- 1College of Animal Science and Technology, Shihezi University, Shihezi, China
- 2Kazakh National Agrarian Research University, Almaty, Kazakhstan
- 3Department of Forest, College of Agriculture, Shihezi University, Shihezi, China
- 4NHC Key Laboratory of Prevention and Treatment of Central Asia High Incidence Diseases, School of Medicine, Shihezi University, Shihezi, China
Tick-borne pathogens (TBPs) are a global public health issue. However, there have been few reports on the prevalence of piroplasms, Anaplasma, and Ehrlichia in Kazakhstan. To understand the distribution of piroplasms, Anaplasma, and Ehrlichia pathogens carried by ticks in Kazakhstan, a total of 10,461 ticks were collected from natural hosts (e.g., cattle, sheep, and horses) in six oblasts in eastern, southern, and western Kazakhstan between 2022 and 2024. After morphological identification, 272 representative ticks were further used for species-level detection and partial genotyping analysis of TBPs. Two Babesia species (Babesia occultans and Babesia caballi), four Theileria species (Theileria orientalis, Theileria equi, Theileria annulata, and Theileria ovis), two Anaplasma species (Anaplasma phagocytophilum and Anaplasma ovis), and three Ehrlichia species were detected. Furthermore, genotype B of B. caballi, genotype 1 (Chitose) of T. orientalis, and genotype A of T. equi were confirmed. For the first time, A. phagocytophilum, three phylogeny-independent Ehrlichia spp., genotype B of B. caballi, and genotype A of T. equi were found in Kazakhstan. These findings expand our understanding of the geographical distribution of piroplasms, Anaplasma, and Ehrlichia in Central Asia.
1 Introduction
As hematophagous ectoparasites, ticks can transmit a variety of zoonoses (1, 2). Babesia, Theileria, Anaplasma, and Ehrlichia are tick-borne pathogens (TBPs) that infect a variety of reservoir animals, including domestic animals (e.g., cattle, sheep, and horses) and wildlife. Babesia and Theileria, belonging to the order of Piroplasmida, can cause babesiosis and theileriosis in animals and occasionally in humans. To date, more than 50 species piroplasmida in domestic and wild species have been reported (3–7). Anaplasma and Ehrlichia belong to the order of Anaplasmataceae, with at least eight validated Anaplasma species and eight identified Ehrlichia species.
Kazakhstan, which covers 2,724,900 km2 in Central Asia, is listed as the ninth largest country in the world. TBPs play a vital role in veterinary medicine and public health. Some TBPs, such as Crimean–Congo hemorrhagic fever virus, spotted fever rickettsia, and tick-borne encephalitis virus, have already been reported in Kazakhstan (8–10). However, there have been few reports on the prevalence of piroplasms, Anaplasma, and Ehrlichia in Kazakhstan. In the present study, we aimed to detect Babesia, Theileria, Anaplasma, and Ehrlichia in ticks parasitizing cattle, horses, sheep, pet dogs, and hens in the east, south, and west regions of Kazakhstan.
2 Materials and methods
2.1 Tick sampling
From March to May in 2022, 2023, and 2024, an extensive tick sampling program was conducted in six oblasts of Kazakhstan (Jetysu, Jambyl, Almaty, Turkistan, Kyzylorda, and Aktobe oblasts). Parasitic ticks were collected from the whole body of cattle, horses, sheep, pet dogs, and hens.
2.2 Identification of ticks
Morphological identification was conducted on all of the collected ticks (n = 10,461) (11, 12). The ticks’ morphological features were examined under a stereoscopic dissecting microscope. After the morphological identification, 272 representative ticks were selected for DNA extraction using the TIANamp Genomic DNA Kit (TIANGEN, Beijing, China) following the manufacturer’s instructions. The obtained genomic DNAs from these representative ticks were then subjected to molecular identification using the fragments of cytochrome c oxidase subunit 1 (cox1) and 16S rDNA genes (Appendix Table 1).
2.3 Isolation and identification of piroplasms, Anaplasma, and Ehrlichia pathogens
The detection of piroplasms, Anaplasma, and Ehrlichia was performed by nested PCR. We used the universal primers of 18S rRNA gene to detect Theileria and Babesia. Anaplama and Ehrlichia were detected using a partial 16S rRNA gene (Appendix Table 1). The DNAs of Theileria equi, Babesia caballi, Anaplasma ovis and Ehrlichia spp. in our laboratory were used as positive controls (13–15). Double-distilled water was used as a negative control. The amplified products were cloned into the pGEM-T Easy Vector (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions and then subjected to Sanger sequencing. To gain insights into the evolutionary relationships and taxonomic affiliations of the identified pathogens, the obtained nucleotide sequences were queried against the GenBank database using BLASTn.1 Additionally, phylogenetic trees were constructed employing the Neighbor-Joining (NJ) algorithm within MEGA11 software (bootstrap replicates 1,000).
3 Results
Five tick species belonging to three genera were identified from 272 representative ticks, namely Hyalomma scupense (n = 126), Hyalomma asiaticum (n = 34), Hyalomma anatolicum (n = 75), Rhipicephalus turanicus (n = 24), and Argas persicus (n = 13). A total of 11 TBPs were detected: Theileria orientalis, Theileria equi, Theileria ovis, Theileria annulata, Babesia occultans, Babesia caballi, Anaplasma phagocytophilum, Anaplasma ovis, and three phylogeny-independent Ehrlichia spp. (shown in Figures 1, 2 and Table 1). The information on the pathogens’ sequence similarities and their geographical distribution in this study is presented in Appendix Table 2.

Figure 1. Phylogenetic analysis of Babesia spp. and Theileria spp. in ticks collected in Kazakhstan. The tree was constructed using the Neighbor-Joining (NJ; bootstrap replicates: 1000) method based on the sequence data for 18S rRNA genes with MEGA11.0. The sequences of the Theileria species from ticks obtained in this study are indicated by solid circles (●), and those of the Babesia species are indicated by solid squares (■).
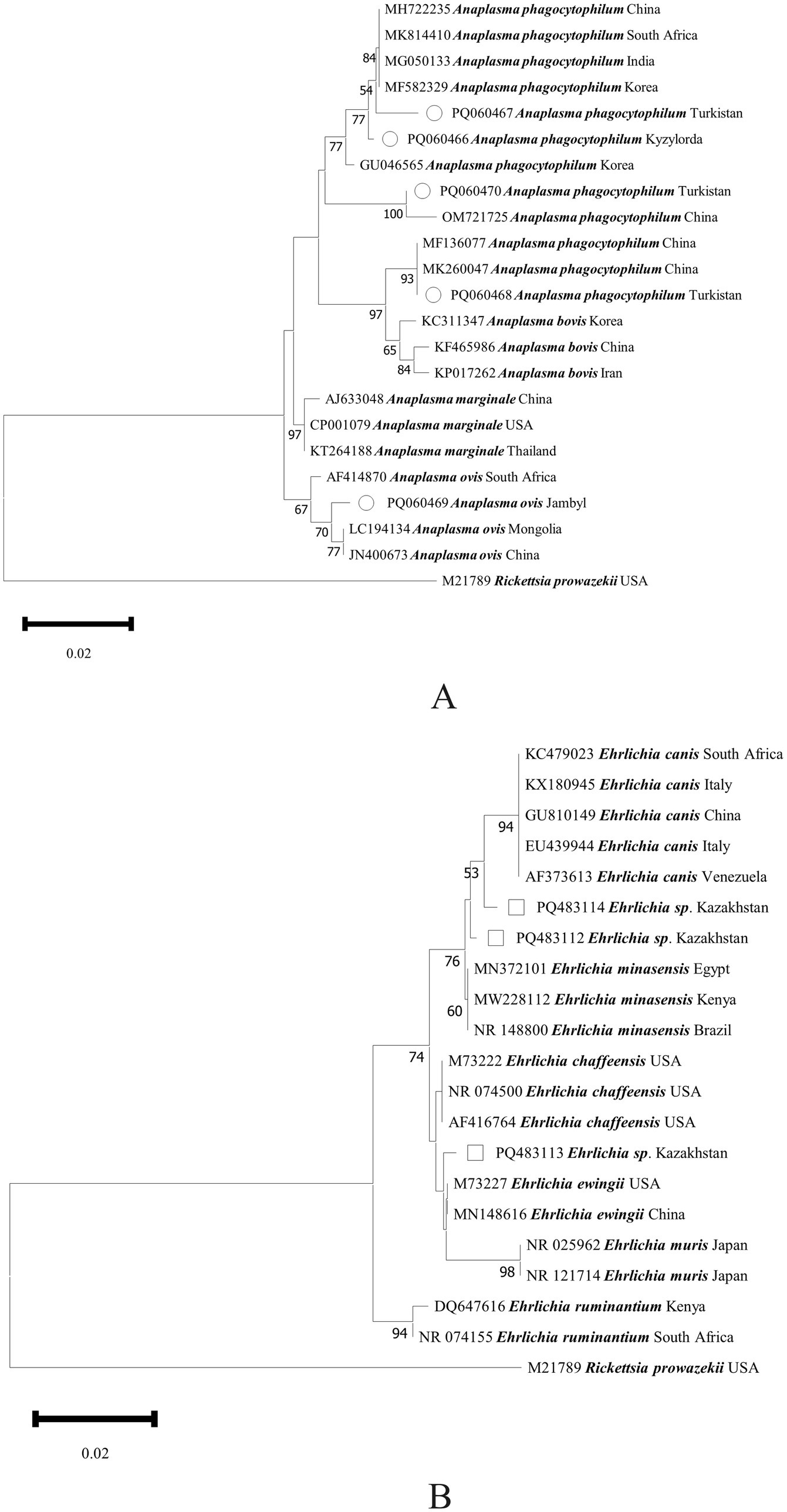
Figure 2. Phylogenetic analysis of Anaplasma spp. (A) and Ehrlichia spp. (B) in ticks collected in Kazakhstan. The tree was constructed using the Neighbor-Joining (NJ; bootstrap replicates: 1000) method based on the sequence data for 16S rRNA genes with MEGA11.0. Anaplasma spp. are indicated by hollow circles (○), and Ehrlichia spp. are indicated by hollow squares (□).
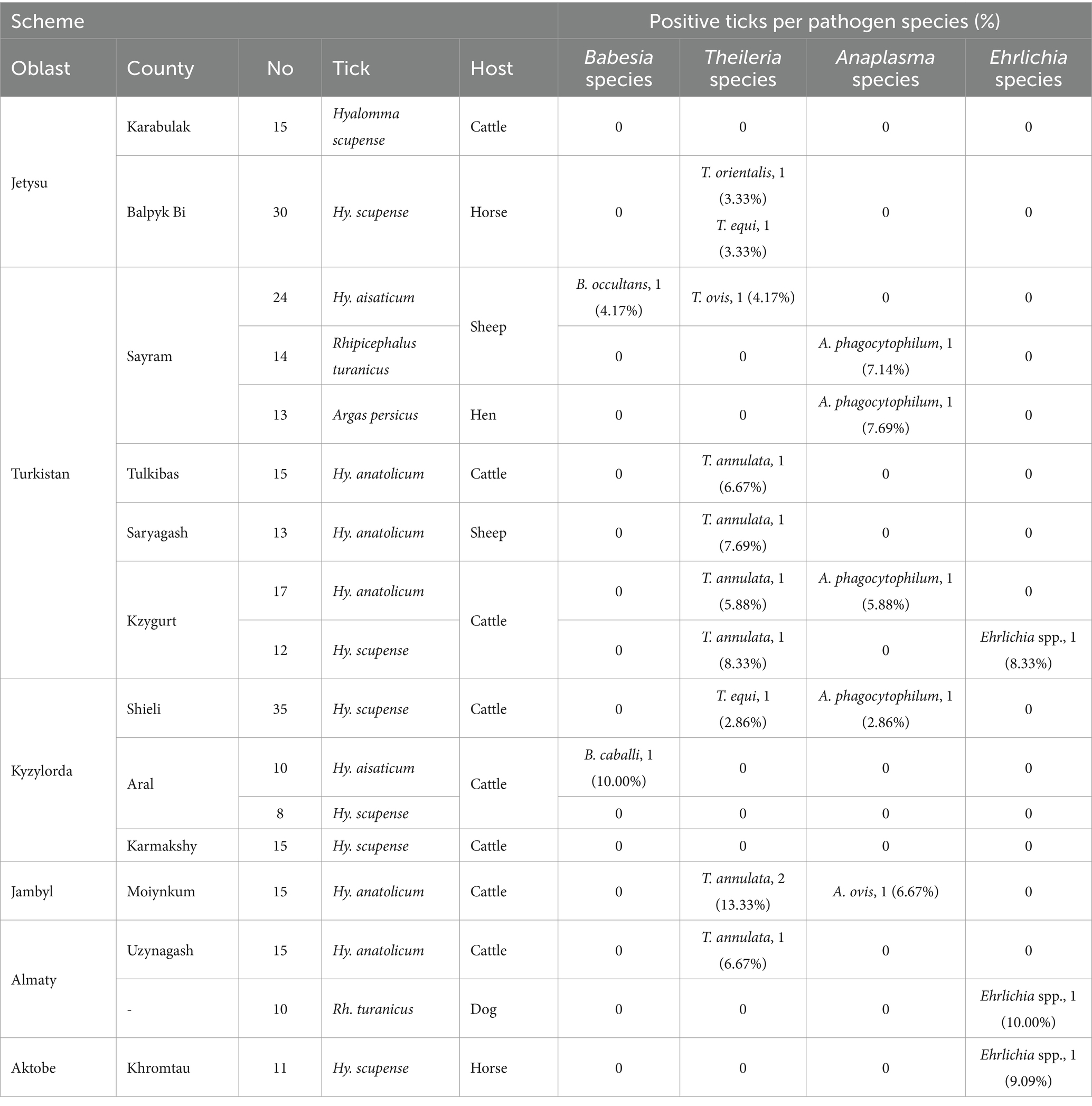
Table 1. Detection of piroplasms, Anaplasma, and Ehrlichia spp. in ticks sampled from six oblasts of Kazakhstan.
Furthermore, T. orientalis genotype 1 (Chitose) (PQ056491), T. equi genotype A (PQ056492, PQ056499), and B. caballi genotype B (PQ056500) were confirmed (Figure 3), which were clustered with those from Australia (AB520953), the United States (JX177671), and South Africa (Z15104), respectively.
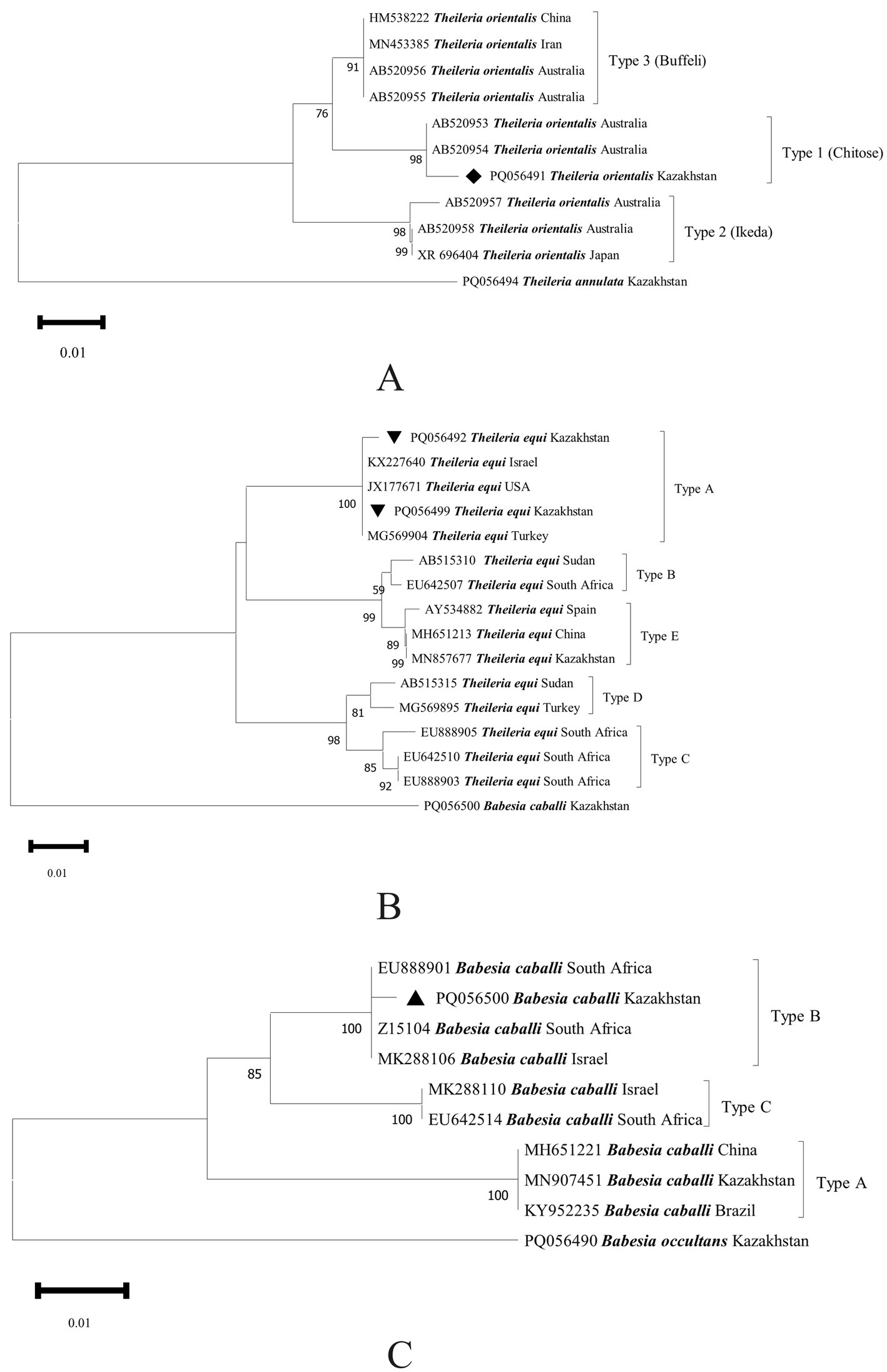
Figure 3. Phylogenetic tree of Theileria orientalis (A), T. equi (B), and B. caballi (C) genotypes inferred from the partial sequences of the 18S rRNA gene. The sequences of T. orientalis obtained in this study are indicated by solid diamonds (◆), those of T. equi are indicated by inverted triangles (▼), and those of B. caballi are indicated by solid triangles (▲).
4 Discussion
Kazakhstan is located in Central Asia, bordered by countries such as China, Russia, Kyrgyzstan, and Turkmenistan. International trade of domestic animals and their products is common. In this study, two Babesia species, four Theileria species, two Anaplasma species, and three independent Ehrlichia species were molecularly identified in hard ticks collected in six oblasts of southern and western Kazakhstan. Piroplasms, Anaplasma, and Ehrlichia are tick-borne pathogens of economically and medically important diseases (16–18). Domestic and wild animals play the roles of reservoirs, carriers, and disseminators in the epidemiology of many tick-borne pathogens. When vertebrates become infected, they may develop babesiosis, theileriosis, anaplasmosis, and ehrlichiosis (18–20). These diseases restrict livestock production and even impact public health in developing countries, including Kazakhstan.
In summary, the prevalence rate of both B. occultans and B. caballi in Hy. asiaticum stands at 2.93%. In Hy. scupense, the prevalence rate for T. orientalis and T. annulata is 0.79%, whereas that for T. equi and Ehrlichia spp. is 1.59%. In Rhipicephalus turanicus, the prevalence rate for both A. phagocytophilum and Ehrlichia spp. is 4.17%. In Hy. anatolicum, the prevalence rate for A. phagocytophilum and A. ovis is 1.33%, with T. annulata having a prevalence rate of 8.00%. And in Argas persicus, the prevalence rate for A. phagocytophilum is 7.69%. Furthermore, genotypes 1 (Chitose) of T. orientalis, genotype A of T. equi, and genotype B of B. caballi were confirmed.
Previously, T. annulata and B. caballi were detected in hard ticks in Turkistan oblast (South Kazakhstan) (5). T. annulata, T. orientalis, B. bigemina, B. major, and B. occultans were detected in bovine blood from Turkistan and Jambyl oblasts, and genotypes 1 (Chitose) and 3 (Buffeli) of T. orientalis were further confirmed (21). B. caballi, T. annulata, T. equi, B. occultans, and T. ovis were detected in hard ticks in Almaty and Turkistan oblasts, and T. equi genotype E and B. caballi genotype A were also confirmed (22). In this study, Babesia species (B. caballi genotype B) and two Theileria species (T. orientalis genotype 1 [Chitose] and T. equi genotype A) were found in Kyzylorda and Jetysu oblasts (southern and eastern Kazakhstan) for the first time. These findings indicate more genetic diversity among piroplasms in Kazakhstan.
Only three Anaplasma species were previously reported in bovine blood samples in Kazakhstan, namely A. ovis in Turkistan oblast, A. marginale in Kyzylorda oblast, and A. centrale in North Kazakhstan oblast (GenBank accession nos.: PQ133423, PQ038050, and PQ038051). In 2015, our team detected A. phagocytophilum in Hy. asiaticum ticks in Almaty oblast (KU723458). Here, A. phagocytophilum was first screened out in Turkistan and Kyzylorda oblasts. In Kazakhstan’s neighboring countries, A. ovis strains were detected in Hy. marginatum, Rh. turanicus, and Dermacentor spp. ticks in Kyrgyzstan and clustered with those in China (MG869525) (23). A. ovis strains in Rh. turanicus and Hy. anatolicum ticks were detected in China and clustered with those in Tunisia (KY659323), Pakistan (MT311202), Italy (GQ130291), and Turkey (OQ167969) (24). In this study, A. ovis was detected in Hy. anatolicum in Jambyl oblast, and it showed an independent clade, although it is comparatively close to those found in sheep blood in Mongolia (LC194134) and China (JN400673). A. phagocytophilum was commonly detected in hard ticks. Meanwhile, it was rarely found in soft ticks, including A. lahorensis, A. japonicus, and A. persicus in China (GenBank accession nos.: MG668811, MN795629, and ON807566). Here, A. phagocytophilum strains were detected in A. persicus and hard ticks (e.g., Hy. anatolicum, Hy. scupense, and Rh. turanicus), and they showed high genetic diversity, especially in the 74–84 bp fragment with U02528 as the original sequence for comparison (Appendix Table 3). To date, 14 genotypes have been reported in A. phagocytophilum. Given the lack of data on Anaplasma in Central Asia, more investigation on Anaplasma should be done in the future.
To date, Ehrlichia includes eight validated species, such as E. chaffeensis, E. ewingii, and E. canis, along with numerous indeterminate species reported. Previously, multiple indeterminate Ehrlichia strains were detected in Amblyomma longirostre, Am. cajennense, Am. romitii, Rh. microplus, and Rh. pusillus ticks (25, 26). In the present study, three phylogeny-independent Ehrlichia strains were detected. One strain originated from pet dog ticks (Rh. turanicus) from a veterinary clinical hospital in Almaty oblast, the second one was from cattle ticks (Hy. scupense) in Turkistan oblast, and the third one was from horse ticks (Hy. scupense) in Aktobe oblast. The discovery and distribution of Ehrlichia species are closely related to their natural hosts and geographical locations. Expanding the sampling to include more tick species, domestic animals, wildlife, and additional sites will be important for future investigations of Ehrlichia species in Central Asia.
Interestingly, we detected A. ovis, B. caballi, and T. equi in ticks collected from cattle, despite the fact that A. ovis is generally considered to be primarily detected in sheep (27, 28), while B. caballi and T. equi are typically found in horses or equines (29, 30). Domestic animals such as cattle, horses, sheep, and camels are natural hosts for species such as Hy. asiaticum, Hy. scupense, Hy. anatolicum, and Rh. turanicus (31, 32). Occasionally, these animals may also host Argas persicus (23). According to reports, ticks infected with T. equi and A. ovis are unable to directly transmit the pathogens to the offspring, while ticks infected with B. caballi can directly pass it on to the next tick generation (6, 33, 34). Moreover, A. ovis does not have strict host specificity and has been detected in cattle in addition to sheep and goats (35–37), which is consistent with our findings. In this study, T. equi and A. ovis were detected in ticks collected from cattle. This could be due to the ticks migrating to cattle after feeding on infected animals. As for B. caballi, it may be carried by the ticks or the cattle themselves. Regarding these findings, future research may delve deeper into aspects such as expanding the range of hosts for tick – borne diseases, the interaction between hosts and pathogens, and the migration of tick vectors and hosts. Therefore, we speculate that cross-species transmission may have occurred as ticks fed on the blood of different hosts. Such cross-species transmission has the potential to cause unknown diseases or symptoms in new hosts, posing a potential threat to public health and animal welfare. Furthermore, these finding underscores the need for further research into the host range and transmission patterns of these pathogens to better understand their distribution and epidemiology in nature.
In this study, although we could not determine whether these TBPs originated from the engorged ticks or their hosts, we still believe that multiple piroplasms, Anaplasmas, and Ehrlichia exist in Kazakhstan. Due to the lack of more data in Kazakhstan and its neighboring countries (especially in Central Asian countries), the taxonomy of TBPs at the level of species and genotype needs further research.
5 Conclusion
Two species of Babesia (B. occultans and B. caballi), four species of Theileria (T. annulata, T. ovis, T. equi, and T. orientalis), two species of Anaplasma (A. phagocytophilum and A. ovis), and three phylogeny-independent Ehrlichia species were detected in 259 hard ticks and 13 soft ticks in six oblasts in Kazakhstan. The genotype 1 (Chitose) of T. orientalis, genotype B of B. caballi, and genotype A of T. equi were further confirmed. These findings expand the geographical distribution and knowledge of TBPs in Central Asia, especially in Kazakhstan.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/ (Babesia caballi 18S rRNA: PQ056500; Babesia occultans 18S rRNA: PQ056490; Theileria equi 18S rRNA: PQ056499; Theileria annulata 18S rRNA: PQ056488-89; PQ056494-98; Theileria ovis 18S rRNA: PQ056493; Theileria orientalis 18S rRNA: PQ056491; Anaplasma phagocytophilum 16S rRNA: PQ060466-68; PQ060470; Anaplasma ovis 16S rRNA: PQ060469; Ehrlichia species 16S rRNA: PQ483112-14).
Ethics statement
The animal study was approved by the Animal Ethics Committee of Shihezi University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
WZ: Conceptualization, Investigation, Methodology, Writing – original draft. ZK: Investigation, Methodology, Resources, Writing – review & editing. MA: Investigation, Validation, Writing – original draft. SA: Formal analysis, Writing – review & editing. KS: Data curation, Methodology, Writing – review & editing. MY: Data curation, Methodology, Writing – review & editing. YW: Methodology, Writing – review & editing. WH: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by the Kazakhstan Republic of scientific-technical projects for Sustainable development of the agro-industrial complex (AP23489750), 2024-2026уу, the National Natural Science Foundation of China (82260399 and 82260414), National Key Research and Development, the Program of China (2022YFC2304000), the Natural Science Key Project of Xinjiang Uygur Autonomous Region (2022B03014), and the Science & Technology Innovation Team Project of TIANSHAN Elite (2023TSYCTD0020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1533589/full#supplementary-material
APPENDIX TABLE 1
PCR protocol for the detection of ticks specimens and pathogens.
APPENDIX TABLE 2
Data of piroplasm, Anaplasma and Ehrlichia species identified in ticks including 18S rRNA and 16S rRNA genes with NCBI BLAST maximum identity percentages.
APPEND IX TABLE 3
A. phagocytophilum genetic variants of 16S rRNA gene fragments.
Footnotes
References
1. Elhelw, R, Elhariri, M, Hamza, D, Abuowarda, M, Ismael, E, and Farag, H. Evidence of the presence of Borrelia burgdorferi in dogs and associated ticks in Egypt. BMC Vet Res. (2021) 17:1–9. doi: 10.1186/s12917-020-02733-5
2. Otranto, D, Dantas-Torres, F, and Breitschwerdt, EB. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol. (2009) 25:157–63. doi: 10.1016/j.pt.2009.01.003
3. Antunes, S, Rosa, C, Couto, J, Ferrolho, J, and Domingos, A. Deciphering Babesia-vector interactions. Front Cell Infect Microbiol. (2017) 7:429. doi: 10.3389/fcimb.2017.00429
4. Mans, BJ, Pienaar, R, and Latif, AA. A review of Theileria diagnostics and epidemiology. Int J Parasitol Parasites Wildl. (2015) 4:104–18. doi: 10.1016/j.ijppaw.2014.12.006
6. Schnittger, L, Ganzinelli, S, Bhoora, R, Omondi, D, Nijhof, AM, and Florin-Christensen, M. The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: species compilation, molecular phylogeny, and evolutionary insights. Parasitol Res. (2022) 121:1207–45. doi: 10.1007/s00436-022-07424-8
7. Fanelli, A. A historical review of Babesia spp. associated with deer in Europe: Babesia divergens/Babesia divergens-like, Babesia capreoli, Babesia venatorum, Babesia cf. odocoilei. Vet Parasitol. (2021) 294:109433. doi: 10.1016/j.vetpar.2021.109433
8. Kulyaisan, S, Gaukhar, O, Shynybekova, NK, Mukhami, NN, Chervyakova, OV, Yerbol Sultankulova, KT, et al. The prevalence and genetic variants of the CCHF virus circulating among ticks in the southern regions of Kazakhstan. Pathogens. (2022) 11:841. doi: 10.3390/pathogens11080841
9. Dong, Q, Yang, M, Li, F, Jia, Y, Rizabek, K, Kairullayev, K, et al. Spotted fever group rickettsiae in hard ticks in eastern and southern Kazakhstan. Ticks Tick Borne Dis. (2023) 14:102238. doi: 10.1016/j.ttbdis.2023.102238
10. Maukayeva, S, and Karimova, S. Tick-borne encephalitis in Kazakhstan: a case report. J Clin Pract Res. (2020) 42:226. doi: 10.14744/etd.2019.70431
11. Estrada-Peña, A, Bouattour, AJ, Camicas, JL, and Walker, AR. Ticks of domestic animals in the Mediterranean region. Spain: University of Zaragoza (2004). 131 p.
12. Estrada-Peña, A, Mihalca, AD, and Petney, TN. Ticks of Europe and North Africa: a guide to species identification, vol. 404. Switzerland: Springer (2017).
13. Li, E, Wu, X, Tang, L, Yang, M, Hornok, S, Zhang, C, et al. Molecular-phylogenetic analyses of Babesia and Theileria species from small mammals and their ticks in northern China suggest new reservoirs of bovine and equine piroplasms. Vet Parasitol. (2024) 332:110304. doi: 10.1016/j.vetpar.2024.110304
14. Song, R, Wang, Q, Guo, F, Liu, X, Song, S, Chen, C, et al. Detection of Babesia spp., Theileria spp. and Anaplasma ovis in border regions, northwestern China. Transbound Emerg Dis. (2018) 65:1537–44. doi: 10.1111/tbed.12894
15. Wu, X, Xu, J, Su, L, Li, E, Wang, S, Hornok, S, et al. First molecular evidence of Babesia caballi and Theileria equi in imported donkeys from Kyrgyzstan. Pathogens. (2024) 13:713. doi: 10.3390/pathogens13090713
16. Mahlobo, SI, and Zishiri, OT. A descriptive study of parasites detected in ticks of domestic animals in Lesotho. Vet Parasitol Reg Stud Rep. (2021) 25:100611. doi: 10.1016/j.vprsr.2021.100611
17. Dahmana, H, Amanzougaghene, N, Davoust, B, Normand, T, Carette, O, Demoncheaux, JP, et al. Great diversity of Piroplasmida in Equidae in Africa and Europe, including potential new species. Vet Parasitol Reg Stud Rep. (2019) 18:100332. doi: 10.1016/j.vprsr.2019.100332
18. Remesar, S, Castro-Scholten, S, Morrondo, P, Díaz, P, Jiménez-Martín, D, Muñoz-Fernández, L, et al. Occurrence of Anaplasma spp. in wild lagomorphs from southern Spain: molecular detection of new Anaplasma bovis lineages. Res Vet Sci. (2024) 166:105093. doi: 10.1016/j.rvsc.2023.105093
19. Chisu, V, Alberti, A, Zobba, R, Foxi, C, and Masala, G. Molecular characterization and phylogenetic analysis of Babesia and Theileria spp. in ticks from domestic and wild hosts in Sardinia. Acta Trop. (2019) 196:60–5. doi: 10.1016/j.actatropica.2019.05.013
20. Buysse, M, Koual, R, Binetruy, F, de Thoisy, B, Baudrimont, X, Garnier, S, et al. Detection of Anaplasma and Ehrlichia bacteria in humans, wildlife, and ticks in the Amazon rainforest. Nat Commun. (2024) 15:3988. doi: 10.1038/s41467-024-48459-y
21. Kuibagarov, M, Makhamed, R, Zhylkibayev, A, Berdikulov, M, Abdrakhmanov, S, Kozhabayev, M, et al. Theileria and Babesia infection in cattle–first molecular survey in Kazakhstan. Ticks Tick Borne Dis. (2023) 14:102078. doi: 10.1016/j.ttbdis.2022.102078
22. Sang, C, Yang, M, Xu, B, Liu, G, Yang, Y, Kairullayev, K, et al. Tick distribution and detection of Babesia and Theileria species in Eastern and Southern Kazakhstan. Ticks Tick Borne Dis. (2021) 12:101817. doi: 10.1016/j.ttbdis.2021.101817
23. Kim, YJ, Seo, JY, Park, JS, Kim, SY, Aknazarov, B, Atabekova, N, et al. Molecular analysis of tick-borne bacterial pathogens from ticks infesting animal hosts in Kyrgyzstan, 2021. Microorganisms. (2024) 12:1046. doi: 10.3390/microorganisms12061046
24. Li, Y, Li, J, Xieripu, G, Rizk, MA, Macalanda, AM, Gan, L, et al. Molecular detection of Theileria ovis, Anaplasma ovis, and Rickettsia spp. in Rhipicephalus turanicus and Hyalomma anatolicum collected from sheep in Southern Xinjiang, China. Pathogens. (2024) 13:680. doi: 10.3390/pathogens13080680
25. Remesar, S, Castro-Scholten, S, Morrondo, P, Díaz, P, Jiménez-Martín, D, Rouco, C, et al. Molecular detection of Ehrlichia spp. in ticks parasitizing wild lagomorphs from Spain: characterization of a novel Ehrlichia species. Parasit Vectors. (2022) 15:467. doi: 10.1186/s13071-022-05600-4
26. Dye-Braumuller, KC, Lynn, MK, Rivas, PM, Lee, C, Aquino, MS, Chandler, JG, et al. First report of multiple Rickettsia sp., Anaplasma sp., and Ehrlichia sp. in the san Miguel Department of El Salvador from zoonotic tick vectors. Acta Trop. (2023) 242:106909. doi: 10.1016/j.actatropica.2023.106909
27. Shabana, II, Alhadlag, NM, and Zaraket, H. Diagnostic tools of caprine and ovine anaplasmosis: a direct comparative study. BMC Vet Res. (2018) 14:1–8. doi: 10.1186/s12917-018-1489-x
28. Bauer, BU, Răileanu, C, Tauchmann, O, Fischer, S, Ambros, C, Silaghi, C, et al. Anaplasma phagocytophilum and Anaplasma ovis–emerging pathogens in the German sheep population. Pathogens. (2021) 10:1298. doi: 10.3390/pathogens10101298
29. Bělková, T, Bártová, E, Řičařová, D, Jahn, P, Jandová, V, Modrý, D, et al. Theileria equi and Babesia caballi in horses in the Czech Republic. Acta Trop. (2021) 221:105993. doi: 10.1016/j.actatropica.2021.105993
30. Scoles, GA, and Ueti, MW. Vector ecology of equine piroplasmosis. Annu Rev Entomol. (2015) 60:561–80. doi: 10.1146/annurev-ento-010814-021110
31. Zhang, B, Zhang, N, Zheng, T, Lu, M, Baoli, B, Jie, R, et al. Tick-borne bacterial agents in Hyalomma asiaticum ticks from **njiang Uygur autonomous region, Northwest China. Parasit Vectors. (2024) 17:167. doi: 10.1186/s13071-024-06256-y
32. Bakkes, DK, Chitimia-Dobler, L, Matloa, D, Oosthuysen, M, Mumcuoglu, KY, Mans, BJ, et al. Integrative taxonomy and species delimitation of Rhipicephalus turanicus (Acari: Ixodida: Ixodidae). Int J Parasitol. (2020) 50:577–94. doi: 10.1016/j.ijpara.2020.04.005
33. de la Fuente, J, Kocan, KM, Blouin, EF, Zivkovic, Z, Naranjo, V, Almazán, C, et al. Functional genomics and evolution of tick–Anaplasma interactions and vaccine development. Vet Parasitol. (2010) 167:175–86. doi: 10.1016/j.vetpar.2009.09.019
34. Dahmani, M, Davoust, B, Tahir, D, Raoult, D, Fenollar, F, and Mediannikov, O. Molecular investigation and phylogeny of Anaplasmataceae species infecting domestic animals and ticks in Corsica, France. Parasites Vectors. (2017) 10:1–2. doi: 10.1186/s13071-017-2233-2
35. Kadyrova, M, Ostrovskii, A, Mukanov, K, Kassen, A, Shevtsova, E, Berdikulov, M, et al. Molecular characterization of Anaplasma spp. in Cattle from Kazakhstan. Pathogens. (2024) 13:894. doi: 10.3390/pathogens13100894
36. Yan, Y, Jiang, Y, Tao, D, Zhao, A, Qi, M, and Ning, C. Molecular detection of Anaplasma spp. in dairy cattle in southern **njiang, China. Vet Parasitol Reg Stud Rep. (2020) 20:100406. doi: 10.1016/j.vprsr.2020.100406
Keywords: ticks, tick-borne pathogens, morphological identification, genotype, Kazakhstan
Citation: Zeng W, Kairat Z, Awulibieer M, Abylay S, Serik K, Yang M, Wang Y and Hazihan W (2025) Molecular detection of piroplasms, Anaplasma, and Ehrlichia species in Kazakhstan. Front. Vet. Sci. 12:1533589. doi: 10.3389/fvets.2025.1533589
Edited by:
Abdul Jabbar, The University of Melbourne, AustraliaReviewed by:
ThankGod Emmanuel Onyiche, University of Maiduguri, NigeriaGabriela Aguilar Tipacamú, Autonomous University of Queretaro, Mexico
Copyright © 2025 Zeng, Kairat, Awulibieer, Abylay, Serik, Yang, Wang and Hazihan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wurelihazi Hazihan, MTUwODIxNzM2NkBxcS5jb20=; Yuanzhi Wang, d2FuZ3l1YW56aGk2MjFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Weixin Zeng
Weixin Zeng Zhumanov Kairat1†
Zhumanov Kairat1† Yuanzhi Wang
Yuanzhi Wang