- Anicura Kleintierspezialisten Augsburg, Augsburg, Germany
Transthoracic fine-needle aspiration of the lungs (TFNA) combined with positive bacterial culture is used less frequently than airway lavage in diagnosing bacterial pneumonia in dogs and cats. This retrospective study evaluated TFNA results and bacterial culture findings in 14 dogs (1 dog twice) and two cats with bacterial pneumonia. Bacterial culture yielded positive results in 9/16 (56.3%) of samples. Only 1/11 (9.1%) of isolated bacterial species showed resistance to empiric treatment (amoxicillin with clavulanate). Six patients without prior antimicrobial treatment had negative bacterial cultures, despite the presence of intracellular bacteria on cytology samples. For patients with suspected bacterial pneumonia, we should consider performing both cytological examination and bacterial culture of lung aspirates, with careful interpretation of the results. Bacterial culture should be considered regardless of ongoing or previous antibiotic treatment, and even in cases where intracellular bacteria are not identified on cytology. Based on our antimicrobial susceptibility findings, first-line empirical treatment with amoxicillin with clavulanate might be an appropriate choice in most of cases regardless of the previous treatment.
1 Introduction
Bacterial pneumonia in dogs and cats is traditionally diagnosed through a combination of airway lavage cytology showing intracellular bacteria and positive bacterial culture results (1, 2). While bronchoalveolar lavage and transtracheal wash are considered gold standard techniques for airway sampling, transthoracic fine-needle aspiration of the lungs (TFNA) represents an alternative diagnostic approach that so far has received less attention in the literature. Previous studies have demonstrated that TFNA can effectively identify bacterial infections through cytological examination (3, 4).
TFNA offers several potential advantages over airway lavage techniques, including the ability to directly sample affected lung regions, and the option to perform the procedure without general anesthesia. However, the relationship between cytological findings and bacterial culture results from TFNA samples has not been studied extensively in clinical cases of canine or feline bacterial pneumonia.
The primary aim of this retrospective study was to evaluate the diagnostic utility of TFNA by comparing cytological findings with bacterial culture results in dogs and cats suffering from bacterial pneumonia. Additionally, we assessed the antimicrobial susceptibility patterns of isolated bacterial species to provide insights into potential antimicrobial resistance.
2 Materials and methods
2.1 Study design and inclusion criteria
Medical records of dogs and cats diagnosed with bacterial pneumonia between 2009 and 2021 at a small animal referral hospital in Germany were reviewed retrospectively. The cases were included if they met the following criteria: TFNA of the lungs was performed, and bacterial pneumonia was confirmed either by obtaining positive bacterial culture from the aspirate, or by identifying intracellular bacteria on cytology samples. Dual-view thoracic radiographs were performed for all patients. Data collected included patient signalment, prior antimicrobial treatment, clinical signs, cytological findings, results of aerobic and anaerobic bacterial culture with antimicrobial susceptibility testing, and details of antimicrobial treatment (Table 1).
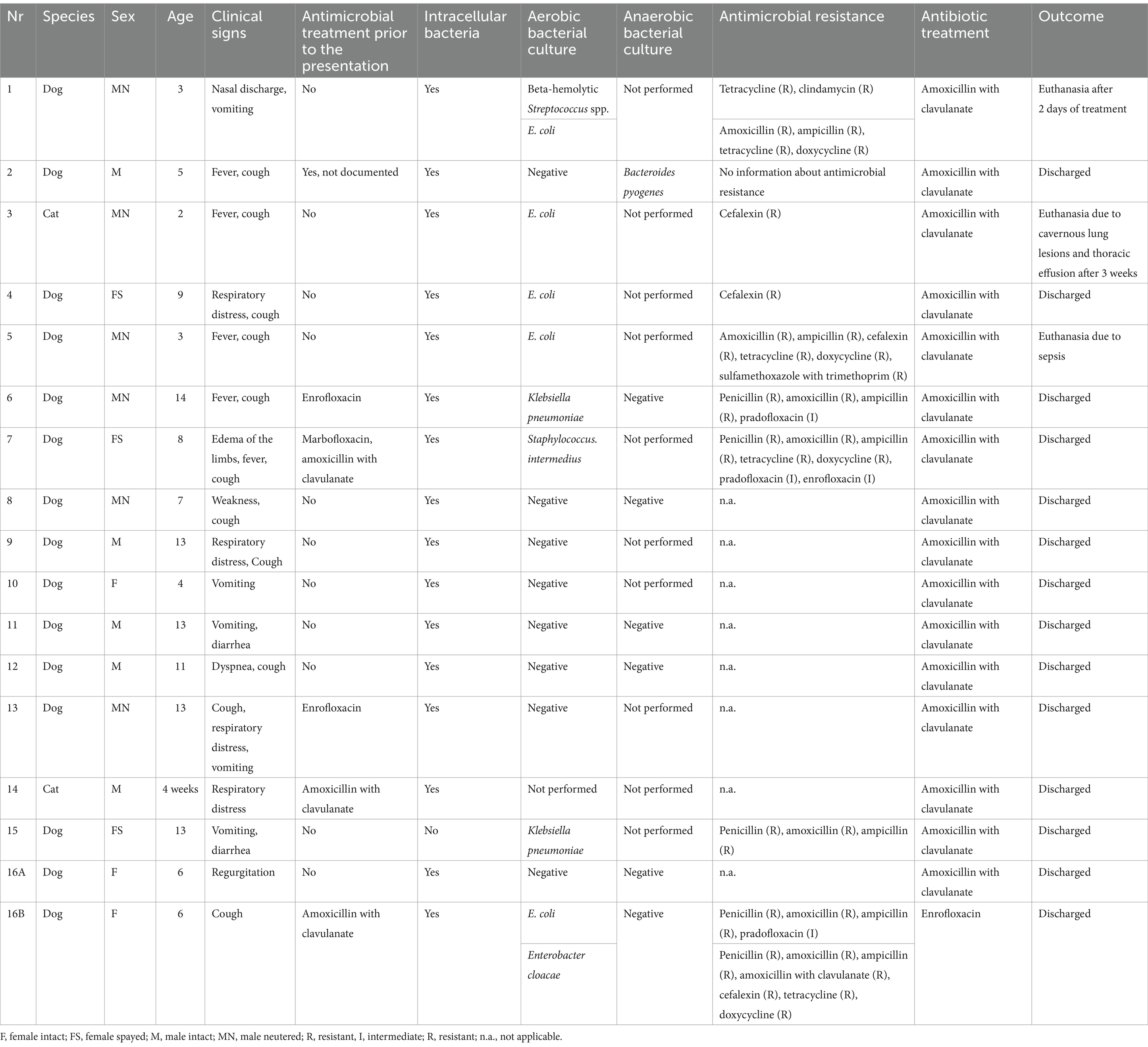
Table 1. Clinical characteristics of the assessed dogs and cats with bacterial pneumonia, including signalment, clinical signs, prior antimicrobial treatment, cytological results, aerobic and anaerobic bacterial culture, in vitro bacterial susceptibility testing results and antimicrobial treatment.
2.2 Transthoracic fine-needle aspiration procedure
TFNA was performed within 6 h of admission. Lesion localization was guided by thoracic radiographs and ultrasound examination. When visible on ultrasound, consolidated lung regions appearing as hypoechoic areas (“shred sign”) adjacent to the pleural surface were targeted. In cases where peripheral lesions were not ultrasonographically visible, TFNA was performed based on radiographic findings. The applied method of the TFNA (blindly performed or ultrasound-guided) was not recorded in all cases. 25G hypodermic needle was used for all aspiration procedures Flow-by oxygen supplementation was provided during the procedure for all the patients. Sedation with butorphanol (Dolorex® 10 mg/mL; MSD; 0.2–0.3 mg/kg IM or IV) was administered only to uncooperative patients. Samples for cytology and bacterial culture were collected from the same anatomical site.
2.3 Sample processing and analysis
Cytological samples were air-dried, stained with Diff-Quick (LABOR+TECHNIK Eberhard Lehmann GmbH, Germany), and evaluated by a board-certified small animal internal medicine specialist. A diagnosis of bacterial pneumonia was made when samples showed increased numbers of neutrophils with intracellular bacteria. Cases with increased neutrophils, but no visible bacteria were classified as purulent inflammation.
2.4 Bacterial culture and antimicrobial susceptibility testing
Samples with increased neutrophils were submitted to the external laboratory (IDEXX Laboratories, Germany) for bacterial culture within 24 h of collection. Both aerobic and anaerobic cultures were performed when sample volume was sufficient. In only 7/17 cases (41%), the TFNA yielded adequate volume for both aerobic and anaerobic bacterial culture procedures. Antimicrobial susceptibility testing was conducted on all bacterial isolates. The exact MIC breakpoints for each testing usually were not provided by the laboratory.
2.5 Post-procedure monitoring
All the animals were monitored in the intensive care unit for at least 24 h following TFNA to assess for potential complications. Clinical outcome (discharge or euthanasia) was recorded for all patients.
3 Results
3.1 Clinical characteristics of dogs and cats under evaluation
The study included 16 animals (14 dogs and 2 cats), with one dog (case nr 16) undergoing TFNA twice, due to recurrent aspiration pneumonia associated with megaesophagus, resulting in 17 samples. The median age was 8.5 years (range: 3–14 years) for the dogs, whereas the two cats were 4 weeks and 2 years old, respectively.
6/17 of samples (35.3%) were obtained from patients that had received prior antimicrobial treatment, including enrofloxacin (n = 2), marbofloxacin with amoxicillin with clavulanate (n = 1), amoxicillin with clavulanate (n = 2) and unknown antimicrobial drug (n = 1). The duration of previous antimicrobial treatment was not documented. The most common clinical presentations included respiratory signs (cough, respiratory distress), gastrointestinal signs (vomiting, regurgitation), and systemic signs (fever). In three dogs aspiration pneumonia associated with megaesophagus (n = 1), surgical arytenoid lateralization (n = 1), and seizures (n = 1) were confirmed. Most patients tolerated TFNA without sedation; only one dog required sedation with butorphanol (0.2 mg/kg IM).
3.2 Cytological examination and bacterial culture results
Cytological examination revealed intracellular bacteria in 16/17 samples (94.1%). Bacterial culture was performed on 16/17 samples (94.1%), with both aerobic and anaerobic cultures conducted in 8/16 cases (50%). Only the sample obtained from 4-week-old kitten was insufficient for performing bacterial culture. 9/16 cultured samples (56.3%) yielded positive results, including the presence of Escherichia coli (n = 5), Klebsiella pneumoniae (n = 2), Staphylococcus intermedius (n = 1), β-hemolytic Streptococcus spp. (n = 1), Enterobacter cloacae (n = 1), and Bacteroides pyogenes (n = 1). 2/9 (22.2%) of positive cultures showed polymicrobial infection. 6/17 of the samples (35.3%) were obtained from patients that had received prior antimicrobial treatment. In 4 of these 6 patients, their bacterial culture results returned positive although they received antimicrobial therapy before the TFNA. The remaining 2 pre-treated patients had either no bacterial culture performed or received a negative result. 6/11 (54.5%) patients without prior antimicrobial treatment had negative bacterial cultures, despite the intracellular bacteria in cytology. Additionally, one sample resulted in a positive culture (Klebsiella pneumoniae) without cytological evidence of bacteria.
3.3 Antimicrobial susceptibility
Antimicrobial susceptibility testing revealed widespread resistance to pure β-lactams (penicillin, amoxicillin, ampicillin) and tetracyclines, as well as frequent resistance to cefalexin. Some isolates showed intermediate susceptibility to fluoroquinolones (pradofloxacin, enrofloxacin). Only 1/11 (9.1%) of the isolates showed resistance to amoxicillin with clavulanate.
3.4 Outcome
Among the 16 animals, 14 (87.5%) were discharged (12 dogs and both cats). Two dogs were euthanized: one shortly after sampling due to severe pneumonia and sepsis, and another one after 2 days of treatment. The 2-year-old cat was readmitted 3 weeks post-discharge with thoracic effusion and cavitary lung lesions (suspected pulmonary abscess). No immediate complications were reported following TFNA procedures in any patient.
4 Discussion
This study demonstrated that TFNA of the lungs is a valuable diagnostic tool for bacterial pneumonia in dogs and cats. In patients with negative bacterial cultures, we frequently observed intracellular bacteria on TFNA cytology. Patients that received antimicrobial therapy before the TFNA, had often positive bacterial culture results.
Bacterial species (Escherichia coli, Staphylococcus intermedius, Streptococcus spp.) isolated in our study are pathogens commonly isolated from dogs and cats with bacterial pneumonia (2, 5–11). Klebsiella pneumoniae, isolated in two of our patients, is likewise commonly isolated (5, 6, 8–11). Isolation of obligate anaerobes like Bacteroides pyogenes in our study highlights the importance of anaerobic culture. One study reported that from the samples obtained from dogs with suspected lower respiratory tract disease, 21.6% were obligate anaerobes, with Bacteroides species being among the most common anaerobic isolates (9). However, since only half of our samples underwent anaerobic culture, we may have underestimated the true prevalence of anaerobic infections in our patients. Our study did not isolate Bordetella bronchiseptica, which differs from findings in other studies (5–7, 9–12). The reason for this could be performing TFNA in patients with bacterial pneumonia instead of bronchoalveolar lavage or tracheal wash reported in the literature.
We found polymicrobial infections in 2/17 (11.8%) samples, in dogs with either confirmed or suspected aspiration pneumonia. Polymicrobial infections were reported frequently in other studies (6, 7, 9–12) but could not be linked solely to aspiration pneumonia. While six of our patients presented with vomiting or regurgitation, aspiration could be confirmed in only three cases. Interestingly, four of the remaining cases yielded single bacterial isolates, which may have made aspiration pneumonia less likely, though did not exclude it at the same time.
The discrepancies we observed between cytology and bacterial culture results (negative cultures with positive cytology and vice versa) have been reported previously, though are relatively uncommon (2, 6) and thus should be interpreted carefully due to possible contamination. However, the study in experimentally infected guinea pigs showed that bacterial culture could identify the infectious agent in only 45.5% of confirmed cases, suggesting that cytological examination positive for bacteria should not be dismissed as sample contamination when bacterial culture is negative (13).
The discrepancy between cytology and bacterial culture could be caused by several factors. Firstly, bacterial growth could be compromised by prior antimicrobial treatment, or the causative agent could be difficult to culture. Secondly, separate aspirations for cytology and culture could sample different areas of the lungs. Thirdly, culture cannot differentiate between intra-and extracellular bacteria, whereas our cytological criterion for bacterial pneumonia was the presence of intracellular bacteria. Lastly, although samples were taken aseptically, there was always some risk of contamination with opportunistic pathogens.
The 35.3% incidence of prior antimicrobial treatment in our population resulted in relatively low bacterial resistance (9.1%), compared to another study reporting 57.4% resistance to previously administered antimicrobials (5). The substantial discrepancy in antimicrobial resistance could be caused by different bacterial susceptibility patterns in North America and Europe. This discrepancy could result from the differences in antibiotic use in these geographic regions. However, studies in human medicine did not confirm increased antimicrobial resistance due to increased antibiotic use (14). We observed minimal bacterial resistance to amoxicillin-clavulanate. Although the study (15) showed a good clinical response to this antimicrobial drug in dogs with aspiration pneumonia, it should be noted that no bacterial culture was performed, so antimicrobial susceptibility was not evaluated. It is important to emphasize that our sample of 11 isolates may be insufficient to formulate general recommendations for an empirical treatment plan.
Study limitations include its retrospective nature with incomplete documentation of prior treatment, small sample size, potentially introducing bias, and inconsistent use of anaerobic culture potentially underestimating anaerobic infections.
While, as demonstrated in several studies (5–12), bronchoalveolar lavage and transtracheal wash are considered gold standard techniques for airway sampling in respiratory infections, TFNA may offer a quick and low-cost diagnostic procedure in unstable patients. In our study, TFNA provided diagnostic samples adequate for both cytology and culture in most of the cases. The only case where bacterial culture was not performed was a 4-week old kitten, with sample size being insufficient for both procedures. What is important, our study showed that TFNA can yield clinically relevant culture and susceptibility results to guide antimicrobial therapy when airway lavage may not be available. However, larger comparative studies are still needed to fully establish the diagnostic utility of these different sampling techniques.
5 Conclusion
This study demonstrated that TFNA is an effective diagnostic tool for bacterial pneumonia in dogs and cats. For patients with suspected bacterial pneumonia, we should consider performing both cytological examination and bacterial culture of lung aspirates, with careful interpretation of the results. Bacterial culture should be considered regardless of ongoing or previous antibiotic treatment, and even in cases where intracellular bacteria are not identified on cytology. Based on our antimicrobial susceptibility findings, first-line empirical treatment with amoxicillin with clavulanate might be an appropriate choice in most of cases regardless of the previous treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving animals in accordance with the local legislation and institutional requirements because it was a retrospective study and only gathered data were analyzed. Written informed consent was not obtained from the owners for the participation of their animals in this study because it was a retrospective study and only gathered data were analyzed.
Author contributions
AP: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. CS: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. We acknowledge the use of Claude 3.5 Sonnet (New) (Anthropic, https://claude.ai/new) to proofread our final draft.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cohn, L. Pulmonary parenchymal disease In: SJ Ettinger and EC Feldman, editors. Textbook of veterinary internal medicine. 8th ed. St. Louis, MO: Elsevier Saunders (2017). 1108–31.
2. Lappin, MR, Blondeau, J, Boothe, D, Breitschwerdt, EB, Guardabassi, L, Lloyd, DH, et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines working Group of the International Society for companion animal infectious diseases. J Vet Intern Med. (2017) 31:279–94. doi: 10.1111/jvim.14627
3. Wood, EF, O'Brien, RT, and Young, KM. Ultrasound-guided fine-needle aspiration of focal parenchymal lesions of the lung in dogs and cats. J Vet Intern Med. (1998) 12:338–42. doi: 10.1111/j.1939-1676.1998.tb02132.x
4. DeBerry, JD, Norris, CR, Samii, VF, Griffey, SM, and Almy, FS. Correlation between fine-needle aspiration cytopathology and histopathology of the lung in dogs and cats. J Am Anim Hosp Assoc. (2002) 38:327–36. doi: 10.5326/0380327
5. Proulx, A, Hume, DZ, Drobatz, KJ, and Reineke, EL. In vitro bacterial isolate susceptibility to empirically selected antimicrobials in 111 dogs with bacterial pneumonia. J Vet Emerg Crit Care (San Antonio). (2014) 24:194–200. doi: 10.1111/vec.12128
6. Johnson, LR, Queen, EV, Vernau, W, Sykes, JE, and Byrne, BA. Microbiologic and cytologic assessment of bronchoalveolar lavage fluid from dogs with lower respiratory tract infection: 105 cases (2001-2011). J Vet Intern Med. (2013) 27:259–67. doi: 10.1111/jvim.12037
7. Peeters, DE, McKiernan, BC, Weisiger, RM, Schaeffer, DJ, and Clercx, C. Quantitative bacterial cultures and cytological examination of bronchoalveolar lavage specimens in dogs. J Vet Intern Med. (2000) 14:534–41. doi: 10.1111/j.1939-1676.2000.tb02272.x
8. Dear, JD. Bacterial pneumonia in dogs and cats: an update. Vet Clin North Am Small Anim Pract. (2020) 50:447–65. doi: 10.1016/j.cvsm.2019.10.007
9. Angus, JC, Jang, SS, and Hirsh, DC. Microbiological study of transtracheal aspirates from dogs with suspected lower respiratory tract disease: 264 cases (1989-1995). J Am Vet Med Assoc. (1997) 210:55–8. doi: 10.2460/javma.1997.210.01.55
10. Lebastard, M, Beurlet-Lafarge, S, Gomes, E, and Le Boedec, K. Association between quantitative bacterial culture of bronchoalveolar lavage fluid and antibiotic requirement in dogs with lower respiratory tract signs. J Vet Intern Med. (2022) 36:1444–53. doi: 10.1111/jvim.16456
11. Radhakrishnan, A, Drobatz, KJ, Culp, WTN, and King, LG. Community-acquired infectious pneumonia in puppies: 65 cases (1993-2002). J Am Vet Med Assoc. (2007) 230:1493–7. doi: 10.2460/javma.230.10.1493
12. Foster, SF, and Martin, P. Lower respiratory tract infections in cats: reaching beyond empirical therapy. J Feline Med Surg. (2011) 13:313–32. doi: 10.1016/j.jfms.2011.03.009
13. Kohno, S, Watanabe, K, Hamamoto, A, Dotsu, Y, Koga, H, Hayashi, T, et al. Transthoracic needle aspiration of the lung in respiratory infections. Tohoku J Exp Med. (1989) 158:227–35. doi: 10.1620/tjem.158.227
14. Veloo, ACM, Tokman, HB, Jean-Pierre, H, Dumont, Y, Jeverica, S, Lienhard, R, et al. Antimicrobial susceptibility profiles of anaerobic bacteria, isolated from human clinical specimens, within different European and surrounding countries. A joint ESGAI study. Anaerobe. (2020) 61:102111. doi: 10.1016/j.anaerobe.2019.102111
15. Fernandes Rodrigues, N, Giraud, L, Bolen, G, Fastrès, A, Clercx, C, Gommeren, K, et al. Antimicrobial discontinuation in dogs with acute aspiration pneumonia based on clinical improvement and normalization of C-reactive protein concentration. J Vet Intern Med. (2022) 36:1082–8. doi: 10.1111/jvim.16405
Keywords: pulmonary FNA, transthoracic FNA, bacterial pneumonia, bacterial infection, pneumonia
Citation: Preibisz A and Schwedes CS (2025) Transthoracic fine-needle aspiration of the lungs, and bacterial culture with antimicrobial susceptibility testing used in diagnosing bacterial pneumonia in dogs and cats: 14 dogs and 2 cats (2009–2021). Front. Vet. Sci. 12:1521793. doi: 10.3389/fvets.2025.1521793
Edited by:
Rachel Jean Derscheid, Iowa State University, United StatesReviewed by:
Dong Chan Moon, Korea National Institute of Health, Republic of KoreaJennifer Granick, University of Minnesota Twin Cities, United States
Copyright © 2025 Preibisz and Schwedes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aleksandra Preibisz, YWxla3NhbmRyYS5wcmVpYmlzekBnbWFpbC5jb20=
 Aleksandra Preibisz
Aleksandra Preibisz Claudia Sabine Schwedes
Claudia Sabine Schwedes