
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Vet. Sci., 24 March 2025
Sec. Oncology in Veterinary Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1546872
This article is part of the Research TopicCancer in Domestic, Exotic and Wild Animals: New Horizons in Tumorigenesis, Diagnosis, Prognosis and Therapeutics through Comparative OncologyView all 11 articles
Canine osteosarcomas (COS) are the most common bone tumors in dogs, characterized by high metastatic rates, poor prognosis, and poor responsiveness to routine therapies, which highlights the need for new treatment targets. In this context, the metabolism of neoplastic cells represents an increasingly studied element, as cancer cells depend on particular metabolic pathways that are also elements of vulnerability. Among these, tumor cells (TCs) show higher iron requirements to sustain proliferation (so-called iron addiction), which are achieved by increasing iron uptake and/or by activating ferritinophagy, a process mediated by the Nuclear receptor Co-Activator 4 (NCOA4) leading to iron mobilization from ferritin (Ft) deposits. Previous studies have shown that COS cells overexpress Transferrin Receptor 1 (TfR1) to increase iron uptake. In this study we evaluated the immunohistochemical expression of ferritinophagy-related proteins, namely Ferritin Heavy chain (FTH1) and NCOA4, and proliferating cell nuclear antigen (PCNA) in canine normal bone and canine osteoblastic osteosarcoma (COOS) samples. Normal samples revealed negative/weak immunoreactivity for FTH1, NCOA4 and PCNA in <10% of osteocytes. In COOS samples the majority of neoplastic cells showed immunoreactivity to FTH1, NCOA4 and PCNA. Our data suggest that the activation of ferritinophagy by COOS cells responds to the need for feed their “iron addiction.” These data, though preliminary, further suggest that targeting iron metabolism represents a new potential strategy worthy of further study to be transferred into clinical practice.
The study of metabolic alterations of neoplastic cells is currently a hot topic, as cancer cells can become addicted to specific metabolic pathways also representing metabolic vulnerabilities against which novel drugs that target them can be developed (1). Among these, the so-called “iron addiction” is one of the most relevant metabolic alterations of neoplastic cells (2). Cancer cells show higher iron requirements than normal cells to sustain proliferation (3) and tissue invasion (4) and tend to satisfy this need by over-expressing a series of proteins involved both in the iron uptake from the bloodstream (5, 6) and in its mobilization from intracellular reserves by so-called “ferritinophagy,” a selective form of autophagy that specifically targets intracellular (Ft) for lysosomal degradation (7, 8). Key molecules in iron metabolism are: (1) TfR1, which uptakes and internalizes iron by binding transferrin (Tf)-Fe3+ complex, which is followed by Fe3+ reduction to Fe2+ by ferrireductases in the cytosol (9); (2) the Ft, which represents the storage site of iron in the cytosol, and which also contributes to the physiological release of iron from reserves to form the cytoplasmic labile iron pool (cLIP) (10–12); and (3) the NCOA4, a selective cargo protein which binds to a conserved C-terminal domain of FTH1 and to autophagy-related proteins to deliver FT to autophagosomes and trigger ferritinophagy (13, 14). Previous studies in human pathology have reported impairment of iron metabolism in different cancers (15–21). This appears to be particularly true in human osteosarcomas (22, 23), the most common primary malignant bone tumor affecting children and adolescents (24, 25). Unfortunately, in veterinary medicine iron metabolism and its alterations connected to cancer are still poorly studied (26–31). The early results presented in a previous study (26) highlighted the relevance of TfR-1 expression in canine osteosarcomas (COS), suggesting therapies involving both TfR-1 and other molecules related to iron metabolisms in dogs with osteosarcoma should be developed, also considering the potential clinical impact for humans. COS represent a well-known preclinical model for human osteosarcoma, particularly for those developing in young people as they share molecular and morphological aspects, as well as prognosis and treatment options (32). COS represent the most frequent primary malignant bone neoplasms of mesenchymal origin in dogs (33, 34), exhibiting local aggressiveness, high metastatic behavior and high mortality rates (35–38). COS originate mainly from appendicular skeleton, with the most frequent localization occurring at the metaphyseal level, while only 20–25% of tumors originate from the axial bone (34). Histological classification of bone tumors of domestic animals describes the presence of six different histotypes, namely: poorly differentiated, osteoblastic (productive and non-productive), chondroblastic, fibroblastic, telangiectatic, giant cell type, with the osteoblastic type being the most frequent (33, 39). To date, therapy is based on surgery (conservative or not) coupled to chemotherapy and radiotherapy, however life expectancy remains low (40–42) and resistance to typical antineoplastic drugs is building up (43–45). Therefore, the need for new targets, new antineoplastic drugs and/or adjuvant antineoplastic compounds for COS is rising. In this context, we recently validated and studied the expression of the NCOA4 and FTH1 in some canine normal and neoplastic tissues (46). In this report, we provide additional evidence for the relevance of iron metabolism alterations in canine osteoblastic osterosacomas (COOS), highlighting the role of ferritinophagy-related molecules NCOA4 and FTH1, thus suggesting that the mechanisms of ferritinophagy could represent a further potential pathway to be targeted to selectively destroy this type of cancer cells.
Three normal bone samples (N1-N3) and 20 COOS samples (COOS1-COOS20) were retrieved from the archives of the Department of Veterinary Medicine – University of Perugia. Ethics committee’s approval and animal testing request were waived since all animal tissue samples examined in this study were retrieved from archives. Samples had been previously decalcified and processed by routing histological techniques, paraffin-embedded and stained with hematoxylin and eosin (H&E). All samples had been observed by light microscopy for morphological classification of histological subtypes according to the World Health Organization’s histologic classification of tumors of domestic (33).
For each paraffin-embedded sample 3 μm sections were processed for immunohistochemistry (IHC) as previously described (47) to evaluate expression of proteins involved in ferritinophagy (FTH1, NCOA4), and PCNA to assess proliferation (46). Antibody specification and dilutions are reported in Table 1. Sections were counterstained with hematoxylin, and immunolabeling was revealed with diaminobenzidine-tetrahydrochloride (DAB).
To evaluate the expression of FTH1, NCOA4 and PCNA a semiquantitative score was applied by analyzing the number of positively labelled cells in 1,000 cells in 10 fields at 400x magnification (40x objective 10x ocular) for each specimen by two independent observers (Leonardo Leornardi and Gionata De Vico) under blinded conditions (48). Results were expressed as percentage.
Breeds, sex, age, tumor localization and histologic classification are summarized in Supplementary Table S1. Normal tissue samples (N1-N3) were characterized by abundant bone matrix in which elliptical osteocytes, showing mildly basophilic cytoplasm and oval nucleus, were immersed (Figure 1A). All COS samples (COOS1-COOS20) were characterized by polyhedral cells with eccentric nuclei and basophilic cytoplasm. Nuclei appeared pleomorphic, presenting hyperchromatic chromatin, and bizarre and atypical mitosis were observed. Osseus matrix was present in moderate to high amounts, often in the pattern of dense sheets (Figure 1B). Considered the histopathological features observed in the COS samples, they were classified as productive COOS.
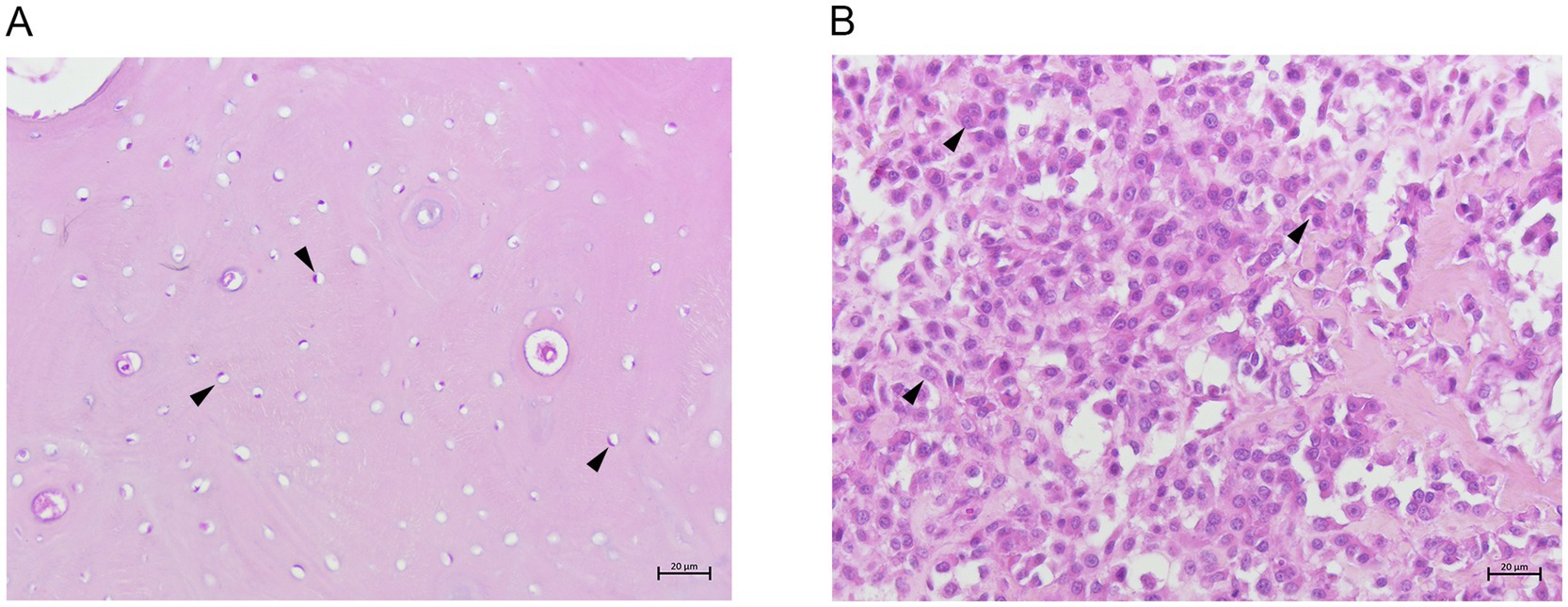
Figure 1. (A) Canine normal bone tissue showing osteocytes (arrow heads) and abundant bone matrix. H&E 20x. (B) Canine productive osteoblastic osteosarcoma showing many polyhedral cells (arrow heads) and osseus matrix. H&E 20x.
Normal bone samples presented less than 10% of cells positive for all the three tested antibodies (Figures 2A,C,E). On the contrary, in COOS samples 85–95% of neoplastic cells showed a strong cytoplasmic immunostaining for FTH1 (Figure 2B) and NCOA4. (Figure 2D). Moreover, 70–80% of neoplastic cells were strongly labelled at the nuclear level by anti-PCNA (Figure 2F).
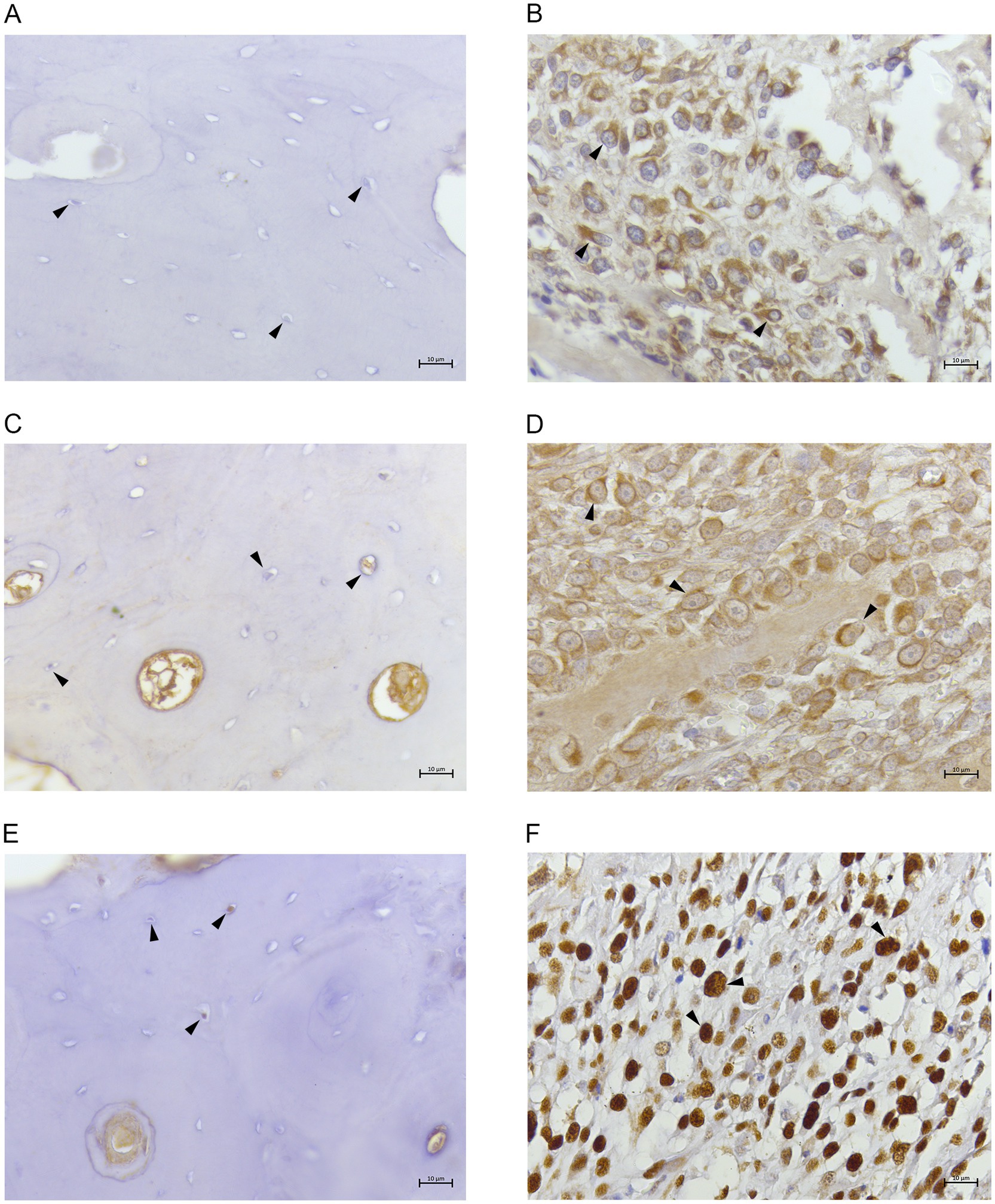
Figure 2. (A) Canine normal bone tissue. FTH1. Osteocytes showing no immunolabeling. 40x; (B) canine productive osteoblastic osteosarcoma. FTH1. Tumoral cells revealed cytoplasmic immunostaining (arrow heads). 40x; (C) canine normal bone tissue. NCOA4. Osteocytes showing no/weak immunolabeling. 40x; (D) canine productive osteoblastic osteosarcoma. NCOA4. Tumoral cells revealed cytoplasmic/perinuclear immunostaining (arrow heads). 40x; (E) canine normal bone tissue. PCNA. Few osteocytes showing weak nuclear immunolabeling. 40x. (F) canine productive osteoblastic osteosarcoma. PCNA. Tumoral cells showing strong nuclear immunolabeling (arrow heads). 40x.
Canine osteosarcomas (COS) are aggressive malignancies of the bone, for which the prognosis of patients still remains relatively poor and survival rates have not significantly improved during the recent decades. COS share biological and clinical similarities with the human counterpart, where a growing research tendency is focusing on the role of iron and its metabolism in both tumor progression and tumor suppression (2, 3, 20). Given the similarities between the two species, we investigated the expression in COOS of key proteins involved in iron metabolism to possibly identify new therapeutical targets for both dogs and possibly humans. Our results show an increased expression of all analyzed proteins in COOS samples compared to normal samples. Previous data on the overexpression of TfR1 in COS (26), supported the idea that iron uptake plays a decisive role in supporting the growth of COOS neoplastic cells and could represent a new therapeutic target. Our study emphasizes for the first time in COOS the role of NCOA4 and FTH1, key molecules involved in ferritinophagy regulation (49). Interestingly, in our study cancer samples showed higher immunoreactivity in neoplastic cells compared to normal ones, in accordance to literature (50, 51). In the classical ferritinophagy pathway NCOA4 interacts with ferritin-heavy chain (FTH1), transferring autophagosomes to lysosomes to degrade FT and release free iron thus increasing cLIP. Physiologically, NCOA4 combined with iron is continuously degraded by ubiquitin-proteasome system or directly by lysosomes (52), explaining why in our study NCOA4 was usually poorly highlighted in normal cells by immunohistochemistry. On the contrary, an intriguing result of our investigation is the strong immunohistochemical detection of NCOA4 coupled with the one of FTH1 in COOS cells, which testify for a deep dysregulation of iron metabolism and in particular of the ferritinophagy pathway. In our case, in fact, it could be hypothesized that the COOS cells are so highly dependent on the availability of iron for their growth and survival (iron addiction), to simultaneously activate different pathways that allow them to maintain high levels of iron in the cytosol, namely iron upload, storage and mobilization from storage. High iron loads and ferritinophagy have also been closely correlated with ferroptosis, a form of iron dependent non-apoptotic programmed cell death linked to oxidation of membrane lipid (53). It is to be believed that COOS cells have developed mechanisms to evade these forms of cell death as already described in other tumor types (54, 55, 69). As a matter of fact, in our cases there was no evidence of characteristic morphological feature of ferroptosis in COS cells, namely cell membrane rupture, cytoplasmic swelling, and moderate chromatin condensation (56). Escaping ferroptotic mechanisms provides further vulnerable possible targets for ferroptosis-based therapy (70). Previous studies in human oncology have described the possibility of using synthetical or natural compounds to target iron metabolism (57–60) and enhance ferroptosis. Artemisin, the main bioactive component of Artemisia annua L, has been proven to activate apoptosis, ferroptosis and induce cancer cell death by producing ROS in human osteosarcoma (61, 62) and also in COS cell lines (63). More recently, two studies by Isani et al. (64) and Colurciello et al. (65) showed that COS cells treated with artemisin showed higher mortality rates and lower iron concentrations compared to untreated ones, probably due to ferroptosis. Furthermore, targeting ferritinophagy pathway can also represent mechanisms for some common anticancer drugs. As examples, low-dose cisplatin combined with ursolic acid inhibits cancer cell growth by activating autophagic degradation of Ft and overloading intracellular iron ions (66). The combination of artesunate and the hepatocellular carcinoma first-line drug sorafenib induces ferritinophagy in hepatocellular carcinoma cells and improve the efficacy of single anticancer drugs (67). The results of our study provide relevant, thought preliminary data on the alteration of the iron-metabolic pathway in COOS. Notably, they suggest an increased uptake of iron (26), release of iron from ferritin-storage coupled to a continuous replacement of the used Ft storage. COS appear as favorable candidates for the use of antineoplastic drugs targeting iron metabolism, ferroptosis and ferritinophagy. Ideally, therapies should on one hand enhance cLIP by increasing NCOA4-induced ferritinophagy and on the other hand use TfR1 as a tool to selectively deliver compounds to tumoral cells and reduce undesired effects on healthy cells. Further studies will help deepen the knowledge about alterations in iron metabolism in COOS. Of particular interest would be correlating the overexpression of these molecules with patient follow-up data to assess their potential prognostic implications, and using 2D cell models hopefully opening the way to possible in vivo studies to be transferred into clinical practice.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The requirement of ethical approval was waived by Institutional review board of the University of Perugia for the studies involving animals because the study was performed on archive paraffine embedded samples, previously used for diagnostic purposes. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
KP: Conceptualization, Writing – original draft. RL: Investigation, Methodology, Writing – original draft. GF: Investigation, Writing – original draft. GV: Supervision, Writing – review & editing. LL: Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
Authors would like to thank Dr. Claire Power for helping editing the figures in the article. The authors acknowledge the Histopathology and Diagnostics Core at Department of Biology of University of Naples “Federico II for their technical and scientific support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1546872/full#supplementary-material
1. Zaal, EA, and Berkers, CR. The influence of metabolism on drug. Resp Cancer Front Oncol. (2018) 8:500. doi: 10.3389/fonc.2018.00500
2. Torti, SV, and Torti, FM. Iron and Cancer: 2020 vision. Cancer Res. (2020) 80:5435–48. doi: 10.1158/0008-5472.CAN-20-2017
3. Steegmann-Olmedillas, JL. The role of iron in tumour cell proliferation. Clin Transl Oncol. (2011) 13:71–6. doi: 10.1007/s12094-011-0621-1
4. Fischer-Fodor, E, Miklasova, N, Berindan-Neagoe, I, and Saha, B. Iron, inflammation and invasion of cancer cells. Clujul Med. (2015) 88:272–7. doi: 10.15386/cjmed-492
5. Aisen, P. Transferrin receptor 1. Int J Biochem Cell Biol. (2004) 36:2137–43. doi: 10.1016/j.biocel.2004.02.007
6. Candelaria, PV, Leoh, LS, Penichet, ML, and Daniels-Wells, TR. Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front Immunol. (2021) 12:607692. doi: 10.3389/fimmu.2021.607692
7. Sun, K, Li, C, Liao, S, Yao, X, Ouyang, Y, Liu, Y, et al. Ferritinophagy, a form of autophagic ferroptosis: new insights into cancer treatment. Front Pharmacol. (2022) 13:1043344. doi: 10.3389/fphar.2022.1043344
8. Wang, J, Wu, N, Peng, M, Oyang, L, Jiang, X, Peng, Q, et al. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. (2023) 9:463. doi: 10.1038/s41420-023-01753-y
9. Knutson, MD. Steap proteins: implications for iron and copper metabolism. Nutr Rev. (2007) 65:335–40. doi: 10.1301/nr.2007.jul.335–340
10. Shesh, BP, and Connor, JR. A novel view of ferritin in cancer. Biochim Biophys Acta Rev Cancer. (2023) 1878:188917. doi: 10.1016/j.bbcan.2023.188917
11. Ford, GC, Harrison, PM, Rice, DW, Smith, JM, Treffry, A, White, JL, et al. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond Ser B Biol Sci. (1984) 304:551–65. doi: 10.1098/rstb.1984.0046
12. Levi, S, Luzzago, A, Cesareni, G, Cozzi, A, Franceschinelli, F, Albertini, A, et al. Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site. A study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem. (1988) 263:18086–92. doi: 10.1016/S0021-9258(19)81326-1
13. Mancias, JD, Wang, X, Gygi, SP, Harper, JW, and Kimmelman, AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. (2014) 509:105–9. doi: 10.1038/nature13148
14. Federico, G, Carrillo, F, Dapporto, F, Chiariello, M, Santoro, M, Bellelli, R, et al. NCOA4 links iron bioavailability to DNA metabolism. Cell Rep. (2022) 40:111207. doi: 10.1016/j.celrep.2022.111207
15. Steinbicker, AU, and Muckenthaler, MU. Out of balance--systemic iron homeostasis in iron-related disorders. Nutrients. (2013) 5:3034–61. doi: 10.3390/nu5083034
16. Tian, Y, Tian, Y, Yuan, Z, Zeng, Y, Wang, S, Fan, X, et al. Iron metabolism in aging and age-related diseases. Int J Mol Sci. (2022) 23:3612. doi: 10.3390/ijms23073612
17. Lee, J, and Hyun, DH. The interplay between intracellular Iron homeostasis and Neuroinflammation in neurodegenerative diseases. Antioxidants. (2023) 12:918. doi: 10.3390/antiox12040918
18. Rosenblum, SL. Inflammation, dysregulated iron metabolism, and cardiovascular disease. Front Aging. (2023) 4:1124178. doi: 10.3389/fragi.2023.1124178
19. Manz, DH, Blanchette, NL, Paul, BT, Torti, FM, and Torti, SV. Iron and Cancer: recent insights. Ann N Y Acad Sci. (2016) 1368:149–61. doi: 10.1111/nyas.13008
20. Chen, Y, Fan, Z, Yang, Y, and Gu, C. Iron metabolism and its contribution to cancer. Inter J Onc. (2019) 54:1143–54. doi: 10.3892/ijo.2019.4720
21. Brown, RAM, Richardson, KL, Kabir, TD, Trinder, D, Ganss, R, and Leedman, PJ. Altered Iron metabolism and impact in Cancer biology, metastasis, and immunology. Front Oncol. (2020) 10:476. doi: 10.3389/fonc.2020.00476
22. Chen, H, Han, Z, Wang, Y, Su, J, Lin, Y, Cheng, X, et al. Targeting Ferroptosis in bone-related diseases: facts and perspectives. J Inflamm Res. (2023) 16:4661–77. doi: 10.2147/JIR.S432111
23. Ma, Y, Cong, L, Shen, W, Yang, C, and Ye, K. Ferroptosis defense mechanisms: the future and hope for treating osteosarcoma. Cell Biochem Funct. (2024) 42:e4080. doi: 10.1002/cbf.4080
24. Brown, HK, Schiavone, K, Gouin, F, Heymann, MF, and Heymann, D. Biology of bone sarcomas and new therapeutic developments. Calcif Tissue Int. (2018) 102:174–95. doi: 10.1007/s00223-017-0372-2
25. Harris, MA, and Hawkins, CJ. Recent and ongoing research into metastatic osteosarcoma treatments. Int J Mol Sci. (2022) 23:3817. doi: 10.3390/ijms23073817
26. De Vico, G, Martano, M, Maiolino, P, Carella, F, and Leonardi, L. Expression of transferrin receptor-1 (TFR-1) in canine osteosarcomas. Vet Med Sci. (2020) 6:272–6. doi: 10.1002/vms3.258
27. McCown, JL, and Specht, AJ. Iron homeostasis and disorders in dogs and cats: a review. J Am Anim Hosp Assoc. (2011) 47:151–60. doi: 10.5326/JAAHA-MS-5553
28. Priest, H, McDonough, S, Erb, H, Daddona, J, and Stokol, T. Transferrin receptor expression in canine lymphoma. Vet Pathol. (2011) 48:466–74. doi: 10.1177/0300985810377074
29. Caro, JT, Marìn, LM, Iazbik, MC, Zaldivar-Lopez, S, Borghese, H, and Couto, CG. Markers of iron metabolism in retired racing greyhounds with and without osteosarcoma. Vet Clin Pathol. (2013) 42:360–3. doi: 10.1111/vcp.12066
30. Ploypetch, S, Rungsipipat, A, Piyaviriyakul, P, Choisunirachon, N, Makoom, P, and Kalpravidh, C. Relationships between transferrin and transferrin receptor (TfR) expression in dogs with malignant Oronasal tumors. Thai J Vet Med. (2017) 47:61–70. doi: 10.56808/2985-1130.2812
31. Marques, O, Canadas, A, Faria, F, Oliveira, E, Amorim, I, Seixas, F, et al. Expression of iron-related proteins in feline and canine mammary gland reveals unexpected accumulation of iron. Biotech Histochem. (2017) 92:584–94. doi: 10.1080/10520295.2017.1369160
32. Simpson, S, Dunning, MD, de Brot, S, Grau-Roma, L, Mongan, NP, and Rutland, CS. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. (2017) 59:71. doi: 10.1186/s13028-017-0341-9
33. Slayter, MV, Boosinger, TR, Pool, RR, Dammrich, K, Misdrop, W, and Larsen, S. World Health Organization, international histologic classification of tumors of domestic animals, histological classification of bone and joint tumors of domestic animals. Washington, DC: Armed Forces Institute of Pathology American Registry of Pathology (1994).
34. Thompson, KG, and Pool, RR. “Tumors of bones” in tumors in domestic animals. 4th. ed. Ames, IA: Iowa State Press (2002).
35. Brodey, RS, and Riser, WH. Canine osteosarcoma. A clinicopathologic study of 194 cases. Clin Orthop Relat Res. (1969) 62:54–64.
36. Culp, WT, Olea-Popelka, F, Sefton, J, Aldridge, CF, Withrow, SJ, Lafferty, MH, et al. Evaluation of outcome and prognostic factors for dogs living greater than one year after diagnosis of osteosarcoma: 90 cases (1997-2008). J Am Vet Med Assoc. (2014) 245:1141–6. doi: 10.2460/javma.245.10.1141
37. Silver, KI, Patkar, S, Mazcko, C, Berger, EP, Beck, JA, and LeBlanc, AK. Patterns of metastatic progression and association with clinical outcomes in canine osteosarcoma: a necropsy study of 83 dogs. Vet Comp Oncol. (2023) 21:646–55. doi: 10.1111/vco.12927
38. Wright, TF, Brisson, BA, Belanger, CR, Tiessen, A, Sabine, V, Skowronski, K, et al. Quantification of circulating tumour cells over time in dogs with appendicular osteosarcoma. Vet Comp Oncol. (2023) 21:541–50. doi: 10.1111/vco.12918
39. Misdorp, W, and Hart, AA. Some prognostic and epidemiologic factors in canine osteosarcoma. J Natl Cancer Inst. (1979) 62:537–45. doi: 10.1093/jnci/62.3.537
40. Schmidt, AF, Nielen, M, Klungel, OH, Hoes, AW, de Boer, A, Groenwold, RH, et al. Prognostic factors of early metastasis and mortality in dogs with appendicular osteosarcoma after receiving surgery: an individual patient data meta-analysis. Prev Vet Med. (2013) 112:414–22. doi: 10.1016/j.prevetmed.2013.08.011
41. Szewczyk, M, Lechowski, R, and Zabielska, K. What do we know about canine osteosarcoma treatment? Rev Vet Res Commun. (2015) 39:61–7. doi: 10.1007/s11259-014-9623-0
42. Griffin, MA, Mastorakis, A, Wustefeld-Janssens, B, Martin, TW, Duda, L, Seguin, B, et al. Outcomes in dogs undergoing surgical stabilization and non-stereotactic radiation therapy for axial and appendicular bone tumors. Front Vet Sci. (2024) 10:1283728. doi: 10.3389/fvets.2023.1283728
43. Shahi, MH, York, D, Gandour-Edwards, R, Withers, SS, Holt, R, and Rebhun, RB. BMI1 is expressed in canine osteosarcoma and contributes to cell growth and chemotherapy resistance. PLoS One. (2015) 10:e0131006. doi: 10.1371/journal.pone.0131006
44. Weinman, MA, Ramsey, SA, Leeper, HJ, Brady, JV, Schlueter, A, Stanisheuski, S, et al. Exosomal proteomic signatures correlate with drug resistance and carboplatin treatment outcome in a spontaneous model of canine osteosarcoma. Cancer Cell Int. (2021) 21:245. doi: 10.1186/s12935-021-01943-7
45. Hodge, MA, Miller, T, Weinman, MA, Wustefeld-Janssens, B, Bracha, S, and Davis, BW. Cellular Transcriptomics of carboplatin resistance in a metastatic canine osteosarcoma cell line. Genes. (2023) 14:558. doi: 10.3390/genes14030558
46. Leandri, R, Power, K, Buonocore, S, and De Vico, G. Preliminary evidence of the possible roles of the Ferritinophagy-Iron uptake Axis in canine testicular Cancer. Animals. (2024) 14:2619. doi: 10.3390/ani14172619
47. Paciello, O, Passantino, G, Costagliola, A, Papparella, S, and Perillo, A. Histiocytic sarcoma of the nasal cavity in a horse. Res Vet Sci. (2013) 94:648–50. doi: 10.1016/j.rvsc.2013.01.005
48. Martano, M, Altamura, G, Power, K, Restucci, B, Carella, F, Borzacchiello, G, et al. Evaluation of hypoxia-inducible Factor-1 alpha (HIF-1α) in equine sarcoid: an Immunohistochemical and biochemical study. Pathogens. (2020) 9:58. doi: 10.3390/pathogens9010058
49. Tang, M, Chen, Z, Wu, D, and Chen, L. Ferritinophagy/ferroptosis: Iron-related newcomers in human diseases. J Cell Physiol. (2018) 233:9179–90. doi: 10.1002/jcp.26954
50. Santana-Codina, N, Del Rey, MQ, Kapner, KS, Zhang, H, Gikandi, A, Malcolm, C, et al. NCOA4-mediated Ferritinophagy is a pancreatic Cancer dependency via maintenance of Iron bioavailability for Iron-sulfur cluster proteins. Cancer Discov. (2022) 12:2180–97. doi: 10.1158/2159-8290.CD-22-0043
51. Feng, Z, Luan, M, Zhu, W, Xing, Y, Ma, X, Wang, Y, et al. Targeted ferritinophagy in gastrointestinal cancer: from molecular mechanisms to implications. Arch Toxicol. (2024) 98:2007–18. doi: 10.1007/s00204-024-03745-y
52. Le, Y, Liu, Q, Yang, Y, and Wu, J. The emerging role of nuclear receptor coactivator 4 in health and disease: a novel bridge between iron metabolism and immunity. Cell Death Discov. (2024) 10:312. doi: 10.1038/s41420-024-02075-3
53. Dixon, SJ, Lemberg, KM, Lamprecht, MR, Skouta, R, Zaitsev, EM, Gleason, CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. (2012) 149:1060–72. doi: 10.1016/j.cell.2012.03.042
54. Huang, R, Dong, R, Wang, N, He, Y, Zhu, P, Wang, C, et al. Adaptive changes allow targeting of Ferroptosis for glioma treatment. Cell Mol Neurobiol. (2022) 42:2055–74. doi: 10.1007/s10571-021-01092-5
55. Hong, X, Roh, W, Sullivan, RJ, Wong, KHK, Wittner, BS, Guo, H, et al. The Lipogenic regulator SREBP2 induces transferrin in circulating melanoma cells and suppresses Ferroptosis. Cancer Discov. (2021) 11:678–95. doi: 10.1158/2159-8290.CD-19-1500
56. Tang, D, Chen, X, Kang, R, and Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. (2021) 31:107–25. doi: 10.1038/s41422-020-00441-1
57. Lv, H, Zhen, C, Liu, J, and Shang, P. β- Phenethyl Isothiocyanate induces cell death in human osteosarcoma through altering Iron metabolism, disturbing the redox balance, and activating the MAPK signaling pathway. Oxidative Med Cell Longev. (2020) 2020:5021983. doi: 10.1155/2020/5021983
58. Lin, H, Chen, X, Zhang, C, Yang, T, Deng, Z, Song, Y, et al. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed Pharmacother. (2021) 136:111202. doi: 10.1016/j.biopha.2020.111202
59. Xue, Y, Zhang, G, Zhou, S, Wang, S, Lv, H, Zhou, L, et al. Iron Chelator induces apoptosis in osteosarcoma cells by disrupting intracellular Iron homeostasis and activating the MAPK pathway. Int J Mol Sci. (2021) 22:7168. doi: 10.3390/ijms22137168
60. Zhao, J, Zhao, Y, Ma, X, Zhang, B, and Feng, H. Targeting ferroptosis in osteosarcoma. J Bone Oncol. (2021) 30:100380. doi: 10.1016/j.jbo.2021.100380
61. Bhaw-Luximon, A, and Jhurry, D. Artemisinin and its derivatives in cancer therapy: status of progress, mechanism of action, and future perspectives. Cancer Chemother Pharmacol. (2017) 79:451–66. doi: 10.1007/s00280-017-3251-7
62. Li, Z, Ding, X, Wu, H, and Liu, C. Artemisinin inhibits angiogenesis by regulating p38 MAPK/CREB/TSP-1 signaling pathway in osteosarcoma. J Cell Biochem. (2019) 120:11462–70. doi: 10.1002/jcb.28424
63. Hosoya, K, Murahari, S, Laio, A, London, CA, Couto, CG, and Kisseberth, WC. Biological activity of dihydroartemisinin in canine osteosarcoma cell lines. Am J Vet Res. (2008) 69:519–26. doi: 10.2460/ajvr.69.4.519
64. Isani, G, Bertocchi, M, Andreani, G, Farruggia, G, Cappadone, C, Salaroli, R, et al. Cytotoxic effects of Artemisia annua L. and pure Artemisinin on the D-17 canine osteosarcoma cell line. Oxidative Med Cell Longev. (2019) 2019, 2019:1615758. doi: 10.1155/2019/1615758
65. Culurciello, R, Bosso, A, Di Fabio, G, Zarrelli, A, Arciello, A, Carella, F, et al. Cytotoxicity of an innovative pressurised cyclic solid-liquid (PCSL) extract from Artemisia annua. Toxins. (2021) 13:886. doi: 10.3390/toxins13120886
66. Liu, X, Zhang, Y, Wu, X, Xu, F, Ma, H, Wu, M, et al. Targeting Ferroptosis pathway to combat therapy resistance and metastasis of Cancer. Front Pharmacol. (2022) 13:909821. doi: 10.3389/fphar.2022.909821
67. Cui, Z, Wang, H, Li, S, Qin, T, Shi, H, Ma, J, et al. Dihydroartemisinin enhances the inhibitory effect of sorafenib on HepG2 cells by inducing ferroptosis and inhibiting energy metabolism. J Pharmacol Sci. (2022) 148:73–85. doi: 10.1016/j.jphs.2021.09.008
68. Ersoy, T, and Ozmen, O. Immunohistochemical detection of caspase 3 and proliferating cell nuclear antigen in the intestines of dogs naturally infected with parvovirus. Vet Res Forum. (2022) 13:127–131. doi: 10.30466/vrf.2020.116534.2772
69. Li, Z, Hu, Y, Zheng, H, Li, M, Liu, Y, Feng, R, et al. LPCAT1-mediated membrane phospholipid remodelling promotes ferroptosis evasion and tumour growth. Nat Cell Biol. (2024) 26:811–824. doi: 10.1038/s41556-024-01405-y
Keywords: bone cancer, canine osteosarcoma, immunohistochemistry, iron metabolism, therapy
Citation: Power K, Leandri R, Federico G, De Vico G and Leonardi L (2025) Ferritinophagy: a possible new iron-related metabolic target in canine osteoblastic osteosarcoma. Front. Vet. Sci. 12:1546872. doi: 10.3389/fvets.2025.1546872
Received: 17 December 2024; Accepted: 06 March 2025;
Published: 24 March 2025.
Edited by:
Helen C. Roberts, Middlesex University, United KingdomReviewed by:
Carmelo Iaria, University of Messina, ItalyCopyright © 2025 Power, Leandri, Federico, De Vico and Leonardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen Power, a2FyZW4ucG93ZXJAdW5pbmEuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.