
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Virol. , 08 December 2021
Sec. Translational Virology
Volume 1 - 2021 | https://doi.org/10.3389/fviro.2021.780298
This article is part of the Research Topic Translational Virology in Pregnancy View all 12 articles
 Dakshnapriya Balasubbramanian1
Dakshnapriya Balasubbramanian1 Sathish Dharani2
Sathish Dharani2 Mohammad Tauseef3
Mohammad Tauseef3 Mansoor A. Khan2
Mansoor A. Khan2 Ziyaur Rahman2†
Ziyaur Rahman2† Brett M. Mitchell1*†
Brett M. Mitchell1*†The maternal innate immune system plays a central role in preeclampsia (PE). Toll-like receptors (TLRs) are innate immune system receptors that recognize characteristics of extracellular endogenous ligands or pathogens, and their activation leads to a pro-inflammatory immune response. We and others have reported that excessive activation of TLRs causes pregnancy-dependent hypertension in animals and is associated with PE in women. Activation of TLR3 by poly I:C mimics the innate immune system activation by viruses that women who develop PE encounter during pregnancy. Vardenafil was approved by the FDA for erectile dysfunction but has recently been examined as a potential PE medication due to studies done with a similar drug, sildenafil. Preclinical as well as recent clinical studies demonstrate the potential effectiveness of sildenafil for PE. However, vardenafil is more potent than sildenafil and acts by increasing expression of placental growth factor in addition to increasing cGMP levels. We hypothesized that vardenafil will be more potent and effective in reducing the negative health effects in a mouse model of virus-induced PE. Pregnant mice were injected with the TLR3 agonist poly I:C (PPIC) on gestational days 13, 15, and 17. We treated PPIC mice with a high dose of vardenafil (50 mg human equivalent), a lower dose of vardenafil (20 mg human equivalent), or sildenafil (50 mg human equivalent) on gestational days 15–17 after hypertension was established. Daily i.p. injections of either high dose or low dose vardenafil significantly decreased systolic blood pressure in PPIC mice whereas sildenafil had no effect. There were no differences in body weight between the groups. The splenomegaly induced in PPIC mice was ameliorated in high dose vardenafil-treated PPIC mice, while low dose vardenafil-treated and sildenafil-treated PPIC mice still exhibited splenomegaly. High dose vardenafil-treated PPIC mice also did not exhibit any fetal demise characteristic of PPIC mice, while low dose vardenafil-treated and sildenafil-treated PPIC mice still had significantly increased incidences of fetal demise. These data support the notion that high dose vardenafil may be safe and effective at reducing blood pressure during a virus-associated hypertensive pregnancy.
Hypertensive disorders of pregnancy, including preeclampsia (PE), are high-risk conditions diagnosed in the latter stage of pregnancies (1, 2). Due to the limited availability of effective therapeutic options, PE often leads to a high rate of fetal, neonatal, and maternal morbidity and mortality (2, 3). The underlying pathophysiology of PE is poorly understood and a continued search for novel and effective drugs to manage the condition is needed (4, 5). PE is multifactorial in its pathophysiology, but is widely associated with reduced placental perfusion, immune system activation, and systemic vascular endothelial dysfunction (6). The resultant placental ischemia induces the renin-angiotensin-aldosterone system (RAAS), inflammation, and oxidative stress that may propagate PE. With respect to immune system activation, we and others have reported viral activation of the maternal immune system during pregnancy is associated with PE in women and induces a PE-like syndrome in rodents (7–11). Given the recent rise in viral pandemics, therapies to aid in managing PE associated with a viral infection are needed.
In the clinic a variety of therapeutic agents have been used to acutely lower blood pressure in PE including calcium channel blockers, methyldopa, diazoxide, hydralazine, prostacyclin, prazosin, and isosorbide (5). In recent years oral extended release nifedipine, oral labetalol, and methyldopa are the generally accepted first-line agents for non-severe hypertension in women with PE. Beta-blockers and diuretics are acceptable, while RAAS inhibitors remain contraindicated (5). However, these agents have their own side effect profile and have been shown to be relatively ineffective (12). Thus, the clinical management of PE is still a challenge and needs effective treatment options (13). One of the quickest ways to develop an effective drug for the treatment of PE is to identify new effects of the drugs that are already approved for other indications and have been found clinically safe (14).
Vardenafil is a phosphodiesterase 5 (PDE5) inhibitor that is closely related in function to sildenafil, a PDE5 inhibitor commonly used to treat male erectile dysfunction and pulmonary vascular diseases (15–17). During pregnancy, nitric oxide (NO) is synthesized in in utero placental tissues and endothelial cells and activates the intracellular second messenger cyclic guanosine monophosphate (cGMP). cGMP causes vasodilation and thus maintains low vascular resistance in the uterus and fetoplacental circulations (12, 18). However, cGMP is metabolized by PDE5 and leads to vasoconstriction and may contribute to the development and maintenance of PE. Thus, inhibition of PDE5 may enhance the vasodilatory effect of NO by preventing cGMP degradation (12). It has been demonstrated that PE is, in part, related to decreased NO-mediated vasodilation of the uterine circulation (18). Earlier studies demonstrated that sildenafil treated PE in mouse models (19–21). However, in clinical studies, it was reported to be relatively ineffective as a treatment for PE, although it was shown that it could be used safely during pregnancy (22). Since vardenafil is a potent PDE5 inhibitor and available clinically as a safe and effective drug (17, 23, 24), we hypothesized that vardenafil will decrease blood pressure and improve fetal outcomes in a mouse model of PE induced by a viral mimetic.
Male and female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal use protocols were approved by the Texas A&M University Institutional Animal Care and Use Committee and were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Pregnant (P) mice were injected i.p. with the TLR3 agonist and viral mimetic poly I:C 20 mg/kg body weight (PPIC) on gestational days 13, 15, and 17 as described previously (8–10, 25–30). These PPIC mice develop hypertension by day 14, which remains when poly I:C is injected every other day until parturition. We treated PPIC mice with a high dose of vardenafil (50 mg human equivalent), a low dose of vardenafil (20 mg human equivalent), or sildenafil (50 mg human equivalent) on gestational days 15–17 after hypertension was established. All PDE5 inhibitors were injected i.p. Mice had free access to normal chow (Teklad 8604, with NaCl content roughly 0.5%) and drinking water. Systolic blood pressure was determined using the tail-cuff method after acclimatization and training as described previously (31). All mice were anesthetized and euthanized on gestational day 18 and body weight, spleen weight, and the number of fetuses and morphology were counted and noted. The P and PPIC groups had 10 mice in each, while the PPIC groups treated with vardenafil or sildenafil had six mice in each group.
A one-way ANOVA followed by a Student–Newman–Keuls post-hoc test was used to compare groups and the significance level was set at p < 0.05 compared to P.
Daily i.p. injections of either high dose (n = 6) or low dose (n = 6) vardenafil significantly decreased systemic blood pressure in PPIC (n = 10) mice to P (n = 10) levels, whereas sildenafil (n = 6) had no effect (Figure 1). There were no differences in body weight between the groups (Figure 2). The splenomegaly induced in PPIC mice was ameliorated in high dose vardenafil-treated PPIC mice, while low dose vardenafil-treated and sildenafil-treated PPIC mice still exhibited splenomegaly (Figure 3). High dose vardenafil-treated PPIC mice also did not exhibit any fetal demise characteristic of PPIC mice, while low dose vardenafil-treated and sildenafil-treated PPIC mice still had significantly increased incidences of fetal demise (Figure 4). The fetal demise incidence in sildenafil-treated PPIC mice was significantly increased compared to that of PPIC mice (Figure 4).
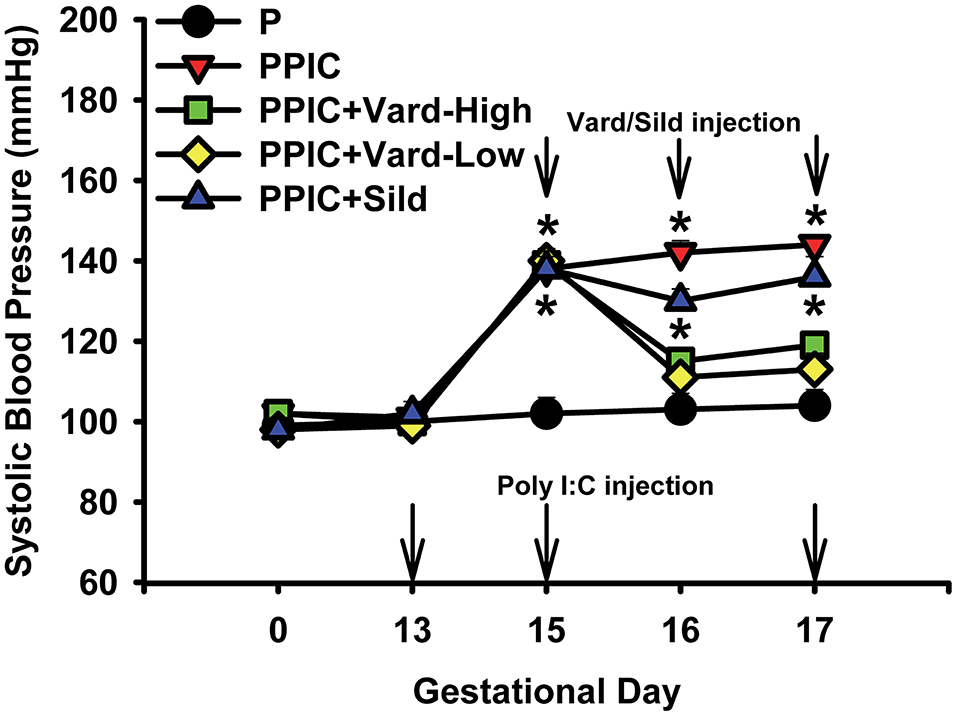
Figure 1. Systemic blood pressures of control pregnant (P) mice (n = 10), P mice treated with the TLR3 agonist and viral mimetic poly I:C (PPIC) (n = 10), PPIC mice treated with a high dose (n = 6) and low dose (n = 6) of vardenafil (Vard), and PPIC mice treated with sildenafil (Sild) (n = 6). *p < 0.05 by ANOVA compared to P mice.
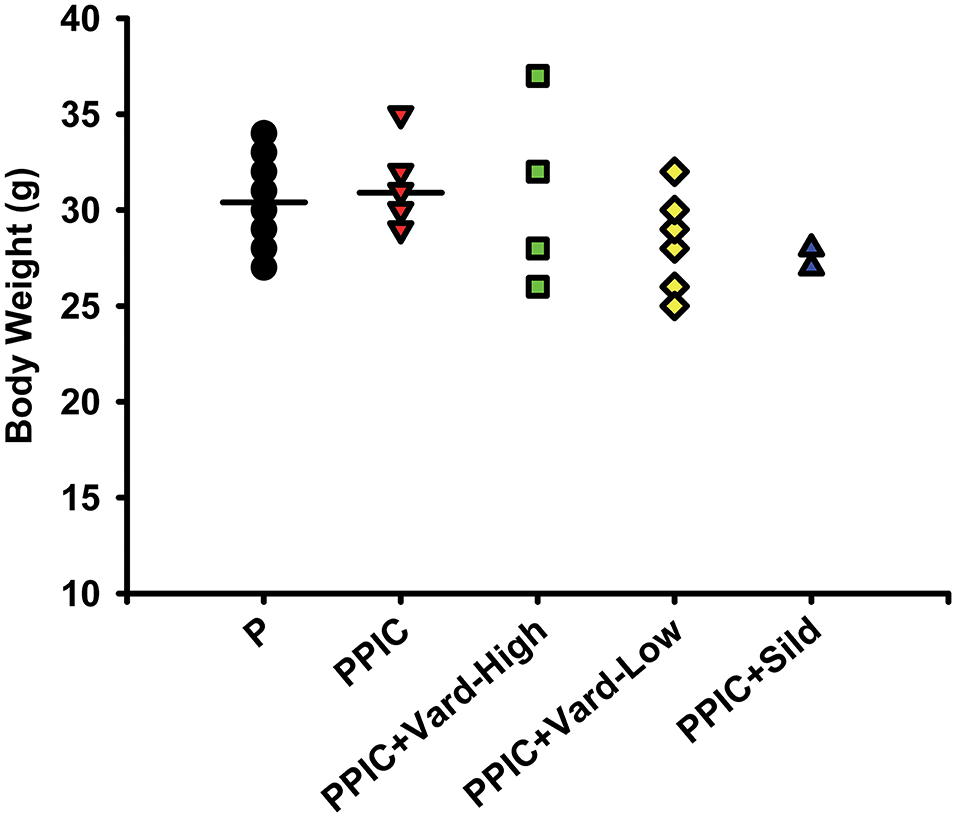
Figure 2. Body weight measures of control pregnant (P) mice (n = 10), P mice treated with the TLR3 agonist and viral mimetic poly I:C (PPIC) (n = 10), PPIC mice treated with a high dose (n = 6) and low dose (n = 6) of vardenafil (Vard), and PPIC mice treated with sildenafil (Sild) (n = 6).
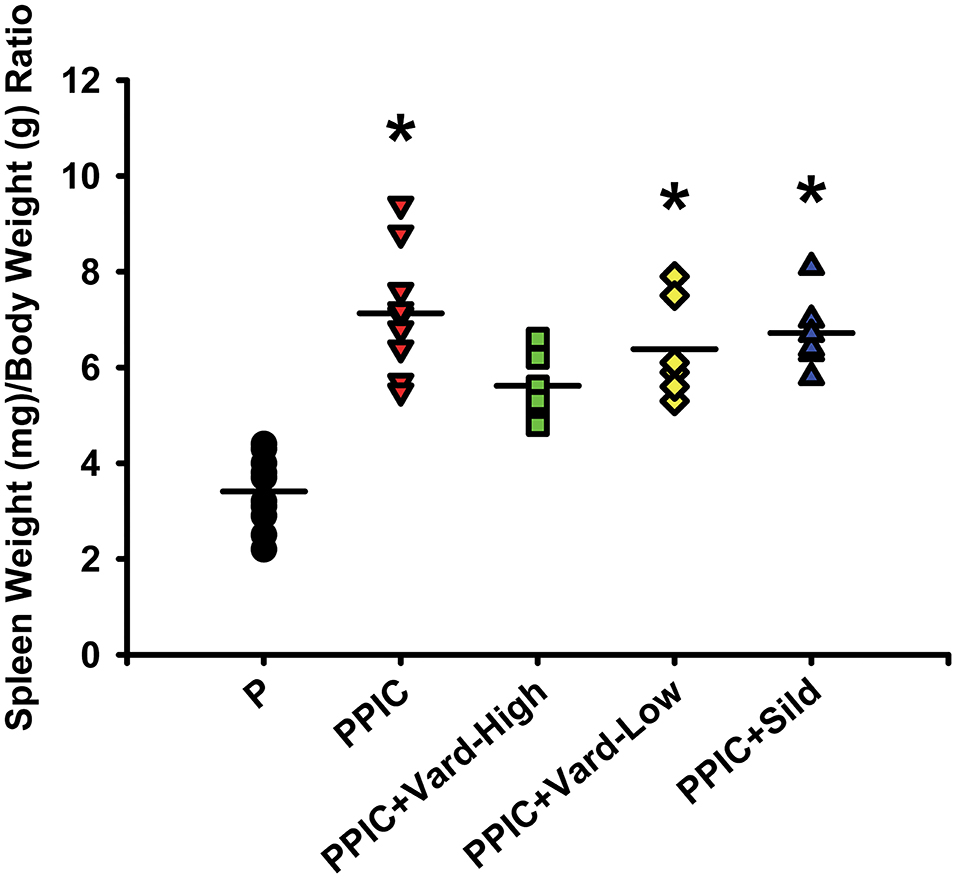
Figure 3. Spleen weight to body weight ratios of control pregnant (P) mice (n = 10), P mice treated with the TLR3 agonist and viral mimetic poly I:C (PPIC) (n = 10), PPIC mice treated with a high dose (n = 6) and low dose (n = 6) of vardenafil (Vard), and PPIC mice treated with sildenafil (Sild) (n = 6). *p < 0.05 by ANOVA compared to P mice.
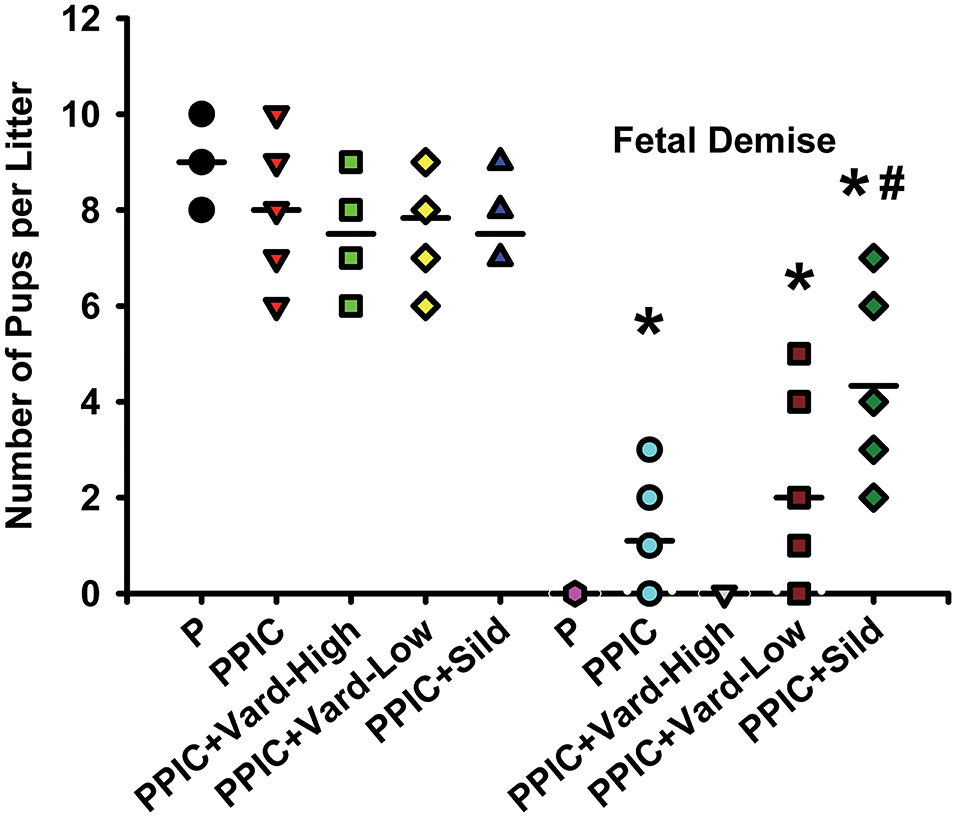
Figure 4. Number of pups per litter as well as number of pups undergoing fetal demise per litter of control pregnant (P) mice (n = 10), P mice treated with the TLR3 agonist and viral mimetic poly I:C (PPIC) (n = 10), PPIC mice treated with a high dose (n = 6) and low dose (n = 6) of vardenafil (Vard), and PPIC mice treated with sildenafil (Sild) (n = 6). *p < 0.05 by ANOVA compared to P mice, #p < 0.05 by ANOVA compared to PPIC mice.
In the current study, like our previous reports (7–11), we confirm that administration of a viral mimetic during pregnancy induces hypertension, immune system activation as measured by splenomegaly, and fetal demise in mice. These results are consistent with PE in women of which there are known associations with various viruses, and these are associated with hypertension, immune system activation, and intrauterine growth restriction. We also report that treatment with a high dose of vardenafil, but not sildenafil, was able to attenuate these negative effects in pregnant mice.
A PubMed search of “vardenafil preeclampsia” only reveals 2 papers. In 2011, Karasu et al. (32) examined vardenafil and sildenafil effects on relaxation responses of human umbilical arteries taken from women with PE and healthy pregnant women. They reported that in all sets of experiments, including the absence and presence of various NO pathway inhibitors, vardenafil induced a maximal relaxation response while sildenafil did not. These results suggest vardenafil acts through NO/cGMP dependent and independent mechanisms. The other paper reported in 2015 by Kakigano and colleagues found that vardenafil markedly increased placental growth factor through various endothelial cell screens and suggested that vardenafil may be useful to treat PE given its pro-angiogenic and vasodilator properties (1).
A number of previous reports demonstrate beneficial effects of the PDE5 inhibitors sildenafil and tadalafil in isolated vessels from women with PE and animal models of PE (32–35). Others have also reported beneficial blood pressure and/or fetal effects of sildenafil in pregnant hypertensive animals (21, 36–42). However, these promising pre-clinical data have not translated smoothly into clinical trials (19, 43). Earlier studies reported no beneficial effects of sildenafil treatment on pregnancy duration, isolated vessels, or fetal outcomes (22, 44, 45). The more recent STRIDER (Sildenafil TheRapy In Dismal prognosis Early-onset fetal growth Restriction) clinical trial not only reported no beneficial effect of sildenafil on pregnancy duration but that treatment increased the risk of neonatal pulmonary hypertension and mortality and was subsequently ended (46–48). While the negative effect of sildenafil on neonatal pulmonary pressure has not been directly determined, it is possible that too much NO at certain time points of gestation may be harmful, similar to that found with high doses of antioxidants during pregnancy. Together, these would support the notion that a proper NO-ROS balance is needed for a healthy fetal outcome.
The significantly increased incidence of fetal demise in our sildenafil-treated PPIC mice supports these findings. Additionally, our results in sildenafil-treated PPIC mice showing no beneficial effects on fetal demise, blood pressure, or splenomegaly contrast those in other animal models of PE such as the COMT –/– mouse, Dahl salt-sensitive rat, L-NAME-treated mice, sFlt-1-treated mice, and others (19–21, 39, 42, 49). This may suggest that viral infection during pregnancy resulting in a PE-like syndrome does not affect the NO pathways that sildenafil targets, but rather that vardenafil improves both NO and placental growth factor and together these contribute to the beneficial effects.
Cellular calcium handling may also play a role in the detrimental effects of a viral infection during a hypertensive pregnancy, as well as the beneficial effects of vardenafil but not sildenafil. Viral infections have been reported to alter calcium dynamics (50). Vardenafil, but not sildenafil, was reported to block caclium channels resulting in vasorelaxation (51, 52). This mechanism may also contribute to the decreased blood pressure and improved fetal development.
Taken together, the beneficial maternal and fetal effects of vardenafil treatment during a virus-induced hypertensive pregnancy in mice may overcome the once promising effects of sildenafil for treating PE that turned out to be somewhat harmful. In pregnancies complicated by viral infection, vardenafil therapy may be beneficial in decreasing blood pressure, dampening immune system activation, and improving fetal outcomes.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Texas A&M University IACUC.
MK, ZR, and BM: study concept, design, and obtained funding. DB and SD: acquisition, analysis, and interpretation of data. DB, MT, ZR, and BM: drafting of the manuscript. All authors carried out critical revision of the manuscript for important intellectual content, read, and approved the final manuscript.
This study was provided by the Texas A&M College of Medicine and the Texas A&M Rangel College of Pharmacy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kakigano A, Tomimatsu T, Mimura K, Kanayama T, Fujita S, Minato K, et al. Drug repositioning for preeclampsia therapeutics by in vitro screening: phosphodiesterase-5 inhibitor vardenafil restores endothelial dysfunction via induction of placental growth factor. Reprod Sci. (2015) 22:1272–80. doi: 10.1177/1933719115574340
2. Wilkerson RG, Ogunbodede AC. Hypertensive disorders of pregnancy. Emerg Med Clin North Am. (2019) 37:301–16. doi: 10.1016/j.emc.2019.01.008
3. Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. (2014) 2:e323–e33. doi: 10.1016/S2214-109X(14)70227-X
4. Anthony J, Damasceno A, Ojjii D. Hypertensive disorders of pregnancy: what the physician needs to know. Cardiovasc J Afr. (2016) 27:104–10. doi: 10.5830/CVJA-2016-051
5. Townsend R, O'Brien P, Khalil A. Current best practice in the management of hypertensive disorders in pregnancy. Integr Blood Press Control. (2016) 9:79–94. doi: 10.2147/IBPC.S77344
6. Tomimatsu T, Mimura K, Matsuzaki S, Endo M, Kumasawa K, Kimura T. Preeclampsia: maternal systemic vascular disorder caused by generalized endothelial dysfunction due to placental antiangiogenic factors. Int J Mol Sci. (2019) 20:4246. doi: 10.3390/ijms20174246
7. Bounds KR, Newell-Rogers MK, Mitchell BM. Four pathways involving innate immunity in the pathogenesis of preeclampsia. Front Cardiovasc Med. (2015) 2:20. doi: 10.3389/fcvm.2015.00020
8. Chatterjee P, Chiasson VL, Kopriva SE, Bounds KR, Newell-Rogers MK, Mitchell BM. Both maternal and placental toll-like receptor activation are necessary for the full development of proteinuric hypertension in mice. Pregnancy Hypertens. (2018) 13:154–60. doi: 10.1016/j.preghy.2018.06.011
9. Chatterjee P, Chiasson VL, Pinzur L, Raveh S, Abraham E, Jones KA, et al. Human placenta-derived stromal cells decrease inflammation, placental injury and blood pressure in hypertensive pregnant mice. Clin Sci (Lond). (2016) 130:513–23. doi: 10.1042/CS20150555
10. Chatterjee P, Chiasson VL, Seerangan G, De Guzman E, Milad M, Bounds KR, et al. Depletion of MHC class II invariant chain peptide or gamma-delta T-cells ameliorates experimental preeclampsia. Clin Sci (Lond). (2017) 131:2047–58. doi: 10.1042/CS20171008
11. Rustveld LO, Kelsey SF, Sharma R. Association between maternal infections and preeclampsia: a systematic review of epidemiologic studies. Matern Child Health J. (2008) 12:223–42. doi: 10.1007/s10995-007-0224-1
12. Braunthal S, Brateanu A. Hypertension in pregnancy: pathophysiology and treatment. SAGE Open Med. (2019) 7:1–15. doi: 10.1177/2050312119843700
13. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. (2013) 20:229–39. doi: 10.1053/j.ackd.2013.01.014
14. Tejera E, Perez-Castillo Y, Chamorro A, Cabrera-Andrade A, Sanchez ME. A multi-objective approach for drug repurposing in preeclampsia. Molecules. (2021) 26:777. doi: 10.3390/molecules26040777
15. Huang SA, Lie JD. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. P T. (2013) 38:407–19.
16. Butrous G. The role of phosphodiesterase inhibitors in the management of pulmonary vascular diseases. Glob Cardiol Sci Pract. (2014) 2014:257–90. doi: 10.5339/gcsp.2014.42
17. Gao C, Liu J, Zhang R, Zhao M, Wu Y. The efficacy of bosentan combined with vardenafil in the treatment of postoperative pulmonary hypertension in children with congenital heart disease: a protocol of randomized controlled trial. Medicine (Baltimore). (2021) 100:e23896. doi: 10.1097/MD.0000000000023896
18. Larre AB, Sontag F, Pasin DM, Paludo N, do Amaral RR, da Costa BEP, et al. Phosphodiesterase inhibition in the treatment of preeclampsia: what is new? Curr Hypertens Rep. (2018) 20:83. doi: 10.1007/s11906-018-0883-x
19. Paauw ND, Terstappen F, Ganzevoort W, Joles JA, Gremmels H, Lely AT. Sildenafil during pregnancy: a preclinical meta-analysis on fetal growth and maternal blood pressure. Hypertension. (2017) 70:998–1006. doi: 10.1161/HYPERTENSIONAHA.117.09690
20. Stanley JL, Sulek K, Andersson IJ, Davidge ST, Kenny LC, Sibley CP, et al. Sildenafil therapy normalizes the aberrant metabolomic profile in the Comt(-/-) mouse model of preeclampsia/fetal growth restriction. Sci Rep. (2015) 5:18241. doi: 10.1038/srep18241
21. Burke SD, Zsengeller ZK, Khankin EV, Lo AS, Rajakumar A, DuPont JJ, et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J Clin Invest. (2016) 126:2561–74. doi: 10.1172/JCI83918
22. Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertens Pregnancy. (2009) 28:369–82. doi: 10.3109/10641950802601278
23. Kendirci M, Bivalacqua TJ, Hellstrom WJ. Vardenafil: a novel type 5 phosphodiesterase inhibitor for the treatment of erectile dysfunction. Expert Opin Pharmacother. (2004) 5:923–32. doi: 10.1517/14656566.5.4.923
24. Wang H, Guo B, Huang Z, Zhao X, Ji Z. Vardenafil in the treatment of male erectile dysfunction: a systematic review and meta-analysis. Adv Ther. (2021) 38:1301–13. doi: 10.1007/s12325-020-01559-9
25. Balasubbramanian D, Gelston CAL, Mitchell BM, Chatterjee P. Toll-like receptor activation, vascular endothelial function, and hypertensive disorders of pregnancy. Pharmacol Res. (2017) 121:14–21. doi: 10.1016/j.phrs.2017.04.018
26. Chatterjee P, Chiasson VL, Bounds KR, Mitchell BM. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front Immunol. (2014) 5:253. doi: 10.3389/fimmu.2014.00253
27. Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, et al. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension. (2011) 58:489–96. doi: 10.1161/HYPERTENSIONAHA.111.172114
28. Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, et al. Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens. (2015) 28:135–42. doi: 10.1093/ajh/hpu100
29. Chatterjee P, Kopriva SE, Chiasson VL, Young KJ, Tobin RP, Newell-Rogers K, et al. Interleukin-4 deficiency induces mild preeclampsia in mice. J Hypertens. (2013) 31:1414–23; discussion 23. doi: 10.1097/HJH.0b013e328360ae6c
30. Chatterjee P, Weaver LE, Doersch KM, Kopriva SE, Chiasson VL, Allen SJ, et al. Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLoS One. (2012) 7:e41884. doi: 10.1371/journal.pone.0041884
31. Balasubbramanian D, Baranwal G, Clark MC, Goodlett BL, Mitchell BM, Rutkowski JM. Kidney-specific lymphangiogenesis increases sodium excretion and lowers blood pressure in mice. J Hypertens. (2020) 38:874–85. doi: 10.1097/HJH.0000000000002349
32. Karasu E, Kayacan N, Sadan G, Dinc B. Different effects of different phosphodiesterase type-5 inhibitors in pre-eclampsia. Pregnancy Hypertens. (2011) 1:231–7. doi: 10.1016/j.preghy.2011.04.002
33. Wareing M, Myers JE, O'Hara M, Kenny LC, Warren AY, Taggart MJ, et al. Effects of a phosphodiesterase-5 (PDE5) inhibitor on endothelium-dependent relaxation of myometrial small arteries. Am J Obstet Gynecol. (2004) 190:1283–90. doi: 10.1016/j.ajog.2003.12.024
34. Wareing M, Myers JE, O'Hara M, Kenny LC, Taggart MJ, Skillern L, et al. Phosphodiesterase-5 inhibitors and omental and placental small artery function in normal pregnancy and pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. (2006) 127:41–9. doi: 10.1016/j.ejogrb.2004.06.014
35. Turgut NH, Temiz TK, Bagcivan I, Turgut B, Gulturk S, Karadas B. The effect of sildenafil on the altered thoracic aorta smooth muscle responses in rat pre-eclampsia model. Eur J Pharmacol. (2008) 589:180–7. doi: 10.1016/j.ejphar.2008.04.034
36. Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. (2006) 12:642–9. doi: 10.1038/nm1429
37. Ueki N, Kanasaki K, Kanasaki M, Takeda S, Koya D. Catechol-O-methyltransferase deficiency leads to hypersensitivity of the pressor response against angiotensin II. Hypertension. (2017) 69:1156–64. doi: 10.1161/HYPERTENSIONAHA.117.09247
38. Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. (2008) 453:1117–21. doi: 10.1038/nature06951
39. Stanley JL, Andersson IJ, Poudel R, Rueda-Clausen CF, Sibley CP, Davidge ST, et al. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension. (2012) 59:1021–8. doi: 10.1161/HYPERTENSIONAHA.111.186270
40. Roberts RP, Refuerzo JS, Ferrari F, Ontiveros AE, Tamayo EH, Sibai BM, et al. Sildenafil treatment in a nonsevere hypertensive murine model lowers blood pressure without reducing fetal growth. Am J Obstet Gynecol. (2016) 215:386.e1–e8. doi: 10.1016/j.ajog.2016.05.002
41. George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol. (2013) 305:R397–R403. doi: 10.1152/ajpregu.00216.2013
42. Gillis EE, Mooney JN, Garrett MR, Granger JP, Sasser JM. Sildenafil treatment ameliorates the maternal syndrome of preeclampsia and rescues fetal growth in the dahl salt-sensitive rat. Hypertension. (2016) 67:647–53. doi: 10.1161/HYPERTENSIONAHA.115.06071
43. Simon-Tillaux N, Lecarpentier E, Tsatsaris V, Hertig A. Sildenafil for the treatment of preeclampsia, an update: should we still be enthusiastic? Nephrol Dialysis Transp. (2019) 34:1819–26. doi: 10.1093/ndt/gfy328
44. Samangaya RA, Wareing M, Skillern L, Baker PN. Phosphodiesterase inhibitor effect on small artery function in preeclampsia. Hypertens Pregnancy. (2011) 30:144–52. doi: 10.3109/10641955.2010.484083
45. Trapani A Jr, Goncalves LF, Trapani TF, Vieira S, Pires M, et al. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: a randomized controlled trial. Obstet Gynecol. (2016) 128:253–9. doi: 10.1097/AOG.0000000000001518
46. Ganzevoort W, Alfirevic Z, von Dadelszen P, Kenny L, Papageorghiou A, van Wassenaer-Leemhuis A, et al. STRIDER: Sildenafil Therapy In Dismal prognosis Early-onset intrauterine growth Restriction–a protocol for a systematic review with individual participant data and aggregate data meta-analysis and trial sequential analysis. Syst Rev. (2014) 3:23. doi: 10.1186/2046-4053-3-23
47. Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC, et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health. (2018) 2:93–102. doi: 10.1016/S2352-4642(19)30020-3
48. Hawkes N. Trial of Viagra for fetal growth restriction is halted after baby deaths. Bmj. (2018) 362:k3247. doi: 10.1136/bmj.k3247
49. Motta C, Grosso C, Zanuzzi C, Molinero D, Picco N, Bellingeri R, et al. Effect of sildenafil on pre-eclampsia-like mouse model induced by L-name. Reprod Domest Anim. (2015) 50:611–6. doi: 10.1111/rda.12536
50. Saurav S, Tanwar J, Ahuja K, Motiani RK. Dysregulation of host cell calcium signaling during viral infections: emerging paradigm with high clinical relevance. Mol Aspects Med. (2021) 81:101004. doi: 10.1016/j.mam.2021.101004
51. Toque HA, Teixeira CE, Priviero FB, Morganti RP, Antunes E, De Nucci G. Vardenafil, but not sildenafil or tadalafil, has calcium-channel blocking activity in rabbit isolated pulmonary artery and human washed platelets. Br J Pharmacol. (2008) 154:787–96. doi: 10.1038/bjp.2008.141
Keywords: vardenafil, immunity, virus, pregnancy, hypertension
Citation: Balasubbramanian D, Dharani S, Tauseef M, Khan MA, Rahman Z and Mitchell BM (2021) High Dose Vardenafil Blunts the Hypertensive Effects of Toll-Like Receptor 3 Activation During Pregnancy. Front. Virol. 1:780298. doi: 10.3389/fviro.2021.780298
Received: 20 September 2021; Accepted: 11 November 2021;
Published: 08 December 2021.
Edited by:
Kristina M. Adams Waldorf, University of Washington, United StatesReviewed by:
Marina Durward-Diioia, West Virginia School of Osteopathic Medicine, United StatesCopyright © 2021 Balasubbramanian, Dharani, Tauseef, Khan, Rahman and Mitchell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brett M. Mitchell, YnJldHRtaXRjaGVsbEB0YW11LmVkdQ==; orcid.org/0000-0002-2575-8761
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.