
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 10 March 2025
Sec. Animal Nutrition and Metabolism
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1533872
This article is part of the Research TopicUnlocking Algae's Potential in Animal Nutrition and Sustainable AgricultureView all articles
 Yuliang Wu1,2
Yuliang Wu1,2 Yuxin Li1,2
Yuxin Li1,2 Mengli Chen1,2
Mengli Chen1,2 Juan Zhao3
Juan Zhao3 Xia Xiong2,4,5*
Xia Xiong2,4,5* Chen Guang Olnood3
Chen Guang Olnood3 Yundi Gao3
Yundi Gao3 Fei Wang3
Fei Wang3 Can Peng2,4
Can Peng2,4 Miao Liu6,7
Miao Liu6,7 Chunxia Huang5
Chunxia Huang5 Jianzhong Li1
Jianzhong Li1 Liuqin He1
Liuqin He1 Huansheng Yang1*
Huansheng Yang1* Yulong Yin1,2,4
Yulong Yin1,2,4The intestine is the largest immune and barrier organ in the body, and diarrhea and even death during piglet development are related to dysfunction caused by intestinal barrier damage and inflammation. A water-soluble β-glucan produced by Agrobacterium ZX09 has been shown to have a beneficial effect on gastrointestinal health. The main objective of this study was to investigate whether pre-feeding β-glucan has a protective effect on LPS-induced immune stress in piglets. In this study, 24 weaned piglets (21-day-old; 6.64 ± 0.16 kg) were assigned to 4 treatments in a two × two factorial design with diet (with or without β-glucan) and immunological challenge (saline or LPS). Piglets were challenged with saline or LPS after 39 days of feeding 0 or 200 mg/kg β-glucan. The results demonstrated that β-glucan supplementation increased the average daily weight gain and daily feed intake, and decreased diarrhea rate of piglets. Intestinal inflammation symptoms and histological changes in LPS-challenged piglets were alleviated by pre-feeding of β-glucan. β-glucan supplementation reduced serum IL-1β (interleukin-1β) and NO (nitric oxide) secretion in piglets after LPS challenge (0.01 < p < 0.05). Supplementation with β-glucan downregulated the mRNA expression of IL-6 in piglets after LPS challenge (0.01 < p < 0.05). β-glucan supplementation enriched the short-chain fatty acid-producing bacteria, such as Agathobacter and Subdoligranulum (0.01 < p < 0.05), and increased the concentrations of propionate and butyrate (0.01 < p < 0.05). In conclusion, pre-feeding β-glucan can enhance piglet immunity and promote piglet growth by influencing gut microbiota composition and metabolism, and alleviate intestinal damage after LPS challenge.
The intestinal tract is the largest immune and barrier organ in the mammalian body, and its function is to effectively prevent the invasion of foreign antigens, microorganisms, and toxins from the external environment into the interior of the organism. Piglet stress reduces feed intake, leads to intestinal dysfunction and diarrhea, and ultimately hinders growth (1, 2). The health of the piglet gut is critical to pig production, but the underlying mechanisms of intestinal epithelial cell injury currently require further investigation. Studies have shown a subtle correlation between disruption of intestinal epithelial barrier function and the development of an inflammatory state in the gastrointestinal tract (3–5). In addition, intestinal microorganisms may also be involved in the latent mechanisms of intestinal injury and repair (6, 7). In the presence of inappropriate epithelial injury and repair processes, the gut microbiota may deviate from a state of ecological imbalance or undergo migration. Therefore, it is critical to reduce intestinal inflammation or optimize the GI microbiota to maintain normal GI tract function.
Contemporary means of regulating gut health include promoting intestinal immunity and modifying the gut microbiota (8–10). β-glucan is a linear polysaccharide composed of D-glucose monomers linked by glycosidic bonds. It has been shown to selectively promote the growth or activity of intestinal bacteria, thereby impacting host health and the immune system (11, 12). Our previous study revealed that a water-soluble β-glucan (molecular weight 2000 kDa, purity 60%) from Agrobacterium zeylanicum ZX09 improved the growth performance of weaned piglets by altering the gut microbiota (13). In addition, this β-glucan reduced obesity in mice by enriching the beneficial flora, increasing short-chain fatty acid content in the cecum (14, 15), and alleviating dextrose sodium sulfate-induced colonic inflammation (16). Thus, this water-soluble β-glucan may be beneficial for the gastrointestinal health of animals. It has potential uses as an immunomodulator and for the development of functional foods. However, the role and mechanism of β-glucan in the treatment of intestinal inflammation in piglets is unknown.
In this study, we challenged piglets with LPS (lipopolysaccharide) after 4 weeks of β-glucan supplementation and investigated the effects of β-glucan on growth, intestinal immunity, and gut microbiology of piglets after LPS challenge.
The experimental design and procedures used in this study were approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (ISA-2021-00-20).
Twenty-four 21-day-old weaned piglets (Duroc × (Landrace × Yorkshire); 6.64 ± 0.16 kg) of similar body weight were randomly divided into four treatments in a 2 × 2 factorial design with diet (with or without β-glucan) and immunological challenge (saline or LPS). LPS was purchased from Sigma-Aldrich (E. coli serotype 055: B5; purity >99%; REF: L2880; St Louis, MO, USA). Following a 4-day pre-feeding period, two of the groups were provided with a basal diet, while the remaining two groups received a basal diet supplemented with 200 mg/kg of β-glucan (2,000 kDa; 50% β-glucan with 50% maltodextrin as carrier; Sichuan Synlight Biotech Ltd., Chengdu, China). Five weeks later, half of the piglets were injected intraperitoneally with LPS at 80 μg/kg body weight (17). The other half was injected with the same amount of sterilized saline. Within 4 h after the injection of LPS or normal saline, the piglets were fasted with free access to water before blood and intestinal samples were collected (18, 19) (Appendix Figure 1). The piglets were initially fed with the first-phase diet for the first 14 days, and then transitioned to the second-phase diet for the ensuing 21 days. The basal diet (Table 1) was formulated according to the National Research Council 2012 (NRC2012).
The piglets were given intravenous injections of 4% pentobarbital sodium solution after being given LPS or saline injections for 4 h. 15 mL of blood was collected from the jugular vein. Among them, 5 mL was placed for a routine blood test and flow cytometry after 0.5 h, and the other 10 mL was stained at 4°C for 4 h. The serum was centrifuged (3,500 g, 10 min) and separated in a 1.5 mL centrifuge tube and stored at −80°C. The digest was obtained from the colon. The ileum was cut longitudinally with scissors, and the digest and mucus in the intestinal cavity were washed with pre-cold saline. Furthermore, 2 cm intestinal segments were washed with pre-cold saline to remove the digesta before being fixed in a formalin fixation solution.
The piglets’ health status and daily feed intake for each replicate were monitored throughout the entire experiment. For the aim of calculating ADG (average daily gain), ADFI (average daily feed intake), and F/G (feed/gain), body weight was measured every week, and feed intake was noted every day. The diarrhea rate and diarrhea score were calculated according to Equation 1 and Equation 2 below, and the piglets’ feces were scored during the test period using the following criteria: 0 for normal, solid feces; 1 for soft, looser than normal feces, mild diarrhea; 2 for moderately diarrhoeic feces; 3 for liquid, severely diarrhoeic feces (20).
Blood was collected and placed into 5 mL Ethylenediamine Tetraacetic Acid collection tubes. Complete blood counts were performed using a Siemens hemology analyzer (Munich, Germany). Another 0.5 mL of blood sample was added to 5 mL of lysis buffer and incubated for 10 min. The reaction was stopped by diluting the lysis buffer with 10 mL of PBS. The cells were centrifuged at 4°C, and the pellet was resuspended in phosphate buffered saline. The following antibodies were used for flow cytometric analysis: anti-pig CD3 (PE, BD Biosciences, San Jose, CA, USA), anti-pig CD4 (PerCP-Cy5.5, BD Biosciences, San Jose, CA, USA), and anti-pig CD8 (Alexa 647, BD Biosciences, San Jose, CA, USA). Primary antibodies were diluted at 1:20 in PBS, after which 50 μL was added to the resuspension samples, and the mixtures were incubated for 30 min at room temperature. Cells were washed twice in PBS with 2% BSA and immediately analyzed on a FlowJo™ Software v10.8.1 (BD Life Sciences, USA). A total of 100,000 leukocytes were collected, and absolute cell counts were calculated directly by FlowJo Software.
The concentrations of proinflammatory cytokines in plasma and ileal mucosa were determined using porcine ELISA kits (TNF-α [tumor necrosis factor-α], REF: CSB-E16980; IL-1β [interleukin-1β], REF: CSB-E06782; IL-6, REF: CSB-E06786; sIgA [secretory immunoglobulin A], REF: CSB-E06786; Cusabio Biotech Co., Ltd., Hubei, China; NO [Nitric Oxide], REF: A013-2-1 Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
After being quickly frozen in liquid nitrogen, the ileal tissue was washed with cold saline and was embedded for cryosectioning. Frozen sections (8–10 μm) were stained with ROS (reactive oxygen species) staining solution at 37°C for 30 min in a light and humidified chamber. Stained sections were assessed using fluorescence microscopy. The fluorescence quantification was performed using Caseviewer on three fields per section and five sections per animal.
Histological evaluation was conducted according to the following procedures: The ileal and colon specimens were dehydrated, embedded in paraffin, cut into slices, and stained with hematoxylin and eosin after being fixed for 24 h. The degree of monocyte or neutrophil infiltration, histomorphology injury, intestinal epithelial cell dysplasia, or erosion was measured using Image-Pro Plus software to evaluate the morphological changes in the intestine. The histological evaluation result was divided into four grades: grade 1 (normal morphology scored 1–3); grade 2 (slight morphological injury scored 4–6); grade 3 (moderate morphological damage scored 7–9); and grade 4 (severe morphological damage scored 10–12).
RNA extraction and RT-qPCR were performed according to a previous study (21). Briefly, total RNA was isolated from the ileal mucosa with the RNeasy Kit (Accurate Biotechnology [Hunan] Co., Ltd.) in accordance with the instructions provided by the manufacturer. Then, by utilizing a PrimeScript RT reagent kit with a gDNA Eraser (Accurate Biotechnology [Hunan] Co., Ltd.), the RNA was reverse transcribed into cDNA. The SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology [Hunan] Co., Ltd.) was used for qRT-PCR, which was carried out with the employment of a LightCycle 480 real-time PCR system (Roche Diagnostics, Germany). The relative expression of each gene in the ileal mucosa was calculated by the 2−ΔΔCT method, with β-actin serving as the internal reference. Appendix Table 1 contains all primer sequences.
The ileum tissue’s total protein was extracted using Radio Immunoprecipitation Assay (Beyotime Institute of Biotechnology), which was then stored at −80°C for further study. Following the measurement of total protein concentration with Bicinchoninic acid assays (Beyotime Institute of Biotechnology), Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis separation, and transfer to PVDF membranes for western blotting. After being blocked for at least an hour, the membrane was incubated with primary antibodies for an entire night. p-NF-κB p65 (1:500; REF:3033S; Cell Signaling Technology, USA), NF-κB p65 (1:1000; REF:6956S; Cell Signaling Technology, USA), p-IκB (1:500; REF: 9246S; Cell Signaling Technology, USA), IκB (1:1000; REF: 4814S; Cell Signaling Technology, USA), β-actin (1:1000; REF: 4970S; Cell Signaling Technology, USA) were used as primary antibodies. After a 2-h incubation period with the goat anti-mouse or rabbit IgG (H + L) secondary antibody (1:5,000, Abiowell, Hunan, China), imprinting was detected by chemiluminescence. Using Image Lab software, the protein content was normalized to β-actin.
The microbial diversity of the colon was investigated following previously described procedures (22). The 16S V3 + V4 specific primers were used to extract and amplify bacterial DNA from colonic contents. Paired-end sequencing was carried out on the Illumina HiSeq 2,500 platform (Illumina, San Diego, California). Using Uchime1 and Cutadapt v.1.9.1, raw tags were assembled and filtered to produce clean data. Based on UPARSE (v7.0.1001) (23), sequences were grouped into the same OTU (operational taxonomic units) at a 97% similarity level. Through OTUs (Chao1, Shannon, and Simpson), alpha diversity and richness were calculated to analyze the complexity of species diversity for a sample. The differences in bacterial composition between groups were graphically displayed using the partial least squares discriminant analysis, which was carried out in the R language package “mixOmics.” The PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) software2 was additionally utilized to predict the function of the microflora. The assembled HiSeq sequences obtained in this study were submitted to the NCBI’s Sequence Read Archive (SRA, no. PRJNA899839).
The quantitative analysis of SCFAs (short chain fatty acids) was performed by gas chromatography in line with previous research (22). Briefly, the frozen digest was thawed and approximately 1.00 g of the sample was weighed. The samples were thoroughly mixed with ddH2O before centrifugation at 10,000 g for 10 min to obtain the supernatant. Metaphosphoric acid (25% w/v) was supplemented to the extracts at a ratio of 1:9. The supernatant was subjected to SCFA analysis after centrifugation at 10,000 g using an Agilent 7890A (Agilent Technologies, Santa Clara, CA, USA).
For comparisons between two groups, the data on the growth performance and diarrhea scores were analyzed using two-tailed t-tests (SPSS Inc., Chicago, IL, USA). The other data were analyzed using two-factor analysis of variance (SPSS Inc., Chicago, IL, USA) for a two × two factorial design with diet (0 or 200 mg/kg β-glucan), immunological challenge (saline or LPS) and their interactions as sources of variation. Tukey’s test was used to evaluate the difference between treatment groups, and p < 0.05 was used as the criterion for significance of the difference. 0.05 ≤ p < 0.10 was considered a significant trend. Where there was a significant trend for interaction, data were further analyzed using one-way ANOVA with Duncan’s multiple range tests. Correlations between bacterial abundance (at the general level) and proinflammatory cytokine levels in plasma and ileal mucosa SCFA levels were evaluated by Spearman’s correlation test using the R language package ‘Pheatmap.’
There was no significant difference in F:G between the groups from day 1 to 28 (p > 0.05). Dietary supplementation with β-glucan significantly increased the final body weight of piglets. Moreover, such supplementation significantly increased the ADG and ADFI of piglets (0.001 < p < 0.01; Table 2). In comparison to the control group, the β-glucan group had lower diarrhea scores on days 2, 3, 5 and 6 after weaning, and had lower diarrhea scores and rate on day 5 after weaning (0.01 < p < 0.05; Figures 1A,B).
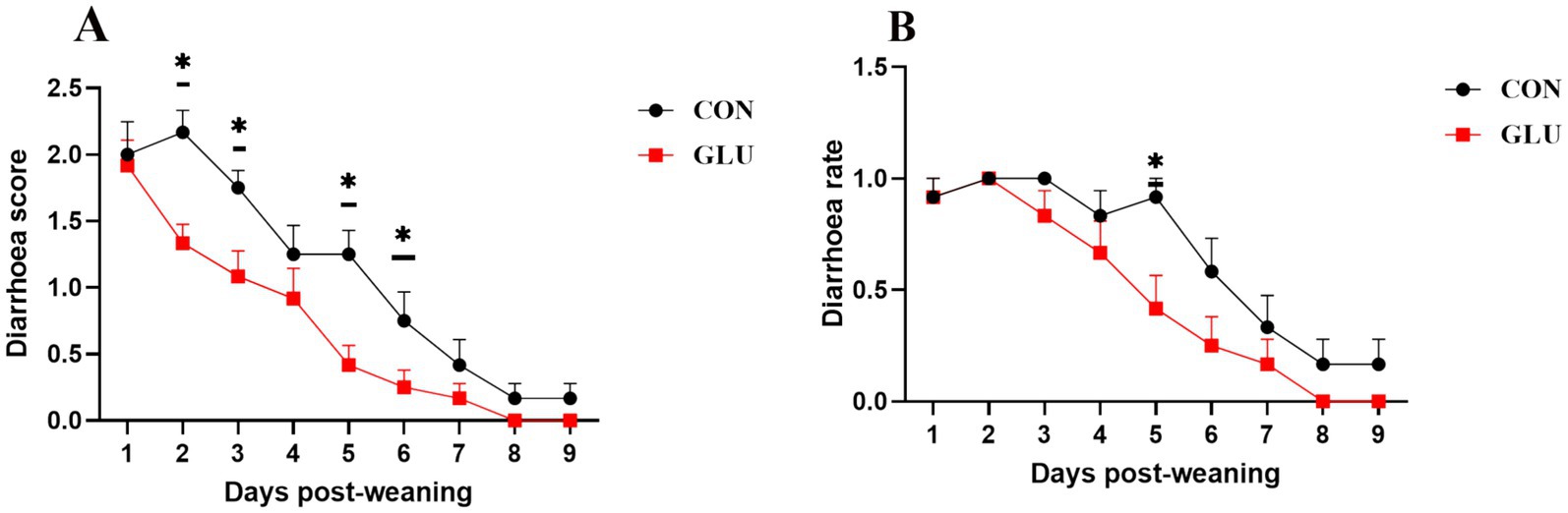
Figure 1. Dietary supplementation with β-glucan alleviate diarrhea of piglet. (A) Diarrhea score. (B) Diarrhea rate. Data were expressed as means ± SEM (n = 12). Significance was presented as *p < 0.05, **p < 0.01, and ***p < 0.001.
Intestinal inflammation in piglets was induced by intraperitoneal injection of LPS for 4 h, including an increase in leukocytes and the percentage of monocytes, eosinophils, basophils and CD8+ T cells in the whole blood and a decrease in the ratio of CD4+/CD8+ T cells (p < 0.05; Figures 2A,D–F,H,I). Dietary supplementation with β-glucan could attenuate the increase in lymphocytes, eosinophils, and CD8+ T cells after the LPS challenge (p < 0.001; Figures 2A,E,H).
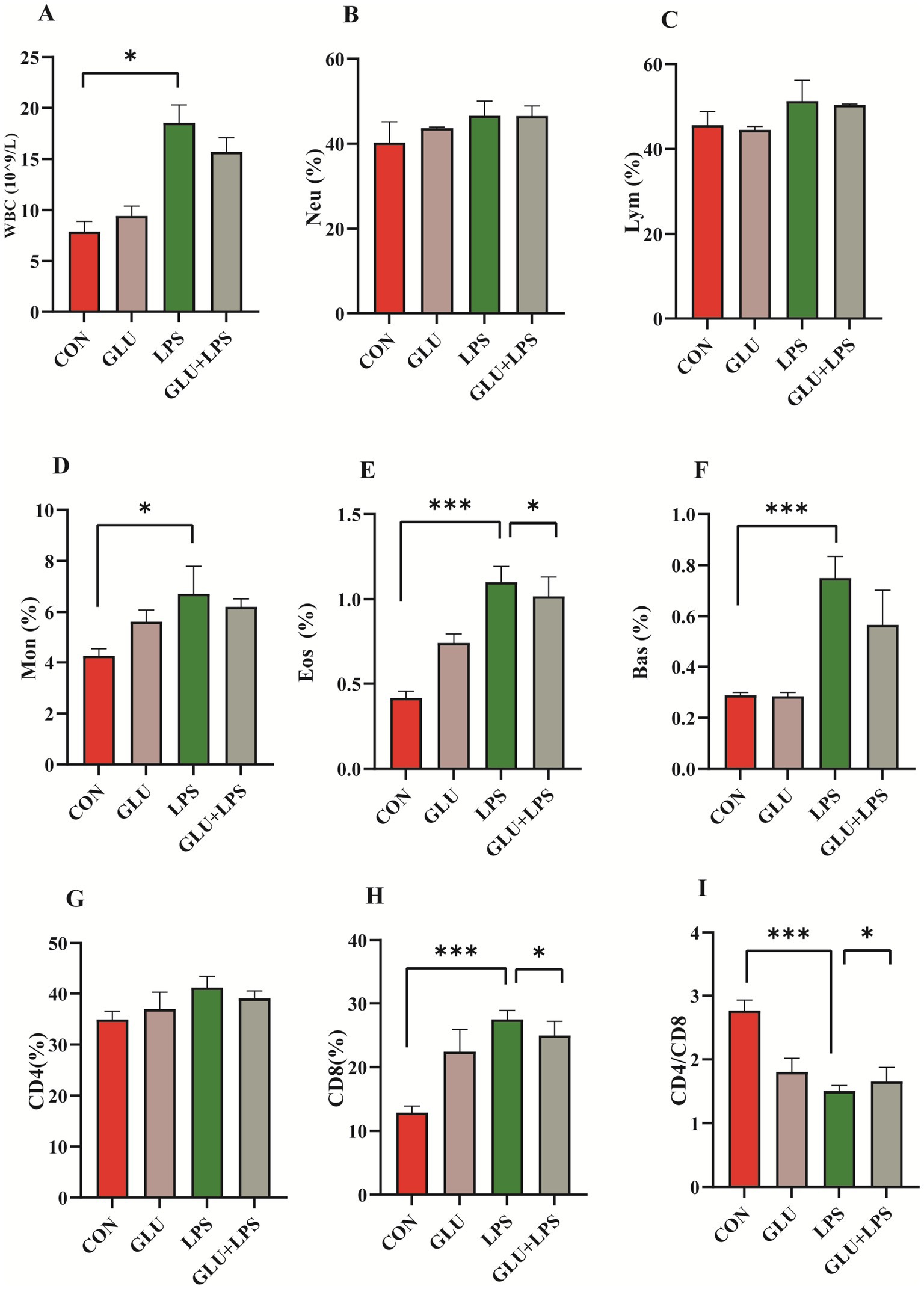
Figure 2. Dietary supplementation with β-glucan alleviate LPS-induced inflammation of piglet. (A–F) Blood cell composition. (G–I) Lymphocyte subsets composition. Data were expressed as means ± SEM (n = 6). Significance was presented as *p < 0.05, **p < 0.01, and ***p < 0.001.
Ileal and colonic tissue images stained with H&E showed significant tissue damage in LPS-treated piglets, including crypt loss, leukocyte infiltration and intestinal epithelial erosion. However, the addition of β-glucan significantly reduced these signs of damage (Figures 3A,B). LPS significantly increased ileal and colonic histological scores, which were significantly reduced by β-glucan supplementation (p < 0.001; Figures 3C,D). LPS and dietary β-glucan supplementation exerted no significant effect on ROS levels (p > 0.05; Figure 3E).
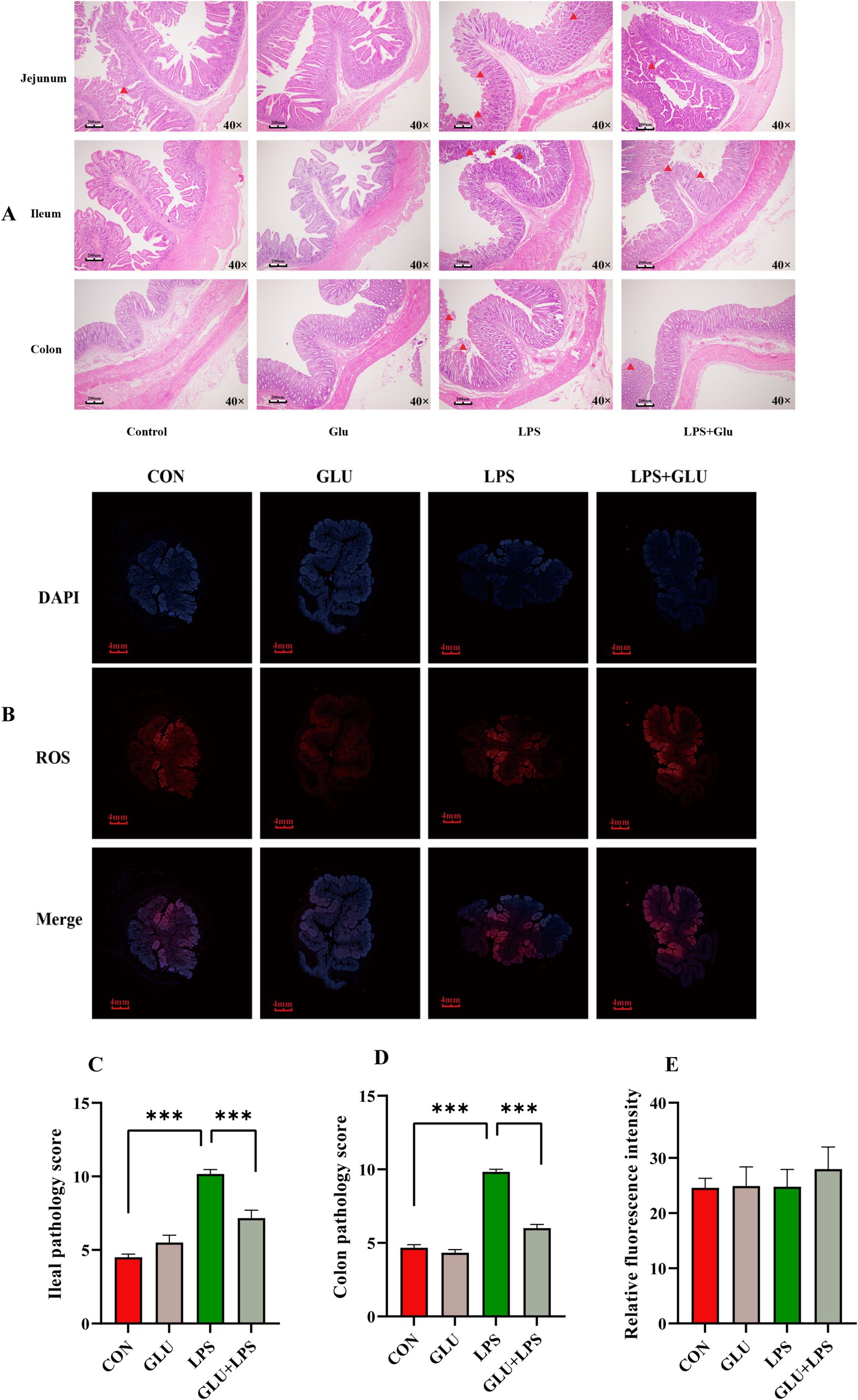
Figure 3. Dietary supplementation with β-glucan alleviate LPS-induced intestinal histological changes of piglet. (A,C,D) The H&E staining of the ileal and colon morphology of piglet. (B,E) The fluorescent micrographs of ROS (red) and DAPI (blue) staining of ileal. Data were expressed as means ± SEM (n = 6). Significance was presented as *p < 0.05, **p < 0.01, and ***p < 0.001.
LPS treatment significantly increased the levels of the cytokines IL-6 in the ileal mucosa (0.01 < p < 0.05; Figure 4B) and IL-1β (0.01 < p < 0.05; Figure 4F), sIgA (0.001 < p < 0.01; Figure 4H), and NO (p < 0.001; Figure 4I) in the serum. β-glucan supplementation significantly rescued the LPS-induced increase in serum IL-1β (0.01 < p < 0.05; Figure 4F) and NO levels (0.001 < p < 0.01; Figure 4I) and protected the piglet from intestinal inflammation.
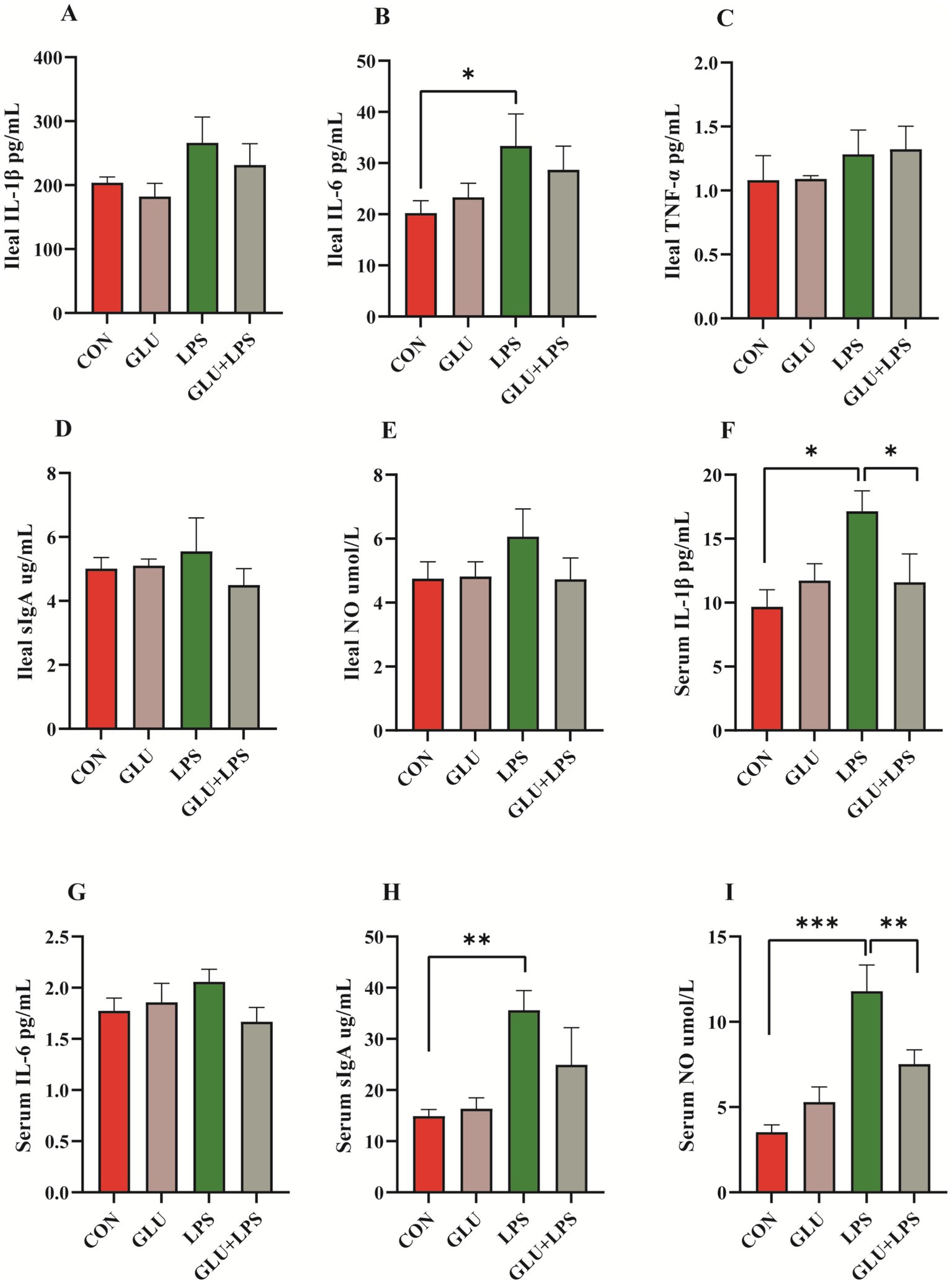
Figure 4. Dietary supplementation with β-glucan decreased the content of pro-inflammatory cytokines and NO of LPS-challenged piglet in plasma and ileal mucosa. (A–E) The concentrations of IL-1β, IL-6, TNF-α, sIgA and NO ileal mucosa. (F–I) The concentrations of IL-1β, IL-6, sIgA and NO in serum. Data were expressed as means ± SEM (n = 6). Significance was presented as *p < 0.05, **p < 0.01, and ***p < 0.001.
Treatment with LPS significantly increased the mRNA expression of NF-κB (nuclear factor kappa-B) (0.01 < p < 0.05; Figure 5A), MYD88 (myeloid differentiation factor 88) (0.001 < p < 0.01; Figure 5A), TLR-2 (Toll-like receptor-2) (0.01 < p < 0.05; Figure 5A), IL-1β, IL-6 and TNF-α (0.01 < p < 0.05; Figure 5B) and the protein expression of phosphorylated NF-κB (0.01 < p < 0.05; Figure 5D) and IκB (inhibitor of nuclear factor kappa-B) (0.05 < p < 0.1; Figure 5E). The mRNA expression of NF-κB, MYD88 (0.05 < p < 0.1; Figure 5A) and IL-6 (0.01 < p < 0.05; Figure 5B) of the NF-κB signaling pathway was reduced by supplementation of β-glucan after LPS treatment.
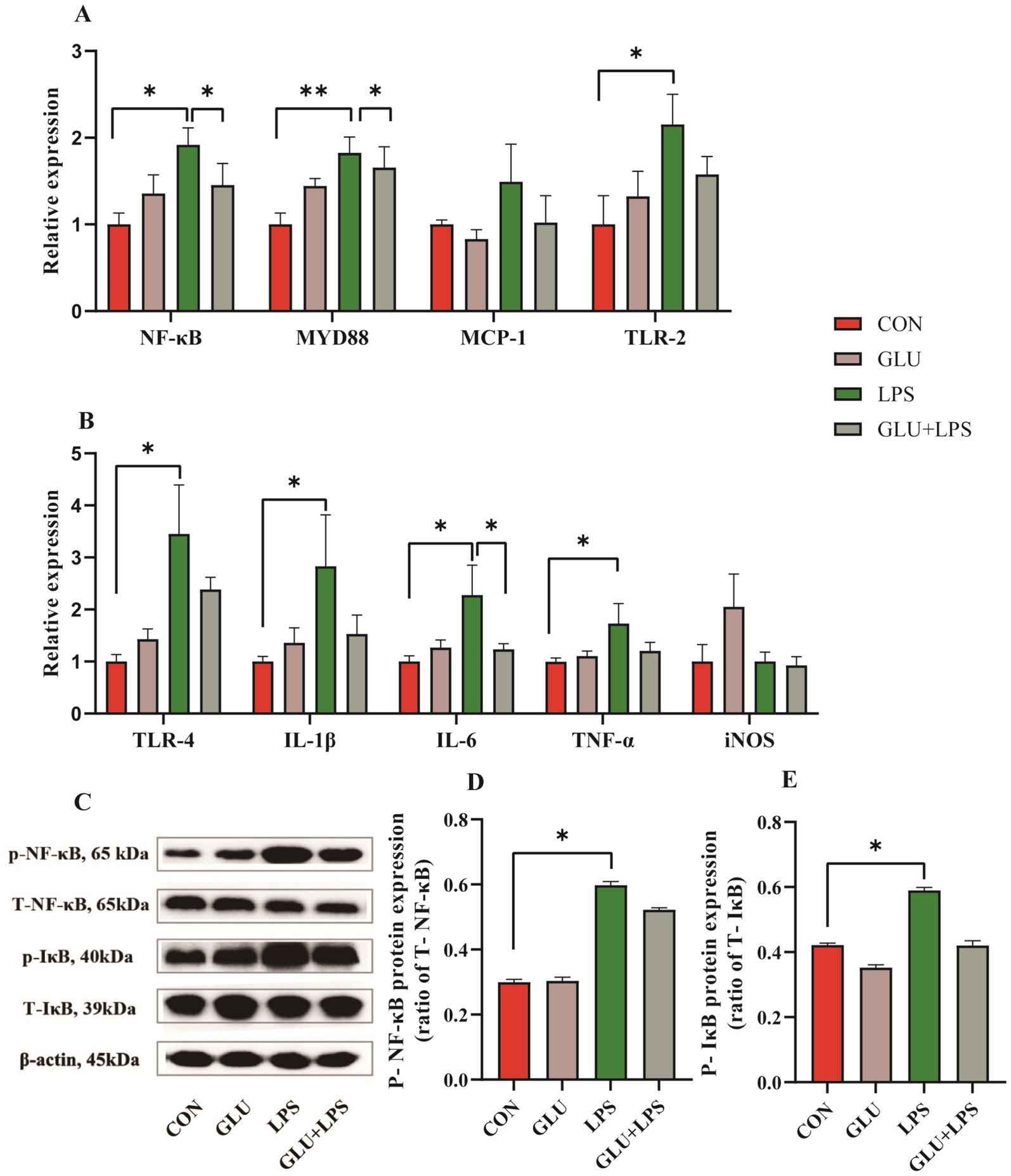
Figure 5. Dietary supplementation with β-glucan inhibited the NF-κB signaling in ileal tissues of piglet. (A,B) The mRNA relative expression of NF-κB, MYD88, MCP-1, TLR-2, TLR-4, IL-1β, IL-6, TNF-α and iNOS. (C) Representative protein bands for p-NF-κB, T-NF-κB65, p-IκB, and T-IκB. (D,E) Statistical analysis of protein bands. Data were expressed as means ± SEM (n = 3). Significance was presented as *p < 0.05, **p < 0.01, and ***p < 0.001.
The colonic microbial community of the piglets with different treatments was evaluated by a 16S rDNA phylogenetic method with OTUs >97% similarity. There was no significant difference between dietary β-glucan and LPS treatments in the abundance or uniformity (Appendix Table 2) of the colonic microflora. However, PLS-DA (Partial Least Squares Discriminant Analysis) (Figure 6A) at the OTU level revealed clear segregation and differences in microbiota composition between the four groups. Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Spirochetes were the dominant flora in the colons of piglets in each group. The relative abundance of the bacterial communities was examined at the phylum and genus level (Figure 6C) (Appendix Table 3). Compared to piglets fed a basal diet, β-glucan supplementation reduced the abundance of Synergistetes at the phylum level (0.01 < p < 0.05). The top 30 genera were selected for comparison. Agathobacter and Subdoligranulum had higher abundances after the addition of β-glucan (0.01 < p < 0.05). LEfSe (The linear discriminant analysis effect size) method (Figures 6D,E) was used to examine the composition of gut microbes. In the GLU group (β-glucan supplementation group), Prevotella, Faecalibacterium, Subdoligranulum, Prevotella-2 and Eubacterium emerged as dominant species.
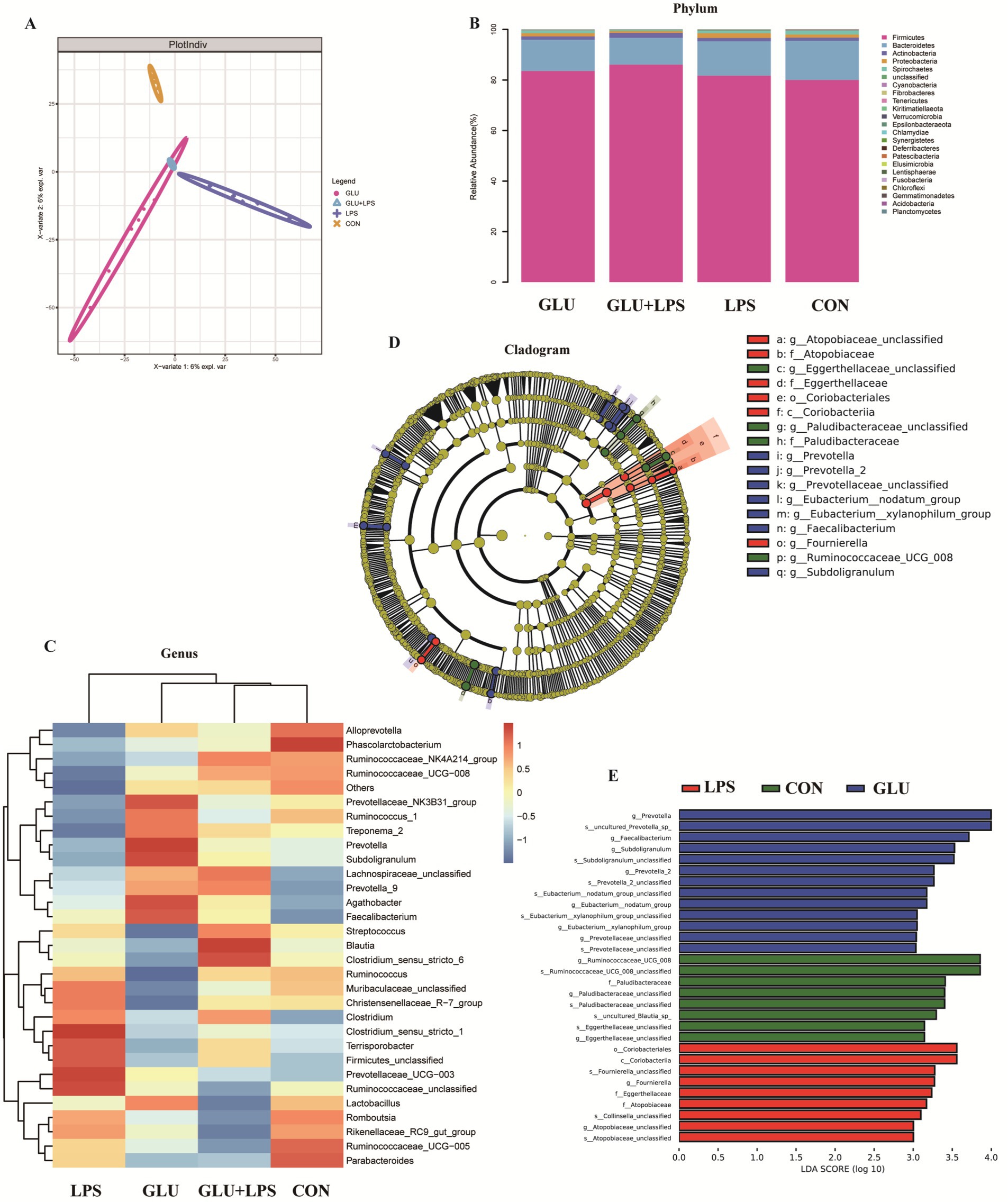
Figure 6. Dietary supplementation with β-glucan altered the composition of the colon microbiota in piglet. (A) Partial least squares discriminant analysis (PLS-DA) of gut microbiota. (B,C) Relative abundance of predominant bacteria was shown at the phylum and genus. (D) Taxonomic cladogram of LEfSe analysis. Different colors indicate the enrichment of the biomarker taxa in the control (green), LPS (red), and Glu (blue) group. The circle from inside to outside means the rank from kingdom to species, and the circle size represents the taxa abundance in the community. (E) Linear discriminant analysis (LDA) score for different taxa abundances. Data were expressed as means ± SEM (n = 6).
The PICRUST software was used to predict the role of the microbial communities, and the outcomes were compared to recognized metabolic pathways (Figures 7A,B). At KEGG level 3, the function of microbial genes involved in starch and sucrose metabolism, porphyrin and chlorophyll pathways were significantly reduced in the LPS group compared to the control group, while the function of microbial genes involved in steroid hormone biosynthesis was significantly increased. The Glu-LPS group (β-glucan supplementation and LPS treatment group) showed significantly lower gene function in the sugar transport system, phenylalanine, tyrosine and tryptophan biosynthesis and significantly higher gene function in the starch and sucrose metabolism pathways compared to the LPS group. The majority of the alterations were connected to the metabolism of carbon, starch, and sucrose. In addition, dietary β-glucan supplementation increased the levels of butyric acid and valeric acid in the colon, which further validated the above results (Figures 7D,E).
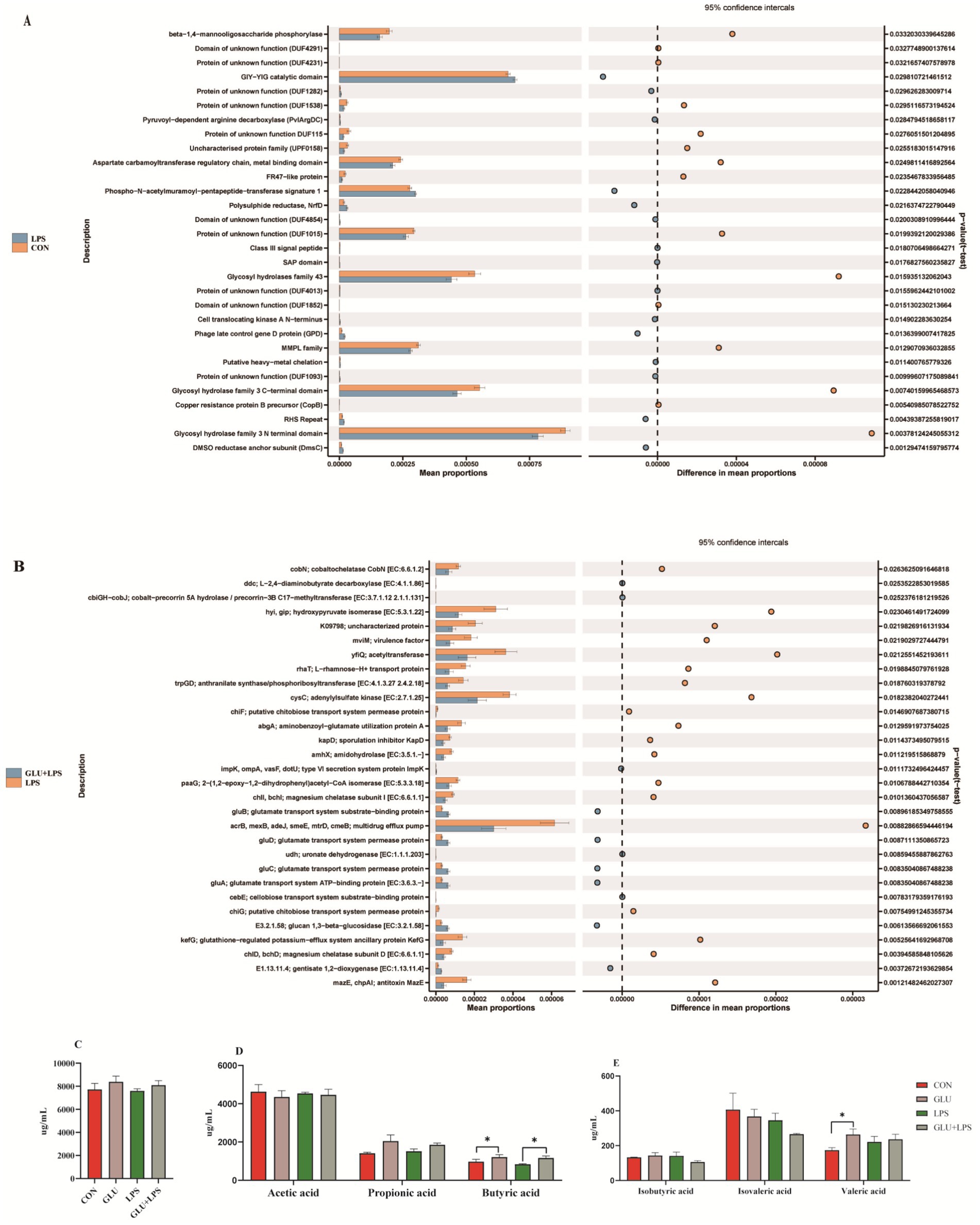
Figure 7. The pathway of different abundances of microflora at STAMP variance analysis (A,B) and short-chain fatty acid composition in colon (C–E). Data were expressed as means ± SEM (n = 6). Significance was presented as *p < 0.05, **p < 0.01, and ***p < 0.001.
The gut microbiota is involved in the regulation of host immunity. Therefore, the correlation between the levels of pro-inflammatory cytokines in serum and ileal mucosa was analyzed, the levels of SCFA in the colon and the abundance of colonic microorganisms. Spearman correlation analysis (Figure 8) was performed on the gut flora of weaned piglets at the genus level using R software. Acetate content was positively correlated with Agathobacter, Blautia, Prevotella_9 and Faecalibacterium and negatively correlated with Ruminococcacea and Muribaculaceae. Propionate content correlated positively with Prevotella_9. Butyrate content was negatively correlated with Ruminococcus_1. Isovalerate content was positively correlated with Ruminococcaceae_NK4A214_group, Treponema_2 and Faecalibacterium. Plasma IL-6 was positively correlated with Alloprevotella, Phascolarctobacterium, Streptococcus and Prevotellaceae_NK3B31_group and negatively correlated with Clostridium_sensu_stricto_1. The content of sIgA in ileal mucosa was positively correlated with Muribaculaceae, Ruminococcaceae_UCG-005, Rikenellaceae_RC9_gut_group and Ruminococcaceae_UCG-008. IL-1β in ileal mucosa was positively correlated with Firmicutes, Ruminococcaceae_NK4A214_group and Christensenellaceae_R-7_group and negatively correlated with Prevotella_9.
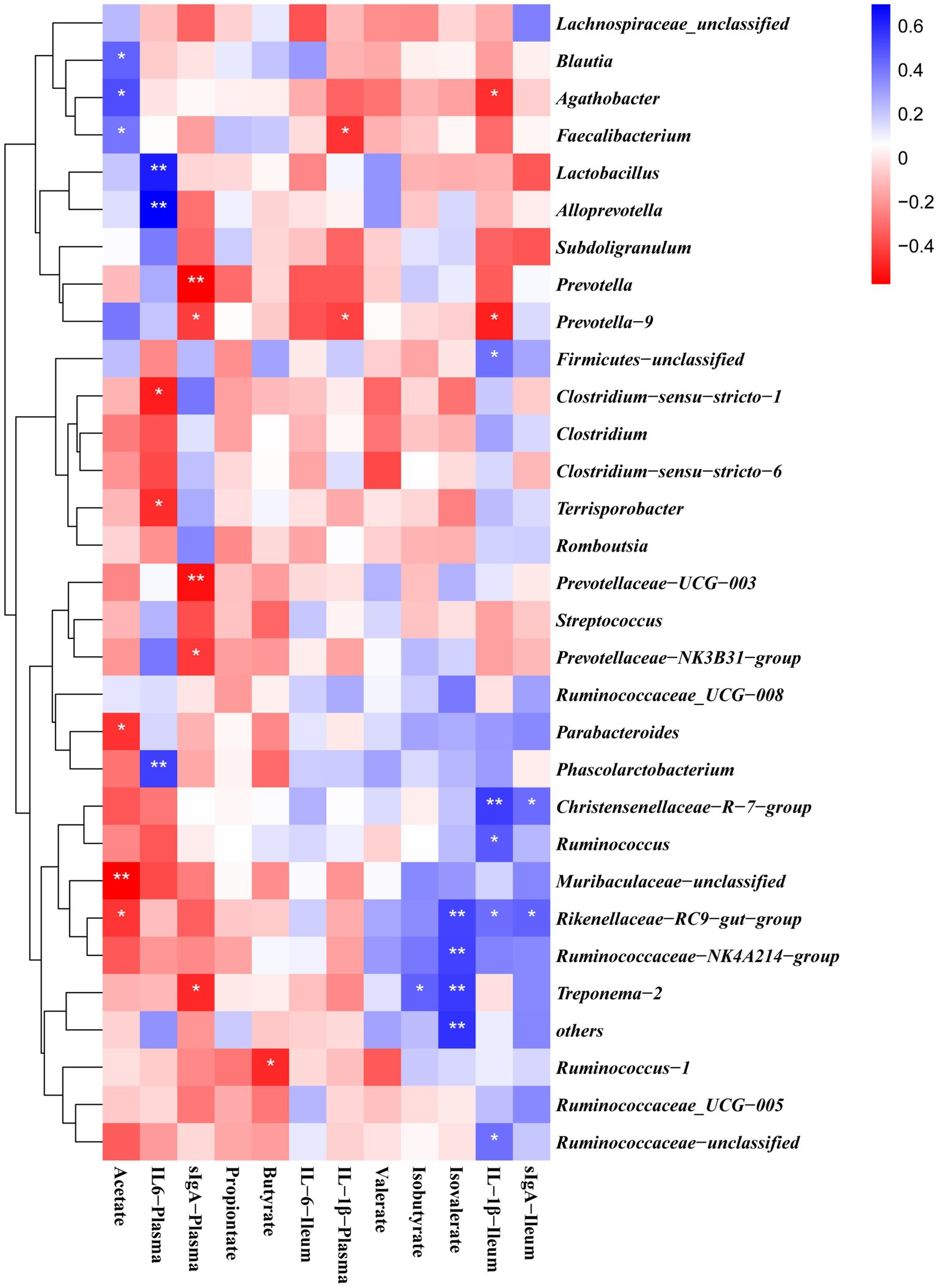
Figure 8. Correlations between bacterial abundance (at the genera level) and pro-inflammatory cytokines contents in plasma and ileal mucosa or colon SCFA content. The red represents a positive correlation, and the blue represents a negative correlation. *and **are used to indicate the statistical significance of the correlation, significance was presented as *p < 0.05, ** p < 0.01.
The small intestine is the main digestive and absorptive organ, as well as the largest immune and barrier organ in the body (24). The results of this study indicate that feeding β-glucan can enhance piglet immunity, alleviate intestinal damage after LPS challenge, and promote piglet growth through modulating gut microbiota composition and metabolism. Intraperitoneal injection of LPS, which is commonly used to induce intestinal inflammation in piglets (25, 26), and the composition of lymphocyte subsets, often regarded as an effective indicator to determine whether the body is producing inflammation (27). In this study, LPS was injected intraperitoneally to induce intestinal inflammation in piglets. The results showed a significant decrease in the CD4+/CD8+T cell ratio and a significant increase in the number of neutrophils and lymphocytes in the blood of piglets, indicating the successful establishment of a piglet inflammation model.
Intestinal morphology is one of the key indicators of intestinal health (28). LPS challenge can induce morphological changes in the intestine, including villus shedding, vacuolization and necrosis of intestinal epithelial cells (20, 29, 30). In this study, LPS caused intestinal epithelial erosion, villous atrophy, and monocyte and lymphocyte infiltration in the ileum and colon of piglets, but the addition of β-glucan reduced the histopathological scores of the ileum and colon tissues in piglets. Cytokines IL-1 β, IL-6, and TNF-α are involved in the occurrence of intestinal inflammation (31). Previous studies have shown that supplementation of feed with β-glucan reduces the TNF-α concentration in plasma of LPS-attacked piglets (32). Our current study revealed that LPS challenge significantly increased the levels of IL-6 in the ileal mucosa and IL-1 β in serum of piglets, whereas supplementation with β-glucan reduced the levels of IL-1 β in serum. In addition, NO produced by the body under normal conditions activates protective inflammatory pathways, which regulates the host response to exogenous pathogens, but overproduction of NO leads to its accumulation under abnormal conditions and can induce oxidative stress (33). In the current study, serum NO levels were significantly higher in LPS-treated piglets, whereas β-glucan significantly reduced serum NO levels. These results suggest that glucan may inhibit intestinal inflammation by mediating inflammatory factors.
The TLR/NF-κB signaling pathway is crucial for both innate and adaptive immune responses, and NF-κB is a major target of the inflammatory response (34). Activation of Toll like receptors leads to the production of IL-1 β, IL-6, and TNF-α, which participate in the immune response of bacteria and pathogens (35, 36). Studies have shown that pectin supplementation attenuates endotoxin attacks by inhibiting Toll-like receptor activation (20, 37). This study indicates that LPS exposure increased the mRNA expression levels of NF-κB, TLR-2, TLR-4, MYD88, IL-1β, IL-6 and TNF-α in piglet ileal mucosa as well as the protein expression levels of p-NF-κB and p-IκB in ileal tissue. Dietary supplementation with β-glucan reduced the mRNA expression of NF-κB and inflammatory cytokines, while having no effect on NF-κB protein expression. This might be attributed to the fact that β-glucan could reduce NF-κB mRNA expression either by inhibiting the DNA-binding activity of NF-κB or by inhibiting IKKbeta kinase activity (38, 39). However, due to the influence of post-translational transcriptional regulatory mechanisms of proteins or stress-induced compensatory mechanisms, β-glucan reduced the LPS-induced NF-κB mRNA expression but had no significant effect on protein levels (40, 41). This result is consistent with previous reports in that β-glucan supplementation effectively suppressed the elevated TLR-4 mRNA expression caused by LPS treatment (42). These findings suggest that β-glucan may inhibit LPS-induced inflammation by suppressing TLR/NF-κB activation.
Microorganisms mediate gastrointestinal metabolism, mucosal inflammation, and immune processes in the body, affecting gastrointestinal diseases such as inflammatory bowel disease and colorectal cancer (2, 43, 44). Indeed, the ecological imbalance of gut microbiota can lead to host immune dysfunction, and regulating the composition of gut microbiota can affect gut immunity (10). Some studies have shown that β-glucan feeding alters the cecal microbiota of rats by increasing the abundance of Bifidobacteria and Lactobacillus (32). Hence, we hypothesized that β-glucan supplementation may influence the microbiota composition in the piglet colon following LPS challenge. 16S rRNA analysis of colonic digest revealed that piglets administered with β-glucan had significantly altered gastrointestinal microbiota after LPS treatment, according to the current study. LPS challenge reduced the abundance of Ruminococcus_1 and Subdoligranulum, whereas β-glucan increased the relative abundance of beneficial bacteria such as Prevotella, Agathobacter, Faecalibacterium, Prevotella_9 and Subdoligranulum. β-glucan supplementation restored the colonic microbial community after LPS challenge.
We used PICRUST2 to examine the functional characteristics of the gut microbiota. The LPS challenge significantly lowered the functions of starch and sucrose metabolism, porphyrin and chlorophyll metabolism, and manno-oligosaccharide phosphorylation. In contrast, supplementing with β-glucan significantly improved the processes of tyrosine, starch, and sucrose metabolism. Thus, β-glucan supplementation may aid in regulating abnormal intestinal flora function brought on by LPS and maintaining intestinal homeostasis during weaning. In addition, we discovered that the most of the characteristically predicted changes in biological functions were associated with sugar metabolism, including the metabolism of starch, sucrose, and carbon. There was a significant increase in propionate and butyrate concentrations after β-glucan supplementation, which could be due to β-glucan promoting the development of microbial fermentation processes associated with an increased abundance of certain butyrate-producing bacteria, such as Subdoligranulum and Prevotella-9. Previous studies have shown that SCFAs can promote the maturation of the gastrointestinal tract, improve intestinal barrier function, and regulate body immunity, thereby reducing diarrhea rates and improving piglet growth performance (45–47). Correlation analysis revealed that the impact of β-glucan on the gut flora of piglets was strongly related to changes in the composition of SCFAs and inflammatory cytokines, and that enhancing the composition of the gut flora and its metabolites improved the gut function and growth performance of the host.
These findings indicate that β-glucan can enhance piglet immunity, alleviate intestinal damage after LPS challenge, and promote piglet growth by affecting gut microbiota composition and metabolism.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
YW: Writing – original draft. YL: Data curation, Writing – original draft. MC: Data curation, Formal analysis, Writing – original draft. JZ: Resources, Writing – original draft. XX: Funding acquisition, Writing – review & editing. CO: Resources, Writing – original draft. YG: Resources, Writing – original draft. FW: Resources, Visualization, Writing – original draft. CP: Methodology, Software, Writing – review & editing. ML: Methodology, Supervision, Writing – review & editing. CH: Formal analysis, Validation, Writing – review & editing. JL: Investigation, Supervision, Writing – review & editing. LH: Supervision, Writing – review & editing. HY: Conceptualization, Writing – review & editing. YY: Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This present study was jointly supported by National Natural Science Foundation of China (32130099), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28110100), and Sichuan Synlight Biotech Ltd.
JZ, CO, YG, and FW are currently employed by Sichuan Synlight Biotech Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study has received funding from Sichuan Synlight Biotech Ltd. The funder has the following involvement in the study: The funder was involved in aspects of research design and manuscript writing during the course of the study.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1533872/full#supplementary-material
1. Campbell, JM, Crenshaw, JD, and Polo, J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. (2013) 4:19. doi: 10.1186/2049-1891-4-19
2. Gresse, R, Chaucheyras-Durand, F, Fleury, MA, Van de Wiele, T, Forano, E, and Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. (2017) 25:851–73. doi: 10.1016/j.tim.2017.05.004
3. Liu YuLan, LY, Chen Feng, CF, Odle, J, Lin Xi, LX, Jacobi, S, Zhu HuiLing, ZH, et al. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr. (2012) 142:2017–24. doi: 10.3945/jn.112.164947
4. Barbara, G, Barbaro, MR, Fuschi, D, Palombo, M, Falangone, F, Cremon, C, et al. Corrigendum: inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. (2021) 8:718356. doi: 10.3389/fnut.2021.790387
5. Fasano, A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. (2011) 91:151–75. doi: 10.1152/physrev.00003.2008
6. Cheng, S, Ma, X, Geng, S, Jiang, X, Li, Y, Hu, L, et al. Fecal microbiota transplantation beneficially regulates intestinal mucosal autophagy and alleviates gut barrier injury. mSystems. (2018) 3:5. doi: 10.1128/msystems.00137-18
7. Reid, G, Younes, JA, Van der Mei, HC, Gloor, GB, Knight, R, and Busscher, HJJNRM. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. (2011) 9:27–38. doi: 10.1038/nrmicro2473
8. Wang, X, Zhang, P, and Zhang, XJM. Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules. (2021) 26:6076. doi: 10.3390/molecules26196076
9. Gareau, MG, Sherman, PM, and Walker, WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. (2010) 7:503–14. doi: 10.1038/nrgastro.2010.117
10. Zheng, D, Liwinski, T, and Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. (2020) 30:492–506. doi: 10.1038/s41422-020-0332-7
11. Xiu, A, Kong, Y, Zhou, M, Zhu, B, Wang, S, and Zhang, J. The chemical and digestive properties of a soluble glucan from Agrobacterium sp. ZX09. Carbohydr Polym. (2010) 82:623–8. doi: 10.1016/j.carbpol.2010.05.027
12. Zhou, Y, Luo, Y, Yu, B, Zheng, P, Yu, J, Huang, Z, et al. Agrobacterium sp. ZX09 β-Glucan attenuates enterotoxigenic Escherichia coli-induced disruption of intestinal epithelium in weaned pigs. Int J Mol Sci. (2022) 23:10290. doi: 10.3390/ijms231810290
13. Wu, Y, Li, X, Liu, H, Du, Y, Zhou, J, Zou, L, et al. A water-soluble β-glucan improves growth performance by altering gut microbiome and health in weaned pigs. Anim Nutr. (2021) 7:1345–51. doi: 10.1016/j.aninu.2021.04.006
14. Zhou, M, Pu, C, Xia, L, Yu, X, Zhu, B, Cheng, R, et al. Salecan diet increases short chain fatty acids and enriches beneficial microbiota in the mouse cecum. Carbohydr Polym. (2014) 102:772–9. doi: 10.1016/j.carbpol.2013.10.091
15. Zhou, M, Wang, Z, Chen, J, Zhan, Y, Wang, T, Xia, L, et al. Supplementation of the diet with Salecan attenuates the symptoms of colitis induced by dextran sulphate sodium in mice. Br J Nutr. (2014) 111:1822–9. doi: 10.1017/S000711451300442X
16. Chen, M, Tian, S, Li, S, Pang, X, Sun, J, Zhu, X, et al. β-Glucan extracted from Highland barley alleviates dextran sulfate sodium-induced ulcerative colitis in C57BL/6J mice. Molecules. (2021) 26:5812. doi: 10.3390/molecules26195812
17. Wang, X, Xiao, K, Yu, C, Wang, L, Liang, T, Zhu, H, et al. Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities. Anim Nutr. (2021) 7:609–20. doi: 10.1016/j.aninu.2020.11.008
18. Wu, H, Liu, Y, Pi, D, Leng, W, Zhu, H, Hou, Y, et al. Asparagine attenuates hepatic injury caused by lipopolysaccharide in weaned piglets associated with modulation of toll-like receptor 4 and nucleotide-binding oligomerisation domain protein signalling and their negative regulators. Br J Nutr. (2015) 114:189–201. doi: 10.1017/S0007114515001476
19. Nordgreen, J, Munsterhjelm, C, Aae, F, Popova, A, Boysen, P, Ranheim, B, et al. The effect of lipopolysaccharide (LPS) on inflammatory markers in blood and brain and on behavior in individually-housed pigs. Physiol Behav. (2018) 195:98–111. doi: 10.1016/j.physbeh.2018.07.013
20. Sun, Z, Li, H, Li, Y, and Qiao, J. Lactobacillus salivarius, a potential probiotic to improve the health of LPS-challenged piglet intestine by alleviating inflammation as well as oxidative stress in a dose-dependent manner during weaning transition. Front Vet Sci. (2020) 7:547425. doi: 10.3389/fvets.2020.547425
21. Qi, M, Tan, B, Wang, J, Li, J, Liao, S, Yan, J, et al. Small intestinal transcriptome analysis revealed changes of genes involved in nutrition metabolism and immune responses in growth retardation piglets1. J Anim Sci. (2019) 97:3795–808. doi: 10.1093/jas/skz205
22. Qi, M, Tan, B, Wang, J, Liao, S, Li, J, Cui, Z, et al. Postnatal growth retardation is associated with deteriorated intestinal mucosal barrier function using a porcine model. J Cell Physiol. (2021) 236:2631–48. doi: 10.1002/jcp.30028
23. Edgar, RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. (2013) 10:996–8. doi: 10.1038/nmeth.2604
24. König, J, Wells, J, Cani, PD, García-Ródenas, CL, MacDonald, T, Mercenier, A, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. (2016) 7:e196. doi: 10.1038/ctg.2016.54
25. Liu, Y, Huang, J, Hou, Y, Zhu, H, Zhao, S, Ding, B, et al. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. (2008) 100:552–60. doi: 10.1017/S0007114508911612
26. Liu, Y, Xu, Q, Wang, Y, Liang, T, Li, X, Wang, D, et al. Necroptosis is active and contributes to intestinal injury in a piglet model with lipopolysaccharide challenge. Cell Death Dis. (2021) 12:62. doi: 10.1038/s41419-020-03365-1
27. Wang, Z, Shao, Y, Guo, Y, and Yuan, J. Enhancement of peripheral blood CD8+T cells and classical swine fever antibodies by Dietaryβ-1,3/1,6-glucan supplementation in weaned piglets. Transbound Emerg Dis. (2008) 55:369–76. doi: 10.1111/j.1865-1682.2008.01049.x
28. Zhang, Y, Wang, Y, Chen, D, Yu, B, Zheng, P, Mao, X, et al. Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets. Food Funct. (2018) 9:4968–78. doi: 10.1039/C8FO01126E
29. Fan, C, Han, J, Liu, X, Zhang, F, Long, Y, and Xie, Q. Modulation of hypoxia-inducible factor-1α/cyclo-oxygenase-2 pathway associated with attenuation of intestinal mucosa inflammatory damage by Acanthopanax senticosus polysaccharides in lipopolysaccharide-challenged piglets. Br J Nutr. (2019) 122:666–75. doi: 10.1017/S0007114519001363
30. Xu, B, Yan, Y, Yin, B, Zhang, L, Qin, W, Niu, Y, et al. Dietary glycyl-glutamine supplementation ameliorates intestinal integrity, inflammatory response, and oxidative status in association with the gut microbiota in LPS-challenged piglets. Food Funct. (2021) 12:3539–51. doi: 10.1039/D0FO03080E
31. Zhu, W, Ren, L, Zhang, L, Qiao, Q, Farooq, MZ, and Xu, QJ. The potential of food protein-derived bioactive peptides against chronic intestinal inflammation. Mediators Inflamm. (2020) 2020:1–15. doi: 10.1155/2020/6817156
32. Zhou, TX, Jung, JH, Zhang, ZF, and Kim, IH. Effect of dietary β-glucan on growth performance, fecal microbial shedding and immunological responses after lipopolysaccharide challenge in weaned pigs. Anim Feed Sci Technol. (2013) 179:85–92. doi: 10.1016/j.anifeedsci.2012.10.008
33. Kim, YJ, Kim, E-H, and Hahm, KB. Oxidative stress in inflammation‐based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol. (2012) 27:1004–10. doi: 10.1111/j.1440-1746.2012.07108.x
34. Atreya, I, Atreya, R, and Neurath, MF. NF-κB in inflammatory bowel disease. J Intern Med. (2008) 263:591–6. doi: 10.1111/j.1365-2796.2008.01953.x
35. Sabroe, I, Parker, L, Dower, S, and Whyte, M. The role of TLR activation in inflammation. J Pathol. (2008) 214:126–35. doi: 10.1002/path.2264
36. Fukata, M, Vamadevan, AS, and Abreu, MT. Toll-like receptors (TLRs) and nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. (2009) 21:242–53. doi: 10.1016/j.smim.2009.06.005
37. Wen, X, Zhong, R, Dang, G, Xia, B, Wu, W, Tang, S, et al. Pectin supplementation ameliorates intestinal epithelial barrier function damage by modulating intestinal microbiota in lipopolysaccharide-challenged piglets. J Nutr Biochem. (2022) 109:109107. doi: 10.1016/j.jnutbio.2022.109107
38. Luhm, J, Langenkamp, U, Hensel, J, Frohn, C, Brand, JM, Hennig, H, et al. β-(1→3)-D-glucan modulates DNA binding of nuclear factors κB, AT and IL-6 leading to an anti-inflammatory shift of the IL-1β/IL-1 receptor antagonist ratio. BMC Immunol. (2006) 7:5. doi: 10.1186/1471-2172-7-5
39. Williams, DL, Ha, T, Li, C, Laffan, J, Kalbfleisch, J, and Browder, WJS. Inhibition of LPS-induced NFkappaB activation by a glucan ligand involves down-regulation of IKKbeta kinase activity and altered phosphorylation and degradation of IkappaBalpha. Shock. (2000) 13:446–52. doi: 10.1097/00024382-200006000-00005
40. Singh, R, Chandel, S, Ghosh, A, Matta, T, Gautam, A, Bhattacharya, A, et al. Glucogallin attenuates the LPS-induced signaling in macrophages and protects mice against Sepsis. Int J Mol Sci. (2022) 23:11254. doi: 10.3390/ijms231911254
41. Takasuka, N, Matsuura, K, Yamamoto, S, and Akagawa, KS. Suppression of TNF-alpha mRNA expression in LPS-primed macrophages occurs at the level of nuclear factor-kappa B activation, but not at the level of protein kinase C or CD14 expression. J Immunol. (1995) 154:4803–12. doi: 10.4049/jimmunol.154.9.4803
42. Luo, J, Cheng, L, Du, Y, Mao, X, He, J, Yu, B, et al. The anti-inflammatory effects of low-and high-molecular-weight beta-glucans from Agrobacterium sp. ZX09 in LPS-induced weaned piglets. Food & Function. (2020) 11:585–95. doi: 10.1039/C9FO00627C
43. Ashida, H, Ogawa, M, Kim, M, Mimuro, H, and Sasakawa, C. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol. (2012) 8:36–45. doi: 10.1038/nchembio.741
44. Ni, J, Wu, GD, Albenberg, L, and Tomov, VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. (2017) 14:573–84. doi: 10.1038/nrgastro.2017.88
45. Koh, A, De Vadder, F, Kovatcheva-Datchary, P, and Bäckhed, FJC. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. (2016) 165:1332–45. doi: 10.1016/j.cell.2016.05.041
46. Chen, T, Chen, D, Tian, G, Zheng, P, Mao, X, Yu, J, et al. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Anim Feed Sci Technol. (2020) 260:114335. doi: 10.1016/j.anifeedsci.2019.114335
Keywords: β-glucan, piglet, diarrhea, intestinal inflammation, lipopolysaccharide, intestinal microbiota
Citation: Wu Y, Li Y, Chen M, Zhao J, Xiong X, Olnood CG, Gao Y, Wang F, Peng C, Liu M, Huang C, Li J, He L, Yang H and Yin Y (2025) The effect of a water-soluble β-glucan on intestinal immunity and microbiota in LPS-challenged piglets. Front. Vet. Sci. 12:1533872. doi: 10.3389/fvets.2025.1533872
Received: 25 November 2024; Accepted: 07 February 2025;
Published: 10 March 2025.
Edited by:
Mohamad Padri, King Abdullah University of Science and Technology, Saudi ArabiaReviewed by:
Zheng Niu, Northwest A&F University, ChinaCopyright © 2025 Wu, Li, Chen, Zhao, Xiong, Olnood, Gao, Wang, Peng, Liu, Huang, Li, He, Yang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Xiong, eHhAaXNhLmFjLmNu; Huansheng Yang, eWhzQGh1bm51LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.