- 1Institut Pasteur de Dakar, Pôle de Virologie, Dakar, Senegal
- 2Institut Pasteur de Dakar, Unité d’Épidémiologie, de Recherche Clinique et de Science des Données, Dakar, Senegal
- 3Ministère de la Santé et de l’Action Sociale, Direction de la Prévention, Dakar, Senegal
- 4Ministère de la Santé et de l’Action Sociale, Centre d’Opérations des Urgences Sanitaires (COUS), Dakar, Senegal
- 5Ministère de la Santé et de l’Action Sociale, Région Médicale de Diourbel, Diourbel, Senegal
- 6Institut Pasteur de Dakar, Unité de Zoologie Médicale, Dakar, Senegal
On 10th September 2018, the Syndromic Sentinel Surveillance network that monitors febrile illnesses in all 14 regions of Senegal detected a peak of fever in the Fatick region. On 13 September 2018, 10 samples were sent to the WHO Collaborating Centre for Arboviruses and Viral Haemorrhagic Fevers at the Institut Pasteur de Dakar (IPD). Laboratory investigations revealed an epidemic of dengue 1 genotype V and dengue 3 genotype III. Fatick neighbors the Holy City of Touba where 3.5 million people from all over the word gather every year for the Grand Magal pilgrimage. This article discusses the impact of mass gatherings and their role in the recent introduction of dengue serotypes in Senegal. Dengue is now endemic in Senegal and across many countries in Africa, highlighting the need for early detection, control measures and prevention of severe dengue cases in highly connected urban settings.
Introduction
Dengue virus (DENV) is responsible for the majority of arthropod-borne viral infections globally (1) and is transmitted between humans by the Aedes mosquito species (2). DENV is comprised of four distinct serotypes (DENV1-4) belonging to the Flavivirus genus. Although most dengue cases are asymptomatic, severe illness accompanied with fever, muscles spasms and joint pains or death may occur. Individuals with a prior exposure to another flavivirus or a different dengue serotype may have a greater risk of developing Dengue haemorrhagic fever (DHF) or Dengue shock syndrome (DSS) when DHF progresses to a critical state of associated with multi-organ failure (3). Dengue was first isolated in Senegal in 1970 from a febrile young girl at Bandia village (4). A dengue urban epidemic was later detected in Dakar in 2009 with DHF and DSS cases (5).
Ethical consideration
In this study, we used samples collected as part of approved ongoing surveillance conducted by the Institut Pasteur de Dakar (a World Health Organization Collaborating Centre for Arboviruses and Haemorrhagic Fever Reference and Research). The Senegalese national ethical committee approved the protocol as a less than minimal risk research, and written consent forms were not required. All samples from humans were de-identified before we performed virus characterization and analysis.
Results and discussions
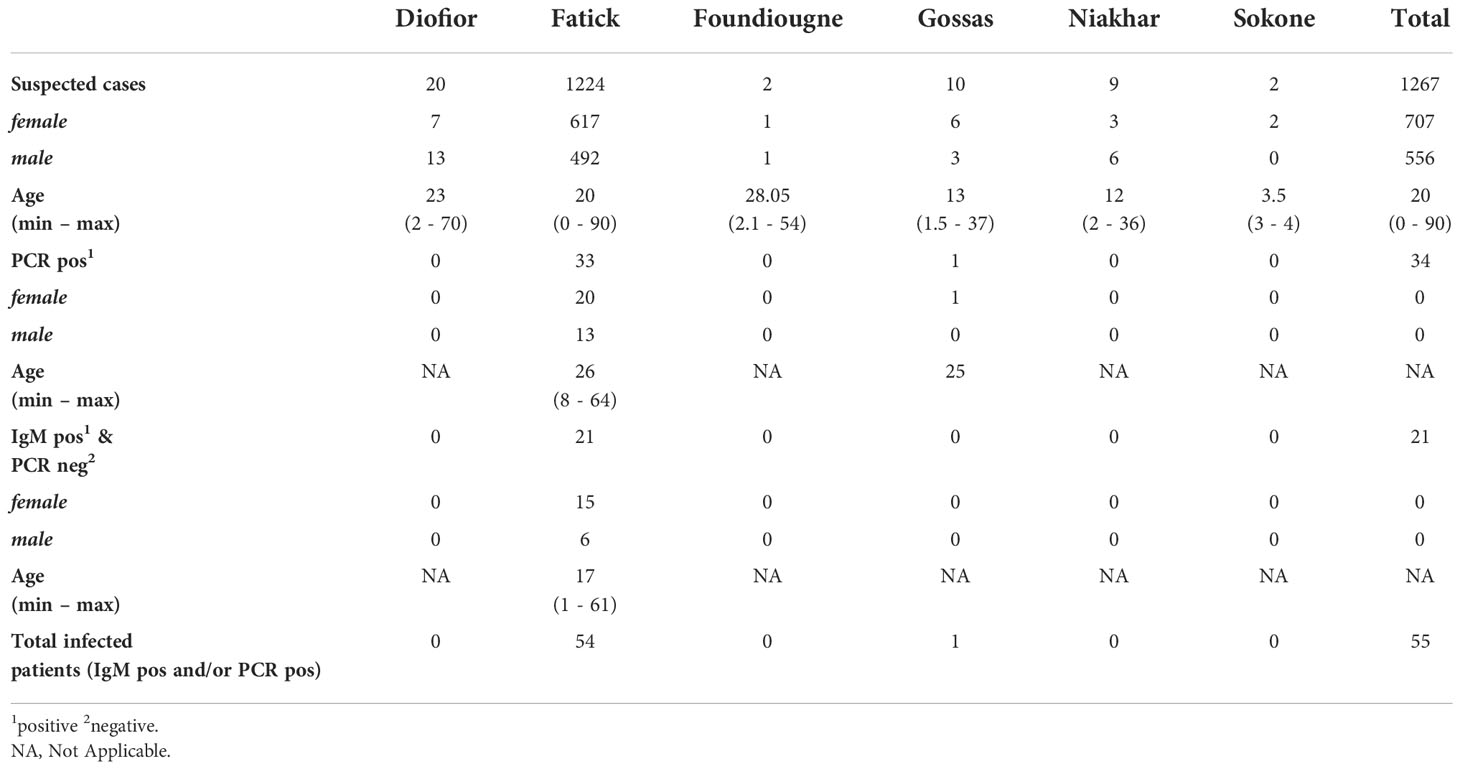
On 13th September 2018, IPD’s reference laboratory confirmed a case of dengue to the sentinel surveillance site of Ndiaye Ndiaye in Fatick, Senegal, that notified a suspect case. A taskforce from IPD composed of a rapid response team was deployed with a RT-PCR mobile laboratory in the regional hospital for widespread testing. Results were delivered to physicians within two hours of sampling. Patients presenting acute febrile illness with two or more of the following clinical signs and symptoms (arthralgia, myalgia, headache, vomiting, asthenia, retro-orbital pain, rash and haemorrhagic manifestations) were defined as suspected cases and tested onsite for DENV serotypes 1-4 virus using the LightMix® Modular DENV RT-PCR kit (TIBMOLBIOL-Germany) and the SmartCycler® II (Cepheid-USA) powered by the mobile laboratory. Samples were also sent to IPD for further investigations for differential arbovirus and viral haemorrhagic fever viruses by MAC-ELISA and Plaque Reduction Neutralization tests (PRNT). 1,267 patients coming from Diofior (20), Fatick (1224), Foundiougne (2), Gossas (10), Niakhar (9), Sokone (2) were tested and among them, 55 patients (4.3%) were found to be infected with DENV. A total of 34 patients were dengue RT-PCR positive (33 confirmed cases in Fatick district and 1 confirmed case in Gossas) and 21 patients were serologically positive by IgM (PCR negative) in Fatick district. The M:F sex ratio for the case-patients was 0.62 (13 male, 20 female) and the median age was 26 years old (see Table 1). All dengue IgM positive samples were confirmed by PRNT.
Of the 34 RT-PCR positive samples, 20 with pre-defined serotypes using a Dengue typing kit (Cat-No. 40-0700-24) (TIBMOLBIOL-Germany) were randomly selected to attempt viral isolation using mosquito C6/36 continuous cell lines at IPD according to a previously described protocol (6). 14 DENV isolates were successfully recovered. Full DENV-E gene coding regions were amplified by RT-PCR with serotype specific E gene primers, amplicons of expected size were purified after excision from agarose gels after electrophoresis using QIAquick Spin PCR Purification kit (Qiagen-Germany), and Sanger sequenced for dengue serotypes identification and phylogenetic analyses. Results based on the E gene sequencing followed by Maximum Likelihood phylogenetic analysis revealed that two distinct serotypes co-circulated during this outbreak: DENV-1 genotype V closely related to isolates having circulated in Singapore in 2013 and in the Thies region of Senegal in 2018 with 98.91% and 99.73% sequence similarity respectively (Figure 1 coloured in green). In addition, the DENV-3 sequences belonged to genotype III and were closely related to isolates obtained during the dengue outbreak in Burkina Faso in 2017 (MT2611979) and Thies region in Senegal during an outbreak in 2018 (MW288031) (Figure 1 coloured in red) with 99.31% and 99.68% sequence similarity, respectively. Interestingly, all sequences from Senegal (Fatick and Thies outbreaks) are closely related and fall on a monophyletic cluster suggesting a link between both epidemics.
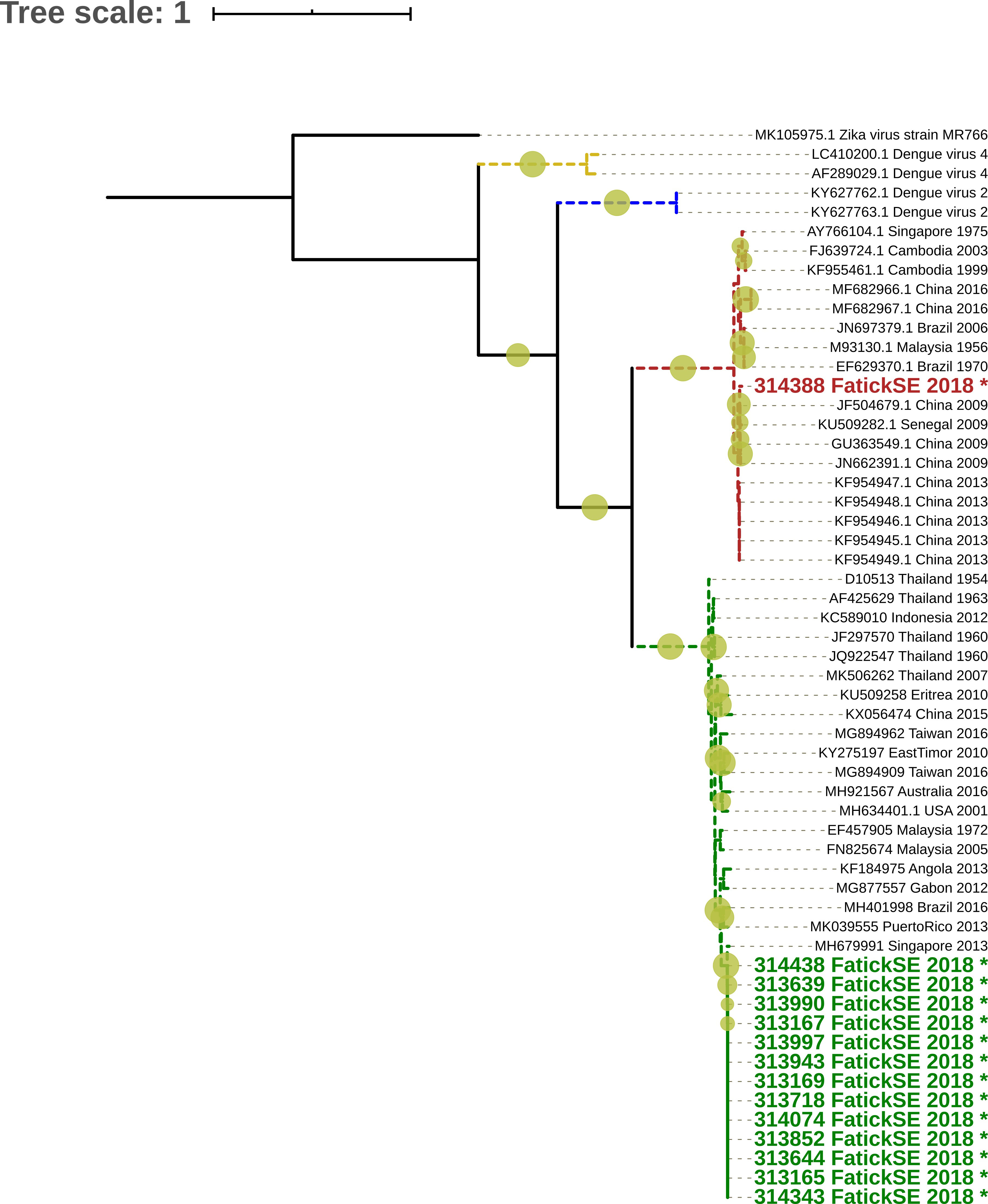
Figure 1 Maximum Likelihood (ML) tree based on DENV full E gene of DENV strains detected in Fatick, multiple sequence alignment was obtained by using Mafft (7) and the phylogenetic tree was constructed using the maximum likelihood method implemented in IQ-TREE (8). 1,000 replicates of the sequences data were used for the robustness. The tree shows relationship between isolated virus in this study label names colored respectively in Red (DENV-3) and in Green (DENV-1) and indicated by (*) sign with 62 global sequences of Dengue virus belonging to described serotypes (DENV1-4), TIM2+F+I+G4 nucleotide substitution model were used based on BIC criterion. It show that Strains from Fatick belong to DENV-1 and DENV-3 serotypes.
This study highlighted an additional co-circulation of at least two different serotypes in a single outbreak in Senegal during the Louga region dengue outbreak in 2017 with the co-circulation of DENV-1 and DENV-2 (7), Thies region dengue outbreak in 2018 which reveals co-circulation of 3 serotypes (DENV 1-3) (8). The co-circulation of many DENVserotypes in the same area is known to be a risk factor for the emergence of severe dengue infections (9). Based on published data, severe dengue cases are not very common in Africa. Published studies suggest that individuals of African descent are less susceptible to severe dengue compared to other ancestral groups. However, the dengue situation in Africa is likely underestimated due to poor access to healthcare, underreporting and misdiagnosis (10).
Beyond diagnostic testing, the rapid response team localized and monitored suspect and confirmed cases and investigated their contacts through detailed surveys. Vector investigation was conducted between 22nd - 30th September 2018 after the second human case was reported on September 10th, 2018. Investigations were initiated at the geographic location of confirmed cases and their contacts. Next, investigations were carried out step by step around the foci of confirmed cases over a radius of 200 meters, and folowing the testimonies of people who had knowledge of suspected cases at the community level during the two months preceding the investigation. Active case finding was also performed in primary health care centre records. Epidemiological and clinical information from all these cases were obtained using structured questionnaires.
The IPD entomology team looked for mosquitos and larvae presence in the contacts’ environment in order to deliver effective vector control strategies. Single methods of vector control are insufficient for all mosquitoes, therefore a combination of methods was used. Sampling methods for the adult mosquito population included the use of a backpack aspirator (BioQuip Products-Inc), pyrethrum spray catches, Biogent sentinel Traps and Biogent Gravid Adult Traps. A total of 16 mosquito species belonging to four genera were caught over the investigation period. The species previously found to be associated with DENV in Senegal (Aedes aegypti, Aedes metallicus, Aedes luteocephalus and Aedes vittatus) represented 8.5% of the 5,449 female mosquitoes collected. The epidemic risk indices from the investigated localities were over the risk threshold (23.4 ≤ BI ≤ 77.1 et 26.6 ≤ RI ≤ 53.2), suggesting that sampled places at Fatick area were at risk of DENV transmission. The heavy precipitation may promote the formation of aquatic habitats favourable to vector breeding which increases vector population density as well as transmission risk. However, dengue cases reported to the surveillance system represent a small proportion of true infections, with numerous infections are unrecognized due to both asymptomatic infections as well as symptomatic cases not being reported or misdiagnosis with other malaria like illnesses.
Conclusions
On 13 September 2018, a dengue case was detected in Fatick regions in Senegal. Full DENV E sequencing revealed a co-circulation of DENV-1 genotype V and DENV-3 genotype III. Based on the phylogeny trees, we believe these strains have been introduced in Senegal by travellers from Asia, Australia, America or Western Africa. In Fact, Fatick region is located 50 km far from Diourbel region and the Holy City of Touba. Every year, around 3.5 million people coming from Senegal and the different continent gathers in Touba for the Grand Magal pilgrimage. This important mass gathering may contribute to the introduction of dengue serotypes in Touba and its vicinity (11).
The burden of dengue is underestimated in Africa due to the misdiagnosis related to a high number of causes of fever and the limited access to health care (12). To date, increasingly regular dengue outbreaks have been reported in Senegal and evidence of active co-circulation with malaria have been documented in Kedougou (southeastern Senegal) between 2009 and 2013 (5, 11, 13, 14). Even if the introduction of mobile laboratories allows rapid dengue case management during epidemics, we believe scalable and cost-effective resources such as rapid diagnostic tests alongside the already existing data management and reporting system should be implemented in the different health centres in order to 1) efficiently deal with dengue as an endemic disease in Senegal through a robust surveillance system and 2) better understand the epidemiology and dissemination of the virus. Improving the understanding of dengue in Africa is critically important, especially in the context of co-circulating serotypes to minimise the risk of severe dengue in settings with poor access to the necessary healthcare facilities to manage the disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
In this study, we used samples collected as part of an ongoing surveillance conducted by the Institut Pasteur de Dakar (a World Health Organization Collaborating Centre for Arboviruses and Haemorrhagic Fever Reference and Research) and approved by the Senegalese Ministry of Health. As part of this routine surveillance, written consent forms were not required and all human samples were de-identified before any sample characterization and data anaylysis.
Author contributions
DI, DC, FC, DM: Performed experiements, Data analysis and write the manuscript. BA and BY: Collected the samples. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Mr. Moussa Dia and Arame Ba for their technical support with laboratory diagnosis. This work was funded by Institut Pasteur de Dakar, Senegal.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jentes ES, Lash RR, Johansson MA, Sharp TM, Henry R, Brady OJ, et al. Evidence-based risk assessment and communication: a new global dengue-risk map for travellers and clinicians. J Travel Med (2016) 23:1–5. doi: 10.1093/jtm/taw062
2. Diallo M, Ba Y, Faye O, Soumare ML, Dia I, Sall AA. Vector competence of aedes aegypti populations from Senegal for sylvatic and epidemic dengue 2 virus isolated in West Africa. Trans R Soc Trop Med Hyg (2008) 102:493–8. doi: 10.1016/j.trstmh.2008.02.010
4. Robin Y, Cornet M, Heme G, Le Gonidec G. Isolement du virus de la dengue au sénégal. Annales l’Institut Pasteur/Virologie (1980) 131:149–54. doi: 10.1016/0769-2617(80)90029-5
5. Faye O, Ba Y, Faye O, Talla C, Diallo D, Chen R, et al. Urban epidemic of dengue virus serotype 3 infection, Senegal, 2009. Emerging Infect Dis (2014) 20:456–9. doi: 10.3201/eid2003.121885
6. Dieng I, Hedible BG, Diagne MM, El Wahed AA, Diagne CT, Fall C, et al. Mobile laboratory reveals the circulation of dengue virus serotype I of Asian origin in Medina gounass (Guediawaye), Senegal. Diagnostics (2020) 10:408. doi: 10.3390/diagnostics10060408
7. Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res (2002) 30:3059–66. doi: 10.1093/nar/gkf436
8. Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol (2015) 32:268–74. doi: 10.1093/molbev/msu300
9. World Health Organization, Regional Office for Africa. Weekly bulletin on outbreak and other emergencies: Week 43: 20 - 26 October 2018. Brazzavile, Republic of Congo: Health Emergency Information and Risk Assessment (2018) p. 1–18.
10. Gaye A, Ndiaye T, Sy M, Deme AB, Thiaw AB, Sene A, et al. Genomic investigation of a dengue virus outbreak in thiès, Senegal, in 2018. Sci Rep (2020) 11:10321. doi: 10.1038/s41598-021-89070-1
11. Halstead SB. Dengue antibody-dependent enhancement: Knowns and unknowns. Microbiol Spectr (2014) 2:30–48. doi: 10.1128/microbiolspec.AID-0022-2014
12. Gainor EM, Harris E, LaBeaud AD. Uncovering the burden of dengue in Africa: Considerations on magnitude, misdiagnosis, and ancestry. Viruses (2022) 14:233. doi: 10.3390/v14020233
13. Diagne CT, Barry MA, Ba Y, Faye O, Sall AA. Dengue epidemic in touba, Senegal: Implications for the grand magal pilgrimage for travelers. J Travel Med (2018) 1:1–2. doi: 10.1093/jtm/tay123
14. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature (2013) 496:504–7. doi: 10.1038/nature12060
15. Sokhna C, Goumballa N, Gautret P. The grand magal of touba in the time of a dengue outbreak in Senegal. Travel Med Infect Dis (2019) 28:107–8. doi: 10.1016/j.tmaid.2018.11.002
Keywords: Dengue, arbovirus, epidemics, public health, Senegal, Fatick
Citation: Diagne CT, Dieng I, Faye O, Fall C, Barry MA, Diarra M, Ndiaye O, Dior Ndione MH, Ndiaye M, Diop B, Bousso A, Sall A, Fall G, Loucoubar C, Ba Y, Sall AA, Diallo M and Faye O (2023) Co-circulation of dengue virus serotypes 1 and 3 in the Fatick region of senegal 2018. Front. Virol. 2:1009382. doi: 10.3389/fviro.2022.1009382
Received: 01 August 2022; Accepted: 07 November 2022;
Published: 30 January 2023.
Edited by:
John H.-O. Pettersson, Uppsala University, SwedenReviewed by:
Ernest Gould, Aix Marseille Université, FranceRosa Maria Sanchez Casas, Autonomous University of Nuevo León, Mexico
Copyright © 2023 Diagne, Dieng, Faye, Fall, Barry, Diarra, Ndiaye, Dior Ndione, Ndiaye, Diop, Bousso, Sall, Fall, Loucoubar, Ba, Sall, Diallo and Faye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Idrissa Dieng, SWRyaXNzYS5ESUVOR0BwYXN0ZXVyLnNu
 Cheikh Tidiane Diagne1
Cheikh Tidiane Diagne1 Idrissa Dieng
Idrissa Dieng Oumar Faye
Oumar Faye Abiboulaye Sall
Abiboulaye Sall Amadou Alpha Sall
Amadou Alpha Sall