
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Vet. Sci. , 05 February 2025
Sec. Oncology in Veterinary Medicine
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1535432
This article is part of the Research Topic Cancer in Domestic, Exotic and Wild Animals: New Horizons in Tumorigenesis, Diagnosis, Prognosis and Therapeutics through Comparative Oncology View all 11 articles
Neuroendocrine tumors of the nasal cavity are rare in both animals and humans. This report describes the macroscopic, histopathological and immunohistochemical characteristics of a neuroendocrine tumor in a three-year-old female roe deer (Capreolus capreolus) that was shot due to a facial deformity caused by an oval, firm, exophytic lesion effacing the left frontal and parietal regions. Longitudinal sectioning of the skull revealed a nasal cavity tumor that had invaded the cribriform plate, the rostral bones of the skull, the rostral aspect of the cranial cavity and the frontal sinuses and extended through the lacrimal, sphenoid and zygomatic bones into the subcutaneous tissue. Histopathologically, the tumor consisted of neoplastic cells forming sheets, nests, trabecular and cribriform structures separated by a delicate fibrovascular stroma. Mitoses were rare. Based on the histopathological and immunohistochemical findings, a neuroendocrine carcinoma was diagnosed. Based on thorough database searches, this is the first known case of a nasal neuroendocrine carcinoma in a roe deer.
Tumors in the nasal cavity or paranasal sinuses (i.e., sinonasal tumors) are uncommon in animals. Among the epithelial tumors, adenocarcinomas predominate, whereas chondrosarcoma is the most common malignant mesenchymal tumor (1). Sinonasal tumors with neuroendocrine differentiation, such as neuroendocrine carcinomas (NECs) and olfactory neuroblastomas (ONBs) (formerly known as esthesioneuroblastoma) have been occasionally reported in animals. Namely, NECs have been reported in dogs (Canis lupus familiaris) (2), horses (Equus ferus caballus) (3) and free-living Japanese raccoon dog (Nyctereutes procyonoides viverrinus) (4), whereas ONBs have been described in cats (Felis catus) and dogs (2, 5–10), horse (11), cattle (Bos taurus) (12), axolotl (Ambystoma mexicanum) (13, 14) and goldfish (Carassius auratus) (15).
During passive disease surveillance of roe deer in Switzerland (16), Slovenia (17), Sweden (18) and in the Netherlands (19), tumors were detected in 32, 19, 19 and four cases, respectively. The most frequently diagnosed tumor in Slovenia (17) was a fibropapilloma, in Switzerland and Sweden a lymphoma (16, 18), while reports from other researchers suggest that liver neoplasms are the most common tumors in roe deer (20, 21). Other neoplasms of various origins have been described in several case reports, such as pulmonary adenocarcinoma (22), mandibular ossifying fibroma and oral papillomas (23), leukemia (24), neuroblastoma (25), oral squamous cell carcinoma (26), cutaneous teratoma (27), suggesting that this species is particularly prone to developing neoplastic diseases (16).
This report describes the macroscopic, histopathological and immunohistochemical findings of an NEC in a three-year-old female roe deer (Capreolus capreolus).
A three-year-old female roe deer was shot by a local hunter in June 2023 in Nova vas in the Inner Carniola hunting area in southern Slovenia, Europe (45°45′42.89 “N, 14°30′11.43 “E), due to a facial deformity. The age of the animal was estimated by hunter and authorized hunting committees during the mandatory annual inspection of hunted ungulates at the end of the year. Eruption patterns and tooth wear were used to estimate the age of the animal. The animal was submitted to the Veterinary Faculty of the University of Ljubljana for necropsy. At necropsy, the animal was in good body condition (19.5 kg), defined by normally developed skeletal muscles and adequate subcutaneous and internal fat reserves. Gross examination of the head revealed an oval, apparently well-circumscribed, firm lesion in the left frontal and parietal region of the head (Figures 1A,B), which was covered with intact skin. Examination of the cut surface of the head revealed a poorly circumscribed, unencapsulated, oval, grey-white tumor mass measuring 14 × 11.5 × 13 cm, which originated from the ethmoid region of the left nasal cavity and had invaded and destroyed the cribriform plate and the rostral bones of the skull. The tumor occupied the rostral part of the cranial cavity and affected the frontal lobe of the brain. The tumor mass also occupied the frontal sinuses, invaded the frontal, lacrimal, sphenoid and zygomatic bones and grew into the subcutis of the frontal and parietal regions (Figure 1). As a result of the ingrowth of the tumor mass into the orbit, proptosis of the left eye occurred. No metastases and no other lesions were detected at necropsy.
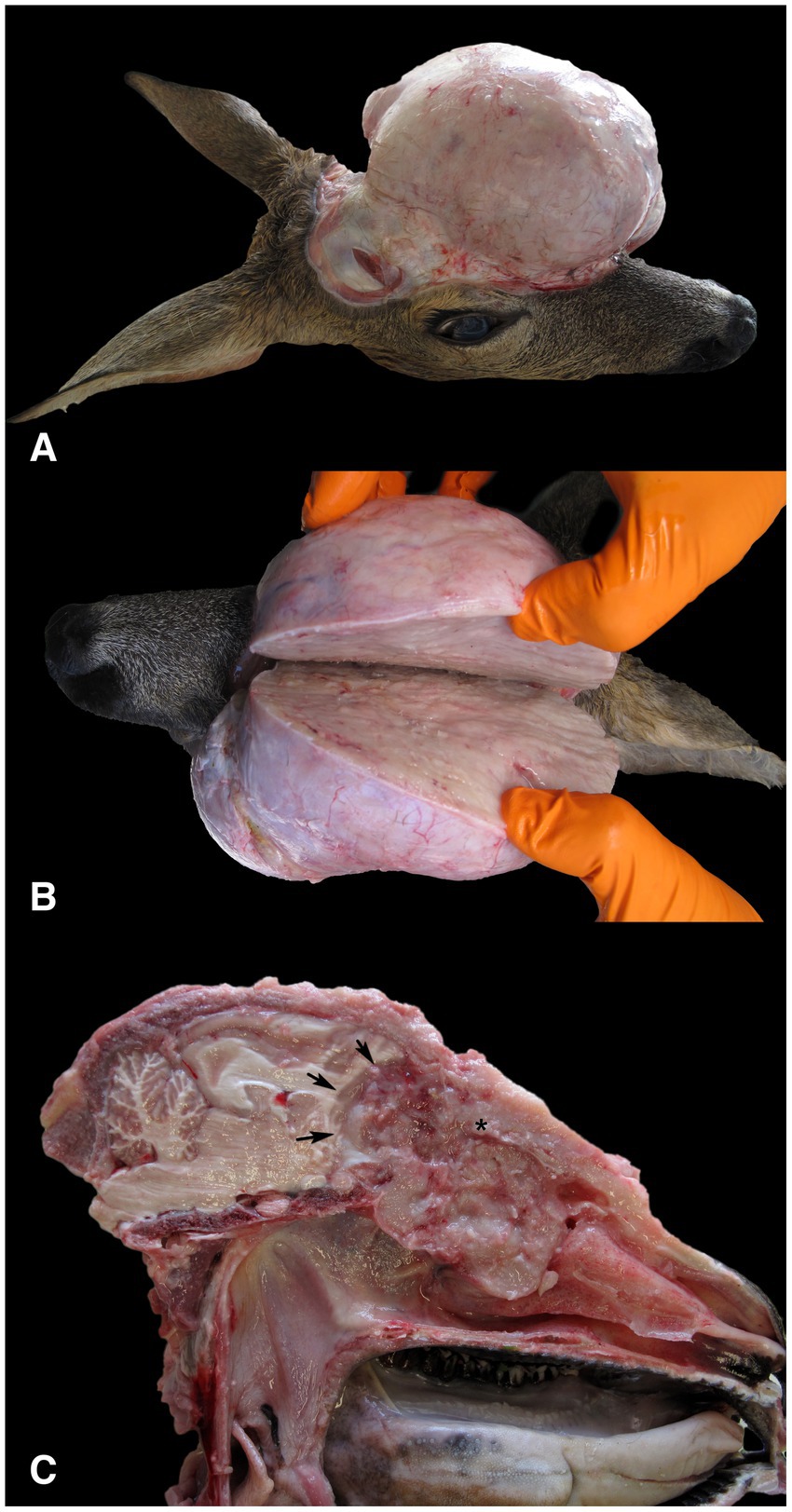
Figure 1. Gross findings in a female roe deer (Capreolus capreolus) with neuroendocrine carcinoma. (A) The surface of the tumor mass after removal of the skin showed a highly exophytic, grey-white, firm mass. (B) The cut surface of the tumor mass was grey-white, smooth and moderately bulged. (C) The tumor, which originated from the ethmoidal region of the left nasal cavity (*), had invaded and destroyed the cribriform plate and the rostral bones of the skull. The tumor occupied the rostral part of the cranial cavity and affected the frontal lobe of the brain (arrows). The exophytic portion of the tumor, which protruded above the parietal and nasal regions, was removed.
For histopathology, several samples of the tumor were collected, immediately fixed in 10% buffered formalin and routinely embedded in paraffin. For light microscopic examination, 4 μm thick sections were stained with hematoxylin and eosin and with the modified Grimelius stain (28). Histopathological examination revealed predominantly a well-demarcated, partially encapsulated tumor that was multifocally infiltrative and was composed of sheets, nests, trabeculae, rosettes, and cribriform structures separated by a small to moderate amount of fibrovascular stroma. The neoplastic cells were polygonal, with indistinct cell borders and small to moderate amounts of eosinophilic non-granular cytoplasm demonstrating mild anisocytosis. The modified Grimelius staining revealed numerous dark cytoplasmic granules within the neoplastic cells. Single round to oval nuclei were evident, exhibiting mild anisokaryosis and up to one small nucleolus (Figures 2A,B). Three mitoses per 10 high-power fields (2.37 mm2) were counted. Multifocally, small to medium sized necrotic foci, hemorrhages and lytic bone fragments were present within the neoplasm. Invasion of blood and lymph vessels was not observed.
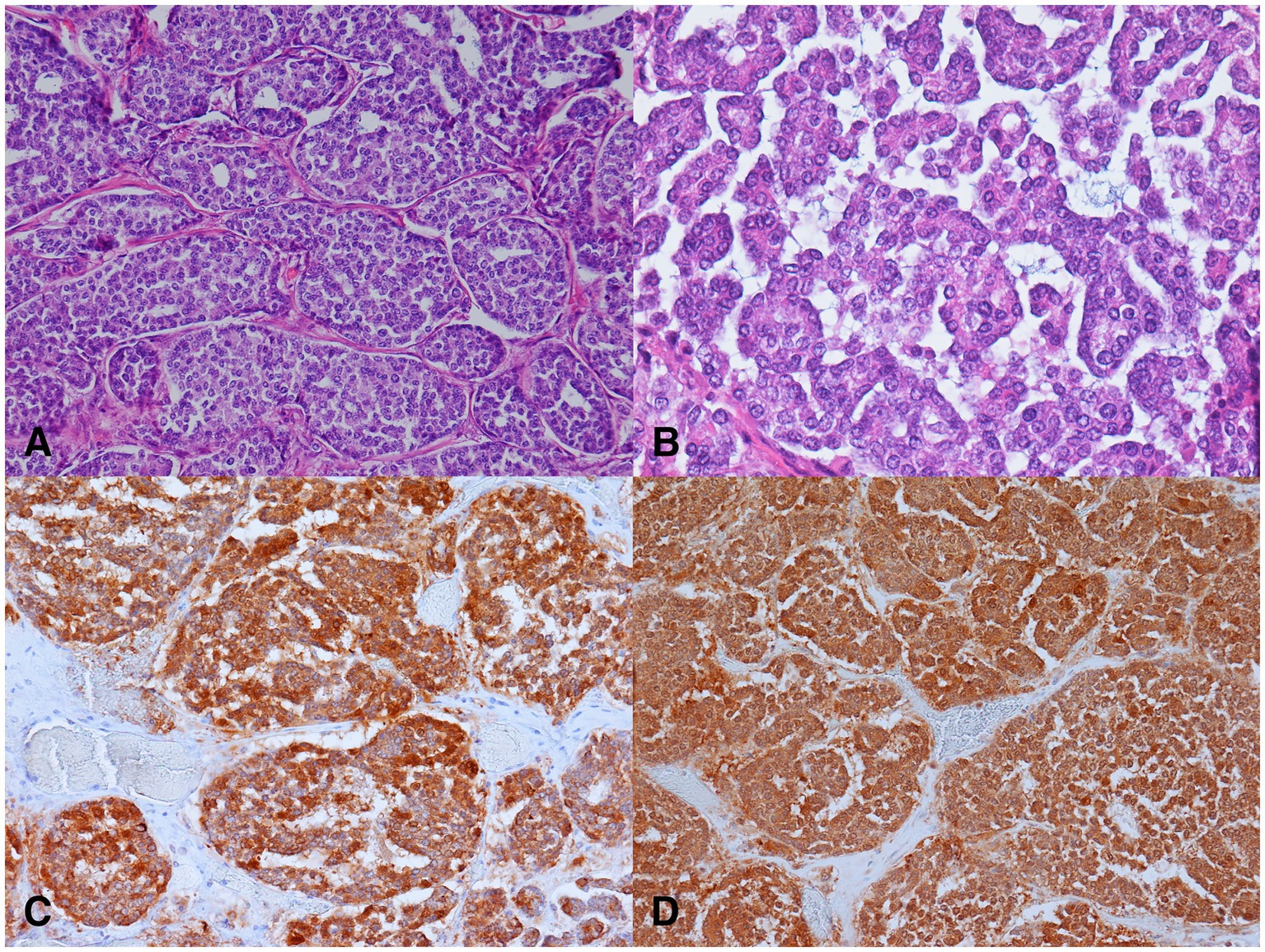
Figure 2. Microscopic findings of the neuroendocrine carcinoma in a female roe deer (Capreolus capreolus). (A) Nests of neoplastic cells separated by delicate fibrovascular stroma. H&E, 200x. (B) Nests of small to medium sized neoplastic cells exhibiting mild anisocytosis and anisokaryosis. H&E, 400x. (C) Neoplastic cells demonstrated strong cytoplasmic immunolabeling for cytokeratins. Pancytokeratin AE1/AE3 immunohistochemistry (IHC), 200x. (D) Neoplastic cells exhibited strong cytoplasmic immunolabeling for neuron specific enolase (IHC), 200x.
Immunohistochemistry was performed to confirm the origin of the neoplastic cells. The procedure was performed on selected 4 μm thick paraffin sections, which were first deparaffinized. The antigens were retrieved by boiling the slides in EDTA buffer (pH 9.0) in a microwave oven for 10 min [for immunolabeling of neuron-specific enolase (NSE), chromogranin A, synaptophysin, and microtubule-associated protein 2 (MAP-2)] or in citrate buffer for 10 min [for immunolabeling of neurofilament H (NFH)] or 20 min [for immunolabeling of cytokeratins, S-100 protein, glial fibrillary acidic protein (GFAP) and calcitonin]. The slides were then incubated with the primary antibodies for 1 h at room temperature in a humidified chamber (Table 1). The remaining immunohistochemistry was performed according to a previously described protocol (29). Tissues from the roe deer without lesions were used as positive controls: pancreas served as a positive control for immunoreactivity for cytokeratins, synaptophysin, chromogranin A, and NSE; thyroid gland served as a positive control for calcitonin; and brain tissue was used for S-100 protein, GFAP, NFH and MAP-2.
Most of the neoplastic cells were immunoreactive for cytokeratin AE1/AE3, and NSE. The immunolabeling was intense and localized in the cytoplasm (Figures 2C,D). The neoplastic cells were negative for cytokeratin MNF116, synaptophysin, chromogranin A, calcitonin, S-100 protein, GFAP, and NFH.
Based on the gross, histopathological and immunohistochemical features of the tumor, a nasal NEC was diagnosed.
Based on thorough database searches (PubMed Central, Google Scholar, CAB Abstract) covering the years (1950–2024), and using mixed keyword combinations of “neuroendocrine,” “tumor,” “neoplasm,” “neoplasia,” “carcinoma,” “roe deer,” and “Capreolus capreolus,” this is the first description of NEC in a roe deer and the second reported case in wild animals. The first case was previously described in a raccoon dog (4).
Histologically, the neuroendocrine differentiation of the tumor was suspected based on the architecture of the tumor and the morphology of the tumor cells, which were consistent with an endocrine tumor (1). This was confirmed by histochemical and immunohistochemical stainings. The tumor cells showed argyrophilic staining and expressed NSE and cytokeratins. The Grimelius stain is a silver stain that shows neuroendocrine granules with specific peptide hormones and biogenic amines. Although it stains almost all neuroendocrine tumors with few exceptions, it is not specific for a single neuroendocrine tumor type (30). Immunohistochemistry is valuable for the confirmation of tumors of neuroendocrine origin in both humans and animals (2, 4, 31, 32). Cytokeratins, synaptophysin, NSE, and chromogranin A are usually expressed in sinonasal tumors with neuroendocrine differentiation (3, 32). In addition, tumor cells can also express somatostatin, vasoactive intestinal polypeptide (VIP), protein gene product 9.5 (PGP 9.5) (2), and S-100 (4).
In this case, two differential diagnoses were considered: NEC and ONB, based on histopathologic and immunohistochemical confirmation of neuroendocrine differentiation in the tumor cells. Their phenotypic characteristics overlap considerably, which makes differentiation and diagnosis difficult (5, 33).
Grossly, ONB often grows more invasively and causes more extensive destruction of adjacent bony structures (34, 35). Extension of the neoplasm into the olfactory bulb and orbital wall causing proptosis has been described in a cattle with ONB (12). Compression of the olfactory bulb and frontal lobe with caudal displacement of both cerebral hemispheres and the cerebellum has been described in a dog with ONB (36), and osteolysis of the cribriform plate and extension of the tumor into the brain was observed in two dogs and one cat with ONB (7). In the axolotl, the tumor replaced part of the maxillary bone tissue (13). On the other hand, there have been some other cases of ONB in dogs, cats, and horses, in which the tumor showed no invasive growth into the bone and/or brain cavity (6, 11, 37).
In a case of NEC in a Japanese raccoon dog, Kubo et al. (4) reported destruction of the maxilla, extension of the tumor to the subcutis leading to swelling of the ridge of the nose, and extrusion of the eyeball. Sako et al. (2) found neoplastic infiltration of the nasal septum and frontal sinus in two dogs, and osteolysis of the maxilla and frontal bone in two of the ten dogs with NEC included in the study (2). Three horses with NEC were also found to have exophthalmos (3). In the case described here, the tumor also invaded the frontal sinuses, frontal bone, lacrimal bone, sphenoid bone and zygomatic bone as well as the rostral part of the cranial cavity and the orbit, resulting in proptosis of the left eye.
Both NEC and ONB form microscopic rosettes (1), and electron microscopic or immunohistochemical examination is often necessary to differentiate between them. In contrast to NEC, ONB has cell extensions that contain microtubules (1, 4). While the WHO classification requires electron microscopy for a definitive diagnosis in humans (37), these ultrastructural features have not yet been clearly demonstrated in animals (2, 5, 38).
The immunohistochemical findings of NEC and ONB also overlap considerably, and some researchers have even suggested that these two tumors are different manifestations of the same entity (11). Cytokeratins, chromogranin, and synaptophysin, as well as several peptides such as calcitonin and VIP, which are more regularly expressed in NEC (1) and the lack of immunolabeling for some cytoskeletal proteins such as neurofilament, class III beta-tubulin isotype and MAP-2 in human NEC are suggested to be the main differences between NEC and ONB (2, 39). In a study, cytokeratin AE1/AE3 was expressed in all 10 cases of NEC in the nasal cavity of dogs (2). Expression of MAP-2 has been shown to be a potentially reliable and sensitive marker for ONB in dogs and cats, as all but one case of ONB in cats were immunoreactive for MAP-2 (5). MAP-2 expression has also been described in case of ONB in horse (11).
NSE expression is used to support the diagnosis of ONB in human pathology and has also been described in cases of ONB in animals (5, 10). However, in animals, NSE is also expressed in NEC (2).
In conclusion, NEC in a roe deer was diagnosed based on the histopathological and immunohistochemical characteristics of the tumor. The tumor cells expressed NSE and cytokeratin AE1/AE3, but were immunohistochemically negative for synaptophysin, chromogranin A, calcitonin, S-100 protein, GFAP, NFH, and MAP-2, which in our opinion supports the diagnosis of NEC.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements as all samples were taken post-mortem.
GV: Conceptualization, Data curation, Investigation, Writing – original draft. DV: Data curation, Investigation, Writing – original draft. CC: Data curation, Investigation, Writing – review & editing. MG: Data curation, Investigation, Writing – review & editing. KT: Data curation, Investigation, Writing – review & editing. TŠ: Data curation, Investigation, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Slovenian Research and Innovation Agency (research core funding No. P4-0092 ‘Animal health, environment and food safety’), Administration of the Republic of Slovenia for Food Safety, Veterinary Service and Plant Protection, and Hunting Association of Slovenia (No. 403–114/2024).
The authors would like to thank the Slovenian Research Agency (program P4-0092), hunters, Administration of the Republic of Slovenia for Food Safety, Veterinary Service and Plant Protection and the Slovenian Hunting Association for supporting the research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Meuten, DJ. Tumors in domestic animals In: DJ Meuten, editor. Tumors in domestic animals. 4th ed. Ames: John Wiley & Sons, Ltd (2016)
2. Sako, T, Shimoyama, Y, Akihara, Y, Ohmachi, T, Yamashita, K, Kadosawa, T, et al. Neuroendocrine carcinoma in the nasal cavity of ten dogs. J Comp Pathol. (2005) 133:155–63. doi: 10.1016/j.jcpa.2005.04.002
3. Van Maanen, C, Klein, WR, Dik, KJ, and Van Den Ingh, TSGAM. Three cases of carcinoid in the equine nasal cavity and maxillary sinuses: histologic and immunohistochemical features. Vet Pathol. (1996) 33:92–5. doi: 10.1177/030098589603300114
4. Kubo, M, Matsuo, Y, Okano, T, Sakai, H, Masegi, T, Asano, M, et al. Nasal neuroendocrine carcinoma in a free-living Japanese raccoon dog (Nyctereutes procyonoides viverrinus). J Comp Pathol. (2009) 140:67–71. doi: 10.1016/j.jcpa.2008.09.007
5. Brosinski, K, Janik, D, Polkinghorne, A, Von Bomhard, W, and Schmahl, W. Olfactory neuroblastoma in dogs and cats – a histological and Immunohistochemical analysis. J Comp Pathol. (2012) 146:152–9. doi: 10.1016/j.jcpa.2011.06.002
6. Parker, VJ, Morrison, JA, and Yaeger, MJ. Olfactory neuroblastoma in a cat. J Feline Med Surg. (2010) 12:867–71. doi: 10.1016/j.jfms.2010.09.009
7. Söffler, C, Hartmann, S, Gorgas, D, Ludewig, E, Von Pückler, K, Kramer, M, et al. Magnetic resonance imaging features of esthesioneuroblastoma in three dogs and one cat. Tierarztl Prax Ausg K Kleintiere Heimtiere. (2016) 44:333–40. doi: 10.15654/TPK-150963
8. Church, ME, Veluvolu, SM, Durham, AC, and Woolard, KD. Clinical outcomes, ultrastructure and immunohistochemical features of canine high-grade olfactory neuroblastoma. Vet Comp Oncol. (2019) 17:578–84. doi: 10.1111/vco.12512
9. Romano, AM, and Frank, CB. Olfactory ganglioneuroblastoma in a dog: case report and literature review. J Vet Diagn Invest. (2021) 33:1013–7. doi: 10.1177/10406387211022864
10. Martí-García, B, Priestnall, SL, Holmes, E, and Suárez-Bonnet, A. Olfactory neuroblastoma in a domestic cat and review of the literature. Vet Clin Pathol. (2023) 52:521–6. doi: 10.1111/vcp.13255
11. Yamate, J, Izawa, T, Ogata, K, Kobayashi, O, Okajima, R, Kuwamura, M, et al. Olfactory neuroblastoma in a horse. J Vet Med Sci. (2006) 68:495–8. doi: 10.1292/jvms.68.495
12. Anderson, BC, and Cordy, DR. Olfactory neuroblastoma in a heifer. Vet Pathol. (1981) 18:536–40. doi: 10.1177/030098588101800411
13. Shioda, C, Uchida, K, and Nakayama, H. Pathological features of olfactory neuroblastoma in an axolotl (Ambystoma mexicanum). J Vet Med Sci. (2011) 73:1109–11. doi: 10.1292/jvms.11-0105
14. Modesto, F, Nicolier, A, Hurtrel, C, and Benoît, J. Excisional biopsy and radiotherapy for management of an olfactory neuroblastoma in an axolotl (Ambystoma mexicanum). J Am Vet Med Assoc. (2021) 260:436–41. doi: 10.2460/javma.20.09.0498
15. Vigliano, FA, Marcaccini, AJ, Sarradell, J, Bermúdez, R, and Quiroga, MI. First description of an olfactory neuroblastoma in goldfish Carassius auratus: a case report. Dis Aquat Org. (2011) 96:61–8. doi: 10.3354/dao02383
16. Pewsner, M, Origgi, FC, Frey, J, and Ryser-Degiorgis, MP. Assessing fifty years of general health surveillance of roe deer in Switzerland: a retrospective analysis of necropsy reports. PLoS One. (2017) 12:e0170338. doi: 10.1371/journal.pone.0170338
17. Žele Vengušt, D, Kuhar, U, Jerina, K, and Vengušt, G. Twenty years of passive disease surveillance of roe deer (Capreolus capreolus) in Slovenia. Animals. (2021) 11:407. doi: 10.3390/ani11020407
18. Aguirre, AA, Bröjer, C, and Mörner, T. Descriptive epidemiology of roe deer mortality in Sweden. J Wildl Dis. (1999) 35:753–62. doi: 10.7589/0090-3558-35.4.753
19. Hoekman, ED. Dutch roe deer (Capreolus capreolus), review of cases presented at the Dutch wildlife health Centre. Utrecht, The Netherlands: Facultad de Veterinaria (2013).
20. Munro, R, and Youngson, RW. Hepatocellular tumours in roe deer in Britain. Vet Rec. (1996) 138:542–6. doi: 10.1136/vr.138.22.542
21. Brunk, R. Wildpathologische Untersuchungen der Jahre 1939 bis 1959. Z Für Jagdwiss. (1960) 6:121–85. doi: 10.1007/BF01890162
22. Bednarski, M, Wimonć, M, Kolenda, R, Wlekliński, E, Król, D, and Houszka, M. Primary pulmonary adenocarcinoma in roe deer-a case report. Med Weter. (2019) 75:6206–2019. doi: 10.21521/mw.6206
23. Zürcher-Giovannini, S, Ruder, TD, Pool, R, Erdelyi, K, and Origgi, FC. Mandibular ossifying fibroma and multiple oral papillomas in a roe deer (Capreolus capreolus). Front Vet Sci. (2020) 7:166. doi: 10.3389/fvets.2020.00166
24. Elvestad, K, and Henriques, UV. Leukaemic neoplasia in free-living mammals in Denmark. Acta Vet Scand. (1985) 26:61–71. doi: 10.1186/BF03546564
25. Kleinschmidt, S, Peters, M, and Wohlsein, P. Central nervous system neuroblastoma in a wild deer (Capreolus capreolus). J Comp Pathol. (2012) 146:283–7. doi: 10.1016/j.jcpa.2011.07.001
26. Domenis, L, Campanella, C, Rubini, D, Parovel, E, Orusa, R, and Robetto, S. Oral squamous cell carcinoma in a free-ranging roe deer (Capreolus capreolus). J Vet Med Animal Sci. (2020) 3:1024.
27. Barlow, AM, and Couper, D. Cutaneous teratoma in a wild roe deer (Capreolus capreolus) in the UK. Vet Rec. (2006) 159:211–2. doi: 10.1136/vr.159.7.211
28. Lack, EE, and Mercer, L. A modified Grimelius argyrophil technique for neurosecretory granules. Am J Surg Pathol. (1977) 1:275–8. doi: 10.1097/00000478-197709000-00009
29. Cociancich, V, Švara, T, and Pogačnik, M. Malignant mesenchymoma of the aortic valve in a dog. Slov Vet Res. (2013) 50:83–8.
30. Grimelius, L. Methods in neuroendocrine histopathology, a methodological overview. Ups J Med Sci. (2008) 113:243–60. doi: 10.3109/2000-1967-238
31. Kulke, MH, and Mayer, RJ. Carcinoid tumors. N Engl J Med. (1999) 340:858–68. doi: 10.1056/NEJM199903183401107
32. Kuwata, K, Shibutani, M, Kemmochi, Y, Taniai, E, Morita, R, Ogawa, B, et al. A neuroendocrine carcinoma of undetermined origin in a dog. J Toxicol Pathol. (2010) 23:151–5. doi: 10.1293/tox.23.151
33. Ninomiya, F, Suzuki, S, Tanaka, H, Hayashi, S, Ozaki, K, and Narama, I. Nasal and paranasal adenocarcinomas with neuroendocrine differentiation in dogs. Vet Pathol. (2008) 45:181–7. doi: 10.1354/vp.45-2-181
34. Pickuth, D, and Heywang-Köbrunner, SH. Imaging of recurrent esthesioneuroblastoma. Br J Radiol. (1999) 72:1052–7. doi: 10.1259/bjr.72.863.10700820
35. Siudak, K, Klingler, M, Schmidt, MJ, and Herden, C. Metastasizing Esthesioneuroblastoma in a dog. Vet Pathol. (2015) 52:692–5. doi: 10.1177/0300985814559402
36. Hara, K, Shimada, A, Morita, T, Sawada, M, and Umemura, T. Olfactory neuroepithelioma in a dog: an immunohistochemical and electron microscopic study. J Vet Med Sci. (2002) 64:391–3. doi: 10.1292/jvms.64.391
37. Dungworth, DL, and Donald, L. Histological classification of tumors of the respiratory system of domestic animals. Washington, DC: Armed Forces Institute of Pathology (1999).
38. Döpke, C, Gröne, A, Borstel, MV, Oppen, TV, Boéve, MH, and Baumgärtner, W. Metastatic esthesioneuroblastoma in a horse. J Comp Pathol. (2005) 132:218–22. doi: 10.1016/j.jcpa.2004.07.003
Keywords: roe deer, nasal cavity, neuroendocrine carcinoma, tumor, immunohistochemistry
Citation: Vengušt G, Vengušt DŽ, Cantile C, Gombač M, Tekavec K and Švara T (2025) Case report: Neuroendocrine carcinoma of the nasal cavity in a roe deer (Capreolus capreolus). Front. Vet. Sci. 12:1535432. doi: 10.3389/fvets.2025.1535432
Received: 27 November 2024; Accepted: 20 January 2025;
Published: 05 February 2025.
Edited by:
Yasunaga Yoshikawa, Kitasato University, JapanReviewed by:
Andrew F. Rich, International Zoo Veterinary Group, United KingdomCopyright © 2025 Vengušt, Vengušt, Cantile, Gombač, Tekavec and Švara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gorazd Vengušt, Z29yYXpkLnZlbmd1c3RAdmYudW5pLWxqLnNp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.