- 1Henan Key Laboratory of Rare Diseases, Endocrinology and Metabolism Center, The First Affiliated Hospital, and College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 2Department of Pathology, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 3Monogastric Science Research Centre, Scotland's Rural College (SRUC), Edinburgh, United Kingdom
- 4Institute of Zoology, University of Punjab, Lahore, Pakistan
- 5Institute of Pharmaceutical Sciences, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 6Department of Parasitology and Microbiology, Faculty of Veterinary and Animal PMAS-ARID Agriculture University, Rawalpindi, Pakistan
- 7Department of Pharmacology and Toxicology, University of Veterinary and Animal Sciences, Lahore, Pakistan
- 8Institute of Biochemistry and Biotechnology, University of Veterinary Animal Sciences, Lahore, Pakistan
Background: Amid growing concern about antimicrobial resistance due to the irrational use of antibiotics in treating common poultry diseases, particularly Salmonella which is a foodborne pathogen in humans. This study investigates the effects of ethnoveterinary supplementation of Rauwolfia serpentina (L. Benth. ex Kurz) powder (RSP) on three key immune-related genes; Suppressor of cytokine signaling 3 (SOCS3), the quiescence-related gene P20K (P20K), and the major histocompatibility complex Class IIβ (MHC class IIβ), gut morphology and growth performance of broiler chicks infected with Salmonella Gallinarum.
Methods: Two hundred and forty day-old Hubbard classic chickens were randomly assigned to four groups: non-challenged control (NC), and Salmonella Gallinarum challenge group (SGC), and two treatment groups fed a basic diet supplemented with 1.5% Rauwolfia serpentina powder (RSP) with SGC (RSP-1) and 3% RSP with SGC (RSP-2), respectively, from day 3 till 28 days of age. Each treatment was replicated 4 times with 15 bird/replicate pen. On day 7, all the birds in the RSP-1, RSP-2 and SGC groups received 1 ml of BHI broth containing 2 × 108 CFU of Salmonella Gallinarum via oral gavage. While control birds received an equivalent volume of sterile BHI broth. Gene expression analysis was conducted using real-time PCR to measure the expression of key immune-related genes: SOCS3, P20K, and MHC Class IIβ in spleen, liver, and caeca. Additionally, histopathological assessments of gut and growth performance parameters including feed intake, body weight gain, and feed conversion ratio (FCR) were monitored throughout the experimental period.
Result: The gene expression analysis at 3 and 21 days post-challenge revealed that SGC birds had significantly higher SOCS3, P20K, and lower MHC class IIβ expression (p < 0.001) in the caecum, liver, and spleen of broiler chickens. In contrast, the RSP-1 and RSP-2 groups showed significantly lower SOCS3 and P20K expression (p < 0.001), alongside improved gut morphology, weight gain, and FCR compared to the SGC group, with these benefits increasing over time.
Conclusion: In conclusion, these findings suggest that Rauwolfia serpentina supplementation modulates key immune-related gene expression (SOCS3, P20K, and MHC class IIβ), enhances intestinal health, and improves growth performance in broilers challenged with Salmonella Gallinarum.
1 Introduction
Salmonella enterica subspecies enterica serovar Gallinarum biotype Gallinarum (Salmonella Gallinarum) causes a severe septicemic disease known as fowl typhoid in broiler chickens and various bird species. Fowl typhoid leads to significant economic losses in commercial poultry production (1). Clinically, Salmonella Gallinarum infection presents with diarrhea, dehydration, weakness, and unpredictable morbidity and mortality with lesions developing in multiple organs, and the highest bacterial counts in the liver, spleen, and intestines, especially the caeca (2). The primary treatment for fowl typhoid includes antibiotics such as ciprofloxacin, furazolidone, ampicillin, and gentamycin. However, frequent antibiotic use for both therapeutic and prophylactic purposes has contributed to the emergence of Salmonella Gallinarum strains resistant to commonly used antibiotics, including β-lactams, quinolones, fluoroquinolones (e.g., nalidixic acid, ciprofloxacin), and tetracycline (3).
The rise in multidrug-resistant bacteria presents a serious threat to both animal and human health. As a result, there is a growing need for alternative strategies in poultry production, particularly approaches that reduce antibiotic use while promoting gut health and pathogen resistance (4). The increasing resistance to antibiotics, particularly quinolones, underscores the urgent need to find alternative strategies for managing fowl typhoid.
Phytochemicals have gained popularity as potential alternatives to antibiotics due to their antibacterial, antioxidant, and antifungal properties (5). One such plant, Rauwolfia serpentina L. Benth. ex Kurz (Rauwolfia serpentina), commonly referred to as snakeroot, has traditionally been used to treat various conditions, including hypertension, intestinal diseases, snake bites, breast cancer, and infections caused by Gram-positive bacteria (6). Studies have shown that Rauwolfia serpentina root extracts possess antibacterial, antifungal, anti-inflammatory, and antioxidant activities (7), suggesting its potential as a candidate for mitigating the effects of Salmonella Gallinarum infection. An in vivo study conducted in rats showed that administering 600 mg/kg of Rauwolfia serpentina root extract reduced edema, while 800 mg/kg exhibited anti-inflammatory activity anti-bacterial (8), along with antifungal activity and bacteriostatic ability against Salmonella typhi (9).
Research findings indicate that reserpine, an alkaloid derived from Rauwolfia serpentina, demonstrates significant antimicrobial properties against Salmonella. Experimental studies have revealed enhanced Salmonella elimination in reserpine-treated explants: In vitro study using tissue explants from 21 day old broiler chicks showed that when treated with reserpine, these samples exhibited a higher rate of Salmonella destruction compared to untreated controls. This suggests that reserpine may augment the tissue's innate defense mechanisms against this pathogen. In vivo studies involving oral reserpine treatment demonstrated a notable decrease in the population of Salmonella Typhimurium within the intestinal tract. Moreover, this treatment appeared to have a broader impact, reducing the overall levels of Enterobacteriaceae in the gut (10). These findings underscore reserpine's potential as an antimicrobial agent, particularly in combating Salmonella infections. Rauwolfia serpentina has numerous pharmacological properties, including antimicrobial, anti-inflammatory, and stress reliever and immune-modulating effects, which may be beneficial in controlling infections and various conditions (11–13).
The intestinal mucosa plays a crucial role as a barrier against both commensal and pathogenic organisms and provides an innate immune response to invading pathogens (14). Salmonella infections typically occur via the oral route, with bacteria invading the intestines, through Peyer's patches and caecal tonsils (3). The bacteria can persist in the intestines by suppressing the host's inflammatory responses, primarily through T-cell modulation particularly regulatory T cells (Treg). Anti-inflammatory Treg cytokines play important role in maintaining balanced immune response. P20K mRNA expression levels variation were observed in spleen of chickens challenged with S. Enteritidis (15). Suppressor of cytokine signaling 3 (SOCS3) protein, a member of SOCS family plays a crucial role in regulating inflammatory and immune responses through multiple mechanisms, negative feedback regulation of the Janus kinase-signal transducer and activator of transcription (JAK/STAT) signaling pathway, which leads to the suppression of immune responses by inhibiting T cell differentiation. Additionally, SOCS3 exerts an anti-inflammatory effect by repressing the M1 proinflammatory macrophage phenotype and deactivating inflammatory responses in macrophages. This dual action on both adaptive and innate immune cells underscores SOCS3's importance in modulating inflammation (16, 17). The major histocompatibility complex (MHC) Class IIβ molecules are crucial for antigen presentation to CD4+ T cells, playing a central role in adaptive immunity. All three genes (SOCS3, P20K, and MHC Class IIβ) were differentially expressed in a microarray experiment and has shown variation in their expression in response to Salmonella challenge (15, 58).
There is limited understanding of the immunomodulatory effects and gene expression modulation by Rauwolfia serpentina in broiler chickens challenged with Salmonella Gallinarum. While previous studies have highlighted the antimicrobial, antifungal, and anti-inflammatory properties of Rauwolfia serpentina and its alkaloid reserpine (7, 9), most of this research has been conducted in non-avian models, such as rats (8), or has focused on different Salmonella serovars like S. Typhi (10), rather than Salmonella Gallinarum. This study aims to examine the effects of Rauwolfia serpentina on growth performance, gene expression of SOCS3, P20K and MHC Class IIβ as well as the immune response in broilers challenged with Salmonella Gallinarum. We hypothesized that supplementation with Rauwolfia serpentina will modulate gene expression, improve intestinal health and enhance growth performance in broilers infected with Salmonella Gallinarum.
2 Materials and methods
2.1 Ethical approval
Animal Ethics Committee of the University of Veterinary and Animal Sciences, Lahore, approved all the experimental procedures (Ref. No. DR/350 dated 24-07-2023).
2.2 Collection and authentication of Rauwolfia Serpentina root material
Rauwolfia serpentina root material was purchased from Dr. Masood Pharmaceuticals (Pvt.) Ltd., Lahore, and authenticated by a taxonomist at Government College University, Lahore (specimen voucher: GC.Herb.Bot.2324). The roots were washed, dried and milled into a powder that was used in the birds' diet. Two experimental diets were used one with 15 and 30 g/kg Rauwolfia serpentina root powder in the diet. A proximate analysis (18) and phytochemical analysis of Rauwolfia serpentina were performed for qualitative and quantitative screening of the plant.
2.3 Phytochemical analysis
2.3.1 Qualitative screening
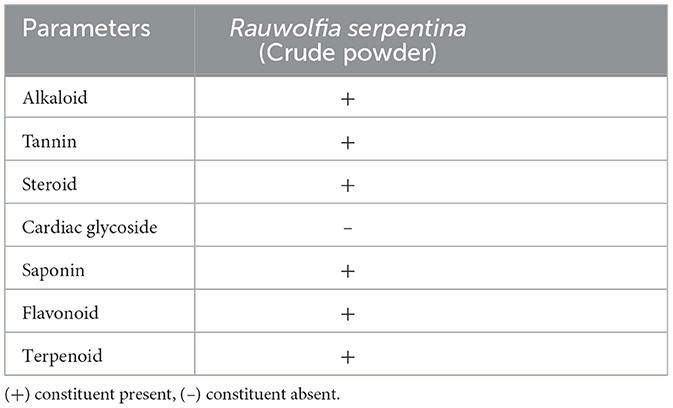
The presence of steroids, alkaloids, glycosides, tannins, flavonoids, terpenoids, and phenols in Rauwolfia serpentina root was assessed by qualitative tests following the procedure described by Gul et al. (19) and Lawal et al. (20).
2.3.2 Quantitative analysis
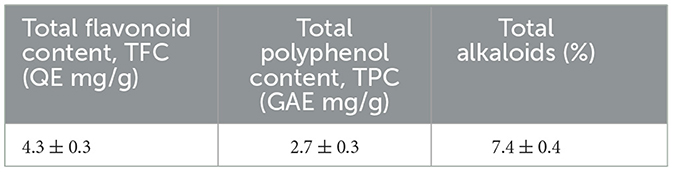
Total flavonoid content (TFC) was quantified using the method described by Chang et al. (21), with quercetin as the standard. Total polyphenol content (TPC) was estimated using the Slinkard and Singleton (22) method, with Gallic acid as the standard. Alkaloid content was assessed using the method outlined by Kokate et al. (23).
2.4 Gas chromatography-mass spectrometry (GC-MS) analysis
Gas Chromatography-Mass Spectrometry (GC-MS) analysis was performed using an Agilent Technologies GC-MS system (California, USA) with a 6850 Network GC system and 5973-mass selective detector. Compound identification was done by comparing with the NIST 05 Mass Spectral Library and published spectra (24).
2.5 Experimental design and animal husbandry
A total of 240 Hubbard Classic chickens were randomly assigned to four experimental groups, each consisting of four replicates with 15 birds per replicate. The treatment groups included: non-challenge control (NC), Salmonella Gallinarum challenge only (SGC), 1.5% Rauwolfia serpentina powder + challenge (RSP-1), and 3% Rauwolfia serpentina powder + challenge (RSP-2). Bacteriological analysis of fecal samples and serum plate agglutination test using Salmonella Gallinarum antigen was performed to make sure Salmonella-free status of chickens (25). On day 7, all the birds in the SGC group, RSP-1 and RSP-2 received the Salmonella Gallinarum challenge. In addition, challenged birds in RSP-1 and RSP-2 groups received a diet supplemented with 1.5% (15 g/kg) and 3% (30 g/kg) of Rauwolfia serpentina root powder respectively from day 3 till day 28 of age. During the experiment, all birds were housed in floor pens within climate-controlled rooms. The temperature was maintained between 33 and 30°C during the first 14 days, after which it was gradually reduced to 21°C by day 28. Throughout the study, all birds had unrestricted access to feed and water.
2.6 Salmonella Gallinarum challenge
Salmonella Gallinarum stock, previously used in Liaqat et al. (26), was thawed and cultured on Salmonella Shigella Agar (Oxoid, UK) and Brilliant Green Agar (Oxoid, UK). An individual bacterial colony was transferred into Brain Heart Infusion Broth (Oxoid, UK) and allowed to grow overnight in an incubator maintained at 37°C. Birds in the challenge groups received 1 ml of BHI broth containing 2 × 108 CFU of Salmonella Gallinarum via oral gavage on day 7, while control birds received an equivalent volume of sterile BHI broth. Following the challenge, chickens were monitored twice daily for signs and symptoms of fowl typhoid, as described by Saleem et al. (2) till the end of the experiment.
2.7 Sample collection and processing
On days 3 and 21 post-challenge, five birds from each replicate were humanely euthanized by cervical dislocation for post-mortem examination. Following necropsy, tissue samples from the caeca, spleen and liver were aseptically collected to evaluate expression levels of SOCS3, P20K and MHC CLASSIIβ genes that represent classical immune response, and stored in RNAlater® solution (Thermo Fisher Scientific, Waltham, MA, USA) at −80°C until further processing.
2.8 RNA extraction and gene expression analysis
Total RNA was extracted using TRIzol® Reagent (27) according to the manufacturer's recommendation. Quantity and quality of RNA were assessed using a NanoDrop™ 2000 spectrophotometer and Bioanalyzer (Agilent 2100). Samples with an RNA Integrity Number (RIN) above 8.0 were utilized for subsequent analyses.
For the synthesis of complementary DNA (cDNA), 1 μg of RNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Quantitative real-time PCR (qPCR) was conducted in technical triplicate with SYBR Green chemistry using a Thermal Cycler (Applied Biosystems™ Veriti). The reactions were carried out in a total volume of 20 μl, containing 10 μl of 2 × SYBR Green Master Mix (Thermo Fisher Scientific), 0.5 μl of each forward and reverse primer (10 μM), 2 μl of cDNA template, and 7 μl of nuclease-free water. GAPDH was used as the endogenous control. The thermal cycling conditions included an initial denaturation at 95°C for 10 min, followed by 45 cycles of a three-step touchdown protocol (15). Melt curve analysis was performed to confirm amplification specificity. Gene expression levels for SOCS3, P20K, and MHC CLASSIIβ were quantified using previously validated primers (15). Primer sequences and gene accession numbers are provided (in Supplementary Table 1). Relative gene expression was determined using the 2−ΔΔCt formula (28).
2.9 Histopathological and morphometric analysis
Caecal tissue samples from five birds from each replicate were collected on day 21 post-infection (in addition to gene analysis), washed with sterile saline solution to remove the digesta immersed in fixative (10% buffered formalin) followed by embedding in paraffin and cutting into 4 μm sections (29) and stained with H&E and Periodic Acid–Schiff (PAS). The slides were analyzed using a light microscope (Olympus CX31) with a digital imaging system (Olympus DP20, Olympus USA). The morphometric analysis included measurements of villus height (VH), crypt depth (CD), VH and CD ratio, and surface area calculated using the formula (2π) (VW/2) (VL). Goblet cell numbers, acidic and neutral mucin were also assessed (30).
2.10 Growth performance
Body weights (BW) were recorded for each replicate at day 0, 7 and 28 days of age. Body weight gain was calculated using the initial BW at the beginning and on days 7 and 28 days of age. Feed intake was also recorded at day 7 and 28 days of age. FCR were calculated to assess the growth performance of birds.
2.11 Statistical analysis
Data were analyzed using Genstat 11 for Windows (VSN International Ltd, Hemel Hempstead, UK). For qPCR data, relative gene expression was calculated using the 2−ΔΔCt method. The pen served as the experimental unit for growth performance data, while the individual bird was the unit for histopathological, morphometric, and gene expression analyses. Morphometric parameters, including villus height (VH), crypt depth (CD), VH:CD ratio, surface area, and goblet cell counts, as well as mucin composition (acidic and neutral), were analyzed using analysis of variance (ANOVA) to evaluate the effects of treatments on caecal histomorphometry and mucin production. No outliers were removed prior to analysis. Gene expression data were then analyzed using a one-way analysis of variance (ANOVA). Post-hoc comparisons were conducted using Bonferroni's test to determine significant differences between specific groups. Differences were considered statistically significant at p < 0.05. Results are presented as means ± standard error of the mean (SEM).
3 Results
The proximate analysis of Rauwolfia serpentina root showed the sample contained 5% moisture, 7% total ash, 4% water-soluble ash, 2% acid-insoluble ash, and 1% sulphated ash. Alcoholic and aqueous extractions yielded 22% and 34%, respectively. Qualitative and quantitative analysis of the crude powder of Rauwolfia serpentina root revealed the phytochemical composition (Tables 1, 2). Preliminary phytochemical analysis showed the presence of alkaloids, tannin and glycosides. Quantitative analysis indicated that alkaloids were present in significantly higher concentrations compared to polyphenols and flavonoids (Table 2). The GC-MS analysis identified various compound of Rauwolfia serpentina root powder and their respective immunomodulatory and biological activities, such as Reserpine, 7-Hydroxycoumarin, and Oleic acid. Each compound is referenced with supporting literature, highlighting their diverse therapeutic potentials (Table 3).
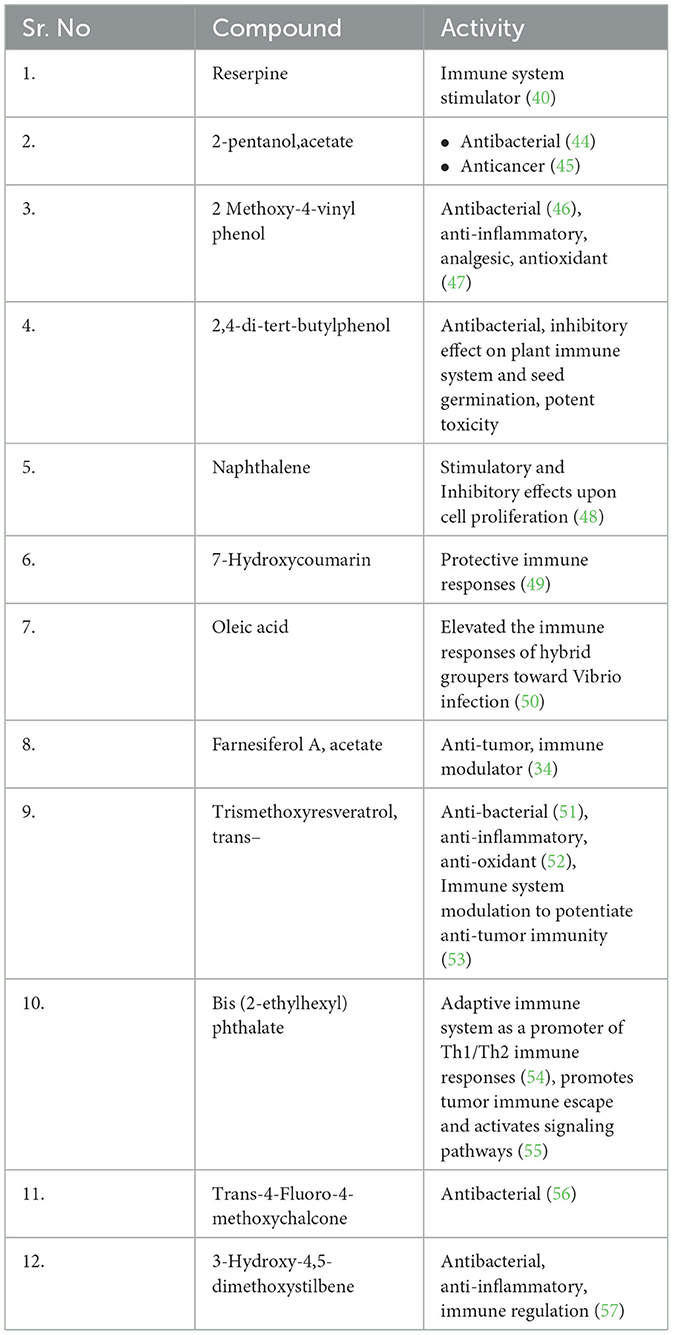
Table 3. Identified compounds in GC-MS analysis in Rauwolfia serpentina root powder with immunomodulatory activity.
3.1 Clinical observations
During daily clinical observations, ~15% of the birds in the SGC group exhibited symptoms consistent with fowl typhoid, including ruffled feathers, rapid respiration, emaciation, and yellowish-white diarrhea. During the experiment, 1% of the chickens died. None of these deaths showed evidence of being caused by the Salmonella Gallinarum infection. On day 10 (3 days post-challenge), a post-mortem examination revealed moderate liver discolouration with hemorrhages and caeca lesions, predominantly inflammation, along with splenomegaly in the SGC group (Figure 1). This indicated a moderate severity of the challenge. In contrast, birds supplemented with Rauwolfia serpentina at 1.5% and 3%, exhibited milder effects with only three out of 10 and two out of 10 birds, respectively, showing mild liver discolouration with necrotic foci. Additionally, only a very mild degree of enteritis was observed in these birds.
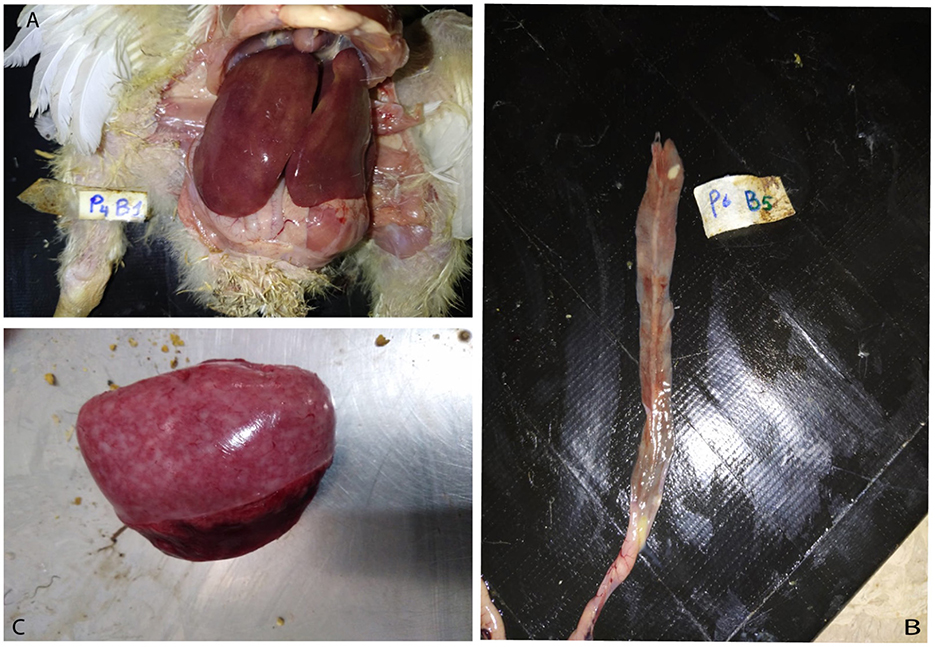
Figure 1. Gross lesions in broiler chicks experimentally infected with Salmonella Gallinarum. (A) Enlarged and hemorrhagic liver with characteristic swelling and bronze discoloration. (B) Enteritis with areas of necrosis. (C) Splenomegaly.
3.2 Gene expression analysis
The expression levels of three key immune-related genes-SOCS3, P20K, and MHC class IIβ were analyzed in the caecum, liver, and spleen of broiler chicks at 3 and 21 days post-challenge against Salmonella Gallinarum. The effects of Rauwolfia serpentina supplementation were compared to both NC and SGC (Figure 2).
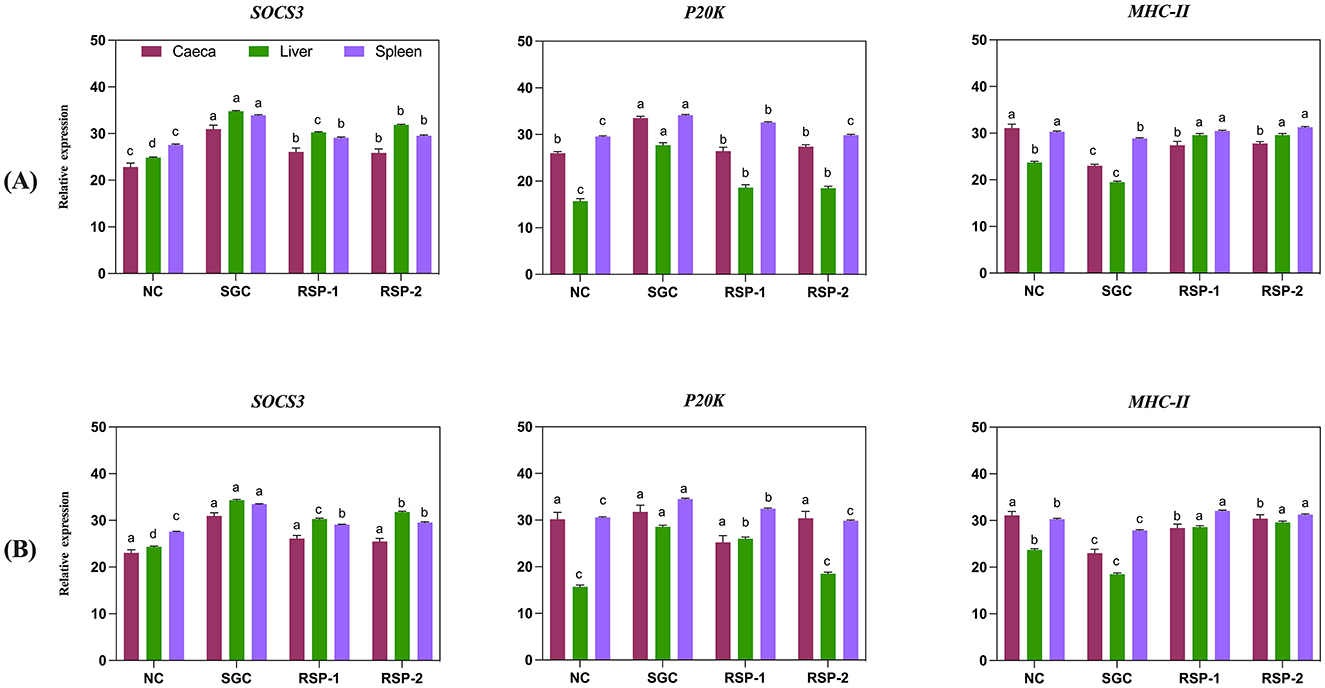
Figure 2. Effect of Rauwolfia serpentina root supplementation on expression levels of SOCS3, P20K and MHC class IIβ gene in caecum, liver and spleen of broiler chicks experimentally challenged with Salmonella Gallinarum (A) 3 days post-challenge (B) 21 days post-challenge. (NC) Non-challenge control group (SGC), Salmonella Gallinarum challenge group (RSP-1) group fed basic diet + 1.5% Rauwolfia serpentina root along with Salmonella Gallinarum challenge (RSP-2) group fed basic diet + 3% Rauwolfia serpentina root + Salmonella Gallinarum challenge. Values are represented as means and SEM are represented by vertical bars and different letters (a-b-c-d) indicate a significant difference.
On day 3 post-challenge, SOCS3 gene expression varied significantly across treatments and tissue (caeca, liver, and spleen: p < 0.05). The SGC birds exhibited the highest SOCS3 expression levels in the caecum, liver, and spleen, while birds supplemented with RSP-1 and RSP-2 showed significantly lower expression levels than the SGC group (Figure 2A). In the spleen, SOCS3 expression in RSP-1 and RSP-2 groups was still significantly higher than in the NC group (p < 0.05).
Supplementation of Rauwolfia serpentina had a significant effect on SOCS3 gene expression in the liver and spleen (p < 0.001) on day 21 post-challenge, while no significant differences (p = 0.061). were observed in the caeca (Figure 2B). SGC birds exhibited the highest SOCS3 expression in both the liver and spleen, with significantly lower expression observed in birds supplemented with Rauwolfia serpentina (RSP-1 and RSP-2) compared to the SGC group. However, SOCS3 levels in the supplemented groups remained higher than in the unchallenged birds. Both supplementation levels (1.5 and 3%) resulted in similar SOCS3 expression in the spleen, significantly lower than the SGC group but higher than the unchallenged birds (Figure 2B).
On day 3 post-challenge, P20K gene expression showed tissue-specific variations across treatment groups (Figure 2A). In all three organs, both RSP-1 and RSP-2 groups exhibited significantly lower P20K expression than SGC groups (p < 0.05). Expression of P20K was highest in the SGC group, significantly higher than all other groups (p < 0.05). In the liver, supplementation of Rauwolfia serpentina resulted in intermediate expression levels, significantly higher than NC but lower than SGC (p < 0.05). In the spleen, P20K expression was significantly elevated in all SGC group compared to the other treatment group (p < 0.05). At 21 days post-challenge, caecal P20K expression showed no statistically significant differences among groups (p = 0.111, Figure 2B).
MHC class IIβ gene expression patterns varied across tissues and treatment groups on day 3 post challenge (Figure 2A). SGC groups showed significantly lowest expression in all three tissues examined compared to all other treatment groups (p < 0.05). Liver MHC class IIβ expression was significantly elevated in all treatment groups compared to the NC group (p < 0.05). The SGC group showed the lowest expression, followed by RSP-1 and RSP-2, all significantly different from the NC group (p < 0.05). On day 21 post-challenge, significant differences were observed in MHC Class IIβ expression across all tissues (caeca: p = 0.008, liver and spleen: p < 0.001). SGC birds exhibited the lowest expression levels in the caecum, liver, and spleen, whereas birds supplemented with RSP-2 and RSP-1 showed significantly higher expression levels than SGC group (Figure 2B).
3.3 Histopathological and morphometrical analysis of caeca
Histopathological examination of caecal tissue revealed marked disruption of normal architecture and abnormal mucin production in SGC birds (Figure 3B). Alcian Blue staining showed intense mucin production, accompanied by areas of necrosis, tissue degeneration and inflammatory infiltrates (Figure 3F). These findings suggest a progressive pathological process affecting the tissue, likely impairing nutrient absorption and mucosal defense. In contrast, birds supplemented with RSP-2 and RSP-1 showed only light to moderate changes, with mucin production and tissue architecture largely intact. Cellular morphology appeared mostly regular, with only slight nuclear size and shape variations, suggesting that Rauwolfia serpentina supplementation contributed to tissue repair.
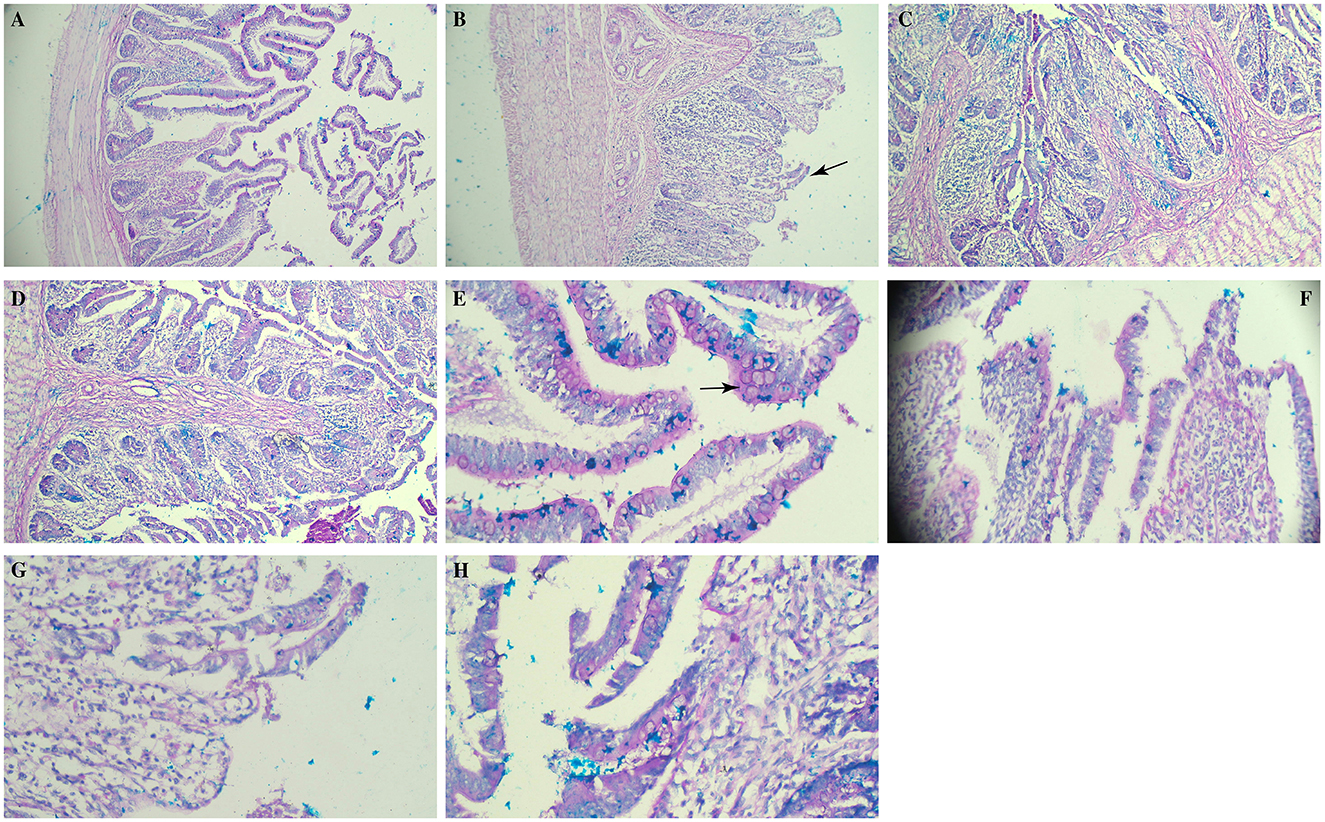
Figure 3. Photomicrograph showing the effect of Rauwolfia serpentina root supplementation on caeca of broiler chicks experimentally challenged with Salmonella Gallinarum (21 days post-challenge). (A, E) Non-challenge control group, (B, F) Salmonella Gallinarum challenge group, (C, G) group fed a basic diet + 1.5% Rauwolfia serpentina root along with challenge, (D, H) group fed a basic diet + 3% Rauwolfia serpentina root + Salmonella Gallinarum challenge. (B) Arrow shows ruptured villi (E) arrow shows goblet cells in the villous (PAS), separation in the epithelium of villous. Upper row—10×; lower row–40×.
Morphometric analysis indicated that Rauwolfia serpentina supplementation had a significant impact on caecal morphology (Table 4). Villus height (VH) was significantly greater in the RSP-2 (707 μm) and RSP-1 (706 μm) groups compared to the SGC group (267 μm; p = 0.008). Crypt depth was also preserved in the RSP-2 (173 μm) and RSP-1 (167 μm) groups, significantly higher than in the SGC group (74.3 μm; p = 0.002). Although the VH ratio did not differ significantly among the groups (p = 0.675), the surface area of the caecum was significantly larger in the RSP-2 (738 μm) and RSP-1 (743 μm) groups compared to the SGC group (145 μm; p = 0.005). The number of goblet cells in the RSP-2 group was significantly higher (p = 0.01), with an average of 12 cells per section, compared to five cells in the SGC group. Acidic and neutral mucin levels did not differ significantly between treatments (p = 0.079 and p = 0.222, respectively).
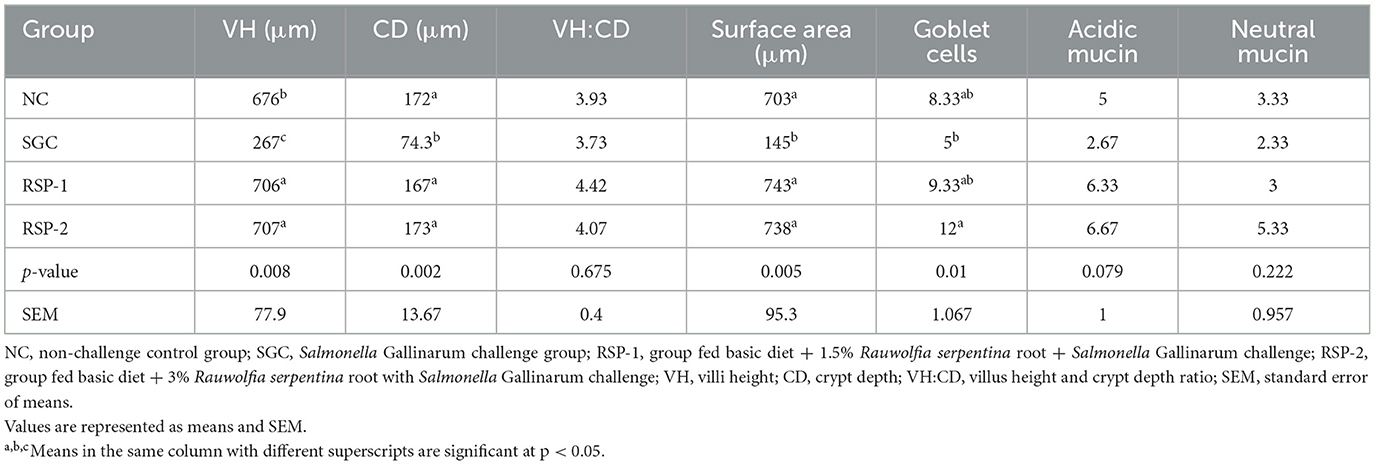
Table 4. Effect of Rauwolfia serpentina root powder supplementation on morphometric analysis of caecum of broiler chicks experimentally challenged with Salmonella Gallinarum (21 days post-challenge).
3.4 Growth performance
During the first week of age, no significant differences in weight gain, FCR, or feed intake were observed among the treatment groups. However, by day 28 (21 days post-challenge), Rauwolfia serpentina supplementation had marked effects on growth performance (Table 5). Birds in the RSP-2 group showed significantly higher weight gain (1,687 g) compared to other groups received SGC (p = 0.006) except NC group. The SGC group showed lower overall weight gain (1,455 g) compared to all other treatment groups (p = 0.001). The RSP-2 group also had significantly lower feed intake (2,379 g) than the other groups received SGC (p = 0.003), indicating better feed efficiency. In contrast, feed intake in the RSP-1 group (2,456 g) was comparable to the control groups. These results suggest that Rauwolfia serpentina supplementation at both levels offered protective effects against SGC, with the effects becoming more pronounced over time.

Table 5. Effect of Rauwolfia serpentina root powder supplementation on weight gain, FCR and feed intake of broiler chicks experimentally challenged with Salmonella Gallinarum.
4 Discussion
The GC-MS analysis of the methanol extract of Rauwolfia serpentina identified 30 distinct compounds based on their retention time, percentage area, molecular weight, and molecular formula (Supplementary Table 2). Among these, 12 compounds have been reported in the literature to exhibit various biological activities, including antibacterial, anticancerous, anti-inflammatory, and antioxidant properties (Table 3). Notably, compounds such as 2,4-di-tert-butylphenol, naphthalene, 7-hydroxycoumarin, oleic acid, farnesiferol A acetate, trismethoxyresveratrol (trans-), bis (2-ethylhexyl) phthalate, and 3-hydroxy-4,5-dimethoxystilbene, reserpine have been shown to influence the immune system (Table 3).
4.1 Gene expression analysis
Present study revealed significant variation in the expression of three key immune-related genes- SOCS3, P20K, and MHC class IIβ in broiler chicks supplemented with Rauwolfia serpentina and challenged with Salmonella Gallinarum. Supplementation with Rauwolfia serpentina, significantly modulated the tissue specific expression of these genes in response to Salmonella Gallinarum challenge.
In particular, gene expression patterns varied across the caecum, liver, and spleen. SOCS3 was significantly upregulated in the SGC group, followed by P20K, while MHC class IIβ expression was lowest in these tissues. The differential expression of immune-related genes in these organs following pathogen exposure underscores the importance of a coordinated immune response during the early stages of systemic infection (15). Following the invasion, Salmonella activates the host immune responses by invading macrophages and subsequent activation of pro-inflammatory cytokines such as IL-6 and TNF-α, responsible for the bird's innate and adaptive immune responses.
Suppressor of cytokine signaling 3 (SOCS3) plays a crucial role in regulating TNF-α, IL-6, and IL-12 (31, 32). In mouse dendritic cells, SOCS3 has been found to inhibit IL-12 expression, which can shift the immune response toward a Th2 profile along with decreased expression of MHC class IIβ (33). This explains the down-regulation of MHC class IIβ expression observed in SGC chicks. MHC class II, consisting of alpha and beta chains, is predominantly expressed on antigen-presenting cells such as macrophages, dendritic cells, and phagocytes. The variability of avian MHC haplotypes influences host susceptibility or resistance to various pathogens (34). In the current study, significant upregulation of SOCS3 in the caecum, liver, and spleen of birds challenged with Salmonella Gallinarum likely indicates an attempt to moderate the inflammatory response against infection (35). As the immune response of birds to Salmonella infection primarily involves a pro-inflammatory reaction, Salmonella is known to evade host immunity by upregulating the expression of SOCS3, thereby inhibiting the JAK/STAT pathway, leading to reduced production of cytokines like IFN-γ responsible for bacterial clearance and inhibition of MHC class IIβ expression to avoid detection of the immune response and persist in infection (36, 59).
A balanced adaptive immune response relies on the proper coordination and regulation of Th1 and Th2 cell activation, facilitated by Treg cells (37). The quiescence-related gene P20K (P20K) was significantly elevated in the liver and spleen of Rauwolfia serpentina-supplemented birds. This upregulation might indicate a Treg response, crucial in balancing the immune response to Salmonella Gallinarum. This differential expression of P20K across different tissues suggests that Rauwolfia serpentina might modulate host responses in a tissue-specific manner. This is particularly evident by an exhibition of different patterns of MHC Class IIβ expression across caeca, liver and spleen on days 3 and 21 post-challenge. MHC Class IIβ gene has a crucial role in initiating adaptive immune responses by presenting antigens to CD4+ T cells. On day 21 post-challenge, SGC birds exhibited the lowest expression of MHC class IIβ levels, whereas birds supplemented with Rauwolfia serpentina showed significantly higher expression levels indicating that Rauwolfia serpentina supplementation upregulated MHC class IIβ expression, in the spleen and caeca. This early immune response in current study is likely due to the initial activation of the immune system in response to both the dietary supplementation and Salmonella Gallinarum challenge. The downregulation of MHC class IIβ expression in the caecum of SGC birds aligns with previous findings (15), suggesting that Salmonella Gallinarum may evade host defense by suppressing MHC class IIβ expressions. Studies have shown that Salmonella Enteritidis can evade the Th1 immune response by inducing SOCS3 up-regulation, which in turn inhibits IFN-γ, leading to reduced MHC class IIβ expression (15, 38, 39). In contrast, the upregulated MHC class IIβ expression in the Rauwolfia serpentina-supplemented groups suggests that phytochemicals in the plant may promote persistent antigen presentation and enhance immune responses, even in the absence of active infection. This may be attributed to immune-boosting and anti-inflammatory properties of Rauwolfia serpentina (40). This tissue-specific difference observed in this study highlights the complexity of the immune responses to systemic pathogen challenges and aligns with previous studies of variable immune responses between systemic and localized immune systems in broiler chicks challenged with Salmonella Gallinarum (15). The immunomodulatory effects of Rauwolfia serpentina against Salmonella Gallinarum observed in the current study may be attributed to its potential chemical compounds, reserpine, saponins, tannins, flavonoids, and terpenoids. It particularly affects the release of norepinephrine with subsequent elevation of cytokine production and cell proliferation. We hypothesized that reserpine, along with other potential compounds present in Rauwolfia serpentina, may interplay host immune system, therefore activating several metabolic pathways. These pathways likely to enhance the production of antimicrobial peptides, enhanced IL-2 expression with reduced CTLA-4 expression therefore promoting T cell activation and proliferation in chickens (41). Additionally, reserpine has been shown to improve gut health by influencing antimicrobial pathways, mainly epidermal growth factor receptor (EGFR) and mammalian target of rapamycin (mTOR) and elevating AMP gene expression while decreasing the number of Enterobacteriaceae and Salmonella counts (10).
4.2 Intestinal morphology and gut health
The results of the current study revealed mucosal damage, as indicated by a reduced VH and CD in broiler birds challenged with Salmonella Gallinarum. However, supplementation with Rauwolfia serpentina, especially in the RSP-2 group, resulted to an increase in goblet cell numbers in the caeca, suggesting improved gut health. Goblet cells are essential for mucus secretion, forming a protective barrier against pathogens and facilitating nutrient absorption (42). The current study further revealed a significant suppressive effect of the Salmonella challenge on goblet cell numbers, as indicated by a notable decrease in goblet cell numbers in the caeca of SGC broiler chickens. This finding aligns with observations of gut morphology, where a reduced VH to CD ratio was evident in broiler chicks that received Salmonella Gallinarum challenge. Prior research has similarly demonstrated a significant decrease in VH and CD in the intestines of Salmonella Typhimurium-infected birds, indicating potential mucosal damage caused by this pathogen.
Intestinal morphology, measured through duodenal and ileal VH and CD, as well as the VH to CD ratio, serves as an indicator of gut health in broilers (42). In addition to increase in goblet cells as evident by histomorphology (Figure 3D) coupled with improved villus morphology (Figure 3; Table 4), mainly increase in surface area that helps in better nutrient absorption, indicating a larger surface area for enzymatic activity and nutrient uptake (43) suggests that Rauwolfia serpentina root may enhance the intestinal barrier function and overall gut health in broiler chicks against Salmonella Gallinarum.
4.3. Growth performance
In the present study, growth performance parameters further support the potential benefits of Rauwolfia serpentina supplementation against Salmonella Gallinarum. During the first week of the trial, no significant differences were observed in feed intake, weight gain, or FCR across different treatment groups. However, by the fourth week, the RSP-2 birds demonstrated the best FCR (lower values) among all treatment groups, suggesting a more efficient conversion of feed into body mass. The enhanced growth performance in the RSP-2 group could be attributed to improved gut health, as evidenced by increased VH and goblet cell numbers, potentially leading to better nutrient absorption. Another possible explanation for better FCR could be attributed to a well-regulated immune response, as indicated by the upregulation of key immune-related genes, allowing birds to combat Salmonella Gallinarum challenge more effectively without excessively diverting energy from growth. The potential effects of Rauwolfia serpentina phytochemicals include promoting beneficial gut microbiota that contributes to improved nutrient utilization (41).
The observation that the birds in RSP-2 group showed slightly better performance in terms of better weight gain and FCR than RSP-1 could be due to the presence of additional beneficial bioactive compounds in the whole root powder or more release of active ingredients and more prolonged action of active compounds in the gut, promoting more sustained immune modulation and better growth performance. The finding that RSP supplementation results in better weight gain and FCR compared to Salmonella Gallinarum challenge may be due to multiple reasons, such as improved intestinal morphology leading to better nutrient absorption, reduced pathogen load, and lower inflammation, which allows more energy to be directed toward growth rather than immune responses.
Our findings suggest that Rauwolfia serpentina supplementation may offer a multifaceted approach to improving the health and productivity of broiler chicks challenged with Salmonella Gallinarum. The combination of enhanced immune gene expression, improved gut morphology, and better growth performance presents a promising picture for the use of this phytogenic as a natural growth promoter and immunomodulator.
5 Conclusions
The study suggests that supplementation with Rauwolfia serpentina has effectively modulated the expression of key immune-related genes, enhanced gut morphology, and improved growth performance in broiler chicks challenged with Salmonella Gallinarum. Notably, Rauwolfia serpentina supplementation influenced the expression of key immune genes such as SOCS3, P20K, and MHC class IIβ, with differential patterns observed depending on the specific gene and treatment group. These findings highlight its potential as a phytogenic compound capable of modulating immune responses, reducing inflammation, and counteracting the immune evasion strategies employed by Salmonella Gallinarum. The varied changes in gene expression underscore the complexity of the immune-modulatory effects and warrant further investigation to elucidate the underlying mechanisms.
The significant increase in goblet cell numbers and improved villus morphology observed in the Rauwolfia serpentina-supplemented groups underscores the plant's positive effects on gut health, which may contribute to enhanced nutrient absorption and overall better growth performance. The findings suggest that the immune-modulatory and antimicrobial properties of Rauwolfia serpentina, particularly through compounds like reserpine, could play a crucial role in promoting beneficial gut microbiota and improving feed conversion ratios.
These results contribute to the growing body of evidence supporting the use of phytogenic compounds as immune booster in poultry production. Unlike previous studies that focused primarily on antimicrobial properties, our study is among the first to demonstrate Rauwolfia serpentina's tissue-specific modulation of key immune genes in broilers challenged with Salmonella Gallinarum. While our study provides valuable insights, several limitations should be addressed in future research: the study duration was relatively short. Long-term studies could provide insights into the sustained effects of Rauwolfia serpentina supplementation on bird health and performance. We focused on a limited number of immune-related genes. A more comprehensive gene expression analysis, possibly using RNA-seq, could provide a broader understanding of the immunomodulatory effects of Rauwolfia serpentina.
While these findings are promising, future research should address the limitations of the current study, including the relatively short duration and the focus on a limited number of immune-related genes. Investigating the expression of pro-inflammatory and anti-inflammatory cytokine genes could be particularly valuable in elucidating the balance of immune responses mediated by Rauwolfia serpentina supplementation. Long-term studies and comprehensive gene expression analyses could provide deeper insights into the sustained effects and broader immunomodulatory mechanisms of this phytogenic compound.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The animal study was approved by Animal Ethics Committee of the University of Veterinary and Animal Sciences, Lahore. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YZ: Funding acquisition, Writing – review & editing, Methodology, Formal analysis. HR: Writing – original draft. FK: Writing – review & editing. BK: Writing – review & editing, Formal analysis, Validation. MT: Data curation, Formal analysis, Validation, Visualization, Software, Writing – review & editing. SC: Writing – original draft, Methodology, Investigation. AR: Writing – review & editing, Methodology, Validation. MO: Writing – review & editing. AA: Writing – review & editing, Methodology. Qu: Writing – review & editing. MM: Writing – review & editing. GS: Conceptualization, Methodology, Writing – review & editing, Resources, Formal analysis, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by joint construction project of Henan Provincial Medical Science and Technology Research Program (LHGJ20190550) and National Health Commission Scientific Research Fund (SBGJ202301010).
Acknowledgments
Thanks to the laboratory staff for helping collecting and analyzing samples. Moreover, we appreciated to the editors and reviewers for their valuable contributions, which significantly enhanced the quality of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1534347/full#supplementary-material
References
1. Barrow PA, Neto OF. Pullorum disease and fowl typhoid—new thoughts on old diseases: a review. Avian Pathol. (2011) 40:1–13. doi: 10.1080/03079457.2010.542575
2. Saleem G, Farooq U, Naseer R, Aslam HB, Mustafa G, Omar MO, et al. Pathobiological and immunohistochemical findings in broiler chickens naturally infected with Salmonella Enterica Serotype Gallinarum biotype Gallinarum. Pak Vet J. (2022) 42:88–94. doi: 10.29261/pakvetj/2021.083
3. Filho Penha RAC, Ferreira JC, Kanashiro AMI, Darini ALDCA. Antimicrobial susceptibility of Salmonella Gallinarum and Salmonella Pullorum isolated from ill poultry in Brazil. Ciência Rural. (2016) 46:513–8. doi: 10.1590/0103-8478cr20150398
4. Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: a developing country-perspective. Front Microbiol. (2016) 7:1881. doi: 10.3389/fmicb.2016.01881
5. Selaledi Andrew L, Mohammed Hassan Z, Manyelo TG, Mabelebele M. The current status of the alternative use to antibiotics in poultry production: an African perspective. Antibiotics. (2020) 9:594. doi: 10.3390/antibiotics9090594
6. Bhardwaj N, Yadav M. Evaluation of the chemical composition of Rauwolfia serpentina and Leucas aspera–a comparative study. World J Pharm Pharm Sci. (2016) 5:914–20. doi: 10.20959/wjpps201610-7812
7. Bhanwaria R, Singh B, Gochar R. Indian snake root and devil root as distinctive medicinal plant for curing human disease: biology, chemistry and cultivation practices of Rauwolfia serpentina and Rauwolfia tetraphylla. In: Singh B, , editor. Plants for Novel Drug Molecules: Ethnobotany to Ethnopharmacology. New Delhi: New India Publishing Agency. (2021), p. 445–65.
8. Owk AK, Lagudu MN. In-vitro antimicrobial activity of roots of Rauwolfia serpentina L. Benth Kurz. Not. Sci. Biol. (2016) 8:312–6. doi: 10.15835/nsb839831
9. Singh HK, Charan AA, Charan AI, Prasad SM. Antifungal and antibacterial activity of methanolic, ethanolic and acetonic leaf extracts of sarpagandha (Rauwolfia serpentina). J Pharmacogn Phytochem. (2017) 6:152–6.
10. Redweik GA, Kogut MH, Arsenault RJ, Lyte M, Mellata M. Reserpine improves Enterobacteriaceae resistance in chicken intestine via neuro-immunometabolic signaling and MEK1/2 activation. Commun Biol. (2021) 4:1359. doi: 10.1038/s42003-021-02888-3
11. Kulkarni RC, Mandal AB, Dey SSS, Salunke VM. Rauwolfia serpentina: a stress alleviator of coloured broilers using incentive to feed in extreme summer. Indian J Poult Sci. (2017) 52:85–8.
12. Alshahrani MY, Rafi Z, Alabdallah NM, Shoaib A, Ahmad I, Asiri M, et al. A comparative antibacterial, antioxidant, and antineoplastic potential of Rauwolfia serpentina (L) leaf extract with its biologically synthesized gold nanoparticles (R-AuNPs). Plants. (2021) 10:2278. doi: 10.3390/plants10112278
13. Paul S, Thilagar S, Nambirajan G, Elangovan A, Lakshmanan DK, Ravichandran G, et al. Rauwolfia serpentina: a potential plant to treat insomnia disorder. Sleep Vigil. (2022) 6:31–40. doi: 10.1007/s41782-021-00192-y
14. Sansonetti PJ, Di Santo JP. Debugging how bacteria manipulate the immune response. Immunity. (2007) 26:149–61. doi: 10.1016/j.immuni.2007.02.004
15. Kaiser MG, Hsieh J, Kaiser P, Lamont SJ. Differential immunological response detected in mRNA expression profiles among diverse chicken lines in response to Salmonella challenge. Poult Sci. (2022) 101:101605. doi: 10.1016/j.psj.2021.101605
16. Ma X, Tian Y, Zhang W, Zhang R, Xu X, Han J, et al. Stress-induced immunosuppression inhibits immune response to infectious bursal disease virus vaccine partially by miR-27b-3p/SOCS3 regulatory gene network in chicken. Poult Sci. (2023) 102:103164. doi: 10.1016/j.psj.2023.103164
17. Qin H, Holdbrooks AT, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN, et al. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. (2012) 189:3439–48. doi: 10.4049/jimmunol.1201168
18. United States Pharmacopeia Convention. United States Pharmacopeia National Formulary, 26th Edn. Rockville, MD: United States Pharmacopeia Convention (2003), p. 2086.
19. Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J. (2017) 2017:5873648. doi: 10.1155/2017/5873648
20. Lawal AM, Abdullahi R, Ibrahim MS, Kurfi MY, Khalid A, Nuhu M, et al. Phytochemical analysis and thin layer chromatography profiling of crude extracts from Senna occidentalis (leaves). J Biotechnol Biomed Sci. (2019) 2:12–21. doi: 10.14302/issn.2576-6694.jbbs-19-2791
21. Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. (2002) 10:2748. doi: 10.38212/2224-6614.2748
22. Slinkard K, Singleton V L. Total phenol analyses: automation and caparison with manual methods. Am J Enol Viticult. (1997) 28:49–55. doi: 10.5344/ajev.1974.28.1.49
23. Kokate CK, Purohit AP, Gokhale SB. Pathway to screen phytochemical nature of natural drugs. In: Pharmacognosy, 39th ed. Pune: Nirali Prakashan (2007). pp. 607–11.
24. Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th online Edn. Gruver, TX: Texensis Publishing (2017).
25. Lee YJ, Kang MS, Woo YK, Mo IP, Tak RB. Competitive exclusion against Salmonella Gallinarum of Salmonella Enteritidis infected chickens. J Vet Sci. (2001) 2:33–6. doi: 10.4142/jvs.2001.2.1.33
26. Liaqat I, Hussain T, Qurashi AW, Saleem G, Bibi A, Qamar MF, et al. Antibiofilm activity of proteolytic enzymes against Salmonella Gallinarum isolates from commercial broiler chickens. Pakistan J Zool. (2021) 53:1111–8. doi: 10.17582/journal.pjz/20191029131040
27. Rio DC, Ares M, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. (2010) 6:5439. doi: 10.1101/pdb.prot5439
28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2– ΔΔCT method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
29. Khan RU, Naz S, Javdani M, Nikousefat Z, Selvaggi M, Tufarelli V, et al. The use of turmeric (Curcuma longa) in poultry feed. Worlds Poult Sci J. (2012) 68:97–103. doi: 10.1017/S0043933912000104
30. Santos DL, Donoghue FS, Farnell AM, Huff MB, Huff GR, Donoghue WE, et al. Gastrointestinal maturation is accelerated in turkey poults supplemented with a mannan-oligosaccharide yeast extract (Alphamune). Poul Sci. (2007). 86:921–30. doi: 10.1093/ps/86.5.921
31. Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. (2000) 18:143–64. doi: 10.1146/annurev.immunol.18.1.143
32. Fujimoto M, Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. (2003) 24:659–66. doi: 10.1016/j.it.2003.10.008
33. Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol. (2006) 177:1679–88. doi: 10.4049/jimmunol.177.3.1679
34. Miller KD, O'Connor S, Pniewski KA, Kannan T, Acosta R, Mirji G, et al. Acetate acts as a metabolic immunomodulator by bolstering T-cell effector function and potentiating antitumor immunity in breast cancer. Nature Cancer. (2023) 4:1491–507. doi: 10.1038/s43018-023-00636-6
35. Tang Y, Foster N, Jones MA, Barrow PA. Model of persistent Salmonella infection: Salmonella enterica serovar Pullorum modulates the immune response of the chicken from a Th17-type response towards a Th2-type response. Infect Immun. (2018) 86:10–1128. doi: 10.1128/IAI.00307-18
36. Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. (2002) 13:413–21. doi: 10.1016/S1359-6101(02)00026-6
37. Degen WG, van Daal N, Rothwell L, Kaiser P, Schijns VE. Th1/Th2 polarization by viral and helminth infection in birds. Vet Microbiol. (2005) 105:163–7. doi: 10.1016/j.vetmic.2004.12.001
38. Bertholet S, Dickensheets HL, Sheikh F, Gam AA, Donnelly RP, Kenney RT, et al. Leishmania donovani-induced expression of suppressor of cytokine signaling 3 in human macrophages: a novel mechanism for intracellular parasite suppression of activation. Infect Immun. (2003) 71:2095–101. doi: 10.1128/IAI.71.4.2095-2101.2003
39. He H, Genovese KJ, Kogut MH. Modulation of chicken macrophage effector function by TH1/TH2 cytokines. Cytokine. (2011) 53:363–9. doi: 10.1016/j.cyto.2010.12.009
40. Park BK, Kim YR, Kim YH, Yang C, Seo CS, Jung IC, et al. Antidepressant-like effects of Gyejibokryeong-Hwan in a mouse model of reserpine-induced depression. Biomed Res Int. (2018) 2018:5845491. doi: 10.1155/2018/5845491
41. Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. (2018) 49:1–18. doi: 10.1186/s13567-018-0562-6
42. Shao Y, Guo Y, Wang Z. β-1, 3/1, 6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella Enterica serovar Typhimurium. Poult Sci. (2013) 92:1764–73. doi: 10.3382/ps.2013-03029
43. Prakatur I, Miskulin M, Pavic M, Marjanovic K, Blazicevic V, Miskulin I, et al. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals. (2019) 9:301. doi: 10.3390/ani9060301
44. Yano T, Miyahara Y, Morii N, Okano T, Kubota H. Pentanol and benzyl alcohol attack bacterial surface structures differently. Appl Environ Microbiol. (2016) 82:402–8. doi: 10.1128/AEM.02515-15
45. Buakaew W, Krobthong S, Yingchutrakul Y, Khamto N, Sutana P, Potup P, et al. In vitro investigation of the anti-fibrotic effects of 1-phenyl-2-pentanol, identified from moringa oleifera lam, on hepatic stellate cells international. J Mol Sci. (2024) 25:8995. doi: 10.3390/ijms25168995
46. Rubab M, Chelliah R, Saravanakumar K, Barathikannan K, Wei S, Kim JR, et al. Bioactive Potential of 2-Methoxy-4-vinylphenol and Benzofuran from Brassica oleracea L. var capitate f, rubra (Red Cabbage) on oxidative and microbiological stability of beef meat. Foods. (2020) 9:568. doi: 10.3390/foods9050568
47. Sajid-Ur-Rehman M, Ishtiaq S, Khan MA, Alshamrani M, Younus M, Shaheen G, et al. Phytochemical profiling, in vitro and in vivo anti-inflammatory, analgesic and antipyretic potential of Sesuvium sesuvioides (fenzl) verdc(aizoaceae). Inflammopharmacology. (2021) 29:789–800. doi: 10.1007/s10787-021-00824-9
48. Connelly H, Means JC. Immunomodulatory effects of dietary exposure to selected polycyclic aromatic hydrocarbons in the bluegill (Lepomis macrochirus). Int J Toxicol. (2010) 29:532–45. doi: 10.1177/1091581810377518
49. Cai L, Mu YR, Liu MM, Zhou MY, Meng B, Liu FY, et al. Penta-acetyl Geniposide suppresses migration, invasion, and inflammation of TNF-α-stimulated rheumatoid arthritis fibroblast-like synoviocytes involving wnt/β-catenin signaling pathway. Inflammation. (2021) 44:2232–45. doi: 10.1007/s10753-021-01495-y
50. Natnan ME, Low CF, Chong CM, Jasmany MSM, Baharum SN. Oleic acid enriched diet affects the metabolome composition of the hybrid grouper infected with vibriosis. Fish Physiol Biochem. (2024) 50:1–16. doi: 10.1007/s10695-024-01389-4
51. Cebrián R, Li Q, Peñalver P, Belmonte-Reche E, Andrés-Bilbao M, Lucas R, et al. Chemically tuning resveratrol for the effective killing of gram-positive pathogens. J Nat Prod. (2022) 85:1459–73. doi: 10.1021/acs.jnatprod.1c01107
52. Choo QY, Yeo SCM, Ho PC, Tanaka Y, Lin HS. Pterostilbene surpassed resveratrol for anti-inflammatory application: potency consideration and pharmacokinetics perspective. J Funct Foods. (2014) 11:352–62. doi: 10.1016/j.jff.2014.10.018
53. Mu Q, Najafi M. Modulation of the tumor microenvironment (TME) by melatonin. Eur J Pharmacol. (2021) 907:174365. doi: 10.1016/j.ejphar.2021.174365
54. Adamovsky O, Buerger AN, Vespalcova H, Sohag SR, Hanlon AT, Ginn PE, et al. Evaluation of microbiome-host relationships in the zebrafish gastrointestinal system reveals adaptive immunity is a target of Bis (2-ethylhexyl) Phthalate (DEHP) exposure. Environ Sci Technol. (2020) 54:5719–28. doi: 10.1021/acs.est.0c00628
55. Xu Y, Jia W, Hu A, Wang J, Huang Y, Xu J, et al. Co-occurrence of light microplastics and phthalate esters in soils of China. Sci Total Environ. (2022) 852:158384. doi: 10.1016/j.scitotenv.2022.158384
56. Marques BC, Santos MB, Anselmo DB, Monteiro DA, Gomes E, Saiki MF, et al. Methoxychalcones: effect of methoxyl group on the antifungal, antibacterial and antiproliferative activities. Med Chem. (2020) 16:881–91. doi: 10.2174/1573406415666190724145158
57. Zhou D, Chang W, Liu B, Chen G, Yang Y, Hao Y, et al. Stilbenes from the tubers of Bletilla striata with potential anti-neuroinflammatory activity. Bioorg Chem. (2020) 97:103715. doi: 10.1016/j.bioorg.2020.103715
58. Zhou H, Lamont SJ. Global gene expression profile after Salmonella enterica Serovar enteritidis challenge in two F8 advanced intercross chicken lines. Cytogenet Genome Res. (2007) 117:131–8. doi: 10.1159/000103195
Keywords: Rauwolfia serpentina, Salmonella Gallinarum, immunity, gene expression, gut morphology, broiler chicks
Citation: Zhang Y, Rehman H, Khattak F, Tariq M, Khan BN, Chaman S, Riaz A, Ovais Omer M, Ali A, un Nisa Q, Muddassir Ali M and Saleem G (2025) Immunomodulatory and growth-promoting effects of Rauwolfia serpentina root powder in broiler chicks challenged with Salmonella Gallinarum. Front. Vet. Sci. 12:1534347. doi: 10.3389/fvets.2025.1534347
Received: 25 November 2024; Accepted: 06 January 2025;
Published: 03 February 2025.
Edited by:
Deji Abiodun Ekunseitan, North Carolina Agricultural and Technical State University, United StatesReviewed by:
Yawei Sun, Henan Institute of Science and Technology, ChinaAli Raza Jahejo, Guangxi University, China
Odinaka C. Iwuozo, North Carolina Agricultural and Technical State University, United States
Copyright © 2025 Zhang, Rehman, Khattak, Tariq, Khan, Chaman, Riaz, Ovais Omer, Ali, un Nisa, Muddassir Ali and Saleem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gulbeena Saleem, Z3VsYmVlbmEuc2FsZWVtQHV2YXMuZWR1LnBr
 Yingyu Zhang1
Yingyu Zhang1 Gulbeena Saleem
Gulbeena Saleem