- College of Animal Science and Technology, Shihezi University, Shihezi, China
Hybrid Broussonetia papyrifera shows great promise for use in antibiotic-free feed, potentially contributing to the green and sustainable development of the animal husbandry industry. In this study, we investigated the impact of Broussonetia papyrifera silage on the intestinal health of Kazakh sheep. Forty healthy male Kazakh sheep, aged 5 months and weighing an average of 28.28 ± 1.14 kg, were randomly assigned to either a control or an experimental group, each comprising four replicates, with five sheep per replicate. The control group was fed a basal diet, while the experimental group received a diet supplemented with 20% Broussonetia papyrifera silage (dry matter basis). The 70-day experiment included a 10-day adaptation phase followed by a 60-day feeding trial. The results showed that there was no significant difference in growth performance or apparent nutrient digestibility between the experimental and control groups (p > 0.05). However, the experimental group exhibited significantly greater total antioxidant capacity, alongside higher contents of superoxide dismutase, catalase, glutathione peroxidase, immunoglobulins A, M, and G, and interleukins-2, −6, and −8 in the intestinal mucosa; in contrast, malondialdehyde and interleukin-4 contents were significantly reduced (p < 0.01). Furthermore, the dietary inclusion of Broussonetia papyrifera silage resulted in a reduction in the relative abundance of the bacterial genera Turicibacter and Romboutsia (p < 0.05). In conclusion, the feeding of Broussonetia papyrifera silage to Kazakh sheep significantly enhanced immune function, increased antioxidant capacity, and reduced the relative abundance of potentially pathogenic bacteria in the sheep without negatively impacting their growth or nutrient digestion, thus supporting the overall health of the animals.
1 Introduction
Livestock husbandry represents a critical source of meat for humans. The use of antibiotics in livestock feed has been banned in many countries to mitigate the risk of animal-derived antibiotic resistance, thereby safeguarding human health (1). Accordingly, research interest has increasingly focused on the development of alternative, antibiotic-free feed additives that can enhance nonspecific immunity in animals (2, 3). Additionally, the advancement of “green, efficient, and safe” feeds, free not only from antibiotics but also from hormones and exogenous chemical agents, represents a significant trend for the future development of the feed industry.
Broussonetia papyrifera, a cultivar recently developed by the Institute of Botany at the Chinese Academy of Sciences, is rich in diverse bioactive compounds such as flavonoids, polysaccharides, and terpenoids (4). It was recently shown that B. papyrifera ensilage treatment enhances its assimilation and absorption by animals (5). The silage derived from B. papyrifera holds significant potential for applications related to animal health and is being explored as a potential alternative to antibiotics (6–11). B. papyrifera silage has been shown to benefit animal health by improving growth performance, immune function, and antioxidant capacity, as well as through its modulatory effects on ruminal bacterial communities (12–18). While numerous studies have investigated the effects of B. papyrifera silage on ruminal bacteria, few have addressed its impact on fecal bacteria in animals. Bacteria in feces reflect the broader microbial ecosystem of the digestive tract of ruminants and offer insights into the effects of diet, digestive efficiency, microbial health, and immune status (19). B. papyrifera silage has shown different effects on the health of different animals such as sheep, goats, rabbits, cows, donkeys, and piglets (12–16). In summary, incorporating Broussonetia papyrifera silage into livestock diets can reduce reliance on antibiotics and exogenous chemicals, thereby promoting more sustainable green development by enhancing feed efficiency and minimizing environmental pollution in agricultural cycles.
Kazakh sheep, originating in China, are highly valued for their ability to endure harsh environments, a quality that is particularly beneficial for nomadic herders in arid and semi-arid regions. To the best of our knowledge, research on the effects of B. papyrifera silage on the health of Kazakh sheep is limited. Therefore, in this study, we explored the effects of B. papyrifera silage on growth performance, intestinal health, and fecal bacterial composition in these animals, aiming to provide a scientific foundation for its application in green, antibiotic-free ruminant breeding.
2 Materials and methods
The animal research protocol used in this study was approved by the biological ethics committee of Shihezi University (Shihezi, China) in March 2023, under approval number A2023-129.
2.1 Preparation of silage from Broussonetia papyrifera
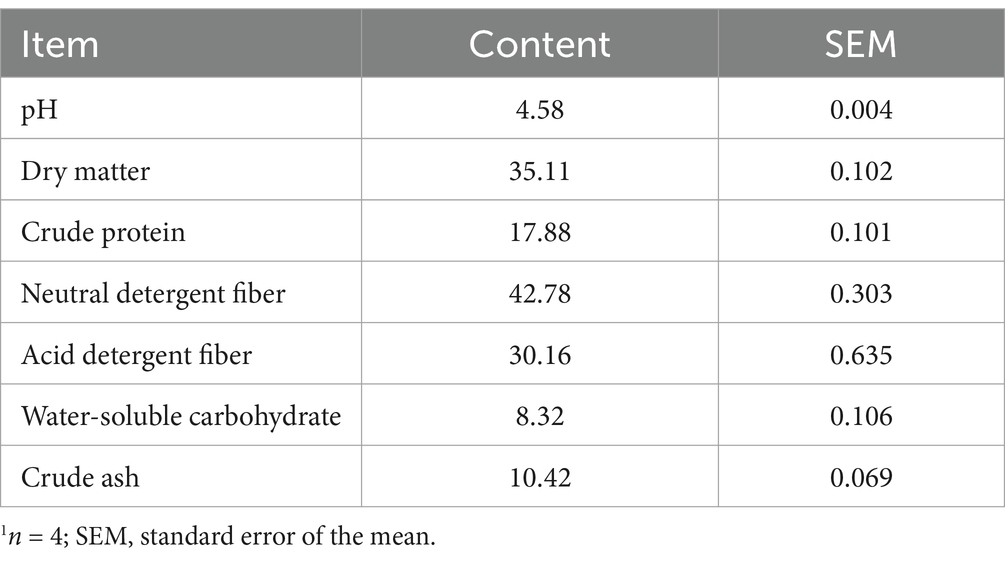
The experimental site was located at the B. papyrifera demonstration base of the Seventh Agricultural Science Institute in Xinjiang Province (N 44°20′, E 83°51′, elevation 450 m). B. papyrifera was harvested on April 20, 2023, at a height of 120 cm, leaving a stubble of approximately 10 cm, and the entire plant was cut into 2–3 cm-long pieces. The plants were then inoculated with 1 × 105 CFU/g Lactiplantibacillus plantarum (isolated from B. papyrifera silage) and thoroughly mixed (4). The B. papyrifera silage was then wrapped and sealed, with each package weighing approximately 80 kg. The silage underwent fermentation for 60 days at a storage temperature of 24°C. Nutrient analysis was performed on the dry matter of Broussonetia papyrifera silage after fermentation, and the results are presented in Table 1.
2.2 Animal experimentation and experimental design
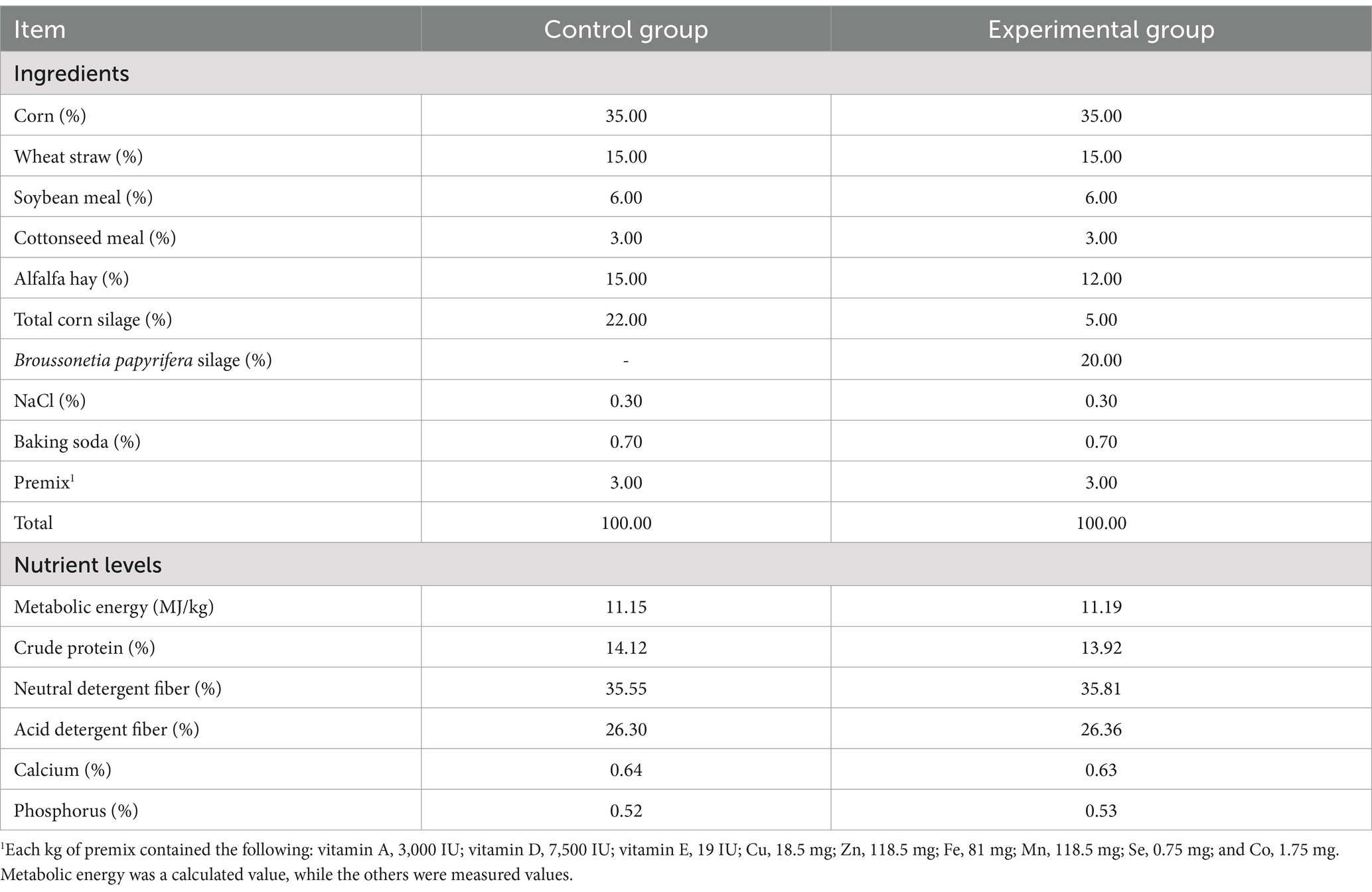
A total of 40 male Kazakh sheep (castrated rams), approximately 5 months old and weighing an average of 28.28 ± 1.14 kg, were selected for the study. The sheep were randomly divided into a control group (CK) and an experimental group (GS), each with four replicates (five sheep per replicate). The control group was fed a basal diet, while the experimental group received a total mixed ration containing B. papyrifera silage (20% dry matter). The formulation of the experimental diet followed the Chinese Sheep Feeding Standard (NY/T 816–2021) and was designed to meet the nutritional requirements for the growth phase of sheep. Detailed information on the composition and nutrient levels of the experimental diet is provided in Table 2. The crude-to-refined ingredient ratio was 4:6. The feeding period lasted for 10 days, followed by a 60-day trial period. The experiment was conducted in Jinghe County, Xinjiang Province (N 82°30′49.20″, E 44°31′19.58″, elevation 384 m). Each sheep was individually housed in pens and had ad libitum access to water throughout the study. The sheep were fed twice daily at 08:00 and 18:00 h, with feed leftovers restricted to approximately 5%.
2.3 Sample collection
On the final day of the experimental period, five sheep from each group with body weights closest to the average weight of the group were randomly selected for fecal sample collection. Samples were obtained from the sheep 4 h after the morning feeding. Rectal fecal samples (approximately 0.2 g each) were stored in a liquid nitrogen tank for microbial community analysis. Subsequently, 3 sheep from each group (6 sheep in total) were randomly selected from those that provided fecal samples for slaughter. After slaughter, the abdominal cavity was quickly opened, and the middle sections of the duodenum, jejunum, and ileum were extracted and stored at −80°C for subsequent analysis (20).
2.4 Measurement of indicators
2.4.1 Feed nutrient levels
Dry matter content was determined according to the AOAC standard procedure. Calcium (Ca) content was determined using AOAC official method 968.08, phosphorus (P) content was determined using AOAC official method 965.17, acid detergent fiber (ADF) content was determined using AOAC official method 973.18, the nitrogen content of the feed was measured using the Kjeldahl method, and the neutral detergent fiber (NDF) content was determined according to Van Soest (21).
2.4.2 Growth
On days 1 and 60 of the experiment, the body weight of the sheep was measured in the morning after an overnight fast.
2.4.3 Apparent nutrient digestibility
On day 53 of the experiment, five sheep were randomly selected from each group, and fecal samples were continuously collected from these sheep for 7 days using the total feces collection method. The collected feces were thoroughly mixed, and 10% of the sample was treated with 10% sulfuric acid for nitrogen preservation. The samples were then dried at 65°C and stored for subsequent determination of fecal nutrient contents. During this period, feed and leftover feed samples were also collected, and their nutrient content was determined using the method outlined in Section 2.4.1. The apparent digestibility of nutrients in the experimental animals was calculated based on the acid-insoluble ash (AIA) content (21).
2.4.4 Determination of antioxidant, immunoglobulin, and cytokine contents
Duodenal, jejunal, and ileal mucosal tissues were individually weighed to 0.1 g. Subsequently, 0.9 mL of prechilled 0.9% physiological saline was added to each sample, and the mixture was homogenized using a homogenizer to obtain a 10% tissue homogenate. A 0.5-mL aliquot of the resulting homogenate was then centrifuged at 2000 rpm for 10 min at 4°C and the resulting supernatant was stored at −20°C for subsequent analysis (22). Intestinal tissue indices in the supernatants were analyzed using commercial kits (Shanghai Meilian Biological Technology Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. The antioxidant indices measured were total antioxidant capacity (T-AOC) and superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) contents. The immunoglobulins assessed were immunoglobulin (IgA), IgG, and IgM. The cytokines evaluated included tumor necrosis factor-alpha (TNF-α), interleukin-2 (IL-2), IL-4, IL-6, and IL-8.
2.4.5 Fecal bacteria
The collected (cryogenically frozen) fecal samples were placed in 200 mL of sterilized triangular flasks, mixed with 50 mL of PBS (pH 7.2), shaken at 200 rpm for 30 min, ultrasonicated at 50 W for 2 min, and subsequently shaken at 150 rpm for another 30 min. The supernatant was then transferred to 50-mL sterilized centrifuge tubes that had been sterilized under high pressure and centrifuged at 1,500 rpm for 1 min. After transferring to new 50-mL sterilized high-speed centrifuge tubes, the samples were centrifuged at 12,000 rpm for 10 min, and the isolated bacteria were collected. DNA was extracted from the fecal samples using a DNA extraction kit (QIAamp PowerFecal DNA Kit, Qiagen, Hilden, Germany) and assessed for concentration and purity using 1% agarose gel electrophoresis.
The V4–V5 region of the bacterial 16S rRNA gene was amplified using the primers 515F (5′-GTGYCAGCMGCCGCGGTAA-3′) and 926R (5′-CCGYCAATTYMTTTRAGT-3′). PCR amplification was performed in 20-μL volumes using the following parameters: 95°C for 3 min, followed by 30 cycles of 94°C for 20 s, 55°C for 20 s, and 72°C for 30 s, with a final extension at 72°C for 10 min. Amplicons were verified by agarose gel electrophoresis, purified, and sent to Meij Biotech for library construction. Sequencing was performed on the Illumina MiSeq platform using paired-end reads (300 bp). Raw reads underwent quality filtering with fastp (v0.19.6) and merging with FLASH (v1.2.11). Denoising was performed using the DADA2 plugin in QIIME2 (default parameters). Denoised sequences (ASVs) were rarefied to 20,000 sequences per sample, yielding an average Good’s coverage of 99.09%. Taxonomic classification was conducted using the Naive Bayes classifier in QIIME2 with the SILVA 16S rRNA database (version 138). Subsequent analyses were performed on the Majorbio Cloud Platform.1 The original data relating to fecal bacteria obtained in this study can be accessed at https://www.ncbi.nlm.nih.gov/sra/PRJNA1167466, with SRA accession number PRJNA1167466.
2.5 Statistical analysis
Data were preprocessed using Excel 2018 and analyzed with SPSS 20.0. Prior to applying independent samples t-tests or one-way ANOVA, data normality was assessed using the Shapiro–Wilk test and homogeneity of variances with Levene’s test. Non-parametric tests were applied if assumptions were not met. Post-hoc comparisons were performed using Tukey’s test (p < 0.05). The classification and abundance of bacteria in the fecal samples were analyzed using the Majorbio Cloud Platform. All of data analysis in the biological cloud platform (see Footnote 1), specific as follows: Alpha is obtained by using the mothur software2 diversity sobs, chao, shannon index, and USES the Wilxocon rank-sum test for Alpha diversity analysis of differences between groups; The similarity of microbial community structure among samples was tested by PCoA analysis (principal coordinate analysis) based on bray-curtis distance algorithm, and the PERMANOVA non-parametric test was used to analyze whether the difference in microbial community structure between sample groups was significant. Species were selected for correlation network analysis based on spearman correlation |r| > 0.6 and p < 0.05.
3 Results
3.1 Growth performance and the apparent digestibility of nutrients
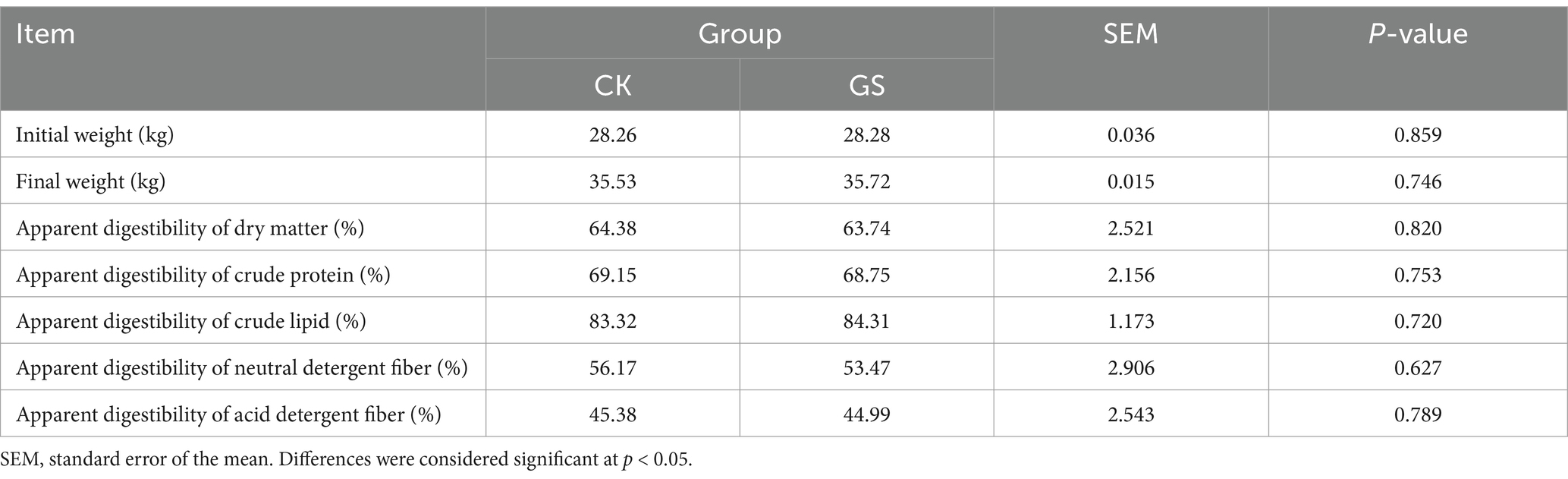
As shown in Table 3, no significant difference in growth performance or apparent nutrient digestibility was detected between the GS and CK groups (p > 0.05).
3.2 Antioxidant capacity
As shown in Table 4, T-AOC and the levels of SOD, CAT, and GSH-Px in duodenal, jejunal, and ileal tissues in the GS group were significantly elevated compared to those in the CK group (p < 0.01), whereas the MDA content was significantly reduced (p < 0.01).
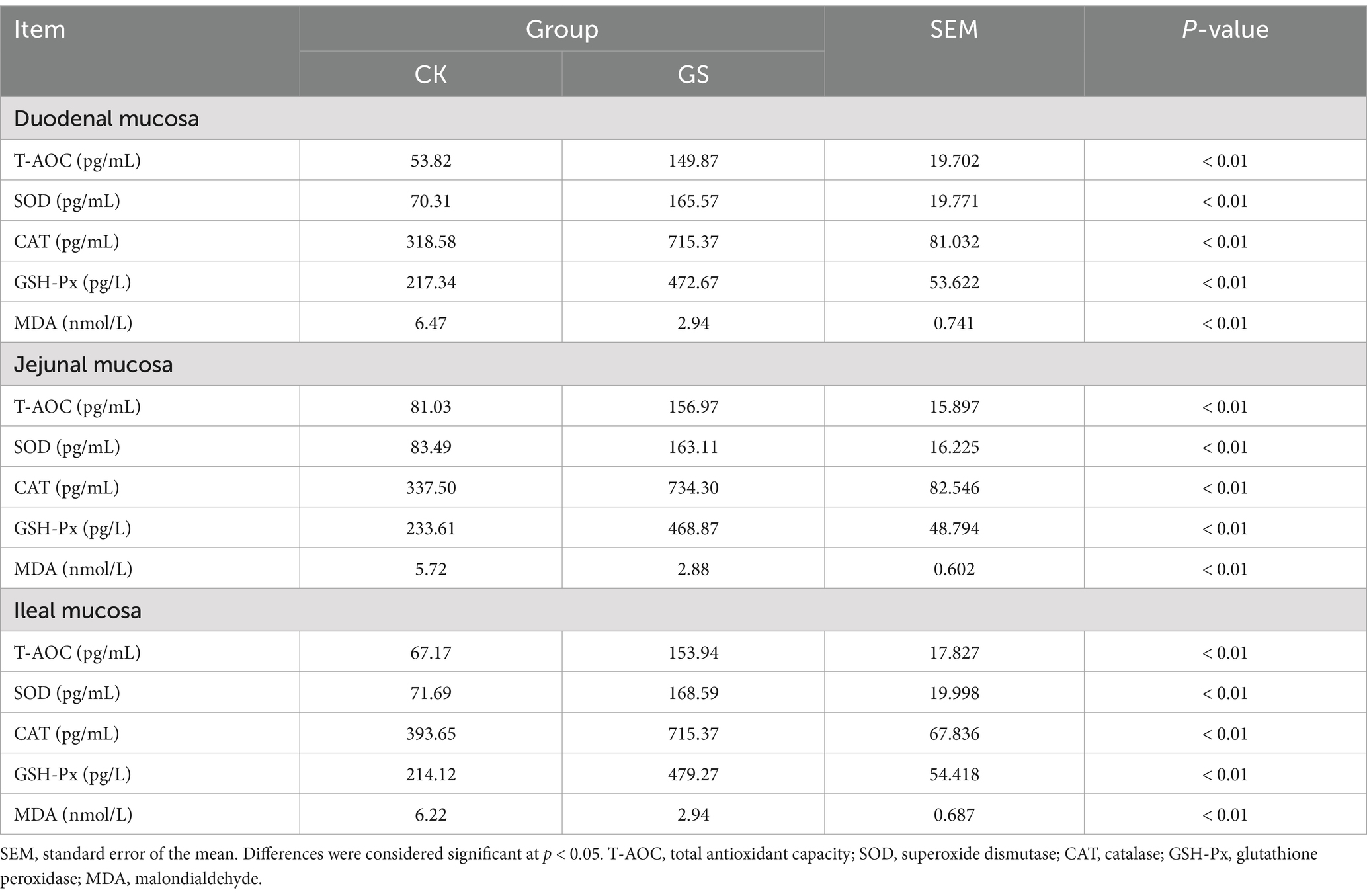
Table 4. The effect of Broussonetia papyrifera silage on the antioxidant capacity of intestinal tissues in Kazakh sheep.
3.3 Immunoglobulin concentrations
As shown in Table 5, the contents of IgA, IgM, and IgG in the duodenal, jejunal, and ileal tissues were all significantly higher in the GS group than in the CK group (p < 0.01).
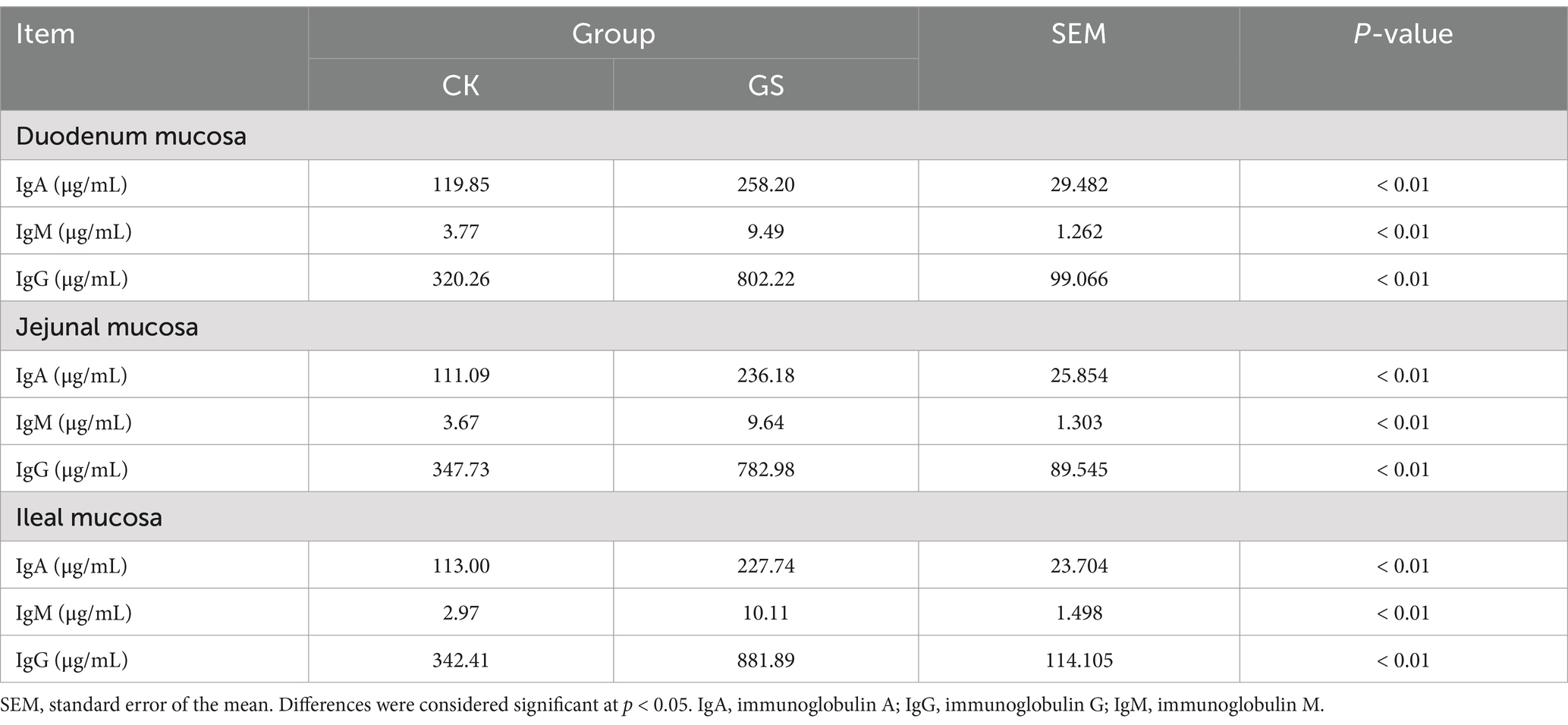
Table 5. The effect of Broussonetia papyrifera silage on the immunoglobulin content in the intestinal tissues of Kazakh sheep.
3.4 Cytokine contents
As listed in Table 6, the levels of TNF-α, IL-2, IL-6, and IL-8 in the duodenal, jejunal, and ileal tissues of the GS group were significantly higher than those in the CK group (p < 0.01), with only IL-4 contents being significantly lower in the GS group than in the control group (p < 0.01).
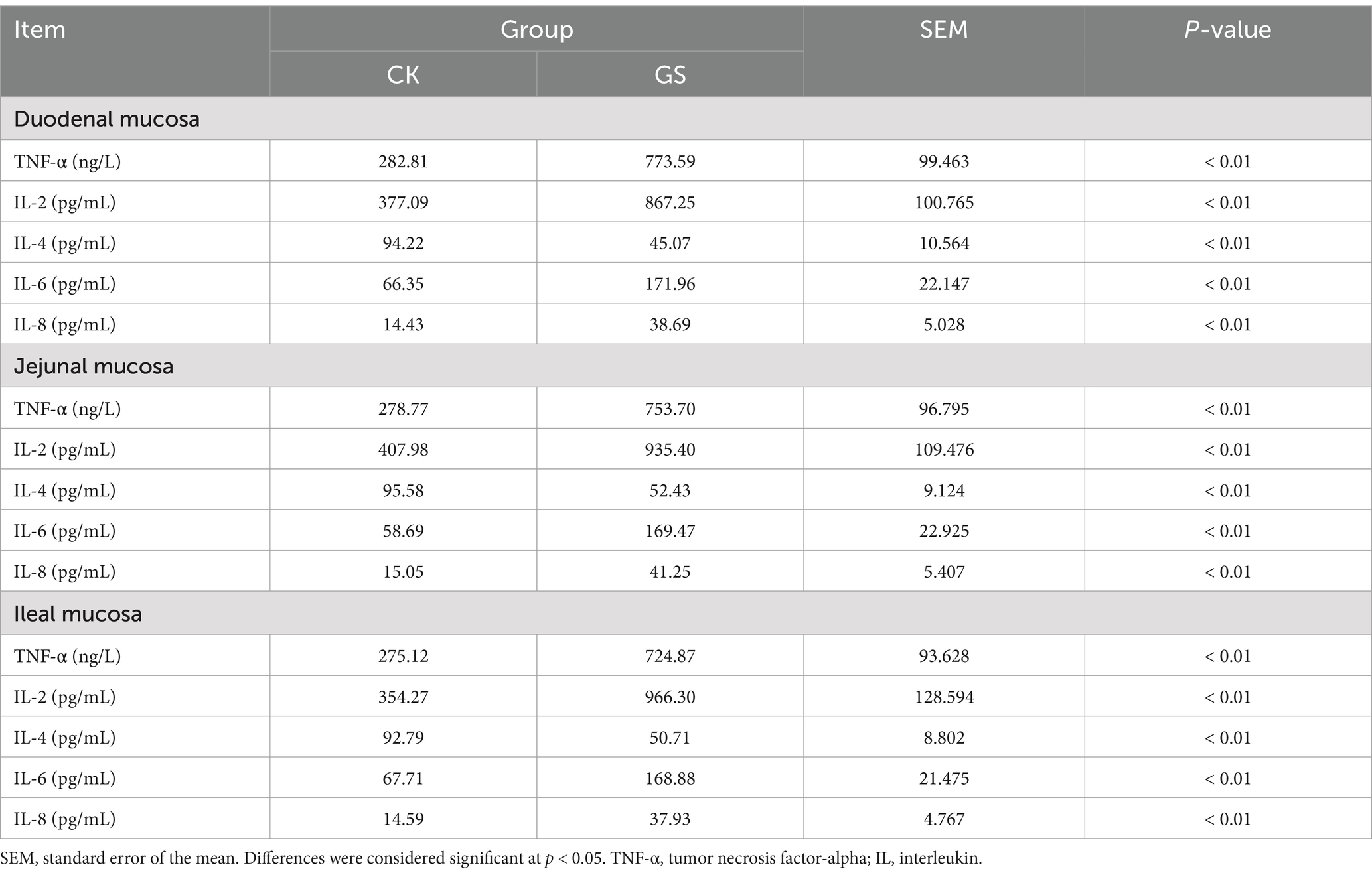
Table 6. The effect of Broussonetia papyrifera silage on the cytokine contents of intestinal tissue in Kazakh sheep.
3.5 Fecal bacteria
As shown in Figure 1, the Sobs, Chao, and Shannon indices were all higher in the GS group than in the CK group; however, the differences were not significant (p > 0.05).

Figure 1. Box plots of alpha diversity analysis. FCK, fecal bacterial samples collected from the control group; FGS, fecal bacterial samples collected from the Broussonetia papyrifera silage treatment group; OTU, operational taxonomic unit.
Principal coordinate analysis was used for the evaluation of beta diversity among fecal microbes (Figure 2). The results showed that microbial composition differed significantly between the treatment groups (R = 0.4480, p < 0.05). The R-value indicates the correlation between sample groups, with higher R-values indicating greater dissimilarity between groups.
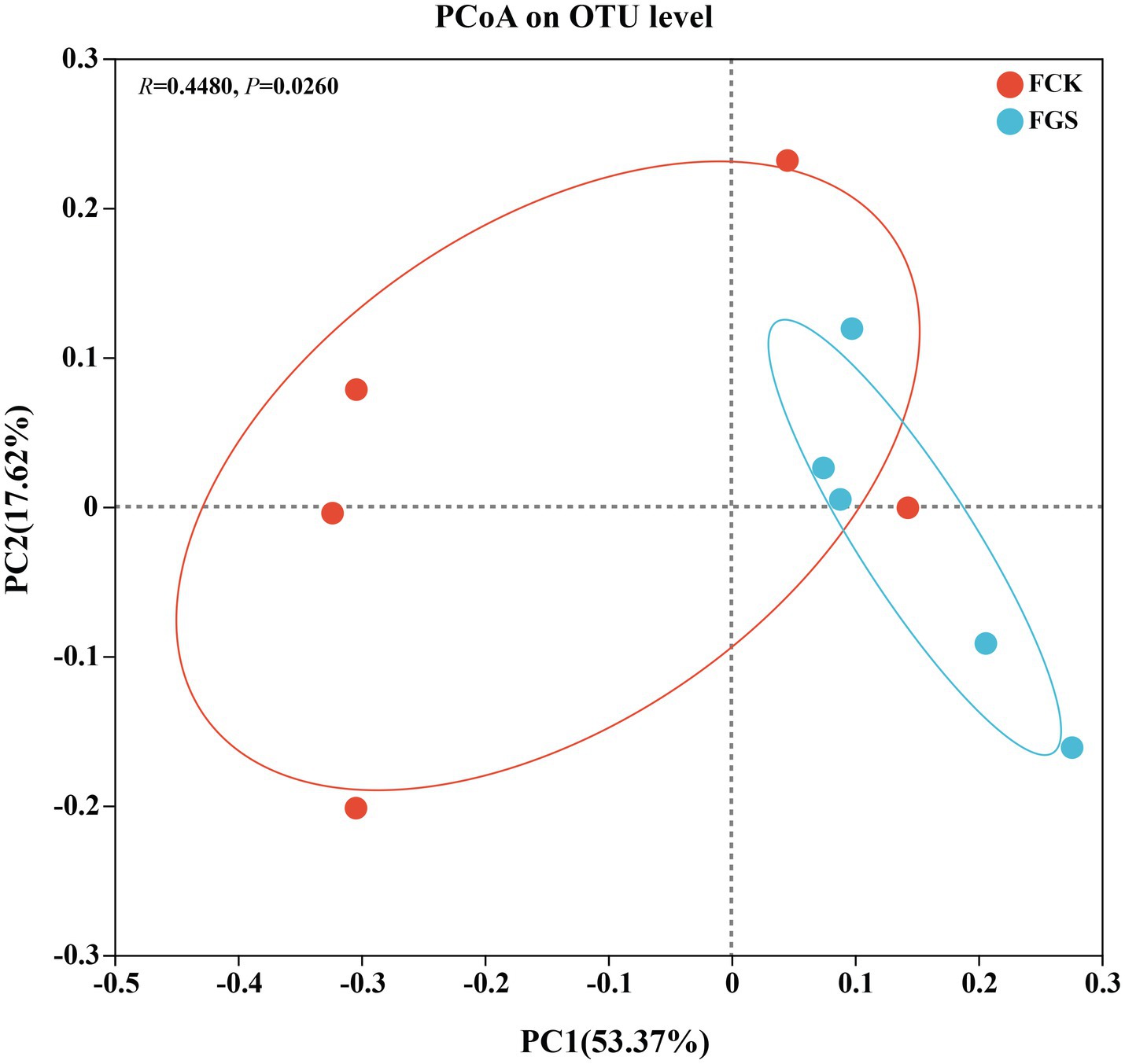
Figure 2. Principal coordinate analysis (PCoA). The horizontal and vertical coordinates represent the two selected principal coordinate components. The percentage represents the contribution of each principal coordinate component to the difference in sample composition. The closer the R-value is to 1, the greater the between-group difference relative to the within-group difference; conversely, the smaller the R-value is, the less significant the between- and within-group differences.
As shown in Figure 3A, Firmicutes was the dominant phylum in fecal bacterial samples collected from both the control (FCK) and Broussonetia papyrifera silage treatment (FGS) groups, with relative abundances of 86.34–93.92% and 90.21–94.47%, respectively, followed by Bacteroidota, with relative abundances of 2.58–9.28% and 3.10–5.68%, respectively.

Figure 3. Fecal bacterial communities at the phylum (A) and genus (B) levels. FCK, fecal bacterial samples collected from the control group; FGS, fecal bacterial samples collected from the Broussonetia papyrifera silage treatment group.
At the genus level, Solibacillus was the dominant bacterial genus in both the FCK and FGS groups (relative abundances: 15.39–26.05 and 15.78% ~ 41.50%, respectively), followed by Clostridium_sensu_stricto_1 (relative abundances: 5.17% ~ 21.17 and 7.00% ~ 15.28%, respectively), Turicibacter (relative abundances: 8.00% ~ 17.24 and 3.07% ~ 7.10%, respectively), and Romboutsia (relative abundances: 9.38% ~ 12.28 and 4.32% ~ 7.09%, respectively) (Figure 3B).
Compared with the FCK group, the abundance of Acinetobacter, norank_f__Oscillospiraceae, and Rhabdanaerobium was significantly higher in the FGS group (p < 0.05), whereas that of Turicibacter, Romboutsia, CPla-4_termite_group, Treponema, Blautia, Mogibacterium, and Lachnospiraceae_NK3A20_group were significantly lower (p < 0.05) (Figure 4).
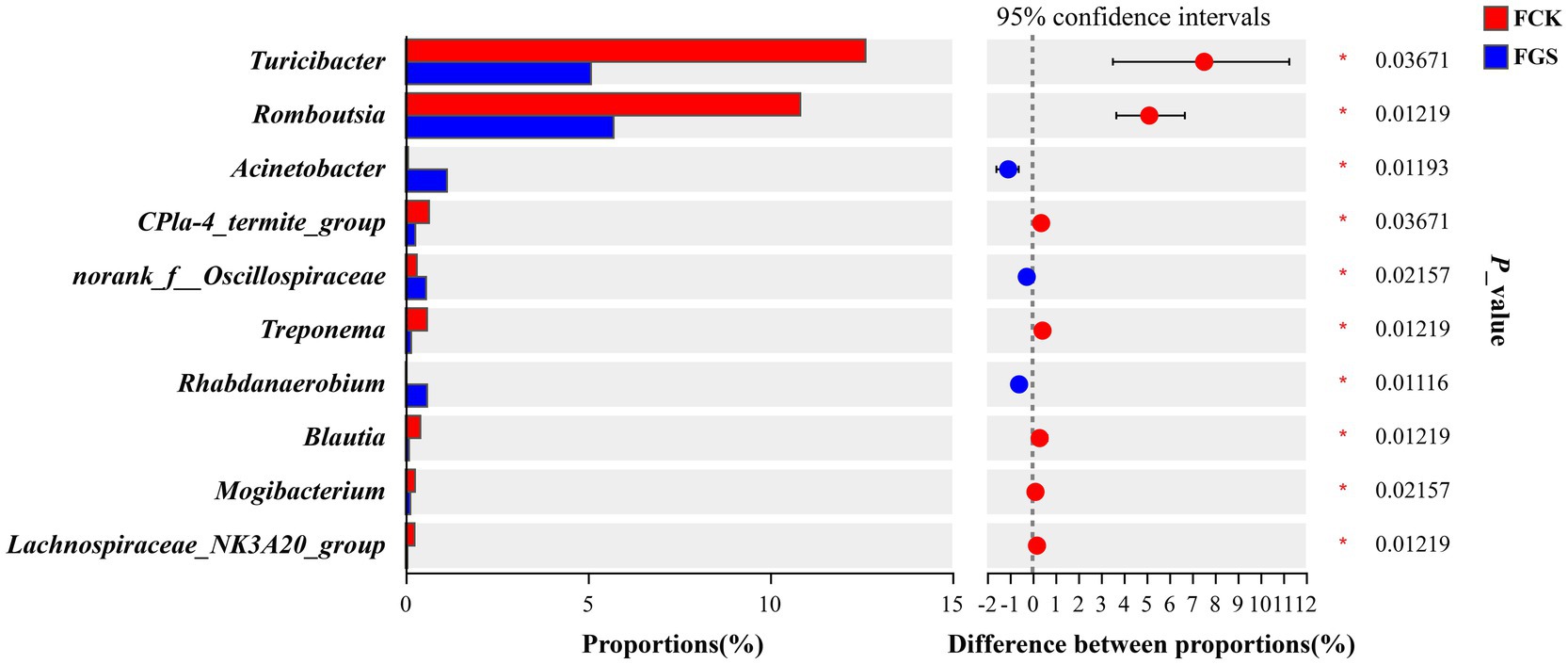
Figure 4. Bar chart for the multi-genera difference analysis. The y-axis represents different genera and the colored boxes represent the FCK (red) and FGS (blue) groups. The x-axis represents the average relative abundance of a particular genus in the different groups. FCK, fecal bacterial samples collected from the control group; FGS, fecal bacterial samples collected from the Broussonetia papyrifera silage treatment group.
As shown in Figure 5, fecal bacteria such as Turicibacter and Romboutsia exhibited marked positive correlations with TNF-α, IL-2, IL-6, IL-8, IgA, IgM, IgG, T-AOC, SOD, CAT, and GSH-Px (p < 0.05), while manifesting significant negative correlations with IL-4 and MDA (p < 0.05).
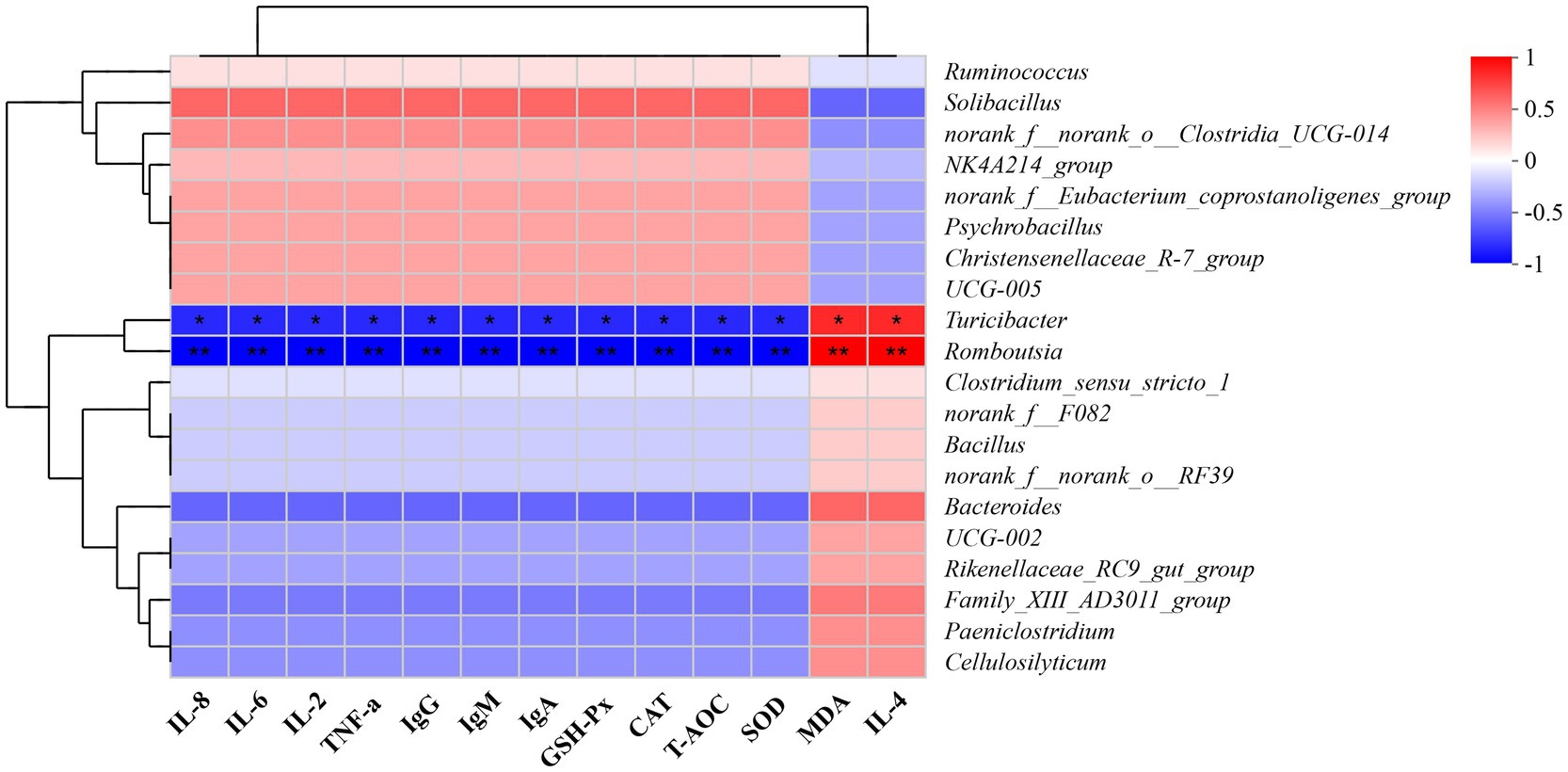
Figure 5. A correlation heatmap of the association of fecal bacteria with antioxidant indexes, immunoglobulin concentrations, and cytokine levels in mucosal tissue of the duodenum, jejunum, and ileum. Antioxidant indexes: T-AOC, total antioxidant capacity; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase; MDA, malondialdehyde. Immunoglobulins: IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M. Cytokines: TNF-α, tumor necrosis factor-alpha; IL, interleukin. The x- and y-axes represent environmental factors and species, respectively. Correlation coefficients (R-values) and p-values were calculated. The R-values are displayed in different colors on the chart; the color range for the R-values is shown on the right of the map. *p ≤ 0.05, **p ≤ 0.01.
4 Discussion
4.1 Growth performance and apparent digestibility of nutrients
In this study, B. papyrifera silage did not affect growth performance or apparent nutrient digestibility in Kazakh sheep. Apparent nutrient digestibility serves as an indicator of both feed nutritional value and the digestive and absorptive capacity of animals. Notably, some studies have suggested that feeding B. papyrifera silage can enhance the growth performance of ruminants, potentially due to the resulting variations in dietary nutrient composition (12, 23). However, other research has indicated that feeding B. papyrifera silage significantly decreases apparent nutrient digestibility, likely due to the presence of antinutritional factors in B. papyrifera that inhibit nutrient absorption (20, 24). Our results differ from these previous reports. We found no significant difference in growth performance between the two groups, which may be attributed to the uniform nutritional level in the diets used in this study. Similarly, no notable differences in apparent nutrient digestibility were observed between the dietary groups, possibly due to the specific lactic acid bacteria preparation used, which may have mitigated the effects of the antinutritional factors associated with B. papyrifera silage (4).
4.2 Immunoglobulins, antioxidants, and cytokines
Relatively few studies to date have examined the impact of the dietary addition of B. papyrifera silage on the intestinal mucosal immune barrier in sheep. The intestine is the largest immune organ in the body and also harbors the highest density of immune cells. In animals, it plays a critical role in mucosal immunity and serves as the first line of defense against infection (25). The levels of immunoglobulins and cytokines can reflect the functionality of the intestinal immune barrier (26). IgA is the primary antibody type in the intestinal mucosa, with IgG and IgM concentrations being present at only relatively low levels. IgA, secreted by intestinal mucosal epithelial cells, plays a protective role during inflammatory responses in the intestinal mucosa (27). Cytokines are key regulators of inflammation and the immune responses triggered by infection or injury (28). T-AOC and SOD, CAT, and GSH-Px contents are essential indicators of the antioxidant capacity of animals, while MDA levels reflect the extent of lipid peroxidation and cellular damage (29–31). The regulatory mechanisms governing cytokine production are complex. The variations in cytokine concentrations observed in this work may be attributed to the multiple antinutritional factors present in B. papyrifera silage, which induce intestinal stress in sheep (22). Additionally, some constituents of B. papyrifera silage may influence cytokine activity, a possibility that warrants further investigation. The increase in immunoglobulin concentrations (IgA, IgG, and IgM) in the experimental group may not necessarily indicate pathogen exposure but rather reflects the modulation of the intestinal immune system by bioactive compounds present in B. papyrifera silage. The dietary incorporation of B. papyrifera silage, which contains flavonoids, polyphenolic compounds, and alkaloids, can enhance intestinal health by improving intestinal morphology (16, 32, 33). Although a rise in antioxidant enzymes could suggest mild stress due to bioactive compounds in B. papyrifera silage, the overall reduction in MDA levels indicates that these compounds help mitigate oxidative damage, thereby supporting the beneficial antioxidant effects rather than harmful stress. Our results corroborate previous research findings, namely, that the addition of B. papyrifera silage in the diet enhances both immunity and antioxidant capacity in animals. Our findings further suggest that such dietary inclusion may bolster the intestinal immune function of sheep and promote overall intestinal health.
4.3 Fecal bacteria
Variations in the intestinal bacterial community can reflect the health status and production performance of the host. These communities interact through various signaling pathways, influencing their host’s nutrient metabolism and immune function (34, 35). Several studies have established that the gastrointestinal microbiota of ruminants is predominantly composed of Firmicutes and Bacteroidetes, in line with the results of this study (36–39). Firmicutes primarily facilitate cellulose decomposition, while Bacteroidetes enhance carbohydrate utilization by animals (40, 41). In the present study, we noted an increasing trend in the relative abundance of Firmicutes in sheep of the GS group, indicative of an improvement in their fiber-decomposing capacity. Conversely, there was a decreasing trend in the abundance of Bacteroidetes, suggesting that structural changes within the intestinal bacterial community of the sheep may be linked to antinutritional factors present in B. papyrifera silage.
In this work, the relative abundances of Turicibacter and Romboutsia demonstrated a significant negative correlation with the health of their hosts. Turicibacter has been positively associated with inflammation and identified as a target microbe for colitis (42). Additionally, research has shown that mice engaged in treadmill running exhibit a lower fecal abundance of Turicibacter, whereas sedentary or forced-exercise mice display the opposite pattern (43). Literature relating to Romboutsia remains limited; however, its abundance is significantly elevated in the colonic chyme of rats with irritable bowel syndrome (44). The abundance of lactobacilli was increased, whereas that of Romboutsia was decreased, in the feces of hens fed diets supplemented with astragalus polysaccharides, indicative of a potential competitive exclusion relationship between these two genera (45). The B. papyrifera silage used in this study contained substantial quantities of lactobacilli, which may account for the observed decrease in the relative abundance of Romboutsia. Notably, the feeding of hybrid mulberry silage led to a significant reduction in the relative abundance of both Turicibacter and Romboutsia, suggesting that such a dietary intervention can mitigate the prevalence of potentially pathogenic bacterial genera in the host.
5 Conclusion
The incorporation of B. papyrifera silage in the diet did not impact growth performance or apparent nutrient digestibility in Kazakh sheep. Notably, B. papyrifera silage enhanced the immune response and antioxidant capacity of the animals, while concurrently reducing the relative abundance of potentially pathogenic bacteria (Turicibacter and Romboutsia) in fecal matter. Given the comprehensive bans on antibiotics being introduced within the livestock sector worldwide, B. papyrifera silage represents a viable green functional feed for ruminants.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA1167466.
Ethics statement
The animal study was approved by Biology Ethics Committee of Shihezi University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XZ: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. YS: Software, Writing – original draft. SG: Writing – original draft. JY: Conceptualization, Investigation, Writing – original draft. RH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. FZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the South Xinjiang Key Industry Innovation and Development Support Plan Project (grant no. 2022DB017) and the China Agriculture Research System of the MOF and MARA (grant no. CARS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Sneeringer, S, Short, G, MacLachlan, M, and Bowman, M. Impacts on livestock producers and veterinarians of FDA policies on use of medically important antibiotics in food animal production. Appl Econ Perspect Pol. (2020) 42:674–94. doi: 10.1002/aepp.13057
2. Jarlov, KJ, Hansen, MS, Skovgaard, K, Svensson, E, Larsen, LE, Heegaard, PMH, et al. Immunogenicity of Bacillus Calmette-Guérin in pigs: potential as a translational model of non-specific effects of BCG. Front Immunol. (2023) 14:1219006. doi: 10.3389/FIMMU.2023.1219006
3. Fonseca, A, Kenney, S, Van Syoc, E, Bierly, S, Dini-Andreote, F, Silverman, J, et al. Investigating antibiotic free feed additives for growth promotion in poultry: effects on performance and microbiota. Poult Sci. (2024) 103:103604. doi: 10.1016/J.PSJ.2024.103604
4. Yulin, Z, Hanjun, Y, Rongzheng, H, Xuzhe, W, Chunhui, M, and Fanfan, Z. Effects of Lactiplantibacillus plantarum and Lactiplantibacillus brevis on fermentation, aerobic stability, and the bacterial community of paper mulberry silage. Front Microbiol. (2022) 13:1063914. doi: 10.3389/FMICB.2022.1063914
5. Hao, Y, Huang, S, Zhang, J, Gong, Y, Liu, G, Sun, X, et al. PSVIII-23 effects of different growth height on the yield, chemical composition, silage fermentation profile, in vitro and in situ digestibility of Broussonetia papyrifera. J Anim Sci. (2020) 98:325–7. doi: 10.1093/jas/skaa278.582
6. Wu, Z, Liang, C, Huang, R, Ouyang, J, Zhao, L, and Bu, D. Replacing alfalfa hay with paper mulberry (Broussonetia papyrifera L.) silage in diets do not affect the production performance of the low lactating dairy cows. Anim Feed Sci Technol. (2022):294. doi: 10.1016/J.ANIFEEDSCI.2022.115477
7. Han, K, Zhang, B, and Cui, Y. Effect of Broussonetia papyrifera leaf meal on growth performance, antioxidant capacity, and gut health status of growing rabbits. Czeh J Anim Sci. (2023) 68:87–97. doi: 10.17221/146/2022-CJAS
8. Niu, KM, Wang, YF, Liang, X, Zhai, Z, Liu, J, Wang, R, et al. Impact of fermented Broussonetia papyrifera on laying performance, egg quality, lipid metabolism, and follicular development of laying hens. Poult Sci. (2023) 102:102569. doi: 10.1016/J.PSJ.2023.102569
9. Wang, R, Wang, X, Xiong, Y, Cao, J, Nussio, LG, Ni, K, et al. Dietary paper mulberry silage supplementation improves the growth performance, carcass characteristics, and meat quality of Yangzhou goose. Animals. (2024) 14:359. doi: 10.3390/ANI14030359
10. Sindhu, S, Saini, T, Rawat, HK, Chahar, M, Grover, A, Ahmad, S, et al. Beyond conventional antibiotics approaches: global perspectives on alternative therapeutics including herbal prevention, and proactive management strategies in bovine mastitis. Microb Pathog. (2024) 196:106989. doi: 10.1016/J.MICPATH.2024.106989
11. Abdallah, A, Zhang, P, Zhong, Q, and Sun, Z. Application of traditional Chinese herbal medicine by-products as dietary feed supplements and antibiotic replacements in animal production. Curr Drug Metab. (2019) 20:54–64. doi: 10.2174/1389200219666180523102920
12. Xiong, Y, Guo, C, Wang, L, Chen, F, Dong, X, Li, X, et al. Effects of paper mulberry silage on the growth performance, rumen microbiota and muscle fatty acid composition in Hu lambs. Fermentation. (2021) 7:286. doi: 10.3390/FERMENTATION7040286
13. Chen, Y, Dong, B, Qu, H, Cheng, J, Feng, Y, Liu, L, et al. Evaluating the effects of replacing alfalfa with Broussonetia papyrifera branch/leaf powder on growth and serum indicators in Dezhou donkeys. Animals. (2023) 14:123. doi: 10.3390/ani14010123
14. Wen, Z, Chen, Y, Wu, L, Tian, H, Zhu, N, Guo, Y, et al. Effects of Broussonetia papyrifera silage on rumen fermentation parameters and microbes of Holstein heifers. AMB Express. (2022) 12:62. doi: 10.1186/S13568-022-01405-X
15. Tang, T, Bai, J, Ao, Z, Wei, Z, Hu, Y, and Liu, S. Effects of dietary paper mulberry (Broussonetia papyrifera) on growth performance and muscle quality of grass carp (Ctenopharyngodon idella). Animals. (2021) 11:1655. doi: 10.3390/ANI11061655
16. Guoshun, C, Shengzhang, S, Mingjie, C, Dong, W, Yingyu, S, Hongbin, W, et al. Effects of paper mulberry (Broussonetia papyrifera) leaf extract on growth performance and fecal microflora of weaned piglets. Biomed Res Int. (2020) 2020:6508494. doi: 10.1155/2020/6508494
17. Hao, Y, Huang, S, Si, J, Zhang, J, Gaowa, N, Sun, X, et al. Effects of paper mulberry silage on the milk production, apparent digestibility, antioxidant capacity, and fecal bacteria composition in Holstein dairy cows. Animals. (2020) 10:1152. doi: 10.3390/ani10071152
18. Si, B, Tao, H, Zhang, X, Guo, J, Cui, K, Tu, Y, et al. Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian-Australas. J Anim Sci. (2018) 31:1259–66. doi: 10.5713/ajas.17.0847
19. Guo, W, Zhou, M, Li, F, Neves, ALA, Ma, T, Bi, S, et al. Seasonal stability of the rumen microbiome contributes to the adaptation patterns to extreme environmental conditions in grazing yak and cattle. BMC Biol. (2024) 22:240. doi: 10.1186/S12915-024-02035-4
20. Zhang, JP, Wei, QX, Li, QL, Liu, RF, Tang, LQ, Song, YX, et al. Effects of hybrid Broussonetia papyrifera silage on growth performance, visceral organs, blood biochemical indices, antioxidant indices, and carcass traits in dairy goats. Anim Feed Sci Technol. (2022):292. doi: 10.1016/J.ANIFEEDSCI.2022.115435
21. Van Soest, PJ, Robertson, JB, and Lewis, BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.s0022-0302(91)78551-2
22. Youkuan, H, Yaoxin, L, Hao, L, Zhichao, H, Shengguo, T, Yulong, Y, et al. Effects of Broussonetra papyrifera silage on growth performance, nutrient apparent digestibility and intestinal health of mutton goats. Chin J Anim Nutr. (2021) 33:5131–41. doi: 10.3969/j.issn.1006-267x.2021.09.033
23. Tao, H, Si, B, Xu, W, Tu, Y, and Diao, Q. Effect of Broussonetia papyrifera L. silage on blood biochemical parameters, growth performance, meat amino acids and fatty acids compositions in beef cattle. Asian Australas J Anim Sci. (2020) 33:732–41. doi: 10.5713/ajas.19.0150
24. Wang, N, Xiong, Y, Wang, X, Guo, L, Lin, Y, Ni, K, et al. Effects of lactobacillus plantarum on fermentation quality and anti-nutritional factors of paper mulberry silage. Fermentation. (2022) 8:144. doi: 10.3390/FERMENTATION8040144
25. Chunmei, Y, Shuiping, W, Kefyalew, G, Xin, Y, Shaoxun, T, Chuanshe, Z, et al. A low-carbon high inulin diet improves intestinal mucosal barrier function and immunity against infectious diseases in goats 13. Front Vet Sci. (2023) 9:1098651. doi: 10.3389/FVETS.2022.1098651
26. Sha, J, Liu, Z, Yu, H, Huo, X, Wang, J, Duan, Y, et al. Dietary supplementation with American ginseng dietary fiber ameliorates intestinal mucosal barrier injury in immunosuppressed mice. Food Biosci. (2023):56. doi: 10.1016/J.FBIO.2023.103237
27. Pardo-Camacho, C, González-Castro, AM, Rodiño-Janeiro, BK, Pigrau, M, and Vicario, M. Epithelial immunity: priming defensive responses in the intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. (2018) 314:G247–55. doi: 10.1152/ajpgi.00215.2016
28. Jiao, LF, Zhang, QH, Wu, H, Wang, CC, Cao, ST, Feng, J, et al. Influences of copper/zinc-loaded montmorillonite on growth performance, mineral retention, intestinal morphology, mucosa antioxidant capacity, and cytokine contents in weaned piglets. Biol Trace Elem Res. (2018) 185:356–63. doi: 10.1007/s12011-018-1259-4
29. Hu, Y, Tang, S, Zhao, W, Wang, S, Sun, C, Chen, B, et al. Dietary ferulic acid improves growth performance of broilers via enhanced intestinal antioxidant capacity and barrier function. Anim Biosci. (2024). doi: 10.5713/AB.23.0487
30. Li, Z, Chen, L, Huang, Z, Jia, G, Zhao, H, Liu, G, et al. Supplementation with L-theanine promotes intestinal antioxidant ability via Nrf2 signaling pathway in weaning piglets and H2O2-induced IPEC-J2 cells. J Funct Foods. (2024) 121:106433. doi: 10.1016/J.JFF.2024.106433
31. Ruan, D, Fouad, AM, Fan, QL, Huo, XH, Kuang, ZX, Wang, H, et al. Dietary L-arginine supplementation enhances growth performance, intestinal antioxidative capacity, immunity and modulates gut microbiota in yellow-feathered chickens. Poult Sci. (2020) 99:6935–45. doi: 10.1016/j.psj.2020.09.042
32. Lee, D, and Kinghorn, AD. Bioactive compounds from the genus Broussonetia. Stud Nat Prod Chem. (2003) 28:3–33. doi: 10.1016/S1572-5995(03)80137-0
33. Park, MH, Jung, S, Yuk, HJ, Jang, HJ, Kim, WJ, Kim, DY, et al. Rapid identification of isoprenylated flavonoids constituents with inhibitory activity on bacterial neuraminidase from root barks of paper mulberry (Broussonetia papyrifera). Int J Biol Macromol. (2021) 174(prepublish:61–8. doi: 10.1016/J.IJBIOMAC.2021.01.140
34. Worley, MJ. Immune evasion and persistence in enteric bacterial pathogens. Gut Microbes. (2023) 15:2163839. doi: 10.1080/19490976.2022.2163839
35. Taglialegna, A. A gut bacterium trims mucosal immunity. Nat Rev Microbiol. (2024) 22:1. doi: 10.1038/S41579-024-01117-Y
36. Wang, D, Tang, G, Wang, Y, Yu, J, Chen, L, Chen, J, et al. Rumen bacterial cluster identification and its influence on rumen metabolites and growth performance of young goats. Anim Nutr. (2023) 15:34–44. doi: 10.1016/J.ANINU.2023.05.013
37. Jiachong, L, Sikandar, A, Chunrong, L, Hongyuan, Y, Xiaoqi, Z, Xiaojun, N, et al. Dietary protein levels modulate the gut microbiome composition through fecal samples derived from lactating ewes. Front Endocrinol. (2023) 14:1194425. doi: 10.3389/FENDO.2023.1194425
38. Parnian-Khajehdizaj, F, Noel, SJ, Johansen, M, Weisbjerg, MR, Hellwing, ALF, Højberg, O, et al. Methane emission, nutrient digestibility, and rumen microbiota in Holstein heifers fed 14 different grass or clover silages as the sole feed. J Dairy Sci. (2023) 106:4072–91. doi: 10.3168/JDS.2022-22638
39. Gruninger, R, Min, ZX, O'Hara, E, Maik, K, and Beauchemin, K. 3-nitrooxypropanol supplementation of a forage diet decreased enteric methane emissions from beef cattle without affecting feed intake and apparent total-tract digestibility. J Anim Sci. (2023):101. doi: 10.1093/JAS/SKAD001
40. Meng, Q, Tang, Z, Yang, F, Shi, J, Liu, T, and Cheng, S. Functional analysis of microorganisms and metabolites in the cecum of different sheep populations and their effects on production traits. Front Microbiol. (2024) 15:1437250. doi: 10.3389/FMICB.2024.1437250
41. Wu, Y, Hou, D, Zhan, S, Wang, L, Cao, J, Guo, J, et al. Colonization profiles of gut microbiota in goat kids from neonatal to weaning period. Front Microbiol. (2024) 15:1467205. doi: 10.3389/FMICB.2024.1467205
42. Shang, L, Liu, H, Yu, H, Chen, M, Yang, T, Zeng, X, et al. Core altered microorganisms in colitis mouse model: a comprehensive time-point and fecal microbiota transplantation analysis. Antibiotics. (2021) 10:643. doi: 10.3390/ANTIBIOTICS10060643
43. Woods, JA, Allen, JM, Berg Miller, MEB, White, BA, Gaskins, H, and Nehra, V. Exercise alters the gut microbiome and microbial metabolites: implications for colorectal cancer and inflammatory bowel disease. Brain Behav Immun. (2015) 49:e7-e. doi: 10.1016/j.bbi.2015.06.046
44. Enqi, W, Jingzhu, S, Lingpeng, P, and Yaqin, L. Comparison of the gut microbiota disturbance in rat models of irritable bowel syndrome induced by maternal separation and multiple early-life adversity. Front Cell Infect Microbiol. (2021) 10:581974. doi: 10.3389/FCIMB.2020.581974
Keywords: Broussonetia papyrifera, antibiotic-free feed, immunity, antioxidation, fecal bacteria
Citation: Zheng X, Sun Y, Guo S, Yu J, Huang R and Zhang F (2025) The effect of Broussonetia papyrifera silage on intestinal health indicators and fecal bacterial composition in Kazakh sheep. Front. Vet. Sci. 12:1543302. doi: 10.3389/fvets.2025.1543302
Edited by:
Moyosore Joseph Adegbeye, University of Africa, Bayelsa State, NigeriaReviewed by:
Ravikanth Reddy Poonooru, University of Missouri, United StatesSadarman Sadarman, State Islamic University of Sultan Syarif Kasim Riau, Indonesia
Copyright © 2025 Zheng, Sun, Guo, Yu, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongzheng Huang, aHVhbmdyejIwMTNAMTYzLmNvbQ==; Fanfan Zhang, emhhbmdmYW5mYW5Ac2h6dS5lZHUuY24=
 Xiaokai Zheng
Xiaokai Zheng Yingchao Sun
Yingchao Sun Fanfan Zhang
Fanfan Zhang