- 1Department of Medical Microbiology, Makerere University, College of Health Sciences, Kampala, Uganda
- 2Department of Medical Microbiology, Habib Medical School, Faculty of Health Sciences, Islamic University in Uganda, Kampala, Uganda
- 3Department of Plant Sciences, Microbiology and Biotechnology, College of Natural Sciences, Makerere University, Kampala, Uganda
- 4Department of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda
- 5Department of Biomolecular Resources and Biolab Sciences (BBS), College of Veterinary Medicine, Animal Resources and Biosecurity (COVAB), Makerere University, Kampala, Uganda
- 6Department of Molecular Biology and Immunology, College of Health Sciences, Makerere University, Kampala, Uganda
- 7Department of Physiology, Habib Medical School, Faculty of Health Sciences, Islamic University in Uganda, Kampala, Uganda
Background: Little is known about treatment eligibility in Africa for the hepatitis B virus (TREAT-B) algorithm. We investigated the treatment eligibility among the HBV chronically infected patients in a low and a high endemic region using the TREAT-B algorithm.
Methods: We recruited 227 treatment-naïve HBV-infected hospital attendees from the low and high HBV endemic regions. We assessed the treatment eligibility by testing for HBeAg serostatus and ALT levels. Socio-demographic data were collected with a structured questionnaire. The accessory correlates of treatment eligibility (AST, ALP, ALB, GGT, and TBIL) and the socio-demographic factors were analyzed by both univariate and multinomial logistic regression using the SPSS and Medcalc. The analysis was done at 95% CI and a p < 0.05 was considered statistically significant.
Results: Overall, 56.8% of the participants qualified for treatment at TREAT-B cutoffs of ≥2, with those from the low endemic region (90, 69.8%) having significantly higher treatment eligibility indication than those from the high endemic region (p < 0.05). Alcohol use and household contact with an HBV-infected person were independent socio-demographic factors significantly associated with treatment eligibility for both low and high endemic regions (p < 0.05). However, birth place was only indicated for treatment eligibility among the high endemic participants (p < 0.05). AST, GGT, and total bilirubin were the liver-related parameters significantly associated with treatment eligibility (p < 0.05), with GGT and AST being significantly elevated among the eligible low endemic dwellers compared to high endemic dwellers (p < 0.05).
Conclusion: Using TREAT-B algorithm can be a plausible alternative to the orthodox methods to specify treatment eligibility with the potential to scale up interventions targeting HBV management and elimination.
Background
Hepatitis B virus (HBV) is the causative agent for liver inflammatory diseases, which, if not diagnosed timely and subsequently treated, are likely to progress to liver fibrosis, cirrhosis, and cancer. Despite the presence of a safe and highly efficacious vaccine, the HBV infection is one of the major public health problems (1) with 257 million people chronically living with the virus globally (2). In Africa, the burden stands at 6.1% (3) in the general population but varies with the study cohort (4). In east Africa, a prevalence rate of 6.025% has been recently reported (5). Locally, approximately 1.845 million Ugandans are chronically infected with the virus and are disproportionately distributed in the 10 regions of the country. These range from low through moderate to high endemicity with a national prevalence rate of 4.1% (6) (7). A genetic component has been implicated in regional difference in burden (8, 9). The lowest cases of HBV have been reported in western, Kampala, central, and mid-eastern regions with prevalence rates of 1.8%, 1.9%, 2.0%, and 2.1%, respectively, conventionally taken as low endemic regions. On the other hand, the highest cases have been noted in the mid-north and north-east with prevalence rates of 4.6% and 4.4%, respectively (6). To achieve the ambitious sustainable development goal (SDG) of eliminating HBV infection by 2030 (2), scaling up antiviral treatment becomes inevitable. Unfortunately, accessing the diagnostic procedures to determine treatment eligibility is inadequate and not economically affordable in resource-constrained settings like ours.
Traditionally, liver biopsy has been used as the gold standard in staging the liver diseases and determining the therapeutic relevancy. However, the assay is expensive for implementation in resource constrained parts of Africa (10). Besides, progressive biopsies to monitor the extent of liver damage and stage fibrosis appears not to be clinically feasible (11). Elastography has been endorsed by the World Health Organization (WHO) as a plausible alternative (12). However, it requires sophisticated and expensive equipment restricting its extensive application to countries with developed economies (13). Alternatively, the viral load-based polymerase chain reaction (PCR) testing approach has been adopted, but still, it is very expensive for use in resource-limited settings (14). Notwithstanding the limitations surrounding the current diagnostic procedures to establish treatment eligibility among the chronic hepatitis B (CHB) patients, the WHO has recommended the screening of the general populations for the HBV in the moderate to high endemic countries in order to come up with the number of chronically infected patients that need to be enrolled on treatment (15). Accordingly, the WHO is targeting 80% of the chronic HBV-infected patients that need the anti-viral therapy to be indeed enrolled on treatment by 2030 (16). Besides, the United Nations (UN) Sustainable Development Goal-3 (SDG-3) target 3.3 highlights the combating of hepatitis B virus by 2030 (17). Nonetheless, the rapid diagnostic tests for the HBsAg and HBeAg with high sensitivity have bridged the screening gap to establish the HBV serostatus and virus replication kinetics, respectively, in communities and hospital outpatient settings (18). Unfortunately, these do not determine treatment eligibility. However, incorporation of other correlates of liver damage including AST (aspartate amino transferase), ALT (alanine amino transferase), GGT (gamma glutamyl transferase), ALP (alkaline phosphatase), ALB (albumin), PT (platelet count), and TB (total bilirubin) (19) has been ordinarily used to establish the HBV treatment candidature (20) (21). For example, the APRI (aspartate aminotransferase:platelet ratio) scores have for long guided the treatment decisions. Unfortunately, the liver function tests (LFTs) are non-specific since many cases of drug-induced or alcohol-persuaded liver diseases have been implicated in their alterations (22, 23). Therefore, these cannot be used in isolation to establish treatment eligibility in chronic HBV patients. The currently used algorithm for treatment eligibility that is recommended by the Uganda ministry of health is based on the following parameters: complete blood count to get the platelet count, LFTs particularly ALT and AST, as well HIV serological testing. The HBV viral load and abdominal ultrasound scan are recommended depending on their availability. In addition, the HBeAg test may be done but the guidelines do not advocate for it as a basis for the decision to treat HBV chronic patients in Uganda (24). The disclaimer given by the Ministry of Health pertaining to the inclusion of HBeAg in the algorithm for the decision making to treat or not to treat the HBV chronically infected patients underestimates its potential in scaling up treatment candidacy in communities.
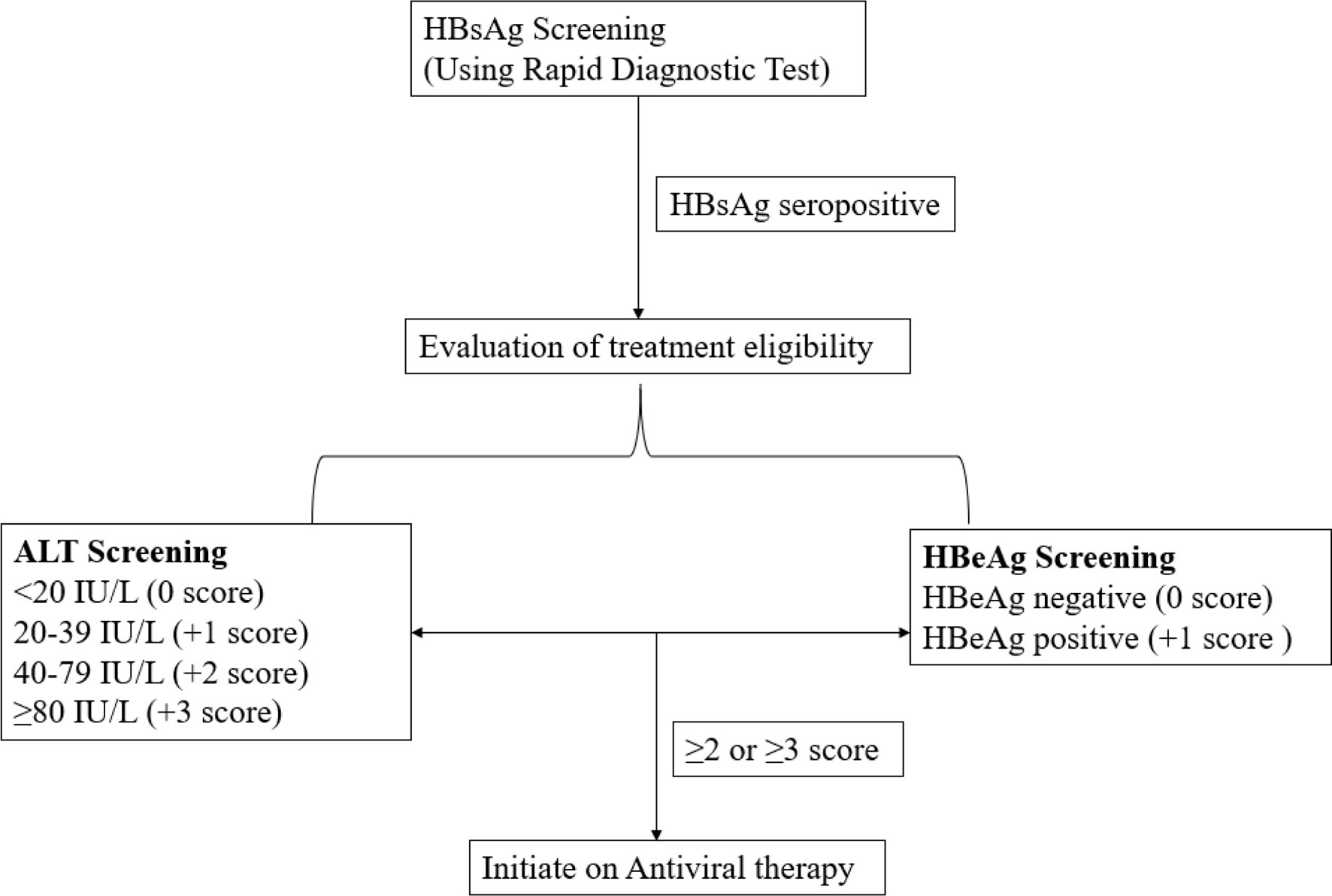
Nonetheless, the ALT in synergy with HBeAg has proved to be a suitable option for the treatment eligibility and has been exploited in the development and validation of a novel HBV infection treatment eligibility algorithm called treatment eligibility in Africa for the hepatitis B virus (TREAT-B) (14, 25). It is a simple score designed based on the ordinary laboratory tests extensively available in the peripheral laboratories in the low- to medium-income countries (LMICs), without depending on the viral load (HBV DNA levels), Fibroscan (elastography) or liver histopathology (liver biopsy). In this model (Figure 1), the total points of TREAT-B are obtained by summing up the HBeAg score as negative (0 point) or positive (1 point) and ALT score in the ranges; <20 IU/L = 0 point, 20–39 = 1 point, 40–79 = 2 points, or ≥80 = 3 points. Thus, TREAT-B scores range from a minimum of 0 for HBeAg-negative and ALT <20 IU/L to a maximum of 4 for HBeAg-positive and ALT ≥80 IU/L (25).
The TREAT-B score strategy snugly fits into the resource-constrained setting unlike other treatment approaches (26) that require specially trained staff and the sophisticated equipment because both ALT and HBeAg are readily accessible and are economically viable in nearly all LMICs (16). Besides, the TREAT-B algorithm requires measurement of ALT using one blood sampling unlike other diagnostic procedures that require frequent visits to the hospital for assessment of ALT (25). Moreover, TREAT-B can use values of ALT adapted from the local laboratory and the cutoff can be adjusted from ≥2 to ≥3 at a relatively lowered sensitivity without compromising specificity in extreme resource poor setting limiting the number of chronic HBV-infected patients under treatment and minimizing those who may not need the lifelong treatment (27). Our study therefore sought to estimate the proportion of the chronic HBV patients eligible for treatment among a sample of HBsAg seropositive treatment-naïve hospital attendees screened at Kibuku health center IV and Kigtum general hospital based on the TREAT-B algorithm using the cutoff of ≥2.
Methods
Study Sites
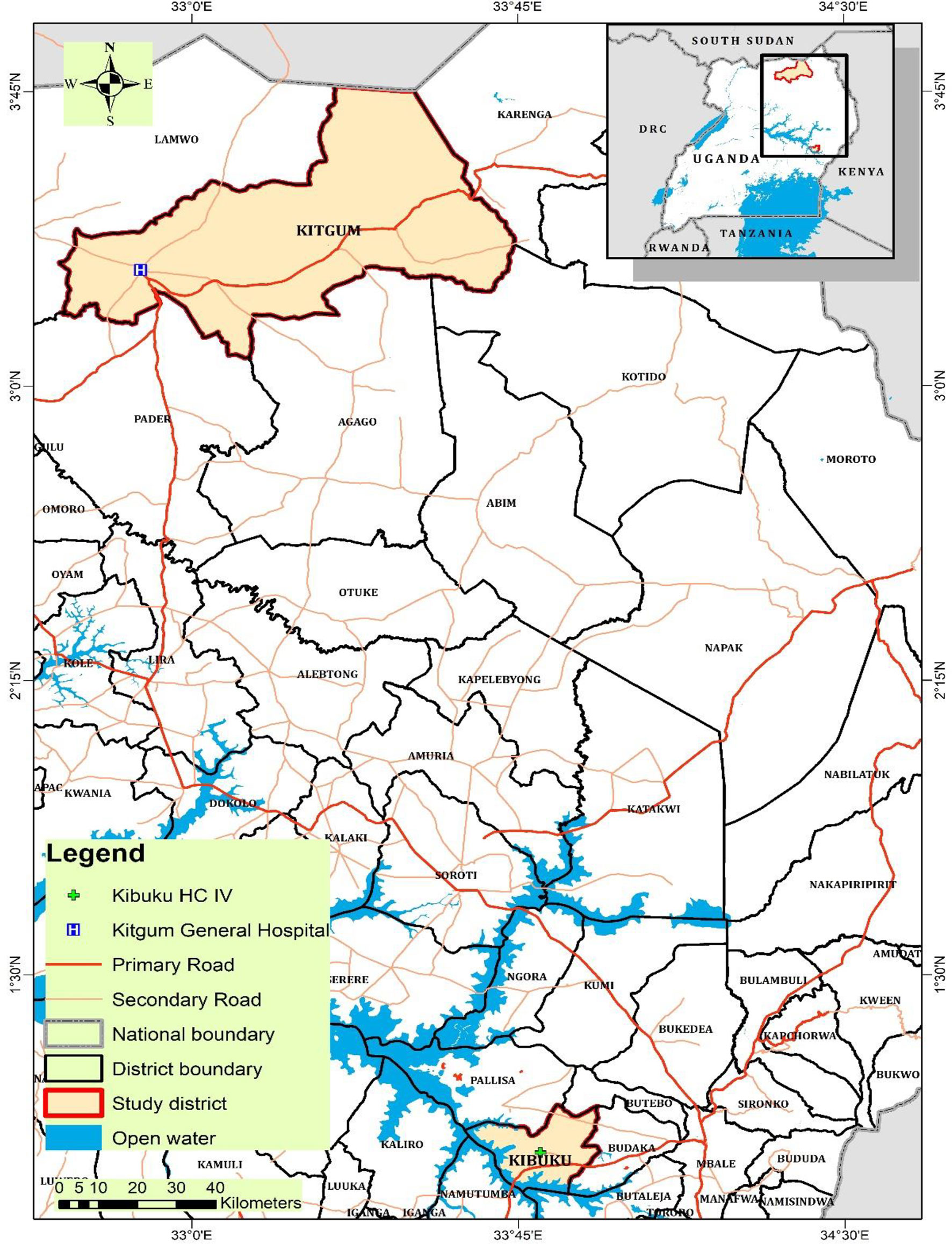
Two sites were purposively chosen for sample collection. Kibuku health center IV and Kitgum General Hospital. Kibuku health center IV was chosen for the low endemic site because it was a pilot site for HBV vaccination in the eastern region during the study period. Similarly, Kitgum General Hospital was selected for the high endemic site because it had just completed massive screening for HBV and vaccination, so the hospital administration was monitoring HBsAg seropositive patients prior to enrollment onto the antiviral therapy through the Ministry of Health hub system of viral evaluation. Kibuku district has a population of 250,600 with an area of 489.1 km2 and a population density of 512.4/km2 (28) at an elevation of 1,100 m above sea level. Kitgum district has a population of 204,012 with a total area of 3,960 km2 and a population density of 62.6/km2 (28) at an elevation of 760 m above sea level.
Study Design and Population
This was a cross-sectional hospital-based study with quantitative methods of data collection and analysis. The study population was the treatment-naïve HBsAg seropositive persons screened for HBV HBsAg serostatus prior to vaccination during the 2019–2020 HBV immunization program in eastern Uganda and the HBsAg seropositive cohort plus volunteer hospital attendees in northern Uganda. The sampling was done for a period of 12 months from September 2019 to September 2020. For the eastern low endemic region, the study site was Kibuku district Health Center IV, while for the northern high endemic region, the study site was Kitgum General Hospital. From either study site, the subjects included in the study were adult HBsAg seropositive. From the low endemic eastern region, the subjects screened for inclusion in the study were coming from the Kibuku district and from the neighboring districts of Butebo, Budaka, Butaleja, Namutumba, and Pallisa. From the high endemic northern region, participants were coming to Kitgum district General Hospital from Kitgum district and the neighboring catchment areas that included Lamwo, Karenga, Pader, Agago, and Kotido districts (Figure 2).
Sample Size Determination
The sample size (n) was estimated by using the formula described by Cochran (29). The national HBV prevalence of 4.1% (6) was used when calculating the sample size. A standard normal deviate corresponding to the critical region of 1.96 at 5% precision was used. After correcting for the 10% loss due to unclear sample, a sample size of 227 treatment-naïve individuals, 132 (58.15%) from the low endemic region and 95 (41.85%) from the high endemic region, was used. We had proportionately more participants from the low endemic eastern region compared to the high endemic northern region because the eastern region is more populated than the northern region (28) and required more representation.
Eligibility Criteria
Inclusion was based on HBsAg seropositivity, being treatment-naïve, having attained 18 years and above at the time of sampling, and being a resident of Kibuku or Kitgum districts and the surrounding areas. Exclusion was based on exposure to treatment for HBV, being under the age of 18 years, or turning down the request to participate in the study.
Measurements
In this study, being infected with HBV was determined by HBsAg seropositivity. Household contact with an HBV-infected patients was defined as the presence of unprotected contact with an apparently healthy person at home. Blood transfusion was defined as any previous incidence of exposure to foreign blood through a transfusion. Infection with a sexually transmitted disease was defined as any kind of previous diagnosis and treatment with such a disease. Alcohol intake, injection drug use, and birth place were measured as self-reported use of alcohol, self-injection with any drug, and self-reported site of birth (hospital or not).
Data Collection, Blood Sampling, and Ethical Approval
The demographic characteristics and the predictors of HBV infection were collected by using a closed-ended questionnaire administered by a nurse on site. For the laboratory investigations, 4 ml of blood was drawn by vein puncture into anticoagulant vacutainers from which serum was immediately obtained to avoid hemolysis. The serum was kept in sterile viols and stored at −20°C till further use. The study protocol was approved by the Research and Ethics Committee of the School of Biomedical Sciences, Makerere University (reference number SBS-REC-708) and the Uganda National Council for Science and Technology (UNCST) (reference number HS575ES).
Serological Testing
We used the 5-panel HBV One Step Hepatitis B Virus Combo Test Device (FastepR, HBV-P43M) on serum samples following the manufacturer’s instructions. The test is a rapid chromatographic immunoassay for the qualitative detection of hepatitis B virus markers including the HBsAg and HBeAg, in serum or plasma.
Data Presentation and Statistical Analysis
Categorical data were presented as frequency and percentages. Univariate analysis was used to determine the crude odds ratio (COR) whereas multinomial logistic regression analysis was used to determine the adjusted odds ratio (AOR). All the analyses were done at 95% level of significance and a p < 0.05 was considered to be statistically significance. Data were analyzed using SPSS version 26 and Medcalc version 20.010.
Liver enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (γ-GT), alkaline phosphatase (ALP), as well as other chemistry parameters including albumin (ALB) and total bilirubin (TB) were evaluated by standard methods using the Chemistry analyzer B120 (Mindray, China). The screening for HBsAg and HBeAg was done on the spot during sample collection by the laboratory technician whereas the chemistry parameters were analyzed at Kibuli Muslim Hospital, the teaching hospital of the Habib Medical School (HMS), Faculty of Health Sciences (FHS), Islamic University in Uganda (IUIU).
Results
Socio-Demographic Characteristics
In the present study, we have recruited 227 treatment-naïve chronic hepatitis B virus patients. 132 (58.15%) were recruited from the low endemic region whereas 95 (41.85%) were from the high endemic region. Majority of our participants were 30 years and above (50.7%), married (63.4%), women (63.9%), and with basic primary education (42.3%) (Table 1).
Treatment Eligibility
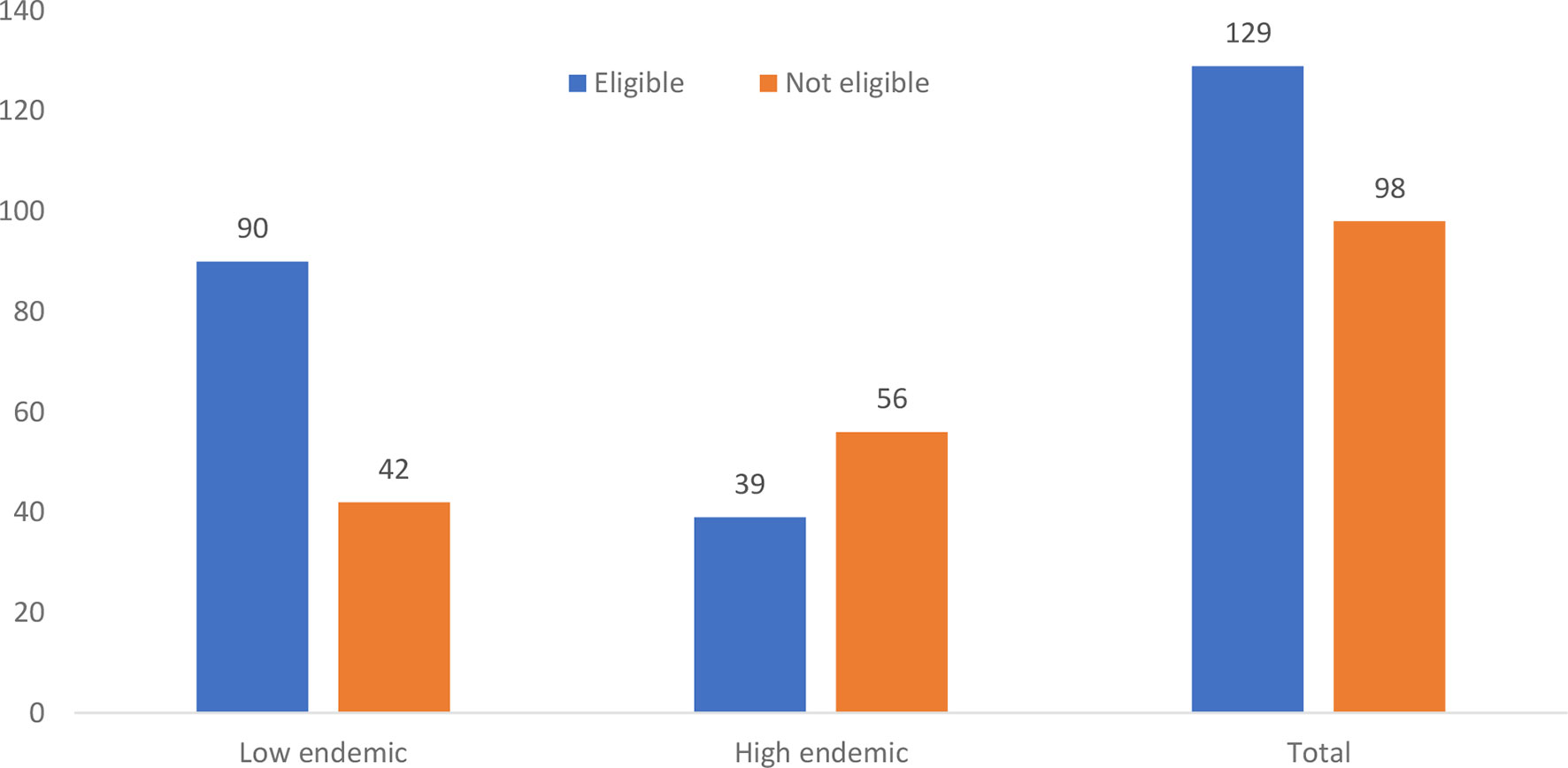
Overall, 129 (56.8%) of the chronically HBV-infected participants were eligible for treatment according to the TREAT-B score of ≤2.0. Of these, 90 (69.8%) were from the low endemic region and 39 (30.2%) were from the high endemic region (Figure 3). On analysis of the relationship between the treatment eligibility and endemicity, the participants from the low endemic region were more likely to be eligible for treatment than the participants from the high endemic region (χ2 = 16.6, dof = 1, N = 227, p < 0.000) (Table 2).

Table 2 2 × 2 table showing the analysis of treatment eligibility by endemicity using the TREAB-B score of ≥2.0 for both low and high endemic regions.
Most of the participants eligible for treatment were female (77, 59.7%), 30 years and above (70, 54.3%), married (83, 64.3%), and of primary level of education (63, 48.8%) (Figure 4).
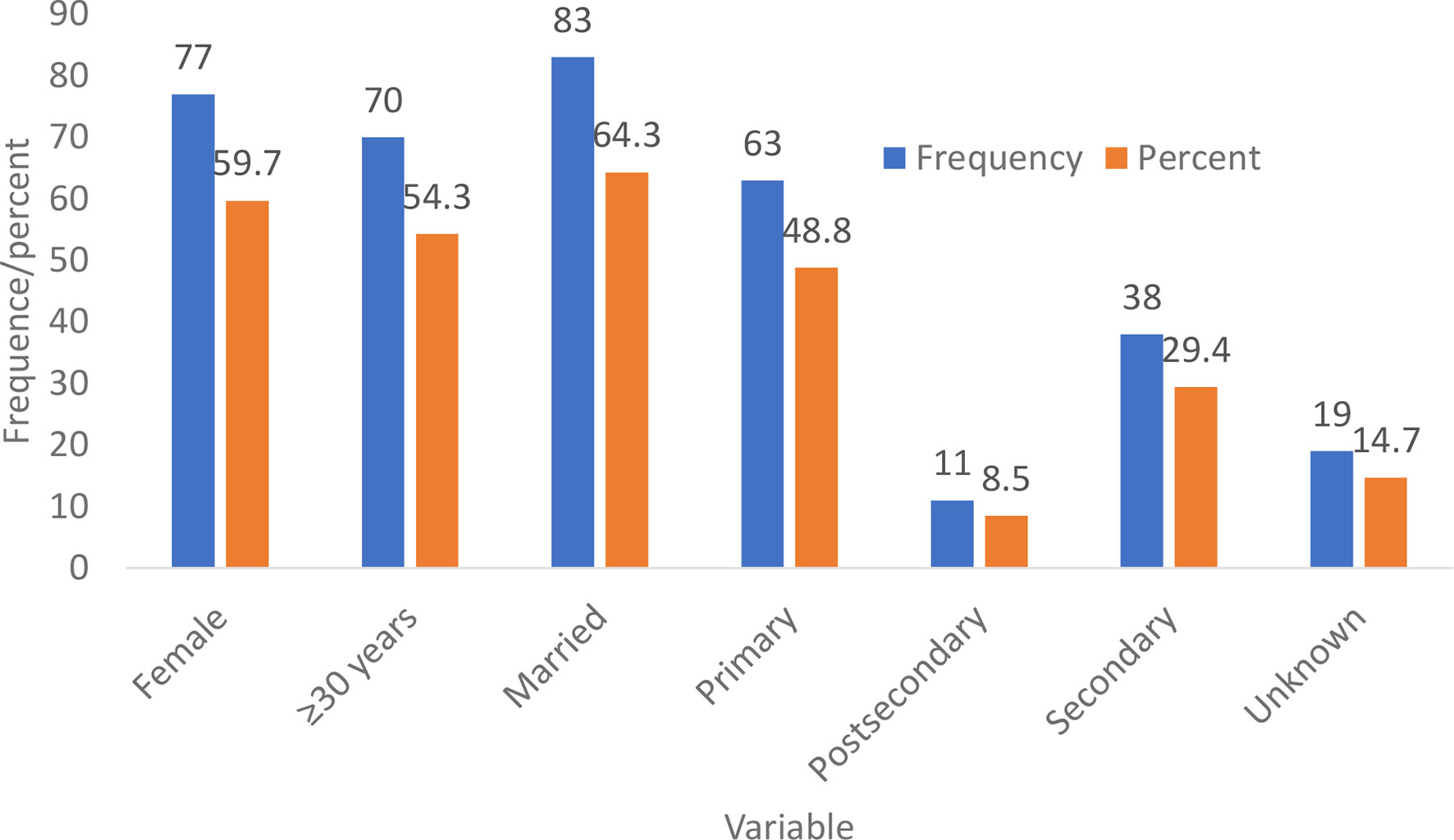
Figure 4 Demographic characteristics of the participants who were eligible for treatment indication using the TREAT-B score ≥2.0 from both the low and high endemic regions.
When we disaggregated data on the predictors of treatment eligibility (socio-demographic and LFTs) with treatment eligibility among participants from the low endemic region, multinomial logistic regression analysis showed that only alcohol use and household contact with an HBV-infected person were the socio-demographic factors associated with the treatment indication. Similarly, only AST and ALB were the LFTs significantly associated with the treatment eligibility (Table 3). Thus, non-alcohol use was associated with reduced odds of treatment eligibility (AOR = 0.17, 95% CI = [0.05 to 0.56], p = 0.004). Similarly, people who reported not to have lived with an HBV-infected person had reduced odds or being eligible for treatment (AOR = 0.143, 95% CI = [0.059 to 0.344], p < 0.000) (Table 3). Thus, our results showed that people who did not use alcohol and those who did not have any contact with an infected person were only 17% and 14.3% eligible for treatment, respectively. On the contrary, persons eligible for treatment from the low endemic region had their AST significantly elevated (AOR = 9.45, 95% CI = [2.93 to 30.7], p < 0.000) compared to their counterparts. Hence, our findings illustrated that those persons eligible for treatment had their AST 9.45 times elevated when compared to those without treatment indication. As regards the albumin and treatment indication, persons with hypoalbuminemia were associated with reduced odds of treatment eligibility (AOR = 0.22, 95% CI = [0.07 to 0.68], p = 0.009). Thus, persons with hypoalbuminemia were only 22% more likely to be eligible for treatment.
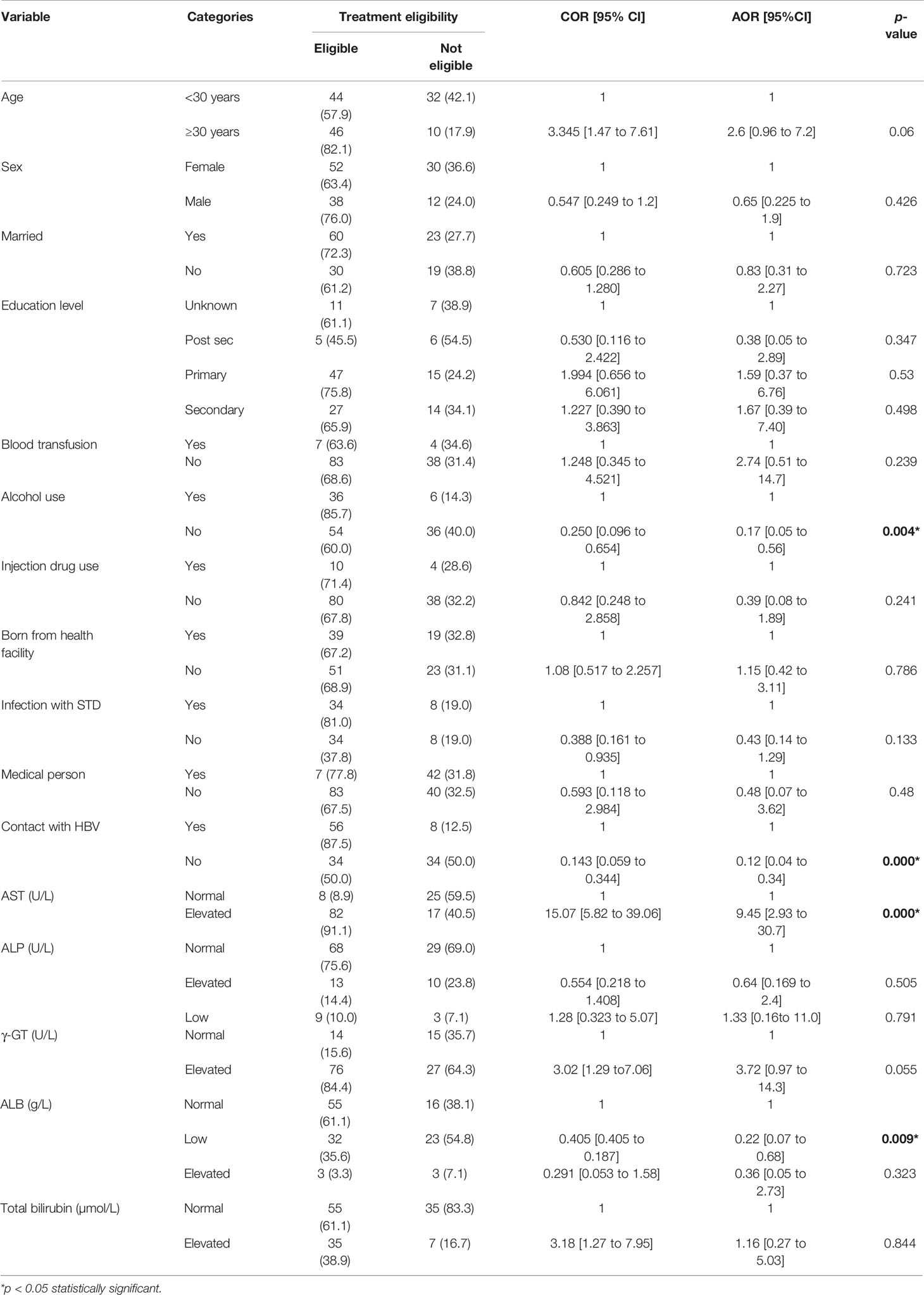
Table 3 Socio-demographic factors and liver function tests associated with treatment eligibility among the HBsAg seropositive participants from the low endemic region.
Further still, for the high endemic region, alcohol use (AOR = 23.2, 95% CI= [3.4 to 157.8] p = 0.001) and birth place (AOR = 10.4, 95% CI= [1.7 to 62.9], p = 0.011) were significantly associated with treatment eligibility. Thus, the persons who reported to be using alcohol were 23.2 times more likely to be eligible for treatment compared to those who did not. Similarly, the persons who were born from home were 10.4 times more likely to be eligible for treatment than their counterparts who were born from the hospital. In contrast, persons who reported not to have had prior contact with an HBV-positive family member were at a reduced odds of treatment indication (AOR = 0.068, 95% CI = [0.01 to 0.5], p = 0.008). Therefore, the likelihood that a person without prior contact with an HBV-infected is eligible for treatment is 6.8% compared to those who have had contact (Table 4). Regarding the LFTs, persons eligible for treatment had elevated total bilirubin (AOR = 18.5, 95% CI= [3.55 to 96.9], p = 0.001) and ALP (AOR = 0.14, 95% CI = [0.024 to 0.8], p = 0.031) and were significantly eligible for treatment. Consequently, persons eligible for treatment from the high endemic region were 18.5 times more likely to have elevated bilirubin and a 14% likelihood of having low ALP.
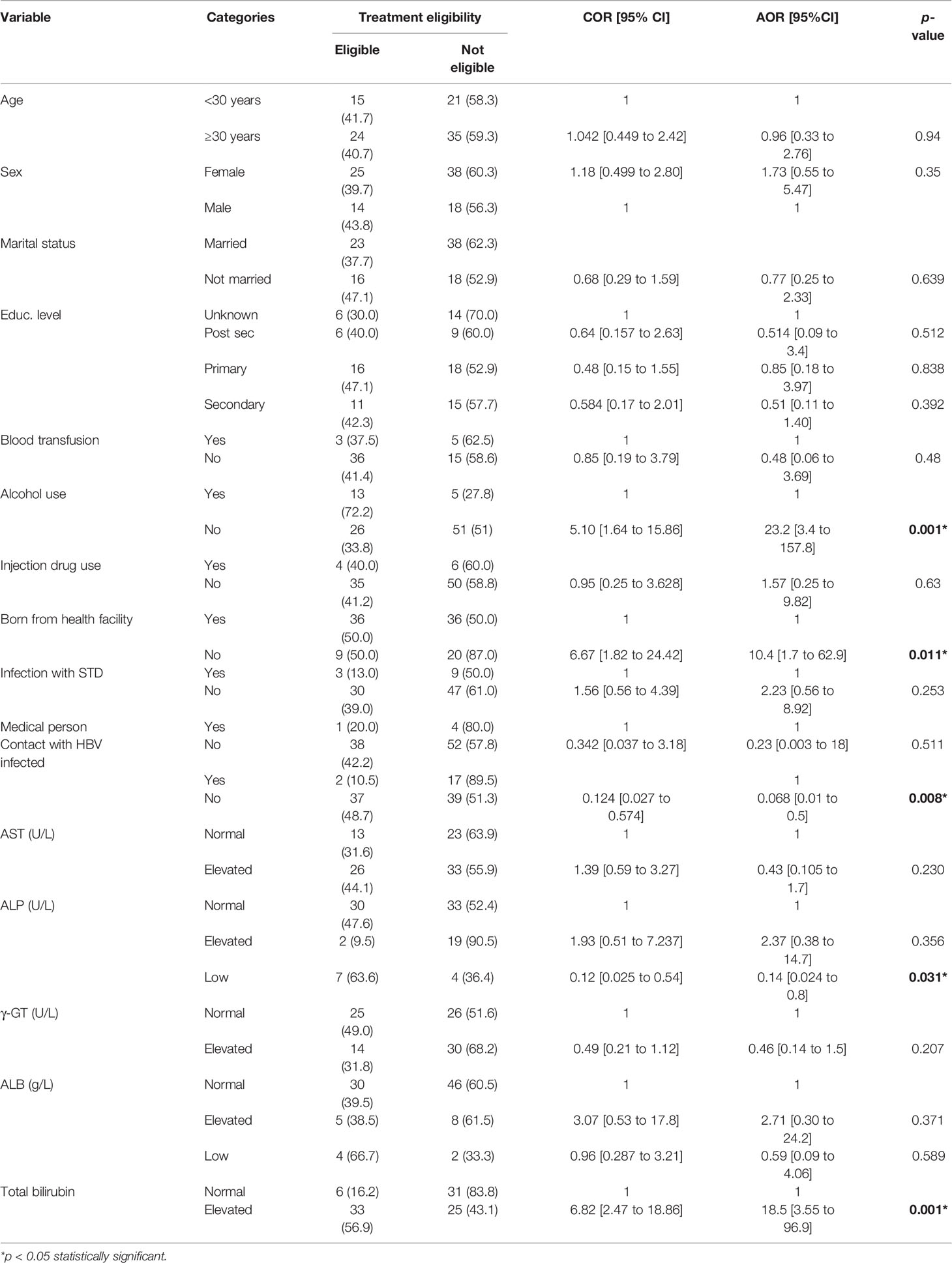
Table 4 Socio-demographic factors and liver function tests associated with treatment eligibility among the HBsAg seropositive participants from the high endemic region.
In addition. when we compared the socio-demographic factors that were significantly associated with the treatment eligibility using the TREAT-B score among the eligible participants for the low and high endemic participants, interesting results were obtained. Only birth place (AOR = 17.1, 95% CI= [4.2 to 69.4], p < 0.000) and household contact (AOR = 0.03, 95% CI= [0.007 to 0.2], p < 0.000) were the socio-demographic factors that remained significantly associated with treatment indication. Regarding the LFTs, AST (AOR-14.5, 95% CI = [1.5 to 143.8], p = 0.022), GGT (AOR = 22.4, 95% CI = [3.1 to 162.8], p = 0.002), and albumin (AOR = 0.01, 95% CI = [0.001 to 0.12], p < 0.000) were significantly associated with treatment eligibility by endemicity. Thus, people who were born from home and eligible for treatment were 17.1 times more likely to be from the low endemic region as opposed to the high endemic region. In addition, people eligible for treatment and having elevated AST and γ-GT were 14.5 and 22.4 times, respectively, more likely to be from the low endemic region.
In contrast, people without prior contact with an HBV-infected person but eligible for treatment were 97.0% least likely to be from the high endemic region but rather more likely to be from the low endemic region. Similarly, people who were eligible for treatment from the low endemic region were 90.0% least likely to have normal bilirubin (Table 5).
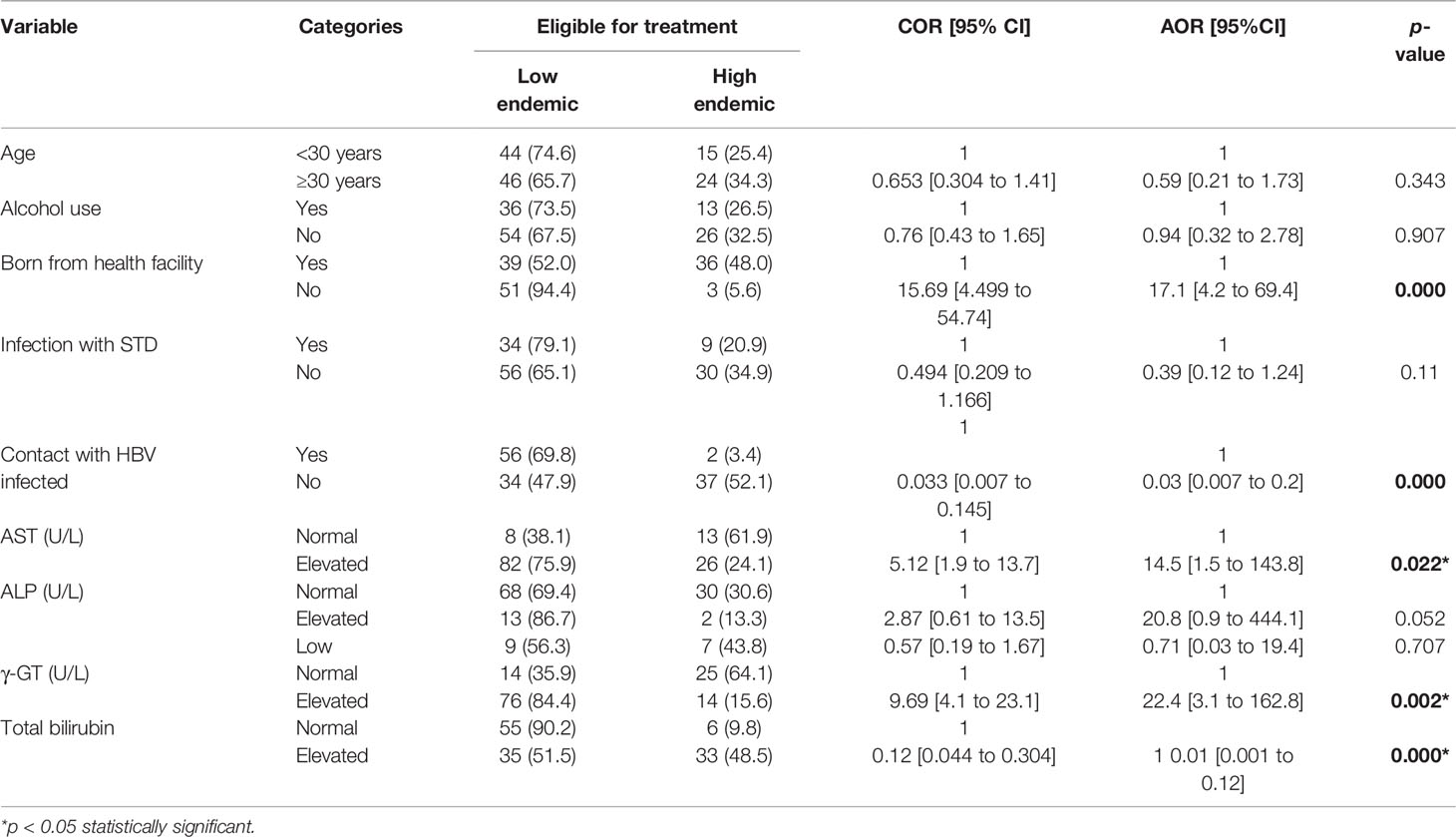
Table 5 Socio-demographic factors and liver function tests associated with eligibility for treatment among the HBsAg seropositive participants from the low and high endemic regions.
Discussion
Uganda is a country of moderate to high endemicity for the HBV infection with a projected prevalence of over 4.1% in the general adult population ranging from 0.8% in the south-west to 4.6% in the mid-north (6). The burden is highest among persons living with HIV (7.4%) (30) and is disproportionately distributed in the different regions ranging from 4.7%–8% in Southern Uganda to 6%–12% in Northern Uganda (6, 12). Unfortunately, treatment eligibility candidature for HBV infection is not well documented in our communities. In 2019, the Uganda Ministry of Health developed a national strategic plan for viral hepatitis and released guidelines for the diagnosis and treatment of chronic hepatitis B virus infection. These guidelines are based on the complex and expensive tests and are hence difficult to implement in remote and rural areas (31). With an estimated 1,845,000 people chronically infected with HBV in Uganda (6), based on the Polaris Observatory Collaborators (POC) data (32), it is projected that 647,000 (34.97%) people require antiviral therapy against the hepatitis B virus. However, only 3,000 people (<1%) are currently receiving the antiviral therapy (32) because of the mainly asymptomatic presentation of CHB infection making case detection challenging (33). The WHO target on eradication of the HBV requires a drastic shift from 8% in 2015 to 80% in 2030 of those eligible for treatment to definitely get the therapy (16). To realize this ambitious target, 517, 600 HBV-infected individuals in Uganda should be enrolled on treatment by 2030. Therefore, over the next decade, an additional 517,600 people will have to be enrolled on the antiviral therapy against HBV. Achieving this will not be plausible in the absence of cost-effective and resource-constrained compatible treatment eligibility algorithms. The appraisal of the LFTs as non-invasive markers dates back from the 1970s (20). However, many of them lack specificity since they are produced by multiple organs in response to altered function (34, 35). Presently, the control of the HBV epidemic in Uganda largely depends on collective infant hepatitis B virus immunization program called the Uganda National Program on Immunization (UNEPI) introduced in 2002 (31). However, studies elsewhere have shown that HBV immunization alone will be inadequate to achieve the ambitious United Nations (UN) and World Health Organization (WHO) goal of HBV eradication in the next decade (25). Thus, it is essential to scale up interventions that target screening and treatment at a manageable cost in resource-limited settings to reduce the transmission by suppressing viral load (36). Consequently, the TREAT-B algorithm as an accurate alternative to the current complex treatment criteria that rely on invasive liver biopsy, HBV viral load testing, and elastography is believed to have a profound potential in complementing the current immunization strategy in order to eliminate HBV by 2030.
Here we report the estimation of treatment eligibility among the CHB-infected patients using the TREAT-B strategy previously described by Yusuke et al. (25). Overall, in a sample of 227 CHB-infected treatment-naïve patients that participated in our study from both low and high endemic regions, 56.8% were eligible for treatment using the TREAT-B cutoff ≥2. Comparing the treatment eligibility by HBV endemicity, in a sample of 132 from low endemic and 95 from the high endemic, we found a large proportion of patients in need of antiviral treatment using the cutoff ≥2 of 69.8% and 30.2%, respectively. To the best of our knowledge, this is the first study in Uganda to analyze treatment eligibility using the TREAT-B algorithm among CHB-infected treatment-naïve persons attending the outpatient clinic in a large government hospital from a high endemic region and those coming for screening during the Ministry of Health screening program prior to vaccination from the district health center IV in a low endemic region.
The TREAT-B strategy uses only ALT and HBeAg tests, both of which are easily accessible and can be carried out in resource-limited and rural areas of Uganda making their incorporation in district hospital and health centers plausible. Using the TREAT-B score cutoff ≥2, a large proportion of patients from the low endemic and high endemic region obligated treatment indication. Consequently, using the TREAT-B score, some CHB-infected patients may receive unnecessary antiviral therapy posing significant medical and cost implication burdens since once initiated on antiviral therapy, the treatment is for life with potential toxicity (25, 14). In our study, nonetheless, this can be mitigated by using a score of ≥3, which though compromises the sensitivity has no effect on specificity and can be applied in areas where resources are severely constrained, restricting the number of patients enrolled onto antiviral therapy and minimizing cases of unnecessary lifelong treatment (25). Unfortunately, although the TREAT-B uses both ALT and HBeAg for a decision to treat or not, which are readily available and can potentially be measured as point-of-care (POC) tests, the detection limit of HBeAg rapid diagnostic tests has been questioned and needs to be improved (37).
Our result of 56.8% of HBV-infected patients indicated for treatment is higher than the estimate of 32% reported elsewhere in Asia, 31.1% in Zambia (38), 41.6% in Vietnam (39), and 41.0% among the Burkinabe of West Africa (14) while slightly lower than the eligibility of 64% reported in Hong Kong (40). The differences in the proportion of chronic HBV patients eligible for treatment reported in our study and other studies reported in Zambia and Gambia may be due to differences in the implantation of the prevention and control strategies by the local and regional governments. In Uganda, the data on the circulating genotypes is scanty (41) but in Zambia and Gambia, the predominant genotypes are A1 (38) and E, respectively. These differ in terms of disease severity. Genotype A1 has been allied with more severe disease compared to E (42).
In Uganda, 647,000 CHB patients are qualified for treatment eligibility (32). Therefore, our results suggest that using the simplified TREAT-B score, 1,047,960 hepatitis B chronically infected persons among our study populations will receive treatment, suggesting that 400,960 (21.7%) would be unnecessarily enrolled on the antiviral therapy. Certainly, this has financial implications to already poorly funded health systems in Uganda especially at a time when most of the resources have been diverted to COVID-19 management. However, widening antiviral therapy coverage has a profound potential towards reducing infection kinetics (43). Notwithstanding, our study gave hospital-based estimates whose findings should be interpreted with caution and may overestimate the proportion of persons eligible for treatment. Hence, population-based studies on the TREAT-B eligibility are warranted for more robust results.
Our findings suggest a significantly large number of patients eligible for treatment by the TREAT-B algorithm from the low endemic region compared to the high endemic region (p < 0.05). This is contrary to the study by Veldhuijzen et al. (33) who reported a profound need for treatment eligibility among high endemic migrants compared to those low endemic parts. The high treatment indication for participants from the low endemic region can be accounted for by several observations from our results and reports from literature, the first being the observed significant association between treatment eligibility and alcohol use among the low endemic participants. The odds of treatment eligibility were increased among alcohol users from the low endemic region (p < 0.05). This could be in part responsible for the elevation of ALT as earlier reported by Lin et al. (44) and Vanderlinde et al. (22). Secondly, the participants eligible for the TREAT-B indication from the low endemic region had their AST 9.45 times elevated compared to those not eligible for treatment. The AST has been implicated in alcoholic liver injury. Moreover, alcohol and hepatitis B virus infection are the top causal agents of liver cancer globally (45). This finding is consistent with the earlier reports on the prevalence of alcohol hepatitis in Uganda by Opio et al. (46) in a national referral hospital based study and from the Uganda Non-Communicable Diseases (NCDs) prevalence survey that highlighted a 10% prevalence of liver diseases due to alcohol misuse (47). Besides, alcohol use in Uganda is very high and available data show that Uganda is one of the top countries globally with the highest consumption of alcohol (48). For the high endemic region, the results are inconsistent with the reported trend of alcohol use and liver-related disease (49). This could be attributed to the self-reported alcohol consumption. The participants could have been dishonest with their alcohol intake status. Thirdly, the participants eligible for treatment from the low endemic region had their γ-GT 22.4 times more elevated than those from the high endemic region. This is consistent with the findings by Ban et al. (50). The elevations in γ-GT observed among the exposed subjects to HBV could be predictive of liver-related damage. The γ-GT is the most specific and thus accurate surrogate marker of liver disease, also originating from the kidney as well as from the intestines. Despite this, most of the γ-GT in serum is from hepatocytes (51). In the current study, the γ-GT levels were significantly higher among the low endemic eligible participants for treatment compared to their counterparts from the high endemic region (p < 0.0001). Nonetheless, sclerosing cholangitis, cholecystitis, and alcoholism have also been implicated in causing elevations in γ-GT giving false-positive rate when γ-GT is used alone (52, 53, 54, 55). Fourthly, the participants eligible for treatment from the low endemic region reported significantly higher odds of being born from home rather than from a health facility. This could probably have increased the chance of infection during birth that has over the years contributed to liver diseases (56). Besides, despite the Ugandan Ministry of Health’s ban on the traditional birth attendants in 2010, 80% of the women in rural areas still prefer their services to the formal health services (57). Thus, our results suggest that the Ministry of Health should strengthen and implement the ban imposed upon the traditional birth attendants to curb the vice and reduce the risks of mother-to-child HBV transmission. Fifthly, the observed significant hypoalbuminemia among the low endemic participants eligible for treatment is a marker of liver disease (58) while the reduced odds of hypoalbuminemia among the high endemic participants is indicative of reduced likelihood of liver damage confirming the low treatment eligibility observed in our study. Finally, eligible participants for treatment indication from the high endemic region had significantly low ALP (p < 0.05). The ALP has been implicated in drug-induced liver injury (23). Thus, our results suggest that in the high endemic region, the relative importance of drug abuse in liver disease indication is minimal. In addition, the hyperbilirubinemia observed among the treament indicated that participants from the high endemic region could be attributed to other conditions other than viral hepatitis including dietary toxins (59) and parasitic infections (60) like fascioliasis and hydatidosis (61, 62, 63, 64, 65).
Additionally, treatment indication using the TREAT-B score was significantly higher among those participants who reported household contact with an HBV-infected person for both the low and high endemic regions (p < 0.05). This finding is consistent with earlier reports from related studies by Shedain et al. (66) in Nepal, Muljono (67) in Indonesia, Pereira et al. (68) in Brazil, and Sofian et al. (69) in Iran, which implicated HBV infection to HBV seropositivity of a family member including the mother, father, or a sibling. Moreover, presence of an HBV carrier member in the family has been reported to increase the risk of HBV transmission by 11%–57% in studies by Ragheb et al. (70) in Egypt, Lobato et al. (71) in Brazil, and Mohammad Alizadeh et al. (72) and Sofian et al. (69) in Iran. This is due to the highly contagious nature of the virus, being 100 times more contagious than HIV, and its ability to remain on surfaces for 7 days (56). Thus, our results suggest that HBV carriers should be screened at the household level to reduce intra-familial transmissions. Finally, our advocacy for TREAT-B algorithm appears to be in fair conformity with the observed treatment indication from literature for age 30 years and above (73), AST elevation (74), alcohol use (75), and γ-GGT (76) for both participants from the low and high endemic regions.
The results from our study have thoughtful relevance to global HBV management strategy by filling the knowledge gap pertaining to treatment eligibility for chronically infected HBV patients. Being cheap, the method is economically viable with profound potential for use in resource-limited settings. Accordingly, because of the already weak health systems in most of the Sub-Saharan African countries that have been constrained by the COVID-19 pandemic, these economic compatible approaches should be prioritized.
Our study could not go without limitations. Firstly, the study findings are not representative of the Ugandan general population because we confined the study to outpatients in two hospitals: one in Eastern Uganda and another in Northern Uganda. Secondly, the cross-section nature of the study with only one assessment of the LFTs could have provided an overestimation of people eligible for treatment. Besides, one-off elevation of liver enzymes could not have been quite informative because factors other than HBV can be attributed to liver inflammation. Thirdly, hepatitis Delta could be prevalent among the study participants. However, we were not able to screen for HDV. Fourthly, the intake of alcohol was self-reported and there is a likelihood of under-reporting alcohol intake. Moreover, the influence of other drug abuse was not investigated. Fifthly, the retrospective nature of the study could have been associated with recall bias. Finally, we did not establish other causes of liver inflammation such as drug persuaded injury and exposure to afro toxins, which can potentially elevate the predictors of liver inflammation.
Conclusion
There is a need for screening of the exposed patients to HBV using point-of-care tests in order to establish treatment eligibility in a bid to eliminate HBV in the next decade as projected by the sustainable development goal 4. The concordance observed between the TREAT-B eligibility scores and the correlates of liver damage justify the appropriateness of this technique in bridging the diagnostic gap between the orthodox liver assessment methods like liver biopsy and expensive methods like magnetic resonance imaging (MRI) and computed tomography (CT).
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research and Ethics Committee of the School of Biomedical Sciences, College of Health Sciences, Makerere University (reference number SBS-REC-708) and the Uganda National Council for Science and Technology (UNCST) (reference number HS575ES). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HuK and HS conceived the idea. HuK, AW, and AK participated in the data presentation, analysis, and discussion. HuK wrote the final manuscript draft. PO, DN, HK, CK, EW, HS, JE and DK reviewed the manuscript draft. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Islamic University in Uganda (IUIU) through the Islamic Development Bank (IsDB) Grant (Grant number: IsDB-IUIUI-001) awarded to HuK by the Research Publications and Innovations (RPI) Department of the Islamic University in Uganda (IUIU) for his doctoral studies. We also received funding from Makerere University, Research and Innovation Fund (Mak-RIF) awarded to HS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the Makerere University, Research and Innovation Fund (Mak-RIF) for the grant awarded to HS for funding the study. We are also grateful to the Islamic Development Bank through the Research Publications and Innovations Department of The Islamic University in Uganda for the funding of the study through the grant awarded to HuK. Finally, we thank Dr. Ntanda K Moses, Makerere University College of Computing and Information Sciences for the ICT technical support.
Abbreviations
HBV, hepatitis B virus; ALT, alanine aminotransferase; APRI, aspartate aminotransferase:platelet ratio; TREAT-B, treatment eligibility in Africa for the hepatitis B virus; WHO, World Health Organization; LMICs, low- and medium-income countries; USD, United States dollar; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; ALP, alkaline phosphatase; ALB, albumin; TB, total bilirubin; HBeAg, hepatitis B pre-core antigen; CHB, chronic hepatitis B; CD4, cluster of differentiation-4; LFTs, liver function tests; CBC, complete blood count; UNHL, Uganda National Health Laboratory services; PCR, polymerase chain reaction; REC, research and ethics committee; UNCST, Uganda National Council for Science and Technology; HBsAg, hepatitis B surface antigen; RDT, rapid diagnostic test; γ-GT, γ-glutamyl transferase; CHBV, chronic hepatitis B virus; POC, Polaris Observatory Collaborators; TBA, traditional birth attendants; UN, United Nations; HDV, hepatitis D virus.
References
1. Ganem D, Prince A. Hepatitis B Virus Infection–Natural History and Clinical Consequences. N Engl J Med (2004) 250:118–29. doi: 10.1056/NEJMra031087
2. WHO. “WHO. Global Health Sector Strategy on Viral Hepatitis 2016– 2021.,” Vol. 2016. Switz. Geneva:World Health Organization (2016).
4. Kafeero H, Ndagire D, Ocama P, Walusansa A, Sendagire H. Sero-Prevalence of Human Immunodeficiency Virus–Hepatitis B Virus (HIV–HBV) Co-Infection Among Pregnant Women Attending Antenatal Care (ANC) in Sub-Saharan Africa (SSA) and the Associated Risk Factors: A Systematic Review and Meta-Analysis. Virol J (2020) 17(1):19. doi: 10.1186/s12985-020-01443-6
5. Kafeero HM, Ndagire D, Ocama P, Kudamba A, Walusansa A, Sendagire H. Prevalence and Predictors of Hepatitis B Virus ( HBV ) Infection in East Africa: Evidence From a Systematic Review and Meta-Analysis of Epidemiological Studies Published From 2005 to 2020. Arch Public Heal (2021) 79(167):1–19. doi: 10.1186/s13690-021-00686-1
6. UPHIA. “UPHIA. Uganda Population HIV Impact Assesment, August,”. Kampala, Uganda:Ministry of Health (2019).
7. Kafeero HM, Ndagire D, Ocama P, Kato CD, Wampande E, Kajumbula H, et al. Disproportionate Distribution of HBV Genotypes A and D and the Recombinant Genotype D / E in the High and Low HBV Endemic Regions of Uganda: A Wake-Up Call for Regional Specific HBV Management. Int J Hepatol (2022) 2022:1–15. doi: 10.1155/2022/3688547
8. Kafeero HM, Ndagire D, Ocama P, Walusansa A, Sendagire H. “Tumor Necrosis Factor-α-863c/A and 1031T/C Single Nucleotide Polymorphic Sites (SNPs) may be Putative Markers of HBV Disease Prognosis Among Caucasoids: Evidence From a Systematic Review With Meta-Analysis,”. Gene Rep (2022) 26:101486. doi: 10.1016/j.genrep.2021.101486
9. Kafeero M, Sendagire H, Ocama P, Ndagire D. Host and Viral Factors Associated With Hepatitis B Clinical Outcomes in Chronic Infection-Review Article. Int J Pure Med Res (2019) 4(3):9–15.
10. Friedrich-Rust M, Poynard T, Castera L. Critical Comparison of Elastography Methods to Assess Chronic Liver Disease. Nat Rev Gastroenterol Hepatol (2016) 13:402–11. doi: 10.1038/nrgastro.2016.86
11. Yu J, Lee J. Current Role of Transient Elastography in the Management of Chronic Hepatitis B Patients. Ultrasonography (2017) 36:86–94. doi: 10.14366/usg.16023
12. WHO. “World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons With Chronic Hepatitis B Infection. 2015;,” (2015). Available at: http://apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng.
13. Dong X, Wu Z, Zhao H. Evaluation and Comparison of Thirty Noninvasive Models for Diagnosing Liver Fibrosis in Chinese Hepatitis B Patients. J Viral Hepat (2018) 26:297–307. doi: 10.1111/jvh.13031
14. Shimakawa Y, Boucheron P, Nguyen LBL, Lemoine M, Sombié R. Performance of Two Simplified HBV Treatment Criteria (TREAT-B Score and WHO Guidelines) in Burkina Faso, West Africa. J Hepatol (2019) 71(4):842–4. doi: 10.1016/j.jhep.2019.06.024
15. WHO. “World Health Organization; Geneva: 2016. Guidelines on Hepatitis B and C Testing, Policy Brief.,” . Available at: http://apps.who.int/iris/bitstream/10665/251330/1/WHO-HIV-2016.23-eng.pdf?ua=1.
16. WHO. “WHO. Global Hepatitis Report, 2017. Switzerland: Geneva; 2017.,”. Geneva:World Health Organization (2017).
17. WHO. “World Health Organization. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals.,”. Switzerland: Geneva (2016).
18. Njai H, Shimakawa Y, Sanneh B, Ferguson L, Ndow G, Mendy M. Validation of Rapid Point-of-Care (POC) Tests for the Detection of Hepatitis B Surface Antigen (HBsAg) in Field and Laboratory Settings in The Gambia, West Africa. J Clin Microbiol (2015) 53:1156–63. doi: 10.1128/JCM.02980-14
19. Wong G-H, Chan H-Y, Wong C-Y, Leung C, Chan C. Liver Stiffness-Based Optimization of Hepatocellular Carcinoma Risk Score in Patients With Chronic Hepatitis B. J Hepatol (2014) 60:339–45. doi: 10.1016/j.jhep.2013.09.029
20. De Ritis F, Coltorti M, Giusti G. Serum Transaminase Activities in Liver Disease.,”. Lancet (1972) 299:685–7. doi: 10.1016/S0140-6736(72)90487-4
21. Mahoney FJ. Update on Diagnosis, Management, and Prevention of Hepatitis B Virus Infection. Clin Microbiol Rev (1999) 12(2):351–66. doi: 10.1128/CMR.12.2.351
22. Vanderlinde R. Review of Pyridoxal Phosphate and the Transaminases in Liver Disease. Ann Clin Lab Sci (1986) 16(2):79–93.
23. Velayudham L, Farrell G. Drug-Induced Cholestasis. Expert Opin Drug Saf (2003) 2:287–304. doi: 10.1517/14740338.2.3.287
24. MOH. Uganda Guidelines for Prevention, Testing, Care and Treatment of Hepatitis B and C Virus Infection. Uganda Minist Heal (2019).
25. Yusuke S, Njie R, Ndow G, Vray M, Mbaye PS, Bonnard P, et al. Development of a Simple Score Based on HBeAg and ALT for Selecting Patients for HBV Treatment in Africa. J Hepatol (2018) 69:776–84. doi: 10.1016/j.jhep.2018.05.024
26. EASL. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of Chronic Hepatitis B Virus Infection. J Hepatol (2012) 57:167–85. doi: 10.1016/j.jhep
27. Clement F, Dewint P, Leroux-Roels G. Evaluation of a New Rapid Test for the Combined Detection of Hepatitis B Virus Surface Antigen and Hepatitis B Virus E Antigen. J Clin Microbiol (2002) 40:4603–6. doi: 10.1128/JCM.40.12.4603-4606.2002
28. UBOS. National Population and Housing Census 2014 -Area Specific Profiles. Repuloc Uganda Kampala (2017).
30. WHO. “World Health Organisation 2017, Global Hepatitis Report,” (2017). Available at: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/.
31. MOH. Uganda Guidelines for Prevention, Testing, Care and Treatment of Hepatitis B and C Virus Infection, November 2019. Repub Uganda (2019).
32. Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, et al. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol Hepatol (2018) 3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6
33. Veldhuijzen IK, Toy M, Hahné SJ, De Wit GA, Schalm SW, Robert A, et al. Screening and Early Treatment of Migrants for Chronic Hepatitis B Virus Infection is Cost-Effective. Gastroenterology (2010) 138(2):522–30. doi: 10.1053/j.gastro.2009.10.039
34. Fishman W. Alkaline Phosphatase Isoenzymes: Recent Progress. Clin Biochem (1990) 23(2):99–104. doi: 10.1016/0009-9120(90)80019-F
35. Dufour D, Lott J, Nolte F, Gretch D, Koff R, Seeff L. Diagnosis and Monitoring of Hepatic Injury. I. Performance Characteristics of Laboratory Tests. Clin Chem (2000) 46(12):2027–49. doi: 10.1093/clinchem/46.12.2027
36. Nayagam S, Thursz M, Sicuri E, Conteh L, Wiktor S, Low-Beer D. Requirements for Global Elimination of Hepatitis B: A Modelling Study. Lancet Infect Dis (2016) 16(12):1399–408. doi: 10.1016/S1473-3099(16)30204-3
37. Seck A, Ndiaye F, Maylin S, Ndiaye B, Simon F, Funk A. Poor Sensitivity of Commercial Rapid Diagnostic Tests for Hepatitis B E Antigen in Senegal, West Africa. Am J Trop Med Hyg (2018) 99:428–34. doi: 10.4269/ajtmh.18-0116
38. Vinikoor MJ, Sinkala E, Kanunga A, Muchimba M, Zanolini A, Saag M, et al. Eligibility for Hepatitis B Antiviral Therapy Among Adults in the General Population in Zambia. PloS One (2020) 15(1):e0227041. doi: 10.1371/journal.pone.0227041
39. Observatory PC. Polaris Observatory C. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol Hepatol (2018) 3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6
40. Fung J, Seto W, Lai C, Yuen J, Wong D, Yuen M. Profiles of HBV DNA in a Large Population of Chinese Patients With Chronic Hepatitis B: Implications for Antiviral Therapy. J Hepatol (2011) 542:195–200. doi: 10.1016/j.jhep.2010.06.031
41. Zirabamuzaale J, Ocama P. Hepatitis B Virus Genotypes A and D in Uganda. J Virus Erad (2016) 2:19–21. doi: 10.1016/S2055-6640(20)30693-2
42. Lemoine M, Shimakawa Y, Njie R, Taal M, Ndow G, Chemin I. Acceptability and Feasibility of a Screen-and-Treat Programme for Hepatitis B Virus Infection in The Gambia: The Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) Study. Lancet Glob Heal (2016) 4(8):e559–e67. doi: 10.1016/S2214-109X(16)30130-9
43. Hai VV, Shimakawa Y, Kim J, Do Ngoc H, Le Minh Q, Laureillard D, et al. Assessment and Simplification of Treatment Eligibility Among Patients With Chronic Hepatitis B Infection in Vietnam. Clin Infect Dis (2020) 73(15):e1072–7. doi: 10.1093/cid/ciaa1814
44. Lin Z, Xin Y, Dong Q. Performance of the Aspartate Aminotransferase-to-Platelet Ratio Index for the Staging of Hepatitis C-Related Fibrosis: An Updated Meta-Analysis. Hepatology (2011) 53:726–36. doi: 10.1002/hep.24105
45. Akinyemiju T, Abera S. Global Burden of Disease Liver Cancer Collaboration. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. AMA Oncol J (2017) 3:1683. doi: 10.1001/jamaoncol.2017.3055
46. Opio C, Seremba E, Ocama P. Diagnosis of Alcohol Misuse and Alcoholic Liver Disease Among Patients in the Medical Emergency Admission Service of a Large Urban Hospital in Sub-Saharan Africa; a Cross Sectional Study. Pan Afr Med J (2013) 1523. doi: 10.11604/pamj.2013.15.23.2040
47. Kabwama S, Ndyanabangi S, Mutungi G. Alcohol Use Among Adults in Uganda: Findings From the Countrywide Non-Communicable Diseases Risk Factor Cross-Sectional Survey. Glob Heal Action (2016) 9:31302. doi: 10.3402/gha.v9.31302
48. WHO. “Gender, Alcohol and Culture: An International Study (GENACIS).,” (2012). WHO (Accessed 26 Aug 2012).
49. Mabry-Hernandez I, Lewis P. Screening for Hepatitis B Virus Infection in Nonpregnant Adolescents and Adults. Am Fam. Phys (2015) 92(4):301–2.
50. Ban M. A. A.-K., Mushtak T. S. A.-O., Khalid F. A. A.-R. Comparative Study of the Molecular, Biochemical, and Other Parameters in Iraqi Hepatitis B Patients. Drug Invent Today (2020) 14(6):870–6.
51. UHG. United Healthcare Group. Gamma Glutamyl Transferase (GGT). Policy Number: CMP-021. United States: United Healthcare Group; 2018. United Healthc Group (2018).
52. Thapa B. Serum Gamma Glutamyl Transferase and Alkaline Phosphatase in Acute Cholecystitis. J Nepal Health Res Counc (2010) 8(2):78–81.
53. Ferri P. The Role of Genetic and Immune Factors for the Pathogenesis of Primary Sclerosing Cholangitis in Childhood. Gastroenterol Res Pr (2016) 2016. doi: 10.1155/2016/3905240
54. Rusine J, Ondoa P, Asiimwe JKB, Boer KR, Uwimana JM, Mukabayire O, et al. High Seroprevalence of HBV and HCV Infection in HIV-Infected Adults in Kigali, Rwanda. PloS One. PloS One (2013) 8(5):e63303. doi: 10.1371/journal.pone.0063303
55. Whitfield J. Gamma Glutamyl Transferase. Crit Rev Clin Lab Sci (2001) 38:263–355. doi: 10.1080/20014091084227
57. Kabayambi J. Why TBAs are Still Preferred by Pregnant Mothers. Uganda Heal Commun Alliance Matern Heal (2015).
58. Lodenyo H, Schoub B, Ally R, Kairu S, Segal I. Hepatitis B and C Virus Infections and Liver Function in AIDS Patients at Chrishanibaragwanath Hospital Johannesburg. East Afr Med January (2000) 77(1):13–5. doi: 10.4314/eamj.v77i1.46369
59. Jayeeta S, Saha D, Bandyopadhyay B, Saha B, Kedia D, Guha Mazumder DN, et al. Baseline Characteristics of HIV & Hepatitis B Virus (HIV/HBV) Co-Infected Patients From Kolkata, India,”. Indian J Med Res (2016) 143(5):636–42. doi: 10.4103/0971-5916.187113
60. Jose-Abrego A, Roman S, Rebello Pinho J, de Castro V, Panduro A. Hepatitis B Virus (HBV) Genotype Mixtures, Viral Load, and Liver Damage in HBV Patients Co-Infected With Human Immunodeficiency Virus. Front Microbiol (2021) 12:640889(640889). doi: 10.3389/fmicb.2021.640889
61. Rahimi M, Kheiandish F, Arab-Mazar Z, Mirzapour A. Level of Liver Enzymes in Patients With Mono-Parasitic Infections. Infect Epidemiol Microbiol (2017) 3(4):137–42. doi: 20.1001.1.25884107.2017.3.4.2.5
62. Jalilzadeh-Amin G, Esmaeilnejad B, Farhang-Pajuh F. Study on the Relationship Between Liver Parasitic Infections and Serum Vitamin A and β-Carotene Status in Cattle. Türkiye Parazitolojii Derg (2017) 41(4):198. doi: 10.5152/tpd.2017.5364
63. Millán JC, Mull R, Freise S, Richter J. The Efficacy and Tolerability of Triclabendazole in Cuban Patients With Latent and Chronic Fasciola Hepatica Infection. Am J Trop Med Hyg (2000) 63(5):264–9. doi: 10.4269/ajtmh.2000.63.264
64. Bektaş M, Dökmeci A, Cinar K, Halici I, Oztas E, Karayalcin S, et al. Endoscopic Management of Biliary Parasitic Diseases. Dig Dis Sci (2010) 55:1472–8. doi: 10.1007/s10620-009-0850-0
65. Gulsen M, Savas M, Koruk M, Kadayifci A, Demirci F. Fascioliasis: A Report of Five Cases Presenting With Common Bile Duct Obstruction. Neth J Med (2006) 64:17–9.
66. Shedain P, Devkota M, Banjara M, Ling H, Dhital S. Prevalence and Risk Factors of Hepatitis B Infection Among Mothers and Children With Hepatitis B Infected Mother in Upper Dolpa, Nepal. BMC Infect Dis (2017) 17(1):1–9. doi: 10.1186/s12879-017-2763-4
67. Muljono D. Epidemiology of Hepatitis B and C in Republic of Indonesia. Euroasian J Hepato-Gastroenterol (2017) 7(1):55–9. doi: 10.5005/jp-journals-l0018-1212
68. Pereira V, Wolf J, Luz C. Risk Factors for Hepatitis B Transmission in South Brazil. Mem Inst Oswaldo Cruz (2017) 112(8):544–50. doi: 10.1590/0074-02760170043
69. Sofian M, Banifazl M, Ziai M, Aghakhani A, Farazi A-A, Ramezani A. Intra-Familial Transmission of Hepatitis B Virus Infection in Arak, Central Iran. Iran J Pathol (2016) 11(4):328–33.
70. Ragheb M, Elkady A, Tanaka Y, Murakami S, Attia F, Hassan A. Multiple Intra-Familial Transmission Patterns of Hepatitis B Virus Genotype D in North-Eastern Egypt. J Med Virol (2012) 84(4):587–95. doi: 10.1002/jmv.23234
71. Lobato C, Tavares-Neto J, Rios-Leite M, Trepo C, Vitvitski L, Parvaz P. Intrafamilial Prevalence of Hepatitis B Virus in Western Brazilian Amazon Region:Epidemiologic and Biomolecular Study. J Gastroenterol Hepatol (2006) 21(5):863–8. doi: 10.1111/j.1440-1746.2006.04298.x
72. Mohammad Alizadeh A, Ranjbar M, Ansari S, Alavian S, Shalmani H, Hekmat L. Intra-Familial Prevalence of Hepatitis B Virologic Markers in HBsAg Positive Family Members in Nahavand, Iran. World J Gastroenterol (2005) 11(31):4857–60. doi: 10.3748/wjg.v11.i31.4857
73. Barut S, Gemici Ü, Güneş F, Demir O, Duygu F. Predictors of Histological Indication for Treatment in HBeAg Negative Chronic HBV Infection. J Med Virol (2017), 1–17. doi: 10.1002/jmv.24879
74. Nalpas B, Vassault A, Charpin S, Lacour B, Berthelot P. Serum Mitochondrial Aspartate Aminotransferase as a Marker of Chronic Alcoholism: Dia Gnostic Value and Interpretation in a Liver Unit. Hepatology (1986) 6:608–14. doi: 10.1002/hep.1840060410
75. Cohen J, Kaplan M. The SGOTISGPT Ratio: An Indicator of Alcoholic Liver Disease. Dig Dis Sci (1979) 24:435–8. doi: 10.1007/BF01324898
Keywords: TREAT-B, eligibility, endemicity, hepatitis B core antigen, alanine aminotransferase
Citation: Kafeero HM, Ndagire D, Ocama P, Kato CD, Wampande E, Kajumbula H, Kateete DP, Walusansa A, Kudamba A, Ssenku JE and Sendagire H (2022) TREAT-B Algorithm for Treatment Eligibility Among Chronically Infected Hepatitis B Virus Persons in a Low and a High Endemic Region: A Potential Strategy Towards Virus Elimination by 2030. Front.Virol. 2:754711. doi: 10.3389/fviro.2022.754711
Received: 06 August 2021; Accepted: 14 March 2022;
Published: 11 April 2022.
Edited by:
Javier Buesa, University of Valencia, SpainReviewed by:
Edford Sinkala, University of Zambia, ZambiaSlim Fourati, Emory University, United States
Copyright © 2022 Kafeero, Ndagire, Ocama, Kato, Wampande, Kajumbula, Kateete, Walusansa, Kudamba, Ssenku and Sendagire. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hussein Mukasa Kafeero, aHVzc2Vpbm11a2FzYWthZmVlcm9AZ21haWwuY29t
 Hussein Mukasa Kafeero
Hussein Mukasa Kafeero Dorothy Ndagire3
Dorothy Ndagire3 Abdul Walusansa
Abdul Walusansa