
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 01 April 2025
Sec. Veterinary Infectious Diseases
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1556965
This article is part of the Research TopicResearch Advances toward One Health in BrucellosisView all 9 articles
 Tessa E. LeCuyer1*
Tessa E. LeCuyer1* Rebecca Franklin-Guild2
Rebecca Franklin-Guild2 Cassandra Guarino2
Cassandra Guarino2 Alexandra Fox3
Alexandra Fox3 Kelli Maddock4
Kelli Maddock4 Rebecca Barber5
Rebecca Barber5 David H. Baum6
David H. Baum6 Felipe Bustamante7
Felipe Bustamante7 Joshua Daniels8
Joshua Daniels8 David M. de Avila9
David M. de Avila9 Dubraska Diaz-Campos10
Dubraska Diaz-Campos10 Lisa G. Glick5
Lisa G. Glick5 Jessica Haley3
Jessica Haley3 Sheila Heinen6
Sheila Heinen6 Laura Leger11
Laura Leger11 John Dustin Loy11
John Dustin Loy11 Deepti Pillai12
Deepti Pillai12 Korakrit Poonsuk9
Korakrit Poonsuk9 Michael M. Russell8
Michael M. Russell8 Mithila Shukla12
Mithila Shukla12 Erika R. Schwarz13
Erika R. Schwarz13 Matthew Stempien10
Matthew Stempien10 Deepanker Tewari7
Deepanker Tewari7 Joany C. van Balen10
Joany C. van Balen10 Stephen R. Werre14
Stephen R. Werre14 Julie T. Cecere15
Julie T. Cecere15Brucella canis is a zoonotic pathogen of dogs that poses diagnostic challenges. While direct detection of B. canis by PCR or culture is ideal, serologic diagnosis is necessary for identification of carrier animals and can support a clinical diagnosis of brucellosis. Prior to 2022, B. canis seroscreening in the United States was primarily performed using a commercially available rapid slide agglutination test. However, the kit was discontinued by the manufacturer in early 2022, leaving a gap in the availability of commercial B. canis seroassays. The goal of this study was to compare the performance of three B. canis serologic tests that are currently available: VMRD Brucella ovis ELISA, Bionote Anigen Rapid C.Brucella Ab immunochromatographic lateral flow assay, and VMRD B. canis indirect fluorescent antibody (IFA) assay. A panel of 56 banked serum specimens originally submitted to the Cornell University Animal Health Diagnostic Center (the study reference laboratory) for B. canis seroscreening was distributed to 12 testing laboratories. Each sample was run on three assays developed at the reference lab: rapid slide agglutination test with 2-mercaptoethanol (2-ME RSAT), agar gel immunodiffusion test using cytoplasmic antigen (AGID II), and Canine Brucella Multiplex. Five testing labs ran the ELISA, six ran the lateral flow, and six ran the IFA. When evaluated as a screening assay, we compared the assays to the 2ME-RSAT. The ELISA had the highest sensitivity (96.8, 95%CI 83.8–99.9) but the lowest specificity (79.3, 95%CI 57.9–92.9). The sensitivity of the lateral flow was 90.6% (95%CI 75–98%) and the IFA was 87.5% (95%CI 71–96.5). Specificity for the lateral flow was 95.8% (95%CI 78.9–99.9) and IFA was 97.5% (95%CI 67.6–97.3). When compared to AGID II and Canine Brucella Multiplex, the test assays were all highly sensitive, but specificity was <90%. Interrater reliability was highest for IFA (Κ = 0.92) and lowest for the lateral flow (Κ = 0.82). Serial testing of positive samples with a more specific test, such as AGID II, will continue to be necessary when using any of the three assays tested in this study.
Brucella canis, the primary etiologic agent of canine brucellosis, is a zoonotic pathogen that is facultatively intracellular and can persist in macrophages for months to years (1, 2). Clinical signs of infection are non-specific and highly variable, ranging from testicular enlargement and abortion to ocular abnormalities and discospondylitis (1–6). This combination of inconsistent clinical signs and the intracellular, chronic persistence of the organism makes B. canis a diagnostic challenge (1, 3). The gold standard diagnostic method is direct detection of B. canis by culture, although use of PCR is increasingly described (2, 4, 5). However, direct detection is not always possible in infected animals because bacterial shedding in secretions and bacteremia may be intermittent and short-lived, with low levels of organism in circulation (1, 3).
Because of the difficulties with direct detection of B. canis, serologic tests are important for B. canis screening, as dogs remain seropositive after bacteremia has waned (2). Unfortunately, serologic tests for B. canis tend to have relatively low sensitivity and specificity (1, 7–9). However, they remain useful for detecting subclinical and chronic infections that may not be otherwise recognized as well as screening breeding animals (4). The serologic tests that are typically recommended for B. canis diagnosis and screening in the United States are the rapid slide agglutination test (RSAT), rapid slide agglutination test with 2-mercaptoethanol (2ME-RSAT), and agar gel immunodiffusion test using cytoplasmic antigen (AGID II). A commercially available RSAT (with optional 2ME step), the Zoetis D-Tec CB, was available until 2022. There is currently no commercially available source of RSAT, 2ME-RSAT, or AGID II tests or assay components in the United States, leaving a gap in diagnostic assays available for B. canis screening and diagnosis (5).
The goal of this study was to evaluate the performance of three methods of B. canis serologic testing that were available at the time of the study in the United States for use by veterinary diagnostic laboratories or as a point-of-care test by performing an interlaboratory comparison of a panel of 56 clinical serum specimens. The assays that were tested were VMRD Brucella ovis ELISA, Bionote Anigen Rapid C.Brucella Ab immunochromatographic lateral flow assay, and VMRD B. canis indirect fluorescent antibody assay (IFA). The assays were compared to the 2ME-RSAT, AGID II and Cornell University Animal Health Diagnostic Center’s (AHDC) Canine Brucella Multiplex (CBM).
The participating testing laboratories were: Colorado State University Veterinary Diagnostic Laboratory (Fort Collins, CO), Indiana Animal Disease Diagnostic Laboratory – Purdue University (West Lafayette, IN), Montana Veterinary Diagnostic Laboratory (Bozeman, MT), Nebraska Veterinary Diagnostic Center (Lincoln, NE), North Dakota State University Veterinary Diagnostic Laboratory (Fargo, ND), Ohio State University Veterinary Medical Center Clinical Microbiology Laboratory (Columbus, OH), Pennsylvania Veterinary Laboratory (Harrisburg, PA), University of Florida Veterinary Hospital Clinical Microbiology Laboratory (Gainesville, FL), Virginia Tech Animal Laboratory Services (Blacksburg, VA), Washington Animal Disease Diagnostic Laboratory (Pullman, WA), Wyoming State Veterinary Laboratory (Laramie, WY). The reference laboratory was Cornell University AHDC (Ithaca, NY), which provided 2ME-RSAT, AGID II, and CBM test results. Testing laboratories volunteered to participate in the study and self-selected one or two diagnostic assays to test that were considered candidate assays for use in each respective laboratory. The ELISA was tested at five laboratories, the IFA at six laboratories, and the lateral flow assay at six laboratories. Specimens were tested September–November 2023. All laboratories had established quality assurance systems, and the staff was experienced in performing serological assays.
Samples for the test panel were provided by Cornell University AHDC from banked clinical specimens and were distributed to participating laboratories. Serum samples were frozen at −20°C at Cornell University AHDC after diagnostic testing and were selected for use in the study based on the availability of sufficient volumes and prior B. canis serologic test results. The test panel consisted of 56 samples, which consisted of 24 specimens that were negative on 2ME-RSAT and AGID II, 7 specimens that were 2ME-RSAT positive and AGID II negative, and 25 samples that were both 2ME-RSAT and AGID II positive (20 were high positive on the CBM and 5 were low positive or equivocal on CBM). This panel was designed to have a composition appropriate for studies of assay repeatability, reproducibility, and laboratory and method comparison exercises because of the size of the panel and range of potential test results (10).
Specimens were tested on AGID II, 2ME-RSAT and CBM prior to aliquoting and shipping the specimens to the participating laboratories. Serum was shipped to the participating laboratories frozen on dry ice and stored frozen (−20°C or below) at each laboratory until testing occurred. Specimens were tested September–November 2023.
Specimens for the dilution series were prepared at Virginia Tech Animal Laboratory Services (Blacksburg, VA). Two aliquots of NVSL reagent 212-H “high positive B. canis Tube Agglutination Tests (TAT) control serum” (National Veterinary Services Laboratory, Ames, IA) were pooled and serial 2-fold dilutions were made using sterile 0.9% saline as the diluent. Specimens generated ranged from neat, undiluted control serum to a 1:2048 dilution. Aliquots were prepared and frozen at −20°C until shipping. Specimens were shipped to the participating laboratories on ice within 72 h of generation. The specimens were stored frozen (−20°C or below) at each laboratory until testing occurred. The dilution series specimens were run with the test panel by the same operators.
Assays performed at the Cornell University AHDC included the 2ME-RSAT, AGID II, and CBM. The 2ME-RSAT was performed using a killed whole-cell antigen derived at Cornell University from B. canis M- strain and stained with Rose Bengal. Patient serum was mixed with 0.2 M 2-mercaptoethanol (2-ME) as previously described (11). The AGID II was performed using a B. canis M-strain-derived cytoplasmic antigen and was performed as previously described (11). The CBM was performed using a multiplex fluorescent bead-based platform (Luminex) with capture reagents composed of recombinant B. canis proteins including BP26 and a peptide derived from Omp31 (6, 12).
Assays performed at the testing laboratories included B. ovis ELISA test components (VMRD, Pullman, WA), B. canis IFA assay (VMRD, Pullman, WA) and Rapid C.Brucella Ab immunochromatographic lateral flow assay (Bionote Anigen, Gentaur, San Jose, CA). Eleven testing laboratories ran each assay in duplicate with each run generated by a different operator. One testing laboratory ran their test assay in singlet.
The B. canis IFA and Bionote lateral flow were performed following manufacturer kit insert instructions. For the IFA, specimens were run at a screening dilution of 1:50. In brief, the protocol for the B. ovis ELISA was to dilute specimens 1:50 in serum diluting buffer and load 100 μL of diluted specimen into the antigen coated plate. Specimens were incubated for 30 min at room temperature, and then the plate was washed four times with wash buffer. Peroxidase conjugate was added, followed by another 30-min room temperature incubation and four washes. Finally, plates were developed with peroxidase substrate solution for 15 min at room temperature and then stop solution was added. Plates were read at 450 nm and optical density values were converted to S/P ratios. The cutoff for positive was S/p ≥ 0.8, which was based on canine data provided by the manufacturer.
Results from each laboratory were compiled by the study lead (TEL). Sensitivity and specificity of each assay were determined with respect to each of the Cornell University ADHC assays: 2ME-RSAT, AGID II, and CBM. Sensitivity and specificity calculations were based on the first result for each assay that was submitted by each laboratory. The CBM assay is semi-quantitative and therefore results were recategorized into negative (negative results) and positive (equivocal, low positive, and high positive) categories for sensitivity/specificity calculations. Exact 95% confidence intervals were calculated based on binomial probabilities. Interrater reliability was determined by Cohen’s kappa. These calculations were performed in SAS (Cary, NC). Youden’s indices were manually calculated from the sensitivity/specificity calculations. ELISA S/P ratios were compared to 2ME-RSAT results for receiver operating characteristics (ROC) analysis. The optimal ELISA cut-off was calculated by maximizing the Youden’s index with the online tool easyROC (v.1.3.1) (13).
Sensitivity and specificity of each assay with respect to the reference B. canis seroassays 2ME-RSAT and AGID II are provided in Table 1. Interrater reliability for the test assays was highest for the IFA assay, Κ = 0.92 (95%CI: 0.88–0.96). The kappa scores for interrater reliability for the other assays were: ELISA Κ = 0.87 (95%CI: 0.82–0.93), lateral flow Κ = 0.82 (95%CI 0.79–0.86).
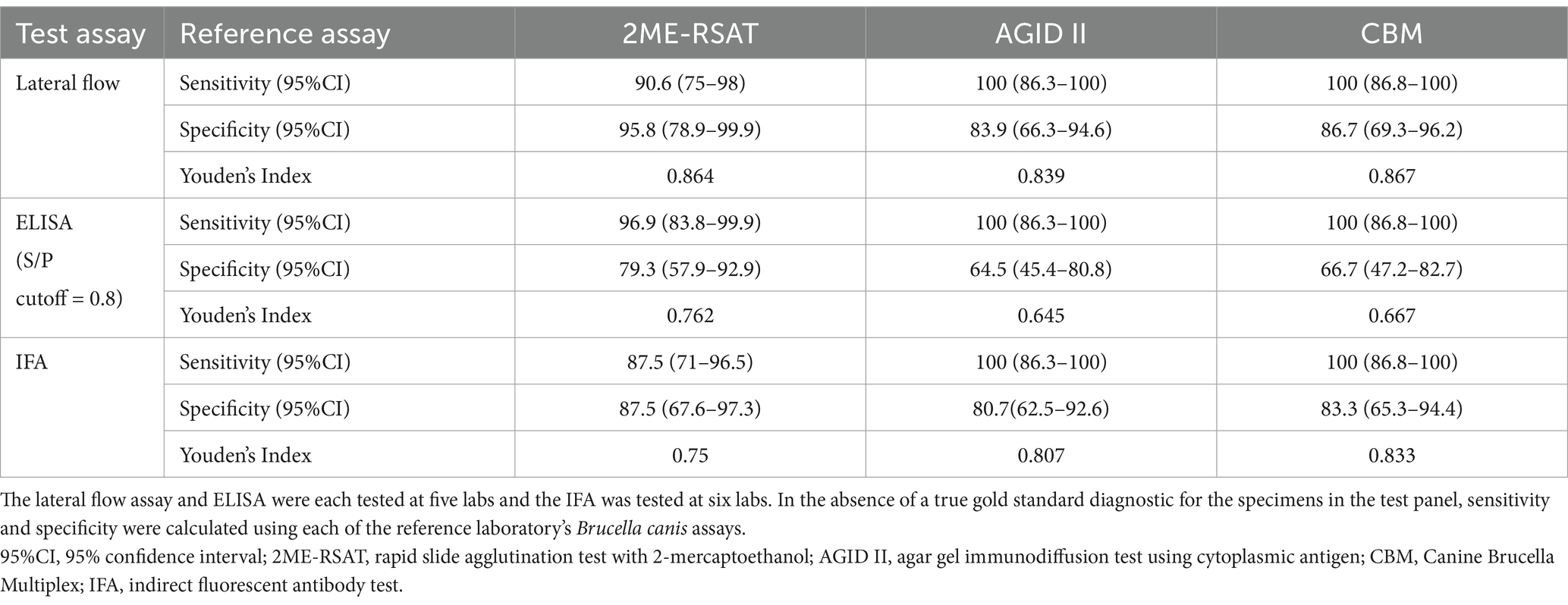
Table 1. Sensitivity and specificity of each test assay in the study, based on the test panel of 56 serum samples that were tested on each assay.
We determined how many samples on the panel had results that were discordant with all three of the reference laboratory tests (2ME-RSAT, AGID II, and CBM) based on the results of at least one test assay in the study. The lateral flow had the highest number of serum samples that tested negative (n = 6) when all the reference lab tests were non-negative. The ELISA had the highest number of samples that tested positive when all the reference tests were negative (n = 8) (Supplemental Figure 1).
Based on ROC analysis comparing the ELISA S/P ratios to reference 2ME-RSAT results, the optimal cut-off for ELISA for the data generated in this study was 0.913. Using this cutoff, the revised sensitivity of the ELISA with respect to the 2ME-RSAT was 93.1% (95%CI: 89.8–95.6) and the revised specificity was 86.7% (95%CI: 81.7–90.7). Using the revised S/P cutoff, the number of samples that tested positive on ELISA when all the reference tests were all negative was reduced to 7 samples.
The relative limit of detection for each assay was assessed by testing a serial dilution panel of B. canis TAT control serum that is available from the National Veterinary Services Laboratories (NVSL, Ames, IA) (Table 2).
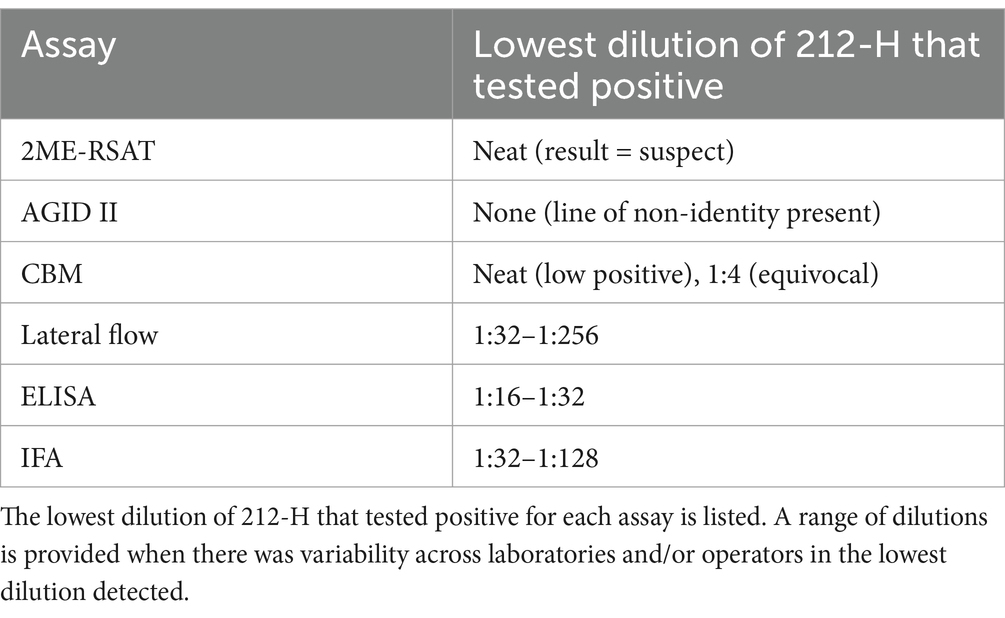
Table 2. Two-fold serial dilutions of NVSL reagent 212-H Brucella canis high positive TAT control were run on each assay to compare the limit of detection of each assay on a standardized serum specimen.
It is crucial for clinicians and veterinary diagnostic laboratories to have access to reliable diagnostic tests for B. canis. Because B. canis remains a diagnostic challenge with no single diagnostic approach that is best for every case, we performed this interlaboratory comparison to improve the understanding of the strengths and limitations of currently available B. canis test options in the United States. Because neither culture nor PCR status of the animals that provided serum for the test panel used in the study were available, it was not possible to determine a true “gold standard” for sensitivity and specificity calculations. Therefore, we determined the sensitivity and specificity with respect to each of the assays that is currently offered at Cornell University AHDC, the lab that has become the B. canis reference laboratory in the US and the only laboratory to currently offer the CBM, 2ME-RSAT, and AGID II assays for diagnostic testing.
The Bionote B. canis immunochromatographic lateral flow assay has not been well-characterized to date, although the manufacturer reports in the kit insert that the assay’s “overall accuracy is greater or equal to 90%” when compared to 2ME-RSAT (14). The antigen composition has not been well-described but is presumably a whole cell extract of the M (−) strain of B. canis based on previously published data from Bionote’s parent company, Animal Genetics, Inc., South Korea (15). Despite the lack of verified performance information for the current iteration of the test, it was adopted by many veterinarians as a point-of-care screening assay for B. canis upon discontinuation of the Zoetis D-tec RSAT. Several veterinary diagnostic laboratories have also started to offer this lateral flow assay after performing internal assessments of assay performance. The assay was evaluated under the name “Anigen Rapid Canine Brucella Ab Test” (manufactured by Bioeasy, South Korea) in Brazil. While the test overall had higher sensitivity than RSAT, 2ME-RSAT, and a B. ovis AGID, 10.4% of infected dogs (confirmed by culture and/or PCR) tested negative (8). However, of the five infected dogs in the study that tested negative, 3 had clinical signs of active brucellosis when they tested negative and likely had not seroconverted yet, as they did not test positive on RSAT or B. ovis AGID either. In populations in the United States in which the prevalence of B. canis is relatively low, such as many breeding kennels, the use of the lateral flow assay may be adequate as a screening assay but follow-up testing using another method is still required for confirmation due to the specificity of the assay when compared to the confirmatory AGID II (16, 17).
The lateral flow assay had the lowest interrater reliability of the assays tested in this study despite having the highest Youden’s index, a measure of overall diagnostic test performance. Some of the laboratories reported difficulty in reading the results for some specimens on the lateral flow assay due to the presence of non-specific colorless bands that appeared on the test kit. Although there was no color change, these areas had a slightly different color from the background of the test device and therefore could be misinterpreted as non-negative results. This, as well as the qualitative evaluation for color change in the lateral flow assay, may have led to the lower inter-rater reliability for this assay. Despite the lower agreement between test operators, the agreement for this assay could still be interpreted as “almost perfect” (18). All the test operators in this study were experienced technicians at veterinary diagnostic laboratories; it is possible that interrater agreement in a clinic environment may be lower due greater variability in experience of test operators in that setting. Due to the zoonotic implications of B. canis infection, it would be prudent to consider any test result that is not clearly negative as indication for confirmatory testing.
The antigen for the ELISA used in this study is B. ovis whole cell lipopolysaccharide extract and was designed for B. ovis testing. The ELISA reagents are currently available for research use only. Like B. canis, B. ovis is also a rough strain and the two species have serologic cross-reactivity (19). The ELISA components have also been tested by the manufacturer on canine serum samples to test for B. canis exposure. The manufacturer’s ROC analysis of ELISA S/P ratios, compared to the IFA assay, showed that a S/P cutoff of 0.8 was ideal. Our ROC analysis using the 2ME-RSAT as the reference test showed that a slightly higher S/P cutoff of 0.931 improved the specificity of the ELISA without greatly reducing the sensitivity. Follow-up testing by a more specific test such as AGID II is needed if the B. ovis ELISA is used as a screening test. Although ELISA results are read by an automated reader and interpreted using standard cut-off values, thereby increasing objectivity in result interpretation, this assay did not have the highest interrater reliability observed in this study.
The IFA assay uses B. canis strain RM666 antigen, which is the American type-strain of B. canis (20). We used the 1:50 screening dilution, but the manufacturer’s validation data shows a 1:100 dilution can decrease false positive reactions (21). We used a 1:50 sample dilution for this study because it was the lowest dilution regularly used by any of the labs using the IFA for diagnostic purposes. The manufacturer kit insert suggests that samples that are positive at 1:100 are “suspect” and follow-up testing with a confirmatory test such as AGID II is recommended (22).
Most B. canis serologic assays detect antibody against lipopolysaccharide (LPS) which may lead to cross reactions with antibody to Gram-negative bacteria (6, 23). In addition, antibodies generated against antigens on the surface of other bacteria can lead to non-specific B. canis serologic test results (1). The AGID II assay can avoid non-specific reactions by using cytoplasmic antigens and the CBM assay uses non-LPS antigens to avoid non-specific anti-LPS antibody binding (1, 12). Therefore, it is not surprising that the specificity of the three test assays was <90% when compared to the AGID II and CBM due to differences in the antigens across assays.
The high titer B. canis TAT control serum 212-H was generated as a control for the tube agglutination test, and therefore its performance has been optimized for that assay only. The reagent was obtained from a naturally infected dog and consists of lyophilized serum that contains 0.02% thimerosal as a preservative. The finding that the reference assays 2ME-RSAT and AGID II have a lower ability to detect antibody in this serum generated for use in a different test system highlights the difficulties in B. canis serodiagnosis. It is likely that there are antibodies to antigenic targets present in this serum sample that minimally interact with the antigens in the 2ME-RSAT and AGID II.
In our evaluation of three B. canis serologic assays with different test formats and antigens, each at five or six different veterinary diagnostic laboratories, we found that interrater reliability of all the assays was good. Each test assay has its own limitations and there was no single assay that had the best overall performance. The lateral flow had the lowest interrater agreement. The ELISA had the lowest specificity, but this could be mitigated in part by optimizing the S/P ratio cutoff for positive/negative results. The IFA had the lowest sensitivity when compared to the 2ME-RSAT, which has historically been used as the primary B. canis screening assay in the US. With knowledge of these limitations, all three of the test assays could reasonably be incorporated into a diagnostic testing algorithm for B. canis, but for all, a positive result on any of the tests would be an indication for follow-up testing with a more specific test (4, 5).
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because the study used leftover specimens submitted to a veterinary diagnostic laboratory for clinical diagnostic purposes. Samples submitted for testing became the property of the diagnostic laboratory upon submission. Written informed consent was not obtained from the owners for the participation of their animals in this study because the study used leftover specimens submitted to a veterinary diagnostic laboratory for clinical diagnostic purposes.
TL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. RF-G: Conceptualization, Methodology, Project administration, Resources, Writing – original draft. CG: Conceptualization, Methodology, Project administration, Resources, Writing – original draft. AF: Investigation, Project administration, Resources, Writing – review & editing. KM: Conceptualization, Data curation, Project administration, Writing – review & editing. RB: Investigation, Writing – review & editing. DB: Project administration, Writing – review & editing. FB: Investigation, Project administration, Writing – review & editing. JD: Conceptualization, Methodology, Project administration, Writing – review & editing. DA: Investigation, Project administration, Writing – review & editing. DD-C: Conceptualization, Methodology, Project administration, Writing – review & editing. LG: Investigation, Project administration, Writing – review & editing. JH: Investigation, Writing – review & editing. SH: Project administration, Writing – review & editing. LL: Project administration, Writing – review & editing. JL: Conceptualization, Methodology, Project administration, Writing – review & editing. DP: Writing – review & editing, Project administration. KP: Investigation, Project administration, Writing – review & editing. MR: Investigation, Writing – review & editing. MiS: Investigation, Writing – review & editing. ES: Project administration, Writing – review & editing. MaS: Investigation, Writing – review & editing. DT: Project administration, Writing – review & editing. JV: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. SW: Formal analysis, Methodology, Writing – review & editing. JC: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. Funding for this study was provided in part through the Dr. JoAnne S. O’Brien Endowment Fund at Virginia Polytechnic Institute and State University through the Virginia-Maryland College of Veterinary Medicine. Individual laboratories purchased diagnostic supplies and reagents for their component of the testing.
We would like to thank the laboratory staff who assisted with this project, including Tucker Bean, Sharon Wilson, and Lynae Hansen-Lardy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
VMRD and Gentaur provided discounted (10-15% off retail price) reagents to the testing laboratories, but the manufacturers had no control over the design, implementation, or analysis of this study.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1556965/full#supplementary-material
1. Cosford, KL. Brucella canis: an update on research and clinical management. Can Vet J. (2018) 59:74–81.
2. Santos, RL, Souza, TD, Mol, JPS, Eckstein, C, and Paíxão, TA. Canine brucellosis: an update. Front Vet Sci. (2021) 8:594291. doi: 10.3389/fvets.2021.594291
3. Hollett, RB. Canine brucellosis: outbreaks and compliance. Theriogenology. (2006) 66:575–87. doi: 10.1016/j.theriogenology.2006.04.011
4. Bramlage, DJ, Fortney, W, Kesler, R, Mabray, CJ, Mason, JW, Reinhold, H, et al. Best practices for Brucella canis prevention and control in dog breeding facilities. USDA APHIS. (2015) https://www.aphis.usda.gov/sites/default/files/brucella_canis_prevention.pdf
5. Sebzda, MK, and Kauffman, LK. Update on Brucella canis: understanding the past and preparing for the future. Vet Clin. (2023) 53:1047–62. doi: 10.1016/j.cvsm.2023.05.002
6. Pinn-Woodcock, T, Frye, E, Guarino, C, Franklin-Guild, R, Newman, AP, Bennett, J, et al. A one-health review on brucellosis in the United States. JAVMA. (2023) 261:451–62. doi: 10.2460/javma.23.01.0033
7. Carmichael, LE, and Shin, SJ. Canine brucellosis: A diagnostician’s dilemma. Semin Vet Med Surg. (1996) 11:161–5. doi: 10.1016/s1096-2867(96)80028-4
8. Keid, L, Diniz, J, Oliveira, T, Ferreira, H, and Soares, R. Evaluation of an Immunochromatographic test to the diagnosis of canine brucellosis caused by Brucella canis. Reprod Domestic Animals. (2015) 50:939–44. doi: 10.1111/rda.12612
9. Mol, JPS, Guedes, ACB, Eckstein, C, Quintal, APN, Souza, TD, Mathias, LA, et al. Diagnosis of canine brucellosis: comparison of various serologic tests and PCR. J Vet Diagn Invest. (2020) 32:77–86. doi: 10.1177/1040638719891083
10. World Organisation for Animal Health (OIE). WOAH terrestrial manual. Chapter 2.2.6. Paris, France: WOAH; (2024). Available online at: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/2.02.06_REFERENCE_SAMPLES.pdf (Accessed May 30, 2024).
11. Helms, AB, Balogh, O, Franklin-Guild, R, Lahmers, K, Caswell, CC, and Cecere, JT. Presumptive identification of smooth Brucella strain antibodies in canines. Front Vet Sci. (2021) 8:697479. doi: 10.3389/fvets.2021.697479
12. Guarino, C, Wagner, B, and Altier, C. A multiplex assay for the diagnosis of Brucella canis infection. United States Patent. (2022)
13. Goksuluk, D, Korkmaz, S, Zararsiz, G, and Karaagaoglu, AE. easyROC: an interactive web-tool for ROC curve analysis using R language environment. R Journal. (2016) 8:213. doi: 10.32614/RJ-2016-042
15. Kim, JW, Lee, YJ, Han, MY, Bae, DH, Jung, SC, Oh, JS, et al. Evaluation of Immunochromatographic assay for Serodiagnosis of Brucella canis. J Vet Med Sci. (2007) 69:1103–7. doi: 10.1292/jvms.69.1103
16. Hubbard, K, Wang, M, and Smith, DR. Seroprevalence of brucellosis in Mississippi shelter dogs. Prev Vet Med. (2018) 159:82–6. doi: 10.1016/j.prevetmed.2018.09.002
17. Hensel, ME, Negron, M, and Arenas-Gamboa, AM. Brucellosis in dogs and public health risk. Emerg Infect Dis. (2018) 24:1401–6. doi: 10.3201/eid2408.171171
18. Landis, JR, and Koch, GG. The measurement of observer agreement for categorical data. Biometrics. (1977) 33:159. doi: 10.2307/2529310
19. Nielsen, K, Smith, P, Conde, S, Draghi de Benitez, G, Gall, D, Halbert, G, et al. Rough lipopolysaccharide of Brucella abortus RB51 as a common antigen for serological detection of B. ovis, B. canis, and B. abortus RB51 exposure using indirect enzyme immunoassay and fluorescence polarization assay. J Immunoass Immunochem. (2004) 25:171–82. doi: 10.1081/ias-120030526
20. Forbes, LB, and Pantekoek, JF. Brucella canis isolates from Canadian dogs. Can Vet J. (1988) 29:149–52.
22. VMRD. Brucella canis FA substrate slide technical data sheet. VMRD. (2016) https://d556be5e-f357-482c-8cb2-93c70893ed9d.usrfiles.com/ugd/fa6b8d_cd5ea433878d421ab6f8288adbf486b7.pdf
Keywords: brucellosis, Brucella canis , serology, canine, diagnosis
Citation: LeCuyer TE, Franklin-Guild R, Guarino C, Fox A, Maddock K, Barber R, Baum DH, Bustamante F, Daniels J, de Avila DM, Diaz-Campos D, Glick LG, Haley J, Heinen S, Leger L, Loy JD, Pillai D, Poonsuk K, Russell MM, Shukla M, Schwarz ER, Stempien M, Tewari D, van Balen JC, Werre SR and Cecere JT (2025) Performance characteristics of three Brucella canis serological assays in the United States. Front. Vet. Sci. 12:1556965. doi: 10.3389/fvets.2025.1556965
Received: 07 January 2025; Accepted: 12 March 2025;
Published: 01 April 2025.
Edited by:
Claire Ponsart, Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (ANSES), FranceReviewed by:
Roberto Senas Cuesta, University of Arkansas, United StatesCopyright © 2025 LeCuyer, Franklin-Guild, Guarino, Fox, Maddock, Barber, Baum, Bustamante, Daniels, de Avila, Diaz-Campos, Glick, Haley, Heinen, Leger, Loy, Pillai, Poonsuk, Russell, Shukla, Schwarz, Stempien, Tewari, van Balen, Werre and Cecere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tessa E. LeCuyer, dGxlY3V5ZXJAdWNkYXZpcy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.