dis. (2018) 18:469. doi: 10.1186/s12879-018-3372-6
47. Tozan Y, Capasso A, Sun S, Neilands TB, Damulira C, Namuwonge F, et al. The efficacy and cost-effectiveness of a family-based economic empowerment intervention (Suubi + Adherence) on suppression of HIV viral loads among adolescents living with HIV: results from a Cluster Randomized Controlled Trial in southern Uganda. J Int AIDS Soc. (2021) 24:e25752. doi: 10.1002/jia2.25752
48. Weiser SD, Hatcher AM, Hufstedler LL, Weke E, Dworkin SL, Bukusi EA, et al. Changes in Health and Antiretroviral Adherence Among HIV-Infected Adults in Kenya: Qualitative Longitudinal Findings from a Livelihood Intervention. AIDS Behav. (2017) 21:415–27. doi: 10.1007/s10461-016-1551-2
49. Mbizvo MT, Bonduelle MM, Chadzuka S, Lindmark G, Nystrom L. Unplanned pregnancies in Harare, Zimbabwe: what is the contraceptive history and awareness of the mothers? Cent Afr J Med. (1997) 43:200–5.
50. Schwartz SR, Bassett J, Mutunga L, Yende N, Mudavanhu M, Phofa R, et al. HIV incidence, pregnancy, and implementation outcomes from the Sakh'umndeni safer conception project in South Africa: a prospective cohort study. Lancet HIV. (2019) 6:e438–46. doi: 10.1016/S2352-3018(19)30144-4
51. Simms V, Bernays S, Chibanda D, Chinoda S, Mutsinze A, Beji-Chauke R, et al. Risk factors for HIV virological non-suppression among adolescents with common mental disorder symptoms in Zimbabwe: a cross-sectional study. J Int AIDS Soc. (2021) 24:e25773. doi: 10.1002/jia2.25773
52. Mills JC, Pence BW, Todd JV, Bengtson AM, Breger TL, Edmonds A, et al. Cumulative Burden of Depression and All-Cause Mortality in Women Living With Human Immunodeficiency Virus. Clin Infect Dis. (2018) 67:1575–81. doi: 10.1093/cid/ciy264
53. Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. (2001) 285:1466–74. doi: 10.1001/jama.285.11.1466
54. Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. (2011) 58:181–7. doi: 10.1097/QAI.0B013E31822D490A
55. Joska JA, Andersen LS, Smith-Alvarez R, Magidson J, Lee JS, O'Cleirigh C, et al. Nurse-Delivered Cognitive Behavioral Therapy for Adherence and Depression Among People Living With HIV (the Ziphamandla Study): Protocol for a Randomized Controlled Trial. JMIR Res Protoc. (2020) 9:e14200. doi: 10.2196/14200
56. Viljoen L, Wademan D, Hoddinott G, Bond V, Seeley J, Bock P, et al. The act of telling: South African women's narratives of HIV status disclosure to intimate partners in the HPTN 071 (PopART) HIV prevention trial. Womens Health (Lond). (2021) 17:1745506521998204. doi: 10.1177/1745506521998204
57. Gabagaya G, Rukundo G, Amone A, Wavamunno P, Namale-Matovu J, Lubega I, et al. Prevalence of undetectable and suppressed viral load in HIV-infected pregnant women initiating Option B+ in Uganda: an observational study nested within a randomized controlled trial. BMC Infect Dis. (2021) 21:907. doi: 10.1186/s12879-021-06608-4
Keywords: antenatal plasma HIV RNA load, pre-/post-conception ART initiation, lack of viral suppression, undetectable HIV plasma viral load, days to viral suppression after ART initiation, option B plus era in a resource limited setting, low level viremia
Citation: Duri K, Munjoma PT, Mazhandu AJ, Marere T, Gomo E, Banhwa S, Jordi SBU, Misselwitz B and Mazengera LR (2022) Predictors and Timing to Viral Suppression in HIV-Infected Pregnant Women in the University of Zimbabwe Birth Cohort Study During the Era of Lifelong Antiretroviral Therapy (Option B+ Treatment Strategy). Front. Virol. 2:838234. doi: 10.3389/fviro.2022.838234
Received: 17 December 2021; Accepted: 25 January 2022;
Published: 06 April 2022.
Edited by:
Caroline T. Tiemessen, National Institute of Communicable Diseases (NICD), South AfricaReviewed by:
Ria Lassauniere, Statens Serum Institut (SSI), DenmarkDoreen Donald Kamori, Muhimbili University of Health and Allied Sciences, Tanzania
Copyright © 2022 Duri, Munjoma, Mazhandu, Marere, Gomo, Banhwa, Jordi, Misselwitz and Mazengera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerina Duri, a2R1cmlAbWVkc2NoLnV6LmFjLnp3; a2VyaW5hLmR1cmlAZ21haWwuY29t
†These authors have contributed equally to this work and share last authorship
 Kerina Duri
Kerina Duri Privilege Tendai Munjoma1
Privilege Tendai Munjoma1 Arthur John Mazhandu
Arthur John Mazhandu Exnevia Gomo
Exnevia Gomo