
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 21 March 2025
Sec. One Health
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1536861
Parasitic infections remain a global health concern, affecting human populations worldwide. However, comprehensive studies evaluating human, animal, and environmental interactions driven transmission of parasites are limited. We conducted a One Health study in an urban area of Valdivia, Chile. Human participants provided fecal and blood samples for parasitological and serological analysis. Environmental soil samples were collected from public parks, and fecal samples from owned and stray dogs were analyzed. Detection of intestinal parasites employed microscopy and molecular techniques, including next-generation sequencing (NGS), while anti-Toxocara canis antibodies in humans were assessed using ELISA. Socioeconomic surveys explored risk factors associated with parasitism. Parasite prevalence was 28% in humans, 26% in owned dogs, and 44% in environmental dog feces. Anti-T. canis IgG antibodies were present in 33% of humans. Soil contamination was identified in up to 30.5% of park samples, harboring zoonotic parasites such as Toxocara sp. and Trichuris vulpis, the same species identified in environmental dog feces. Zoonotic subtypes of Giardia duodenalis and Blastocystis sp. were detected in humans. Our findings highlight significant zoonotic and environmental transmission contributing to human parasitic infections in urban settings, underscoring the need for integrated public health interventions. This study demonstrates the importance of adopting an OneHealth approach in the study of parasitology. The complex ecology of parasites requires an integrated perspective to fully understand their transmission pathways and develop effective control strategies. By emphasizing the interconnectedness of human, animal, and environmental health, we aim to contribute to the management and mitigation of this persistent public health issue.
Parasitic diseases remain a major global health challenge, affecting at least one in six people worldwide, with the highest burden in economically vulnerable regions (1). Many of these infections are classified as Neglected Tropical Diseases (NTDs), disproportionately impacting marginalized populations and reinforcing cycles of poverty (2).
Parasite transmission is influenced not only by host-pathogen interactions but also by environmental and urban conditions. Climate change, for instance, alters key factors such as temperature, humidity, and precipitation, potentially facilitating parasite persistence and spread in both endemic and non-endemic regions (3, 4). Among intestinal parasites, protozoa such as Blastocystis sp., Giardia duodenalis (syn. Giardia lamblia), Cryptosporidium spp., and Entamoeba spp. are widely distributed and primarily transmitted through contaminated food and water (5–7).While more prevalent in developing countries, these parasites are also detected in developed nations, often linked to contaminated water sources, travel, or migration (8). Risk factors for infection include inadequate sanitation, poor water quality, and contact with infected animals, many of which act as reservoirs for zoonotic parasites (5, 7).
Although sanitation improvements have significantly reduced intestinal parasite prevalence, alternative transmission routes must be considered. Studies in Chile and Argentina have shown that prevalence rates range from 37.5 to 50.7% in populations with access to drinking water, rising to 68.1–92.9% in those without (9, 10). In Chile, a long-term study in Talca documented a decline in pathogenic parasites like G. duodenalis (from 18.5 to 5.5%) and Ascaris lumbricoides (from 10 to 0.1%). However, a dramatic increase was observed in the prevalence of Blastocystis sp., from 7.6% (1990–1994) to 73% (2005–2007), probably associated with an improvement in its diagnosis (11). Similar trends have been observed in Valdivia and Puerto Montt, where Blastocystis sp. and Entamoeba coli—both with zoonotic potential—are highly prevalent (10, 12).
Dogs, particularly strays, are key reservoirs of zoonotic parasites and contribute to environmental contamination. Studies in Chilean cities reveal high levels of soil contamination with parasite-laden dog feces: in Los Ángeles and Temuco, 60% of soil samples harbored eggs of Toxocara sp., Ancylostoma sp., Dipylidium caninum, and Taenia sp. (13, 14). In areas near Valdivia, such as Niebla and Corral, contamination rates reach 85.1 and 92.3%, respectively (15). One of the most concerning parasites is Toxocara canis, a nematode found in dogs that can cause visceral and ocular larva migrans in humans (16). Diagnosis is typically based on antibody detection, with reported seroprevalence rates reaching as high as 50.6% (17). In 2012, a 25.4% seroprevalence of antibodies against Toxocara spp. was reported in Niebla, Chile, alongside 15% soil contamination with Toxocara spp. eggs in urban and rural areas (18). In Valdivia, Toxocara canis eggs were found in 100% of households with dogs (19).
Since parasite transmission occurs at the intersection of human, animal, and environmental health, a holistic approach is essential (20). This study applies a One Health framework to assess intestinal parasite prevalence in humans and dogs, determine T. canis IgG seroprevalence in humans, and evaluate environmental contamination in an urban area of Valdivia, Chile. By integrating these components, we aim to provide a comprehensive understanding of parasite transmission dynamics and associated risk factors, generating evidence to support effective control strategies.
In Chile, public health centers serve a geographic area within each city. The study was conducted at the Community Family Health Center “Mulato” in the city of Valdivia, Chile (39°48′51″S 73°14′45″W), from October 2021 to August 2022. The study obtained the necessary permissions for its realization in humans given by Research Ethics Committee Los Ríos Health Service (Ord. N°354 14.10.2021). Subsequently, to determine the spatial distribution of parasites, the territory was divided into three sectors A, B and C, each with a public park, respectively (Figure 1).
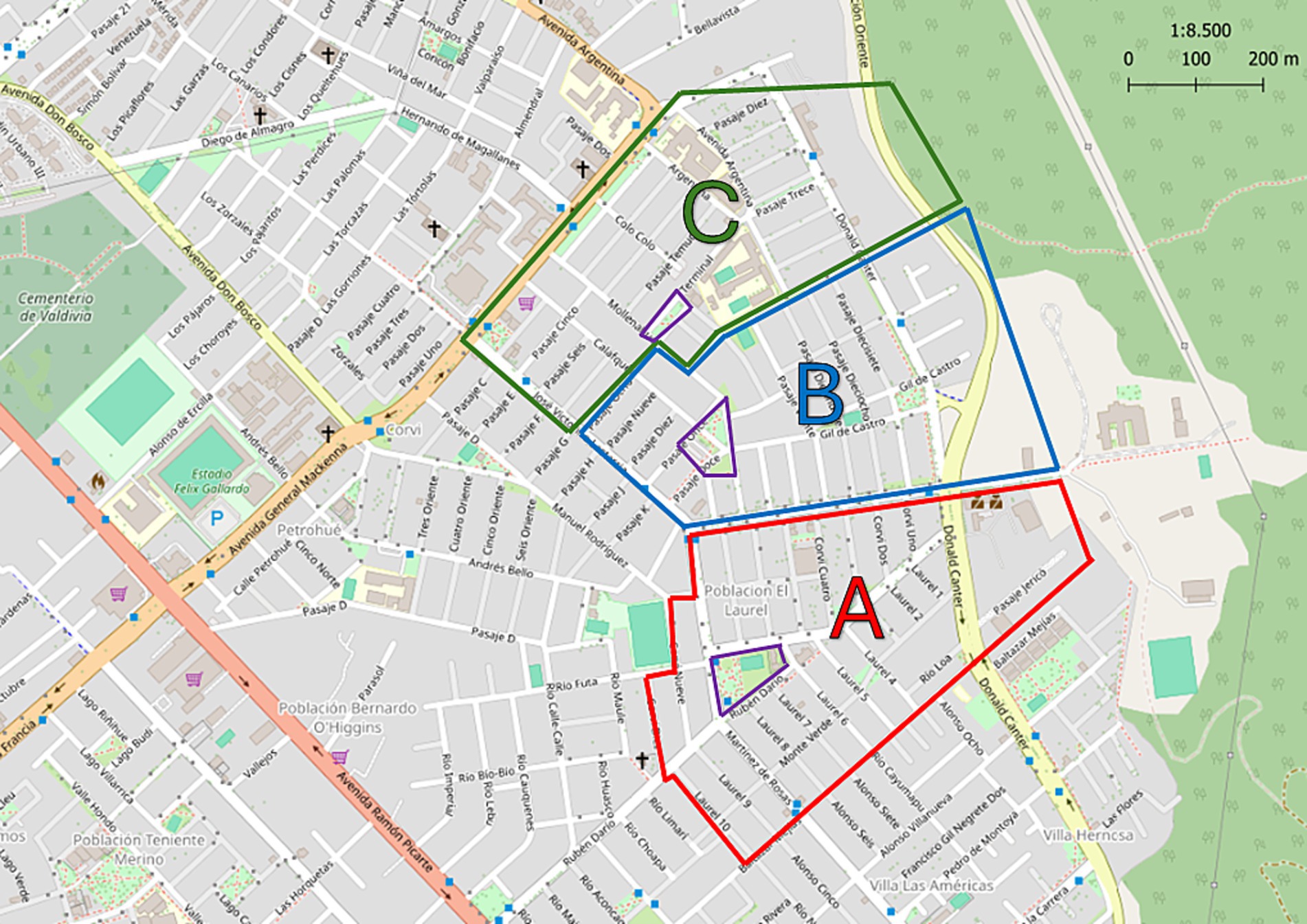
Figure 1. Map of territory covered by Public Health Center “Mulato,” Valdivia, Los Ríos, Chile. The division into three sectors is indicated: A, B, and C with the colors red, blue and green, respectively. The park of each sector is indicated in yellow. Modified from ©OpenStreetMap contributors.
The estimated population of the study area is approximately 5,000 people. Recruitment was conducted through informational posters at the health center and community meetings. A total of 157 residents participated, providing informed consent for blood and stool sample collection. While all participants provided blood samples, 16 individuals did not return stool samples, resulting in a total of 141 stool samples collected. A survey was carried out on each participant to collect information on their socioeconomic conditions, potentially linked to parasite prevalence (survey details are provided in Supplementary material). Participants, who owned dogs as pets, were offered a diagnostic test to identify intestinal parasites in their dogs.
All samples were collected at the health center and then transported to the Institute of Parasitology of the Universidad Austral de Chile. For the diagnosis of parasitosis in fecal samples, each participant received a container with 35 mL of PAF (Phenol, Alcohol, and Formaldehyde) fixative and a 15 mL conical-bottom tube with 8 mL of 70% ethanol, along with oral and written instructions for proper sample collection. Participants deposited fecal samples from three different days (every other day) into PAF containers (21). A single sample from the final collection day was placed in the 70% ethanol tube for molecular analysis. Containers with PAF were stored at 2–5°C, while the ethanol tube were stored at −20°C until processing. To obtain serum, blood samples were collected by health center staff by venipuncture in tubes without anticoagulant and centrifuged at 3,000 rpm for 5 min. The serum was extracted and then frozen at −20°C until the ELISA test was performed.
Fecal samples, from both human and dog, were processed using the Modified Burrows Method (PAFS) (21) and following the recommendations of the National Reference Laboratory of the Instituto de Salud Pública de Chile.
A commercial ELISA kit (NovaLisa from Novatec) was used to detect IgG anti-Toxocara canis antibodies. The test was performed according to the manufacturer’s instructions. Indeterminate results were sent for confirmation to the national reference laboratory at the Institute of Public Health, Santiago, Chile.
Ten human stools samples, previously diagnosed with Blastocystis sp. or with G. duodenalis by microscopy, were further analyzed using next-generation sequencing (NGS) at the Austral-OMICS laboratory (specialized unit for supporting scientific research). Samples were selected to represent each of the sampling sectors. For G. duodenalis the β-Giardina gene was targeted, while for Blastocystis sp. the 18rRNA gene was analyzed. A detailed description of the technique is provided in Supplementary material.
Soil samples were collected from parks A, B, and C during two periods: October 2021 (spring) and May 2022 (autumn). In each park, areas of interest were defined as two meters perimeter the children’s playgrounds, as these locations have the highest presence of both humans and dogs. Within these sampling areas, three parallel imaginary lines were drawn, spanning from one end to the other. Sampling points were established at 1 m intervals and 5 g soil sample was collected from a depth of 3–5 cm. The collected soil samples were dried for 48 h at room temperature. The whole sample was then sieved with a grid into a metal container and processed using the zinc sulfate method (22, 23). For the physicochemical analysis, 300 g of soil from each sector was collected in a re-sealable plastic bag. In the case of parks A and B parks, one sample was taken for each study (parasitological and physicochemical), while in park C there were two soil types, therefore, one sample was taken from each soil type (C1 and C2).
The physicochemical analysis of the soil was carried out by the Forest Soil and Nutrition Laboratory of the Universidad Austral de Chile. The parameters measured were pH, % Total Carbon (TC), % Soil Organic Matter (SOM) and % Humidity.
A walking tour of each of the streets and passageways in the sector was conducted twice, in October 2021 (spring) and May 2022 (autumn). All samples of dog feces that were not dry for collection were obtained in each sector. For each sample, 5 g of stool were placed in containers with 15 mL of PAF fixative. Samples were then transported to the laboratory and processed using the Modified Burrows Method (PAFS) (21).
Participants who agreed to include their dogs in the research received a container with 15 mL of PAF fixative and were asked to collect 5 grams of feces. Finally, the collected stool samples were processed by the Modified Burrows Method (21).
A descriptive analysis of the data was performed using Chi-Square and a statistical significance level of 0.05 was established. To determine the magnitude of the observed relationship, the Odds Ratio (OR) was used, and a 95% confidence interval (CI) was established. Graphpad Prism 9 and IBM SPSS Statistics (Statistical Package for the Social Sciences) were used in the analysis of the data obtained.
One hundred forty-one people participated in the study, of whom 67.4% were women. The participants age ranged from 5 to 89 years, with an average age of 64 years, with most of them (76.6%) between 50 and 79 years old. In terms of educational level, 35.5% completed basic education (8 years), 47.5% completed secondary education (12 years) and 10.6% completed high education (more than 12 years). In addition, 65.3% of the participants are retired or housewives. In terms of income, 95.8% of the participants declare a monthly income less than or equal to US$ 660. Ninety-two point 9 % of the population declares to have at least one chronic non-transmissible disease. Also, 89.4% of the participants report having consumed only drinking water in the last year and 97.9% having access to sewerage.
The overall prevalence of parasites was 27.7% (CI: 20.9–35.6) (Figure 2A). Sixty-one point 5 % of positive patients had 1 parasitic agent, 33.3% had two parasitic agents, and 5.1% had 3 or more (Figure 2B). Among the positive patients, 61.5% correspond to women. The age distribution of patients with parasites shows that the majority correspond to patients over 60 years of age (Figure 3).
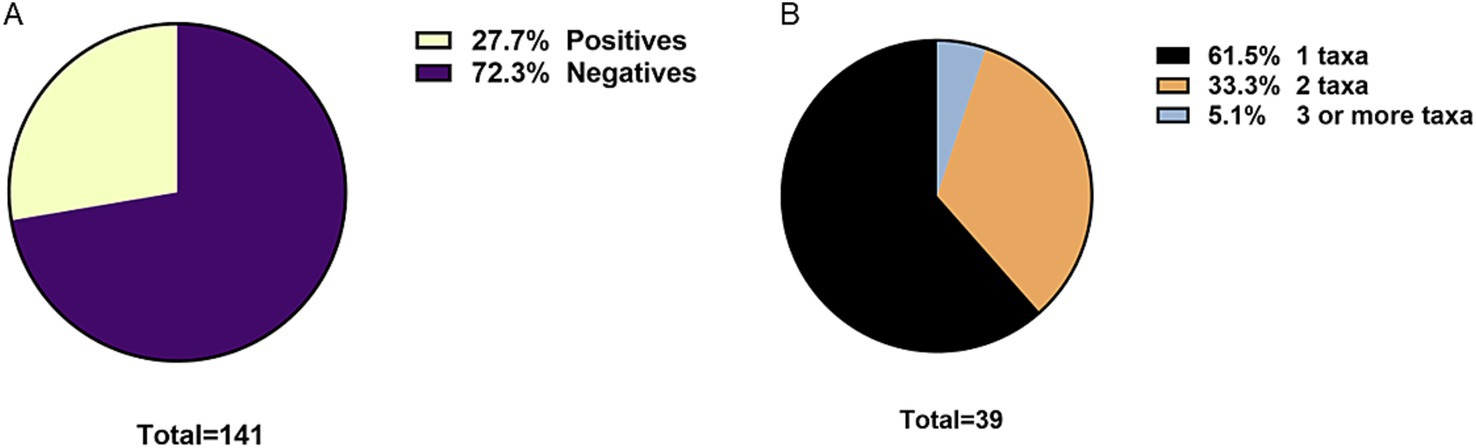
Figure 2. (A) Overall prevalence of intestinal parasites in humans. (B) Frequency of mono or polyparasitism in humans.
In terms of prevalence by species or taxa, the most prevalent parasites were Blastocystis sp. with 22% (CI: 15.9–29.5), followed by Ent. coli with 11.3% (CI: 7.1–17.6) and Endolimax nana with 3.5% (CI: 1.5–8.0) (Figure 4A). As previously mentioned, in our study almost 40% of polyparasitism was observed (Figure 2B). Thus, Figure 4B described in detail the frequency of observation of each parasite related to other parasites taxa. Blastocystis sp. is the most frequently described singly (19 times) but also accompanied by Ent. coli (10 times).
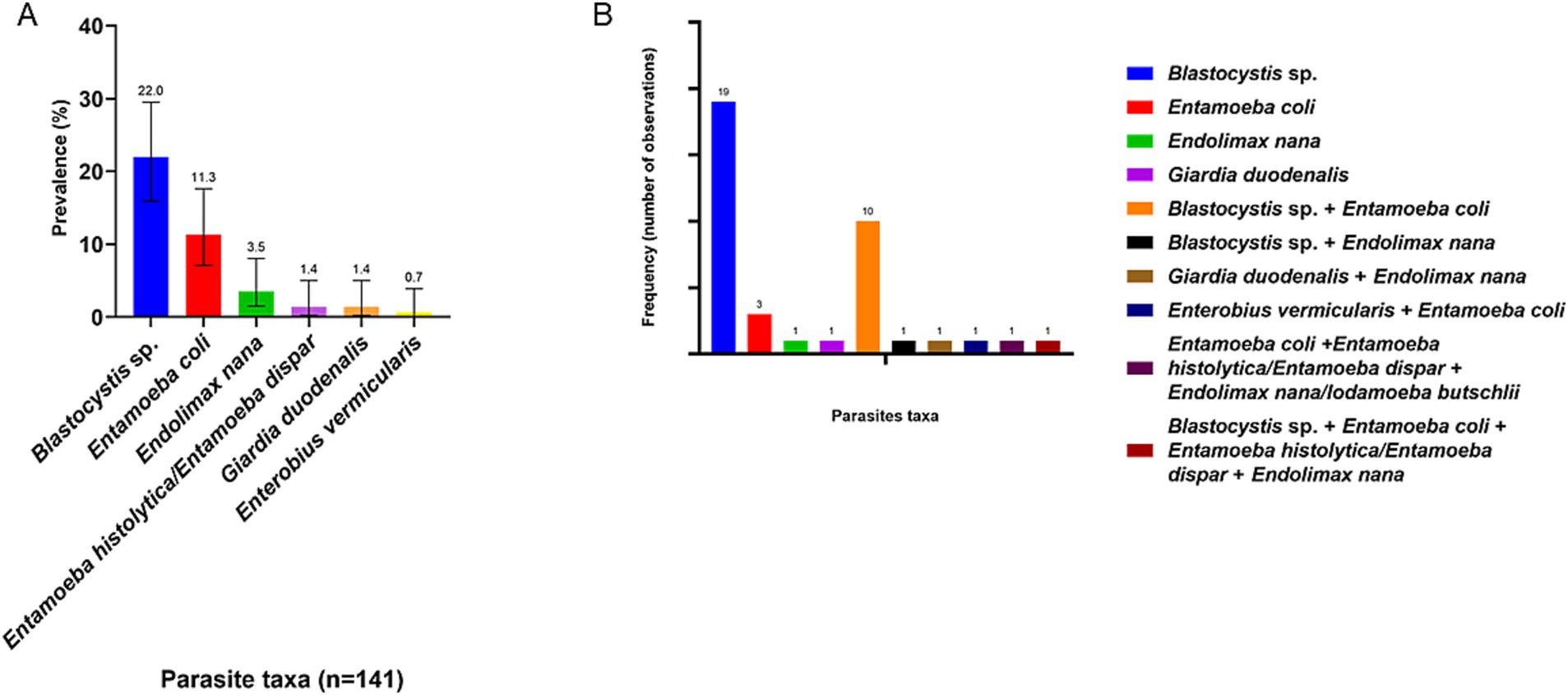
Figure 4. (A) Prevalence of parasite taxa in human population. The number indicated above the bars refers to the prevalence of each parasite. (B) Frequency of observation of each parasite taxa, whether singly or in association with other parasites.
Comparing each of the components of the socioeconomic survey with the presence of parasites, it was found that living with pets is a statistically significant factor (χ2 = 4.9, p = 0.03). Thus, having pets increases the risk of harboring parasites almost threefold (OR: 2.9, [1.1–7.5]). No statistically significant differences were observed in any of the other socioeconomic factors studied (p > 0.05).
A total of 157 human serum samples were analyzed. The average age of the participants was 61 years, with a maximum of 89 years and a minimum of 4 years. Among them, 110 were women and 47 were men.
There was a global prevalence of 33% of anti-Toxocara canis IgG. Thus, 52 participants were positive, 101 negative and four undetermined. The undetermined samples were sent to the Institute of Public Health of Chile, and due to the detection limitations of the ELISA technique used, the result remained indeterminate.
Figure 5 shows the age distribution of the participants who were reactive to the ELISA, with the range between 51 and 80 years showing the highest prevalence with 85% of the cases.
A statistically significant difference was found between the prevalence of anti-Toxocara canis IgG and the educational level of the participants (p = 0.03), with a lower risk of being serologically reactive at a higher educational level (OR: 0.5; CI: 0.2–0.9).
No statistically significant differences were observed based on gender, income, chronic disease status, outdoor activities, symptoms, living with minors, pet ownership, or having a garden (p > 0.05). Similarly, there were no significant associations between anti-Toxocara canis IgG prevalence and prior parasite history, treatment, or living with treated individuals (p = 0.83), nor with dog ownership (p = 0.99) or enteroparasite presence (p = 0.65).
The samples analyzed by NGS correspond to samples positives by microscopy to G. duodenalis or Blastocystis sp. In 8 of the 10 samples, there was amplification, and the resulting sequences were compared with database built based on the sequences available in NCBI. For both parasites only one subtype was identified in each sample.
Three samples were sequenced for G. duodenalis, in two of them the A2 assembly was identified (~140,000 reads each). In the third, G. muris was identified, a parasite usually described in rodents, but with a very low number of reads (9,364 reads). However, due to the technique used in both samples identified as G. duodenalis assembly A2, a marginal number of reads corresponding to G. duodenalis assemblies D and E was also observed (~130 reads each), both corresponding to less than 0.1% of the total reads (Figure 6A).
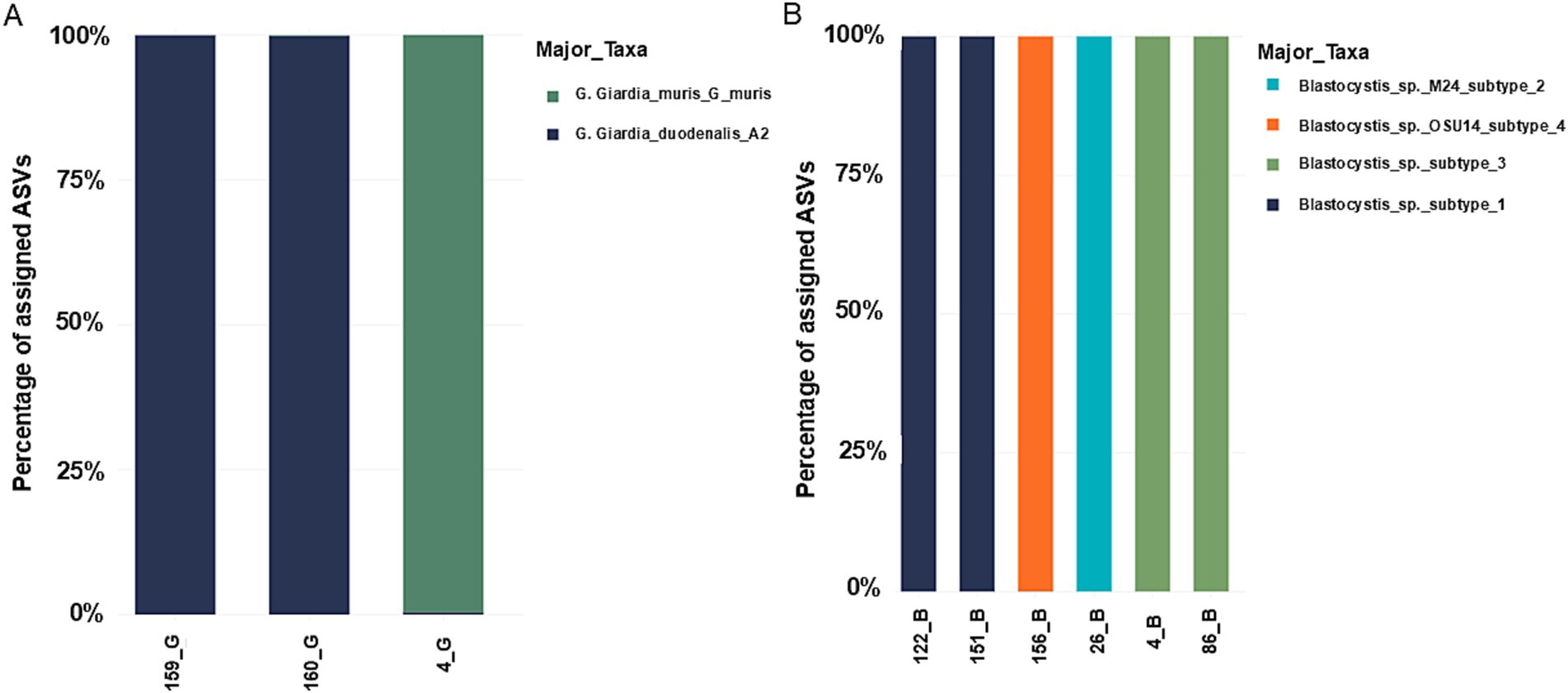
Figure 6. Taxonomic mapping using the database built based on the sequences available in NCBI. (A) Giardia duodenalis. (B) Blastocystis sp. Note that in the case of Giardia duodenalis, due to the small number of readings of subtypes D and E, they are not observable in the graph.
For Blastocystis sp. six samples were sequenced with a total of 800,087 reads. Subtype identification was as follows: two samples with subtype 1; two samples for subtype 1, one sample with subtype 2, two samples with subtypes 3 and one sample with subtype 4 (Figure 6B).
A total of 8 soil samples were collected, 4 in spring and 4 in autumn. Of these, 7 contained at least 1 parasitic element. They showed a diversity of parasitic elements composed exclusively of nematode eggs. In spring, eggs of Trichuris vulpis, Toxocara sp. and Toxascaris leonina were found. While, in autumn, eggs of T. vulpis, Toxocara sp. and Uncinaria stenocephala were observed (Figure 7).
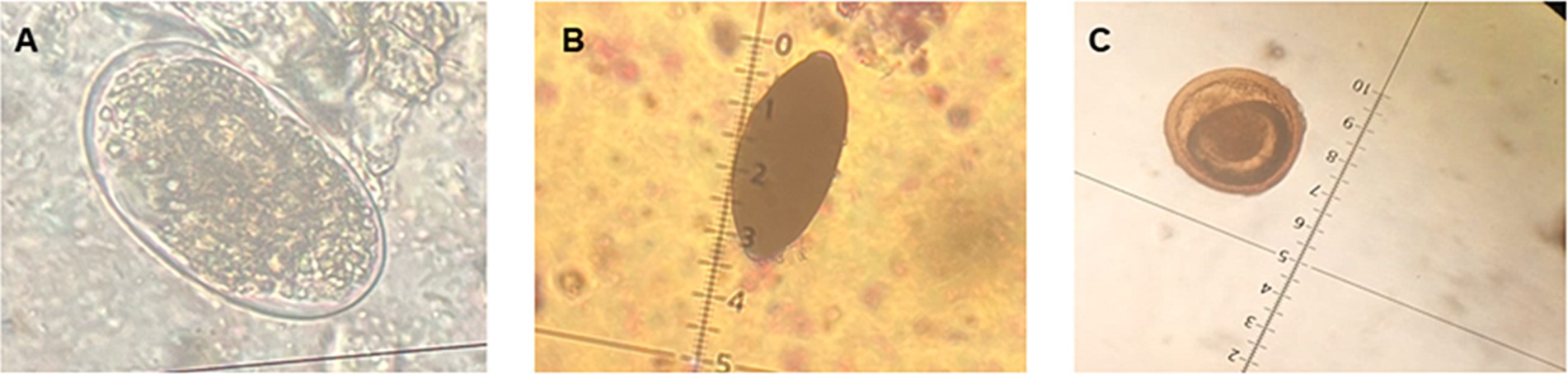
Figure 7. Parasites observed in soil samples. (A) Egg of Uncinaria stenocephala. (B) Egg of Trichuris vulpis. (C) Egg of Toxocara sp. (40x).
The results of the physicochemical analysis of soil samples indicate that parks A and B maintained similar characteristics in total carbon (TC) (%), soil organic matter (SOM) (%) and humidity (%) across both sampling periods. However, the pH in park A increased from 5.2 in spring to 6.3 in autumn. In contrast, significant changes were observed in park C, especially in C1, where TC (%) increased from 4.3% in spring to 8.2% in autumn, and SOM (%) increased from 7 to 14% during the same period.
A total of 180 samples were collected, of which 44.4% (CI: 37.4–51.7) showed the presence of parasites. The seasonal variations show that in spring there were 48.4% and in autumn 40% positive samples. The percentage of positive samples analyzed by sector and per season varied from 16.7% (sector B in autumn) to 79.2% (sector A autumn). In spring no statistically significant difference was observed by the chi-square test (χ2) between the frequency of observation of parasitic elements in dog feces and the sector in which the samples were collected (p > 0.05). In autumn a statistically significant difference was observed using chi-square (χ2) between the frequency of observation of parasitic elements in dog feces and the sector in which the samples were collected. (A vs. B p < 0.05, χ2 = 18.8 and A vs. C p < 0.05, χ2 = 14.2). Additionally, it was observed using the Odds Ratio (OR) that in sector A there was 19 times more probability of finding a positive dog stool sample compared to sector B (OR: 19; CI: 4.4–81.6) and 8.9 times more than in sector C (OR: 8.9; CI: 2.7–30.2). Finally, by performing a chi-square test (χ2), it was observed that only in sector A there is a statistically significant difference between the season of sampling (spring or autumn) and the percentage of positive samples (p < 0.05, χ2 = 4.0). This shows that the spatial distribution of parasites is not homogeneous within the study area.
The parasite most present in these samples was T. vulpis with 27.2% (CI: 21.2–34.1) of positive samples, followed by U. stenocephala with 15.6% (CI: 11.0–21.6). It is important to point out, due to their zoonotic importance and the clinical presentation in humans, the presence of Toxocara sp. in 4.4% (CI: 2.3–8.5) and G. duodenalis in 1.7% (CI: 0.5–4.8) of the collected samples. Most of the parasitic elements were observed in both spring and autumn, except for T. leonina and Capillariidae gen. sp. which were only observed in spring (Figure 8).
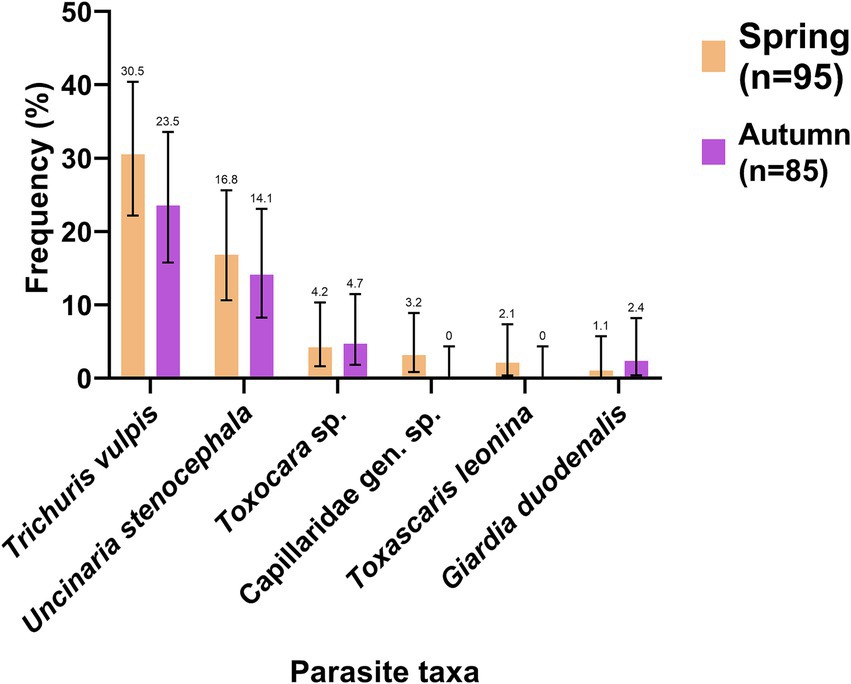
Figure 8. Frequency of observation (%) of parasites in dog feces collected in the environment in spring (orange) and autumn (purple). The number indicated above the bars refers to the frequency of each parasite.
According to the sector, T. vulpis and U. stenocephala were the most frequent species in all sectors (A, B and C). In Sector A, T. vulpis was found in 30.6% (CI: 19.5–44.5) and U. stenocephala in 18.4% (CI: 10.0–31.4) of the samples. In Sector B, T. vulpis was found in 32% (CI: 17.2–51.6) and U. stenocephala in 12% (CI: 4.2–30.0) of the samples. In Sector C, T. vulpis was found in 28.6% (CI: 13.8–50.0) and U. stenocephala in 19% (CI: 7.7–40.0) of the samples. All details about the frequency of parasites found for each sector and period are shown in Supplementary material. When performing the chi-square test (χ2), no statistically significant difference was observed between the total positive samples in spring versus the total positive samples in autumn (χ2 = 1.3; p > 0.05) nor between the sample collection season (spring or autumn) and the frequency of any parasitic species (p > 0.05).
A total of 38 samples were collected from dogs with owners. A prevalence of 25.6% (CI: 14.6–41.1) was described. Four zoonotic species were observed D. caninum (2.6%; CI: 0.1–13.2), G. duodenalis (2.6; CI: 0.1–13.2), T. vulpis (15.4%; CI: 7.2–29.7) and T. leonina (5.1%; CI: 0.9–16.9) (Figure 9).
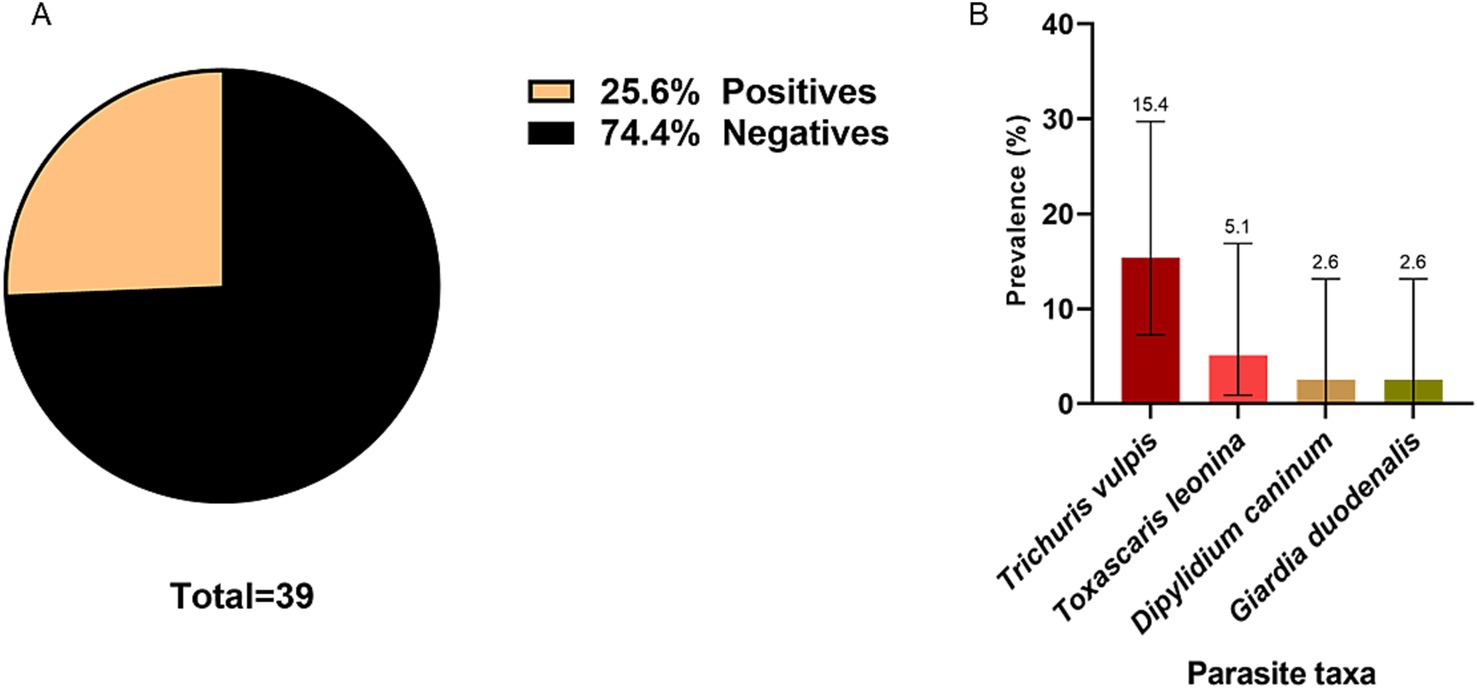
Figure 9. (A) Global prevalence of intestinal parasites in samples collected from owned dogs. (B) Prevalence of parasite species in samples collected from owned dogs. The number indicated above the bars refers to the prevalence of each parasite.
No statistically significant difference was found between the presence of parasites and whether pets go outside (χ2 = 3.3, p = 0.07), nor between the presence of parasites and deworming within the last 6 months (χ2 = 0.3, p = 0.57).
In Chile, recent studies on parasites in the general population are scarce, with the last study in the Los Rios region conducted over 20 years ago. The present study provides an update of the epidemiological data for intestinal parasitosis in humans. Thus, the global prevalence of intestinal parasitism observed in this study was 27.7%, a lower prevalence compared to previous studies in Chile, such as the 1997 study in Santiago, which reported a prevalence of 37.7% (24).
Blastocystis sp. was the most prevalent agent identified (22%), followed by Ent. coli (11.4%). In contrast to our results, a previous study carried out in Valdivia in 1987 reported higher prevalences, with 61.8% of Blastocystis sp. and 30% of Ent. coli (12). While Ent. coli and other non-pathogenic organisms identified directly cause disease, their presence indicates exposure to fecal contamination, often through contaminated food or water with feces. This is relevant despite high levels of potable water and sanitation services in Chile. Compared to other Latin American countries, Chile demonstrate a lower prevalence of intestinal parasites and polyparasitism. A possible explanation for this could be related to the quality of water sanitation (25). To support this claim, a study analyzed the Environmental Performance Index (EPI) in the drinking water category of 180 different countries. The results concluded that Chile is the leader in Latin America in sanitation and drinking water quality (26). However, even if our study demonstrated a lower prevalence of intestinal parasitosis and polyparasitism in Chile compared to past data and neighboring countries, likely reflecting improvements in sanitation and water quality, the persistence of intestinal protozoa like Blastocystis sp. and Ent. coli underscores ongoing exposure to fecal contamination, suggesting the need for continued public health efforts.
The causes of enteroparasitosis are multifactorial, influenced by factors such as basic sanitation, drinking water sources, overcrowding, environmental temperature, among others. These factors can significantly impact the probability of parasitosis and explain the variability in prevalence report across different studies, which are highly dependent on the characteristics of the population studied (27, 28). At the local level, the difference in prevalence described in a study performed in 1987 and the present study may be attributed to contrasting living conditions. The 1987 study focused on a rural population, where many lacked accesses to potable water, sanitary excreta disposal, and regulated garbage collection, conditions that are not present in the urban population of this study. Here, 98% of participants reported having access to sewerage system, 91% use only drinking water over the past year and 100% had garbage collection services (12).These improved conditions suggest that the parasitized patients in our study would likely acquire parasitosis through alternative routes, such as the consumption of poorly washed vegetables or coming into contact with soil contaminated with infective stages (28). Furthermore, the statistical analysis associating the presence of parasites with socioeconomic factors showed that living with pets is a statistically significant factor (χ2 = 4.9, p = 0.03). Participants living with pets were found to be almost three times more likely to have parasites (OR: 2.9, [1.1–7.5]). This highlights zoonotic transmission as the most critical route of infection for intestinal parasites in this urban setting.
In the present study the prevalence of anti-Toxocara canis IgG obtained was 33%. In Chile, a prevalence of 8.8% was previously reported in 1989, 10% in 1998, and 24.4% in 2012 (18, 29, 30). A statistically significant difference was found between the prevalence of anti-Toxocara canis IgG and the level of education of the participants (p = 0.03) and a higher risk of having anti-Toxocara canis antibodies was found with a lower educational level (OR: 0.5; CI: 0.2–0.9). Although education has been associated as a predisposing factor in enteroparasitosis, in toxocariosis its relationship is not clearly established, thus an analysis performed in Pakistan on different human populations, found a statistical association in one group of human and no relationship with others depending mainly with the regular contact of each group with pet animals or livestock, a key factor in the transmission (31). In addition, most serological studies against T. canis have focused on children, as they are considered to be the largest population at risk, so education has not been a fully investigated as a factor.
All the other 17 factors studied in our socioeconomic survey showed no statistically significant difference associated to the prevalence of antibodies anti-Toxocara canis. Between them, no significant difference was described related to the presence of a garden or patio in the home or with pet ownership (p > 0.05). This is consistent with a study conducted in Niebla, Chile, in 2016, which found no statistical association with these variables (18, 32). Studies in Argentina and Venezuela have found statistical significance with dog ownership (33, 34). Since the dog is the definitive host of T. canis, this lack of association may indicate that the origin of infection comes from other sources such as the consumption of water or food contaminated with T. canis eggs, onychophagia, geophagy, etc. (35). In our study, we observed T. canis eggs throughout the sampling period, in urban parks and also in dog feces collected from the environment. These results, associated with the high prevalence of anti-Toxocara canis antibodies in humans, allow us to hypothesize that humans are in contact with the parasite from the environment in their own neighborhood and the high presence of the parasite in urban areas (parks and streets) is a source of human infection and should therefore be considered as an important public health issue for local authorities.
Giardia duodenalis assembly A2 was found in 2 sequenced samples. The A group has zoonotic potential, it is frequently found in companion animals (dogs, cats and horses) and livestock (cattle, sheep, goats, alpacas, pigs, etc.). This group can be divided into subsets, which are A1, A2, and A3. The A2 subset is usually found in human hosts, but has also been found in cats, dogs, horses, and other animals (36–38). Most human infections around the world are caused by the A2 and B genotypes (39). Several studies have reported an association between assembly A and intermittent diarrhea, while other studies have reported a correlation between subset B and severe or persistent diarrhea (40). In this case, the A2 subset found in the samples of our study suggests the possibility of zoonotic transmission involving domestic animals such as cats or dogs.
Regarding the diversity of subtypes in each patient, a single majority subtype was successfully identified in each case. Specifically, one patient had G. duodenalis subtype A2 with 137,999 reads, plus a minimal proportion of reads for other subtypes: 165 reads for subtype D and 101 reads for subtype E of G. duodenalis. In another patient with G. duodenalis subtype A2 (146,476 reads), 87 reads for Giardia muris were detected. These readings of other subtypes are attributed to the high sensitivity of the technique used, which can detect even single nucleotide variations, which may result in residual readings representing less than 0.1% of the total readings. These residual readings are not considered reliable for subtype identification, but artifacts generated by the technique. This situation may be due to sequencing errors, chimera formation during PCR, and/or the technology’s high sensitivity. Several strategies could be implemented to mitigate these artifacts in future analyses, such as increasing sequencing depth, applying stricter thresholds to filter out spurious reads, or improving quality controls during sample processing.
Among the samples analyzed by NGS containing Blastocystis sp., subtype (ST) 1 was found in 2 samples, 1 sample with ST 2, 2 samples with ST 3 and one sample with ST 4, while in 2 samples (20%) there was no amplification due to the low quality of the DNA extracted. The most common subtypes worldwide are ST 1, 2 and 3, while ST 4 is regularly detected in symptomatic patients in Spain and other European countries, but is rare in South American countries, although it has been previously reported in countries such as Colombia and Brazil (41, 42). Although some studies indicate that Blastocystis sp. may be associated with diarrhea, abdominal pain, irritable bowel syndrome, constipation and abdominal distension, it has not been determined whether these symptoms belong to a specific ST, it has also been described that subtypes 1 and 3 of Blastocystis sp. are frequently found in patients with irritable bowel syndrome (IBS). whose prevalence is not well documented in Chile. The 4 subtypes present in this study have also been detected in animals such as dogs, pigs, cows, rodents and others (41). In our study, most of the identified subtypes of Blastocystis sp. and G. duodenalis found in humans are potentially zoonotic parasites, which is consistent with the previously discussed finding that ownership domestic animal increases the risk of having intestinal parasites threefold.
Eggs of T. canis, U. stenocephala, T. vulpis, and T. leonina were found in the surveyed parks; all of which are associated with zoonotic transmission. The contamination of public spaces with parasitic elements is most likely due to the large number of stray dogs observed in the sector. Dogs can excrete up to 600 grams of feces per day on average and cover distances of up to 15 km per day (43), so it is very likely that they have had a significant impact on soil contamination. Similar findings were reported in a study carried out in Temuco (Chile), where eggs of Toxocara sp. and Trichuris sp. were found. However, in contrast to our study, the presence of Taenia sp. eggs was detected (14). This last difference could be related to differing levels of interaction between peri-urban or rural and urban environments (44). In urban settings, the dissemination of cestodes parasitic elements occurs mainly through the feces of carnivorous animals, such as stray dogs (45).
Interestingly, a greater diversity of zoonotic parasitic elements, particularly nematodes, was observed in spring compared to autumn. This is consistent with findings in New Zealand, where soil nematode diversity is higher during the spring season (46). The increased diversity of parasitic nematodes can be attributed to the improved weather conditions, which increases circulation of dogs in public areas, raising the possibility of soil contamination and the potential for parasite transmission (47, 48).
The physicochemical analysis of the soil showed that the pH range was from 5.3 in spring to 6.4 in autumn. The infective stages of geohelminths tolerate soil pH ranges between 4.6 and 9.4 (49). Other studies conducted in Ghana and Egypt have described a significant relationship between soil pH and the number of parasitic stages concluding that the highest number of parasitic elements are found in soils with pH between 5 and 8 (50, 51). Also, in the present study have been described a concentration of total carbon up to 14%, and it has been demonstrated that soils containing a higher amount of total carbon and soil organic matter are associated with a higher number of geohelminth eggs because it affects the porosity of the soil (52), therefore, species such as those belonging to the Ancylostomatidae family are favored in their development due to a soil with greater porosity that allows the infective larva to be kept close to the surface, providing greater oxygenation and facilitating contact with a susceptible host (53). Overall, the results suggest that the parks in our city possess physicochemical properties conducive to the survival and development of various zoonotic parasite species, highlighting the need for targeted public health measures.
In the study of feces of dogs with owners, a total of 39 samples were analyzed, revealing an overall prevalence of parasitosis of 25.6%. Among these, T. vulpis was observed in 15.4% of the samples, while G. duodenalis was found in 2.6%. Similar studies conducted in Chile, specifically in the cities of Talca and Cabrero, reported a predominance of T. canis eggs in Talca, with a frequency of 14%, and eggs from the Ancylostomatidae family in Cabrero, with frequency of 43%. Notably, in both studies T. vulpis ranked second in frequency with percentages ranging from 5 to 12.9% (54, 55). There was no statistically significant difference between parasitosis and dogs going outside their house freely (p > 0.05). Over half of the surveyed pets regularly went outside, contrasting with 27% reported in urban area of the Coquimbo region, Chile, but similar to the 50% reported in rural areas in the same region (56). The absence of a statistically significant difference between parasitosis and factors such as deworming or outdoor activities habits may be attributing to the limitations of this research. A limitation of the study could be the selection bias that may have occurred during the selection of the participants by the pet owners. Furthermore, the small number of samples analyzed (n = 39) could reduce the strength of the statistical analysis (57).
The frequency of one or more parasitic elements in these samples was 48.4% in spring and 40% in autumn, with no statistically significant differences between the two periods (p > 0.05). These frequencies were higher than those reported in a similar study conducted in Santiago de Chile, where 31.7% of the samples contained parasitic elements (58).
In our study the parasitic species did not vary significantly between the two sampling periods, with T. vulpis and U. stenocephala being the most frequently observed species, ranging from 23.5 to 30% and from 14.1 to 16.8%, respectively. In a study carried out in urban areas of Niebla and Corral, it was also found that these two species were the most common, with a difference in the frequency of observation, since in this study the percentages for T. vulpis ranged from 50.7 to 54.8%, while for U. stenocephala were between 65.7 and 83.4% (15). Additionally, our study detected Toxascaris leonina during the spring at a frequency of 2.1%, consistent with previous studies in the Los Ríos region, which reported frequencies of 3.3–8.0% (59, 60). It has also been documented in other areas in the country, such as Los Angeles (Chile), where it was detected in 1.33% of the samples (13), and in Santiago (Chile), where it was observed in 7.7% of the samples (58, 61). The frequency of protozoan parasites with zoonotic potential where only the presence of G. duodenalis cysts was found in 1.1% in spring and 2.4% in autumn. In various studies carried out in Italy, Germany, Poland, Colombia and Mexico, it has been seen that the prevalence of these parasite in dog feces ranges from 0 to 25% (61–66). In general, the presence of protozoan parasites such as G. duodenalis in dog feces is underestimated (66–68). This could be attributed to several factors, as the intermittent elimination of infective stages of parasites can lead to false negatives when observing an isolated sample microscopically (61, 65).
In summary, our study identified a 28% prevalence of intestinal parasites and a 33% prevalence of anti-T. canis antibodies in humans; also, a 26% prevalence in owned dogs and 44% frequency of parasites in dog stools collected from the street during spring and fall. Additionally, up to 30.5% (spring and fall) of soil samples from parks in the study area was found to contain parasitic elements. Thus, using a OneHealth approach, our study highlights that the prevalence of parasites in humans is influenced non only by human-to-human transmission, but also and significantly, by zoonotic and environmental transmission. The most striking example of this is the high presence prevalence of anti-T. canis antibodies in humans (over 30%) alongside the detection of Toxocara sp. eggs in both parks soil and environmental dog feces. The absence of T. canis in the feces of owned dogs suggests environmental transmission as a primary pathway. In addition, zoonotic subtypes of G. duodenalis (A2 assembly) and Blastocystis sp. subtypes 1,2,3 and 4 were detected in humans, which have also been reported in dogs and cats, reinforcing the role of pets in parasite transmission.
This study highlights the importance of adopting a OneHealth approach to parasitology research. The complex ecology of parasitic organisms demands an integrated perspective to fully understand their transmission pathways and develop effective control strategies. By emphasizing the interconnectedness of human, animal, and environmental health, we aim to contribute to the management and mitigation of this persistent public health issue.
The data presented in the study are deposited in NCBI repository, accession number PRJNA1231441, https://www.ncbi.nlm.nih.gov/bioproject/1231441.
The studies involving humans were approved by the Research Ethics Committee Los Ríos Health Service (Ord. N°354 14.10.2021). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
DS: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. OC: Visualization, Writing – review & editing. CC: Visualization, Writing – review & editing. DC: Formal analysis, Investigation, Writing – review & editing. PC: Formal analysis, Investigation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Agencia Nacional de Investigación y Desarrollo (ANID), Chile, Fondecyt 11220065: Data collection, laboratory analysis, results interpretation. Vicerrectoria de Investigación, Desarrollo y Creación Artistica (VIDCA) Universidad Austral de Chile: Data collection, patient recruitement, laboratory analysis.
Acknowledgement to the Community Health Centre (CECOSF) of Mulato for allowing us to work in their facilities and for their collaboration in promoting this study in the community of the sector.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1536861/full#supplementary-material
1. Torgerson, PR, Devleesschauwer, B, Praet, N, Speybroeck, N, Willingham, AL, Kasuga, F, et al. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. (2015) 12:e1001920. doi: 10.1371/journal.pmed.1001920
2. Maurice, AP, Jenkin, A, Norton, RE, Hamilton, A, and Ho, YH. Epidemiology of parasitic diseases In: G Tsoulfas, JJ Hoballah, GC Velmahos, and YH Ho, editors. The surgical management of parasitic diseases. Cham: Springer International Publishing (2020). 3–21.
3. Macpherson, CNL . The epidemiology and public health importance of toxocariasis: a zoonosis of global importance. Int J Parasitol. (2013) 43:999–1008. doi: 10.1016/j.ijpara.2013.07.004
4. Short, EE, Caminade, C, and Thomas, BN. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect Dis Res Treat. (2017) 10:117863361773229. doi: 10.1177/1178633617732296
5. Theel, ES, and Pritt, BS. Parasites. Diagnostic microbiology of the immunocompromised host. Microbiol Spectr. (2016) 4:411–66. Available from:. doi: 10.1128/microbiolspec.dmih2-0013-2015
6. Fletcher, SM, Stark, D, Harkness, J, and Ellis, J. Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev. (2012) 25:420–49. doi: 10.1128/CMR.05038-11
7. Ahmed, M . Intestinal parasitic infections in 2023. Gastroenterol Res. (2023) 16:127–40. doi: 10.14740/gr1622
8. Alum, A, Rubino, JR, and Ijaz, MK. The global war against intestinal parasites—should we use a holistic approach? Int J Infect Dis. (2010) 14:e732–8. doi: 10.1016/j.ijid.2009.11.036
9. Soriano, S, Manacorda, A, Pierangeli, N, Navarro, M, Giayetto, A, Barbieri, L, et al. Parasitosis intestinales y su relacion con factores socioeconómicos y condiciones de habitat en niños de Neuquén, Patagonia, Argentina. Rev. Ibero Latinoam Parasitol. (2005) 60:154–61. doi: 10.4067/S0717-77122005000200009
10. Barra, M, Bustos, L, and Ossa, X. Inequality in the prevalence of intestinal parasitic infections among schoolchildren from urban and rural schools. Rev Med Chile. (2016) 144:886–93. doi: 10.4067/s0034-98872016000700009
11. Vidal, S, Toloza, L, and Cancino, B. Evolution of the prevalence the enteroparasitoses in Talca-Chile. Rev Chil Infect. (2010) 27:336–40. doi: 10.4067/S0716-10182010000500009
12. Torres, P, Miranda, JC, Flores, L, Riquelme, J, Franjola, R, Perez, J, et al. Blastocistosis y otras infecciones por protozoos intestinales en comunidades humanas ribereñas de la cuenca del rio Valdivia, Chile. Rev Inst Med Trop Sao Paulo. (1992) 34:557–64. doi: 10.1590/S0036-46651992000600010
13. Luzio, A, Espejo, S, Troncoso, I, Fernández, I, and Fischer, C. Determinación coproscópica de formas parasitarias en heces de "Canis lupus familiaris" diseminadas en playas de la comuna de Tomé, Región del Bío Bío. Chile Rev Ibero Latinoam Parasitol. (2013) 72:88–94. Available at: https://dialnet.unirioja.es/servlet/articulo?codigo=5969420 ISSN 0718-8730
14. Armstrong, W, Oberg, C, and Orellana, J. Presencia de huevos de parásitos con potencial zoonótico en parques y plazas públicas de la ciudad de Temuco, Región de La Araucanía, Chile. Arch Med Vet. (2011) 43:127–34. doi: 10.4067/S0301-732X2011000200005
15. Subiabre, Á, and Torres, P. Eukaryotic parasites in feces of dogs collected in the streets of the urban zone of the coastal localities of corral and Niebla in the south of Chile. Rev Invest Vet Peru. (2022) 33:e20772. doi: 10.15381/rivep.v33i1.20772
16. Bowman, DD . History of Toxocara and the associated larva migrans. Adv. Parasitol. (2020) 109:17–38. doi: 10.1016/bs.apar.2020.01.037
17. Schoenardie, ER, Scaini, CJ, Brod, CS, Pepe, MS, Villela, MM, McBride, AJA, et al. Seroprevalence of Toxocara infection in children from southern Brazil. J Parasitol. (2013) 99:537–9. doi: 10.1645/GE-3182
18. Vargas, C, Torres, P, Jercic, MI, Lobos, M, Oyarce, A, Miranda, JC, et al. Frequency of anti- Toxocara spp. antibodies in individuals attended by the Centro de Salud Familiar and environmental contamination with Toxocara canis eggs in dog feces, in the coastal Niebla town, Chile. Rev Inst Med Trop Sao Paulo (2016);58:62, doi: 10.1590/S1678-9946201658062, PMCID: PMC5048633.
19. Sievers, G, Amenábar, A, and Gádicke, P. Comparación de cuatro sistemas de muestreo de tierra para determinar contaminación de áreas con huevos de Toxocara canis. Parasitol Latinoam. (2007) 62:67–71. doi: 10.4067/S0717-77122007000100011
20. Prata, JC, Ribeiro, AI, and Rocha-Santos, T. An introduction to the concept of One Health In: JC Prata, AI Ribeiro, and T Rocha-Santos, editors. One Health : Elsevier (2022). 1–31. Available at: https://www.sciencedirect.com/science/article/pii/B9780128227947000046 (Accessed October 10, 2024).
21. Torres, P, and Navarrete, N. Comparación entre los métodos del fijador PAFS y del Telemann modificado en el diagnóstico de protozoos intestinales del hombre. Bol Chil Parasitol. (1972) 27:90–5.
22. Mizgajska-Wiktor, H . Recommended method for recovery of Toxocara and other geohelminth eggs from soil. Wiad Parazytol. (2005) 51:21–2. Available at: https://pubmed.ncbi.nlm.nih.gov/16841685/
23. Sievers, G, Concha, C, and Gadicke, P. Prueba de una técnica para recuperar huevos de Toxocara canis de muestras de tierra. Parasitol Latinoam. (2007) 62:61–6. doi: 10.4067/S0717-77122007000100010
24. Chen, S, Codoceo, A, Carrasco, O, and Torres, M. Enteroparasitosis en la poblacion de la tercera edad consultante en centros medicos de la Pontificia Universidad Catolica de Chile, 1997. Parasitología al día. (1998) 22:114–116.
25. Bourli, P, Eslahi, AV, Tzoraki, O, and Karanis, P. Waterborne transmission of protozoan parasites: a review of worldwide outbreaks–an update 2017–2022. J Water Health. (2023) 21:1421–47. doi: 10.2166/wh.2023.094
26. Block, S, Emerson, JW, Esty, DC, de Sherbinin, A, Wendling, ZA, et al. Environmental performance index. New Haven, CT: Yale Center for Environmental Law & Policy. (2024). Available at: https://epi.yale.edu/
27. Soriano, SV, Manacorda, AM, Pierangeli, NB, Navarro, MC, Giayetto, AL, Barbieri, LM, et al. Intestinal parasitosis in relation to socioeconomic factors and habitat conditions in children of Neuquén, Patagonia, Argentina. Parasitol Latinoam. (2005) 60:154–61. Available at: https://scielo.cl/pdf/parasitol/v60n3-4/art09.pdf
28. Rodríguez-Sáenz, AY . Factores de riesgo para parasitismo intestinal en niños escolarizados de una institución educativa del municipio de Soracá-Boyacá. Univ Salud. (2015) 17:112–20. Available at: https://www.scielo.org.co/scielo.php?pid=S0124-71072015000100010&script=sci_abstract&tlng=es
30. Navarrete, N, and Rojas, E. Toxocarosis seroprevalence in blood donors. Arch Med Vet. (1998) 30:153–6.
31. Said, A, Khattak, I, Abbas, RZ, Khan, MK, Saleemi, MK, Budke, CM, et al. Toxocara canis seropositivity in different exposure groups in the Khyber Pakhtunkhwa province of Northwest Pakistan. Parasitol Res. (2023) 122:1159–66. doi: 10.1007/s00436-023-07816-4
32. Roldán, WH, Espinoza, YA, Atúncar, A, Ortega, E, Martinez, A, and Saravia, M. Frequency of eosinophilia and risk factors and their association with Toxocara infection in schoolchildren during a health survey in the north of Lima, Peru. Rev Inst Med Trop Sao Paulo. (2008) 50:273–8. doi: 10.1590/S0036-46652008000500005
33. Chiodo, P, Basualdo, J, Ciarmela, L, Pezzani, B, Apezteguía, M, and Minvielle, M. Related factors to human toxocariasis in a rural community of Argentina. Mem Inst Oswaldo Cruz. (2006) 101:397–400. doi: 10.1590/S0074-02762006000400009
34. Gallardo, J, Forlano, M, and Ontiveros, Y. Presencia de huevos de Toxocara spp. en el suelo de patios de casas y heces de perros mascotas de la ciudad de Barquisimeto, estado Lara, Venezuela. Gaceta Cienc Vet. (2018) 23:19–23. Available at: https://revistas.uclave.org/index.php/gcv/article/view/1861
35. Alderete, JMS, Jacob, CMA, Pastorino, AC, Elefant, GR, Castro, APM, Fomin, ABF, et al. Prevalence of Toxocara infection in schoolchildren from the Butanta region, Sao Paulo, Brazil. Mem Inst Oswaldo Cruz Rio de Janeiro. (2003) 98:593–7. doi: 10.1590/S0074-02762003000500002
36. Ryan, U, and Cacciò, SM. Zoonotic potential of Giardia. Int J Parasitol. (2013) 43:943–56. doi: 10.1016/j.ijpara.2013.06.001
37. Yaoyu, F, and Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. (2011) 24:110–40. doi: 10.1128/CMR.00033-10
38. Krumrie, S, Capewell, P, McDonald, M, Dunbar, D, Panarese, R, Katzer, F, et al. Molecular characterisation of Giardia duodenalis from human and companion animal sources in the United Kingdom using an improved triosephosphate isomerase molecular marker. Curr Res Parasitol Vect. (2022) 2:100105. doi: 10.1016/j.crpvbd.2022.100105
39. Adam, RD, Dahlstrom, EW, Martens, CA, Bruno, DP, Barbian, KD, Ricklefs, SM, et al. Genome sequencing of Giardia lamblia genotypes A2 and B isolates (DH and GS) and comparative analysis with the genomes of genotypes A1 and E (WB and pig). Genome Biol Evol. (2013) 5:2498–511. doi: 10.1093/gbe/evt197
40. Read, C, Walters, J, Robertson, ID, and Thompson, RCA. Correlation between genotype of Giardia duodenalis and diarrhoea. Int J Parasitol. (2002) 32:229–31. doi: 10.1016/S0020-7519(01)00340-X
41. del Coco, VF, Molina, NB, Basualdo, JA, and Córdoba, MA. Blastocystis spp.: avances, controversias y desafíos futuros. Rev Argent Microbiol. (2017) 49:110–8. doi: 10.1016/j.ram.2016.08.004
42. Domínguez-Márquez, MV, Guna, R, Muñoz, C, Gómez-Muñoz, MT, and Borrás, R. High prevalence of subtype 4 among isolates of Blastocystis hominis from symptomatic patients of a health district of Valencia (Spain). Parasitol Res. (2009) 105:949–55. doi: 10.1007/s00436-009-1485-y
43. Jiménez, P, Muñoz, M, and Ramírez, JD. An update on the distribution of Blastocystis subtypes in the Americas. Heliyon. (2022) 8:e12592. doi: 10.1016/j.heliyon.2022.e12592
44. Craig, PS, Hegglin, D, Lightowlers, MW, Torgerson, PR, and Wang, Q. Echinococcosis In: RCA Thompson, P Deplazes, and AJ Lymbery, editors. Echinococcus and echinococcosis, part B : Elsevier (2017). 55–158. Available at: https://www.sciencedirect.com/science/article/pii/S0065308X16300859 (Accessed October 10, 2024).
45. Alvarez Rojas, CA, Mathis, A, and Deplazes, P. Assessing the contamination of food and the environment with Taenia and Echinococcus eggs and their zoonotic transmission. Curr Clin Microbiol Rep. (2018) 5:154–63. doi: 10.1007/s40588-018-0091-0
46. Yeates, GW . Variation in soil nematode diversity under pasture with soil and year. Soil Biol Biochem. (1984) 16:95–102. doi: 10.1016/0038-0717(84)90098-1
47. Shimizu, T . Prevalence of Toxocara eggs in sandpits in Tokushima City and its outskirts. J Vet Med Sci. (1993) 55:807–11. doi: 10.1292/jvms.55.807
48. Avcioglu, H, and Burgu, A. Seasonal prevalence of Toxocara ova in soil samples from public parks in Ankara, Turkey. Vect Borne Zoonot Dis. (2008) 8:345–50. doi: 10.1089/vbz.2007.0212
49. Hassan, AA, and Oyebamiji, DA. Intensity of soil transmitted helminths in relation to soil profile in selected public schools in Ibadan metropolis. J Epidemiol Infect Dis. (2018) 1:73–7. doi: 10.15406/jeid.2018.01.00011
50. Chammartin, F, Scholte, RGC, Guimarães, LH, Tanner, M, Utzinger, J, and Vounatsou, P. Soil-transmitted helminth infection in South America: a systematic review and geostatistical meta-analysis. Lancet Infect Dis. (2013) 13:507–18. doi: 10.1016/S1473-3099(13)70071-9
51. Etewa, SE, Abdel-Rahman, SA, Abd El-Aal, NF, Fathy, GM, El-Shafey, MA, and Ewis, AMG. Geohelminths distribution as affected by soil properties, physicochemical factors and climate in Sharkyia governorate Egypt. J Parasit Dis. (2016) 40:496–504. doi: 10.1007/s12639-014-0532-5
52. Christensen, BT . Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur J Soil Sci. (2001) 52:345–53. doi: 10.1046/j.1365-2389.2001.00417.x
53. Brooker, S, and Michael, E. The potential of geographical information systems and remote sensing in the epidemiology and control of human helminth infections. Adv Parasitol. (2000) 47:245–88. doi: 10.1016/S0065-308X(00)47011-9
54. Quilodrán-González, D, Gädicke, P, Junod, T, Villaguala-Pacheco, C, and Landaeta-Aqueveque, C. Factores de riesgo asociados con parásitos gastrointestinales zoonóticos en perros de cabrero, región del Biobío, Chile. Chilean J Agric Anim Sci Ex Agro Cienc. (2018) 34:118–25. doi: 10.4067/S0719-38902018005000401
55. Muñoz-Caro, T, Sáez, D, and Aravena, C. Determination of intestinal parasites in owned dogs from the city of Talca, Chile, and its association to epidemiological variables. Rev Invest Vet Peru. (2023) 34:e23590. doi: 10.15381/rivep.v34i2.23590
56. Acosta-Jamett, G, Cleaveland, S, and Cunningham, AABronsvoort BM De C. Demography of domestic dogs in rural and urban areas of the Coquimbo region of Chile and implications for disease transmission. Prev Vet Med. (2010) 94:272–81. doi: 10.1016/j.prevetmed.2010.01.002
57. García, J . La estadística robusta: nuevos caminos en la investigación Universidad de Salamanca. Universidad de Salamanca, Salamanca, España (2015).
58. Alegría-Morán, R, Pastenes, Á, Cabrera, G, Fredes, F, and Ramírez-Toloza, G. Urban public squares as potential hotspots of dog-human contact: a spatial analysis of zoonotic parasites detection in gran Santiago, Chile. Vet Parasitol Reg Stud Rep. Elsevier (2021) 24:100579. doi: 10.1016/j.vprsr.2021.100579
59. Torres, P, Ramos, M, Carrasco, L, Neumann, M, Franjola, R, and Navarrete, N. Protozoos, helmintos y artrópodos parásitos del perro doméstico en la ciudad de Valdivia, Chile. Bol Chil Parasitol. (1974) 29:18–23.
60. Oberg, C, Franjola, R, and Leyán, V. Helmitos del perro domestico (Canis familiaris) en la ciudad de Valdivia, Chile. Bol Chil Parasitol. (1979) 34:47–9.
61. Becker, AC, Rohen, M, Epe, C, and Schnieder, T. Prevalence of endoparasites in stray and fostered dogs and cats in northern Germany. Parasitol Res. (2012) 111:849–57. doi: 10.1007/s00436-012-2909-7
62. Solarte Paredes, LD, and Castañeda Salazar, R, AP Pulido Villamarín . Parásitos gastrointestinales en perros callejeros del centro de zoonosis de Bogotá DC, Colombia. Neotrop Helminthol (2013);7:83–93. doi: 10.24039/rnh201371951
63. Paoletti, B, Traversa, D, Iorio, R, De Berardinis, A, Bartolini, R, Salini, R, et al. Zoonotic parasites in feces and fur of stray and private dogs from Italy. Parasitol Res. (2015) 114:2135–41. doi: 10.1007/s00436-015-4402-6
64. Szwabe, K, and Błaszkowska, J. Stray dogs and cats as potential sources of soil contamination with zoonotic parasites. Ann Agric Environ Med. (2017) 24:39–43. doi: 10.5604/12321966.1234003
65. De Liberato, C, Berrilli, F, Odorizi, L, Scarcella, R, Barni, M, Amoruso, C, et al. Parasites in stray dogs from Italy: prevalence, risk factors and management concerns. Acta Parasitol. (2018) 63:27–32. doi: 10.1515/ap-2018-0003
66. Godínez-Galaz, EM, Veyna-Salazar, NP, Olvera-Ramírez, AM, Milián-Suazo, F, Perea-Razo, CA, Bernal-Reynaga, R, et al. Prevalence and zoonotic potential of giardia intestinalis in dogs of the central region of Mexico. Animals. (2019) 9:325. doi: 10.3390/ani9060325
67. Capelli, G, Frangipane di Regalbono, A, Iorio, R, Pietrobelli, M, Paoletti, B, and Giangaspero, A. Giardia species and other intestinal parasites in dogs in north-east and Central Italy. Vet Rec. (2006) 159:422–4. doi: 10.1136/vr.159.13.422
Keywords: parasites, zoonosis, public health, One Health, Chile, pathogens
Citation: Sanhueza Teneo D, Cerna O, Chesnais CB, Cárdenas D and Camus P (2025) High parasite prevalence driven by the human-animal-environment interface: a One Health study in an urban area in southern of Chile. Front. Vet. Sci. 12:1536861. doi: 10.3389/fvets.2025.1536861
Received: 29 November 2024; Accepted: 12 February 2025;
Published: 21 March 2025.
Edited by:
Kate Worthing, The University of Sydney, AustraliaReviewed by:
Laura Tomassone, University of Turin, ItalyCopyright © 2025 Sanhueza Teneo, Cerna, Chesnais, Cárdenas and Camus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Sanhueza Teneo, ZGFuaWVsLnNhbmh1ZXphMDFAdWFjaC5jbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.